Abstract
Intravenous thrombolysis is a standard of care treatment for patients with acute ischemic stroke. Tissue plasminogen activator (tPA) has been the main thrombolytic agent used since the publication of the seminal National Institutes of Neurological Disorders and Stroke trial in 1995. There is now mounting evidence to support the routine use of Tenecteplase (TNK) to treat acute ischemic stroke. TNK is a genetically modified tPA with higher fibrin specificity, longer half‐life, and reduced systemic coagulopathy. In this illustrated review, we compare the indications, doses, mechanisms of action, efficacy and safety of TNK and tPA. We provide an overview of published clinical trials studying TNK in acute ischemic stroke, including dose‐escalation studies and head‐to‐head comparisons with tPA. Finally, we summarize current acute stroke guideline recommendations and suggest treatment algorithms to manage the two main complications of intravenous thrombolysis: symptomatic intracerebral hemorrhage and angioedema.
Keywords: fibrinolytic agents, hemorrhagic stroke, pharmacological mechanisms of action, stroke, treatment outcome
Essentials.
Thrombolysis, traditionally with Alteplase, is a standard of care treatment for ischemic stroke.
Tenecteplase is a genetically modified version of Alteplase.
Tenecteplase has theoretical benefits over Alteplase: better recanalization and reduced bleeding.
Growing evidence support the routine use of Tenecteplase 0.25mg/kg for acute ischemic stroke.

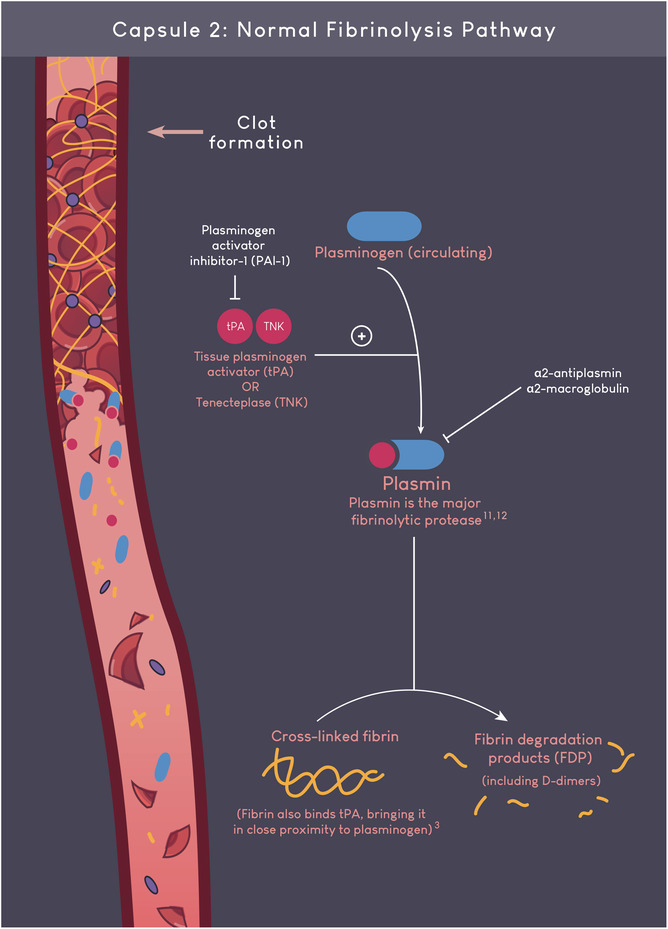
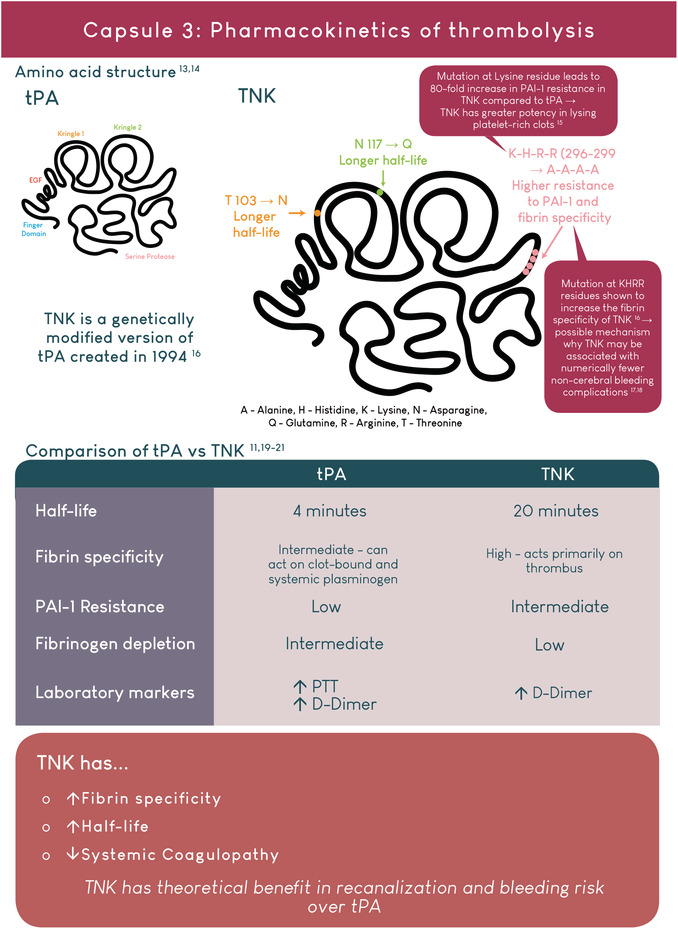

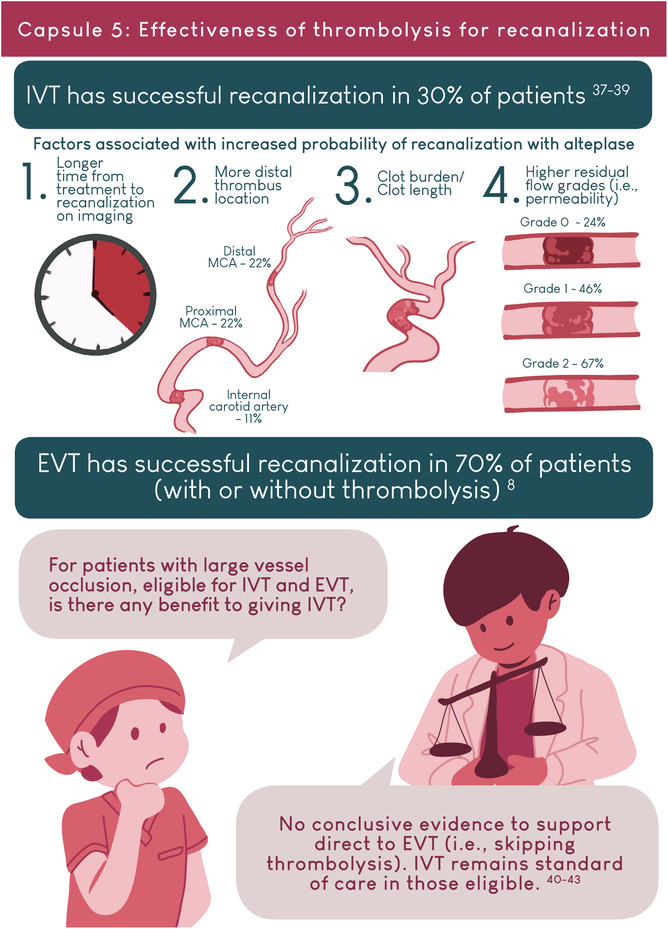
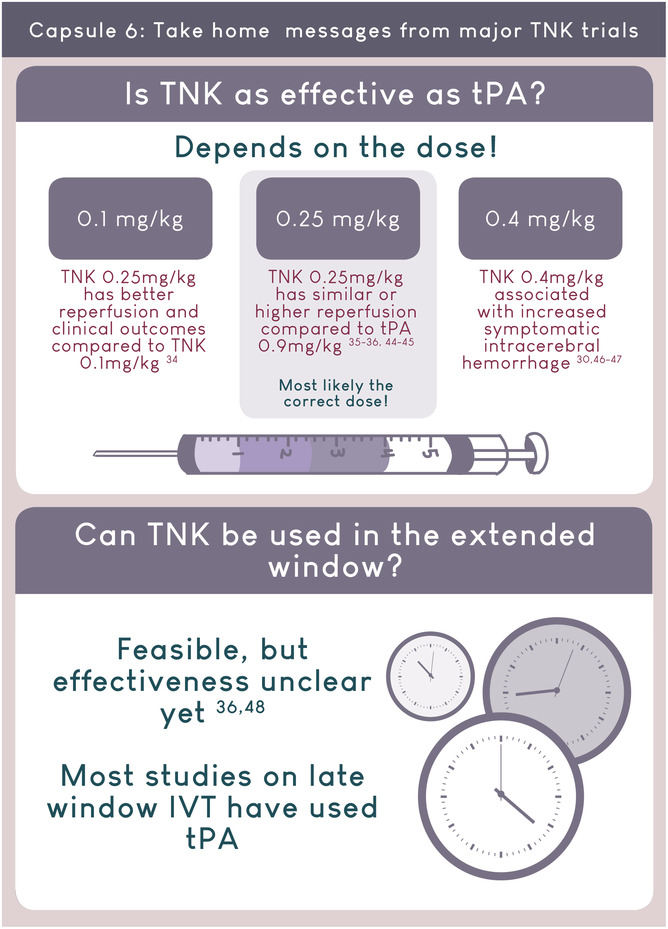



AUTHOR CONTRIBUTIONS
A.Z. contributed to the study design, manuscript drafting, and illustration. P.R. contributed to the study design and manuscript drafting. E.T. contributed to the study design and manuscript revision. S.B.C. contributed to the study design and manuscript revision. A.Y.X.Y. contributed to the study design, manuscript drafting and revision, and supervision.
FUNDING INFORMATION
A.Y. holds a National New Investigator Award from the Heart & Stroke Foundation of Canada.
RELATIONSHIP DISCLOSURE
S.B.C. is the principal investigator of the TEMPO‐2 trial, which is assessing the use of tenecteplase in the treatment of minor stroke. TEMPO‐2 is funded by CIHR and the study drug is provided by Boehringer Ingelheim.
Zhu A, Rajendram P, Tseng E, Coutts SB, Yu AYX. Alteplase or tenecteplase for thrombolysis in ischemic stroke: An illustrated review. Res Pract Thromb Haemost. 2022;6:e12795. doi: 10.1002/rth2.12795
Annie Zhu and Phavalan Rajendram contributed equally to this work
Handling Editor: Dr Suzanne Cannegieter
Contributor Information
Annie Zhu, @cazezhu.
Phavalan Rajendram, @nerdybraindoc.
Eric Tseng, @tsengeric.
Shelagh B. Coutts, @SCouttsMD.
Amy Y. X. Yu, Email: amyyx.yu@utoronto.ca, @amyyu_md.
REFERENCES
- 1. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990‐2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke Statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254‐e743. [DOI] [PubMed] [Google Scholar]
- 4. NINDS and Stroke rt‐PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt‐PA stroke study group. N Engl J Med. 1995;333(24):1581‐1587. [DOI] [PubMed] [Google Scholar]
- 5. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317‐1329. [DOI] [PubMed] [Google Scholar]
- 6. Coutts SB, Berge E, Campbell BC, Muir KW, Parsons MW. Tenecteplase for the treatment of acute ischemic stroke: a review of completed and ongoing randomized controlled trials. Int J Stroke. 2018;13(9):885‐892. [DOI] [PubMed] [Google Scholar]
- 7. Thomalla G, Boutitie F, Ma H, et al. Intravenous alteplase for stroke with unknown time of onset guided by advanced imaging: systematic review and meta‐analysis of individual patient data. Lancet. 2020;396(10262):1574‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723‐1731. [DOI] [PubMed] [Google Scholar]
- 9. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 10. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baker WF Jr. Thrombolytic therapy. Clin Appl Thromb Hemost. 2002;8:291‐314. [DOI] [PubMed] [Google Scholar]
- 12. Cesarman‐Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307‐321. [DOI] [PubMed] [Google Scholar]
- 13. Behrouz R. Intravenous tenecteplase in acute ischemic stroke: an updated review. J Neurol. 2014. Jun;261(6):1069‐1072. [DOI] [PubMed] [Google Scholar]
- 14. Tanswell P, Modi N, Combs D, Danays T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41(15):1229‐1245. [DOI] [PubMed] [Google Scholar]
- 15. Keyt BA, Paoni NF, Refino CJ, et al. A faster‐acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci U S A. 1994;91(9):3670‐3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paoni NF, Chow AM, Peña LC, Keyt BA, Zoller MJ, Bennett WF. Making tissue‐type plasminogen activator more fibrin specific. Protein Eng. 1993;6(5):529‐534. [DOI] [PubMed] [Google Scholar]
- 17. Davydov L, Cheng JW. Tenecteplase: a review. Clin Ther. 2001;23(7):982‐997. [DOI] [PubMed] [Google Scholar]
- 18. Huang X, MacIsaac R, Thompson JL, et al. Tenecteplase versus alteplase in stroke thrombolysis: an individual patient data meta‐analysis of randomized controlled trials. Int J Stroke. 2016;11(5):534‐543. [DOI] [PubMed] [Google Scholar]
- 19. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543‐3546. [DOI] [PubMed] [Google Scholar]
- 20. Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (nor‐test): a phase 3, randomised, open‐label, blinded endpoint trial. Lancet Neurol. 2017;16:781‐788. [DOI] [PubMed] [Google Scholar]
- 21. Tsikouris JP, Tsikouris AP. A review of available fibrin‐specific thrombolytic agents used in acute myocardial infarction. Pharmacotherapy: the journal of human pharmacology and drug. Therapy. 2001;21(2):207‐217. [DOI] [PubMed] [Google Scholar]
- 22. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2019;50:e344‐e418. [DOI] [PubMed] [Google Scholar]
- 23. Boulanger JM, Lindsay MP, Gubitz G, et al. Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6th edition, update 2018. Int J Stroke. 2018;13:949‐984. [DOI] [PubMed] [Google Scholar]
- 24. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I‐LXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minematsu K, Toyoda K, Hirano T, et al. Guidelines for the intravenous application of recombinant tissue‐type plasminogen activator (alteplase), the second edition, october 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc Dis. 2013;22:571‐600. [DOI] [PubMed] [Google Scholar]
- 26. Yamaguchi T, Mori E, Minematsu K, et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J‐ACT). Stroke. 2006;37:1810‐1815. [DOI] [PubMed] [Google Scholar]
- 27. Mori E, Minematsu K, Nakagawara J, et al. Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J‐ACT II). Stroke. 2010;41:461‐465. [DOI] [PubMed] [Google Scholar]
- 28. Nakagawara J, Minematsu K, Okada Y, et al. Thrombolysis with 0.6 mg/kg intravenous alteplase for acute ischemic stroke in routine clinical practice: the Japan post‐marketing alteplase registration study (J‐MARS). Stroke. 2010;41:1984‐1989. [DOI] [PubMed] [Google Scholar]
- 29. Toyoda K, Koga M, Naganuma M, et al. Routine use of intravenous low‐dose recombinant tissue plasminogen activator in japanese patients: general outcomes and prognostic factors from the samurai register. Stroke. 2009;40:3591‐3595. [DOI] [PubMed] [Google Scholar]
- 30. Haley EC Jr, Thompson JL, Grotta JC, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke. 2010;41:707‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haley EC Jr, Lyden PD, Johnston KC, Hemmen TM, Investigators TNKiS . A pilot dose‐escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005;36:607‐612. [DOI] [PubMed] [Google Scholar]
- 32. Coutts SB, Dubuc V, Mandzia J, et al. Tenecteplase‐tissue‐type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke. 2015;46:769‐774. [DOI] [PubMed] [Google Scholar]
- 33. Campbell BC, Mitchell PJ, Churilov L, et al. Determining the optimal dose of tenecteplase before endovascular therapy for ischemic stroke (EXTEND‐IA TNK Part 2): a multicenter, randomized, controlled study. Int J Stroke. 2020;15:567‐572. [DOI] [PubMed] [Google Scholar]
- 34. Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366:1099‐1107. [DOI] [PubMed] [Google Scholar]
- 35. Huang X, Cheripelli BK, Lloyd SM, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open‐label, blinded endpoint study. Lancet Neurol. 2015;14:368‐376. [DOI] [PubMed] [Google Scholar]
- 36. Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378:1573‐1582. [DOI] [PubMed] [Google Scholar]
- 37. Menon BK, Al‐Ajlan FS, Najm M, et al. Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA. 2018;320:1017‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riedel CH, Zimmermann P, Jensen‐Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intrave‐nous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775‐1777. doi: 10.1161/STROKEAHA.110.609693 [DOI] [PubMed] [Google Scholar]
- 39. Shobha N, Bal S, Boyko M, et al. Measurement of length of hyperdense MCA sign in acute ischemic stroke predicts disappearance after IV tPA. J Neuroimaging. 2014;24:7‐10. doi: 10.1111/j.1552-6569.2012.00761.x [DOI] [PubMed] [Google Scholar]
- 40. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382:1981‐1993. [DOI] [PubMed] [Google Scholar]
- 41. Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the skip randomized clinical trial. JAMA. 2021;325:244‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325:234‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. LeCouffe NE, Kappelhof M, Treurniet KM, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385:1833‐1844. [DOI] [PubMed] [Google Scholar]
- 44. Bivard A, Zhao H, Churilov L, et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile stroke unit (TASTE‐A): a phase 2, randomised, open‐label trial. Lancet Neurol. 2022;21:520‐527. [DOI] [PubMed] [Google Scholar]
- 45. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open‐label, registry‐linked, randomised, controlled, non‐inferiority trial. Lancet. 2022;400:161‐169. [DOI] [PubMed] [Google Scholar]
- 46. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND‐IA TNK Part 2 randomized clinical trial. JAMA. 2020;323:1257‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR‐TEST 2, Part A): a phase 3, randomised, open‐label, blinded endpoint, non‐inferiority trial. Lancet Neurol. 2022;21:511‐519. [DOI] [PubMed] [Google Scholar]
- 48. Parsons MW, Miteff F, Bateman GA, et al. Acute ischemic stroke: imaging‐guided tenecteplase treatment in an extended time window. Neurology. 2009;72:915‐921. [DOI] [PubMed] [Google Scholar]
- 49. Stroke Foundation . Clinical guidelines for stroke management 2017. (Chapter 3 of 8: Acute medical and surgical management). Melbourne Australia. 2021. Available from: informme.org.au/Guidelines/Clinical‐Guidelines‐for‐Stroke‐Management. Accessed September 5, 2022.
- 50. NINDS t‐PA Stroke Study Group . Intracerebral hemorrhage after intravenous t‐PA therapy for ischemic stroke. Stroke. 1997;28(11):2109‐2118. [DOI] [PubMed] [Google Scholar]
- 51. Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 52. Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e343‐e361. [DOI] [PubMed] [Google Scholar]
- 53. Zhong CS, Beharry J, Salazar D, et al. Routine use of Tenecteplase for thrombolysis in acute ischemic stroke. Stroke. 2021;52(3):1087‐1090. [DOI] [PubMed] [Google Scholar]
- 54. Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): an observational study. Lancet. 2007;369(9558):275‐282. [DOI] [PubMed] [Google Scholar]
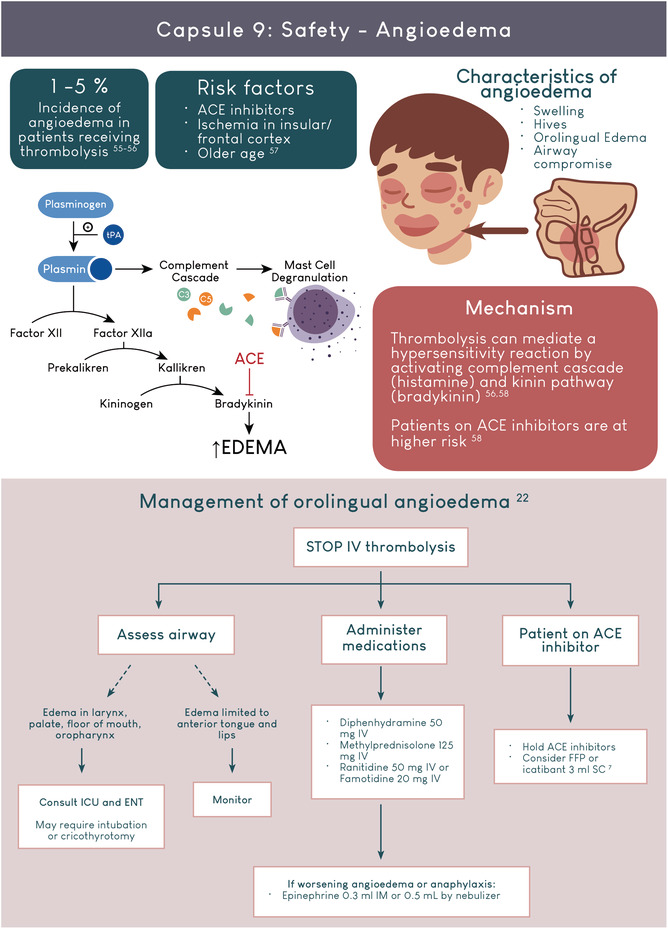
- 55. Pahs L, Droege C, Kneale H, Pancioli A. A novel approach to the treatment of orolingual angioedema after tissue plasminogen activator administration. Ann Emerg Med. 2016;68(3):345‐348. [DOI] [PubMed] [Google Scholar]
- 56. Alakbarzade V, O'Kane D, Pereira AC. Hypersensitivity reactions to recombinant tissue plasminogen activator. Pract Neurol. 2020;20:75‐79. [DOI] [PubMed] [Google Scholar]
- 57. O'Carroll CB, Aguilar MI. Management of postthrombolysis hemorrhagic and orolingual angioedema complications. Neurohospitalist. 2015;5:133‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hill MD, Lye T, Moss H, et al. Hemi‐orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525‐1527. [DOI] [PubMed] [Google Scholar]


