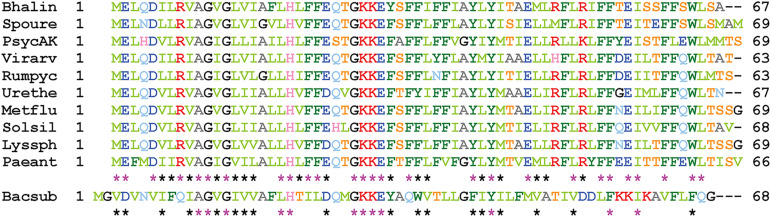ABSTRACT
Sporulation in Firmicutes starts with the formation of two adjacent cells and proceeds with the engulfment of the smaller one, the forespore, by the larger one, the mother cell. This critical step involves a core set of conserved genes, some transcribed in the forespore, such as spoIIQ, and others transcribed in the mother cell, such as the eight-gene spoIIIA operon. A model has been proposed in which the SpoIIIA and the SpoIIQ proteins form a channel connecting the mother cell and the forespore, playing the role of a secretion apparatus allowing the mother cell to nurture the fully engulfed forespore. Exploration of the genomes of Caryophanaceae and Erysipelotrichales has provided informations that are not fully congruent with data from Bacillaceae or Clostridia. The differences observed are correlated with specific physiological features, and alternate, not mutually exclusive views of the function of the SpoIIIA-SpoIIQ complex are presented.
KEYWORDS: engulfment, membrane channel proteins, sporulation
INTRODUCTION
Sporulation in Bacillus subtilis converts a vegetatively growing bacterium into a dormant spore through successive morphological steps and involves hundreds of genes being activated in a precisely timed cascade (1). The first recognizable feature is the formation of a septum at a polar position, creating two cells of unequal size. This step is designated stage II. Next, the larger cell, the mother cell, engulfs the smaller one, the forespore, ultimately isolating it from the external medium. This step is designated stage III. When fully mature, the spore is released into the external medium by lysis of the mother cell.
Since B. subtilis is accessible to classical and reverse genetics, it has been the subject of intense studies aiming at identifying the functions of the various genes involved in the sporulation process. Cloning and sequencing spo genes, whose inactivation interrupts the sporulation morphological program, provided an answer in some cases, such as for spoIIGB/sigE (2), spoIIAC/sigF (3, 4), and spoIIIG/sigG (5, 6), the genes encoding the new sigma factors controlling transcription around stage II and stage III of sporulation (SigF and then SigG in the forespore, and SigE in the mother cell). Sometimes the function, even if already inferred, was demonstrated only years later, as for spoIIGA (7), which encodes the protease activating SigE (8), or spoIIP (9), which encodes an enzyme with both endopeptidase and amidase activities required during engulfment (10, 11). A reverse approach, looking for genes under the control of SigF, led to the identification of loci playing a major role in sporulation but where no mutations had previously been isolated: spoIIR (12, 13), whose product is required for activation of SpoIIGA and therefore of SigE, and spoIIQ (14), which encodes a protein contributing to engulfment from the forespore side.
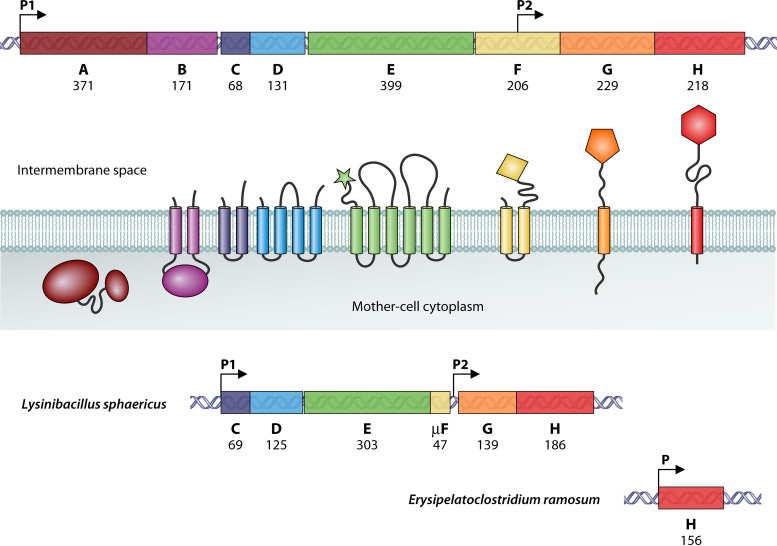
The spoIIIA locus had been noted as harboring a disproportionate number of mutations, suggesting the possible existence of several genes (15). The spoIIIA mutants are able to complete engulfment, but the isolated forespores are often collapsed, a phenotype quite distinct from that of the spoIIIG mutants (6, 15, 16). In order to unravel the function of the spoIIIA product(s), the entire locus was cloned and sequenced by Anne-Marie Guérout-Fleury in our laboratory, and the results were reported at the Spores meeting in May 1992 and summarized in the following book (17). The complete sequence was deposited in GenBank in September 1995 under accession number U35252. As shown in Fig. 1, the spoIIIA locus is an operon of eight cistrons, from spoIIIAA to spoIIIAH. In-frame deletions were created in each of the eight cistrons, and the mutant strains were brought to the Losick laboratory in the summer of 1992 and have since been widely distributed and used, although their origin has fallen into oblivion. Transcription of spoIIIA occurs in the mother cell under the control of SigE (17, 18).
FIG 1.
Genetic organization of the spoIIIA locus in various spore formers. The upper part of the figure shows the spoIIIA operon of B. subtilis. Its eight cistrons are differently colored, and the number of amino acids of their products is indicated. The color-coded SpoIIIA proteins are schematically represented in relation to the mother cell membrane in the middle of the figure. The bottom part of the figure shows the truncated spoIIIA loci found in Caryophanaceae (Lysinibacillus sphaericus) and in Erysipelotrichales (Erysipelatoclostridium ramosum). Arrows indicate the promoters. The operons are drawn to scale.
The sequences of the spoIIIA products did not provide clear-cut clues to their function, apart from suggesting a membrane localization for seven of them. A proper publication was constantly postponed in the hope that new data would shed light on the physiological role of spoIIIA. It required some 15 years and fresh eyes to discover hidden in the SpoIIIAH sequence a pattern shared with a family of pore-forming proteins (19). Building on the previous demonstration of a direct interaction between SpoIIIAH, from the mother cell side, and SpoIIQ, from the forespore side (20, 21), a model was proposed in which the SpoIIIA and the SpoIIQ proteins form a channel connecting the mother cell and the forespore, playing the role of a secretion apparatus allowing the mother cell to nurture the fully engulfed forespore (16, 22, 23). Additional compatible structural data, reviewed recently (24, 25), have contributed to make this model widely accepted.
Because of such a remarkable role, the spoIIIA operon was expected to be present in all spore-forming Bacilli and Clostridia, as confirmed in 2012 (26). In addition, spoIIIAE was chosen as one of the genes whose absence strongly suggested the inability of mycobacteria to produce endospores (27). However, the architecture of the whole complex in the spore membranes associating SpoIIQ and the eight SpoIIIA proteins remains elusive. The molecules transported through the proposed channel are still unidentified. Hundreds of genomes of spore formers are now available. Mining this wealth of data could provide answers that a single sequence could not 30 years ago, identifying the features strictly conserved and presumably essential for the function of the complex, correlating potential changes with specific physiological traits, and searching for covariations that could point to contacts between interacting partners. These were the goals of the work reported here.
THE CANONICAL spoIIIA OPERON, ITS PRODUCTS, AND THEIR PARTNERS
The B. subtilis spoIIIA operon as shown in Fig. 1 is representative of the spoIIIA operon found in Bacillales and Clostridia (Table 1). The eight cistrons, henceforth called A through H for simplicity, are tightly arranged with apparent translational coupling in all cases. A ribosome binding site overlaps or immediately follows the upstream cistron, whereas the B and G reading frames start inside the A and F cistrons, respectively. This specific arrangement was conserved in all constructs designed for complementing the in-frame deletions that were built in each cistron. For instance, the first codons of A, together with its upstream promoter, were fused in frame to the last codons of E, followed by a complete F cistron, and the resulting plasmid used to complement the F mutant. All in-frame deletion mutants were asporogenous, and introducing the proper construct by double recombination at an ectopic locus fully restored sporulation in all mutants, including the H mutant.
TABLE 1.
Presence of the spoIIIA products and their SpoIIQ/GerM partners in various spore formersa
| Phylum | Class | Order | Family | Genus | Species | NCBI txid | Proteins present |
|---|---|---|---|---|---|---|---|
| Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | B. subtilis | 224308 | A B C D E F G H Q M |
| Caryophanales | Caryophanaceae | Bhargavaea | B. lindanitolerans | 550447 | ■ ■ C D E * G H Q ■ | ||
| B. ginsengi | 426757 | ■ ■ C D E * G H Q ■ | |||||
| Sporosarcina | S. ureae | 1571 | ■ ■ C D E * G H Q ■ | ||||
| S. psychrophila | 1476 | ■ ■ C D E * G H Q ■ | |||||
| S. ureilytica | 1421 | ■ ■ C D E * G H Q ■ | |||||
| Bacillus sp. OxB-1 | 98228 | ■ ■ C D E * G H Q ■ | |||||
| Psychrobacillus | P. glaciei | 2283160 | ■ ■ C D E * G H Q ■ | ||||
| Psychrobacillus sp. AK1817 | 2303505 | ■ ■ C D E * G H Q ■ | |||||
| Viridibacillus | V. arvi | 263475 | ■ ■ C D E * G H Q ■ | ||||
| Viridibacillus sp. JNUCC-6 | 2779527 | ■ ■ C D E * G H Q ■ | |||||
| Rummeliibacillus | R. stabekisii | 241244 | ■ ■ C D E * G H Q ■ | ||||
| R. pycnus | 101070 | ■ ■ C D E * G H Q ■ | |||||
| Ureibacillus | U. thermosphaericus | 51173 | ■ ■ C D E * G H Q ■ | ||||
| Metasolibacillus | M. fluoroglycofenilyticus | 1239396 | ■ ■ C D E * G H Q ■ | ||||
| Solibacillus | S. silvestris | 76853 | ■ ■ C D E * G H Q ■ | ||||
| Lysinibacillus | L. fusiformis | 28031 | ■ ■ C D E * G H Q ■ | ||||
| L. sphaericus | 1421 | ■ ■ C D E * G H Q ■ | |||||
| Paenisporosarcina | P. antarctica | 417367 | ■ ■ C D E * G H Q ■ | ||||
| Erysipelotrichia | Erysipelotrichales | Erysipelatoclostridiaceae | Beduini | B. massiliensis | 1585974 | ■ ■ ■ ■ ■ ■ ■ H Q M | |
| Coprobacillus | C. cateniformis | 100884 | ■ ■ ■ ■ ■ ■ ■ H Q M | ||||
| Faecalibacillus | F. intestinalis | 1982626 | ■ ■ ■ ■ ■ ■ ■ H Q M | ||||
| Erysipelatoclostridium | E. spiroforme | 29348 | ■ ■ ■ ■ ■ ■ ■ H Q M | ||||
| E. ramosum | 445974 | ■ ■ ■ ■ ■ ■ ■ H Q M | |||||
| Erysipelotrichaceae | Amedibacillus | A. dolichus | 31971 | ■ ■ ■ ■ ■ ■ ■ H Q M | |||
| Clostridium_AQ | C. innocuum | 1522 | ■ ■ ■ ■ ■ ■ ■ H Q M | ||||
| Longicatena | L. caecimuris | 1796635 | ■ ■ ■ ■ ■ ■ ■ H Q M | ||||
| Holdemania | H. filiformis | 61171 | ■ ■ ■ ■ ■ ■ ■ H Q M | ||||
| Clostridia | Clostridiales | Clostridiaceae | Clostridium | C. acetobutylicum | 272562 | A B C D E F G H Q ■ | |
| Peptostreptococcales | Peptostreptococcaceae | Clostridioides | C. difficile | 1496 | A B C D E F G H Q ■ |
Taxonomy is according to the Genome Taxonomy Database (https://gtdb.ecogenomic.org/). Caryophanales = Bacillales_A. Caryophanaceae = Planococcaceae. Erysipelatoclostridiaceae = Coprobacillaceae. ■, absent; *, μF.
In the course of cloning the spoIIIA operon, the presence of a secondary promoter within the F cistron became apparent. As reported at the 1992 Spores meeting (17) and as fully described in the data deposited in GenBank in 1995, this P2 promoter was precisely mapped by primer extension and characterized as being under the control of SigE, as is P1, the primary promoter (17, 18). This internal promoter was “rediscovered” years later (28) and claimed to be essential. This is definitely not the case, as detailed in the supplemental material.
Recent excellent reviews have thoroughly reported the characteristics of the eight B. subtilis spoIIIA proteins and detailed their remote similarities to proteins from specialized secretion systems (24, 25). The advent of the AlphaFold prediction program (29, 30) allows these descriptions to be updated, as schematized in Fig. 1. The soluble, cytoplasmic protein A is organized in two globules, the larger one containing the four motifs conserved in secretion superfamily ATPases, which have been demonstrated to be essential for A’s activity (16). In full accordance with experimental structural data (31), the soluble cytoplasmic domain of B is a bundle of six helices, two of them extending from two anchoring transmembrane helices. This domain is proposed to interact with A and to bring it to the membrane, a functional interaction in good accordance with the tight organization of the A and B cistrons.
The topology of the bitopic C protein is ambiguous, but comparing the predictions of the orientation of the C orthologs from several spore formers (using the server at https://dtu.biolib.com/DeepTMHMM) and assuming it is conserved leads to the model shown in Fig. 1, where a short C-terminal tail is located in the intermembrane space. The topology of D is a 2-fold duplicate of the topology of C, with four transmembrane helices and short linking segments on the mother cell side and a larger linking central segment on the forespore side. There is something odd with the translation start of D. A common feature in the genomes of many spore formers is a run of Gs overlapping the stop codon of C, followed seven nucleotides downstream by an ATT codon. There is an alternate TTG codon in B. subtilis, but it is too close to the ribosome binding site and it is absent in the genomes of Bacillus licheniformis and Bacillus atrophaeus, close relatives of B. subtilis, where ATT is the only possible start codon. Because of the presence of this unusual start codon, the automatic annotations often miss a part of the D coding sequence. The reason for this anomaly is obscure.
Processing of its signal peptide is a prerequisite for E’s activity (32). The processing signal peptidase acts on the outside of the mother cell membrane, where it releases a large (~70-residue) soluble domain predicted by AlphaFold to be highly structured. It is followed by a series of six transmembrane helices with large helical protrusions extending as far as ~40 Å into the intermembrane space. The presence of these very long helices leads to a smaller number of actual transmembrane segments than originally thought. Conversely, the two helices anchoring F to the membrane are fully confirmed. They are followed by an elongated helix that sets the characteristic ring-building motif (RBM), experimentally demonstrated (33), ~45 Å away from the membrane.
A disordered region (40 residues) links the unique transmembrane helix of G to a highly structured domain located in the intermembrane space. Despite its complexity, both experimental data (34, 35) and computer predictions are in perfect agreement. This domain contains two motifs, an RBM and a unique, triangle-shaped β structure that is required and sufficient for ring formation in vitro (36). The overall structure of H is similar, with a unique transmembrane helix, a long disordered region (75 residues), and a globular domain organized around another RBM that has also been experimentally demonstrated (37, 38).
The genetic organization of the spoIIIA operon seems to reflect the structural data reported above, each cistron encoding the potential partner of the product of the next one. It starts with A, a soluble cytoplasmic protein, eventually brought to the membrane through its interaction with B, a membrane-bound protein facing the mother cell cytoplasm, then becoming part of a complex with C and D, two intrinsic membrane proteins, and finally associating with E, a membrane-bound protein extending toward the intermembrane space where F, G, and H could stack upon each other, being anchored to the mother cell membrane through increasingly longer polypeptide linkers (24, 25). This scaffold could bridge the ~20-nm intermembrane space by reaching SpoIIQ, a protein containing a LytM domain separated by a 35-residue disordered region from a helix embedded in the forespore membrane (37, 38). LytM domains are present in proteins involved, directly or indirectly, in cleavage of peptidoglycan stem peptides or of their cross-links (39). The stoichiometry of the various partners of this putative macromolecular structure is not known, although G has been shown to self-assemble into a 30-mer complex (35). Counterintuitively, the absence of H has a smaller negative effect on sporulation than the absence of any other member of the proposed megastructure, a decrease of 3 orders of magnitude compared to 5 under our laboratory conditions, and an even higher level of residual sporulation has been observed elsewhere (16). Maybe, in a few instances, SpoIIQ is able to interact with G in a similar fashion as with H, which would allow an active complex to form and sporulation to proceed. Another possible explanation is presented below.
The spoIIIA products are well conserved in Bacillales and Clostridia (Table 2), but a distinct form of SpoIIQ is present in Clostridia (40). Two other proteins have been described as involved in the function of the putative IIIA-IIQ complex in Bacillales, one synthesized in the forespore, SpoIIIL (41), and the other in the mother cell, GerM (42). Little is known about SpoIIIL, which is absent from Clostridia. GerM is a lipoprotein containing a duplicate GerMN motif, present in a large number of proteins with a broad phylogenetic distribution and potentially interacting with peptidoglycan (43). A shortened version of GerM containing only the first GerMN motif is present and conserved in several Clostridiaceae (26), whereas Clostridioides difficile harbors a different truncated form that is not conserved in its close relative Paeniclostridium sordellii. Interestingly, a spoIIIAH mutation in C. difficile has the same negative effect on sporulation as a polar mutation in spoIIIAA (44). This difference from B. subtilis points to a possible role for GerM from Bacillales, where it might contribute to maintain a partially functional IIIA-IIQ complex in the absence of H (42).
TABLE 2.
Conservation (E values) of the spoIIIA products and their SpoIIQ/GerM partners in various spore formers compared to B. subtilis
| Species | A | B | C | D | E | F | G | H | Q | M |
|---|---|---|---|---|---|---|---|---|---|---|
| Geobacillus kaustophilus | 1e−119a | 3e−55 | 1e−35 | 4e−58 | 1e−128 | 2e−44 | 1e−53 | 2e−42 | 4e−83 | 1e−146 |
| Brevibacillus brevis | 1e−111 | 3e−35 | 1e−18 | 2e−58 | 1e−112 | 3e−14 | 2e−41 | 1e−21 | 3e−51 | 3e−62 |
| Clostridium acetobutylicum | 3e−79 | 6e−19 | 1e−09 | 1e−29 | 2e−49 | 9e−12 | 5e−21 | 9e−09 | 5e−04 | ■ |
| Clostridioides difficile | 2e−74 | 1e−05 | 5e−11 | 2e−29 | 4e−28 | 5e−07 | 5e−14 | 2e−10 | 3e−05 | ■ |
| Bhargavaea lindanitolerans | ■ | ■ | 2e−07 | 2e−01 | 3e−04 | ■ | 4e−03 | 2e−23 | 6e−37 | ■ |
| Bhargavaea ginsengi | ■ | ■ | 1e−09 | ND | 3e−05 | ■ | 3e−04 | 4e−21 | 2e−42 | ■ |
| Sporosarcina ureae | ■ | ■ | 1e−07 | 2e−05 | 3e−09 | ■ | 7e−04 | 3e−19 | 4e−35 | ■ |
| Sporosarcina psychrophila | ■ | ■ | 7e−08 | 2e−01 | 3e−08 | ■ | 2e−05 | 5e−17 | 7e−34 | ■ |
| Sporosarcina ureilytica | ■ | ■ | 6e−07 | ND | 1e−11 | ■ | 1e−02 | 4e−17 | 1e−30 | ■ |
| Bacillus sp. OxB-1 | ■ | ■ | 7e−08 | 7e−00 | 4e−08 | ■ | 1e−04 | 2e−22 | 8e−28 | ■ |
| Psychrobacillus glaciei | ■ | ■ | 5e−07 | ND | 9e−11 | ■ | 4e−04 | 2e−20 | 2e−26 | ■ |
| Psychrobacillus sp. AK1817 | ■ | ■ | 4e−07 | ND | 8e−12 | ■ | 3e−04 | 9e−21 | 5e−27 | ■ |
| Viridibacillus arvi | ■ | ■ | 2e−07 | ND | 5e−03 | ■ | 7e−08 | 3e−28 | 3e−37 | ■ |
| Viridibacillus sp. JNUCC-6 | ■ | ■ | 2e−07 | ND | 8e−04 | ■ | 7e−08 | 2e−27 | 7e−37 | ■ |
| Rummeliibacillus stabekisii | ■ | ■ | 3e−06 | ND | 1e−07 | ■ | 2e−07 | 2e−24 | 4e−41 | ■ |
| Rummeliibacillus pycnus | ■ | ■ | 1e−06 | ND | 5e−07 | ■ | 5e−10 | 5e−27 | 8e−39 | ■ |
| Ureibacillus thermosphaericus | ■ | ■ | 4e−07 | ND | 5e−03 | ■ | 1e−05 | 3e−27 | 4e−30 | ■ |
| Metasolibacillus fluoroglycofenilyticus | ■ | ■ | 1e−06 | ND | ND | ■ | 7e−06 | 4e−23 | 1e−31 | ■ |
| Solibacillus silvestris | ■ | ■ | 6e−06 | ND | ND | ■ | 5e−09 | 5e−29 | 5e−26 | ■ |
| Lysinibacillus fusiformis | ■ | ■ | 2e−07 | ND | 4e−06 | ■ | 4e−05 | 6e−30 | 1e−32 | ■ |
| Lysinibacillus sphaericus | ■ | ■ | 2e−07 | ND | 4e−06 | ■ | 1e−05 | 9e−28 | 6e−34 | ■ |
| Paenisporosarcina antarctica | ■ | ■ | 6e−07 | ND | 2e−09 | ■ | 3e−04 | 1e−25 | 6e−33 | ■ |
| Beduini massiliensis | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 7e−08 | 7e−31 | 2e−19 |
| Coprobacillus cateniformis | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 5e−06 | 5e−33 | 2e−13 |
| Faecalibacillus intestinalis | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 1e−03 | 2e−31 | 1e−08 |
| Erysipelatoclostridium spiroforme | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 2e−05 | 1e−35 | 4e−11 |
| Erysipelatoclostridium ramosum | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 5e−05 | 1e−34 | 2e−13 |
| Amedibacillus dolichus | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 4e−04 | 4e−17 | 8e−31 |
| Clostridium innocuum | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 2e−08 | 3e−18 | 6e−32 |
| Longicatena caecimuris | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 6e−05 | 5e−20 | 2e−28 |
| Holdemania filiformis | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 4e−03 | 9e−21 | 2e−23 |
E values were obtained by running a BLASTP search against the SubtiList database (http://genolist.pasteur.fr/SubtiList/). Two species of Bacilli belonging to other families than B. subtilis and two species of Clostridia from different orders were used as internal references. ■, gene absent; ND, gene present but has product with no detectable similarity in the B. subtilis proteome.
THE CURIOUS CASE OF LYSINIBACILLUS SPHAERICUS (AND CONSORTS)
Our attention was drawn to Lysinibacillus sphaericus when we discovered that, contrary to all Bacillaceae, it does not contain csfB, the gene encoding Gin (Pfam identification code [ID] PF10764), the inhibitor of SigG (45). It was soon joined by Bacillus sp. B14905, an obscure Bacillus species sequenced at TIGR, which is now known to be a Lysinibacillus fusiformis strain. Searching these two genomes for other potential anomalies, we found a truncated spoIIIA operon, as shown in Fig. 1. As more and more genomes of spore formers became available, this highly specific signature appeared multiple times in a diverse range of Bacilli. It is only recently that a coherent picture emerged with the assignment of all these species to a unique family, the Caryophanaceae, also known as Planococcaceae (46). This family contains 10 distinct genera of spore formers (Table 1), among which is the emended Lysinibacillus genus. What is described below is shared by all spore-forming members of the Caryophanaceae family. The only apparent exceptions, Sporosarcina globispora and Jeotgalibacillus, are misclassified and belong to the Bacillaceae family (46).
Instead of eight cistrons tightly organized in a single operon, the spoIIIA locus of L. sphaericus contains five cistrons split into two operons (Table 1). Both are preceded by sequences sharing all the characteristics of SigE-dependent promoters (Fig. S2 in the supplemental material). The product of the first cistron is very highly conserved among Caryophanaceae and is the ortholog of C in Bacillaceae and Clostridia (Fig. 2). The second cistron encodes a protein with no similarity to D at the amino acid sequence level (Table 2) but with an identical predicted membrane topology. Therefore, given its genomic location, it seems reasonable to assume that this protein plays a similar physiological role as the canonical D and that it is a case of nonorthologous gene displacement, like spoIIQ in Clostridia. This protein, henceforth identified as D, is much less conserved than C among Caryophanaceae (Fig. S3).
FIG 2.
Strong conservation of SpoIIIAC in Caryophanaceae. The 10 sequences can be aligned without any gap or insertion. The 27 positions strictly conserved in the 10 C proteins are indicated by pink asterisks. The additional 17 positions with similar amino acids in the 10 C proteins are indicated by black asterisks. Similarity is color coded using the following groups: ILVM, FYW, KR, DE, ST, and NQ. The sequence of the B. subtilis C protein is aligned at the bottom, with asterisks indicating the overall residue conservation. Bhalin, Bhargavaea lindanitolerans; Spoure, Sporosarcina ureae; PsycAK, Psychrobacillus sp. AK1817; Virarv, Viridibacillus arvi; Rumpyc, Rummeliibacillus pycnus; Urethe, Ureibacillus thermosphaericus; Metflu, Metasolibacillus fluoroglycofenilyticus; Solsil, Solibacillus silvestris; Lyssph, Lysinibacillus sphaericus; Paeant, Paenisporosarcina antarctica; Bacsub, B. subtilis.
The product of the third cistron is well conserved in Caryophanaceae, with seven proline residues appearing to play a major structural role (Fig. S4). It is an ortholog of E in B. subtilis, to which it is more or less strongly related depending on the species (Table 2). The E proteins of Caryophanaceae lack the signal sequence found in E of B. subtilis and the whole 70-residue extracellular domain preceding its first transmembrane domain. Presumably they assume the same conformation as the remaining part of E in B. subtilis.
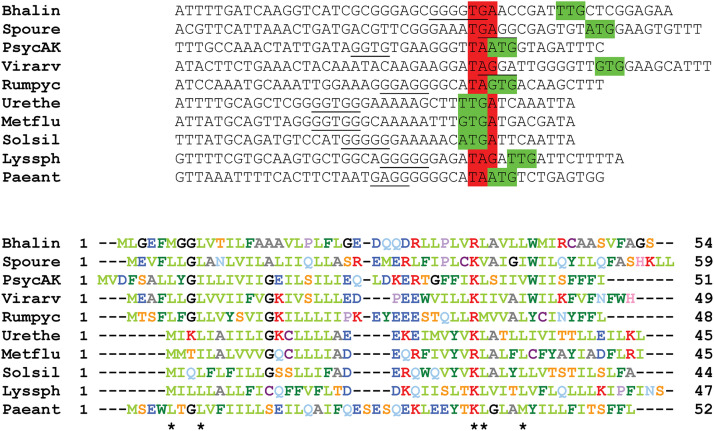
The E cistron is not followed by an F cistron. However, careful examination of the sequences reveals a conserved pattern, with a strong potential translational coupling that could allow the expression of a short open reading frame extending down to the GH promoter (Fig. 3 and Fig. S2). The products of these reading frames share little similarity at the amino acid level, but they are predicted to have a similar conformation: two potential transmembrane helices linked by a short cytoplasmic turn. All currently available genome sequences from the 10 genera of sporogenous Caryophanaceae contain this small open reading frame. Thus, despite the lack of any actual experimental evidence, it seems worth assuming its existence as the fourth cistron of the CDE operon. Its product will be named here μF.
FIG 3.
A short open reading frame downstream from spoIIIAE in Caryophanaceae. The upper part of the figure shows the last 10 codons of E and the first 4 codons of the possible downstream short open reading frame. The stop codons are highlighted in red, and the start codons in green. Potential ribosome binding sites are underlined. The bottom part of the figure shows the aligned sequences of the predicted products of the downstream short open reading frame. The 5 positions with similar amino acids in the 10 proteins are indicated by black asterisks. Similarity is color coded using the following groups: ILVM, FYW, KR, DE, ST, and NQ. Bhalin, Bhargavaea lindanitolerans; Spoure, Sporosarcina ureae; PsycAK, Psychrobacillus sp. AK1817; Virarv, Viridibacillus arvi; Rumpyc, Rummeliibacillus pycnus; Urethe, Ureibacillus thermosphaericus; Metflu, Metasolibacillus fluoroglycofenilyticus; Solsil, Solibacillus silvestris; Lyssph, Lysinibacillus sphaericus; Paeant, Paenisporosarcina antarctica.
The G cistron encodes a protein more compact than its counterpart in B. subtilis (Fig. S5). There is no N-terminal 30-residue extension located in the mother cell cytoplasm, the disordered region linking the highly organized external soluble domain to the membrane is reduced to a dozen residues at most, and the internal segment that folds into a characteristic triangle-shaped β structure is missing. Conversely, all the other structural elements that constitute the RBM motif (α1β1β8α2β9 in B. subtilis) are predicted to be conserved. The few positions highly conserved among Caryophanaceae are also shared with B. subtilis.
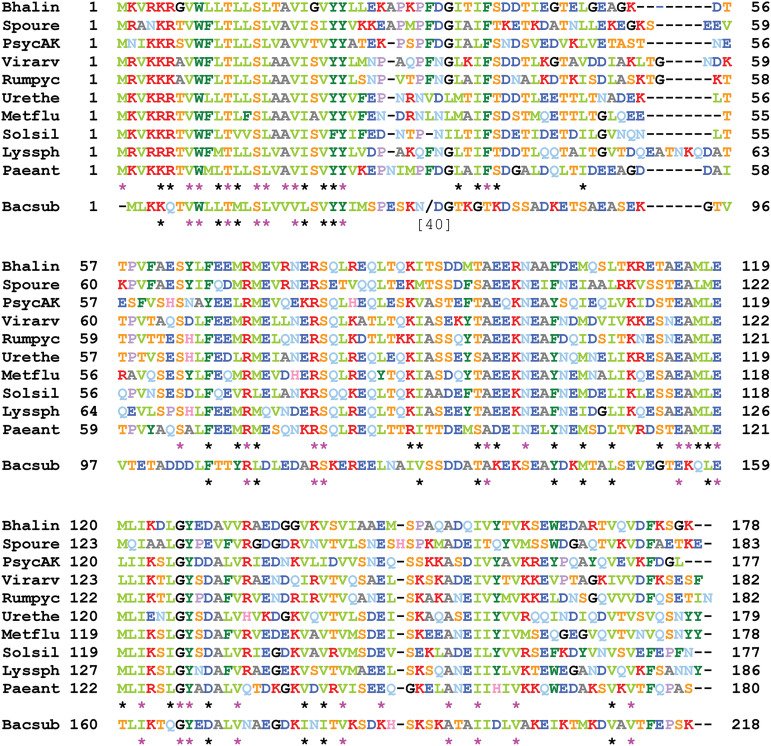
The H cistron encodes a protein that can be aligned with its B. subtilis ortholog throughout its length, with the exception of an ~40-residue-shorter disordered linker region following a strikingly conserved transmembrane domain (Fig. 4). Presumably these alignments reflect a structural organization that is similar for all H proteins. An even higher level of conservation is observed for SpoIIQ (Table 2 and Fig. S6), including the transmembrane helix, a disordered linker region of similar size, and a well conserved LytM domain. The B. subtilis C-terminal 60-residue extension, known to be nonessential for SpoIIQ activity (23), is absent. The distinctive motifs of promoters recognized by SigF are found immediately upstream from the spoIIQ coding sequences, in good accordance with the synthesis of the SpoIIQ orthologs in the forespore (Fig. S6).
FIG 4.
The SpoIIIAH proteins of Caryophanaceae. The 10 sequences have been aligned with ClustalΩ (using the server at https://toolkit.tuebingen.mpg.de/). The 29 positions strictly conserved in the 10 H proteins are indicated by pink asterisks. The additional 30 positions with similar amino acids in the 10 H proteins are indicated by black asterisks. Similarity is color coded using the following groups: ILVM, FYW, KR, DE, ST, and NQ. The sequence of the B. subtilis H protein is aligned at the bottom, with a bracketed number and slash indicating the number of residues omitted to maintain the alignment and asterisks showing the overall residue conservation. Bhalin, Bhargavaea lindanitolerans; Spoure, Sporosarcina ureae; PsycAK, Psychrobacillus sp. AK1817; Virarv, Viridibacillus arvi; Rumpyc, Rummeliibacillus pycnus; Urethe, Ureibacillus thermosphaericus; Metflu, Metasolibacillus fluoroglycofenilyticus; Solsil, Solibacillus silvestris; Lyssph, Lysinibacillus sphaericus; Paeant, Paenisporosarcina antarctica; Bacsub, B. subtilis.
There is no gerM or spoIIIL in the genomes of Caryophanaceae, a feature they share with many Clostridiaceae. But the absence of A, B, and F at the spoIIIA locus (as well as elsewhere in the genomes) is a unique property that hardly fits with the current model for SpoIIIA structure and function. To explore the possibility that these missing genes were replaced by nonhomologous genes, a proteome comparison was performed at PATRIC (47), compiling the proteins shared by 11 spore-forming Caryophanaceae and absent in six asporogenous Caryophanaceae, as well as in B. subtilis (using the server at https://patricbrc.org/). The resulting list did not provide potential candidates that could substitute for A, B, or F, but it pointed out two relevant partners. One conserved and specific protein is encoded by a gene separated from the spoIIA operon by the fur ripX drm pupG cluster, which is the location of spoIIM in B. subtilis. SpoIIM is an intrinsic membrane protein that coordinates peptidoglycan remodeling by the SpoIIP and SpoIID proteins during engulfment (10). There is no bona fide spoIIM gene in Caryophanaceae, whereas spoIID and spoIIP are well conserved (data not shown). Several elements suggest that this conserved protein plays the role of SpoIIM in Caryophanaceae. Not only is its gene as the same genetic location as spoIIM in B. subtilis, but it appears also to be controlled by SigE and therefore expressed in the mother cell, and its product is predicted to contain five transmembrane segments, just like SpoIIM (Fig. S7). The other conserved and specific protein is encoded by a gene also seemingly controlled by SigE, located downstream from the mreC mreD minC minD cluster and upstream from, and apparently in an operon with, a distant ortholog of spoIVFB. This is the location of spoIVFA in B. subtilis, and there is no bona fide spoIVFA gene in Caryophanaceae. There is none in Clostridia either, but the putative clostridial version of SpoIVFA (identified by being located in the same genetic environment) is not the same as the one found in Caryophanaceae (Fig. S8). A cascade of interactions has been shown to be involved in localizing in the engulfing membrane the postulated IIIA-IIQ megastructure, the SpoIVFA protein, and the complex built around SpoIIM (21, 48, 49). It might not be a coincidence that drastic changes have occurred simultaneously in the three partners. We postulate that these are other cases of nonorthologous gene displacement, the same function being assumed by proteins belonging to distinct families. We propose that they share the same names as a convenient mnemonic trick until further analysis.
The very strong conservation of C among Caryophanaceae (Fig. 2) suggests that this protein interacts with another highly conserved partner. The absence of B excludes the possibility, hinted at above, that C is connecting an AB complex to the membrane-embedded D and E proteins. The only highly conserved candidate is the H transmembrane domain (Fig. 4). Interestingly, for the most part, these conservations also extend to the B. subtilis C and H orthologs.
Conversely, if, in B. subtilis, D is the bridge between an AB complex and an external megastructure built above E, the loss of B in Caryophanaceae may have allowed the D sequence to drift away from its canonical form, making it almost unrecognizable (Table 2). The primary sequence of the planococcal D is poorly conserved (Fig. S3), and there are enough variations in E (Fig. S4) to leave open the possibility of maintaining an interaction between D and E.
The parallel loss of F and of the extracytoplasmic N-terminal domain of E (Fig. 1 and Fig. S4) suggests that this globule is a site of interaction between the two proteins in the canonical IIIA complex. Similarly, the parallel loss of F and of the triangle-shaped β structure present in G suggests that this β-triangle region is a site of interaction between G and F in the B. subtilis IIIA complex. The absence of F in Caryophanaceae implies that a new link must exist for G to associate with E. This might be the role of μF, which appears at the genetic level almost as an extension of E and where one of the two predicted membrane-spanning domains could interact with E and the other with the transmembrane helix of G. This hypothetical cascade of interactions is schematized in Fig. 5.
FIG 5.
Proposed interactions between the SpoIIIA proteins. Dumbbells indicate potential interactions between specific domains of the various spoIIIA products. The choice of interacting transmembrane segments for polytopic membrane proteins is arbitrary. Top, B. subtilis; bottom, L. sphaericus.
The disordered linkers of G and H are much smaller in Caryophanaceae than in B. subtilis, suggesting that the characteristic ring structures of G and H are closer to the mother cell membrane in these bacteria. There is no significant size difference in the linker regions of the SpoIIQ orthologs (Fig. S6), indicating that their LytM domains reside at roughly the same distance from the forespore membrane. Then, how can the continuous junction proposed for the IIIA-IIQ megastructure be achieved? An answer can be found in a remarkable article published almost 50 years ago (50), where it is stated that the cell wall of L. sphaericus contains an “unusually low content of peptidoglycan (20%).” This would lead to a shorter distance between the two membranes, which could be spanned by the IIIA-IIQ scaffold with one less element (loss of F) and shorter linkers for the stacked G and H ring-building motifs. Ultrastructural information is currently missing to allow generalization of this proposition to other genera of Caryophanaceae, but the conservation of the spoIIIA locus suggests that they could also share similar envelope characteristics.
Assuming there is some validity in the above suggestions, they do not take into account the loss of A and B. Presumably, some specific feature of Caryophanaceae, beyond a possible shorter intermembrane space, must have led to this reduced version of the spoIIIA operon. One silent companion of the postulated IIIA-IIQ megacomplex is the peptidoglycan, which has to be crossed over to allow a physical contact between proteins anchored in opposing membranes. The peptidoglycan of Caryophanaceae contains l-lysine instead of meso-diaminopimelate (m-DAP) in the third position of the stem peptides, and the bridging between peptides of two glycan strands is of the A4α type (51). Peptidoglycan in Bacillaceae and Clostridia is of the A1γ type, where a d-Ala residue in the fourth position of a stem peptide is directly linked to the m-DAP residue of an adjacent peptide (52). In Caryophanaceae, the A4α linkage between d-Ala and l-Lys involves an interpeptide bridge containing a dicarboxylic amino acid (d-Asp or d-Glu), as well as an additional Gly or l-Ala in some Sporosarcina species (51). The peptidoglycan bridge length is the key factor in determining the peptidoglycan architecture (53). The combination of a reduced number of glycan strands and more relaxed cross-links may facilitate protein insertion through the mesh-like hydrogel cell wall (54). If this is correlated with the loss of a requirement for ATP hydrolysis while maintaining a functional IIIA-IIQ megastructure, it suggests that, in Bacillaceae and Clostridia, ATP-driven reactions contribute to the insertion of the complex itself through a thicker, denser cell wall, in addition to or instead of allowing the secretion of molecules from the mother cell to the forespore.
INSIGHTS FROM GUT MICROBIOTA: THE SHRUNK WORLD OF ERYSIPELOTRICHALES
The advent of large-scale genome sequencing has contributed to a revision of previous classifications and has opened a window on some obscure and neglected bacteria, the members of the gut microbiome. The order of Erysipelotrichales appears now to be constituted of two distinct families, each made of multiple genera, some of them containing sporogenous species (Table 1). The misnamed Clostridium innocuum is now recognized as a member of the Erysipelotrichaceae family. This species was suspected of being misclassified because, unlike other Clostridia, it contains l-Lys instead of m-DAP in its cell wall (52). Since this specific feature is a shared characteristic of Caryophanaceae and might be linked to their atypical spoIIIA operon, it seemed worth exploring the genomes of Erysipelotrichales and analyzing the status of the SpoIIIA proteins and their partners.
Data, including four complete genomes, are currently available for both families of Erysipelotrichales, represented by eight genera (Table 1). In all cases, the spoIIIA operon is reduced to a single gene, spoIIIAH (Fig. 1). The promoters are of the SigE type, and the amino acid sequences are only remotely homologous to the B. subtilis H sequence (Fig. 6 and Table 2). Most of the conservation with B. subtilis occurs in the transmembrane domain, which is almost identical for all Erysipelotrichales. Although distantly related at the amino acid level to their B. subtilis ortholog, the Erysipelotrichales H proteins are predicted to adopt similar secondary structures (Fig. 6) and therefore to fold as a ring-building motif. Compared to B. subtilis, the highly variable region linking the transmembrane domain to the RBM structure is shorter (~30 residues) in some Erysipelotrichaceae and quasi-absent in some Erysipelatoclostridiaceae. Conversely, the SpoIIQ orthologs share strong similarities with their B. subtilis counterpart (Table 2 and Fig. S9), with the exception of its nonessential C-terminal extension. There is little or no difference in the size of the variable linker region following the transmembrane-anchoring helix. Promoter sequences are of the SigF type, as expected.
FIG 6.
The spoIIIAH genes of Erysipelotrichales. The upper part of the figure shows the promoter regions of the Erysipelotrichales spoIIIAH genes. The —10 and —35 motifs recognized by SigE are shown in bold blue type. The critical T in the —35 region is underlined. The start codons are highlighted in green. In the bottom part, the 8 SpoIIIAH sequences have been aligned with ClustalΩ (using the server at https://toolkit.tuebingen.mpg.de/) The 17 positions strictly conserved in the 8 H proteins are indicated by pink asterisks. The additional 19 positions with similar amino acids in the 8 H proteins are indicated by black asterisks. Similarity is color coded using the following groups: ILVM, FYW, KR, DE, ST, and NQ. The sequence of the B. subtilis H protein is aligned at the bottom, with a bracketed number and slash indicating the number of residues omitted to maintain the alignment and asterisks showing the overall residue conservation. Predictions of alpha-helices and beta-strands with Ali2D (using the same server) are shown on top of the alignment. Bedmas, Beduini massiliensis; Copcat, Coprobacillus cateniformis; Faeint, Faecalibacillus intestinalis; Eryram, Erysipelatoclostridium ramosum; Amedol, Amedibacillus dolichus; Cloinn, Clostridium innocuum; Loncae, Longicatena caecimuris; Holfil, Holdemania filiformis; Bacsub, B. subtilis.
A major distinctive feature is the presence of a bona fide gerM gene (Table 1), preceded by a typical SigE-dependent promoter (Fig. S10). Conservation with the B. subtilis GerM protein is stronger in Erysipelotrichaceae than in Erysipelatoclostridiaceae, the opposite of what is observed for the SpoIIQ proteins (Table 2). Alignments of the amino acid sequences and of their predicted secondary structures highlight the conservation of the duplicate GerMN motif present in B. subtilis (Fig. S11). The GerM proteins from Erysipelotrichales do not contain a lipoprotein signal sequence and appear to use their N-terminal alpha-helix to be anchored into the membrane (a potential lipoprotein cleavage site in Erysipelatoclostridium ramosum is not conserved in the closely related Erysipelatoclostridium spiroforme). The disordered linker separating the membrane from the first GerMN motif is much shorter (~12 to 20 residues) than in B. subtilis (55 residues).
Exploration of the four complete genomes available indicates the absence of spoIIIL. Comparing at PATRIC (47) their proteomes with the proteomes of five asporogenous Erysipelotrichales revealed the presence of very distant orthologs of SpoIIM (encoded by a SigE-controlled gene located in the dinB xerD interval) and SpoIVFA (encoded by a SigE-dependent gene located downstream from mreC mreD). Although well conserved among Erysipelotrichales (data not shown), these proteins barely give a positive hit when running a BLASTP search against the SubtiList database (http://genolist.pasteur.fr/SubtiList/). They are not related to the planococcal (nor clostridial) versions described above. An unrelated discovery was the identification of a nonorthologous displacement of the spoIIR gene that is missing in Erysipelotrichales, as detailed in the supplemental material (Fig. S12 and S13).
The very high conservation of the H transmembrane domain (Fig. 6), already observed in Caryophanaceae, is all the more striking given that there is no potential SpoIIIA interacting partner. Since it is obviously not an ordinary transmembrane-anchoring helix, it follows that it must interact either with itself or with a highly conserved protein, such as a component of the division machinery still present after the end of septation. The first proposition should be accessible to modeling and, if validated, opens challenging possibilities. Could packing of H transmembrane segments, each one being shifted from the next one by a fixed angle, lead to a closed circular structure forming a hydrophobic pore? Without the assistance of any other protein? Alternatively, intrinsic properties of these helices might target them to the engulfing membrane because of some specific feature of the mother cell membrane at the forespore interface, be it fluidity or curvature, such as proposed in the supplemental material for the action of SpoIIR on SpoIIGA.
The presence of GerM may compensate for the absence of the other SpoIIIA proteins. However, the low conservation of the GerM transmembrane domain (Fig. S11) excludes its interaction with H within the membrane. Interaction between the extracellular folded domains of the two proteins also seems unlikely since there is no correlation between the length of the disordered regions linking them to the membrane (18 to 47 residues for H but 19 to 20 for GerM in Erysipelotrichaceae). Also, the degree of conservation between Erysipelotrichales and B. subtilis is very high for GerM and SpoIIQ and quite low for H, whereas in Caryophanaceae, both H and SpoIIQ are similarly strongly related to their B. subtilis orthologs (Table 2). Therefore, instead of facilitating the interaction between H and SpoIIQ, GerM might play an independent role, in conjunction with SpoIIQ or without it.
In seven Erysipelotrichales species (the exception being Faecalibacillus intestinalis), gerM is preceded by ldcB, a gene encoding an ld-carboxypeptidase that trims the cell wall peptides to tripeptides. In four cases, the racE/murI gene is inserted between ldcB and gerM. The racE gene, which also immediately precedes gerM in Bacillaceae, encodes the enzyme that converts l-Glu to d-Glu, present at the second position of the cell wall peptides. This genomic vicinity adds to the suspicion that GerM is interacting with peptidoglycan (43). Since SpoIIQ contains a LytM domain and because of the known functions of such domains, it is tempting to speculate that GerM and SpoIIQ, each in close contact with the peptidoglycan from opposite sides, de facto interact with each other, albeit indirectly.
Data about the cell wall of Erysipelotrichales are scarce and sometimes contradictory. Phylogenomics indicate that Erysipelotrichales are neighboring clades of Mycoplasmatales, bacteria that lack a cell wall (55, 56). Therefore, they could be expected to harbor a thin peptidoglycan, which would fit with the shorter disordered linkers observed for H and for GerM compared to B. subtilis, an interpretation discussed above for Caryophanaceae. Regarding its architecture, information available for Erysipelotrichaceae (57) indicates the common existence of interpeptide bridges between a d-Ala residue in the fourth position of a stem peptide and either the l-Lys residue of an adjacent peptide (in C. innocuum, Longicatena caecimuris, and Amedibacillus dolichus) or the d-Glu residue in the second position of another stem peptide (in Holdemania filiformis). Much less is known for Erysipelatoclostridiaceae. Because the special cell wall that is built around the forespore at a later stage always contain m-DAP, a dedicated murE gene must be present and specifically activated in the mother cell. Therefore, bacterial spore formers that contain l-Lys in their vegetative cell wall (and in the primordial cell wall synthesized during engulfment) must harbor two copies of murE in their genome, encoding enzymes with different substrate specificities. This is indeed the case for the Caryophanaceae species described above, whereas their asporogenous relatives contain only one copy of murE. This is also true for Erysipelotrichales, which strongly suggests that the previous observations and discussions can be extended to Erysipelatoclostridiaceae. Hopefully, future ultrastructural studies will clarify this issue.
SpoIIIA, A VERSATILE CHARACTER, OR FIFTY SHADES OF GLUE?
The simplified version of SpoIIIA in Erysipelotrichales, bacteria presumed to harbor a thin cell wall, is reminiscent of an experiment in which H and SpoIIQ were shown to be essential for the engulfment of B. subtilis protoplasts (58). Taken at face value, these data imply that the H-IIQ complex is the engulfase speculated many years ago (59). A zipper model (20) suggests a continuous structure, but this does not need to be the case. The close contacts between the two membranes could be spread out and still be sufficient for completing engulfment when the amount of proteins involved is severely reduced (16). As a transenvelope complex, the engulfase would be expected to adapt to changes in the forespore/mother cell interface, especially its cell wall architecture. Accordingly, engulfment stalls at an intermediate stage in a B. subtilis spoIIQ null mutant when sporulation is induced by medium exhaustion, the laboratory conditions closest to the natural environment (14). Also, mutations in spoIIQ or spoIIIAH prevent engulfment in C. difficile, where the Bacillales version of gerM is lacking (44, 60). Based on these observations, it has been suggested that the ancestral function of the H-IIQ complex is to control engulfment during sporulation (44). The presence of GerM in Bacillaceae may explain the fact that spoIIIA mutants complete engulfment. Conservation of GerM in Erysipelotrichales, where H, the only remnant of the SpoIIIA family, is poorly conserved, points to a similar role in these bacteria where severe gene reduction spared that specific gene.
It is widely accepted that SpoIIIA and SpoIIQ form a channel connecting the mother cell and the forespore and constitute a secretion apparatus. The strong conservation of the H transmembrane domain in the various families of endospore formers hints at its specific and critical role in channel function. A direct involvement at the channel entrance would make it essential. However, as discussed above, the absence of H in B. subtilis has a much less severe effect on sporulation than the absence of any other product of the spoIIIA operon. Similarly, SpoIIQ is located at the channel exit, but its membrane-anchoring segment can be replaced with an unrelated transmembrane helix without any adverse effect on sporulation (23). Thus, SpoIIQ is not a secretin and another, as-yet-unidentified forespore protein must be involved in the final step of secretion (24, 25). In any case, transfer of molecules from the mother cell to the forespore cannot be a passive process because of the higher osmotic pressure in the forespore (61). This is the role attributed to the A protein, but this ATP-binding protein is missing in Caryophanaceae, where the IIIA-IIQ complex might have lost (or not yet acquired) the ability to be a secretion apparatus.
Supplying metabolites to the isolated forespore through a specialized secretion machinery seems an ingenious and beneficial process. It is then puzzling that the B. subtilis IIIA-IIQ complex dissociates shortly after the end of engulfment (62), precisely at the time when the mother cell is supposed to start nurturing the forespore. This untimely behavior is more in line with the completion of a function carried out throughout the engulfment process, such as maintaining a close contact between the growing forespore and mother cell membranes (34, 63). Mechanisms must exist that ensure coordinated incorporation of phospholipids on both sides of the invaginating envelope and in concert with peptidoglycan synthesis. The IIIA-IIQ complex might contribute to this process by acting as both a tether and a topological organizer. A defect in forespore inner membrane synthesis could explain the shrunken, misshaped, collapsed phenotype of the forespores observed in spoIIIA mutants.
Engulfment is successful in a B. subtilis spoIIIE mutant, where most of the chromosome of the forespore remains trapped on the mother cell side. Many genes involved in phospholipid synthesis are absent from the forespore in such a strain. Either their products are stable and do not need to be replenished or phospholipids are provided by the mother cell and reach the forespore inner membrane. A common feature of the genomic environment of the spoIIIA operon, in all its versions, is the presence of the yqhY gene either immediately following it or 2 or 3 genes further downstream. The YqhY protein has been shown to be involved in lipid biosynthesis through a still-obscure mechanism (64). Additionally, in P. sordellii, spoIIIA is immediately preceded by an 8-gene operon encoding most of the enzymes from the pathway leading to phosphatidic acid, the precursor of membrane phospholipids. A physiological relationship between the SpoIIIA proteins and phospholipids might be worth investigating. Presumably the energy barrier due to the higher osmotic pressure in the forespore becomes irrelevant if molecules are not channeled into but onto the forespore.
Following the same tracks as other observant colleagues (55, 65, 66), I have explored genomes in search of answers about the role of the mysterious SpoIIIA machine. From its most sophisticated incarnation in B. subtilis to its evolutionarily most downgraded gut form, are we witnessing the many facets of the same coin or completely independent avatars? Can an elegant feeding tube turn into a simple snap button? Abductive reasoning followed along this work cannot provide a definite conclusion. Moreover, to quote an eminent scientist, “it is very rash to use simplicity and elegance as a guide in biological research” (67).
ACKNOWLEDGMENTS
I am grateful to Anne-Marie Guérout-Fleury for her momentous work on spoIIIA and I deeply regret not to have been able to get her the credit she deserved. Many thanks to Patrick Lane for enhancing the figures. This review would not have been written without the incentive provided by John Helmann in his chronicle of sigma factors and Javier Lopez-Garrido in his anthology of sporulation milestones. I salute them both. I dedicate this article to my crisscross partner, Rich Losick, one year ahead of his 80th birthday.
Biography

Patrick Stragier, after graduating from École Normale Supérieure in Paris, was recruited by CNRS, the French science agency, where he benefited from the freedom that was once granted to its tenured scientists. At the University of Orsay, he discovered the joys of gene cloning and sequencing, first working on the lysine regulon of Escherichia coli and identifying the founding member of the LysR family. Then, he shifted his interests to Bacillus subtilis, the paradigmatic organism for studying sporulation. In Orsay, and then later at the Institut de Biologie Physico-Chimique in Paris and from time to time at Harvard University, Dr. Stragier was lucky enough to live the golden years of the molecular genetics of sporulation, when the puzzling collection of spo genes turned into a coherent and satisfying picture, still valid today. He was awarded the EMBO Gold Medal for his contributions. He is now retired.
Footnotes
Supplemental material is available online only.
Contributor Information
Patrick Stragier, Email: pstragier@phare.normalesup.org.
Michael Y. Galperin, NCBI, NLM, National Institutes of Health
REFERENCES
- 1.Hilbert DW, Piggot PJ. 2004. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol Mol Biol Rev 68:234–262. doi: 10.1128/MMBR.68.2.234-262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stragier P, Bouvier J, Bonamy C, Szulmajster J. 1984. A developmental gene product of Bacillus subtilis homologous to the sigma factor of Escherichia coli. Nature 312:376–378. doi: 10.1038/312376a0. [DOI] [PubMed] [Google Scholar]
- 3.Fort P, Piggot PJ. 1984. Nucleotide sequence of sporulation locus spoIIA in Bacillus subtilis. J Gen Microbiol 130:2147–2153. doi: 10.1099/00221287-130-8-2147. [DOI] [PubMed] [Google Scholar]
- 4.Errington J, Fort P, Mandelstam J. 1985. Duplicated sporulation genes in bacteria: implication for simple developmental systems. FEBS Lett 188:184–188. doi: 10.1016/0014-5793(85)80368-9. [DOI] [Google Scholar]
- 5.Masuda ES, Anaguchi H, Yamada K, Kobayashi Y. 1988. Two developmental genes encoding sigma factor homologs are arranged in tandem in Bacillus subtilis. Proc Natl Acad Sci USA 85:7637–7641. doi: 10.1073/pnas.85.20.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karmazyn-Campelli C, Bonamy C, Savelli B, Stragier P. 1989. Tandem genes encoding sigma-factors for consecutive steps of development in Bacillus subtilis. Genes Dev 3:150–157. doi: 10.1101/gad.3.2.150. [DOI] [PubMed] [Google Scholar]
- 7.Stragier P, Bonamy C, Karmazyn-Campelli C. 1988. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 52:697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- 8.Imamura D, Zhou R, Feig M, Kroos L. 2008. Evidence that the Bacillus subtilis SpoIIGA protein is a novel type of signal-transducing aspartic protease. J Biol Chem 283:15287–15299. doi: 10.1074/jbc.M708962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frandsen N, Stragier P. 1995. Identification and characterization of the Bacillus subtilis spoIIP locus. J Bacteriol 177:716–722. doi: 10.1128/jb.177.3.716-722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chastanet A, Losick R. 2007. Engulfment during sporulation in Bacillus subtilis is governed by a multiprotein complex containing tandemly acting autolysins. Mol Microbiol 64:139–152. doi: 10.1111/j.1365-2958.2007.05652.x. [DOI] [PubMed] [Google Scholar]
- 11.Morlot C, Uehara T, Marquis KA, Bernhardt TG, Rudner DZ. 2010. A highly coordinated cell wall degradation machine governs spore morphogenesis in Bacillus subtilis. Genes Dev 24:411–422. doi: 10.1101/gad.1878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karow ML, Glaser P, Piggot PJ. 1995. Identification of a gene, spoIIR, that links the activation of σE to the transcriptional activity of σF during sporulation in Bacillus subtilis. Proc Natl Acad Sci USA 92:2012–2016. doi: 10.1073/pnas.92.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Londoño-Vallejo JA, Stragier P. 1995. Cell-cell signaling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev 9:503–508. doi: 10.1101/gad.9.4.503. [DOI] [PubMed] [Google Scholar]
- 14.Londoño-Vallejo JA, Frehel C, Stragier P. 1997. spoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol Microbiol 24:29–39. doi: 10.1046/j.1365-2958.1997.3181680.x. [DOI] [PubMed] [Google Scholar]
- 15.Piggot PJ, Coote JG. 1976. Genetic aspects of bacterial endospore formation. Bacteriol Rev 40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doan T, Morlot C, Meisner J, Serrano M, Henriques AO, Moran CP, Jr, Rudner DZ. 2009. Novel secretion apparatus maintains spore integrity and developmental gene expression in Bacillus subtilis. PLoS Genet 5:e1000566. doi: 10.1371/journal.pgen.1000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stragier P. 1994. A few good genes: developmental loci in Bacillus subtilis, p 207–245. In Piggot PJ, Moran CP, Jr, Youngman P (ed), Regulation of bacterial differentiation. ASM Press, Washington, DC. [Google Scholar]
- 18.Illing N, Errington J. 1991. The spoIIIA operon of Bacillus subtilis defines a new temporal class of mother-cell-specific sporulation genes under the control of the σE form of RNA polymerase. Mol Microbiol 5:1927–1940. doi: 10.1111/j.1365-2958.1991.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 19.Camp AH, Losick R. 2008. A novel pathway of intercellular signalling in Bacillus subtilis involves a protein with similarity to a component of type III secretion channels. Mol Microbiol 69:402–417. doi: 10.1111/j.1365-2958.2008.06289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaylock B, Jiang X, Rubio A, Moran CP, Jr, Pogliano K. 2004. Zipper-like interaction between proteins in adjacent daughter cells mediates protein localization. Genes Dev 18:2916–2928. doi: 10.1101/gad.1252704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doan T, Marquis KA, Rudner DZ. 2005. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol Microbiol 55:1767–1781. doi: 10.1111/j.1365-2958.2005.04501.x. [DOI] [PubMed] [Google Scholar]
- 22.Meisner J, Wang X, Serrano M, Henriques AO, Moran CP, Jr.. 2008. A channel connecting the mother cell and forespore during bacterial endospore formation. Proc Natl Acad Sci USA 105:15100–15105. doi: 10.1073/pnas.0806301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camp AH, Losick R. 2009. A feeding tube model for activation of a cell-specific transcription factor during sporulation in Bacillus subtilis. Genes Dev 23:1014–1024. doi: 10.1101/gad.1781709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morlot C, Rodrigues CDA. 2018. The new kid on the block: a specialized secretion system during bacterial sporulation. Trends Microbiol 26:663–676. doi: 10.1016/j.tim.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Zeytuni N, Strynadka NCJ. 2019. A hybrid secretion system facilitates bacterial sporulation: a structural perspective. Microbiol Spectr 7:7.1.08. doi: 10.1128/microbiolspec.PSIB-0013-2018. [DOI] [PubMed] [Google Scholar]
- 26.Galperin MY, Mekhedov SL, Puigbo P, Smirnov S, Wolf YI, Rigden DJ. 2012. Genomic determinants of sporulation in Bacilli and Clostridia: towards the minimal set of sporulation-specific genes. Environ Microbiol 14:2870–2890. doi: 10.1111/j.1462-2920.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traag BA, Driks A, Stragier P, Bitter W, Broussard G, Hatfull G, Chu F, Adams KN, Ramakrishnan L, Losick R. 2010. Do mycobacteria produce endospores? Proc Natl Acad Sci USA 107:878–881. doi: 10.1073/pnas.0911299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guillot C, Moran CP, Jr.. 2007. Essential internal promoter in the spoIIIA locus of Bacillus subtilis. J Bacteriol 189:7181–7189. doi: 10.1128/JB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, Žídek A, Green T, Tunyasuvunakool K, Petersen S, Jumper J, Clancy E, Green R, Vora A, Lutfi M, Figurnov M, Cowie A, Hobbs N, Kohli P, Kleywegt G, Birney E, Hassabis D, Velankar S. 2022. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res 50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeytuni N, Flanagan KA, Worrall LJ, Massoni SC, Camp AH, Strynadka NCJ. 2018. Structural characterization of SpoIIIAB sporulation-essential protein in Bacillus subtilis. J Struct Biol 202:105–112. doi: 10.1016/j.jsb.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serrano M, Vieira F, Moran CP, Jr, Henriques AO. 2008. Processing of a membrane protein required for cell-to-cell signaling during endospore formation in Bacillus subtilis. J Bacteriol 190:7786–7796. doi: 10.1128/JB.00715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeytuni N, Flanagan KA, Worrall LJ, Massoni SC, Camp AH, Strynadka NCJ. 2018. Structural and biochemical characterization of SpoIIIAF, a component of a sporulation-essential channel in Bacillus subtilis. J Struct Biol 204:1–8. doi: 10.1016/j.jsb.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues CDA, Henry X, Neumann E, Kurauskas V, Bellard L, Fichou Y, Schanda P, Schoehn G, Rudner DZ, Morlot C. 2016. A ring-shaped conduit connects the mother cell and forespore during sporulation in Bacillus subtilis. Proc Natl Acad Sci USA 113:11585–11590. doi: 10.1073/pnas.1609604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeytuni N, Hong C, Flanagan KA, Worrall LJ, Theiltges KA, Vuckovic M, Huang RK, Massoni SC, Camp AH, Yu Z, Strynadka NC. 2017. Near-atomic resolution cryoelectron microscopy structure of the 30-fold homooligomeric SpoIIIAG channel essential to spore formation in Bacillus subtilis. Proc Natl Acad Sci USA 114:E7073–E7081. doi: 10.1073/pnas.1704310114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Chan H, Bauda E, Contreras-Martel C, Bellard L, Villard A-M, Mas C, Neumann E, Fenel D, Favier A, Serrano M, Henriques AO, Rodrigues CDA, Morlot C. 2022. Structural insights into ring-building motif domains involved in bacterial sporulation. J Struct Biol 214:107813. doi: 10.1016/j.jsb.2021.107813. [DOI] [PubMed] [Google Scholar]
- 37.Levdikov VM, Blagova EV, McFeat A, Fogg MJ, Wilson KS, Wilkinson AJ. 2012. Structure of components of an intercellular channel complex in sporulating Bacillus subtilis. Proc Natl Acad Sci USA 109:5441–5445. doi: 10.1073/pnas.1120087109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meisner J, Maehigashi T, André I, Dunham CM, Moran CP, Jr.. 2012. Structure of the basal components of a bacterial transporter. Proc Natl Acad Sci USA 109:5446–5451. doi: 10.1073/pnas.1120113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chodisetti PK, Bahadur R, Amrutha RN, Reddy M. 2022. A LytM-domain factor, ActS, functions in two distinctive peptidoglycan hydrolytic pathways in E. coli. Front Microbiol 13:913949. doi: 10.3389/fmicb.2022.913949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stragier P. 2002. A gene odyssey: exploring the genomes of endospore-forming bacteria, p 519–526. In Sonenshein AL, Hoch JA, Losick R (ed), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC. [Google Scholar]
- 41.Meeske AJ, Rodrigues CDA, Brady J, Lim HC, Bernhardt TG, Rudner DZ. 2016. High-throughput genetic screens identify a large and diverse collection of new sporulation genes in Bacillus subtilis. PLoS Biol 14:e1002341. doi: 10.1371/journal.pbio.1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues CDA, Ramírez-Guadiana FH, Meeske AJ, Wang X, Rudner DZ. 2016. GerM is required to assemble the basal platform of the SpoIIIA–SpoIIQ transenvelope complex during sporulation in Bacillus subtilis. Mol Microbiol 102:260–273. doi: 10.1111/mmi.13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rigde DJ, Galperin MY. 2008. Sequence analysis of GerM and SpoVS, uncharacterized bacterial “sporulation” proteins with widespread phylogenetic distribution. Bioinformatics 24:1793–1797. doi: 10.1093/bioinformatics/btn314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fimlaid KA, Jensen O, Donnelly ML, Siegrist MS, Shen A. 2015. Regulation of Clostridium difficile spore formation by the SpoIIQ and SpoIIIA proteins. PLoS Genet 11:e1005562. doi: 10.1371/journal.pgen.1005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karmazyn-Campelli C, Rhayat L, Carballido-Lopez R, Duperrier S, Frandsen N, Stragier P. 2008. How the early sporulation sigma factor σF delays the switch to late development in Bacillus subtilis. Mol Microbiol 67:1169–1180. doi: 10.1111/j.1365-2958.2008.06121.x. [DOI] [PubMed] [Google Scholar]
- 46.Gupta RS, Patel S. 2019. Robust demarcation of the family Caryophanaceae (Planococcaceae) and its different genera including three novel genera based on phylogenomics and highly specific molecular signatures. Front Microbiol 10:2821. doi: 10.3389/fmicb.2019.02821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wattam AR, Brettin T, Davis JJ, Gerdes S, Kenyon R, Machi D, Mao C, Olson R, Overbeek R, Pusch GD, Shukla MP, Stevens R, Vonstein V, Warren A, Xia F, Yoo H. 2018. Assembly, annotation and comparative genomics in PATRIC, the all bacterial bioinformatics resource center. Methods Mol Biol 1704:79–101. doi: 10.1007/978-1-4939-7463-4_4. [DOI] [PubMed] [Google Scholar]
- 48.Rodrigues CDA, Marquis KA, Meisner J, Rudner DZ. 2013. Peptidoglycan hydrolysis is required for assembly and activity of the transenvelope secretion complex during sporulation in Bacillus subtilis. Mol Microbiol 89:1039–1052. doi: 10.1111/mmi.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fredlund J, Broder D, Fleming T, Claussin C, Pogliano K. 2013. The SpoIIQ landmark protein has different requirements for septal localization and immobilization. Mol Microbiol 89:1053–1068. doi: 10.1111/mmi.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holt SC, Gauther JJ, Tipper DJ. 1975. Ultrastructural studies of sporulation in Bacillus sphaericus. J Bacteriol 122:1322–1338. doi: 10.1128/jb.122.3.1322-1338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shivaji S, Srinivas TNR, Reddy GSN. 2014. Family Planococcaceae, p 303–345. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The Prokaryotes. Springer, Berlin, Germany. [Google Scholar]
- 52.Schleifer KH, Kandler O. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SJ, Chang J, Singh M. 2015. Peptidoglycan architecture of Gram-positive bacteria by solid-state NMR. Biochim Biophys Acta 1848:350–362. doi: 10.1016/j.bbamem.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasquina-Lemonche L, Burns J, Turner RD, Kumar S, Tank R, Mullin N, Wilson JS, Chakrabarti B, Bullough PA, Foster SJ, Hobbs JK. 2020. The architecture of the Gram-positive bacterial cell wall. Nature 582:294–297. doi: 10.1038/s41586-020-2236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis JJ, Xia F, Overbeek RA, Olsen GJ. 2013. Genomes of the class Erysipelotrichia clarify the firmicute origin of the class Mollicutes. Int J Syst Evol Microbiol 63:2727–2741. doi: 10.1099/ijs.0.048983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Huang JM, Zhou YL, Almeida A, Finn RD, Danchin A, He LS. 2020. Phylogenomics of expanding uncultured environmental Tenericutes provides insights into their pathogenicity and evolutionary relationship with Bacilli. BMC Genomics 21:408. doi: 10.1186/s12864-020-06807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikeyama N, Toyoda A, Morohoshi S, Kunihiro T, Murakami T, Mori H, Iino T, Ohkuma M, Sakamoto M. 2020. Amedibacterium intestinale gen. nov., sp. nov., isolated from human faeces, and reclassification of Eubacterium dolichum Moore et al. 1976 (Approved Lists 1980) as Amedibacillus dolichus gen. nov., comb. nov. Int J Syst Evol Microbiol 70:3656–3664. doi: 10.1099/ijsem.0.004215. [DOI] [PubMed] [Google Scholar]
- 58.Broder DH, Pogliano K. 2006. Forespore engulfment mediated by a ratchet-like mechanism. Cell 126:917–928. doi: 10.1016/j.cell.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stragier P. 1989. Temporal and spatial control of gene expression during sporulation: from facts to speculations, p 243–254. In Smith I, Slepecky RA, Setlow P (ed), Regulation of procaryotic development. ASM Press, Washington, DC. [Google Scholar]
- 60.Serrano M, Crawshaw AD, Dembek M, Monteiro JM, Pereira FC, Pinho MG, Fairweather NF, Salgado PS, Henriques AO. 2016. The SpoIIQ-SpoIIIAH complex of Clostridium difficile controls forespore engulfment and late stages of gene expression and spore morphogenesis. Mol Microbiol 100:204–228. doi: 10.1111/mmi.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopez-Garrido J, Ojkic N, Khanna K, Wagner FR, Villa E, Endres RG, Pogliano K. 2018. Chromosome translocation inflates Bacillus forespores and impacts cellular morphology. Cell 172:758–770. doi: 10.1016/j.cell.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang X, Rubio A, Chiba S, Pogliano K. 2005. Engulfment-regulated proteolysis of SpoIIQ: evidence that dual checkpoints control sigma activity. Mol Microbiol 58:102–115. doi: 10.1111/j.1365-2958.2005.04811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramírez-Guadiana FH, Meeske AJ, Rodrigues CDA, Barajas-Ornelas RC, Kruse AC, Rudner DZ. 2017. A two-step transport pathway allows the mother cell to nurture the developing spore in Bacillus subtilis. PLoS Genet 13:e1007015. doi: 10.1371/journal.pgen.1007015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tödter D, Gunka K, Stülke J. 2017. The highly conserved Asp23 family protein YqhY plays a role in lipid biosynthesis in Bacillus subtilis. Front Microbiol 8:883. doi: 10.3389/fmicb.2017.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soto-Avila L, Merce RC, Santos W, Castañeda N, Gutierrez-Ríos R-M. 2021. Distribution and preservation of the components of the engulfment. What is beyond representative genomes? PLoS One 16:e0246651. doi: 10.1371/journal.pone.0246651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galperin MY, Yutin N, Wolf YI, Alvarez RV, Koonin EV. 2022. Conservation and evolution of the sporulation gene set in diverse members of the Firmicutes. J Bacteriol 204:e00079-22. doi: 10.1128/jb.00079-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crick F. 1988. What mad pursuit: a personal view of scientific discovery, p. 188. Basic Books, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental text and Fig. S1 to S13. Download jb.00187-22-s0001.pdf, PDF file, 1.0 MB (1,012KB, pdf)