Abstract
Pneumococcal disease can be divided into invasive disease, i.e. when bacteria are detected in normally sterile body fluids, and noninvasive disease. Pneumococcal disease occurs more frequently in younger children and older adults. It is estimated that, in 2050, 30.3% of the European population will be ≥65 yrs old, compared with 15.7% in 2000. Preventive medicine, including vaccination, is essential for the promotion of healthy ageing. Uptake rates for influenza vaccination in the elderly are generally low, despite recommendations in many countries. In addition, it has been reported that influenza infections can make people more susceptible to pneumococcal infections. Despite pneumococcal vaccination, case fatality rates for patients hospitalised with invasive pneumococcal disease have remained at around 12% since the 1950s. Even when effective antibiotic therapy is administered, mortality can be high amongst immunocompetent patients in intensive care. Timely and accurate diagnosis of pneumococcal disease and identification of patients at high risk of poor outcome is essential to ensure that adequate treatment, including hospitalisation when necessary, is implemented as early as possible. Improved diagnostic techniques and more efficacious treatments may help to reduce the burden of pneumococcal disease, but preventive measures, such as influenza and pneumococcal vaccination, should be promoted in order to avoid preventable disease, particularly in the elderly.
Keywords: Adults, community-acquired pneumonia, immunisation programmes, invasive pneumococcal disease, pneumococcal conjugate vaccine, pneumococcal pneumonia
EPIDEMIOLOGY AND BURDEN OF DISEASE
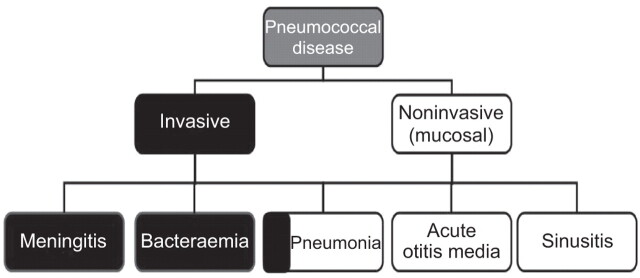
Pneumococcal disease can be broadly grouped into two categories of invasive disease and noninvasive (also termed mucosal) disease (fig. 1) [1]. Invasive disease is usually diagnosed when bacteria are identified in normally sterile body fluids. Noninvasive forms of disease may become invasive (e.g. pneumonia when accompanied by bacteraemia) [2].
Figure 1.
Pneumococcal diseases can be broadly divided into invasive and noninvasive disease. Invasive disease is diagnosed when Streptococcus pneumoniae is detected in normally sterile body fluids. Pneumonia can be invasive (bacteraemic) or noninvasive.
In a 3-month retrospective chart review of all emergency department visits in an inner city hospital in the USA, 1,948 of the 8,811 patients evaluated presented with respiratory complaints. About 10% of those with respiratory complaints were diagnosed as community-acquired pneumonia (CAP) [3]. In more than 40% of cases of CAP, no pathogen is identified, but it is estimated that about two-thirds of bacterial pneumonia is caused by pneumococcus [4, 5]. The clinical burden associated with CAP is, in part, related to its bacteraemic and invasive potential.
The clinical and economic burden of invasive pneumococcal disease (IPD) is particularly high in older adults. For example, in a retrospective study of IPD that examined surveillance data covering a representative section of 25% of the Dutch population from June 2004 to June 2006 (prior to pneumococcal conjugate vaccine (PCV)7 implementation), 1,275 hospitalised cases were identified. The age-specific annual incidence peaked in the 3- to 5-month-old children (63 cases per 100,000 population, annually) and after falling started to increase steadily to 26 cases in those aged 50–64 yrs up to 97 cases per 100,000 population, annually, in those aged ≥90 yrs [6]. More than 80% of IPD in older adults was bacteraemic pneumonia. In 1997, data were reported to the Centers for Disease Control and Prevention from active, population-based surveillance for IPD in seven metropolitan areas in the USA (population 16,073,596). The case fatality rates were highest for the elderly; 29% of IPD was reported in the elderly, but 55% of deaths occurred in this age group [6, 7]. The case fatality rate for patients hospitalised with IPD has remained relatively stable at about 12% since 1952, despite the availability of the pneumococcal polysaccharide vaccine (PPV) (table 1) [8–11].
Table 1. Case fatality rate in patients hospitalised for invasive pneumococcal disease, 1952–2001.
| Period studied [ref.] | Patients n | Case fatality rate % |
| 1952–1962 [8] | 1130 | 13 |
| 1966–1995 [10] | 4432 | 12 |
| 1995–1997 [9] | 5837 | 12 |
| 1999–2001 [11] | 730 | ∼12# |
#: 90-day fatality in intensive care unit versus ward patients, with an average of the two rates ∼12%.
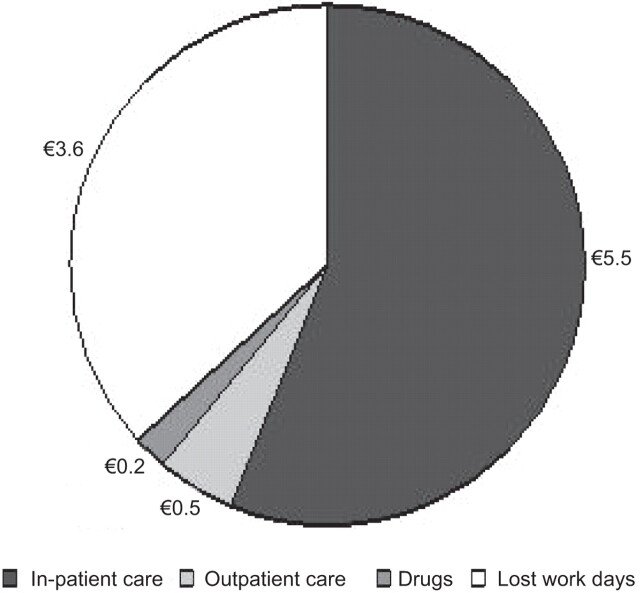
The current annual clinical and economic burden of pneumococcal disease in adults aged ≥50 yrs in the USA was estimated using a model. Although IPD represented only 6% of all pneumococcal pneumonia, the IPD case fatality rate was 24.4% compared with 9.7% for non-bacteraemic pneumonia requiring in-patient care [12]. The total cost (indirect and direct) was estimated to be $5.5 billion, with IPD being responsible for $1.8 billion. The authors concluded that, despite vaccination with PPV23 in this age group and indirect benefits from PCV7 vaccination of young children, pneumococcal disease remains a substantial burden for American adults aged ≥50 yrs. It has been estimated that, in Europe, pneumonia costs globally about €10 billion per year. Direct costs (in-patient care, outpatient care and drugs) account for €6.2 billion and indirect (working days lost) for €3.6 billion (fig. 2) [13].
Figure 2.
Annual costs (in € billion) associated with invasive pneumococcal disease in Europe [13].
In addition, data from Canada (1995–2009) suggest that influenza influences Streptococcus pneumoniae disease incidence by enhancing short-term (1-week lag) risk of invasion in colonised subjects [14]. The mechanism of action is thought to involve influenza virus infecting the epithelial cells of the respiratory tract, causing extensive epithelial damage. This is thought to be due to direct effects of the virus on the cell and possibly also due to the effects of induced interferon. At later times, cell death may also result from the actions of cytotoxic T-cells. The efficiency of ciliary clearance is reduced, leading to impaired function of the mucus and reduced clearance of infectious agents from the respiratory tract. Thus, other pathogens, such as S. pneumoniae, can more easily attach themselves to the cells and cause infections in the lung tissue. In contrast to the risk of invasion in colonised subjects, the lack of correlation between the seasonal wave forms for influenza and S. pneumoniae disease suggests there is less effect on person-to-person transmission [14].
PUBLIC HEALTH POLICY, HEALTHY AGEING AND PREVENTION
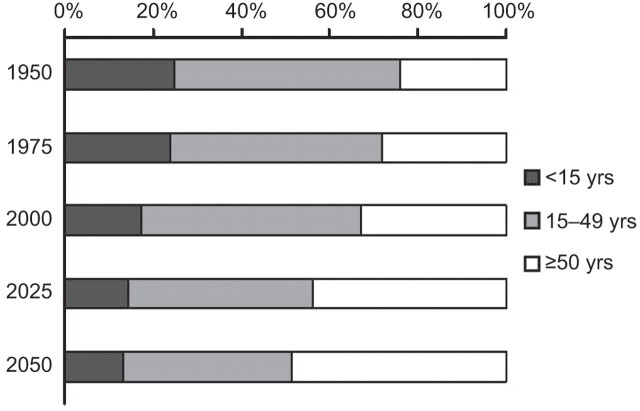
Progress in healthcare, antibiotics and vaccines in a world with improved living standards in most industrial countries has led to an increased lifespan. Over the past century, life expectancy has increased from about 40 yrs old to just over 80 yrs. The European population grew from 350 million in 1950 to 418 million in 1975, and in 2050 it is expected to reach 449 million. It is estimated that, in 2050, 30.3% of the European population will be ≥65 yrs old, compared with 15.7% in 2000 (fig. 3) [15].
Figure 3.
Percentage of people by age group from 1950 and projected up to 2050. Data taken from [15].
As people grow old, their immune system also grows old. This phenomenon, known as immunosenescence, makes older people more susceptible to infectious diseases and less responsive to vaccines. Older people tend to have more chronic comorbidities than younger people, and this makes any infectious disease more serious [16]. Immunosenescence develops through complex changes in the ageing immune system (table 2) [17–20].
Table 2. Summary of the complex changes in the immune system resulting in immunosenescence.
| Innate immunity | Adaptive immunity |
| ↑ inflammatory reactions | Restricted T-cell repertoire |
| ↑ numbers of natural killer cells | ↓ naïve T-cell output |
| ↓ antigen uptake and presentation | ↑ number of effector T-cell |
| ↓ phagocytic capacity and oxidative burst of macrophages | Low-affinity antibody responses |
| ↓ cytotoxic function | ↓ B-cell production and defective isotype switching |
This situation has led to an awareness of programmes to promote healthy ageing. The outcome for one such community-based programme aimed at promoting prevention and reducing risk factors for common diseases among older people was the proposal of 10 keys for healthy ageing [21]. One of these 10 keys concerns vaccination. In many countries, influenza and pneumococcal polysaccharide vaccines are recommended for elderly people (table 3) [22–26]. The effectiveness of influenza and pneumococcal polysaccharide vaccines has been shown in different age groups. In addition, several studies have reported the synergy between influenza and pneumococcal polysaccharide vaccination during the influenza season, even in seasons with low activity [27–29]. Unfortunately, in most countries the vaccine uptake for these two vaccines in the elderly population is below the target set by the World Health Organization (WHO) [30].
Table 3. Summary of World Health Organization (WHO) and the Advisory Committee on Immunization Practices (ACIP) recommendations for pneumococcal polysaccharide and influenza vaccinations.
| WHO recommendations | |
| Pneumococcal polysaccharide vaccination | Influenza vaccination |
| Healthy elderly (>65 yrs of age), particularly those living in institutions | Elderly individuals above a nationally defined age limit, irrespective of other risk factors |
| Patients with chronic organ failure | Residents of institutions for elderly people and the disabled |
| Heart, lung, liver or kidney disease, diabetes mellitus and alcoholism | Elderly, non-institutionalised individuals with chronic heart or lung diseases, metabolic disease including diabetes or renal disease, or immunodeficiencies |
| Children >2 yrs old at high risk for disease (splenectomised children and sickle-cell disease) | All individuals >6 months of age with any of the conditions listed above |
| Patients with immunodeficiencies particularly those with functional or anatomical asplenia | Other groups defined on the basis of national data and capacities, such as contacts of high-risk people, pregnant females, healthcare workers and others with key functions in society, as well as children 6–23 months of age |
| Prevention of subsequent pneumococcal infection in patients recovering from proven or assumed pneumococcal pneumonia | |
| ACIP recommendations | |
| Pneumococcal polysaccharide vaccination | Influenza vaccination |
| Adults aged ≥65 yrs Patients with chronic illness |
Persons aged ≥50 yrs |
| Chronic cardiovascular, pulmonary and liver disease | Adults and children with chronic disorders |
| Diabetes mellitus | Pulmonary (including asthma) and cardiovascular systems (except hypertension), renal, hepatic, haematological or metabolic disorders (diabetes mellitus) |
| Alcoholism | Immunocompromised adults and children, including HIV-infected persons and users of immunosuppressive medications |
| Cerebrospinal fluid leak | Children and teenagers (6 months to 18 yrs of age) receiving long-term aspirin therapy |
| Persons aged 2–64 yrs with physical or functional asplenia | Females who will be pregnant during the influenza season |
| Immunocompromised persons aged ≥2 yrs including those with HIV infection, leukaemia, lymphoma, Hodgkin's disease, multiple myeloma, generalised malignancy, chronic renal failure or nephritic syndrome; those receiving immunosuppressive chemotherapy (including corticosteroids) and those who have received an organ or bone marrow transplant | Residents of nursing homes and other chronic care facilities |
| Persons aged 2–64 yrs living in special environments or social settings | Healthy children aged 6–59 months |
| Persons who live with, or care for, persons at high risk for flu-related complications | |
| Adults and children who have any condition that can compromise respiratory function or the handling of respiratory secretions or that can increase the risk for aspiration (e.g. cognitive dysfunction, spinal cord injuries, seizure disorders or other neuromuscular disorders) | |
In most European Union (EU) countries there are national recommendations for pneumococcal polysaccharide vaccination of risk groups in all ages, but the number of identified risk groups varies from two to 12 in the different countries. The majority of EU countries also have aged-based recommendations for those >65 yrs of age [31]. For influenza vaccination, 22 of the 29 EU countries have recommendations for vaccination of those aged ≥65 yrs; in Germany, Greece, Hungary and Iceland the recommendation is for those aged ≥60 yrs, in Malta the recommendation is for those aged ≥55 yrs and in Poland for those aged ≥50 yrs [31]. The WHO has set a target vaccination uptake for those aged ≥65 yrs in Europe of 50% and 75% in 2006 and 2015, respectively [32]. In January 2008, 11 out of 22 countries surveyed had reached the 2006 target, but only one (the Netherlands) had reached the 2015 target [32].
For healthcare deciders, even when vaccines have shown their efficacy, decisions made on a public health level often differentiate between the choice to vaccinate those who are at highest risk or to base the vaccination strategy on age. In many countries there are risk-based strategies for adults for various vaccines, such as hepatitis A and B, influenza, pneumococcal and varicella [33–35]. However, there are many advantages to having an age-based strategy, since at-risk strategies alone may have sub-optimal uptake, missing those who are targeted (table 4).
Table 4. Comparison of risk-based and age-based vaccination strategies.
| Age-based strategies | At-risk strategies |
| Target group easy to access | High-risk people often difficult to access (e.g. socially disadvantaged) |
| Infrastructures usually exist to administer vaccines | Responsibility for vaccination lies with different healthcare professionals |
| Community protection (herd effect) is possible | Difficult to establish a herd effect |
| Generally higher levels of vaccine uptake achieved | Unlikely to achieve high levels of vaccine uptake |
CURRENT CHALLENGES
Diagnosis
The difficulty of timely diagnosis and the correct assessment of the patient's condition, particularly in the outpatient setting, are major challenges in CAP. It is important to identify patients at risk of poor outcome, and to identify those who should be offered intensive care or adjuvant therapy. Although a new radiograph infiltrate with corresponding clinical symptoms are the only accepted criteria for confirmed pneumonia, the diagnosis is mainly based on clinical findings. The updated British Thoracic Society guidelines for the management of CAP in adults published in 2009 recommend not performing a chest radiograph [36], unless: 1) the diagnosis is not certain and it will help with the differential diagnosis and management of the acute illness; 2) the patient is not responding to treatment; or 3) there is a suspicion of an underlying lung pathology, such as lung cancer.
The recently published European Respiratory Society/European Society of Clinical Microbiology and Infectious Diseases guidelines recommend that “in the case of persisting doubt after C-reactive protein (CRP) testing, a chest X-ray should be considered to confirm or reject the diagnosis” [5]. The evidence for the diagnostic accuracy of CRP was rated as inconsistent, but a systematic review concluded that it could be used to rule out CAP [37]. In practice, the diagnosis of CAP is generally based on clinical symptoms and physical examination, and the decision to send patients to hospital or prescribe outpatient treatment is made on the basis of their general condition. The introduction of clinical scores, such as the CRB-65, has helped to improve identification of patients at risk but about 25% of patients are still misclassified [38]. The consequences of erroneous diagnosis can be serious. In the case of a false-negative classification there will be a delay to treatment; this can occur in young and middle-aged patients, who are otherwise healthy, or in elderly patients without typical signs of pneumonia. Conversely, a false-positive classification will lead to unnecessary hospitalisation and probably excessive antibiotic treatment in patients who have a favourable prognosis. As was mentioned previously, the indication for a chest radiograph in the outpatient setting is based on the clinician's decision [5]. However, because of the unfavourable consequences of a false diagnosis, a chest radiograph should be performed, when available, in order to provide a definitive diagnosis of pneumonia.
The classical microbiological methods, such as Gram-staining and culture have a low sensitivity, and there is a delay before results from culture are available. Isolation of pneumococci from blood provides a specific aetiologicial diagnosis that not only allows effective treatment to be given to the patient, but can also be used for surveillance of the epidemology of pneumococcal infections. This is particularly important in the era of conjugate pneumococcal vaccines to detect any serotype replacement. Unfortunately, blood culture methods only detect pneumococci in about 10% to 20% of pneumococcal pneumonia cases, and this detection rate can be lower, for example, if there has been prior antibiotic treatment.
To overcome these limits to their clinical utility, significant effort has been made to develop new diagnostic tools that give faster results with better sensitivity [39, 40]. One of these tools is the detection of pneumococcal antigen in urine and other body fluids that gives an aetiological diagnosis in a couple of hours. The newly developed BinaxNOW test shows high sensitivity and specificity in adults [40]. In children, nasopharyngeal colonisation leads to poor specificity with this test, but the results from a recently published cohort study in adults suggest that false-positive results for pneumococcal infection detection in adults are unlikely. In the study, the included females had a pneumococcal colonisation rate of 25%; the test sensitivity was 4.2% with a specificity of 97.3% and only 3% of the females with pneumococcal nasopharyngeal colonisation were found to be positive [40].
Another non-culture-based diagnostic approach uses real-time (rt)-PCR techniques. This enables the rapid and accurate quantification of the bacterial load, both viable and nonviable. This approach, which uses the LytA gene, is more sensitive than blood culture [41]. The rt-PCR test was positive for 85% of blood culture positive samples, whereas the blood culture test was positive for only 50% of rt-PCR positive samples [41]. It should also be noted that there was a wide range for the number of copies of S. pneumoniae DNA per mL of whole blood, demonstrating that pneumococcal pneumonia is not an “all or nothing” phenomenon, but more of a continuum. Moreover, in this prospective study, an association between the severity of pneumococcal pneumonia and bacterial genomic load with a cut-off of <103 copies per mL was reported (table 5) [41]. The authors concluded that this method may be a useful tool for the assessment of the severity of pneumococcal pneumonia, since high genomic load was associated with higher likelihood of more severe disease and death.
Table 5. Association between bacterial load (as assessed using real-time PCR techniques) and clinical outcome.
| Low bacterial load# | High bacterial load¶ | OR (95% CI) | |
| <103 copies per mL | ≥103 copies per mL | ||
| Rapid radiological spread | 1.5 | 25.9 | 22.75 (3.36–148.37) |
| Acute respiratory distress syndrome | 1.5 | 18.5 | 14.77 (2.12–99.49) |
| Septic shock | 9.1 | 44.4 | 8.00 (2.65–24.12) |
| Need for mechanical ventilation | 4.5 | 33.3 | 10.50 (2.74–39.63) |
| Hospital case fatality rate | 6.1 | 25.9 | 5.43 (1.52–19.24) |
Data are presented as the percentage of patients for each outcome, unless otherwise stated. #: n=66; ¶: n=27. Data are taken from [41].
The indications for the use of the tests discussed above in everyday clinical practice are not based on results from prospective studies, but are largely based on expert opinions. The present antibiotic treatment strategy that covers the whole pathogen spectrum and incorporates the severity of infection and the patient's general condition works well. It has resulted in an international agreement that microbiological tests should not be performed in the outpatient setting for patients with mild or moderate CAP. In case of severe CAP requiring hospitalisation, blood culture, sputum examination and urine antigen tests are strongly recommended by the European guidelines [5]. It would seem that the use of urine tests and the PCR methods are going to be increasingly used, but large-scale clinical trials are needed to evaluate their clinical utility [42]. Since there is evidence that early introduction of adequate therapy is critical for the final outcome, in addition to the classical methods, we should urge the use of [42]: 1) chest radiograph for the definitive diagnosis of pneumonia; 2) urine antigen tests to reveal rapidly the presence of the most dangerous pathogens in CAP; and 3) rt-PCR to obtain a more accurate assessment of the risk of an unfavourable outcome.
CRP and procalcitonin have been evaluated not only for use in aetiological diagnosis, but recently also as potential prognostic markers in the decision to admit patients to hospital or an intensive care unit (ICU) [43]. Although they have shown to be useful in several settings, there is not enough evidence to draw final conclusions on their utility in the management of CAP.
Risk factors
The assessment of patients with suspected CAP requires identifying the presence of risk factors that are known to be associated with the likelihood of developing pneumococcal disease and poorer outcome. One major risk factor is age, specifically for children aged <2 yrs and adults ≥65 yrs [44]. The presence of underlying medical conditions, such as chronic heart, lung (including asthma), renal or liver disease, are also risk factors for developing pneumococcal disease and poorer outcome [44]. Results from a study on a nationwide database in Germany showed a clear relationship between age and in-hospital case fatality rates for adult patients hospitalised with CAP [45]. In patients <50 yrs old, the case fatality rate was <3.6%, compared with 6.6% (50–59 yrs), 9.6% (60–69 yrs), 13.9% (70–79 yrs), 19.1% (80–89 yrs) and 25.5% (≥90 yrs). Other risk factors for poor outcome in patients with CAP have been identified. These include: multilobar involvement; septic shock; need for ICU or mechanical ventilation; alcohol abuse; chronic cardiac or pulmonary diseases; renal failure; immunosuppression; socioeconomic status; and living in a long-term care facility [46–48].
In a prospective cohort study of 404 patients with CAP admitted to Methodist Healthcare Memphis Hospitals in the USA between November 1998 and June 2001, after a mean follow-up time of 1,058 days, increasing age (41 to 80 yrs) was an independent predictor of medium-term mortality. Medium-term survival was better in younger patients, but it was poor in younger patients with comorbidites [49]. In another prospective cohort study in Barcelona, Spain of non-severely immunosuppressed adults hospitalised between February 1995 and June 2008 with pneumococcal pneumonia, patients with septic shock at admission (defined as a systolic blood pressure <90 mmHg and peripheral hypoperfusion with the need for vasopressors for >4 h after fluid replacement) had longer hospital stays, needed mechanical ventilation more often and had a higher risk of early and overall mortality [50]. Smoking, chronic glucocorticoid treatment and serotype 3 pneumococcal infections were identified as independent risk factors for septic shock.
In a retrospective cohort study of 161 patients admitted to the ICU in one of two American hospitals with a diagnosis of CAP in 1999 to 2001, later admission to ICU with CAP (after day 2 versus direct admission or within 24 h) was associated with higher 30-day mortality, even after adjustment for disease severity [51]. Among those who were admitted early, 53% had severe CAP and the 30-day case fatality rate was 23.4%, compared with those who were admitted later, where 26% had severe CAP with a 30-day case fatality rate of 47.4% [51].
In a prospective cohort study from 2001 to 2009 of 626 adults hospitalised in the Hospital Clinic of Barcelona, Spain with a diagnosis of pneumococcal pneumonia, 38% had pulmonary complications: pleural effusion, empyema and multilobar infiltration [52]. Although the case fatality rate was similar to that for patients without pulmonary complications, these patients presented with higher rates of bacteraemia, shock and need for mechanical ventilation, with associated higher rates of ICU admission and longer hospital stay [52].
Pneumococcal serotypes
Pneumococcal serotype has been reported to be associated with disease severity and invasiveness [53]. In a meta-analysis evaluating the association of serotype with the risk of death, serotypes 1, 7F and 8 were associated with a lower mortality risk. Serotypes 3, 6A, 6B, 9N and 19F were found to be associated with a higher risk of fatality compared with serotype 14 [54]. These serotypes have high carriage rates, low invasiveness and thicker capsules. In a prospective observational study not included in the meta-analysis, it was reported that serotype 3 infections, as well as current smoking and chronic corticosteroid treatment, were independent risk factors for septic shock [50]. In another study, also not included in the meta-analysis, adult patients with serotypes with low invasiveness potential (3, 6A, 6B, 8, 19F and 23F) had poorer outcomes, including higher mortality (table 6) [55].
Table 6. Influence of pneumococcal serotype on outcome in adults with bacteraemic pneumonia.
| Invasive potential | Other serotypes | ||
| High | Low | ||
| Need for vasopressor | 25.8 | 44.8 | 34.0 |
| Mechanical ventilation | 12.9 | 25.6 | 13.8 |
| Severe pneumonia | 26.8 | 51.2 | 34.9 |
| 30-day case fatality rate | 3.2 | 25.6 | 7.3 |
Data are presented as %. High invasive disease potential serotypes included serotypes 1, 5 and 7F; low invasive disease potential serotypes included 3, 6A, 6B, 8, 19F and 23F. Data taken from [55].
Treatment
The primary treatment for patients with CAP is antibiotic therapy [5]. Penicillin has been the treatment of choice since the 1940s but resistance to this and other antibiotics has grown [56, 57]. Nevertheless, beginning in the 1960s, it was reported that penicillin could reduce mortality only in patients who survived more than 5 days [8]. A secondary analysis of the CAPUCI database (a prospective observational multicentre study) showed that mortality was high among immunocompetent patients admitted to the ICU with bacterial pneumonia, despite adequate initial antibiotic therapy and management of comorbidities [58]. In a cohort study assessing in-hospital and 90-day mortality in 125 hospitalised patients with bacteraemic pneumococcal CAP, it was reported that delay to adequate antibiotic therapy (>4 h) was independently associated with higher in-hospital and 90-day fatality [59].
It is obvious that some patients die despite receiving adequate antibiotic therapy, so clinical failure cannot always be attributed to the administration of ineffective antibiotics. The use of combination antibiotic therapy, rather than monotherapy, may improve efficacy for patients with severe CAP and invasive pneumococcal infections. Despite several trials, the beneficial effect of combination therapy remains controversial [56]. One randomised controlled trial evaluated moxifloxacin monotherapy (sequential i.v. and oral) versus ceftriaxone (i.v.) and levofloxacin (sequential i.v. and oral) with stratification on the Pneumonia Severity Index score (III versus IV/V). This trial concluded that monotherapy was not inferior to combination therapy in patients hospitalised for CAP, however, the most severely ill patients (requiring mechanical ventilation or vasopressor therapy) were excluded from the study [60]. The results from several retrospective observational studies suggest that combined β-lactam and macrolide therapy in patients with pneumococcal infections is better than β-lactam monotherapy, particularly in severe CAP, but others have not confirmed this finding [56]. The superiority of the combined macrolide and β-lactam therapy might be due to the well-recognised anti-inflammatory effect of macrolides [61]. In a prospective cohort study in 27 ICUs in nine European countries, combined β-lactam and macrolide was compared with combined β-lactam and fluoroquinolone in 218 intubated patients. The macrolide combination was found to improve survival compared with fluoroquinolone combination. However, no significant differences in mortality rates were found when patients treated with ciprofloxacin, which is clearly significantly less efficacious against pneumococci than fluoroquinolone, were excluded [62]. Analyses from an observational study initiated by the German competent network CAPNETZ suggest also that intravenous β-lactam combined with a macrolide is superior to β-lactam alone [63]. In another study using a case–control design with propensity matching, it was reported that early combination antibiotic therapy improved survival, compared with monotherapy, in patients in the ICU with bacterial septic shock due to both Gram-positive and Gram-negative infections [64]. In a subgroup of patients with pneumococcal bacteraemia, patients receiving combination therapy had a higher survival rate than those receiving monotherapy (55 (39.0%) out of 141 versus 35 (24.8%) out of 141). This additional effect could be due to the beneficial anti-inflammatory effect of macrolides which may be manifested in the most advanced stage of sepsis, but these results should be interpreted with caution [64]. In the recently published updated European guidelines, combination treatment is recommended for patients with severe CAP [5].
Meningitis occurs relatively infrequently in patients with IPD, but it is associated with a high mortality rate in adults (30%) and neurological complications in a significant number of those who survive [65]. S. pneumoniae is one of the most frequent causes of bacterial meningitis; however, since the introduction of conjugated pneumococcal vaccine in 2000, the number of pneumococcal meningitis cases in children has dropped dramatically [66].
β-Lactams have been shown to be efficacious against invasive pneumococcus serotypes causing meningitis. The pneumococcus serotypes isolated from patients with pneumococcal meningitis were shown to be 96% to 98% sensitive to cefotaxime or ceftriaxone and 100% sensitive to vancomycin. Combination of these antibiotics with dexamethasone is the treatment of choice for those with suspected pneumococcal meningitis [67]. After having determined the sensitivity of the isolated S. pneumoniae, the antibiotic treatment should be adapted to the minimal inhibitory concentration (MIC). Vancomycin can be stopped if the MIC is below 1 mg·L−1 and treatment continued with high dose cefotaxime or ceftriaxone.
VACCINES FOR THE PREVENTION OF PNEUMOCOCCAL DISEASE
Currently, two vaccines are available for the prevention of invasive pneumococcal disease in adults: a polysaccharide 23-valent vaccine and a pneumococcal conjugate 13-valent vaccine that has been approved recently by the European Medicines Agency for adults over the age of 50 yrs. Although the two vaccines have different mechanisms to induce immunity, an assay, which measures functional antibodies, is considered to be the best available means to compare them [68]. The immune responses to the pneumococcal conjugate vaccine, as measured with the OPA assay, were shown to be non-inferior to the responses to the polysaccharide vaccine for the 12 common serotypes, and superior for several of them. A conjugate vaccine can be expected to have benefits over a polysaccharide vaccine, due to the characteristics of a T-cell dependent response in terms of affinity, maturation of antibodies with repeated exposure, induction of immunological memory and long lasting immunity. The pneumococcal conjugate 13-valent vaccine has demonstrated all these characteristics in children and fundamental differences in adults are not expected. The efficacy in adults is currently being investigated and results will be available soon [69]. This tool for the prevention of invasive pneumococcal disease in adults is, therefore, now available and will be implemented according to recommendations of national health authorities in Europe.
CONCLUSIONS
In summary, the lack of specific diagnostic tools means that the burden of pneumococcal disease is probably underestimated. More importantly, many patients do not have access to suitable care as early as they should. This delay to treatment results in patients with more severe disease and poorer prognosis.
It has been shown that age is an independent risk factor for pneumococcal disease. Comorbidites have also been shown to be important risk factors for pneumococcal disease. With an ageing population and in those with associated comorbidities, there will be an increasingly high burden of disease, along with the associated costs, so prevention can contribute to the promotion of healthy ageing.
It is important that we concentrate our efforts on improving the management of pneumococcal diseases, so that diagnosis is improved and patients requiring hospital admission for more severe disease are identified in a timely manner. However, it is also important to promote the prevention of pneumococcal diseases through effective vaccination to offer the promise of a prompt and direct impact on the clinical and economic burden of pneumococcal diseases.
Acknowledgments
The authors would like to thank M. Haugh (MediCom Consult) for medical writing assistance funded by Pfizer, France.
Footnotes
Provenance
Publication of this peer-reviewed article was supported by Pfizer, France (article sponsor, European Respiratory Review issue 123).
Statement of Interest
E. Luwig has received support for participation in conferences, and honoraria for lecturing and consulting from Pfizer. P. Bonanni has received financial support for staff involved in studies on pneumococcal vaccination and for taking part as a speaker in symposia sponsored by different vaccine producers. G. Rohde has received fees for speaking and consulting from Pfizer. A. Sayiner has participated as a speaker in a symposium organised by Pfizer. He has also been on the advisory board of Pfizer, Turkey. A. Torres has received research grants from Pfizer and Covidien. He has participated on the advisory boards of Astellas and Sanofi-Aventis.
REFERENCES
- 1.World Health Organization. Acute Respiratory Infections (Update September 2009). www.who.int/vaccine_research/diseases/ari/en/index3.html Date last accessed: December 12, 2011.
- 2.Centers for Disease Control and Prevention. Pneumococcal Disease. Epidemiology and Prevention of Vaccine-preventable Diseases. 12th Edn. Atlanta, Centers for Disease Control and Prevention, 2011; pp. 233–248. [Google Scholar]
- 3.Khalil A, Kelen G, Rothman RE. A simple screening tool for identification of community-acquired pneumonia in an inner city emergency department. Emerg Med J 2007; 24: 336–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67: 71–79. [DOI] [PubMed] [Google Scholar]
- 5.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Clin Microbiol Infect 2011; 17: E1–E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen AG, Rodenburg GD, de Greeff SC, et al. Invasive pneumococcal disease in the Netherlands: syndromes, outcome and potential vaccine benefits. Vaccine 2009; 27: 2394–2401. [DOI] [PubMed] [Google Scholar]
- 7.Butler JC, Schuchat A. Epidemiology of pneumococcal infections in the elderly. Drugs Aging 1999; 15: 11–19. [DOI] [PubMed] [Google Scholar]
- 8.Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med 1964; 60: 759–776. [DOI] [PubMed] [Google Scholar]
- 9.Feikin DR, Schuchat A, Kolczak M, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health 2000; 90: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 1996; 275: 134–141. [PubMed] [Google Scholar]
- 11.Restrepo MI, Mortensen EM, Velez JA, et al. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest 2008; 133: 610–617. [DOI] [PubMed] [Google Scholar]
- 12.Weycker D, Strutton D, Edelsberg J, et al. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine 2010; 28: 4955–4960. [DOI] [PubMed] [Google Scholar]
- 13.European Respiratory Society/European Lung Foundation. European Lung White Book. The first comprehensive survey on respiratory health in Europe. Loddenkemper R, Gibson GJ, Sibille Y, eds. Sheffield, ERSJ, 2003. [Google Scholar]
- 14.Kuster SP, Tuite AR, Kwong JC, et al. Evaluation of coseasonality of influenza and invasive pneumococcal disease: results from prospective surveillance. PLoS Med 2011; 8: e1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turning the age pyramid on its head. RDT Info. Demography and family; the changing face of the family. European Commission, 2006; pp, 11–13. [Google Scholar]
- 16.Christensen K, Doblhammer G, Rau R, et al. Ageing populations: the challenges ahead. Lancet 2009; 374: 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009; 9: 185–194. [DOI] [PubMed] [Google Scholar]
- 18.Weinberger B, Herndler-Brandstetter D, Schwanninger A, et al. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008; 46: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 19.Aspinall R, Del Giudice G, Effros RB, et al. Challenges for vaccination in the elderly. Immun Ageing 2007; 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maggi S. Vaccination and healthy aging. Expert Rev Vaccines;9: 3–6. [DOI] [PubMed] [Google Scholar]
- 21.Robare JF, Bayles CM, Newman AB, et al. The "10 keys" to healthy aging: 24-month follow-up results from an innovative community-based prevention program. Health Educ Behav;38: 379–388. [DOI] [PubMed] [Google Scholar]
- 22.Pneumococcal conjugate vaccine for childhood immunization – WHO position paper. Wkly Epidemiol Rec 2007; 82: 93–104. [PubMed] [Google Scholar]
- 23.Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010; 59: 1102–1106. [PubMed] [Google Scholar]
- 24.Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60: 1128–1132. [PubMed] [Google Scholar]
- 25.Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children – use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine – recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59: 1–18. [PubMed] [Google Scholar]
- 26.World Health Organization. Influenza. Fact Sheet 211. www.who.int/mediacentre/factsheets/fs211/en/ Date last updated: April 2009. Date last accessed: December 6, 2011.
- 27.Hung IF, Leung AY, Chu DW, et al. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis 2010; 51: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 28.Christenson B, Pauksen K, Sylvan SP. Effect of influenza and pneumococcal vaccines in elderly persons in years of low influenza activity. Virol J 2008; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichol KL. The additive benefits of influenza and pneumococcal vaccinations during influenza seasons among elderly persons with chronic lung disease. Vaccine 1999; 17: Suppl. 1, S91–S93. [DOI] [PubMed] [Google Scholar]
- 30.Mereckiene J, Cotter S, Weber JT, et al. Low coverage of season influenza vaccination in the elderly in many European countries. Eurosurveillance 2008; 13: 1–3. [DOI] [PubMed] [Google Scholar]
- 31.Pebody RG, Leino T, Nohynek H, et al. Pneumococcal vaccination policy in Europe. Euro Surveill 2005; 10: 174–178. [PubMed] [Google Scholar]
- 32.Mereckiene J, Cotter S, Nicoll A, et al. National season influenza vaccination survey in Europe, 2008. Eurosurveillance 2008; 13: 1–7. [DOI] [PubMed] [Google Scholar]
- 33.Lam S, Jodlowski TZ. Vaccines for older adults. Consult Pharm 2009; 24: 380–391. [DOI] [PubMed] [Google Scholar]
- 34.Resnick HE, Foster GL, Bardsley J, et al. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care 2006; 29: 531–537. [DOI] [PubMed] [Google Scholar]
- 35.Parkins MD, McNeil SA, Laupland KB. Routine immunization of adults in Canada: review of the epidemiology of vaccine-preventable diseases and current recommendations for primary prevention. Can J Infect Dis Med Microbiol 2009; 20: e81–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009; 64: iii1–iii55. [DOI] [PubMed] [Google Scholar]
- 37.Falk G, Fahey T. C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies. Fam Pract 2009; 26: 10–21. [DOI] [PubMed] [Google Scholar]
- 38.Bauer TT, Ewig S, Marre R, et al. CRB-65 predicts death from community-acquired pneumonia. J Intern Med 2006; 260: 93–101. [DOI] [PubMed] [Google Scholar]
- 39.Klugman KP, Madhi SA, Albrich WC. Novel approaches to the identification of Streptococcus pneumoniae as the cause of community-acquired pneumonia. Clin Infect Dis 2008; 47: Suppl. 3, S202–S206. [DOI] [PubMed] [Google Scholar]
- 40.Turner P, Turner C, Kaewcharernnet N, et al. A prospective study of urinary pneumococcal antigen detection in healthy Karen mothers with high rates of pneumococcal nasopharyngeal carriage. BMC Infect Dis 2011; 11: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rello J, Lisboa T, Lujan M, et al. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest 2009; 136: 832–840. [DOI] [PubMed] [Google Scholar]
- 42.Vernet G, Saha S, Satzke C, et al. Laboratory-based diagnosis of pneumococcal pneumonia: state of the art and unmet needs. Clin Microbiol Infect 2011; 17: 1–13. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez P, Ferrer M, Marti V, et al. Inflammatory biomarkers and prediction for intensive care unit admission in severe community-acquired pneumonia. Crit Care Med 2011; 39: 2211–2217. [DOI] [PubMed] [Google Scholar]
- 44.Vinogradova Y, Hippisley-Cox J, Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract 2009; 59: e329–e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ewig S, Birkner N, Strauss R, et al. New perspectives on community-acquired pneumonia in 388406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax 2009; 64: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kothe H, Bauer T, Marre R, et al. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J 2008; 32: 139–146. [DOI] [PubMed] [Google Scholar]
- 47.Kyaw MH, Rose CE, Jr, Fry AM, et al. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005; 192: 377–386. [DOI] [PubMed] [Google Scholar]
- 48.Lynch J, Zhanel G. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med 2009; 30: 189–209. [DOI] [PubMed] [Google Scholar]
- 49.Waterer GW, Kessler LA, Wunderink RG. Medium-term survival after hospitalization with community-acquired pneumonia. Am J Respir Crit Care Med 2004; 169: 910–914. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Vidal C, Ardanuy C, Tubau F, et al. Pneumococcal pneumonia presenting with septic shock: host- and pathogen-related factors and outcomes. Thorax 2009; 65: 77–81. [DOI] [PubMed] [Google Scholar]
- 51.Restrepo MI, Mortensen EM, Anzueto A. Common medications that increase the risk for developing community-acquired pneumonia. Curr Opin Infect Dis;23: 145–151. [DOI] [PubMed] [Google Scholar]
- 52.Cilloniz C, Ewig S, Polverino E, et al. Pulmonary complications of pneumococcal community-acquired pneumonia: incidence, predictors, and outcomes. Clin Microbiol Infect 2011. [Epub ahead of print DOI: 10.1111/j.1469–0691.2011.03692.x]. [DOI] [PubMed] [Google Scholar]
- 53.Sjostrom K, Spindler C, Ortqvist A, et al. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis 2006; 42: 451–459. [DOI] [PubMed] [Google Scholar]
- 54.Weinberger DM, Harboe ZB, Sanders EAM, et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis 2010; 51: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lujan M, Gallego M, Belmonte Y, et al. Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. Eur Respir J 2010; 36: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 56.Feldman C, Anderson R. Bacteraemic pneumococcal pneumonia current therapeutic options. Drugs 2011; 71: 131–153. [DOI] [PubMed] [Google Scholar]
- 57.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 2009; 374: 1543–1556. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez A, Lisboa T, Blot S, et al. Mortality in ICU patients with bacterial community-acquired pneumonia: when antibiotics are not enough. Intensive Care Med 2009; 35: 430–438. [DOI] [PubMed] [Google Scholar]
- 59.Garnacho-Montero J, García-Cabrera E, Diaz-Martín A, et al. Determinants of outcome in patients with bacteraemic pneumococcal pneumonia: importance of early adequate treatment. Scand J Infect Dis 2010; 42: 185–192. [DOI] [PubMed] [Google Scholar]
- 60.Torres A, Garau J, Arvis P, et al. Moxifloxacin monotherapy is effective in hospitalized patients with community-acquired pneumonia: the MOTIV Study – a randomized clinical trial. Clin Infect Dis 2008; 46: 1499–1509. [DOI] [PubMed] [Google Scholar]
- 61.Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacol Ther 2008; 117: 393–405. [DOI] [PubMed] [Google Scholar]
- 62.Martin-Loeches I, Lisboa T, Rodriguez A, et al. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med 2010; 36: 612–620. [DOI] [PubMed] [Google Scholar]
- 63.Tessmer A, Welte T, Martus P, et al. Impact of intravenous β-lactam/macrolide versus β-lactam monotherapy on mortality in hospitalized patients with community-acquired pneumonia. J Antimicrob Chemother 2009; 63: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 64.Kumar A, Zarychanski R, Light B, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med 2010; 38: 1773–1785. [DOI] [PubMed] [Google Scholar]
- 65.Weisfelt M, van de Beek D, Spanjaard L. Clinical features, complications, and outcomes in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol 2006; 5: 123–129. [DOI] [PubMed] [Google Scholar]
- 66.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 2006; 295: 1668–1674. [DOI] [PubMed] [Google Scholar]
- 67.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39: 1267–1284. [DOI] [PubMed] [Google Scholar]
- 68.European Medicines Agency. Assessment Report. Prevenar 13: Pneumococcal Polysaccharide Conjugate Vaccine (13-valent, Adsorbed). Procedure Number EMEA/H/C/001104/II/0028. London, European Medicines Agency, 2011. Available from: hwww.ema.europa.eu/docs/en_GB/document_library/EPAR__Assessment_Report_-_Variation/human/001104/WC500119784.pdf.
- 69.Hak E, Grobbee DE, Sanders EA, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med 2008; 66: 378–383. [PubMed] [Google Scholar]