ABSTRACT
Taniborbactam, an investigational β-lactamase inhibitor that is active against both serine- and metallo-β-lactamases, is being developed in combination with cefepime to treat serious infections caused by multidrug-resistant Gram-negative bacteria. Anticipating the use of cefepime-taniborbactam in patients with impaired renal function, an open-label, single-dose clinical study was performed to examine the pharmacokinetics of both drugs in subjects with various degrees of renal function. Hemodialysis-dependent subjects were also studied to examine the amounts of cefepime and taniborbactam dialyzed. Single intravenous infusions of 2 g cefepime and 0.5 g taniborbactam coadministered over 2 h were examined, with hemodialysis-dependent subjects receiving doses both on- and off-dialysis. No subjects experienced serious adverse events or discontinued treatment due to adverse events. The majority of adverse events observed were mild in severity, and there were no trends in the safety of cefepime-taniborbactam related to declining renal function or the timing of hemodialysis. Clinically significant and similar decreases in drug clearance with declining renal function were observed for both cefepime and taniborbactam. The respective decreases in geometric mean clearance for subjects with mild, moderate, and severe renal impairment compared to subjects with normal renal function were 18%, 63%, and 78% for cefepime and 15%, 63%, and 81% for taniborbactam, respectively. Decreases in clearance were similar for both drugs and were shown to be proportional to decreases in renal function. Both cefepime and taniborbactam were dialyzable, with similar amounts removed during 4 h of hemodialysis. This study is registered at ClinicalTrials.gov as NCT03690362.
KEYWORDS: taniborbactam, cefepime, cefepime-taniborbactam, VNRX-5133, beta-lactamase inhibitor, drug safety, pharmacokinetics, renal impairment
INTRODUCTION
Taniborbactam (formerly VNRX-5133) is a broad spectrum, cyclic boronate β-lactamase inhibitor that is being developed with cefepime as a partnered antibiotic. Taniborbactam exhibits potent competitive inhibition of the Ambler Class A, C, and D serine-β-lactamases, and the Ambler class B metallo-β-lactamases, such as the Verona integron-encoded metallo-β-lactamases (VIM) and the New Delhi metallo-β-lactamases (NDM) (1–4). Cefepime is a fourth-generation cephalosporin antibiotic with extended spectrum activity against Gram-positive and Gram-negative bacteria. In the United States, cefepime is approved for the treatment of moderate to severe pneumonia, uncomplicated and complicated urinary tract infections, including pyelonephritis, uncomplicated skin and skin structure infections, and complicated intra-abdominal infections that are caused by susceptible strains of designated microorganisms as well as for empirical therapy for febrile neutropenia (5). The combination of cefepime and taniborbactam (cefepime-taniborbactam) is being investigated as a treatment for complicated urinary tract infections (NCT03840148) and for other serious infections in which multidrug resistant Gram-negative pathogens occur, such as hospital-acquired and ventilator-associated pneumonia (6, 7). In nonclinical models of infection, taniborbactam has been shown to potentiate cefepime activity against Gram-negative pathogens with β-lactamase-mediated resistance (8–12).
As an antibiotic combination that will be used to treat life-threatening infections, cefepime-taniborbactam is likely to be administered to patients with various degrees of renal impairment. In clinical studies, following the administration of 0.25 to 2 g cefepime, at least 80% of the cefepime dose was excreted unchanged in urine (13–15). In patients with renal impairment, the cefepime terminal elimination half-life (t1/2) significantly increases up to 6-fold in patients with creatinine clearance (CLCR) values of <10 mL/min, and dosing adjustments are required for patients with various degrees of renal impairment (5, 13, 16). The pharmacokinetics of taniborbactam in healthy volunteers are generally similar to that of cefepime (17). Clinical studies have shown that taniborbactam is primarily eliminated unchanged in urine and at steady-state; approximately 89% of the taniborbactam dose is recovered in urine as unchanged parent compound (18).
The objective of this study was to evaluate the safety and pharmacokinetics of cefepime-taniborbactam in subjects with mild, moderate, or severe renal impairment as well as in subjects with end-stage renal disease (ESRD) requiring hemodialysis.
RESULTS
Subjects.
A total of 33 subjects were enrolled in the study, received the combination treatment of single intravenous infusions of 2 g cefepime and 0.5 g taniborbactam, and had pharmacokinetics assessed. A summary of demographics and baseline estimates of renal function for subjects in each group can be found in Table 1. Subject sex, age, and weight were similar across the nondialysis groups. For subjects in the Normal group, the estimated creatinine clearance (eCLCR) determined by the Cockcroft-Gault equation (19) ranged from 100.7 to 174.0 mL/min. For subjects in the Mild, Moderate, and Severe groups, enrollment was based on estimated glomerular filtration rate (eGFR) as determined by the Modification of Diet in Renal Disease (MDRD) equation (20, 21). Across these renal impairment groups, subject eGFR values ranged between 5.5 and 85.0 mL/min/1.73m2.
TABLE 1.
Subject demographics and baseline renal functiona
| Renal group |
|||||
|---|---|---|---|---|---|
| Variable | Normal (n = 8) |
Mild (n = 6) |
Moderate (n = 6) |
Severe (n = 6) |
Dialysis (n = 7) |
| Age, yr, mean (SD) | 49 (11) | 58 (7) | 62 (11) | 60 (12) | 48 (11) |
| Sex, n (%) | |||||
| Male | 6 (75.0) | 3 (50.0) | 3 (50.0) | 3 (50.0) | 7 (100.0) |
| Female | 2 (25.0) | 3 (50.0) | 3 (50.0) | 3 (50.0) | 0 (0.0) |
| Wt, kg, mean (SD) | 87.0 (13.7) | 84.6 (15.3) | 81.4 (20.1) | 78.3 (20.2) | 87.5 (12.7) |
| BMI, kg/m2, mean (SD) | 30.5 (4.7) | 30.1 (3.1) | 28.2 (3.9) | 28.9 (5.1) | 27.9 (4.1) |
| Race, n (%) | |||||
| White | 4 (50.0) | 5 (83.3) | 5 (83.3) | 5 (83.3) | 0 (0.0) |
| Black or African American | 3 (37.5) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 7 (100.0) |
| Other | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Renal Function, mean (SD) | |||||
| eCLCR, mL/min | 131.0 (25.8) | 94.9 (13.3) | 47.1 (13.7) | 32.6 (19.7) | 10.3 (3.5) |
| eGFR, mL/min/1.73m2 | 102.7 (13.6) | 74.6 (7.9) | 36.1 (5.2) | 22.5 (9.4) | 6.1 (1.3) |
eCLCR = estimated creatinine clearance determined by Cockcroft-Gault; eGFR = estimated GFR determined by MDRD equation.
Subjects in the Dialysis group (n = 7) were all hemodialysis-dependent, male, and 27 to 56 years of age. This group had a mean (SD) weight of 87.5 (12.7) kg.
Safety.
Single doses of 2 g cefepime and 0.5 g taniborbactam, administered in combination as a 2 h intravenous infusion, were found to be safe and well-tolerated in otherwise healthy subjects with various degrees of renal impairment and in otherwise healthy subjects that were hemodialysis-dependent. There were no deaths reported in the study. There were no apparent trends in the incidence, type, or severity of treatment-emergent adverse events with declining renal function or timing of hemodialysis. A total of 7 subjects experienced 8 treatment emergent adverse events; these events were migraine, headache, diarrhea, abdominal pain upper, pain in jaw, drug withdrawal syndrome (caffeine withdrawal), and Clostrioides difficile infection. The majority of adverse events were mild in severity (7 of 8) and considered related to treatment (7 of 8). There were no hematology, chemistry, urinalysis, or coagulation adverse events reported. The majority of postbaseline laboratory and vital sign abnormalities were mild, and there were no apparent trends observed in frequency or severity with declining levels of renal function or with the timing of hemodialysis. There were no treatment-emergent adverse events related to ECG or vital signs. There was no evidence of renal toxicity based on the monitoring of blood urea nitrogen, serum creatinine, eGFR, and microscopic evaluation of urine for renal tubular epithelial casts.
Pharmacokinetics.
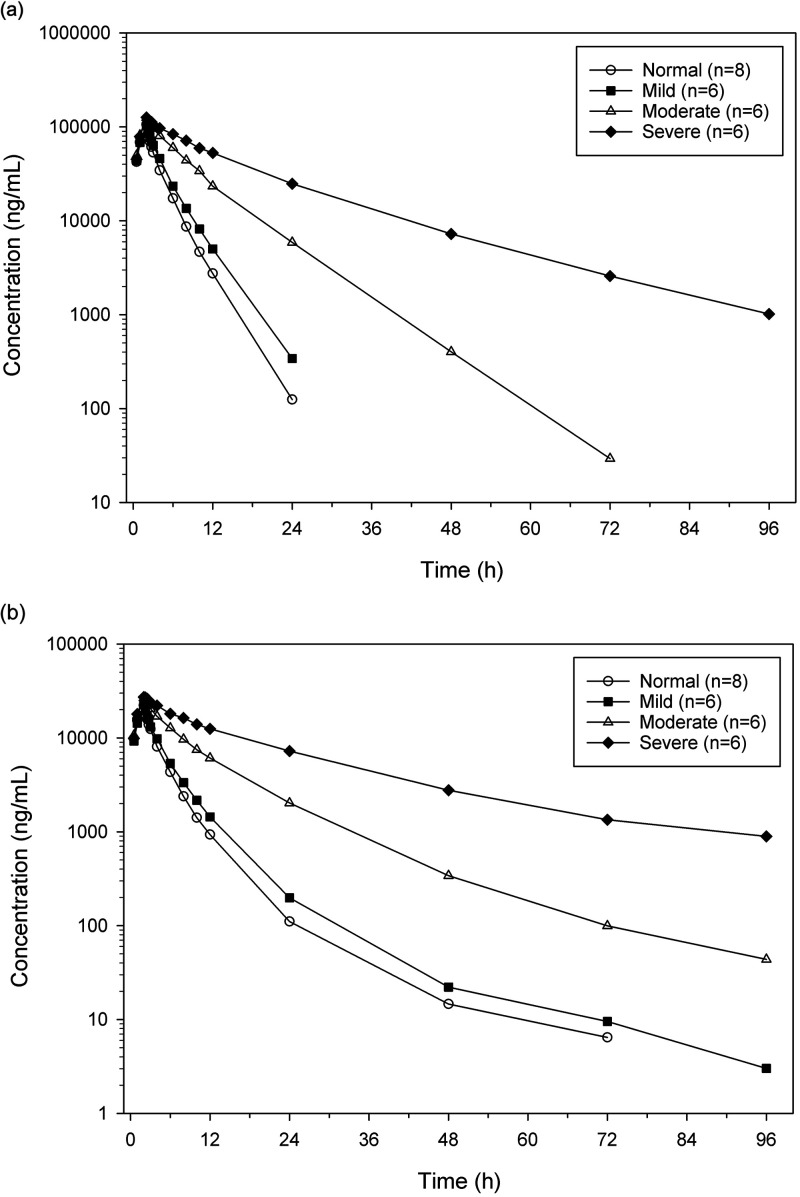
Mean cefepime and taniborbactam plasma concentration-time profiles are compared for the nondialysis renal groups in Fig. 1. Summaries of the cefepime and taniborbactam pharmacokinetic parameters for each of the nondialysis renal groups are shown in Table 2.
FIG 1.
Mean cefepime and taniborbactam plasma concentrations across renal groups (nondialysis). (a) Cefepime, logarithmic concentration scale. (b) Taniborbactam, logarithmic concentration scale.
TABLE 2.
Summary of pharmacokinetic parameters by renal group (nondialysis groups)a
| Renal impairment |
||||
|---|---|---|---|---|
| Parameter, unitb | Normal (n = 8) |
Mild (n = 6) |
Moderate (n = 6) |
Severe (n = 6) |
| Cefepimec | ||||
| Cmax, μg/mL | 102 (25.3) | 101 (19.4) | 124 (20.6) | 129 (21.8) |
| AUCinf, h·μg/mL | 343 (13.2) | 418 (9.0) | 913 (20.0) | 1589 (69.0) |
| t1/2, h | 2.53 (0.53) | 3.03 (0.39) | 5.53 (1.34) | 10.12 (5.16) |
| VZ, L | 20.2 (20.5) | 20.0 (21.2) | 16.3 (25.6) | 16.4 (21.1) |
| CL, L/h | 5.65 (13.8) | 4.61 (11.6) | 2.09 (23.2) | 1.23 (66.6) |
| Taniborbactam | ||||
| Cmax, μg/mL | 22.0 (11.2) | 22.8 (22.6) | 26.9 (24.1) | 27.9 (21.7) |
| AUCinf, h·μg/mL | 83.6 (11.4) | 97.4 (11.5) | 225 (22.5) | 445 (79.3) |
| t1/2, h | 10.2 (2.6) | 19.5 (9.9) | 17.6 (2.6) | 21.3 (10.1) |
| VZ, L | 82.0 (33.4) | 123.0 (59.5) | 53.1 (35.6) | 31.5 (43.5) |
| CL, L/h | 5.79 (11.7) | 4.95 (13.9) | 2.12 (25.8) | 1.10 (76.7) |
| CLR, L/h | 4.37 (17.8)d | 4.23 (22.7) | 1.59 (21.0) | 0.76 (120.3) |
Cmax = maximum plasma concentration; AUCinf = area under the plasma concentration versus time curve, extrapolated through infinity; t1/2 = terminal elimination half-life; Vz = volume of distribution estimated using the terminal phase; CL = total body clearance; CLR = renal clearance.
Geometric mean (geometric coefficient of variation [%]) shown for all parameters except for t1/2, which shows the mean (standard deviation).
Cefepime was not assayed in urine, and cefepime CLR was not estimated in study.
n = 7, as a subject was excluded from the summary statistics because of a missed urine collection.
Statistical comparisons of maximum observed concentration (Cmax), area under the concentration versus time curve (AUC), systemic clearance (CL), and volume of distribution based on the terminal elimination phase (VZ) for each of the renal impairment groups to the Normal group were performed for cefepime and taniborbactam. Least-square geometric mean ratios (GMRs; Renal Impairment Group/Normal [%]) and their 90% confidence intervals (CIs) were calculated. For the Mild/Normal ratio of cefepime and taniborbactam pharmacokinetic parameters, all 90% CIs contained 100% except for taniborbactam VZ, which had a GMR (90% CI) of 149.94% (102.40%, 219.57%). For cefepime and taniborbactam, a statistically significant increase in AUC, extrapolated through infinity (AUCinf), was observed for all nondialysis-dependent renal impairment groups compared with Normal, as assessed using the 90% CIs and the significance boundaries of 80.00% to 125.00%. Inversely, cefepime and taniborbactam CL were significantly decreased in these groups compared with Normal. Similar trends of increased exposure and decreased CL as a function of decreasing renal function were observed for cefepime and taniborbactam (Table 2).
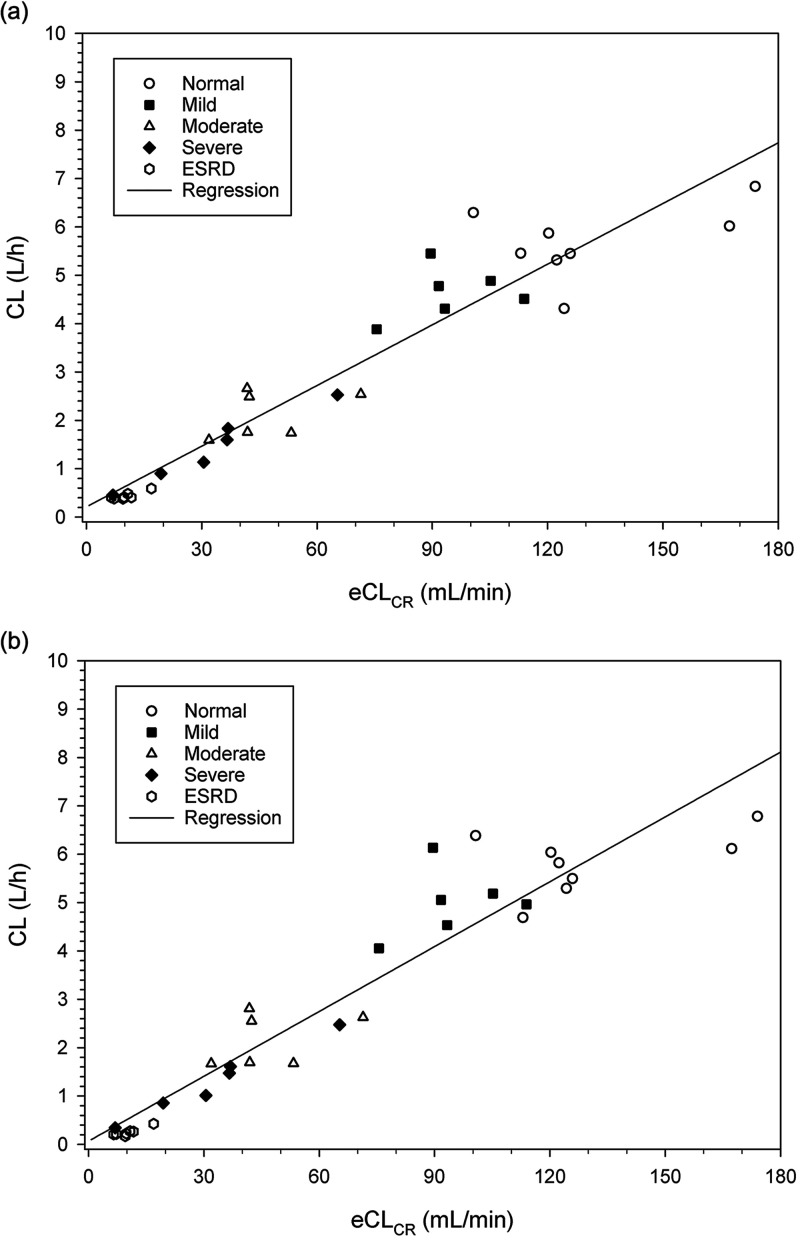
Significant trends in the individual pharmacokinetic parameters as a function of individual measures of renal function were observed for both cefepime and taniborbactam. A robust trend was observed for individual CL as a function of individual eCLCR using linear regression, for both cefepime and taniborbactam (Fig. 2). The data set for these regressions was supplemented with ESRD subjects from the Dialysis group, using data from the Off-dialysis treatment period. This was done to include more subjects with lower degrees of renal function (eGFRMDRD <15 mL/min/1.73 m2). The relationships between drug CL and eCLCR were well-defined by linear regression; the R2 values were 0.9130 and 0.9054 for cefepime and taniborbactam, respectively. The CL values and changes in CL values were similar for cefepime and taniborbactam. The estimated slopes for the relationship of drug CL as a function of eCLCR were 0.0418 and 0.0447 for cefepime and taniborbactam, respectively.
FIG 2.
Cefepime and taniborbactam CL versus eCLCR, showing linear regression. (a) Cefepime. (b) Taniborbactam.
A total of 7 subjects were enrolled in the Dialysis group and received taniborbactam and cefepime in both the On-dialysis and Off-dialysis treatment periods. Hemodialysis was performed in one of the treatment periods (Period 1, On-dialysis) with hemodialysis durations of between 4 and 4.13 h across all subjects. One subject in the Dialysis group was replaced in the study because hemodialysis during the On-dialysis treatment period started almost 8 h after the start of infusion (SOI), compared with the protocol-specified 4 h post-SOI. Pharmacokinetic data were still collected for this subject during both treatment periods, which allowed for a limited assessment of the effect of hemodialysis timing. No obvious differences were noted for this subject, compared with the other Dialysis subjects, as to the amount of cefepime or taniborbactam dialyzed. This subject’s On-dialysis period was excluded from all pharmacokinetic parameter summary statistics and statistical comparisons but was used in the calculation of the dialysis parameters.
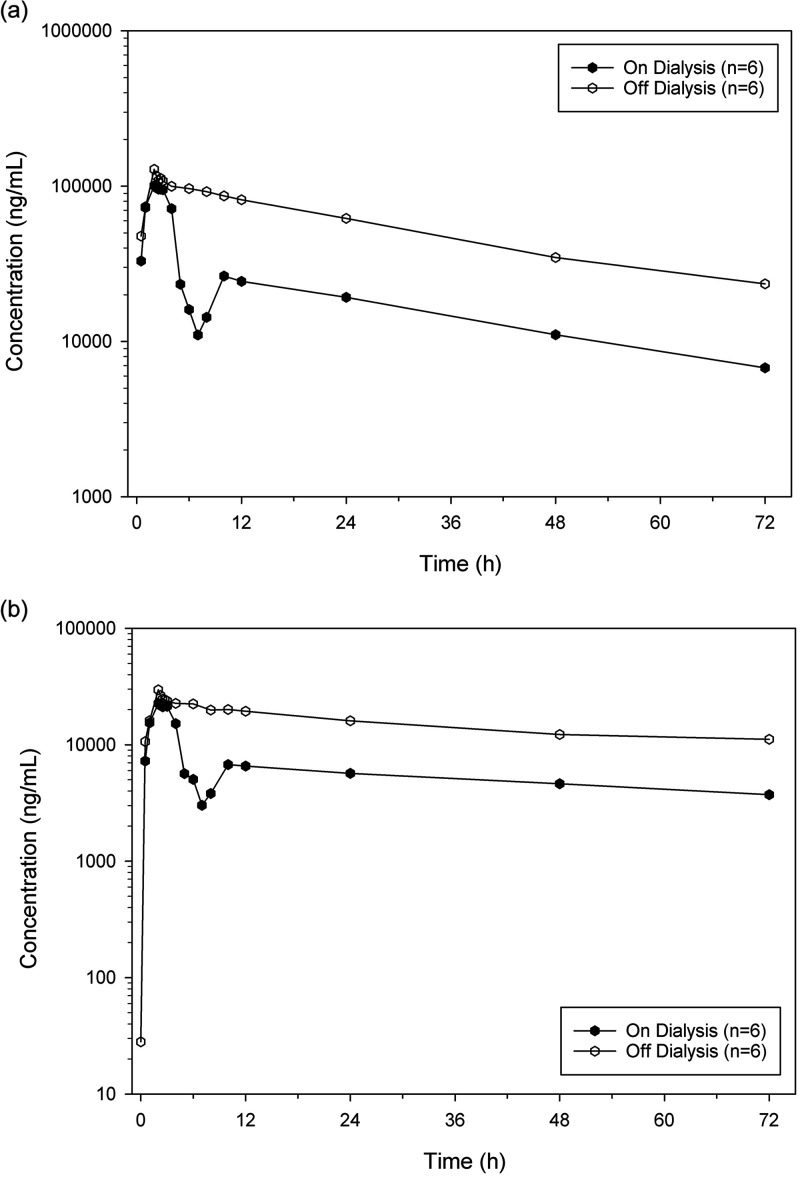
For the Dialysis subjects, the mean cefepime and taniborbactam plasma concentration-time profiles from the On-dialysis and Off-dialysis treatment periods are compared in Fig. 3. Summaries of the cefepime and taniborbactam pharmacokinetic parameters comparing the On-dialysis and Off-dialysis periods are shown in Table 3. All subjects were completely or near completely anuric, and there were no urine-associated pharmacokinetic parameters summarized for Dialysis subjects.
FIG 3.
Comparison of mean cefepime and taniborbactam plasma concentrations in the on-dialysis and off-dialysis treatment periods (dialysis subjects). (a) Cefepime, logarithmic concentration scale. (b) Taniborbactam, logarithmic concentration scale.
TABLE 3.
Comparison of pharmacokinetic parameters in the on-dialysis and off-dialysis treatment periods (dialysis group)a
| Hemodialysis |
||
|---|---|---|
| Parameter, unitb | On-dialysis (n = 6)c |
Off-dialysis (n = 7) |
| Cefepime | ||
| Cmax, μg/mL | 105 (13.0) | 165 (77.2) |
| AUCinf, h·μg/mL | 1597 (16.7) | 4549 (17.7) |
| t1/2, h | 32.0 (4.3) | 29.7 (5.8) |
| VZ, L | 56.5 (9.0) | 18.2 (8.8) |
| CL, L/h | 1.23 (17.6) | 0.432 (15.9) |
| Taniborbactam | ||
| Cmax, μg/mL | 23.4 (9.5) | 37.7 (73.7) |
| AUCinf, h·μg/mL | 851 (23.0) | 2003 (32.1) |
| t1/2, h | 83.7 (21.5) | 70.8 (22.3) |
| VZ, L | 68.1 (15.1) | 23.7 (13.4) |
| CL, L/h | 0.579 (23.0) | 0.245 (29.8) |
Cmax = maximum plasma concentration; AUCinf = area under the plasma concentration versus time curve, extrapolated through infinity; t1/2 = terminal elimination half-life; Vz = volume of distribution estimated using the terminal phase; CL = total body clearance.
Geometric mean (geometric coefficient of variation [%]) shown for all parameters except for t1/2, which shows the mean (standard deviation).
One subject excluded in the summary statistics because dialysis occurred 7.8 h after the start of drug infusion.
Hemodialysis was shown to substantially remove cefepime and taniborbactam from plasma at similar rates. Geometric mean cefepime and taniborbactam AUCs were decreased following hemodialysis by 65% and 58%, respectively. Mean cefepime and taniborbactam Cmax values were comparable between Off-dialysis and On-dialysis treatments when anomalous values from a single subject (thought to be due to a sampling error) were excluded. The mean cefepime and taniborbactam t1/2 remained relatively unaffected by hemodialysis.
Statistical comparisons of treatment periods within the Dialysis group were performed for cefepime and taniborbactam Cmax, AUCinf, CL, and VZ, calculating the least-squares GMR (On-dialysis/Off-dialysis [%]) and their respective 90% CIs. Significant changes were noted in all assessed pharmacokinetic parameters. The GMRs (90% CIs) for cefepime and taniborbactam AUCinf were 33.46% (30.39%, 36.83%) and 39.29% (33.20%, 46.48%), respectively.
The estimated drug-dependent dialysis parameters were similar for both drugs. The mean (SD) dialysis clearances (CLd) were 117 (12.3) mL/min and 102 (5.3) mL/min for cefepime and taniborbactam, respectively. The mean (SD) cefepime and taniborbactam hemodialysis extraction ratios (HER) were 47.4% (7.7%) and 49.7% (7.1%), respectively. 2 of the 7 subjects had On-dialysis venous samples collected from the contralateral arm rather than from the dialysis output, and CLd could not be calculated for these subjects.
DISCUSSION
Cefepime and taniborbactam are both primarily excreted unchanged in urine, and the pharmacokinetics of both drugs are similarly impacted by renal impairment. Generally, the distributions of drug clearance and exposure for both drugs were similar and overlapping for the Normal and Mild groups, indicating only a small effect due to renal impairment for subjects with an eGFR value of >60 mL/min/1.73m2. Continued decreases in renal function led to significant decreases in CL and increases in exposure for both drugs. For subjects in the Moderate group (eGFR = 30 to 59 mL/min/1.73 m2), geometric mean CL decreased 63% for both drugs. In the Severe group (eGFR <30 mL/min/1.73 m2), geometric mean CL decreases of 78% and 81% were observed for cefepime and taniborbactam, respectively. AUCinf increased across groups in an inverse manner. Modest decreases in distributive drug volumes were observed for both drugs with decreasing renal function, with similarly modest and associated increases in Cmax. The mean terminal half-lives (t1/2) of cefepime and taniborbactam in the Normal group were 2.53 h and 10.2 h, respectively, and t1/2 increased with decreasing renal function. The longer taniborbactam t1/2 is due to a longer terminal phase in the drug’s biphasic elimination, which describes only a small fraction of the drug’s overall exposure (Fig. 1).
In the presented study, subjects with normal renal function were enrolled based on eCLCR, and eGFR was used for subjects with renal insufficiency. This was based on regulatory guidance at the time of the study (22). The use of eGFR to quantitate renal insufficiency, typically using the MDRD equation, has become an accepted practice in these types of otherwise healthy volunteer studies. In this study, the enrollment criteria produced a good range and distribution of renally insufficient subjects that allowed for a robust assessment of the relationship between drug pharmacokinetics and impaired renal function, regardless of which serum creatinine-based equation was used as the independent variable. The presented relationship was drug CL versus eCLCR, as determined by the Cockcroft-Gault equation. This estimate of CLCR, which describes renal function, is still a primary method used in drug labels and in clinical practice for renal dose adjustment, and it is the independent variable used in prior studies of cefepime.
Dosage adjustments of cefepime and taniborbactam will need to be further examined using pharmacokinetic data from patient studies, but it appears from these data that similar recommendations can be made with coadministration. Dosage adjustments are recommended in the cefepime prescribing information for CLCR ≤60 mL/min to compensate for decreases in cefepime CL (5). For CLCR between 30 mL/min and 60 mL/min, the cefepime dosing frequency is recommended to be reduced. Decreases in both the cefepime dosing frequency and the dose are recommended for CLCR <30 mL/min, with the adjustments being dependent upon the CLCR level and the prescribed maintenance schedule. Similar adjustments for patients with renal impairment will be examined in data derived from phase 3 studies for cefepime-taniborbactam to minimize the risk of excessive exposure of both drugs, while still ensuring that pharmacokinetic/pharmacodynamic efficacy targets are met.
Cefepime and taniborbactam are dialyzable, with similar amounts removed during hemodialysis in this study. The amount of cefepime removed during a 3 h hemodialysis session has been reported to be approximately 68%, compared with the 47% found in this study during a 4 h session (5, 13, 16). These amounts may vary due to the system and flow rates used for hemodialysis. For patients undergoing hemodialysis, the dosages and timing of coadministered cefepime and taniborbactam with respect to dialysis will need to be taken into consideration. Also, given the degree of dialyzability of both cefepime and taniborbactam and the likelihood of use in critically ill patients with acute kidney injury, dosage adjustment recommendations will need to be developed for other modalities of renal support.
MATERIALS AND METHODS
Study design and subjects.
This was an open-label, single-dose study of the safety, tolerability, and pharmacokinetics of coadministered single doses of taniborbactam and cefepime to subjects with various degrees of renal impairment and matched control subjects with normal renal function. Subjects aged 18 to 80 years were enrolled into 1 of 4 groups based on the level of renal function (renal groups). Renal groups consisted of subjects with normal renal function (“Normal”) with an eCLCR of ≥90 mL/min (Group 1; n = 8), subjects with mild renal impairment (“Mild”) with an eGFR of 60 to 89 mL/min/1.73 m2 (Group 2; n = 6), subjects with moderate renal impairment (“Moderate”) with an eGFR of 30 to 59 mL/min/1.73 m2 (Group 3; n = 6), and subjects with severe renal impairment (“Severe”) with an eGFR of <30 mL/min/1.73 m2 (Group 4; n = 6). Attempts were made in the study to enroll subjects within the Severe group with an eGFR value of <15 mL/min/1.73 m2. Subjects in the Normal group were matched to subjects with renal impairment with regard to gender, age (±10 years), and weight (±10 kg). A single healthy subject may have been the match for more than one subject with renal impairment. For all subjects in Groups 1 to 4, a single 2 h IV infusion of 2 g cefepime and 0.5 g taniborbactam was administered on day 1.
In addition to the groups enrolled by renal function to study the effect of renal impairment, a group of subjects with ESRD undergoing chronic intermittent hemodialysis were enrolled into a separate group (Group 5; “Dialysis”) to determine the amounts of drug removed by filtration. Hemodialysis used standard commercial dialyzers and membranes. The dialyzer was either a Fresenius F160 or F180 (Fresenius Medical Care North America, Waltham, MA) with blood flow rates (Qb) of between 350 and 450 mL/min, dialysate flow rates (Qd) of between 500 and 700 mL/min, and reported urea clearances (CLUREA) of approximately 270 mL/min. Subjects in the Dialysis group received the same dose of cefepime-taniborbactam as did the subjects in Groups 1 to 4 in each of 2 treatment periods, with the treatments separated by a washout period of 7 to 14 days. A fixed sequence of dialysis timing with respect to dose was used, where the dose was administered prior to dialysis in the first treatment period (“On-dialysis”; Period 1) and administered following dialysis in the second treatment period (“Off-dialysis”; Period 2). Hemodialysis for the On-dialysis period started approximately 4 h after the SOI of cefepime-taniborbactam. For the Off-dialysis period, subjects had the SOI begin approximately 6 h after the start of dialysis, which was after the dialysis had been completed.
The study was performed at two sites in the United States. The study protocol was reviewed and approved by an Institutional Review Board, and the study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki. Study conduct complied with the International Council for Harmonisation Guideline for Good Clinical Practice and with applicable regulatory requirements. All subjects provided written informed consent prior to any study-specific procedures.
Safety.
Safety was assessed based on the occurrence of adverse events and on the evaluation of laboratory tests (chemistry, hematology, urinalysis, and coagulation), physical examinations, vital signs, and 12-lead ECGs. Safety was followed through 7 days post-dose.
Pharmacokinetic assessments.
The pharmacokinetics of cefepime and taniborbactam in plasma were assessed over 72 to 96 h, depending on the renal group. Subjects in Groups 1 through 4 had blood samples collected through 96 h after the administration of cefepime-taniborbactam on day 1. The pharmacokinetic time points included: pre-dose (within 30 min before dosing), 0.5, 1, 2, 2.25, 2.5, 2.75, 3, 4, 6, 8, 10, 12, 24, 48, 72, and 96 h after SOI. Subjects in Group 1 were not required to have a collection at 96 h.
Subjects in Group 5 had blood samples collected through 72 h after the administration of cefepime-taniborbactam on day 1 of Period 1 and Period 2. The pharmacokinetic time points included: pre-dose (within 30 min before dosing), 0.5, 1, 2, 2.25, 2.5, 2.75, 3, 4, 6, 8, 10, 12, 24, 48, and 72 h after SOI. Additional blood samples, if not already collected by a defined time point, were collected at 1 h after the start of dialysis and again at the end of dialysis. Samples were not collected from Group 5 subjects at 72 h, should this time point have occurred after the next scheduled dialysis.
Urine was collected to further assess taniborbactam pharmacokinetics. Subjects were instructed to empty their bladders completely before study drug administration and just before the end of each collection interval. Urine from each timed interval was collected in a separate container. The exact start dates, stop dates, times of urine collection, and the weight and volume of each collection were recorded. Urine samples were collected within intervals of 0 to 6 h, 6 to 12 h, 12 to 24 h, 24 to 48 h, 48 to 72 h, and 72 to 96 h following the SOI. Group 1 subjects may not have had a urine collection between 72 to 96 h post-SOI. Group 5 subjects did not have a urine collection between 48 to 72 h post-SOI if this time point occurred after their next scheduled dialysis, nor did these subjects have a urine collection between 72 to 96 h post-SOI. Urine samples were not collected from anuric subjects.
Dialysate samples and hemodialysis-associated arterial/venous (A/V) blood samples were collected from Group 5 subjects during Period 1. Spot dialysate samples were taken immediately before the start of dialysis (blank dialysate fluid) and every 30 min after the start of dialysis. Hemodialysis-associated A/V samples were collected immediately at the start of dialysis and thereafter at 1, 2, 3, and 4 h after the start of hemodialysis.
Bioanalytical methods.
Blood samples collected for pharmacokinetic analysis in the study were processed and assayed for cefepime and taniborbactam in plasma using validated bioanalytical methods. Human dipotassium ethylenediaminetetraacetic acid plasma samples were processed using protein precipitation prior to assay. Urine samples collected for pharmacokinetic analysis in the study were processed and assayed for only taniborbactam using a validated bioanalytical method. Dialysate samples and hemodialysis-associated A/V samples were assayed for taniborbactam and cefepime using validated bioanalytical methods. Acidified urine and dialysate samples were processed using solid-phase extraction prior to assay.
Validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods were used to quantitate the concentrations of cefepime and taniborbactam in plasma. The lower limits of quantitation (LLOQ) for these assays were 100 ng/mL and 5.00 ng/mL for cefepime and taniborbactam, respectively. Validated LC-MS/MS methods (LLOQ = 5.00 ng/mL) were used to quantitate the concentrations of taniborbactam in urine. The validated LC-MS/MS method to assay cefepime and taniborbactam in dialysate had LLOQs of 100 ng/mL and 50.0 ng/mL, respectively.
For cefepime assays, separation was accomplished using a SCX Agilent Zorbax 300 column (Santa Clara, CA) at 40°C and isocratic elution using 35 mM ammonium formate as mobile phase A and acetonitrile as mobile phase B. For the taniborbactam assays, separation was accomplished using a Waters Acquity HSS T3 column (Milford, MA) at 50°C and gradient elution using 10 mM ammonium formate with 1% formic acid as mobile phase A and 50:50:1 acetonitrile:methanol:formic acid vol/vol/vol as mobile phase B. All cefepime and taniborbactam assays used a triple quadrupole mass spectrometer (API Triple Quad 5500, AB Sciex, Framingham, MA) equipped with a turbo-ion spray set that was used for detection in positive ion mode, and quantification was based on multiple reaction monitoring. The internal standards used in the cefepime and taniborbactam assays were d3-cefepime sulfate and d4-taniborbactam, respectively.
Assay accuracy and precision were demonstrated for all assays in the validations, which also included the testing of any required dilutions used in the analysis of study samples. Demonstrated sample stability met the requirements of the sample storage used in the study.
Pharmacokinetic and statistical analyses.
Individual subject plasma cefepime and taniborbactam pharmacokinetic parameters and individual subject urine taniborbactam pharmacokinetic parameters were calculated using NCA methods (Phoenix WinNonlin, Certara, Princeton, NJ). Estimated plasma pharmacokinetic parameters included the Cmax, time to Cmax (Tmax), AUC through the last measurable observed concentration (AUCt), AUCinf, t1/2, CL, and VZ. For taniborbactam, using the urine observations, the amount excreted unchanged in urine (Ae), fraction excreted unchanged in urine as the percentage of administered dose (Fe), renal clearance (CLR), and nonrenal clearance (CLNR) were calculated. The actual sample times were used to calculate the pharmacokinetic parameters. Calculation of the terminal elimination rate (λZ) was based on the best fit of at least 3 concentrations in the observed terminal elimination phase (excluding Cmax) and required the goodness of fit statistic (R2) to be greater than or equal to 0.80 to be considered acceptable. If a good estimate of λZ could not be determined for a concentration-time profile, then none of the pharmacokinetic parameters dependent on λZ were calculated.
The NCA pharmacokinetic parameters were summarized by group using descriptive statistics. Linear regression was used to model changes in pharmacokinetic parameters versus continuous independent variables representative of renal function. Assessed pharmacokinetic parameters included cefepime and taniborbactam Cmax, AUCinf, CL, VZ, and t1/2. Additionally for taniborbactam, CLR was also examined.
For Dialysis subjects (Group 5), the effect of hemodialysis was examined by comparing differences within each subject (On-Dialysis versus Off-Dialysis). The CLd was calculated by taking the individual averaged arterial to venous concentration extraction ratio (CER), multiplying by the reported Qb, and correcting for hematocrit (hct). The hct was estimated as 0.47, an assumption based on the fact that all subjects in the group were male. The amounts of drug removed by hemodialysis (Adial) were calculated using the area under the excretion rate curves, where the rates were determined using the spot dialysis concentrations and estimated dialysate volumes over a 1 min period using the Qd. The fraction of drug removed during hemodialysis, or HER, was then determined by dividing Adial by dose and was expressed as a percentage.
ACKNOWLEDGMENTS
We acknowledge Brooke Geibel, who was an employee of Venatorx Pharmaceuticals, Inc., at the time of the study for her valuable contribution to the operations of the study, and Donna Simcoe for the editing and development of the early manuscript drafts. Funding for this support was provided by Venatorx Pharmaceuticals, Inc., Malvern, PA.
This study was funded in whole or in part by federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (Contract No. HHSN272201300019C), and by Venatorx Pharmaceuticals, Inc., Malvern, PA.
J.A.D. is a principal at Pharmacology Development Services, LLC (Collegeville, PA), T.C.M. is an employee and equity owner of the Orlando Clinical Research Center (Orlando, FL), and W.B.S. is an employee of the Alliance for Multispecialty Research, University of Tennessee Medical Center (Knoxville, TN). All three of these authors performed work as paid contractors for Venatorx Pharmaceuticals, Inc., Malvern, PA. T.H. is an employee of Venatorx Pharmaceuticals, Inc., Malvern, PA.
All authors contributed to the data analysis and interpretation, reviewed the manuscript for intellectual content, and approved the submission of the manuscript.
REFERENCES
- 1.Daigle DM, Burns CJ. 2018. Kinetic mechanism & parameters of inhibition of KPC-2, CTX-M15, p99 AmpC and VIM-2 by the β-lactamase inhibitor VNRX-5133, abstr O0606. Abstr 28th Eur Cong Clin Microbiol Infect Dis, Madrid, Spain. [Google Scholar]
- 2.Liu B, Trout REL, Chu G-H, McGarry D, Jackson RW, Hamrick JC, Daigle DM, Cusick SM, Pozzi C, De Luca F, Benvenuti M, Mangani S, Docquier J-D, Weiss WJ, Pevear DC, Xerri L, Burns CJ. 2020. Discovery of taniborbactam (VNRX-5133): a broad-spectrum serine- and metallo-β-lactamase inhibitor for carbapenem-resistant bacterial infections. J Med Chem 63:2789–2801. doi: 10.1021/acs.jmedchem.9b01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamrick J, Chatwin C, John K, Pevear D, Burns C, Xerri L. 2018. The ability of broad-spectrum beta-lactamase inhibitor VNRX-5133 to restore bactericidal activity of cefepime in Enterobacteriaceae- and P aeruginosa-expressing Ambler class A, B, C and D enzymes is demonstrated using time-kill kinetics, abstr P1545. Abstr 28th Eur Cong Clin Microbiol Infect Dis, Madrid, Spain. [Google Scholar]
- 4.Hamrick JC, Docquier J-D, Uehara T, Myers CL, Six DA, Chatwin CL, John KJ, Vernacchio SF, Cusick SM, Trout REL, Pozzi C, De Luca F, Benvenuti M, Mangani S, Liu B, Jackson RW, Moeck G, Xerri L, Burns CJ, Pevear DC, Daigle DM. 2020. VNRX-5133 (taniborbactam), a broad-spectrum inhibitor of serine- and metallo-β-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother 64:e01963-19. doi: 10.1128/AAC.01963-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxipime (cefepime hydrochloride). 2012. Hospira, Inc., Lake Forest, IL. [Google Scholar]
- 6.Global Antibiotic Research and Development Partnership. 2020. Uniting against antibiotic resistance. https://gardp.org/uploads/2020/05/GARDP-brochure-2020-FINAL.pdf.
- 7.Steiger SN, Comito RR, Nicolau DP. 2017. Clinical and economic implications of urinary tract infections. Expert Rev Pharmacoecon Outcomes Res 17:377–383. doi: 10.1080/14737167.2017.1358618. [DOI] [PubMed] [Google Scholar]
- 8.Vidales A, Hackel M, Wise M, Sahm D. 2020. Antimicrobial activity of cefepime in combination with taniborbactam (formerly VNRX-5133) against clinical isolates of Enterobacterales collected from global 2018–2019 surveillance. Abstr ASM Microbe Online. [Google Scholar]
- 9.Kalamatas J, Hackel M, Wise M, Sahm D. 2020. Antimicrobial activity of cefepime in combination with taniborbactam (formerly VNRX-5133) against a 2018–2019 surveillance collection of Pseudomonas aeruginosa. ASM Microbe Online. [Google Scholar]
- 10.Georgiou P-C, Siopi M, Tsala M, Lagarde C, Kloezen W, Donnelly R, Meletiadis J, Mouton J. 2018. VNRX-5133, a novel broad-spectrum beta-lactamase inhibitor, enhances the activity of cefepime against Enterobacteriaceae and P aeruginosa isolates in a neutropenic mouse thigh infection model, abstr P1540. Abstr 28th Eur Cong Clin Microbiol Infect Dis, Madrid, Spain. [Google Scholar]
- 11.Weiss W, Pulse M, Nguyen P, Valtierra D, Peterson K, Carter K, Pevear D, Burns C, Xerri L. 2018. Efficacy of cefepime/VNRX-5133, a novel beta-lactamase inhibitor combination, against cephalosporin-resistant, ESBL-producing K. pneumoniae in a murine lung-infection model, abstr #O0600. Abstr 28th Eur Cong Clin Microbiol Infect Dis, Madrid, Spain. [Google Scholar]
- 12.Abdelraouf K, Abuhussain SA, Nicolau DP. 2020. In vivo pharmacodynamics of new-generation β-lactamase inhibitor taniborbactam (formerly VNRX-5133) in combination with cefepime against serine-β-lactamase-producing Gram negative bacteria. J Antimicrob Chemother 75:3601–3610. doi: 10.1093/jac/dkaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto MP, Nakahiro RK, Chin A, Bedikian A. 1993. Cefepime clinical pharmacokinetics. Clin Pharmacokinet 25:88–102. doi: 10.2165/00003088-199325020-00002. [DOI] [PubMed] [Google Scholar]
- 14.Barbhaiya RH, Forgue ST, Gleason CR, Knupp CA, Pittman KA, Weidler DJ, Martin RR. 1990. Safety, tolerance and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother 34:1118–1122. doi: 10.1128/AAC.34.6.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nye KJ, Shi YG, Andrews JM, Wise R. 1989. Pharmacokinetics and tissue penetration of cefepime. J Antimicrob Chemother 24:23–28. doi: 10.1093/jac/24.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DR, Pittman KA. 1990. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther 48:268–276. doi: 10.1038/clpt.1990.149. [DOI] [PubMed] [Google Scholar]
- 17.Geibel B, Dowell JA, Dickerson D, Henkel T. 2019. Pharmacokinetics of VNRX-5133 alone and combined with cefepime when co-administered with metronidazole, abstr P1951. Abstr 29th Eur Cong Clin Microbiol Infect Dis, Amsterdam, The Netherlands. [Google Scholar]
- 18.Dowell JA, Dickerson D, Henkel T. 2021. Safety and pharmacokinetics in human volunteers of taniborbactam (VNRX-5133), a novel intravenous β-lactamase inhibitor. Antimicrob Agents Chemother 65:e01053-21. doi: 10.1128/AAC.01053-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration . 2006. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration . 2007. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services. 2010. Guidance for industry, pharmacokinetics in patients with impaired renal function — study design, data analysis, and impact on dosing and labeling. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). [Google Scholar]