Abstract
The Essential Tremor Centralized Brain Repository is the largest repository of prospectively collected essential tremor (ET) brains (n = 231). Hence, we are uniquely poised to address several questions: What proportion of ET cases has Lewy pathology (LP)? What is the nature of that pathology and how does it relate to other comorbidities? Each brain had a complete neuropathological assessment, including α-synuclein immunostaining. We created a 10-category classification scheme to fully encapsulate the patterns of LP observed. Four metrics of cerebellar pathology were also quantified. Mean age at death = 89.0 ± 6.4 years. Fifty-eight (25.1%) had LP and 46 (19.9%) had early to late stages of Parkinson disease (PD). LP was very heterogeneous. Of 58 cases with LP, 14 (24.1%) clinically developed possible PD or PD after a latency of 5 or more years. There was a similar degree of cerebellar pathology in ET cases both with and without LP. In summary, 1 in 4 ET cases had LP—a proportion that seems higher than expected based on studies among control populations. Heterogeneous LP likely reflects clinical associations between ET and PD, and ET with Alzheimer disease-type neuropathology. These data further our understanding of ET and its relatedness to other degenerative diseases.
Keywords: Alzheimer disease, Brain repository, Cerebellar degeneration, Essential tremor, Lewy pathology, Neurodegeneration, Parkinson disease
INTRODUCTION
The Essential Tremor Centralized Brain Repository (ETCBR) was established in 2003 to gain a better understanding of the underlying neuropathological findings in patients with essential tremor (ET). There had been nearly no published postmortem cases at that point and little was understood about the underlying mechanisms of a disease that can be disabling (1), has been linked with increased risk of mortality (2), and affects 2.2% of the entire US population (3). Since then, most of our work (3–7), and that of others (8–12), has centered on the degenerative changes that are evident in the cerebellum of patients with ET. While most studies have focused on the cerebellar pathology in ET (13, 14), there is also a parallel narrative centered on Lewy pathology (LP) in ET (4, 15). This potential link between ET and LP is particularly intriguing because there is a literature, which although controversial (16, 17), presents epidemiological evidence that ET seems to be associated with increased odds and risk of developing Parkinson disease (PD) (18–24). In one of these studies, patients with ET were 4–5 times more likely to develop PD than age-matched individuals without ET (20).
In 2005, we reported an individual with severe and longstanding ET in the absence of clinical parkinsonism (15). Immunohistochemical staining using antibodies directed against α-synuclein demonstrated numerous Lewy bodies (LBs) in the locus coeruleus (LC) and rare LBs in the substantia innominata and dorsal vagal nuclei (DVN), but no LBs in the substantia nigra pars compacta (SNc) (15).
In 2007, we reported the postmortem findings of 33 ET cases. Eight (24.2%) had LP (4). In 6 of 8 cases, LP was abundant in the LC and rare in other brainstem structures and elsewhere; 2 had widespread LP (4).
Over the past 14 years, the ETCBR has steadily expanded, and the number of prospectively banked ET brains has reached 231, which is an order of magnitude greater than that in our previous report in which 20 of 33 had been prospectively collected (4). As such, the ETCBR is the largest repository of ET brains in the world. Hence, we are uniquely poised to address the following scientific questions: (1) What proportion of ET cases exhibit LP? (2) What is the nature (i.e. type, location, severity) of that pathology? (3) How does that pathology relate to various neurodegenerative conditions (e.g. PD, Alzheimer disease [AD]-type changes)? (4) What are the clinical correlates of that pathology?
The pathophysiology of ET remains poorly understood. Thus, these questions have significant value in terms of furthering our understanding of the biological and mechanistic basis for ET as well as its relatedness to other degenerative diseases.
MATERIALS AND METHODS
ET Cases
All ET cases were collected prospectively through the ETCBR, a centralized repository in the New York Brain Bank of brains from cases with ET living in the United States (6). Patient signed informed consent was approved by the University Ethics Board at either Columbia University, Yale University, or University of Texas Southwestern, the sites of the 2 principal investigators (E.D.L. and P.L.F).
ET diagnoses were carefully assigned using 3 sequential methods, as detailed elsewhere (6). First, the vast majority (>95%) were diagnosed clinically with ET by their treating physician; the remaining cases were self-diagnosed, and included healthcare workers and individuals with strong family histories of ET (6). Second, cases completed a series of semi-structured clinical questionnaires that included demographic and medical data as well as tremor-specific and family history data. Each patient then submitted 4 standardized hand-drawn Archimedes spirals and additional clinical information from medical records, treating physicians, and family members (6). ET diagnoses were then confirmed by a senior neurologist specializing in movement disorders (E.D.L.) who used the following criteria: (1) moderate or greater amplitude arm tremor (rating ≥2) in at least one of the submitted Archimedes spirals, (2) no history of PD or dystonia, and (3) no other etiology for tremor (6). Third, ET cases then underwent a standardized, videotaped neurological examination, including a detailed assessment of tremor (25). The videotape protocol included assessments of postural tremor (2 positions with each arm), kinetic tremor (5 activities with each arm), and intention tremor of the arms, as well as neck, voice, and jaw tremors (6) as well as dystonia. Postural or kinetic tremor was rated by E.D.L. (0–3) on 12 items, resulting in a total tremor score (range = 0–36) (26). Intention tremor was assessed during the finger-nose-finger maneuver (10 repetitions per arm) with ratings by E.D.L. including 0 (not present), 0.5 (possibly present), and 1 (definitely present) in each arm, and then summed across both arms (intention tremor score = 0–2) (26, 27). The videotaped neurological examination also included the motor portion of the Unified Parkinson’s Disease Rating Scale (UPDRS), including assessments of speech, facial expression, rest tremor (with arms in 4 positions: resting in the lap, relaxed at sides while standing, while walking, and while lying down), bradykinesia, posture, arising from a chair, and gait while walking and turning (6, 28). Rest tremor was categorized by E.D.L. as present in 1, 2, or 3 or more positions. Each videotaped neurological examination was reviewed (E.D.L.) and, based on the questionnaire and videotape data, the diagnosis of ET was re-examined in each case using published diagnostic criteria—moderate or greater amplitude kinetic tremor (tremor rating ≥2) during 3 or more activities or a head tremor in the absence of PD (as defined below) or other known causes (6, 29). An important methodological feature of the current study is that none of the ET cases had PD at the time of enrollment. More specifically, none had the combination of ET and PD (i.e. 2 diagnoses). There are several reasons for this. First, study advertisements specifically specified that individuals with both ET and PD should not apply for enrollment. Second, during an initial screening process, if diagnoses of both ET and PD were reported, then that individual was not enrolled. Third, if after their videotaped neurological examination, both diagnoses of ET and PD were assigned, then that person was not enrolled. We only included individuals with ET and PD if they developed PD after enrollment (i.e. during the follow-up process).
Every 6–9 months, cases completed a follow-up telephone questionnaire, which included a series of screening questions for PD and dystonia; they also submitted 4 new standardized Archimedes spirals (2 right and 2 left) (6). A follow-up videotaped neurological examination was performed if there was suspicion of new onset of PD or dystonia (6). In addition, a growing number of these cases have enrolled in a prospective study evaluating the clinical progression of cognitive features of ET over time and, as such, receive a detailed assessment, including a videotaped neurological examination every 18 months.
After death, medical records were collected from treating neurologists and internists and from terminal hospital stays. Death certificates were also obtained and reviewed for pertinent information (e.g. diagnoses of PD). There were 231 ET brains.
In ET cases, the age of onset of ET symptoms and signs was defined as the self-reported age of onset of action tremor. The designation of “Parkinsonian features” in ET cases included changes in speech, facial expression, rest tremor, bradykinesia, and changes in posture or gait; it was coded as present even when of questionable diagnostic value (e.g. unilateral reduction in arm swing without any other accompanying signs) either on our videotaped neurological examinations or in medical notes from treating physicians. ET cases who had severe action tremor in the setting of longstanding ET who also had isolated mild rest tremor in an upper limb were not given this designation. The age of onset of Parkinsonian symptoms and signs was the age at which any of these symptoms or signs was first noted in one of our neurological examinations or in medical notes from treating physicians. The designation “ET-ETPD” was assigned when one of our ET cases developed PD (the presence of at least 2 cardinal signs), as previously defined (30), and ET-ETpPD when an ET cases had developed signs of possible PD but not enough to establish a PD diagnosis (e.g. one cardinal sign [other than mild rest tremor in the setting of severe and longstanding ET] or several equivocal signs).
Controls
There were 30 age-matched control brains used for comparative purposes in some of our analyses. Twenty-eight control brains were from the New York Brain Bank. These were individuals who had been prospectively followed at the Alzheimer’s Disease Research Center or the Washington Heights-Inwood Columbia Aging Project, Columbia University. During serial neurological examinations, these individuals were clinically free of ET and other neurodegenerative disorders, including AD, PD, or progressive supranuclear palsy. Two control brains were obtained from the National Institutes of Health NeuroBioBank (University of Miami, Miami, FL).
Neuropathological Evaluation
All brains from the New York Brain Bank had a complete neuropathological assessment at the New York Brain Bank at Columbia University. Eighteen standardized blocks were harvested from each brain and processed; 7-µm-thick paraffin sections were stained with Luxol fast blue/hematoxylin and eosin (LH&E) (6). In addition, selected sections were stained by the Bielschowsky silver impregnation method, and by immunohistochemistry using mouse monoclonal antibodies to α-synuclein (clone KM51, Novocastra), phosphorylated tau (clone AT8, Research Diagnostics, Flanders, NJ), and β-amyloid (clone 6F/3D, Dako, Carpinteria, CA) (6). Sections of frontal cortex, hippocampus, and amygdala were stained with antibody to transactive response DNA binding protein 43 (TDP-43) (#10782-2-AP, ProteinTech Group, Rosemont, IL) in ET cases (85/231 [36.7%]) acquired after the use of this antibody became standard practice for the evaluation of neurodegenerative diseases. All tissues were examined microscopically by a senior neuropathologist (J.P.G.V.), who was blinded to clinical information including age and diagnosis. All brains had standardized measurements of brain weight (g), postmortem interval (PMI = hours between death and placement of brain in a cold room or upon ice), Braak and Braak AD staging for neurofibrillary tangles (31), Consortium to Establish a Registry for AD (CERAD) ratings for neuritic plaques (32), and Thal β-amyloid staging (33). The level of AD neuropathologic change (ADNC) was rated according to National Institute on Aging and Alzheimer's Association (NIA-AA) guidelines using an “ABC” score (33). TDP-43 pathology (loss of nuclear staining with cytoplasmic inclusions or accumulation in cell processes) was categorized into 4 stages (0, 1, 2, 3) of Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) (34).
Lewy Bodies and Lewy Neurites
LH&E-stained sections and α-synuclein-stained sections were used to assess LP, including both LBs and Lewy neurites (LNs) (4). Sections included 3 levels of midbrain containing the SNc, 2 to 4 levels of the pons containing the LC, medulla with DVN, hippocampus and adjacent medial temporal lobe, amygdala with ambient gyrus, lenticular nuclei and nucleus basalis of Meynert, cingulate gyrus, temporal cortex (e.g. insular), prefrontal cortex (BA9), motor cortex (BA4), and parietal cortex (BA9) (4). A semiquantitative scale for quantifying LP, based on previously published criteria, was used (35): Stage 0 (absent or not discernable); Stage 1 (sparse LBs or LNs); Stage 2 (2–4 LBs/100× field and sparse LNs); Stage 3 (5–10 LBs/100× field and scattered LNs); and Stage 4 (>10 LBs/100× field and many LNs). Test re-test reliability using this method was reasonable (for LBs, weighted kappa statistic = 0.80; for LNs, weighted kappa statistic = 0.91) (4).
We created a 10-category classification scheme to categorize the distribution of LP, which included either LBs or LNs, in each ET case. We used a framework of the original Braak staging (neuropathological stage of PD) (36), requiring sequential spread of LP from DVN to LC to SNc in the brainstem (categories 1, 2, 3; corresponding to Braak LP stages 1–3), into mesocortical regions (transentorhinal cortex and temporal occipital cortex); category 4, corresponding to Braak LP stage 4, into higher order sensory association areas and prefrontal areas of neocortex (e.g. insular cortex, gyrus cinguli, superior frontal cortex (BA9); category 5, corresponding to Braak LP stage 5), and into primary motor and sensory areas of neocortex (e.g. frontal cortex [BA4], parietal cortex [BA7]; category 6, corresponding to Braak LP stage 6). Four additional categories (for a total of 10 categories) were created to encompass cases that could not be classified by this system, including the presence of LP predominantly in amygdala (AC; category 7), LP only in the locus coeruleus (category 8), LP in ≥2 regions not matching Braak staging (category 9), or LP only in olfactory bulb (OB; category 10). The 10 categories include: (1) neuropathological stage of Parkinson disease (NSPD) 1; (2) NSPD 2; (3) NSPD 3; (4) NSPD 4; (5) NSPD 5; (6) NSPD 6; (7) AC predominant LP; (8) LP in LC only; (9) LP in ≥2 brain regions not fitting Braak scheme, and (10) LP in OB only.
Cerebellar Changes
We previously reported the postmortem findings of our first 33 ET cases of whom 8 (24.2%) had LP (4). An initial examination of Purkinje cell (PC) counts and torpedoes in that small sample suggested that the ET cases with LP had normal cerebellar histology, whereas those without LP had reduced PC counts and higher torpedo counts than controls (4). Given the small sample size, the current sample (n = 58 with LP) afforded a valuable opportunity to revisit these analyses.
Purkinje Cells
A standard 3 × 20 × 25 mm parasagittal, formalin-fixed, tissue block was harvested from the neocerebellum; the block included the cerebellar cortex, white matter, and dentate nucleus (37). The block contained the anterior quadrangulate lobules in the anterior lobe of the cerebellar cortex, which are involved in motor control (37). Using an LH&E-stained 7-μm-thick section, a senior neuropathologist who was blinded to clinical information (P.L.F.) counted PC bodies in 15 100× fields, as described (37). These counts were divided by the length of the PC layer centered in the microscopic field (cells/mm). This resulted in a measure of PC linear density (37).
Torpedoes
Using an LH&E-stained 7-μm-thick section, as well as a Bielschowsky-stained section, the senior neuropathologist (P.L.F.) quantified torpedoes in the entire section. These counts were divided by the length of the PC layer in the entire section (torpedoes/mm).
Basket Cell Plexus Rating
A semiquantitative rating of the appearance of the basket cell plexus surrounding PC bodies throughout Bielschowsky preparations was carried out by a senior neuropathologist (P.L.F.) who was blinded to all clinical information (5). The following scale was used: 0 (few, or no discernible processes); 1 (sparse number of processes); 2 (moderate number of processes); and 3 (dense tangle of processes). In some instances, the rater used intermediate values (0.5, 1.5, and 2.5). Hence, the rating range included the values 0, 0.5, 1, 1.5, 2, 2.5, and 3, as described (5).
Statistical Analyses
All analyses were performed in SPSS (Version 27.0). For continuous measures, we assessed normality using the Kolmogorov-Smirnov test. When continuous measures were not normally distributed (e.g. torpedo count), we used non-parametric tests (e.g. Kruskal-Wallis test) rather than parametric tests (e.g. analysis of variance [ANOVA]) in our analyses of group differences. Our total sample comprised 231 ET brains. For several analyses, we compared the pathology in the 58 ET cases with LP with 90 age-matched ET cases that did not have LP. These 90 ET cases were drawn from a subsample of 100 of our ET cases who had been the subject of an NIH-funded effort to detail the cerebellar pathology of ET with respect to other disorders of cerebellar degeneration; the 10 oldest of these 100 were removed in order to achieve age-matching with the 58 ET cases with LP. We also used 30 age-matched controls.
RESULTS
There were 231 ET cases with a mean age at death of 89.0 ± 6.4 (median = 90.0) years (Table 1). Their last evaluation with us occurred a median of 0.75 years prior to death and ≤2 years in 73.1%, although their last documented neurological evaluation of any kind (e.g. with an outside physician) occurred a median of 0.25 years prior to death and ≤2 years prior to death in 91.0%. Demographic and clinical data are presented in Table 1.
TABLE 1.
Demographic and Clinical Features of 231 ET Cases
| All ET Cases | ET Cases With LBs | ET Cases Without LBs | Comparison of ET Cases With LBs to ET Cases Without LBs | |
|---|---|---|---|---|
| n | 231 | 58 | 173 | NA |
| Age at death (years) | 89.0 ± 6.4 (90.0) | 88.7 ± 6.3 (89.0) | 89.1 ± 6.4 (90.0) | p = 0.76* |
| Age of action tremor onset (years) | 44.5 ± 23.1 (47.0) | 47.4 ± 23.2 (50.0) | 43.4 ± 23.1 (45.0) | p = 0.18* |
| Tremor duration (years) | 44.5 ± 22.7 (39.0) | 41.3 ± 22.2 (36.0) | 45.6 ± 22.80 (42.0) | p = 0.19* |
| Female gender | 144 (62.3) | 36 (62.1) | 108 (62.8) | p = 0.92† |
| Total tremor score | 24.0 ± 7.3 (23.8) | 24.6 ± 6.4 (24.0) | 23.8 ± 7.6 (23.5) | p = 0.67* |
| Intention tremor score | 0.3 ± 0.7 (0.0) | 0.3 ± 0.6 (0.0) | 0.4 ± 0.7 (0.0) | p = 0.37* |
| Head tremor | 147 (65.9)§ | 38 (69.1) | 109 (64.9) | p = 0.57† |
| Voice tremor | 132 (64.7)§ | 33 (66.0) | 99 (64.3) | p = 0.83† |
| Rest tremor | § | p = 0.02‡ | ||
| Absent | 93 (48.7) | 16 (33.3) | 77 (53.8) | |
| Present in 1 position | 52 (27.2) | 15 (31.3) | 37 (25.9) | |
| Present in 2 positions | 17 (8.9) | 7 (14.6) | 10 (7.0) | |
| Present in ≥3 positions | 29 (15.2) | 10 (20.8) | 19 (13.3) | |
| Rest tremor after removing all subjects with ET-ETpPD or ET- ETPD | § | p = 0.145‡ | ||
| Absent | 91 (50.8) | 14 (37.8) | 77 (54.2) | |
| Present in 1 position | 47 (26.3) | 11 (29.7) | 36 (25.4) | |
| Present in 2 positions | 17 (9.5) | 7 (18.9) | 10 (7.0) | |
| Present in ≥3 positions | 24 (13.4) | 5 (13.5) | 19 (13.4) | |
| First-degree relative with ET | 97 (42.2)§ | 24 (41.4) | 73 (42.4) | p = 0.89† |
All values are mean ± standard deviation (median) or number (percentage) unless otherwise specified.
ET, essential tremor; ET-ETpPD, essential tremor and then possible PD; ET-ETPD, essential tremor and then PD; NA, not applicable.
Mann-Whitney test.
Chi-square test.
Linear-by-linear association.
Number of subjects <231 due to incomplete data.
The level of ADNC rated by ABC score was none (66, 28.6%), low (34, 14.7%), intermediate (99, 43.0%), and high (32, 13.9%) likelihood of AD. A neuropathologic diagnosis of definite primary age-related tauopathy (PART), with neurofibrillary degeneration limited to the temporal lobe (Braak AD stages I–IV), in the absence of amyloid deposits (38), was rendered in 57/231 (24.7%) ET cases, which is consistent with estimates that ∼20% of individuals have this pathology by their ninth decade (39). TDP-43 proteinopathy was detected in 20/85 (23.5%) of ET cases, with LATE-NC stages of 0 (65 [76.5%]), 1 (3 [3.5%]), 2 (13 [15.3%]), and 3 (4 [4.7%]), and only sparse and focal neocortical TDP-43 lesions in all but 1 case with LATE-NC stage 3.
Of 231 ET cases, 58 (25.1%) had LP and 46 (19.9%) had early to late stages of pathological PD. Their mean age at death was 88.7 ± 6.3 (median = 89.0) years (Table 1). Among these 58, ADNC ABC scores were none (16, 27.6%), low (6, 10.3%), intermediate (24, 41.4%), and high (12, 20.7%) likelihood of AD.
It is well-established that the likelihood of having LP is increased in individuals with AD-type dementia. If we were to remove all ET cases with ABC scores of intermediate (n = 99) or high (n = 32), then 22 of 100 (22.0%) ET cases had LP.
Pathologically, cases with LP were stratified into a 10-category staging scheme. The anatomic distribution and severity of LP are shown in Figure 1. Categories 1–6 corresponded to Braak PD stages 1–6 (Table 2) and classified 33/58 (56.9%) of cases. Four of the categories (i.e. categories 7–10), with 25 (43.1%) of the 58 ET cases, did not fit the Braak PD staging scheme; many of these (10/25 = 40.0%) had LP predominantly in the amygdala (Table 2), a recognized LP pattern associated with AD (40). Prior descriptions of LP in ET suggested an enrichment for LP more prominently in the LC (4, 15). In this current larger ET series (n = 231), 2 cases had LBs restricted to the LC (category 8) and 4 other cases in category 9 had LP present in LC but not in the SNc, for a total of only 6/58 (10.3%) cases fitting this prior pattern (Fig. 1). AD-type pathologies (Thal, Braak AD, CERAD, and ABC) were highest in category 7 (amygdala predominant, 10/10 [100.0%] with ABC score = 2–3), followed by category 6 (NSPD = 6, 5/7 [71.4%] with ABC score = 2–3) (Table 2; Fig. 1). AD changes were less prominent in lower Braak PD stages, with ABC score = 3 in 1/4 (25.0%) in category 2 (NSPD 2), and ABC score = 2 in 5/8 (62.5%) in category 3 (NSPD 3), 3/5 (60.0%) in category 4 (NSPD 4), and 4/8 (50.0%) in category 5 (NSPD 5).
FIGURE 1.
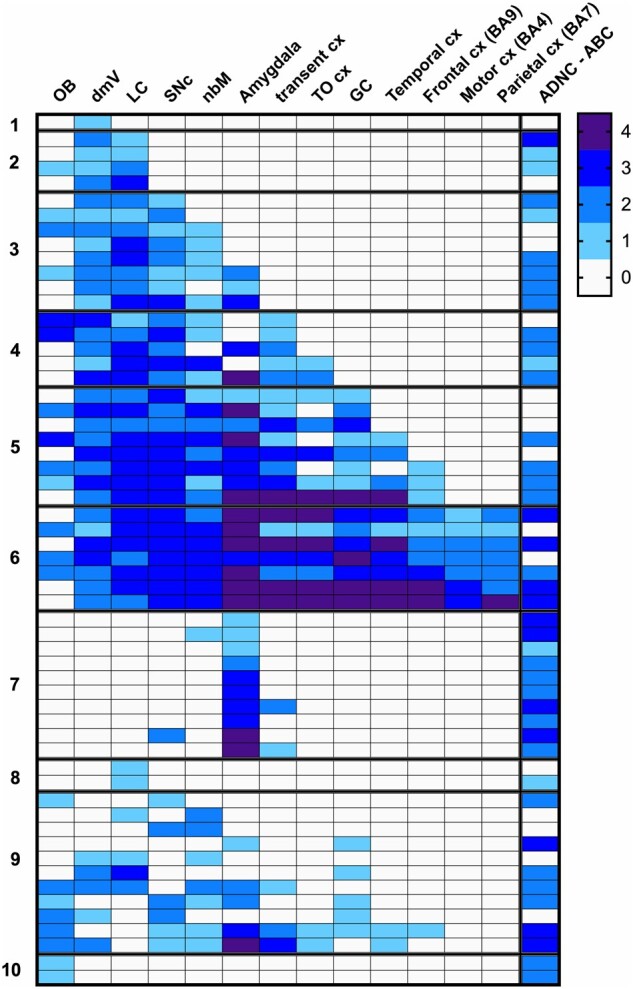
Regional distribution of Lewy pathology in 58 ET cases, with 10-category classification. Severity of Lewy pathology changes is shown by shading, with ordinal gradations from 0 (white) to 4 (darkest shading). ADNC-ABC scores of 0 (white) to 3 (dark shading) scale, corresponding to none, low, intermediate, and high categories. BA, Brodmann area; cx, cortex; dmV, dorsal vagal nucleus; GC, gyrus cinguli; LC, locus coeruleus; nbm, nucleus basalis of Meynert; OB, olfactory bulb; Olf, olfactory; SNc, substantia nigra pars compacta; TO cx, temporal-occipital cortex; transent, transentorhinal.
TABLE 2.
Alzheimer-Type Pathology in 58 ET Cases Stratified into 10 Categories of Lewy Pathology
| Category | Location of LP | N | Age at Death (Years) | Female Sex (%) | Braak PD | Thal A Score | Braak AD | CERAD | ADNC “ABC” Score* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NSPD 1 | 1 | 91.0 | 1 (100) | 1 | 0 | 2 | 0 | 0 |
| 2 | NSPD 2 | 4 | 93.5 ± 5.3 | 3 (75.0) | 2 | 1.25 ± 1.3 | 3.5 ± 1.0 | 1.0 ± 1.4 | 1.25 ± 1.3 |
| 3 | NSPD 3 | 8 | 86.5 ± 6.2 | 5 (62.5) | 3 | 1.6 ± 1.1 | 3.6 ± 0.9 | 1.1 ± 0.8 | 1.4 ± 0.9 |
| 4 | NSPD 4 | 5 | 86.0 ± 6.9 | 5 (83.3) | 4 | 1.8 ± 0.45 | 3.5 ± 1.3† | 1.4 ± 0.9 | 1.4 ± 0.9 |
| 5 | NSPD 5 | 8 | 91.1 ± 4.4 | 5 (62.5) | 5 | 1.0 ± 1.1 | 3.6 ± 0.7 | 0.75 ± 0.9 | 1.0 ± 1.1 |
| 6 | NSPD 6 | 7 | 89.0 ± 4.2 | 4 (57.1) | 6 | 2.0 ± 1.4 | 4.4 ± 1.9 | 1.7 ± 1.4 | 2.0 ± 1.4 |
| 7 | Amygdala predominant | 10 | 88.5 ± 6.6 | 6 (60.0) | UC | 2.3 ± 0.7 | 5.2 ± 0.8 | 2.3 ± 0.8 | 2.3 ± 0.7 |
| 8 | LC only | 2 | 80.5 ± 2.1 | 0 (0.0) | UC | 0.5 ± 0.7 | 2.5 ± 0.7 | 0.0 ± 0.0 | 0.5 ± 0.7 |
| 9 | ≥2 regions not fitting Braak staging | 11 | 89.8 ± 9.5 | 7 (63.4) | UC | 1.7 ± 1.3 | 3.6 ± 1.8† | 1.4 ± 1.3 | 1.55 ± 1.3 |
| 10 | OB only | 2 | 87.0 ± 2.8 | 1 (50.0) | UC | 2.0 ± 0.0 | 4.5 ± 2.1 | 3.0 ± 0.0 | 2.0 ± 0.0 |
All values are mean ± standard deviation or number (percentage) unless otherwise specified.
AD, Alzheimer disease; ADNC, Alzheimer disease neuropathologic change; CERAD, Consortium to Establish a Registry for Alzheimer Disease; LC, locus ceruleus; LP, Lewy pathology; NSPD, neuropathological stage of Parkinson disease; OB, olfactory bulb; PD, Parkinson disease; UC, unclassified.
ADNC “ABC” score is rated as 0 = none, 1 = low, 2 = intermediate, 3 = high.
One case with PSP where Braak AD is not applicable.
Data are shown on the age of onset of ET and the presence of clinical features of parkinsonism in the 58 ET cases, stratified into 10 categories of LP in Table 3. The age of onset of ET tremor ranged from 6 to 80 years (mean = 47.4 ± 23.2 years), with 39 (67.2%) having an onset at or before age 65 years. The time that elapsed between onset of ET tremor and death ranged from 11–87 years (mean = 44.0 ± 22.7 years) and was 15 or more years in 54 (93.1%) of 58 cases. Sixteen (27.6%) cases had parkinsonian features. The age of onset of these parkinsonian features ranged from 73 to 93 years (mean = 82.9 ± 6.3 years). The time that elapsed between onset of ET tremor and onset of these parkinsonian features was 5–78 years (mean = 40.0 ± 23.7 years); in 2 cases, the elapsed time was 5 and 8 years, respectively, and in the remaining cases it was 18 or more years.
TABLE 3.
ET Onset Age and Parkinsonian Features of 58 ET Cases Stratified into 10 Categories of Lewy Pathology
| Category | Location of LP | N | Age at Onset of ET Sx/Sgn (Years) | Years From ET Onset to Death (Years) | Parkinsonian Features | Age at Onset of Parkinsonian Sx/Sgn (Including Rest Tremor) (Years) | Years From Onset of ET Sx/Sgn to Parkinsonian Features (Including Rest Tremor) | Clinical Diagnosis With Respect to ET and PD |
|---|---|---|---|---|---|---|---|---|
| 1 | NSPD 1 | 1 | 41.0 | 50.0 | 0 (0.0) | NA | NA | ET: 1 (100) |
| 2 | NSPD 2 | 4 | 72.3 ± 4.5 | 21.3 ± 5.3 | 0 (0.0) | NA | NA | ET: 4 (100) |
| 3 | NSPD 3 | 8 | 42.1 ± 20.7 | 44.4 ± 16.8 | 4 (50.0) | 81.5 ± 6.1 | 43.9 ± 22.2 | ET: 5 (62.5) |
| ET-ETpPD: 2 (25.0) | ||||||||
| ET-ETPD: 1 (12.5) | ||||||||
| 4 | NSPD 4 | 5 | 52.6 ± 22.5 | 33.4 ± 24.6 | 3 (60.0) | 82.0 ± 10.2 | 24.7 ± 14.7 | ET: 3 (60.0) |
| ET-ETpPD: 1 (20.0) | ||||||||
| ET-ETPD: 1 (20.0) | ||||||||
| 5 | NSPD 5 | 8 | 52.3 ± 23.3 | 38.9 ± 21.9 | 3 (37.5) | 85.7 ± 8.1 | 35.0 ± 38.2 | ET: 5 (62.5) |
| ET-ETpPD: 1 (12.5) | ||||||||
| ET-ETPD: 2 (25.0) | ||||||||
| 6 | NSPD 6 | 7 | 40.3 ± 25.0 | 48.7 ± 24.0 | 4 (57.1) | 81.8 ± 5.1 | 50.3 ± 19.5 | ET: 3 (42.9) |
| ET-ETpPD: 2 (28.6) | ||||||||
| ET-ETPD: 2 (28.6) | ||||||||
| 7 | Amygdala predominant | 10 | 45.4 ± 21.4 | 43.1 ± 21.5 | 1 (10.0) | 83.0 | 67.0 | ET: 9 (90.0) |
| ET-ETpPD: 1 (10.0) | ||||||||
| ET-ETPD: 0 (0.0) | ||||||||
| 8 | LC only | 2 | 29.0 ± 22.6 | 51.5 ± 24.8 | 0 (0.0) | NA | NA | ET: 2 (100) |
| ET-ETpPD: 0 (0.0) | ||||||||
| ET-ETPD: 0 (0.0) | ||||||||
| 9 | ≥2 regions not fitting Braak staging | 11 | 47.0 ± 28.2 | 42.8 ± 28.5 | 1 (9.1) | 88.0 | 18.0 | ET: 10 (90.9) |
| ET-ETpPD: 1 (9.1) | ||||||||
| ET-ETPD: 0 (0.0) | ||||||||
| 10 | OB only | 2 | 45.5 ± 34.7 | 46.5 ± 24.8 | 0 (0.0) | NA | NA | ET: 2 (100) |
| ET-ETpPD: 0 (0.0) | ||||||||
| ET-ETPD: 0 (0.0) | ||||||||
| Entire cohort | 58 | 47.4 ± 23.2 | 41.5 ± 22.0 | 16 (27.6) | 82.9 ± 6.3 | 40.0 ± 23.7 | ET: 44 (75.9) | |
| ET-ETpPD: 8 (13.8) | ||||||||
| ET-ETPD: 6 (10.3) |
All values are mean ± standard deviation or number (percentage) unless otherwise specified.
ET, essential tremor; ET-ETpPD, essential tremor and then possible PD; ET-ETPD, essential tremor and then PD; LC, locus coeruleus; LP, Lewy pathology; NA, not applicable; NSPD, neuropathological stage of Parkinson disease; OB, olfactory bulb; sx/sgn, symptoms and signs.
Of the 231 ET cases, 8 (3.5%) had ET and then developed possible PD (ET-ETpPD), and 6 (2.6%) had ET that later evolved into ET + PD (ET-ETPD). However, it is important to point out that a methodological feature of the current study is that none of the 231 ET cases we selected for enrollment had PD at the time of enrollment. More specifically, none had the combination of ET and PD (i.e. 2 diagnoses). Indeed, 1.65% of potential study applicants were excluded at the time of enrollment because they reported having been diagnosed by and treated for both ET and PD by an outside neurologist. An additional 1.24% were excluded when their detailed videotaped neurological examination, performed at the time of enrollment, was reviewed by the senior movement disorders neurologist (E.D.L.), who noted not only ET but 2 or more hallmark features of PD, making the combined diagnosis clear. Hence, if we had not excluded individuals with ET-ETPD at the time of enrollment, then 5.49% (2.6% + 1.65% + 1.24%) of our sample would have had ET-ETPD. As noted above, an additional 3.5% had ET-ETpPD.
Of the 58 cases with LP, 44 (75.9%) had ET, 8 (13.8%) had ET and then developed possible PD (ET-ETpPD), and 6 (10.3%) had ET that later evolved into ET + PD (ET-ETPD) after a latency of at least 5 years (Table 3). In the 6 with ET-ETPD, the mean latency from the onset of ET tremor to the onset of PD symptoms and signs was 45.75 ± 26.9 years (5 years, 31 years, 36 years, 56.5 years, 68 years, and 78 years).
There were 6 individuals with clinical diagnoses of ET-ETPD; these 6 had Braak PD stages of 3 (n = 1), 4 (n = 1), 5 (n = 2), and 6 (n = 2). The 8 with ET-ETpPD had Braak PD stages of 3 (n = 2), 4 (n = 1), 5 (n = 1), and 6 (n = 2); 2 had LP in the SNc but did not fit into a Braak PD stage—one in category 7 (amygdala predominant) and one in category 9 (≥2 regions not fitting Braak staging) (Table 3).
We compared the 4 metrics of cerebellar pathology in ET cases with LP across the 10 categories of LP (Table 4). These metrics were similar across categories (Table 4: all p values > 0.05). We also compared these 4 metrics in the 58 ET cases with LP versus 90 ET cases without LP and 30 age-matched controls (Table 5). These metrics were higher in both groups of ET cases than in controls (Table 5). ET cases with or without LP had similar PC counts, torpedo counts, and basket plexus ratings (Table 5).
TABLE 4.
Cerebellar Pathology in 58 ET Cases Stratified into 10 Categories of Lewy Pathology
| Category | Location of LP | N | PC/mm | Torpedo/mm (LH&E) | Torpedo/mm (Bielschowsky) | Basket Rating |
|---|---|---|---|---|---|---|
| 1 | NSPD 1 | 1 | 2.90 | 0.198 | 0.222 | 2.0 |
| 2 | NSPD 2 | 4 | 3.65 ± 1.03 | 0.045 ± 0.019 (median = 0.035) | 0.070 ± 0.063 (median = 0.057) | 1.88 ± 0.48 (median = 1.75) |
| 3 | NSPD 3 | 8 | 3.40 ± 0.71 | 0.096 ± 0.059 (median = 0.089) | 0.108 ± 0.077 (median = 0.106) | 2.08 ± 0.92 (median = 2.0) |
| 4 | NSPD 4 | 5 | 3.75 ± 0.90 | 0.075 ± 0.035 (median = 0.056) | 0.103 ± 0.046 (median = 0.086) | 2.20 ± 0.84 (median = 2.0) |
| 5 | NSPD5 | 8 | 4.26 ± 0.33 | 0.062 ± 0.037 (median = 0.043) | 0.094 ± 0.056 (median = 0.074) | 2.19 ± 0.59 (median = 2.0) |
| 6 | NSPD 6 | 7 | 3.96 ± 1.14 | 0.091 ± 0.035 (median = 0.088) | 0.128 ± 0.050 (median = 0.123) | 1.86 ± 0.69 (median = 1.5) |
| 7 | Amygdala predominant | 10 | 3.97 ± 1.53 | 0.099 ± 0.058 (median = 0.103) | 0.118 ± 0.057 (median = 0.109) | 2.30 ± 0.54 (median = 2.0) |
| 8 | LC only | 2 | 4.25 ± 0.48 | 0.025 ± 0.009 (median = 0.025) | 0.057 ± 0.053 (median = 0.057) | 1.75 ± 0.35 (median = 1.75) |
| 9 | ≥2 regions not fitting Braak staging | 11 | 4.14 ± 1.05 | 0.064 ± 0.052 (median = 0.047) | 0.073 ± 0.051 (median = 0.064) | 1.91 ± 0.58 (median = 2.0) |
| 10 | OB only | 2 | 3.34 ± 0.43 | 0.067 ± 0.016 (median = 0.067) | 0.063 ± 0.0001 (median = 0.063) | 2.0 ± 0.0 (median = 2.0) |
| p value | 0.77* | 0.08† | 0.21† | 0.89† | ||
All values are mean ± standard deviation or number (percentage) unless otherwise specified.
LC, locus coeruleus; LH&E, Luxol fast blue hematoxylin & eosin; LP, Lewy pathology; NSPD, neuropathological stage of Parkinson disease; OB, olfactory bulb; PC, Purkinje cell.
Analysis of variance.
Kruskal-Wallis test.
TABLE 5.
Comparison of Cerebellar Pathology in Age-Matched Samples: ET Cases With LBP, ET Cases Without LBP and Controls
| ET Cases With LP (A) | ET Cases Without LP (B) | Controls (C) | Comparison of A, B and C | Comparison of A vs B | Comparison of A vs C | Comparison of B vs C | |
|---|---|---|---|---|---|---|---|
| n | 58 | 90 | 30 | ||||
| Age (years) | 88.7 ± 6.6 (89.5) | 88.7 ± 6.0 (89.5) | 86.6 ± 7.7 (84.5) | p = 0.25* | p = 0.79† | p = 0.13† | p = 0.12† |
| PC count | 3.89 ± 1.00 | 4.06 ± 0.72 | 5.16 ± 1.00 | p < 0.001‡ | p = 0.51§ | p < 0.001§ | p < 0.001§ |
| Torpedo Count (LH&E) | 0.078 ± 0.050 (0.071) | 0.070 ± 0.056 (0.057) | 0.034 ± 0.031 (0.024) | p < 0.001* | p = 0.20† | p < 0.001† | p < 0.001† |
| Torpedo Count (Bielschowsky) | 0.099 ± 0.059 (0.092) | 0.102 ± 0.088 (0.077) | 0.058 ± 0.046 (0.043) | p = 0.003* | p = 0.49† | p < 0.001† | p = 0.005† |
| Basket cell plexus rating | 2.04 ± 0.61 (2.0) | 2.06 ± 0.64 (2.0) | 1.65 ± 0.60 (1.5) | p = 0.008* | p = 0.73† | p = 0.008† | p = 0.003† |
All values are mean ± standard deviation (median).
LH&E, Luxol fast blue hematoxylin and eosin; LP, Lewy pathology; PC, Purkinje cell.
Kruskall-Wallis test.
Mann-Whitney test.
Analysis of variance.
Tukey post doc comparison.
We also assessed whether ET cases with LP differed from those without LP in terms of a range of demographic and clinical features (Table 1). The 2 groups did not differ except with respect to their burden of rest tremor (p = 0.02). However, when the 14 with ET-ETPD and ET-ETpPD were removed, the groups did not differ significantly with respect to their burden of rest tremor (p = 0.145) (Table 1). Furthermore, ET cases with LP versus without LP did not differ in the extent of ADNC (e.g. Braak AD, 3.9 ± 1.4 vs 3.7 ± 1.2, p = 0.38; ABC, 1.55 ± 0.11 vs 1.4 ± 0.3, p = 0.27) or in PART pathology (14/58 [24.1%] vs 43/173 [24.85%]). TDP-43 pathology was more common in ET cases with LP (9/17 [52.9%]) vs ET cases without LP (11/68 [16.2%]), despite similar levels of ADNC in these sampled cases, consistent with emerging data supporting an association between LATE-NC and LB disease (41).
We also examined the age of onset of ET in the 6 ET-ETPD cases, 8 ET-ETpPD cases, and 44 cases who remained as ET, and these ages of onset did not differ (p = 0.48).
DISCUSSION
In this clinical-pathological cohort of 231 ET cases, the proportion with LP was 25.1%, i.e. one-in-four ET cases. Of the 58 ET cases with LP, 8 (13.8%) had ET as well as possible PD (i.e. ET followed by a latency period of at least 5 years, after which they developed early signs of PD but not enough to establish a PD diagnosis) and 6 (10.3%) had ET that had evolved into ET + PD after a latency of at least 5 years. In these 6, the mean latency from the onset of ET tremor to the onset of PD symptoms was 45.75 ± 26.9 years (range = 5–78 years). There is an epidemiological literature demonstrating that prevalent ET is associated with increased odds and risk of PD (18–21); in 1 epidemiological study, ET cases were estimated to be 4–5 times more likely than age-matched counterparts without ET to develop PD (20). Hence, some of the burden of LP in our ET cases is likely due to the enhanced risk of PD among ET cases. Nonetheless, the majority (44 or 75.9%) of our 58 ET cases did not have clinical evidence of possible PD or PD.
We also considered the potential impact of AD-type pathology. Even when we removed all ET cases with ABC categories of intermediate (n = 99) or high (n = 32), 22 of 100 (22.0%) ET cases had LP.
It would be of value to compare the 25.1% we observed among ET cases to other literature on the percentage of normal elderly individuals who have LP on postmortem examination. Data are presented on 14 studies with the range of individuals with LP being 5.0% to 36.5% (42–55) (Table 6). The median value is 14.7%. As these studies varied enormously in terms of their sample sizes (n = 39–1720), we also calculated a weighted average of these values, which took sample size into consideration. That value is 16.4%. These studies for the most part did not systematically exclude PD or ET, inflating their estimates of the proportion of “normal individuals” with LP, and corrections were made for this (Table 6, including footnotes), which used data on the population prevalence of ET and PD (56, 57). Corrected values range from 1.6% to 36.2%, with a median = 12.65% and a weighted average = 14.5%. When the data on these 14 comparison studies are taken together, the value we observed for LP in ET, 25.1%, seems high, being more than 50% higher than the weighted average (16.4%) and nearly 75% higher than the corrected weighted average of comparison studies (14.5%).
TABLE 6.
Published Literature on the Prevalence of LP in Autopsy Series (Ordered by Reported Proportion With LP)
| Country (Author and Year) | n | Mean Age or Approximate Mean Age of Sample (Years) | Reported Proportion With LP* | Fully Excluded PD Cases?† | Excluded ET Cases? | Revised Estimate of Proportion With LP (After Correcting for ET)‡ | Revised Estimate of Proportion With LP (After Correcting for Both ET and PD)§ |
|---|---|---|---|---|---|---|---|
| Austria (Jellinger et al, 2012) | 100 | 81.2 | 5.0% | Uncertain¶ | No | 3.5% | 1.6% |
| USA (Knopman et al, 2003) | 39 | 85 | 12.8% | Uncertain¶ | No | 11.9% | 10.2% |
| Finland (Parkkinen et al, 2003) | 904 | 70–72 | 13.4% | No | No | 12.5% | 11.5% |
| Finland (Parkkinen et al, 2001) | 774 | 70–72 | 14.1% | No | No | 13.3% | 12.5% |
| USA (Dugger et al, 2014) | 119 | 88 | 14.3% | Uncertain¶ | Yes | Not applicable | 12.6% |
| Finland (Parkkinnen et al, 2008) | 1720 | 70–72 | 14.4% | No | No | 13.6% | 12.7% |
| USA (Frigerio et al, 2011) | 235 | 82 | 14.5% | Uncertain¶ | No | 13.7% | 12.0% |
| USA (Fujishiro et al, 2008) | 241 | 79 | 14.9% | Uncertain¶ | No | 14.2% | 12.5% |
| USA (White et al, 2009) | 443 | 80s? | 15.4% | No | No | 14.6% | 13.0% |
| Switzerland (Block et al, 2006) | 98 | 80.7 | 17.3% | Uncertain¶ | No | 16.8% | 15.2% |
| Japan (Saito et al, 2004) | 1,241 | 80.6 | 20.5% | No | No | 20.1% | 18.6% |
| Japan (Wakisaka 2003) | 102 | 80.2 | 22.5% | No | No | 22.4% | 20.9% |
| USA (Markesbery et al, 2009) | 139 | 83.5 | 23.7% | No | No | 23.6% | 22.1% |
| England (Zaccai et al, 2008) | 208 | 80s | 36.5% | No | No | 37.4% | 36.2% |
Ability to exclude a diagnosis of ET would have meant that the study had introduced the term “essential tremor” in the Materials and Methods section, a description of the method of evaluating ET was documented (including the specific items introduced into the neurological examination), details about the rating of tremor on neurological examination and the expertise of the rater were provided, and the specific diagnostic criteria used to assign diagnoses of ET were provided.
†The study did not provide detailed explanations about the methods for excluding individuals with PD. Also see footnote¶ below.
‡In studies that did not systematically exclude individuals with ET from their “normal population,” the estimate of the percentage of normal individuals with LP is likely inflated. To adjust for this, we first examined published data on the prevalence (%) of ET in individuals with the mean age of the sample in the study. Based on the data presented in this article (i.e. that 25.1% of ET cases have LP), we then assumed that 25% of these would have had LP. We then removed from the denominator the expected number of individuals presumed to have had ET and we removed from the numerator 25% of that number.
§In studies that did not systematically exclude individuals with PD from their “normal population,” the estimate of the percentage of normal individuals with LP is likely inflated. To adjust for this, we first examined published data on the prevalence (%) of PD in individuals with the mean age of the sample in the study. We then removed from both the numerator and the denominator the expected number of individuals presumed to have had PD.
Uncertain for one of the following reasons: (1) not clear how many individuals were examined by a neurologist or by a movement disorders neurologist, (2) not clear what type of neurological examination was performed and in how much detail parkinsonian features were assessed, and (3) not clear how many individuals were followed with neurological examinations to a point close in time to death.
In 2007, we reported the postmortem findings of our first 33 ET cases (4). Of those 33, 8 (24.2%) had LP (4), a value that is strikingly similar to that which we now report with a sample that is 7 times greater (n = 231 ET brains). In 6 of these 33 initial ET cases, LBs were abundant in the LC and either absent or infrequent in other brainstem structures (4). In this current larger ET series, 2 cases had LBs restricted to the LC (category 8) and 4 other cases in category 9 had LP present in LC but not in the SNc, for a total of only 6/58 (10.3%) cases fitting this pattern (Fig. 1). In addition, with a larger sample, we now see that the LP in ET cases is more heterogeneous and varied than initially observed. That is, what we termed the “Lewy body variant of ET” has more topographically complex LP than initially envisioned. While 33 (56.9%) of our ET cases follow the Braak staging scheme for LP (36), 25 (43.1%) do not, further indicating a wide variety of patterns of LP (Table 2). While some of our ET cases with LB represent ET plus PD or ET with PD in evolution, others have an amygdala-predominant form of LP associated with AD-type pathology, and yet others appear to have neither (Fig. 1; Table 2). Regardless, the high proportion of observed ET cases with LP represents a pathophysiologically heterogeneous group, likely related in part to the observed association between ET and PD, likely in part due to the observed association between ET and AD-type pathology (18, 58), and likely related to other factors that are unknown (59–64).
Prior epidemiological studies provide evidence for an association between ET and PD (18–21), with one prospective, longitudinal study showing that ET cases have more than a 4-fold increased risk of developing PD (20). With those data, it is credible that the burden of LP in ET exceeds that of the general population, and that LP ranges from mild and early all the way through to higher stages of LP with clinical PD. Stated in another way, it is not suprising that 25.1% of ET cases have LP—this must be the case to explain the reported extraordinarily high conversion rate of ET to ET plus PD.
In this study, we report that 8 (3.5%) of 231 cases had ET and then developed possible PD (ET-ETpPD), and 6 (2.6%) of 231 ET cases had ET that later evolved into ET + PD (ET-ETPD). As noted above, however, an important methodological feature of the current study is we excluded numerous cases with ET-ETPD at the time of enrollment. If we had included those, then 5.49% (2.6% + 1.65% + 1.24%) of our sample would have had ET-ETPD. As noted above, an additional 3.5% had ET-ETpPD, with the majority of these having LP in their SNc. A meta-analysis of 47 epidemiological studies of PD reported that the prevalence of PD among individuals age 80 and older was 1.903% (57). This value, 1.903%, is substantially lower than 5.49% (i.e. approximately one-third of the value) and, if we included those with ET-ETpPD, even further lower than the 8.99% with either ET-ETPD or ET-ETpPD.
In 2007, we reported the postmortem findings of our first 33 ET cases of whom 8 (24.2%) had LP (4). An initial examination of PC counts and torpedoes suggested in that small sample that the ET cases with LP had within normal cerebellar histology, whereas those without LP had reduced PC counts and higher torpedo counts than controls (4). Now, with a sample of ET cases with LP that is substantially higher (58 vs 8), we observed that evidence of cerebellar degeneration is apparent in ET cases with LB as well. Thus, there is evidence of cerebellar degeneration in all of our ET cases rather than just in the subset without LP.
This study was not without limitations. Patients who sign up for a brain donation are not typical ET cases. They tend to have more severe ET. Nonetheless, this is the largest prospectively collected cohort of ET brains reported to date and the most detailed clinical-pathological study of LP in this disease. Phenotyping was performed by a senior movement disorders neurologist and postmortem examination carefully followed a standard protocol.
In summary, in this clinical-pathological cohort of 231 ET cases, the proportion with LP was 25.1% (i.e. 1 in 4) ET cases, a proportion that in the context of other studies among control populations seems high. LP was heterogeneous, and likely a partial function of observed clinical associations between (1) ET and PD and (2) ET and AD, as well as other factors that are unknown. There was evidence of cerebellar degeneration in ET cases both with and without LP, rather than just in those without LP. These data have value in terms of furthering our understanding of the biological and mechanistic basis for ET as well as its relatedness to other degenerative diseases.
ACKNOWLEDGMENTS
Brain tissue was derived from the New York Brain Bank and the National Institutes of Health NeuroBioBank (University of Miami, Miami, FL). We would like to thank all the patients and families that contributed to brain donation.
Contributor Information
Elan D Louis, From the Department of Neurology, University of Texas Southwestern, Dallas, Texas, USA.
Daniella Iglesias-Hernandez, From the Department of Neurology, University of Texas Southwestern, Dallas, Texas, USA.
Nora C Hernandez, From the Department of Neurology, University of Texas Southwestern, Dallas, Texas, USA.
Xena Flowers, Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Columbia University, New York, New York, USA.
Sheng-Han Kuo, Department of Neurology, Vagelos College of Physicians and Surgeons, Columbia University, New York, New York, USA.
Jean Paul G Vonsattel, Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Columbia University, New York, New York, USA; Department of Pathology and Cell Biology, Columbia University Irving Medical Center and the New York Presbyterian Hospital, New York, New York, USA.
Phyllis L Faust, Department of Pathology and Cell Biology, Columbia University Irving Medical Center and the New York Presbyterian Hospital, New York, New York, USA.
This work was supported by the National Institutes of Health (NINDS R01 NS117745, R01 NS086736, and R01 NS088257).
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Louis ED, Vonsattel JP, Honig LS, et al. Neuropathologic findings in essential tremor. Neurology 2006;66:1756–9 [DOI] [PubMed] [Google Scholar]
- 2. Louis ED, Benito-Leon J, Ottman R, et al. ; Neurological Disorders in Central Spain (NEDICES) Study Group. A population-based study of mortality in essential tremor. Neurology 2007;69:1982–9 [DOI] [PubMed] [Google Scholar]
- 3. Lin CY, Louis ED, Faust PL, et al. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 2014;137:3149–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007;130:3297–307 [DOI] [PubMed] [Google Scholar]
- 5. Erickson-Davis CR, Faust PL, Vonsattel JP, et al. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol 2010;69:262–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Babij R, Lee M, Cortes E, et al. Purkinje cell axonal anatomy: Quantifying morphometric changes in essential tremor versus control brains. Brain 2013;136:3051–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Louis ED, Lee M, Babij R, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 2014;137:3142–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology 2008;70:1452–5 [DOI] [PubMed] [Google Scholar]
- 9. Paris-Robidas S, Brochu E, Sintes M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain 2012;135:105–16 [DOI] [PubMed] [Google Scholar]
- 10. Luo C, Rajput AH, Robinson CA, et al. Gamma-aminobutyric acid (GABA)-B receptor 1 in cerebellar cortex of essential tremor. J Clin Neurosci 2012;19:920–1 [DOI] [PubMed] [Google Scholar]
- 11. Delay C, Tremblay C, Brochu E, et al. Increased LINGO1 in the cerebellum of essential tremor patients. Mov Disord 2014;29:1637–47 [DOI] [PubMed] [Google Scholar]
- 12. Louis ED, Kerridge CA, Chatterjee D, et al. Contextualizing the pathology in the essential tremor cerebellar cortex: A patholog-omics approach. Acta Neuropathol 2019;138:859–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Louis ED, Faust PL.. Essential tremor: The most common form of cerebellar degeneration? Cerebellum Ataxias 2020;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Louis ED, Faust PL.. Essential tremor pathology: Neurodegeneration and reorganization of neuronal connections. Nat Rev Neurol 2020;16:69–83 [DOI] [PubMed] [Google Scholar]
- 15. Louis ED, Honig LS, Vonsattel JP, et al. Essential tremor associated with focal nonnigral Lewy bodies: A clinicopathologic study. Arch Neurol 2005;62:1004–7 [DOI] [PubMed] [Google Scholar]
- 16. Adler CH, Shill HA, Beach TG.. Essential tremor and Parkinson's disease: Lack of a link. Mov Disord 2011;26:372–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Algarni M, Fasano A.. The overlap between Essential tremor and Parkinson disease. Parkinsonism Relat Disord 2018;46(Suppl 1):S101–4 [DOI] [PubMed] [Google Scholar]
- 18. LaRoia H, Louis ED.. Association between essential tremor and other neurodegenerative diseases: What is the epidemiological evidence? Neuroepidemiology 2011;37:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan EK, Lee SS, Fook-Chong S, et al. Evidence of increased odds of essential tremor in Parkinson's disease. Mov Disord 2008;23:993–7 [DOI] [PubMed] [Google Scholar]
- 20. Benito-Leon J, Louis ED, Bermejo-Pareja F; on behalf of the Neurological Disorders in Central Spain (NEDICES) Study Group. Risk of incident Parkinson's disease and parkinsonism in essential tremor: A population based study. J Neurol Neurosurg Psychiatry 2008;80:423–5 [DOI] [PubMed] [Google Scholar]
- 21. Thenganatt MA, Jankovic J.. The relationship between essential tremor and Parkinson's disease. Parkinsonism Relat Disord 2016;22(Suppl 1):S162–5 [DOI] [PubMed] [Google Scholar]
- 22. Rocca WA, Bower JH, Ahlskog JE, et al. Increased risk of essential tremor in first-degree relatives of patients with Parkinson's disease. Mov Disord 2007;22:1607–14 [DOI] [PubMed] [Google Scholar]
- 23. Costello S, Bordelon Y, Bronstein J, et al. Familial associations of Alzheimer disease and essential tremor with Parkinson disease. Eur J Neurol 2010;17:871–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spanaki C, Plaitakis A.. Essential tremor in Parkinson’s disease kindreds from a population of similar genetic background. Mov Disord 2009;24:1662–8 [DOI] [PubMed] [Google Scholar]
- 25. Louis ED, Zheng W, Applegate L, et al. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology 2005;65:391–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Louis ED, Huey ED, Cosentino S.. Features of “ET plus” correlate with age and tremor duration: “ET plus” may be a disease stage rather than a subtype of essential tremor. Parkinsonism Relat Disord 2021;91:42–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Louis ED, Frucht SJ, Rios E.. Intention tremor in essential tremor: Prevalence and association with disease duration. Mov Disord 2009;24:626–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Louis ED, Asabere N, Agnew A, et al. Rest tremor in advanced essential tremor: A post-mortem study of nine cases. J Neurol Neurosurg Psychiatry 2011;82:261–5 [DOI] [PubMed] [Google Scholar]
- 29. Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: Methodologic issues in essential-tremor research. Neuroepidemiology 1997;16:124–33 [DOI] [PubMed] [Google Scholar]
- 30. Louis ED, Wise A, Alcalay RN, et al. Essential tremor-Parkinson's disease: A double whammy. J Neurol Sci 2016;366:47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braak B, Braak E.. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 32. Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: A commentary. Neurobiol Aging 1997;18:S91–4 [DOI] [PubMed] [Google Scholar]
- 33. Montine TJ, Phelps CH, Beach TG, et al. ; Alzheimer’s Association. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 2019;142:1503–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKeith IG, Dickson DW, Lowe J, et al. ; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005;65:1863–72 [DOI] [PubMed] [Google Scholar]
- 36. Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211 [DOI] [PubMed] [Google Scholar]
- 37. Choe M, Cortes E, Vonsattel JG, et al. Purkinje cell loss in essential tremor: Random sampling quantification and nearest neighbor analysis. Mov Disord 2016;31:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014;128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braak H, Thal DR, Ghebremedhin E, et al. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011;70:960–9 [DOI] [PubMed] [Google Scholar]
- 40. Hamilton RL. Lewy bodies in Alzheimer’s disease: A neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol 2000;10:378–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang SJ, Guo Y, Ervin JF, et al. Neuropathological associations of limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) differ between the oldest-old and younger-old. Acta Neuropathol 2022;144:45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parkkinen L, Pirttila T, Alafuzoff I.. Applicability of current staging/categorization of α-synuclein pathology and their clinical relevance. Acta Neuropathol 2008;115:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parkkinen L, Soininen H, Alafuzoff I.. Regional distribution of α-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol 2003;62:363–7 [DOI] [PubMed] [Google Scholar]
- 44. Parkkinen L, Soininen H, Laakso M, Alafuzoff I.. Α-synuclein pathology is highly dependent on the case selection. Neuropathol Appl Neurobiol 2001;27:314–25 [DOI] [PubMed] [Google Scholar]
- 45. Fujishiro H, Ahn TB, Frigerio R, et al. Glial cytoplasmic inclusions in neurologically normal elderly: Prodromal multiple system atrophy? Acta Neuropathol 2008;116:269–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bloch A, Probst A, Bissig H, et al. Α-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 2006;32:284–95 [DOI] [PubMed] [Google Scholar]
- 47. Saito Y, Ruberu NN, Sawabe M, et al. Lewy body-related α-synucleinopathy in aging. J Neuropathol Exp Neurol 2004;63:742–9 [DOI] [PubMed] [Google Scholar]
- 48. Dugger BN, Hentz JG, Adler CH, et al. Clinicopathological outcomes of prospectively followed normal elderly brain bank volunteers. J Neuropathol Exp Neurol 2014;73:244–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zaccai J, Brayne C, McKeith I, et al. ; MRC Cognitive Function, Ageing Neuropathology Study. Patterns and stages of α-synucleinopathy: Relevance in a population-based cohort. Neurology 2008;70:1042–8 [DOI] [PubMed] [Google Scholar]
- 50. Frigerio R, Fujishiro H, Ahn TB, et al. Incidental Lewy body disease: Do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiol Aging 2011;32:857–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 2003;62:1087–95 [DOI] [PubMed] [Google Scholar]
- 52. White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: A summary report from the Honolulu-Asia aging study. J Alzheimers Dis 2009;18:713–25 [DOI] [PubMed] [Google Scholar]
- 53. Markesbery WR, Jicha GA, Liu H, et al. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol 2009;68:816–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jellinger KA, Attems J.. Neuropathology and general autopsy findings in nondemented aged subjects. Clin Neuropathol 2012;31:87–98 [DOI] [PubMed] [Google Scholar]
- 55. Wakisaka Y, Furuta A, Tanizaki Y, et al. Age-associated prevalence and risk factors of Lewy body pathology in a general population: The Hisayama study. Acta Neuropathol 2003;106:374–82 [DOI] [PubMed] [Google Scholar]
- 56. Louis ED, McCreary M.. How common is essential tremor? Update on the worldwide prevalence of essential tremor. Tremor Other Hyperkinet Mov (NY) 2021;11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pringsheim T, Jette N, Frolkis A, Steeves TD.. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 2014;29:1583–90 [DOI] [PubMed] [Google Scholar]
- 58. Benito-Leon J, Bermejo-Pareja F, Morales-Gonzalez JM, et al. ; Neurological Disorders in Central Spain (NEDICES) Study Group. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology 2004;62:734–41 [DOI] [PubMed] [Google Scholar]
- 59. Farrell K, Cosentino S, Iida MA, et al. Quantitative assessment of pathological tau burden in essential tremor: A postmortem study. J Neuropathol Exp Neurol 2019;78:31–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pan JJ, Lee M, Honig LS, et al. Alzheimer’s-related changes in non-demented essential tremor patients vs. controls: Links between tau and tremor? Parkinsonism Relat Disord 2014;20:655–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Benito-Leon J, Louis ED, Bermejo-Pareja F; on behalf of the Neurological Disorders in Central Spain (NEDICES) Study Group. Elderly-onset essential tremor is associated with dementia. Neurology 2006;66:1500–5 [DOI] [PubMed] [Google Scholar]
- 62. Bermejo-Pareja F, Louis ED, Benito-Leon J; Neurological Disorders in Central Spain (NEDICES) Study Group. Risk of incident dementia in essential tremor: A population-based study. Mov Disord 2007;22:1573–80 [DOI] [PubMed] [Google Scholar]
- 63. Thawani SP, Schupf N, Louis ED.. Essential tremor is associated with dementia: Prospective population-based study in New York. Neurology 2009;73:621–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim SH, Farrell K, Cosentino S, et al. Tau isoform profile in essential tremor diverges from other tauopathies. J Neuropathol Exp Neurol 2021;80:835–43 [DOI] [PMC free article] [PubMed] [Google Scholar]


