Abstract
In order to clarify the possible role of N-acetylcysteine (NAC) in the treatment of patients with chronic bronchitis and chronic obstructive pulmonary disease (COPD), we have carried out a meta-analysis testing the available evidence that NAC treatment may be effective in preventing exacerbations of chronic bronchitis or COPD and evaluating whether there is a substantial difference between the responses induced by low (≤600 mg per day) and high (>600 mg per day) doses of NAC.
The results of the present meta-analysis (13 studies, 4155 COPD patients, NAC n=1933; placebo or controls n=2222) showed that patients treated with NAC had significantly and consistently fewer exacerbations of chronic bronchitis or COPD (relative risk 0.75, 95% CI 0.66–0.84; p<0.01), although this protective effect was more apparent in patients without evidence of airway obstruction. However, high doses of NAC were also effective in patients suffering from COPD diagnosed using spirometric criteria (relative risk 0.75, 95% CI 0.68–0.82; p=0.04). NAC was well tolerated and the risk of adverse reactions was not dose-dependent (low doses relative risk 0.93, 95% CI 0.89–0.97; p=0.40; high doses relative risk 1.11, 95% CI 0.89–1.39; p=0.58).
The strong signal that comes from this meta-analysis leads us to state that if a patient suffering from chronic bronchitis presents a documented airway obstruction, NAC should be administered at a dose of ≥1200 mg per day to prevent exacerbations, while if a patient suffers from chronic bronchitis, but is without airway obstruction, a regular treatment of 600 mg per day seems to be sufficient.
Short abstract
Evidence that high doses of NAC protect against COPD exacerbations with a favourable risk-benefit ratio http://ow.ly/NeSbl
Introduction
Recently, the large Placebo-Controlled Study on Efficacy and Safety of N-Acetylcysteine High Dose in Exacerbations of Chronic Obstructive Pulmonary Disease (PANTHEON study) [1] has documented that in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD), long-term use of N-acetylcysteine (NAC) at 600 mg twice daily can prevent exacerbations, especially in disease of moderate severity. However, in a later commentary, some of us wrote that, although we were fascinated by these results, the evidence that emerged from currently published studies investigating the effect of NAC in patients with COPD was still not adequate to recommend NAC as an integral component of COPD treatment [2]. In particular, we pointed out that all positive findings on NAC in COPD have come from studies either investigating relatively small numbers of patients, or conducted in China, where patients may not be representative of the wider population. In addition, the large Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS) trial in a largely Caucasian European population showed that NAC is ineffective in preventing deterioration in lung function and exacerbations in patients with COPD [3]. Nonetheless, in the same commentary [2] we highlighted that the PANTHEON study used a higher dose (1200 mg daily) of NAC [1], double the 600 mg daily dose of NAC used in BRONCUS [3].
A previous meta-analysis [4] and a quantitative systematic review [5] have both suggested that a prolonged course of oral NAC prevents acute exacerbations of chronic bronchitis. However, neither analysis, published in 2000, considered COPD as a specific entity; in addition, there was no evidence of any effect of cumulative dosing with NAC on efficacy. However, a more recent meta-analysis showed that long-term high-dose NAC treatment might lead to a lower rate of COPD exacerbations, although the effect of low-dose NAC treatment remained uncertain [6].
In light of the recent PANTHEON study we have carried out a further meta-analysis of whether NAC is effective in preventing COPD exacerbations, and whether the effects are dose-related.
Materials and methods
Searching strategy
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (fig. 1) [7].
FIGURE 1.
Preferred reporting items for systematic reviews and meta-analyses flow diagram for the identification of studies included in the meta-analysis concerning the influence of N-acetylcysteine on chronic bronchitis or chronic obstructive pulmonary disease exacerbations.
We performed a comprehensive literature search for studies concerning the influence of low and high doses of NAC treatment on the rate of exacerbations in patients suffering from COPD detected by pulmonary function testing (PFT) and/or the International Classification of Diseases (ICD)-9 491, 492, 496 categories [8–10]. Low-dose NAC was defined as ≤600 mg daily, whereas high-dose NAC was defined as >600 mg daily. The search terms “chronic obstructive pulmonary disease”, “COPD”, “chronic bronchitis”, “pulmonary emphysema” and “exacerbations” were used for the disease and the terms “acetylcysteine”, “N-acetylcysteine” and “NAC” for the drug. The search was performed on PubMed and Google Scholar in order to provide relevant studies published up to July 31, 2014 [11]. Citations of previous published meta-analyses and relevant reviews were examined to identify further pertinent studies, if any [6, 12].
All studies (both randomised clinical trials (RCTs) and observational investigations) regarding COPD patients receiving oral administration of NAC versus placebo or control subjects have been included in the analysis.
Quality score
The Jadad score, with a scale of 1–5 (5 being the highest score), was used to assess the quality of the papers concerning the likelihood of bias related to randomisation, double blinding, withdrawals and dropouts [13]. A Jadad score ≤3 was used as cut-off value for subgroup analysis of studies.
Data extraction
Data from included studies were extracted and checked for study characteristics and duration, NAC doses, disease characteristics, age, sex, smoking habits, smoking history, use of inhaled corticosteroids, forced expiratory volume in 1 s (FEV1), safety, tolerance and Jadad score.
End-point
The end-point of the meta-analysis was the influence of NAC in modulating the frequency of COPD exacerbations, via normalising data as a function of person-season, where one season lasts 3 months.
Data analysis
The overall analysis conducted on all selected studies and the subgroup analysis of studies sorted according to the doses of NAC (low or high doses) was carried out via a binary random-effects model, in order to balance the study weights and to adequately estimate the confidence interval of the mean distribution of NAC effect on COPD exacerbations [14–16]. A further subgroup analysis was carried out exclusively on RCTs in which the additional inclusion criteria of diagnosis of COPD using PFT according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations (post-bronchodilator FEV1/forced vital capacity (FVC) ≤0.7; FEV1 ≤80% predicted] [8, 10]. In this subgroup analysis, since the selected studies were functionally similar the binary fixed-effect Mantel–Haenszel model was applied to compute the common NAC effect size in COPD patient populations [14–16].
Results of the present analysis are expressed as relative risk (95% CI). The OpenMetaAnalyst software was used to perform this meta-analysis [17, 18].
Results
Study characteristics
Overall, results obtained from 4155 COPD patients (NAC n=1933; placebo or controls n=2222) were selected from 13 studies. Three studies used high-dose NAC [1, 19, 20], nine used low-dose NAC [3, 21–28], and one investigated both low- and high-dose NAC [29]. Seven RCTs in which COPD patients were enrolled using PFT in agreement with ATS/ERS or GOLD recommendations were used for the subgroup analysis [1, 3, 20, 22, 24, 26, 27]. Although the study carried out by the British Thoracic Society Research Committee in 1985 [22] was antecedent to both ATS/ERS and GOLD positions, we added it to those RCTs in which COPD patients were enrolled in agreement with ATS/ERS or GOLD statements [8] because, in that study, FEV1 <50% pred and FEV1/FVC<70% pred were specified as PFT outcomes in the inclusion criteria. In the remaining six studies patients were not included according to ATS/ERS or GOLD positions, since these RCTs were performed before the publication of the international PFT standard guidelines statement for the diagnosis of COPD [19, 21, 23, 25, 28] or because of the retrospective nature of the study [29].
Table 1 shows the definition of exacerbation as reported by studies included in the meta-analysis. All studies were published between 1975 and 2014 and relevant patient demographics, baseline, study characteristics and Jadad score are summarised in table 2.
TABLE 1.
Definition of exacerbation as reported by studies included in the meta-analysis
| Study [ref.], year | Definition of exacerbation |
| Zheng et al. [1], 2014 | “At least a 2 day persistence of two (type II moderate) or all three (type III, severe) major symptoms (worsening dyspnoea, increase in sputum purulence or volume), or of any one major symptom plus at least one minor symptom (type I, mild) (upper airway infection, unexplained fever, and increased wheezing)” |
| Tse et al. [20], 2013 | “Two of the following three symptoms: increase in shortness of breath, volume, or purulence of sputum” |
| Schermer et al. [26], 2009 | “An episode with one or more subsequent unscheduled contacts with either a general practitioner or a chest physician due to worsening of respiratory symptoms” |
| Bachh et al. [27], 2007 | “Increased dyspnea and/or cough associated with a change in quality and quantity of sputum, which led the patient to seek medical attention and lasting for >3 days” |
| Decramer et al. [3], 2005 | “Increase in dyspnoea, cough, or both associated with a change in quality and quantity of sputum, which led the patient to seek medical attention and which lasted for at least 3 days” |
| Gerrits et al. [29], 2003 | “Re-hospitalisation for chronic obstructive pulmonary disease” |
| Pela et al. [24], 1999 | “The worsening of the clinical profile of the patient with increased cough, dyspnea and expectoration with mucopurulent sputum, with or without fever requiring medical intervention. If the total score of the following questionnaire amounted to >3 points, the occurrence of an exacerbation was confirmed: fever: absent=0, present=1; cough: mild=0, moderate=1, severe=2; mucus: unchanged=0, increased=1, increased and purulent=2; dyspnoea: unchanged=0, increased=1, severely increased=2” |
| Hansen et al. [19], 1994 | “A mucopurulent sputum with new or worse cough, plus one or more of the following: new general malaise; new symptoms of cold; fever (38°C or above); increased dyspnoea; increased mucus production; increased viscosity of sputum; new onset of foul-tasting sputum; increased difficulty of expectoration; increase in erythrocyte sedimentation rate in relation to exacerbation; leucocytosis in relation to exacerbation; and/or pneumonia confirmed by X-ray” |
| Rasmussen and Glennow [25], 1988; Boman et al. [23], 1983 | “Mucopurulent or purulent sputum and cough (new or aggravated) plus one or more of the following symptoms: general malaise; new symptoms of common cold; fever (>38°C); breathlessness; increased mucus production; increased sputum-thickness; foul tasting sputum; increased difficulty of expectoration; increase in erythrocyte sedimentation rate; leucocytosis or pneumonia” |
| McGavin et al. [22], 1985 | “New or deteriorating cough with increased sputum purulence lasting for at least 48 hours plus at least one of the following: general malaise, any recorded fever greater than 38°C, increased breathlessness, increased sputum volume or thickness, increased difficulty in expectoration” |
| Babolini et al. [21], 1980 | “A discrete change in the course of the disease marked by rapid increase in coughing and sputum output and purulence, associated with worsening of chest physical signs and possibly dyspnoea but not necessarily with fever” |
| Grassi and Morandini [28], 1976 | “Episode or sudden aggravation of the typical signs and symptoms” |
TABLE 2.
Patient demographics, baseline and study characteristics
| Study [ref.], year | Study characteristics | Duration of study months | Patients (completed study visits) | NAC (daily dose) mg | Disease characteristics | Average age years | Male % | Current smokers % | Smoking history pack-years | ICS treatment % | Post-bronchodilator FEV1 % predicted | Jadad score |
| Zheng et al. [1], 2014 | Multicentre, prospective, randomised, double-blind, placebo-controlled, parallel groups | 12 | 964 | 1200 | COPD diagnosis using PFT, GOLD II–IV | 66.3 | 81.9 | 17.8 | 36.2 | 44.0 | 48.9 | 4 |
| Tse et al. [20], 2013 | Double-blind, randomised placebo-controlled | 12 | 108 | 1200 | COPD diagnosis using PFT, GOLD I–IV | 70.9 | 93.3 | 23.3 | NA | 79.0 | 59.6 | 4 |
| Schermer et al. [26], 2009 | Double blind, randomised, double dummy, placebo-controlled | 36 | 108 | 600 | COPD diagnosis using PFT, GOLD I–IV, chronic bronchitis | 59.4 | 72.9 | 53.6 | 27.3 | 49.0 | 69.8 | 4 |
| Bachh et al. [27], 2007 | Single blind, randomised, placebo-controlled study | 4 | 100 | 600 | COPD diagnosis using PFT, GOLD II and III | 61.4 | 78.0 | NA | 44.1 | 18.0 | 51.7 | 1 |
| Decramer et al. [3], 2005 | Double-blind, randomised, placebo-controlled, parallel groups | 36 | 354 | 600 | COPD diagnosis using PFT, GOLD II and III | 62.0 | 77.0 | 44.0 | NA | 70.0 | 57.0 | 4 |
| Gerrits et al. [29], 2003 | Retrospective cohort investigation | 12 | 1219 | From 200 to >600 | ICD-9: 491, 492, 496 | >55 | 59.6 | NA | NA | 35.9 | NA | NA |
| Pela et al. [24], 1999 | Open, randomised, controlled | 6 | 167 | 600 | COPD diagnosis using PFT and ATS/ERS criteria, FEV1 <70% pred | 66.0 | 75.7 | 24.0 | NA | 39.6 | 58.1 | 2 |
| Hansen et al. [19], 1994 | Multicentre, double-blind, randomised placebo-controlled, parallel groups | 5.5 | 129 | 1200 | Chronic bronchitis, FEV1 >50% pred | 51.4 | 43.1 | 92.2 | NA | NA | NA | 4 |
| Rasmussen and Glennow [25], 1988 | Multicentre, double-blind, randomised placebo-controlled, parallel groups | 6 | 91 | 600 | Chronic bronchitis | 58.8 | 56.9 | NA | NA | NA | NA | 4 |
| McGavin et al. [22], 1985 | Multicentre, double-blind, randomised, placebo-controlled, parallel groups | 5 | 148 | 600 | Chronic bronchitis, FEV1 <50% pred, FEV1/FVC <70% pred | NA | 85.6 | 27.1 | NA | 25.4 | 30.0# | 4 |
| Boman et al. [23], 1983 | Multicentre, double-blind, randomised, placebo-controlled, parallel groups | 6 | 203 | 400 | Chronic bronchitis, FEV1 >50% pred | 52 | NA | 81.3 | NA | NA | 80.0# | 1 |
| Babolini et al. [21], 1980 | Multicentre, double-blind, randomised, placebo-controlled, parallel groups | 6 | 495 | 400 | Chronic bronchitis, FEV1 >40% pred | >50 | 76.6 | 66.3 | NA | NA | NA | 1 |
| Grassi and Morandini [28], 1976 | Double-blind, randomised, placebo-controlled | 6 | 69 | 260 | Chronic bronchitis | 61 | 79.7 | NA | NA | NA | NA | 3 |
Data are presented as n, unless otherwise stated. NAC: N-acetylcysteine; ICS: inhaled corticosteroid; FEV1: forced expiratory volume in 1 s; COPD: chronic obstructive pulmonary disease; PFT: pulmonary function testing; GOLD: Global Initiative for Chronic Obstructive Lung Disease; NA: not available; ICD: International Classification of Diseases; ATS: American Thoracic Society; ERS: European Respiratory Society; FVC: forced vital capacity. #: post-bronchodilator not defined.
The NAC treatment ranged from 4 to 36 months. 10 trials were double-blind, randomised placebo-controlled, one trial was single-blind, randomised placebo-controlled, one trial was open, randomised and controlled and one study was a retrospective cohort investigation.
Influence of NAC on risk of COPD exacerbations
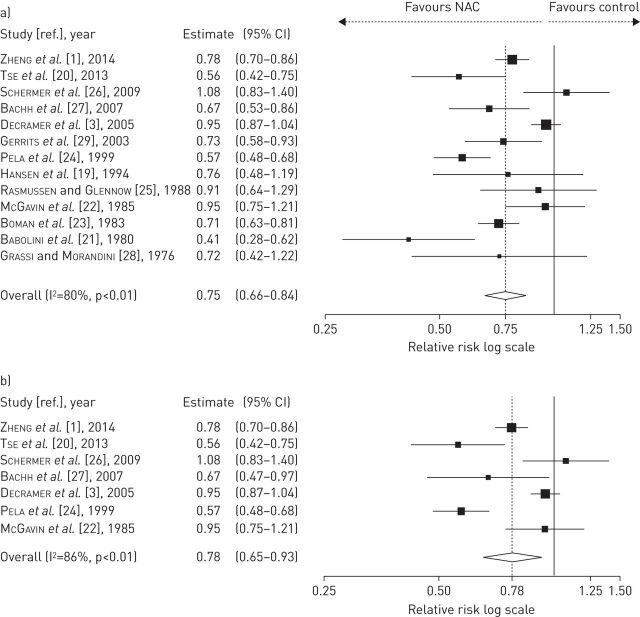
The meta-analysis of all 13 studies showed that overall, the treatment with low and high doses of NAC significantly reduced the frequency of exacerbations (relative risk 0.75, 95% CI 0.66–0.84; p<0.01), and the effectiveness of NAC was also confirmed by RCTs performed in those patients enrolled through PFT in agreement with the ATS/ERS or GOLD guidelines (seven studies: treated n=978, placebo n=971; relative risk 0.78, 95% CI 0.65–0.93; p<0.01), compared with control COPD populations (fig. 2). The subgroup with Jadad score ≤3 signalled a decrease in the risk of exacerbation (six studies: treated n=986, placebo n=1267; relative risk 0.64, 95% CI 0.56–0.74; p=0.07), whereas a significant reduction in the risk of exacerbation was detected in the subgroup of RCTs with Jadad score >3 (seven studies: treated n=957, placebo n=955; relative risk 0.85, 95% CI 0.74–0.98; p<0.01).
FIGURE 2.
Overall forest plot from meta-analysis carried out in all 13 selected studies a) assessing the relative risk of chronic obstructive pulmonary disease (COPD) exacerbations and b) subgroup analysis performed on seven randomised clinical trials in which inclusion criteria required diagnosis of COPD by pulmonary function testing. NAC: N-acetylcysteine.
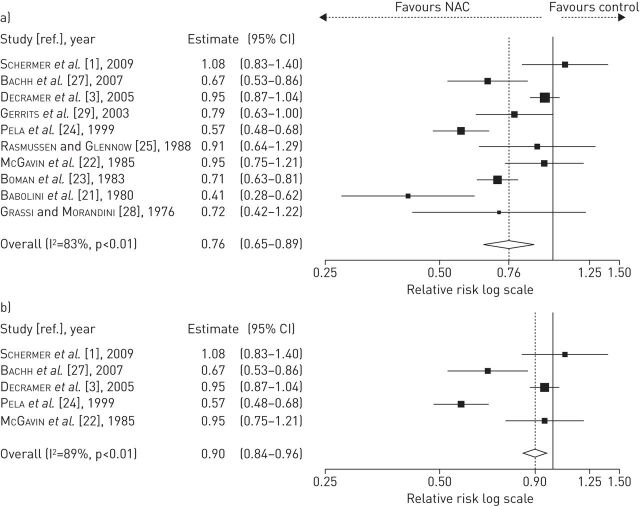
NAC administered at low doses (patients from 10 studies: treated n=1298, placebo or controls n=1614) significantly protected against COPD exacerbations (relative risk 0.76, 95% CI 0.65–0.89; p<0.01), although the reduction of exacerbation risk was less extensive (relative risk 0.90, 95% CI 0.84–0.96; p<0.01) in the analysis of those RCTs where COPD was diagnosed using PFT (patients from five studies: treated n=444, placebo n=433) (fig. 3).
FIGURE 3.
Forest plot from meta-analysis carried out in 10 studies including low-dose N-acetylcysteine (NAC) treatment a) assessing the relative risk of chronic obstructive pulmonary disease (COPD) exacerbations and b) subgroup analysis performed on five randomised clinical trials in which inclusion criteria required diagnosis of COPD by pulmonary function testing.
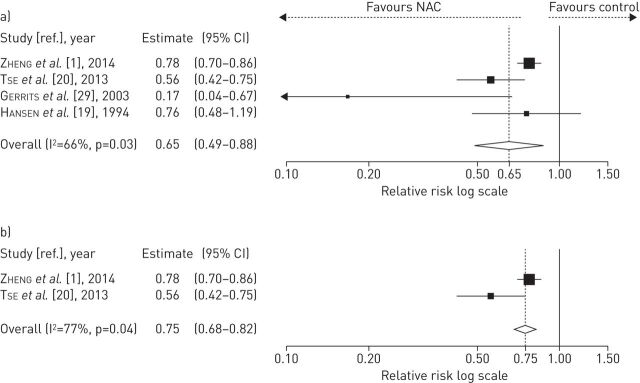
NAC at high doses included patients from four studies (treated n=635, placebo or controls n=1362) and showed a significant reduction of COPD exacerbation rate (relative risk 0.65, 95% CI 0.49–0.88; p=0.03) compared to the untreated COPD population; this was further confirmed by the results of the subgroup of RCTs enrolling COPD patients using PFT according to ATS/ERS or GOLD guidelines (two studies: treated n=534, placebo n=538; relative risk 0.75, 95% CI 0.68–0.82; p=0.04) (fig. 4).
FIGURE 4.
Forest plot from meta-analysis carried out on four studies including high-dose N-acetylcysteine (NAC) treatment a) assessing the relative risk of chronic obstructive pulmonary disease (COPD) exacerbations and b) subgroup analysis performed on two randomised clinical trials in which inclusion criteria required diagnosis of COPD by pulmonary function testing.
Influence of NAC on adverse events
Adverse events, mainly gastrointestinal disorders, were reported in 11 RCTs (treated n=1416, placebo n=1412) (table 3), but NAC did not significantly increase the risk of side-effects (relative risk 0.94, 95% CI 0.88–0.99; p=0.40) compared with placebo and the risk of adverse reactions was not dose-dependent (low doses relative risk 0.93, 95% CI 0.89–0.97; p=0.40; high doses relative risk 1.11, 95% CI 0.89–1.39; p=0.58) (fig. 5). The analysis of RCT subgroups using Jadad scores did not evidence any modulation of the risk of adverse events (Jadad score ≤3, five studies: treated n=521, placebo n=513; relative risk 0.85, 95% CI 0.47–1.53; p=0.21; Jadad score >3, six studies: treated n=895, placebo n=899; relative risk 0.94, 95% CI 0.90–0.98; p=0.58). In addition, NAC did not increase the risk of serious adverse events (eight studies: treated n=1272, placebo n=1267; relative risk 0.95 95% CI 0.67–1.34; p=0.81) compared with placebo. NAC administered at both low and high doses was well tolerated, as reported by six RCTs that included data on drug tolerability [1, 3, 20, 21, 24, 25].
TABLE 3.
Adverse events reported in randomised controlled trials (RCTs) after administration of N-acetylcysteine (NAC) at low and high doses
| NAC dose | ||
| Low | High | |
| RCTs | 8 | 3 |
| Gastrointestinal disorders | ||
| Diarrhoea | 5 | 2 |
| Gastrointestinal pain | 4 | 1 |
| Epigastric discomfort | 3 | 1 |
| Nausea | 3 | 0 |
| Vomiting | 3 | 0 |
| Constipation | 2 | 0 |
| Loss of appetite | 1 | 0 |
| Indigestion | 1 | 0 |
| GORD symptoms | 0 | 1 |
| Dry mouth | 0 | 1 |
| Unpleasant taste | 1 | 0 |
| Respiratory disorders | ||
| Dyspnoea | 2 | 0 |
| Respiratory tract infection | 1 | 1 |
| Cough | 1 | 1 |
| Other disorders | ||
| Pyrosis | 3 | 0 |
| Joint pain and muscle pain | 0 | 2 |
| Dizziness | 0 | 2 |
| Palpitations | 0 | 1 |
| Headache | 1 | 1 |
| Skin disorders | 1 | 0 |
| CNS disorders | 1 | 0 |
| Weight gain | 1 | 0 |
| Oedema | 1 | 0 |
| Urticaria | 1 | 0 |
| Itching | 1 | 0 |
| Tinnitus | 1 | 0 |
| Pruritus | 0 | 1 |
Data are presented as n. GORD: Gastro-oesophageal reflux disease; CNS: central nervous system.
FIGURE 5.
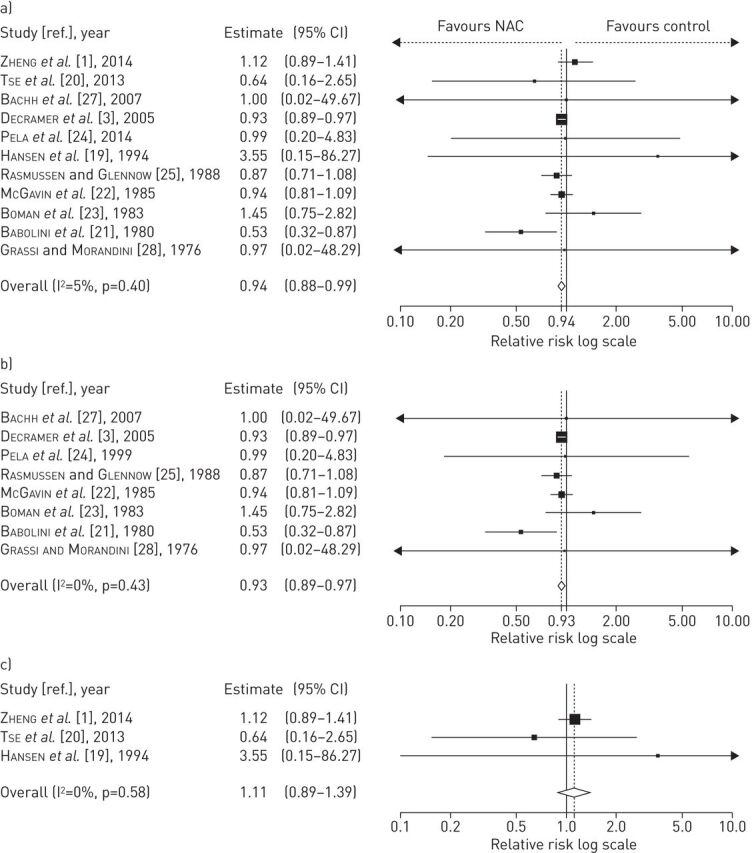
Overall forest plot from meta-analysis carried out in 11 studies a) assessing the relative risk of adverse events; and subgroup analysis performed on studies including b) low and c) high doses of N-acetylcysteine (NAC).
Discussion
The present meta-analysis provides further evidence that patients treated with NAC have significantly and consistently fewer exacerbations of chronic bronchitis or COPD. This is important information, although the variety of definitions of acute exacerbation of chronic bronchitis or COPD that have been used in the different clinical studies makes it difficult to assess and compare these studies.
However, it is interesting that we did not observe a substantial difference in the results when the analysis was focussed only on patients with a diagnosis of COPD using spirometric criteria. Even when the studies focussed only on the effects of a low dose of NAC, we were able to demonstrate that NAC is able to reduce the risk of exacerbations, although this protective effect was more apparent in patients without a diagnosis of COPD made using spirometric criteria. However, NAC at high dose resulted in a significant effect, not only in patients with chronic bronchitis, but also in those with a validated COPD diagnosis. Our meta-analysis has also demonstrated that the administration of NAC at high dosage is characterised by a favourable risk–benefit ratio and that the risk of adverse events was not dose-dependent. This is a relevant finding, since ultimately, the decision to use a drug (or not) is determined by the risk–benefit ratio.
The results of this meta-analysis provide good evidence that NAC can be considered an important, safe and well tolerated therapeutic agent to be integrated into the regular treatment regimens of patients with COPD, but also that NAC should be administered at high dose. Since the administration of NAC at low dose provides a more marked risk reduction for the overall set of studies (without regard to the diagnosis) than that for the subset of studies with a validated COPD diagnosis, this suggests that a low dose is sufficient in providing benefit to patients with chronic bronchitis, but not to patients with COPD. Schermer et al. [26] even found that 3 years’ treatment with NAC administered at low dose increased the exacerbation rate. The authors suggested that, although the higher exacerbation rate in the NAC group may be misinterpreted as a “deleterious” effect of this treatment, this finding is explained by the fact that a small number of patients in the active treatment group experienced very frequent exacerbations [26].
We would point out that chronic bronchitis, characterised by chronic cough and sputum production, is a common clinical feature associated with cigarette smoking and it can precede the development of any spirometric abnormalities indicative of COPD among smokers by many years [30]. However, COPD could be detected earlier if more restrictive limits of normality parameters were used. Furthermore, it is not yet clear whether chronic bronchitis and COPD are two different entities or whether chronic bronchitis is only a phenotype of COPD, although chronic expectoration and airflow obstruction behave largely as independent variables [31]. Therefore, it is possible to have chronic bronchitis without COPD [32].
These data allow us to respond, although still partially, to the fundamental question posed in our commentary [2]: NAC may be beneficial, but for whom? In the commentary, we highlighted that NAC can seemingly play a part in the management of patients with chronic bronchitis, but when this treatment is extended to COPD, the results are less useful [2].
Now, supported by our meta-analysis, we feel confident in saying that the usefulness of NAC in exacerbation risk reduction seems also to be evident in patients with a chronic bronchitis phenotype of COPD, at least when it is administered at high dose.
However, since there is currently a tendency to personalise therapy for patients with COPD [33], it is extremely important to define the category of patients with COPD that would really benefit from a prolonged treatment with NAC, and consequently, look at the whole spectrum of COPD. Miravitlles et al. [34] have proposed four different phenotypes characterised by the combination of the classical types of emphysema, chronic bronchitis, exacerbators and patients with a mixed asthma–COPD phenotype.
A recent retrospective analysis of the High-dose N-acetylcysteine in Stable COPD – a 1-year, Double-Blind, Randomized, Placebo-Controlled Trial (HIACE) study suggests that high-dose NAC is mainly effective in the frequent exacerbator phenotype, regardless of symptoms [35]. The lack of specific information on the topic in the individual papers and our lack of access to the original data did not allow us a specific meta-analysis aimed at confirming this point. Nonetheless, the latest American College of Chest Physicians and Canadian Thoracic Society guideline for the prevention of acute exacerbations of COPD [36] recommends treatment with NAC for those patients with moderate-to-severe COPD who are frequent exacerbators.
Unfortunately, the other two issues highlighted in our commentary remain unresolved. We noted that the only two well-designed trials that have documented an effect of NAC on exacerbations of COPD (HIACE and PANTHEON) were both performed in China, and we pointed out that there might be a population effect (i.e. one linked to genetic, environmental and/or dietary factors) that explains the apparently specific effectiveness of NAC in Chinese patients [1, 20]. However, as correctly stressed by Tse et al. [35], current international guidelines for COPD do not distinguish treatment options (including dosages) for any drug class based on potential racial or cultural differences.
It has been suggested that NAC, which is a mucolytic agent with both antioxidant and anti-inflammatory properties, would be able to reduce acute exacerbations of COPD through an antioxidant effect [1]. Obviously, the mechanism(s) by which NAC reduces the risk of exacerbations cannot be ascertained from this meta-analysis. Clearly, NAC does have some antioxidant potential due to its ability to increase intracellular glutathione, which plays an important role in the control of oxidative–carbonyl stress in COPD [37]. However, NAC is a much weaker antioxidant than glutathione and most, if not all, of the other identified endogenous antioxidant agents and enzymes. For this reason, a high dosage of NAC is required to exert an antioxidant action in patients with COPD [38].
To date there are no clinical studies specifically designed to investigate the interaction between NAC and inhaled corticosteroids in the prevention of exacerbations, and the few available data in the current literature are inconsistent. In fact the results of subgroup analysis of the BRONCUS study [3] showed a decreased risk for exacerbation in patients not taking inhaled corticosteroids assigned to NAC treatment, whereas the PANTHEON study [1] evidenced no interaction between NAC treatment and inhaled corticosteroid use, suggesting that the treatment effect was independent of inhaled corticosteroid use. These inconsistencies might be explained by the fact that the dosage of NAC in PANTHEON (1200 mg per day) was double that used in BRONCUS (600 mg per day), and thus, since a dose-effect association with NAC has been reported, a dose increase might be expected to provide amplified treatment effects [1]. Furthermore, PANTHEON, due to the higher number of enrolled patients compared with BRONCUS, might have provided greater statistical power to detect differences between the study groups [1].
It is likely that several of the studies that we have included in our meta-analysis, having used the diagnosis of chronic bronchitis, will also have recruited patients with COPD. However, the lack of specific information and not being able to access the original data did not allow us to perform this more specific analysis. Nonetheless, the strong signal that comes from our meta-analysis leads us to state that if a patient suffers from COPD with an objective confirmation of airways obstruction, NAC should be administered at a dose of ≥1200 mg per day to prevent exacerbations, while if a patient suffers from chronic bronchitis but is without airways obstruction, a regular treatment with 600 mg per day seems to be sufficient. Finally, since the effectiveness of NAC administered at high dose is slow and progressive [1], prolonged and regular treatment might be necessary to prevent exacerbations.
Supplementary Material
Footnotes
Conflict of interest: Disclosures can be found alongside the online version of this article at err.ersjournals.com
Provenance: Submitted article, peer reviewed.
References
- 1.Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014; 2: 187–194. [DOI] [PubMed] [Google Scholar]
- 2.Cazzola M, Matera MG. N-acetylcysteine in COPD may be beneficial, but for whom? Lancet Respir Med 2014; 2: 166–167. [DOI] [PubMed] [Google Scholar]
- 3.Decramer M, Rutten-van Mölken M, Dekhuijzen PN, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost–Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 2005; 365: 1552–1560. [DOI] [PubMed] [Google Scholar]
- 4.Grandjean EM, Berthet P, Ruffmann R, et al. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther 2000; 22: 209–221. [DOI] [PubMed] [Google Scholar]
- 5.Stey C, Steurer J, Bachmann S, et al. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. Eur Respir J 2000; 16: 253–262. [DOI] [PubMed] [Google Scholar]
- 6.Shen Y, Cai W, Lei S, et al. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD 2014; 11: 351–358. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. International Classification of Diseases. Manual of the International Statistical Classification of Diseases: Injuries and Causes of Deaths. 9th revision. Geneva, World Health Organization, 1977. [Google Scholar]
- 10.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html Date last accessed: March, 2015. Date last updated: January, 2015.
- 11.Shariff SZ, Bejaimal SA, Sontrop JM, et al. Retrieving clinical evidence: a comparison of PubMed and Google Scholar for quick clinical searches. J Med Internet Res 2013; 15: e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutherland ER, Crapo JD, Bowler RP. N-acetylcysteine and exacerbations of chronic obstructive pulmonary disease. COPD 2006; 3: 195–202. [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 14.Borenstein M. Introduction to Meta-analysis. Chichester, John Wiley & Sons, 2009. [Google Scholar]
- 15.DeCoster J. Meta-analysis Notes. www.stat-help.com/notes.html Date last accessed: March, 2015. Date last updated: September, 2004.
- 16.Turner JR, Durham TA. Meta-methodology: conducting and reporting meta-analyses. J Clin Hypertens (Greenwich) 2014; 16: 91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 2012; 49: 1–15. [Google Scholar]
- 18.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2012; 36: 1–48. [Google Scholar]
- 19.Hansen NC, Skriver A, Brorsen-Riis L, et al. Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med 1994; 88: 531–535. [DOI] [PubMed] [Google Scholar]
- 20.Tse HN, Raiteri L, Wong KY, et al. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013; 144: 106–118. [DOI] [PubMed] [Google Scholar]
- 21.Babolini G, Blasi A, Comia G, et al. Long-term oral acetylcysteine in chronic bronchitis. A double-blind controlled study. Eur J Respir Dis Suppl 1980; 111: 93–108. [PubMed] [Google Scholar]
- 22.McGavin CR, MacFarlane JT, Prescott RJ, et al. Oral N-acetylcysteine and exacerbation rates in patients with chronic bronchitis and severe airways obstruction. British Thoracic Society Research Committee. Thorax 1985; 40: 832–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boman G, Bäcker U, Larsson S, et al. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis 1983; 64: 405–415. [PubMed] [Google Scholar]
- 24.Pela R, Calcagni AM, Subiaco S, et al. N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respiration 1999; 66: 495–500. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen JB, Glennow C. Reduction in days of illness after long-term treatment with N-acetylcysteine controlled-release tablets in patients with chronic bronchitis. Eur Respir J 1988; 1: 351–355. [PubMed] [Google Scholar]
- 26.Schermer T, Chavannes N, Dekhuijzen R, et al. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir Med 2009; 103: 542–551. [DOI] [PubMed] [Google Scholar]
- 27.Bachh AA, Shah NN, Bhargava R, et al. Effect of oral N-acetylcysteine in COPD – a randomised controlled trial. JK Practitioner 2007; 14: 12–16. [Google Scholar]
- 28.Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. Eur J Clin Pharmacol 1976; 9: 393–396. [DOI] [PubMed] [Google Scholar]
- 29.Gerrits CM, Herings RM, Leufkens HG, et al. N-acetylcysteine reduces the risk of re-hospitalisation among patients with chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 795–798. [DOI] [PubMed] [Google Scholar]
- 30.Guerra S, Sherrill DL, Venker C, et al. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 2009; 64: 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamal K, Cooney TP, Fleetham JA, et al. Chronic bronchitis. Correlation of morphologic findings to sputum production and flow rates. Am Rev Respir Dis 1984; 129: 719–722. [DOI] [PubMed] [Google Scholar]
- 32.Viegi G, Pistelli F, Sherrill DL, et al. Definition, epidemiology and natural history of COPD. Eur Respir J 2007; 30: 993–1013. [DOI] [PubMed] [Google Scholar]
- 33.Segreti A, Stirpe E, Rogliani P, et al. Defining phenotypes in COPD: an aid to personalized healthcare. Mol Diagn Ther 2014; 18: 381–388. [DOI] [PubMed] [Google Scholar]
- 34.Miravitlles M, Calle M, Soler-Cataluña JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol 2012; 48: 86–98. [DOI] [PubMed] [Google Scholar]
- 35.Tse HN, Raiteri L, Wong KY, et al. Benefits of high-dose N-acetylcysteine to exacerbation-prone patients with COPD. Chest 2014; 146: 611–623. [DOI] [PubMed] [Google Scholar]
- 36.Criner GJ, Bourbeau J, Diekemper RL, et al. Executive summary: prevention of acute exacerbation of COPD: American College of Chest Physicians and Canadian Thoracic Society guideline. Chest 2015; 147: 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther 2014; 141: 150–159. [DOI] [PubMed] [Google Scholar]
- 38.Sadowska AM, Manuel-y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 2007; 20: 9–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.