To the Editors:
A 69-yr-old post-menopausal female with pulmonary nodules and a diagnosis of lymphangioleiomyomatosis (LAM) confirmed elsewhere was referred to our centre (U.O. di Pneumologia, Ospedale San Giuseppe, Milan, Italy) for further evaluation. The patient was a nonsmoker and denied any occupational exposures. There was no family history of cancer. She had no history of pulmonary diseases or respiratory symptoms. Her past medical history included systemic hypertension treated with angiotensin converting enzyme inhibitor, hypercholesterolemia treated with atorvastatin, and diabetes mellitus. The patient had been well until July 2008 when she was evaluated in another hospital and a breast carcinoma was discovered. Multiple bilateral small nodules of the lung were noted on a chest computed tomography performed at the time of breast carcinoma diagnosis (figs 1 and 2). Fluorodeoxyglucose-positron emission tomography did not disclose pathological uptake of the lung nodules. A wedge resection of the lung was performed. The histological diagnosis was LAM. In October 2008 the patient underwent breast conserving surgery (quadrantectomy) with excision of the lesion in the right breast; the pathological diagnosis was infiltrating ductal carcinoma. She was started on tamoxifen treatment.
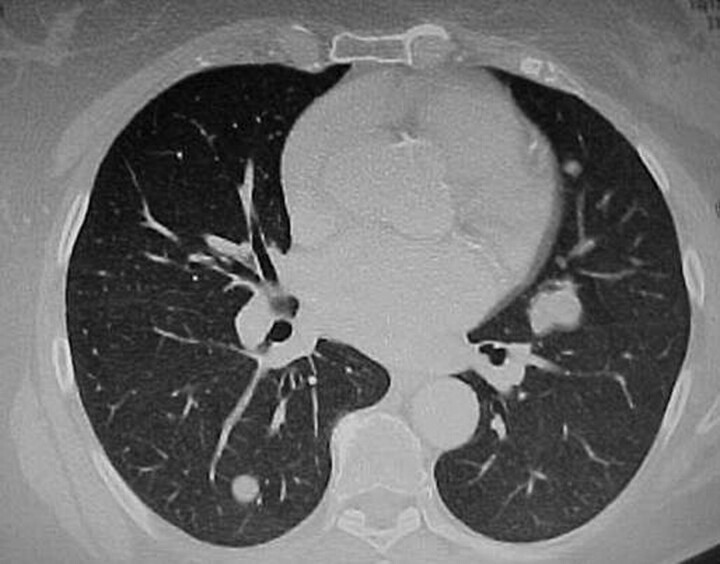
Figure 1.
Chest computed tomography scan showing multiple, bilateral, well-circumscribed pulmonary nodules.
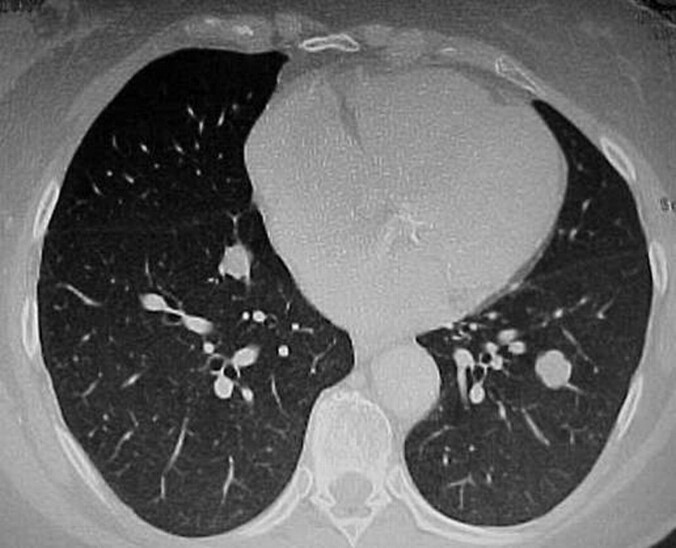
Figure 2.
Another chest computed tomography scan showing multiple, bilateral, well-circumscribed pulmonary nodules.
The patient was evaluated in our department in March 2009. She was completely asymptomatic with no complaints of shortness of breath, cough, chest pain, fever, chills, night sweats or systemic symptoms. On examination the patient appeared well and her vital signs were normal. Pulmonary and general physical examination was normal. Laboratory tests were all in the normal range. The results of pulmonary function studies (lung volumes and diffusing capacity of the lung for carbon monoxide) were normal. Because a previous diagnosis of LAM had been made, a magnetic resonance imaging scan of the brain and abdomen was performed and meningiomas and renal angiomyolipomas were excluded. Lung computed tomography re-evaluation confirmed the presence of bilateral nodules, radiological aspects not characteristic for LAM. A pathological revision of the pulmonary biopsy was performed and revealed a proliferation of bland spindle cells surrounding benign entrapped bronchioloalveolar epithelium (figs 3 and 4). Immunohistochemically, the spindle cells were positive for desmin and smooth-muscle actin, and negative for HMB-45. Immunoreactivity for both oestrogen and progesterone receptors was present in the smooth muscle component. Based on these findings, pulmonary benign metastasising leiomyoma (BML) was diagnosed.
Figure 3.
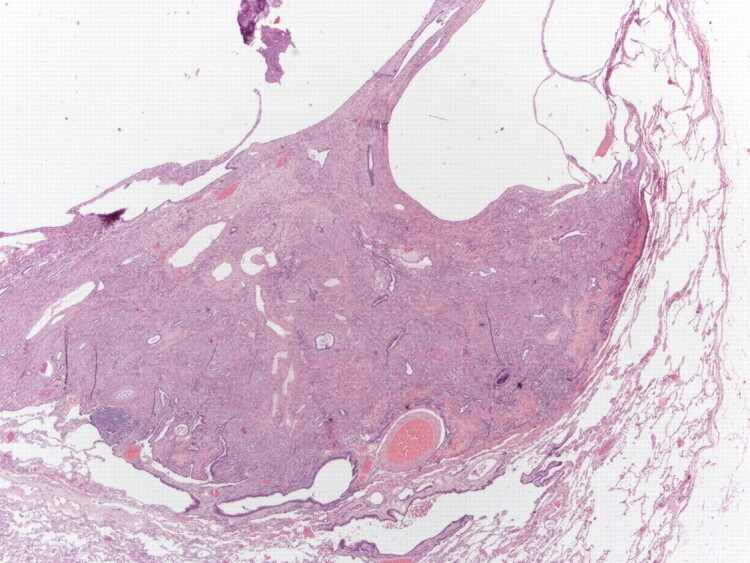
Low-power photomicrograph (haematoxylin–eosin stain) of a lung biopsy showing a well-circumscribed nodule; partially solid and partially cystic (upper part). Magnification: 20x.
Figure 4.
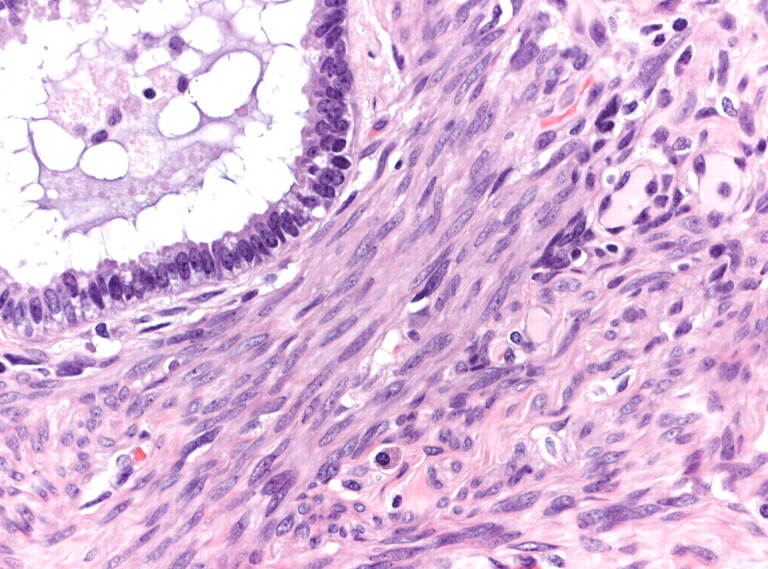
High-power photomicrograph (haematoxylin–eosin stain) of a pulmonary nodule in lung biopsy showing bland appearing smooth muscle proliferation with entrapped bronchiolar epithelium. Magnification: 200x.
A gynaecological examination was performed and multiple uterine leiomyomas were found. A clinical-functional-radiological follow-up was planned. No further treatment was prescribed and the residual nodules have not increased in size during the 18 months of follow-up. The patient remains well and without pulmonary symptoms.
The aetiology of multiple pulmonary nodules is quite complex, with metastatic disease being the most common cause. Other possibilities include sarcoidosis or an inflammatory process, such as fungus, tuberculosis, nocardiosis or septic emboli. However, in asymptomatic patients, further considerations include the presence of rheumatoid nodules, amyloidosis and arteriovenous malformations. The possibility of even less common lesions, including hamartomas and smooth muscle tumours such as leiomyomas, also exists [1]. BML is a rare disorder with ∼100 cases reported in the literature. It affects females who have previously undergone hysterectomies or myomectomies due to histologically benign-appearing uterine leiomyomas. Sometimes uterine leiomyoma may be discovered after pulmonary nodules or other extra-uterine lesions [2, 3]. Although leiomyomas are histologically benign, they can metastasise to distant sites, such as lungs, skin, bones, mediastinum, lymph nodes, muscular tissue, heart and retroperitoneum [4]. The lung is the most common site of involvement. It appears that the tumour metastasises to lungs and other extra-uterine tissues via haematogenous spread [3].
Patients with pulmonary BML are almost always asymptomatic and, as in this case, the radiographic findings are an incidental finding. The radiographic manifestations are usually well-defined nodules ranging in size from 0.2 to 8 cm which may be unilateral or bilateral, scattered among normal interstitium. Typically these nodules are non-calcified and do not enhance with intravenous contrast administration. Pulmonary nodules usually show little change and may even spontaneously regress. Pathologically, pulmonary BML are composed of benign smooth muscle cells that are similar to uterine leiomyoma [5]. In addition, the pulmonary nodules contain glandular appearing structures that have been shown histologically to consist of alveolar or bronchiolar epithelium. These entrapped epithelial elements are commonly observed in the periphery of the metastatic nodules and, sometimes, can be prominent, causing diagnostic confusion [6]. The clinical course of patients with BML varies and seems to depend on the oestrogen status. BML is usually indolent in post-menopausal females, whereas disease progression has been reported in pre-menopausal females [1, 2]. In addition, the effects of natural hormonal changes in females (pregnancy and menopause) on tumour growth have been described previously [7, 8]. Although there have been reports of patient morbidity and mortality from BML, the clinical course is typically indolent, with patient mortality commonly due to an unrelated disease process. There is no standardised treatment for BML because of the limited number of reported cases. Reported treatment methods include careful observation, surgical resection of pulmonary nodules, hysterectomy and bilateral oophorectomy, progesterone, and medical castration using luteinising hormone-releasing hormone analogue [9]. The pathogenesis of BML has been a subject of some controversy over the years. Most pathologists now accept these lesions as haematogenous metastases from morphologically benign uterine tumours, although there is a continuum with lesions that show increased mitotic activity. There has been much debate on the true nature of this lesion, a tumour with benign histological features but having biological behaviour suggesting malignancy [10–12]. The differential diagnosis of BML should include metastatic leiomyosarcoma. However, there is still controversy as to where the line between benign and malignant smooth muscle tumour should be drawn histologically. Further research into the basic biology of this neoplasm might reveal that uterine leiomyoma which gives rise to BML is a different subtype to those that do not “metastasise” [10–12]. BML should not be confused with LAM; they are distinct entities. LAM is characterised by the proliferation of smooth muscle cells from lymphatic walls in the lung and lymph nodes. Young females can present with a spontaneous pneumothorax, chylous pleural effusion or progressive dyspnoea. Characteristic imaging findings include hyperinflation and numerous thin-walled cystic spaces. The histological distinction between these two diseases may be difficult in some cases, however, but clinical and radiographic correlation should help establish the correct diagnosis.
Although it is a rare condition, BML should be considered in females with solitary or multiple pulmonary nodules.
Footnotes
Provenance
Submitted article, peer reviewed.
Statement of Interest
None declared.
REFERENCES
- 1.Maredia R, Snyder BJ, Harvey LAC, et al. Benign metastasizing leiomyoma in the lung. Radiographics 1998; 18: 779–782. [DOI] [PubMed] [Google Scholar]
- 2.Funakoshi Y, Sawabata N, Takeda S, et al. Pulmonary benign metastasizing leiomyoma from the uterus in a postmenopausal woman: report of a case. Surg Today 2004; 34: 55–57. [DOI] [PubMed] [Google Scholar]
- 3.Rao AV, Wilson J, Sylvester K. Pulmonary benign metastasizing leiomyoma following hysterectomy: a clinicopathologic correlation. J Thorac Oncol 2008; 3: 674–676. [DOI] [PubMed] [Google Scholar]
- 4.Moon H, Park SJ, Lee HB, et al. Pulmonary benign metastasizing leiomyoma in a postmenopausal woman. Am J Med Sci 2009; 338: 72–74. [DOI] [PubMed] [Google Scholar]
- 5.Abramson S, Gilkeson RC, Goldstein JD, et al. Benign metastasizing leiomyoma: clinical, imaging, and pathologic correlation. Am J Roentgenol 2001; 176: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 6.Colby TV, Koss MN, Travis WD. Miscellaneous mesenchymal tumors.. In: Sobin LH, Rosai J, eds. Atlas of Tumor Pathology: Tumors of the Lower Respiratory Tract Washington, Armed Forces Institute of Pathology, 1995:pp. 353–356. [Google Scholar]
- 7.Jauthzke G, Muller-Ruchholtz E, Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas). Pathol Res Pract 1996; 192: 215–223. [DOI] [PubMed] [Google Scholar]
- 8.Arai T, Yasuda Y, Takaya T, et al. Natural decrease of benign metastasizing leiomyoma. Chest 2000; 117: 921. [DOI] [PubMed] [Google Scholar]
- 9.Rivera JA, Christopoulos S, Small D, et al. Hormonal manipulation of benign metastasizing leiomyomas: report of two cases and review of the literature. J Clin Endocrinol Metab 2004; 89: 3183–3188. [DOI] [PubMed] [Google Scholar]
- 10.Tietze L, Gunther K, Horbe A, et al. Benign metastasizing leiomyoma: a cytogenetically balanced but clonal disease. Hum Pathol 2000; 31: 126–128. [DOI] [PubMed] [Google Scholar]
- 11.Patton KT, Cheng L, Papavero V, et al. Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis. Mod Pathol 2006; 19: 130–140. [DOI] [PubMed] [Google Scholar]
- 12.Nucci MR, Drapkin R, Dal Cin P, et al. Distinctive cytogenetic profile in benign metastasizing leiomyoma: pathogenetic implications. Am J Surg Pathol 2007; 31: 737–743. [DOI] [PubMed] [Google Scholar]