Abstract
Over the past decade, awareness among the medical profession of pulmonary arterial hypertension (PAH) being a treatable disease has increased. Despite this, approximately one-fifth of newly diagnosed patients are classified as being in the most severely compromised functional class (i.e. New York Health Association/World Health Organization functional class (NYHA/WHO FC) IV). The prognosis for patients in NYHA/WHO FC IV is poor, with 3-yr survival being around 40%, even with treatment. Poor prognosis coupled with severe functional impairment means it is vital that these patients receive optimal treatment. There are also subgroups of patients, who, although classified as NYHA/WHO FC III, may actually be severely haemodynamically compromised and at risk of rapid deterioration. Such subgroups include patients with PAH associated with systemic sclerosis or certain heritable mutations. These patients should be considered as being at the more severe end of the disease spectrum. In this article we will discuss the optimal management of patients with severe PAH. This includes newly emerging evidence from small-scale, open-label studies that use upfront combination therapy with intravenous epoprostenol plus oral PAH-specific drugs. We also review treatment strategies that may offer clinical benefits to patients with more severe PAH.
Keywords: Combination therapy, functional class, pulmonary arterial hypertension, surgical intervention, treat-to-target strategy
The management of patients with pulmonary arterial hypertension (PAH) has improved rapidly over the past decade with the introduction of PAH-specific therapies developed following increasing research into and enhanced knowledge of the pathogenesis of the disease. Meta-analyses of randomised controlled trials (RCTs) suggest that treatment with such targeted therapies improves survival in patients with PAH [1, 2] and data from registries and studies support this, with patients treated in the modern management era showing improved survival compared with historical cohorts [3–5]. There is still considerable room for improvement, however, as despite such improvements, PAH remains a progressive, fatal disease – especially for selected subgroups of patients.
A number of factors have been identified which are associated with mortality in patients with PAH, including: sex; type of PAH; presence of comorbidities; disease severity; exercise capacity; and haemodynamic parameters, such as pulmonary vascular resistance (PVR), mean right atrial pressure (Pra) and cardiac output [4, 6–9]. Of these, disease severity is one of the strongest predictors of outcome, not only at baseline, but also for patients receiving therapy.
The severity of PAH is determined using the World Health Organization (WHO) modification of the New York Heart Association (NYHA) functional classification. The NYHA/WHO functional classification links patient symptoms (dyspnoea, fatigue, syncope and signs and symptoms of right heart failure) with the degree of limitations to their daily activity. Patients are classified in one of four graded functional classes (FC), where those in NYHA/WHO FC I can carry out normal physical activity without undue dyspnoea, fatigue, chest pain or near syncope, while those in NYHA/WHO FC IV are unable to carry out any physical activity without symptoms, which may also be present at rest. The severe symptomatic impairment seen in patients in NYHA/WHO FC IV is associated with significantly reduced exercise capacity, resulting in particularly low 6-min walk distance (6MWD) (table 1) [10]. Haemodynamic parameters are markedly compromised; cardiac index at rest is severely reduced, PVR and Pra are high (table 1), and patients often show signs of right heart failure [10].
Table 1. Characteristics of pulmonary arterial hypertension according to New York Heart Association/World Health Organization functional class (NYHA/WHO FC) at diagnosis.
| Parameter | NYHA/WHO FC | p-value | ||
| I/II | III | IV | ||
| 6MWD n | 134 | 359 | 55 | |
| Distance m | 415±86 | 319±92 | 192±96 | <0.0001 |
| Haemodynamic variables n | 162 | 405 | 77 | |
| Pra mmHg | 6±4 | 9±5 | 11±7 | <0.0001 |
| P̄pa mmHg | 50±17 | 56±15 | 56±13 | 0.0002 |
| Ppcw mmHg | 8±3 | 8±3 | 8±4 | 0.75 |
| Cardiac index L·min−1·m−2 | 2.9±0.9 | 2.4±0.7 | 2.1±0.8 | <0.0001 |
| Sv,O2 | 67±8 | 62±8 | 54±9 | <0.0001 |
| PVRI mmHg·L·min·m−2 | 15.8±9.7 | 21.5±9.8 | 25.5±10.1 | <0.0001 |
Data are presented as mean±sd, unless otherwise stated. 6MWD: 6-min walk distance; Pra: right atrial pressure; P̄pa: mean pulmonary arterial pressure; Ppcw: pulmonary capillary wedge pressure; Sv,O2: venous oxygen saturation; PVRI: pulmonary vascular resistance index. Reproduced from [10] with permission from the publisher.
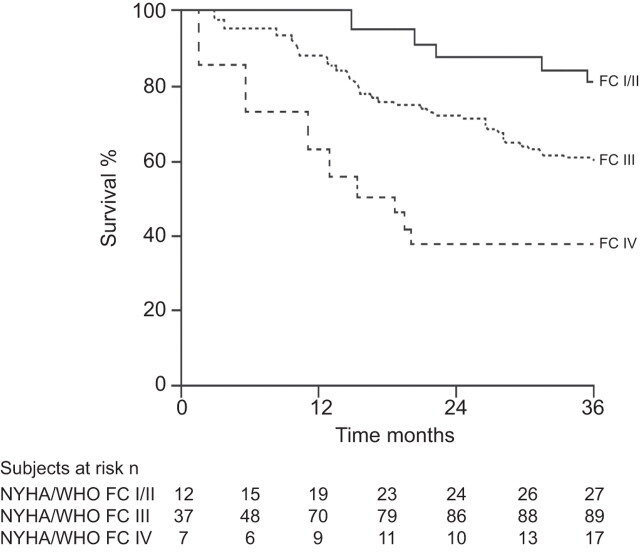
Unsurprisingly, the prognosis of PAH patients in NYHA/WHO FC IV is particularly poor, and if untreated, median survival was only around 6 months, compared with 2.5 yrs for those patients in NYHA/WHO FC III and 6 yrs for those in NYHA/WHO FC I/II in the US historical National Institutes of Health registry [11]. Similarly, baseline NYHA/WHO FC has been shown to be associated with survival in treated patients [9, 12]. NYHA/WHO FC IV patients treated with epoprostenol were shown to have particularly poor outcomes, with survival rates of 76%, 60% and 47% at 1, 2 and 3 yrs, respectively, compared with 90%, 76% and 71% for patients in NYHA/WHO FC III at baseline [9]. Data from registries show that even in the modern management era, patients in NYHA/WHO FC IV continue to have extremely poor survival (fig. 1) [4]. Given this, and bearing in mind the severe impairment seen in NYHA/WHO FC IV PAH, this group of patients by definition can be said to have “severe” disease. However, it should be noted that, patients in NYHA/WHO FC III also have markedly reduced survival relative to those in NYHA/WHO FC II (fig. 1) [4]. Parameters with established importance for assessing disease severity have been identified (table 2) and these form the basis of guideline recommendations for patient status evaluation [13]. Based on these parameters and their association with poor prognosis, patients in NYHA/WHO FC III who have rapid progression of symptoms, or severely compromised haemodynamics (Pra >15 mmHg or cardiac index <2.0 L·min−1·m−2), could also be considered to have severe disease.
Figure 1.
Survival of newly diagnosed (incident) patients with idiopathic, heritable or anorexigen-associated pulmonary arterial hypertension by New York Heart Association/World Health Organization functional class (NYHA/WHO FC) assessed at the time of diagnosis. Reproduced from [4] with permission from the publisher.
Table 2. Parameters with established importance for assessing disease severity, stability and prognosis in pulmonary arterial hypertension.
| Better prognosis | Determinants of prognosis | Worse prognosis |
| No | Clinical evidence of right ventricle failure | Yes |
| Slow | Rate of progression of symptoms | Rapid |
| No | Syncope | Yes |
| I, II | NYHA/WHO FC | IV |
| Longer (>500 m)# | 6MWT | Shorter (<300 m) |
| Peak O2 consumption >15 mL·min−1·kg−1 | Cardio-pulmonary exercise testing | Peak O2 consumption <12 mL·min−1·kg−1 |
| Normal or near normal | BNP/NT-proBNP plasma levels | Very elevated and rising |
| No pericardial effusion TAPSE¶ >2.0 cm | Echocardiographic findings¶ | Pericardial effusion TAPSE¶ <1.5 cm |
| Pra <8 mmHg and CI ≥2.5 L·min−1·m−2 | Haemodynamics | Pra >15 mmHg or CI ≤2.0 L·min−1·m−2 |
NYHA/WHO FC: New York Heart Association/World Health Organization functional class; 6MWT: 6-min walk test; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-B-type natriuretic peptide; TAPSE: tricuspid annular plane systolic excursion; Pra: right atrial pressure; CI: cardiac index. #: depending on age; ¶: TAPSE and pericardial effusion have been selected as they can be measured in the majority of patients. Reproduced from [13] with permission from the publisher.
Patients with PAH associated with systemic sclerosis (SSc) are considered to have the most severe form of the disease with the worse prognosis [14, 15]. In a study carried out prior to the current treatment era, despite similar haemodynamics, patients with PAH-SSc had a three times higher risk of death than those with idiopathic (IPAH), heritable or anorexigen-associated PAH [14]. Although PAH specific treatments have improved survival rates for SSc patients (for example, 1-yr survival rates have increased from 55% [14] to 78% [16] in the pre- versus the post-oral PAH therapy era), data from registries confirm a worse survival for PAH patients with connective tissue disease versus those with IPAH [15]. Even mildly symptomatic PAH-SSc patients can have a severe degree of haemodynamic impairment at diagnosis [17]. Patients with certain types of heritable PAH can also have severe forms of the disease. Patients with heritable PAH associated with the bone morphogenetic protein receptor type 2 (BMPR2) mutation included in the French Network of Pulmonary Hypertension were found to present at a younger age with particularly severe haemodynamic impairment, despite being predominantly in NYHA/WHO FC III, and to show significantly shorter time to death or transplantation compared with IPAH patients [18]. Furthermore, patients with heritable PAH associated with the activin A receptor type II-like kinase-1 (ACVRL1, also known as ALK1) from the same database were found to have more rapid disease progression and even worse prognosis than patients with the BMPR2 mutation, despite initially having less severe haemodynamic and clinical impairment [19]. Therefore, although NYHA/WHO FC IV in itself represents “severe PAH”, given the associated clinical and haemodynamic compromise, other “at risk” patient groups should also be considered to have severe disease, despite possibly having better functional capacity.
It might be assumed that with increased awareness of PAH patients would be identified early in the course of their disease, with few being in the “severe” category at diagnosis. However, studies of data from national registries show that 55.6–88% of patients with PAH are diagnosed in NYHA/WHO FC III, with a substantial proportion (around 20% overall) not being diagnosed until they are in NYHA/WHO FC IV [8, 10, 16, 20, 21]. These severely ill and difficult to manage patients therefore make up a significant proportion of the newly diagnosed PAH population. Given their poor prognosis and high rates of mortality, prompt and optimal management is of considerable importance.
MEDICAL THERAPY IN SEVERE PAH
Monotherapy
Despite the fact that NYHA/WHO FC IV patients make a substantial contribution to the overall population of newly diagnosed PAH patients, the majority of clinical trials include only a small proportion of this patient group (table 3). Even in those trials that do include NYHA/WHO FC IV patients, treatment outcomes for this specific population are not generally reported. Trials of intravenous epoprostenol have included the highest proportion of patients in NYHA/WHO FC IV [23, 24]. In a pivotal study of NYHA/WHO FC III (n=60) and IV (n=21) patients, treatment with epoprostenol compared with conventional therapy (such as anticoagulants, oral vasodilators, diuretic agents, cardiac glycosides or supplemental oxygen) for 12 weeks resulted in significant improvements in 6MWD (mean +32 m versus -29 m, respectively; p=0.002), NYHA/WHO FC IV FC (40% versus 3% improved, respectively; p=0.02) and haemodynamic parameters, including PVR (-21% versus -9%, respectively; p<0.001), and also improved quality of life indices and survival during the 12 weeks of the study [23]. These data are overall figures for the whole study cohort; specific data are not given for FC IV patients. A longer term observational study of 162 patients treated with epoprostenol which included 54% NYHA/WHO FC IV patients also reported improved survival relative to expected (observed 1-, 2- and 3-yr survival rates were 87.8%, 76.3% and 62.8%, respectively versus 58.9%, 46.3% and 35.4%, respectively, based on historical data) [12]. This study found that patients in NYHA/WHO FC IV at the time of presentation had significantly poorer 3- and 5-yr survival (47% and 27%, respectively) compared with NYHA/WHO FC III patients (81% and 70%, respectively), and this significant difference was also seen in patients who remained in NYHA/WHO FC IV during treatment (2- and 3-yr survival 42% and 0%, respectively) compared with those in NYHA/WHO FC III (62% and 35%, respectively) or those who improved to NYHA/WHO FC I or II (89% and 73%, respectively) [12]. Similar results were found in our cohort of 178 patients with IPAH treated with first-line intravenous epoprostenol, with survival rates of 77%, 46% and 33% at 1, 2 and 3 yrs, respectively, in patients persisting in NYHA/WHO FC III or IV after 3 months' epoprostenol, compared with 100%, 93% and 88%, respectively, in those who improved to NYHA/WHO FC I or II [9]. Data for other prostacyclins in severe PAH are limited. The AIR (Aerosolised Iloprost Randomised) study of inhaled iloprost [28] demonstrated improvements of at least one FC in around a quarter of patients. However, although 41% of the study population were defined as NYHA/WHO FC IV, the baseline characteristics of the patients suggest that FC assessment was different to that used in other trials as they were less compromised in terms of exercise capacity and haemodynamics than would be expected; therefore, the patient population in this trial was probably not a true reflection of severe disease. The lack of specific data for NYHA/WHO FC IV patients is particularly apparent for oral therapies, with only 1.7–8.5% of patients in pivotal studies being in NYHA/WHO FC IV [29–32]. Given that the majority of data in NYHA/WHO FC IV patients are derived from studies of epoprostenol, and that an overall survival benefit of treatment has been shown in PAH [33, 34], first-line epoprostenol monotherapy has the highest recommendation based on the strongest evidence in treatment guidelines; other therapies have lower strength recommendations/evidence, largely because of the small number of such patients included in RCTs [13]. However, it is clear that a substantial proportion of patients fail to respond adequately to initial monotherapy and for those patients who persist in NYHA/WHO FC III or IV despite treatment, survival is particularly poor [9, 12].
Table 3. Percentage of New York Heart Association/World Health Organization functional class (NYHA/WHO FC) patients enrolled in randomised controlled trials (RCTs) with monotherapies.
| Study drug | RCT acronym or study group | Patients | Subjects n | NYHA/WHO FC IV | Duration weeks | Primary end-point |
| Epoprostenol | Rubin et al. [22] | IPAH | 23 | NA | 8 | NA |
| Barst et al. [23] | IPAH NYHA/WHO FC III/IV | 81 | 26% | 12 | 6MWD | |
| Badesch et al. [24] | SSc | 111 | 17% | 12 | 6MWD | |
| Treprostinil | Simonneau et al. [25] | IPAH, CTD, CHD | 469 | 7% | 12 | 6MWD |
| Beraprost | ALPHABET [26] | PAH | 130 | 0% | 12 | 6MWD |
| Barst et al. [27] | IPAH, CTD, CHD | 116 | 0% | 52 | TTCW | |
| Iloprost | AIR [28] | IPAH, CTD, CTEPH | 203 | 41% | 12 | Composite |
| Bosentan | BREATHE-1 [29] | IPAH, CTD | 213 | 8.5% | 16 | 6MWD |
| Ambrisentan | ARIES 1 [30] | PAH | 202 | 7% | 12 | 6MWD |
| ARIES 2 [30] | PAH | 192 | 2.3% | 12 | 6MWD | |
| Sildenafil | SUPER 1 [31] | IPAH, CTD, CHD | 277 | 3% | 12 | 6MWD |
| Tadalafil | PHIRST [32] | PAH | 405 | 1.7% | 16 | 6MWD |
PAH: pulmonary arterial hypertension; IPAH: idiopathic PAH; SSc: systemic sclerosis-associated PAH; CTD: connective tissue diseases-associated PAH; CHD: congenital heart disease-associated PAH; CTEPH: chronic thromboembolic pulmonary hypertension; NA: not applicable; 6MWD: 6-min walk distance; TTCW: time to clinical worsening.
Combination therapy and treat-to-target strategy
For patients who fail to show an adequate response to initial monotherapy, guidelines recommend sequential combination therapy using a goal-oriented approach with the aim of improving or maintaining values of prognostic indicators discussed previously (table 2) to levels associated with better prognosis [13]. Combination therapy, using two or more classes of drugs simultaneously, is an attractive option given that current PAH-specific therapies target three separate signalling pathways known to be involved in the pathogenesis of the pulmonary vascular changes characteristic of the disease: 1) the prostacyclin pathway, which plays a role in inducing pulmonary vasodilation and the inhibition of smooth muscle cell growth, is targeted by epoprostenol, iloprost and treprostinil; 2) the endothelin pathway, known to be involved in the proliferation, hypertrophy, fibrosis, inflammation and vasoconstriction is targeted by the oral endothelin receptor antagonists (ERAs), bosentan and ambrisentan; and 3) the nitric oxide pathway, involved in pulmonary vasodilation and inhibition of proliferation smooth muscle cells, which is the target of the phosphodiesterase type-5 inhibitors (PDE5i), sildenafil and tadalafil. Overall, evidence to support the efficacy and safety of combination therapy using a variety of PAH-specific therapies is increasing, although not all studies show benefits for some combinations of agents [35–44]. However, as for monotherapy, studies of combination therapy generally include only small numbers of NYHA/WHO FC IV patients; for example, in the PACES (Pulmonary Arterial hypertension Combination study of Epoprostenol and Sildenafil) study of epoprostenol plus sildenafil [42], which is the largest double-blind placebo-controlled study of combination PAH therapy to have been reported to date, only 16 (6%) of the 267 patients enrolled were in NYHA/WHO FC IV.
Nevertheless, limited data on the use of combination therapy in a treat-to-target strategy are available for patients with severe disease. In one study, 123 patients in NYHA/WHO FC III (n=98) or IV (n=25) were initially treated with bosentan (except for five patients in NYHA/WHO FC IV with haemodynamic instability who were initially treated with intravenous iloprost), with additional therapies being added in a prescribed order if predefined treatment goals were not met [38]. This strategy resulted in improved survival relative to expected and historical controls, and combination therapy was well tolerated with no evidence of additive toxicity. An important finding of this study was that, in order to reach the predefined treatment goals, almost half of all patients required combination treatment during the 3 yrs of the study, with 16% overall requiring triple combination therapy (bosentan, sildenafil and inhaled iloprost) [39]. This is not unexpected as no single PAH-specific therapy “cures” PAH, and the disease eventually progresses in many patients despite monotherapy.
Combination therapy is clearly a promising strategy, but sequential addition of other categories of PAH-specific drugs based on patients showing an inadequate response to monotherapy may not be the best tactic, particularly in patients with severe disease. Given the malignant nature of the clinical course of PAH in many cases and the characteristics of the underlying proliferative vasculopathy, some have argued that an approach resembling that used in cancer chemotherapy may be more appropriate. For example, “induction” therapy at diagnosis with multiple agents followed by a “maintenance phase” of treatment [45].
Initial or “upfront” combination therapy
The use of initial or “upfront” combination therapy has been suggested for NYHA/WHO FC IV PAH patients in recent guidelines [13], although data on the comparative efficacy of this strategy are limited. The Bosentan Randomized trial of Endothelin Antagonist Therapy for PAH (BREATHE-2) trial was the first double-blind RCT combining an ERA with intravenous epoprostenol as initial therapy in NYHA/WHO FC III and IV PAH patients [35]. This study included 33 patients in NYHA/WHO FC III (n=25) or IV (n=8) who were randomised to initial therapy with either epoprostenol alone or in combination with bosentan for 16 weeks. Haemodynamic parameters, exercise capacity and NYHA/WHO FC improved in both treatment groups; however, although there was a trend towards greater improvement in patients treated with combination therapy, the study was not powered to demonstrate statistical significance [35].
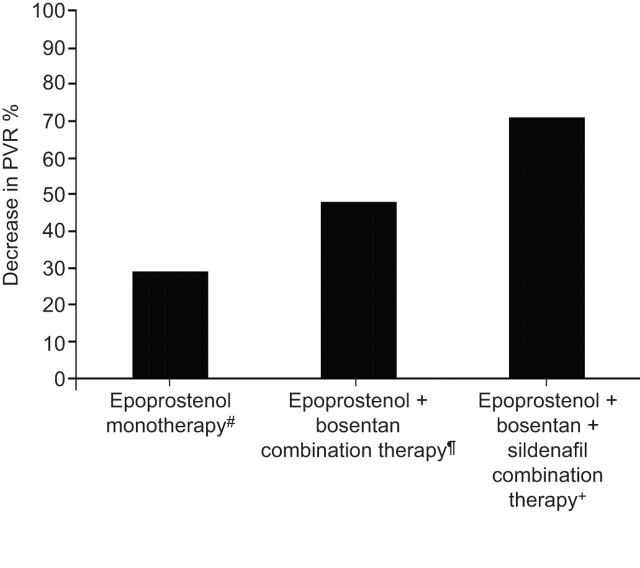
In the absence of data from controlled trials, analysis of treatment and outcomes for patients included in PAH registries or in routine practice can provide important data. Kemp et al. [46] analysed data from 23 patients included in the French National Reference Centre for Pulmonary Hypertension registry who were treated with first-line bosentan and epoprostenol combination therapy. Patients were in NYHA/WHO FC III (70%) and IV (30%) at diagnosis, and had severe disease as evidenced by a mean cardiac index of 1.8±0.3 L·min−1·m−2 and a PVR of 1,514 dyn·s·cm−5 [46]. After 4 months of combination therapy, the majority of patients had significant and marked improvements in exercise capacity, FC and haemodynamics, which were sustained following long-term therapy of over 2 yrs. Furthermore, compared with a haemodynamically matched cohort of patients treated with epoprostenol monotherapy, patients who received combination therapy had a significantly greater reduction in PVR (48% versus 29% on monotherapy). There was also a clear trend towards improved survival in the combination therapy group versus the monotherapy group, although this did not reach statistical significance due to low patient numbers [46]. The French National Reference Centre for Pulmonary Hypertension has recently reported the results of a small study of 10 patients, newly diagnosed with severe PAH in NYHA/WHO FC III (n=4) or IV (n=6) with severely compromised haemodynamics (mean cardiac index 1.6±0.4 L·min−1·m−2; mean PVR 1,798 dyn·s·cm−5), who were treated with upfront triple combination therapy using bosentan, sildenafil and epoprostenol [47]. For the seven patients with available data after 4 months of combination therapy, there was a dramatic improvement in exercise capacity (6MWD 454±67 m versus 290±146 m at baseline) and in haemodynamics, with a reduction of 71% in PVR and normalisation of cardiac index, and all had improved to NYHA/WHO FC II. After a median follow-up period of 18.5 months (range 1–36 months), all patients were alive and in NYHA/WHO FC I or II. Positive treatment effects at 4 months were maintained in those patients (n=5) who were reassessed at 12–28 months. The results of these two studies appears to show increasing treatment benefits of using dual and triple combination therapy compared with monotherapy, in patients with severe disease (fig. 2).
Figure 2.
Comparison of upfront combination therapy with monotherapy in pulmonary arterial hypertension. Percentage decrease in pulmonary vascular resistance (PVR) from baseline to month 4. #: n=46, data from [46]; ¶: n=23, data from [46]; +: n=7, data from [47].
Although encouraging, the validity of initial combination therapy as a strategy in PAH remains to be fully established. A shift in strategy is required away from trials assessing sequential treatment using a two-drug regimen, towards trials aimed at improving management and including initial treatment with various combinations of all three drug classes. A number of new trials are underway or planned which will help to address whether upfront double combination therapy is superior to monotherapy in PAH. AMBITION, A Study of First-Line Ambrisentan and Tadalafil Combination Therapy in Subjects With Pulmonary Arterial Hypertension [48], is a phase III study comparing first-line combination therapy with an ERA and a PDE5i (ambrisentan and tadalafil) versus first-line monotherapy (ambrisentan or tadalafil) in patients with PAH. This large, multicentre study with an estimated enrolment of >350 patients is the first large, randomised trial of combination therapy in PAH. The primary end-point is time to first clinical failure event, with a number of secondary end-points at week 24 including 6MWD, proportion of patients with unsatisfactory clinical response and NYHA/WHO FC. The study is estimated to be completed in 2013. Unfortunately, AMBITION will only enrol patients in NYHA/WHO FC II and III and so the data derived will not directly support the management of patients with severe PAH. A smaller phase IV study, comparing first-line combination therapy with treprostinil and tadalafil versus treprostinil alone, aims to enrol 66 patients in NYHA/WHO FC III and IV [49]. Primary outcome measures include all-cause mortality, hospitalisations and NYHA/WHO FC. A small open-label pilot study of first-line bosentan plus sildenafil is also underway [50].
SURGICAL INTERVENTION
Balloon atrial septostomy
Despite improvements in overall survival with the use of PAH-specific therapies, a proportion of patients show disease progression despite medical intervention. For these patients, surgical intervention is their only remaining option.
Balloon atrial septostomy (BAS) is a transcutaneous intervention in which an atrial defect is created by puncture of the interatrial septum, followed by repetitive balloon dilatation to achieve a haemodynamically predefined inter-atrial right-to-left shunt. The aim of the procedure is to form a “safety valve” in order to decompress the right heart and increase left ventricular pre-load and cardiac output [51]. The rationale behind this technique is based on better survival in patients with Eisenmenger's syndrome relative to patients with IPAH despite similar levels of pulmonary artery pressure, which is attributed to the ability of the failing right ventricle to decompress via the right-to-left shunt [52]. Benefits of successful BAS include a net increase in oxygen tissue delivery despite shunt-induced systemic hypoxaemia, decreased right ventricular filling pressures, and improvements in exercise capacity and NYHA/WHO FC [51]. Guidelines recommend careful pre-procedural risk assessment [13]. Contraindications include severe right ventricular failure on cardiorespiratory support, mean Pra >20 mmHg, PVR index >55 U·m−2, resting oxygen saturation <90% on room air, and left ventricular end diastolic pressure >18 mmHg [13, 53]. It is recommended that patients undergoing BAS should receive optimal medical management, including pre-conditioning with inotropes where required. Timing of BAS is controversial; guidelines recommend that the procedure is reserved for patients with severe PAH (NYHA/WHO FC IV), with right heart failure refractory to medical therapy or with severe syncopal symptoms, patients awaiting transplantation or when medical therapy is not available [13]. However, high mortality rates have been reported for patients with end-stage disease who are referred for BAS, although rates are declining as techniques improve, leading to the suggestion that elective, and not rescue, BAS should be performed [51]. Evidence, in particular long-term survival data, to support this strategy is limited, and it is unknown how early in the course of the disease the technique may be most beneficial [53]. Another potential strategy to improve outcome is to use BAS in combination with PAH-specific therapies. A recent study of 34 patients with severe PAH treated with BAS reported haemodynamic and symptomatic improvement in most patients, with significantly better survival in those patients who also received PAH-specific therapies compared with those who received BAS alone (median 83 months, 95% CI 57–109 months versus 53 months, 95% CI 39–67 months, respectively; p=0.010) [54].
BAS has been shown to result in significant clinical improvements, beneficial and long-lasting haemodynamic effects at rest and a trend toward improved survival; despite the successful implementation of this technique, data are limited to small studies. Therefore, given rates of procedure-related mortality, and the fact that BAS does not affect the disease process itself, guidelines recommend it is used as a palliative procedure or as a bridge to transplantation, or where medical therapy is unavailable [13, 53, 55].
Transplantation
The proportion of transplantations for PAH has decreased over the past decade, in line with the availability of PAH-specific therapies [56]. Nevertheless, for those patients with severe disease who fail to respond despite maximal therapy, transplantation remains the only viable option. Potentially eligible NYHA/WHO FC IV IPAH patients should be referred immediately for transplantation assessment as referral at late stages, where medical therapy has failed and multi-organ failure may be present, is associated with high death rates while on the waiting list and higher rates of post-operative mortality [53]. Any patient who subsequently responds to medical therapy (i.e. moves to NYHA/WHO FC II or better) can be removed from the waiting list and closely followed up. Patients who remain in NYHA/WHO FC III despite combination therapy should also be referred for transplantation assessment and listing as soon as possible, as should any patient with features identifying a worse prognosis profile despite maximal medical therapy. The choice of whether to perform heart–lung (HLTx) or bilateral lung transplantation (BLTx) is largely down to individual centre choice and policy, and donor availability, as the threshold of unrecoverable right ventricular systolic dysfunction and/or left ventricular diastolic dysfunction has not been defined [13]. Both techniques have a range of advantages and disadvantages, but overall survival rates have been reported to be similar, although freedom from obliterative bronchiolitis-related death has been reported to be significantly greater in the HLTx group [57]. There is a proven survival benefit of HLTx in patients with Eisenmenger's syndrome [58]. Overall, patients with IPAH have the lowest 3-month survival of all BLTx recipients (76%), and a diagnosis of IPAH is a risk factor for 1-yr mortality in adult lung transplant recipients [56]. This could be due to a number of factors, including poor patient status at transplantation and post-operative haemodynamic instability caused by residual right and/or left ventricular dysfunction [53]. For those transplant recipients who survive to 1 yr, long-term survival rates are relatively good and around half of these patients are still alive >9 yrs after transplant [56].
SUMMARY
Patients with PAH in NYHA/WHO FC IV have severe symptoms and particularly poor prognosis. Severe disease is not restricted to patients in NYHA/WHO FC IV. Some patients in NYHA/WHO FC III and those in certain subgroups (e.g. patients with PAH associated with SSc or certain heritable mutations) have characteristics which mean they are also at risk, despite relatively better preserved functional capacity. Given the dire prognosis for these patients, aggressive therapy is justified. Current guidelines recommend initial monotherapy with epoprostenol, followed by sequential combination therapy in patients with an inadequate response based on a “treat-to-target” strategy. However, the strategy of waiting to assess response could allow further deterioration of severely ill patients with poor survival, even in the short term. Given increasing data showing benefits of combination therapy, the question arises as to whether it is time for a paradigm shift in strategy, away from the current dogma of sequential therapy using a two-drug regimen based on failure to respond, to a new approach of “upfront” combination therapy. Furthermore, given the fact that we have three classes of PAH-specific drugs in our current armoury targeted at different pathways, should “upfront” triple therapy using a drug from each class be considered? Data from small studies of double and triple upfront combination therapy suggest that such treatment may improve outcome relative to initial monotherapy in patients with severe PAH, but confirmation is required in RCTs. For those patients who fail to respond to therapy, transplantation remains an option, although this is limited by donor availability, with relatively good long-term survival in patients who survive to 1 yr post-transplant. To improve the likelihood of a successful outcome, patients with severe disease at diagnosis, or those who persist in NYHA/WHO FC III despite maximal therapy, should be referred promptly for assessment.
Acknowledgments
We received editorial assistance from L. Thomas (Elements Communications Ltd, Westerham, UK), supported by Actelion Pharmaceuticals Ltd.
Footnotes
Provenance
Publication of this peer-reviewed article was supported by Actelion Pharmaceuticals Ltd, Switzerland (principal sponsor, European Respiratory Review issue 122).
Statement of Interest
O. Sitbon has relationships with drug companies including Actelion, Bayer-Schering, GSK, Lilly, Pfizer and United Therapeutics; in addition to being investigator in trials involving these companies, relationships include consultancy service and membership of scientific advisory boards. He has received reimbursement for attending symposia and funds for research from Actelion, Pfizer, GSK, Lilly and Bayer-Schering. He has received fees for speaking from Actelion, Bayer-Schering, GSK, Lilly, Pfizer and United Therapeutics. G. Simonneau has relationships with pharmaceutical companies including Actelion, BayerHealthCare, GSK, Lilly, Novartis, Pfizer and United Therapeutics. In addition to being investigator in trials involving these companies, relationships include consultancy service, membership of scientific advisory boards, fees for speaking, funds for research and reimbursement for attending symposia.
REFERENCES
- 1.Ryerson CJ, Nayar S, Swiston JR, et al. Pharmacotherapy in pulmonary arterial hypertension: a systematic review and meta-analysis. Respir Res 2010; 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galiè N, Manes A, Negro L, et al. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J 2009; 30: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thenappan T, Shah SJ, Rich S, et al. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J 2010; 35: 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 5.Fischler M, Speich R, Dorschner L, et al. Pulmonary hypertension in Switzerland: treatment and clinical course. Swiss Med Wkly 2008; 138: 371–378. [DOI] [PubMed] [Google Scholar]
- 6.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 7.Launay D, Sitbon O, Le Pavec J, et al. Long-term outcome of systemic sclerosis-associated pulmonary arterial hypertension treated with bosentan as first-line monotherapy followed or not by the addition of prostanoids or sildenafil. Rheumatology (Oxford) 2010; 49: 490–500. [DOI] [PubMed] [Google Scholar]
- 8.Tueller C, Stricker H, Soccal P, et al. Epidemiology of pulmonary hypertension: new data from the Swiss registry. Swiss Med Wkly 2008; 138: 379–384. [DOI] [PubMed] [Google Scholar]
- 9.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002; 40: 780–788. [DOI] [PubMed] [Google Scholar]
- 10.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 11.D′Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002; 106: 1477–1482. [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 14.Kawut SM, Taichman DB, Archer-Chicko CL, et al. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest 2003; 123: 344–350. [DOI] [PubMed] [Google Scholar]
- 15.Humbert M, Sitbon O, Yaici A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010; 36: 549–555. [DOI] [PubMed] [Google Scholar]
- 16.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179: 151–157. [DOI] [PubMed] [Google Scholar]
- 17.Hachulla E, Launay D, Yaici A, et al. Pulmonary arterial hypertension associated with systemic sclerosis in patients with functional class II dyspnoea: mild symptoms but severe outcome. Rheumatology (Oxford) 2010; 49: 940–944. [DOI] [PubMed] [Google Scholar]
- 18.Sztrymf B, Coulet F, Girerd B, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med 2008; 177: 1377–1383. [DOI] [PubMed] [Google Scholar]
- 19.Girerd B, Montani D, Coulet F, et al. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am J Respir Crit Care Med 2010; 181: 851–861. [DOI] [PubMed] [Google Scholar]
- 20.Jing ZC, Xu XQ, Han ZY, et al. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest 2007; 132: 373–379. [DOI] [PubMed] [Google Scholar]
- 21.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010; 137: 376–387. [DOI] [PubMed] [Google Scholar]
- 22.Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med 1990; 112: 485–491. [DOI] [PubMed] [Google Scholar]
- 23.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 1996; 334: 296–302. [DOI] [PubMed] [Google Scholar]
- 24.Badesch DB, Tapson VF, McGoon MD, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med 2000; 132: 425–434. [DOI] [PubMed] [Google Scholar]
- 25.Simonneau G, Barst RJ, Galiè N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002; 165: 800–804. [DOI] [PubMed] [Google Scholar]
- 26.Galiè N, Humbert M, Vachiéry JL, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 2002; 39: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 27.Barst RJ, McGoon M, McLaughlin V, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2003; 41: 2119–2125. [DOI] [PubMed] [Google Scholar]
- 28.Olschewski H, Simonneau G, Galiè N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322–329. [DOI] [PubMed] [Google Scholar]
- 29.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 30.Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008; 117: 3010–3019. [DOI] [PubMed] [Google Scholar]
- 31.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 32.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009; 119: 2894–2903. [DOI] [PubMed] [Google Scholar]
- 33.Barst RJ, Rubin LJ, McGoon MD, et al. Survival in primary pulmonary hypertension with long-term continuous intravenous prostacyclin. Ann Intern Med 1994; 121: 409–415. [DOI] [PubMed] [Google Scholar]
- 34.Badesch DB, McGoon MD, Barst RJ, et al. Long term survival among patients with scleroderma-associated pulmonary arterial hypertension treated with intravenous epoprostenol. J Rheumatol 2009; 36: 2244–2249. [DOI] [PubMed] [Google Scholar]
- 35.Humbert M, Barst RJ, Robbins IM, et al. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J 2004; 24: 353–359. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 174: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 37.Gomberg-Maitland M, McLaughlin V, Gulati M, et al. Efficacy and safety of sildenafil added to treprostinil in pulmonary hypertension. Am J Cardiol 2005; 96: 1334–1336. [DOI] [PubMed] [Google Scholar]
- 38.Hoeper MM, Markevych I, Spiekerkoetter E, et al. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J 2005; 26: 858–863. [DOI] [PubMed] [Google Scholar]
- 39.Hoeper MM, Leuchte H, Halank M, et al. Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2006; 28: 691–694. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz MJ, Escribano P, Delgado JF, et al. Efficacy of sildenafil as a rescue therapy for patients with severe pulmonary arterial hypertension and given long-term treatment with prostanoids: 2-year experience. J Heart Lung Transplant 2006; 25: 1353–1357. [DOI] [PubMed] [Google Scholar]
- 41.Akagi S, Matsubara H, Miyaji K, et al. Additional effects of bosentan in patients with idiopathic pulmonary arterial hypertension already treated with high-dose epoprostenol. Circ J 2008; 72: 1142–1146. [DOI] [PubMed] [Google Scholar]
- 42.Simonneau G, Rubin LJ, Galiè N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008; 149: 521–530. [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55: 1915–1922. [DOI] [PubMed] [Google Scholar]
- 44.Galiè N, Negro L, Simonneau G. The use of combination therapy in pulmonary arterial hypertension: new developments. Eur Respir Rev 2009; 18: 148–153. [DOI] [PubMed] [Google Scholar]
- 45.Provencher S, Jais X, Yaici A, et al. Clinical challenges in pulmonary hypertension: Roger S. Mitchell lecture. Chest 2005; 128: Suppl. 6, 622S–628S. [DOI] [PubMed] [Google Scholar]
- 46.Kemp K, Savale L, O′Callaghan DS, et al. Usefulness of first-line combination therapy with epoprostenol and bosentan in pulmonary arterial hypertension: an observational study J Heart Lung Transplant 2011 [In press]. [DOI] [PubMed] [Google Scholar]
- 47.Sitbon O, Jais X, Savale L, et al. Upfront triple combination therapy of i.v. epoprostenol with oral bosentan and sildenafil in idiopathic and heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2011; 183: A5910. [Google Scholar]
- 48.A study of first-line ambrisentan and tadalafil combination therapy in subjects with pulmonary arterial hypertension (PAH) (AMBITION). http://clinicaltrials.gov/ct2/show/NCT01178073 Date last updated: May 26, 2011. Date last accessed: August 30, 2011.
- 49.Treprostinil combined with tadalafil for pulmonary hypertension (T2). http://clinicaltrials.gov/ct2/show/NCT01302444. Date last updated: June 10, 2011. Date last accessed: August 30, 2011.
- 50.First-line bosentan and sildenafil combination therapy for pulmonary arterial hypertension. http://clinicaltrials.gov/ct2/show/NCT01247116. Date last updated: November 23, 2010. Date last accessed August 30, 2011.
- 51.Kurzyna M, Dabrowski M, Bielecki D, et al. Atrial septostomy in treatment of end-stage right heart failure in patients with pulmonary hypertension. Chest 2007; 131: 977–983. [DOI] [PubMed] [Google Scholar]
- 52.Hopkins WE. The remarkable right ventricle of patients with Eisenmenger syndrome. Coron Artery Dis 2005; 16: 19–25. [DOI] [PubMed] [Google Scholar]
- 53.Keogh A, Benza RL, Corris P, et al. Interventional and surgical modalities of treatment in pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S67–S77. [DOI] [PubMed] [Google Scholar]
- 54.Sandoval J, Gaspar J, Peña H, et al. Effect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertension. Eur Respir J 2011; 38: 1343–1348. [DOI] [PubMed] [Google Scholar]
- 55.Barst RJ, Gibbs JS, Ghofrani HA, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: Suppl. 1, S78–S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report, 2010. J Heart Lung Transplant 2010; 29: 1104–1118. [DOI] [PubMed] [Google Scholar]
- 57.Fadel E, Mercier O, Mussot S, et al. Long-term outcome of double lung and heart–lung transplantation for pulmonary hypertension: a comparative retrospective study of 219 patients. Eur J Cardiothoracic Surg 2010; 38: 277–284. [DOI] [PubMed] [Google Scholar]
- 58.Waddell TK, Bennett L, Kennedy R, et al. Heart–lung or lung transplantation for Eisenmenger syndrome. J Heart Lung Transplant 2002; 21: 731–737. [DOI] [PubMed] [Google Scholar]