Abstract
Introduction
Coronary artery ectasia (CAE) is localized or diffuse enlargement of the coronary artery more than 1.5 times in diameter in comparison with the adjacent normal coronary artery. The etiology and pathophysiology of CAE are not fully elucidated. Resistin is a newly identified adipocyte secreted hormone belonging to a cysteine-rich protein family. Recently it has been found to be relevant to inflammation-related disease and correlated with serum C-reactive protein (CRP). This research aimed to investigate whether the resistin level has a role in CAE etiopathogenesis.
Material and methods
A hundred and three patients with diagnosis of CAE and 122 with normal coronary anatomy (NCA) were included. Details of baseline clinical characteristics and angiographic findings were recorded. Other necessary biochemical parameters were measured with an autoanalyzer. Blood was collected and stored for serum resistin level analysis.
Results
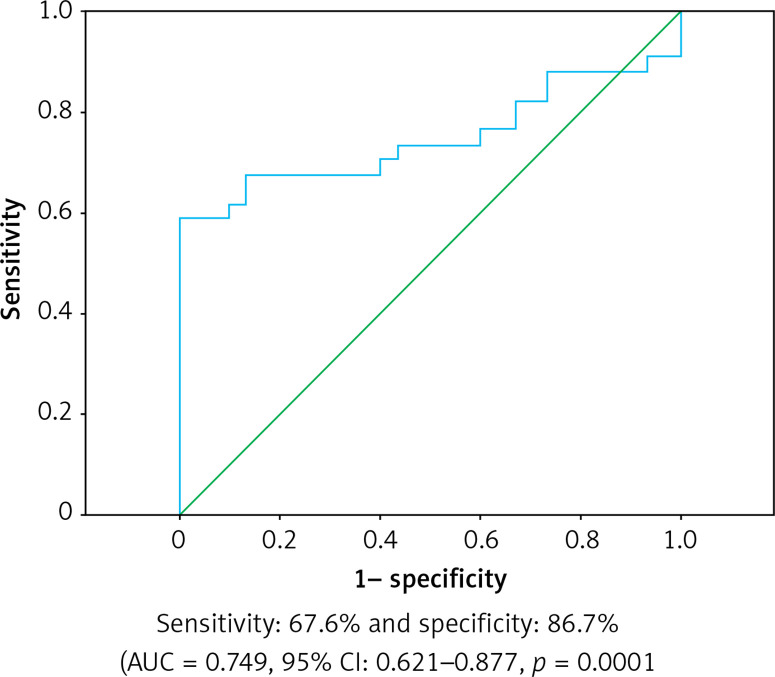
Serum resistin levels in CAE were higher than in the NCA group and were statistically significant (p = 0.001). Hypertension (OR = 1.006, 95% CI: 1.002–1.008, p = 0.025), tobacco use (OR = 1.089, 95% CI: 1.055–1.124, p < 0.001), serum resistin levels (OR = 2.431, 95% CI: 1.100–4.696, p = 0.01), hyperlipidemia (OR = 1.005, 95% CI: 1.000–1.014, p = 0.004), triglyceride (OR = 1.006, 95% CI: 1.001–1.010, p = 0.012) remained as independent factors for CAE. In the subgroup analysis of the CAE group, in patients with ectasia in three coronary arteries, resistin levels were significantly higher and statistically significant (p = 0.001). In ROC analysis, the sensitivity of serum resistin was 67.6% and specificity was 86.7% (AUC = 0.749, 95% CI: 0.621–0.877, p = 0.0001).
Conclusions
Serum resistin level was significantly higher in CAE. In addition this study showed that serum resistin levels are directly proportional to the number of coronary arteries with ectasia. We think that this study will shed light on this subject and encourage further studies in this field.
Keywords: resistin, coronary artery disease, coronary artery ectasia, inflammation
Introduction
Coronary artery disease (CAD) is one of the leading causes of death and morbidity in the world. Coronary artery ectasia (CAE) is among the rare representations of CAD [1]. CAE is an abnormal dilatation of a coronary artery to the extent of 1.5 times or more than the diameter of the adjacent normal segment [1–3]. It may be local or diffuse. It has been reported in the literature that CAE is diagnosed with a frequency of 0.3–4.9% in patients who underwent coronary angiography. The typical clinical symptom in CAE and coronary artery aneurysms is exercise angina, especially observed in diffuse ectasia. Myocardial infarction may develop due to a dilated coronary segment or the thrombotic occlusion of dilated vessels. Thrombus, vasospasm, or spontaneous dissection may occur in ectatic coronary arteries due to structural changes such as arterial wall fragility or dilatation, even in the absence of an occluding lesion [4]. The mortality of CAE alone without obstructive CAD was found to be the same as that of the three-vessel obstructive CAD with an annual mortality rate of 15% [5]. CAE can be congenital or acquired. The majority, namely 50%, of ectasia cases result from coronary atherosclerosis. Many previous studies have suggested the role of widespread inflammation in CAE [6].
Resistin is secreted not only by adipose tissue but also by inflammatory cells. Thus, it is involved in the continuation of inflammation and the release of proinflammatory cytokines (interleukin-2 (IL-2), IL-6, IL-12, tumor necrosis factor α (TNF-α), matrix metalloproteinase (MMP), and endothelin-1. In addition, it produces a prothrombotic effect by inhibiting nitric oxide synthetase (NOS), increasing endothelial permeability [7, 8]. Resistin levels are elevated in patients with atherosclerosis, arthritis, type 1 diabetes mellitus, and inflammatory bowel disease such as Crohn’s disease [9, 10].
The aim of this study is to compare resistin levels between patients with CAE and those with normal coronary arteries (NCA) based on findings in coronary angiography.
Material and methods
This prospective and single-center study was conducted in patients who had undergone coronary angiography between December 2016 and June 2021 at a single center after ethics committee approval from the Non-Interventional Clinical Research Ethics Committee. The study included patients with isolated CAE (without any concomitant stenosis of > 50%) and patients with NCA based on coronary angiography findings. A total of 255 patients were included in the study, consisting of 103 patients with CAE and 122 patients with NCA. The study was conducted in accordance with the Declaration of Helsinki.
As per the exclusion criteria of the study, patients with kidney failure or any kidney disease that impairs renal clearance, malignancy, collagen-connective tissue diseases, congestive heart failure, history of acute coronary syndrome or acute cerebrovascular disease, and CAE with concomitant stenosis of more than 50%, or use of lipid-lowering drugs were excluded.
Demographic, clinical, and angiographic characteristics of all patients included in the study were noted. Chronic kidney failure was defined as having a glomerular filtration rate of less than 60 ml/min for more than 3 months. The diagnosis of hypertension was accepted when patients received antihypertensive therapy or had systolic and diastolic blood pressure values of more than 160 mm Hg and 90 mm Hg respectively at least at three different time points. A diagnosis of diabetes was accepted when patients used antidiabetics or had at least two fasting blood glucose levels of more than 126 mg/dl. Hyperlipidemia was defined as use of lipid-lowering medication or serum containing total cholesterol > 200 mg/dl. Diagnostic criteria for COPD were FEV1/FEVC < 70% or FEV1 < 70% after inhaled bronchodilator.
Coronary angiography evaluation
All patients included in the study had angina or angina-equivalent symptoms and coronary angiography indications were established with positive non-invasive tests (exercise stress test, stress echocardiography, myocardial perfusion scintigraphy) Coronary angiography was performed with a Philips AlluraXper FD10 device.
Angiography was performed through a femoral artery puncture using standard 6FJudkins right and left catheters. At least four and two projections for the left and right coronary system, respectively, were used to obtain and record digital images and cine films. Quantification was performed in patients who were found to have ectatic coronary arteries by inspection. CAE was defined as localized or diffuse dilatation of more than 1.5 times in a segment of or along the whole epicardial coronary artery compared to the diameter of the adjacent normal coronary artery. A normal segment was defined as a coronary artery segment without stenosis and ectasia based on coronary angiography findings.
Measurement of serum resistin levels
After coronary angiography, serum samples collected into plain tubes were centrifuged at 8000 rpm for 5 min and stored at –70°C until the time of the analysis. Serum resistin levels were measured by the enzyme-linked immunosorbent assay method using a Human Resistin enzyme-linked immunosorbent assay kit (BioVendor, Czech Republic).
Statistical analysis
Statistical analysis was performed using the SPSS 18.0 software. The agreement between numerical variables and the normal distribution was examined by the Kolmogorov-Smirnov test. Before statistical analysis, log transformation was applied to non-normally distributed resistin levels. Descriptive statistics for numerical and categorical variables were presented as mean ± standard deviation and as numbers and percentages, respectively. Differences in categorical variables between groups were tested by the χ2 test. The independent sample t-test was used to analyze parametric data. Univariate analysis was used to calculate the association of different variables with CAE. Variables for which the unadjusted p-value in the logistic regression model was < 0.05 were identified as potential risk markers and included in the full multivariate model.
Results
The study included a total of 255 patients, consisting of 103 patients in the CAE group and 122 patients in the NCA group. The mean age was 64.9 ±4.9 years in the CAE group and 63.6 ±4.2 years in the NCA group. There were 55 male and 48 female patients in the CAE group and 70 male and 52 female patients in the NCA group. There were no differences in age and gender between the two groups.
When the groups were examined for risk factors and comorbid diseases, no differences were found in the distribution of diabetes mellitus, cerebrovascular events, and chronic obstructive pulmonary disease (p > 0.05). However, hypertension, tobacco use, and hyperlipidemia were observed significantly more in the CAE group (Table I).
Table I.
Baseline characteristics of patients
| Parameter | Group | P-value | ||||
|---|---|---|---|---|---|---|
| CEA | NCA | |||||
| N | % | n | % | |||
| DM | No | 50 | 48.5 | 58 | 47.5 | 0.55 |
| Yes | 53 | 51.5 | 64 | 52.5 | ||
| HT | No | 59 | 57.2 | 92 | 75.4 | 0.020 |
| Yes | 44 | 42.8 | 30 | 24.5 | ||
| Tobacco use | No | 55 | 53.3 | 95 | 77.8 | 0.012 |
| Yes | 48 | 46.7 | 27 | 22.2 | ||
| CVE | No | 95 | 92.2 | 100 | 81.9 | 0.552 |
| Yes | 8 | 7.8 | 22 | 18.1 | ||
| COPD | No | 85 | 82.5 | 99 | 81.1 | 0.318 |
| Yes | 18 | 17.5 | 23 | 18.9 | ||
| HL | No | 32 | 31 | 85 | 69.6 | 0.036 |
| Yes | 71 | 69 | 37 | 30.4 | ||
| PVD | No | 85 | 82.5 | 98 | 80.3 | 0.235 |
| Yes | 18 | 17.5 | 24 | 19.7 | ||
| Gender | Male | 55 | 53.3 | 70 | 57.3 | 0.251 |
| Female | 48 | 46.7 | 52 | 42.7 | ||
DM – diabetes mellitus, HT – hypertension, CVE – cardiovascular event, HL – hyperlipidemia, PVD – peripheral vascular disease.
When laboratory parameters were evaluated it was determined that serum resistin (4932.78 ±1075.54 vs. 1797.98 ±393.97 pg/dl, p = 0.001), triglyceride (352.1 ±95.2 mg/dl vs. 128.0 ±62.4 mg/dl, p = 0.002) was statistically significantly higher in the CAE group (Table II).
Table II.
Comparison of laboratory parameters in the CAE and NCA groups
| Parameter | CAE | NCA | P-value |
|---|---|---|---|
| Glucose [mg/dl] | 123.2 ±82.5 | 123.7 ±67.8 | 0.889 |
| WBC [μl] | 9.73 ±3.71 | 11.03 ±4.79 | 0.063 |
| Hb [mg/dl] | 12.0 ±1.55 | 11.8 ±1.45 | 0.172 |
| BUN [mg/dl] | 51.2 ±21.2 | 51.0 ±20.9 | 0.937 |
| Cr [mg/dl] | 1.21 ±0.64 | 1.3 ±0.54 | 0.215 |
| Na [mmol/l] | 135.5 ±4.4 | 136.9 ±5.38 | 0.061 |
| K [mmol/l] | 4.27 ±0.52 | 4.24 ±0.62 | 0.587 |
| Gfr [ml/min/m2] | 63.8 ±22.2 | 58.6 ±13.59 | 0.340 |
| Uric acid [mg/dl] | 7.2 ±2.3 | 7.4 ±2.74 | 0.521 |
| Total protein [g/dl] | 6.54 ±1.09 | 6.22 ±1.07 | 0.368 |
| Albumin [g/dl] | 3.5 ±0.58 | 3.38 ±1.0 | 0.108 |
| Calcium [mg/dl] | 8.84 ±0.606 | 8.68 ±0.757 | 0.306 |
| Creatinine [mg/dl] | 1.21 ±0.64 | 1.39 ±0.54 | 0.817 |
| Total cholesterol [mg/dl] | 175.6 ±45.6 | 165.38 ±44.3 | 0.225 |
| HDL [mg/dl] | 37.2 ±13.4 | 36.2 ±12.8 | 0.222 |
| LDL [mg/dl] | 100.8 ±32.8 | 99.2 ±32.1 | 0.302 |
| TAG [mg/dl] | 352.1 ±95.2 | 128.0 ±62.4 | 0.002 |
| Serum resistin levels [pg/dl] | 4932.78 ±1075.54 | 1797.98 ±393.97 | 0.001 |
WBC – white blood cells, Hb – hemoglobin, BUN – blood urea nitrogen, Cr – creatinine, hs-CRP – high-sensitivity C reactive protein, NT-proBNP – N-terminal brain natriuretic peptide, HDL – high-density lipoprotein, LDL – low-density lipoprotein, TAG – triacylglycerol.
Hypertension (OR = 1.006, 95% CI: 1.002–1.008, p = 0.025), tobacco use (OR = 1.089, 95% CI: 1.055–1.124, p < 0.001), serum resistin levels (OR = 2.431, 95% CI: 1.100–4.696, p = 0.01), hyperlipidemia (OR = 1.005, 95% CI: 1.000–1.014, p = 0.004), and triglyceride (OR = 1.006, 95% CI: 1.001–1.010, p = 0.012) remained as independent factors for CAE in univariate and multivariate logistic analysis (Table III). In ROC analysis, the sensitivity of serum resistin was 67.6% and specificity was 86.7% (AUC = 0.749, 95% CI: 0.621–0.877, p = 0.0001) (Figure 1).
Table III.
Effects of different variables on CAE in univariate and multivariate logistic regression analyses
| Variable | Unadjusted OR | 95% CI | P-value | Adjusted OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Resistin levels | 3.317 | 1.487–7.397 | 0.003 | 2.431 | 1,100–4.696 | 0.002 |
| Hyperlipidemia | 1.009 | 1.002–1.016 | 0.014 | 1.005 | 1.000–1.014 | 0.004 |
| Triglyceride | 1.006 | 1.003–1.009 | 0.000 | 1.006 | 1.001–1.010 | 0.012 |
| Hypertension | 1.008 | 1.003–1.014 | 0.004 | 1.006 | 1.002–1.008 | 0.025 |
| Tobacco use | 1.025 | 1.017–1.032 | < 0.001 | 1.089 | 1.055–1.124 | < 0.001 |
Figure 1.
ROC curves of the serum resistin value for detecting the presence of coronary ectasia
A subgroup analysis was performed in the CAE group to identify coronary arteries with ectasia and to determine the numbers of ectatic coronary arteries. Of the 103 CAE patients included in the study, 60.4% had ectasia in the right coronary artery (RCA), 13.3% had ectasia in the left anterior descending artery (LAD), and 26.3% had ectasia in the circumflex artery (CX). Among CAE patients, 40 had ectasia in one coronary artery and 63 had ectasia in two or more coronary arteries. The relationship between resistin levels of patients with ectasia in one coronary artery and those with ectasia in two or more coronary arteries was investigated. In the patient group with ectasia in three coronary arteries, resistin levels were significantly high and the difference was statistically significant (p = 0.001) (Table IV).
Table IV.
Subgroup analysis of CAE group
| Resistin | N | x | Median | Minimum | Maximum | SD | P-value |
|---|---|---|---|---|---|---|---|
| 1 artery | 40 | 1019.33 | 1019.40 | 1001.99 | 1166.66 | 258.90 | 0.001 |
| 2 arteries | 30 | 3442.67 | 3863.75 | 3297.69 | 4256.16 | 1778.18 | |
| 3 arteries | 33 | 6405.73 | 5405.73 | 4133.35 | 8678.10 | 2385.20 |
Discussion
This study showed for the first time that serum resistin level is an independent risk factor for CAE. In addition, a positive correlation was found between resistin levels and CAE, which is an indicator of CAE severity.
Similar to previous studies, it was observed that hypertension, tobacco use, hyperlipidemia, and triglyceride were independent risks factors for CAE in our study [10–12]. Many studies have shown that hypertension is an independent risk factor for CAE due to shear stress, increased inflammatory mediators, and oxidative stress [13, 14]. Markıs and Joffe in their study observed that the majority of CAE patients have hypertension. This situation is associated with destruction of the arterial media layer [15]. Qin et al. found 135% more ectasia in individuals with hypertension than in individuals without hypertension [16]. Kundi et al. found that patients with HT were had 66% more ectasia than detected in patients without it [17]. Increased Inflammatory mediators, MMP levels, and atherosclerotic processes resulting from tobacco use cause devastating effects on the vascular wall [18, 19]. Dastgir and Masood and Fariba and Moradi found that tobacco use is an independent risk factor for CAE [20, 21].
Many studies have shown that dyslipidemia, which is a major risk factor for endothelial dysfunction and atherosclerosis, is an independent risk factor for CAE [22, 23]. Sudhir et al. reported that patients with familial hyperlipidemia had CAE more frequently than patients without it (15% vs. 2.5%) [24]. Fang et al. in an epidemiological study observed the presence of dyslipidemia 2 times more in CAE patients compared to the normal population [25]. Baman et al. found that the presence of hyperlipidemia in CAE patients was a strong predictor of mortality [26].
In many studies on CAE, elevated triglyceride has been shown to be an independent risk factor for CAE. Qin et al. reported that hypertriglyceridemia is an independent risk for CAE [27]. Nyamu et al. in their epidemiological study on CAE patients reported that lipid abnormalities were found in more than half of the patients, and the most common anomaly was found to be hypertriglyceridemia [28].
Resistin, which is cysteine-rich peptide with a molecular weight of 12.5 kDa, is secreted by adipose tissue, the gastrointestinal tract, macrophages, and leukocytes [29]. Although generally considered very important for metabolic hemostasis, resistin plays a fundamental role in the initiation and continuation of the inflammatory reaction. Resistin causes the release of inflammatory mediators such as TNF-α, IL-6, and IL-12 from macrophages and monocytes by activating NF-κB (nuclear factor κ light-chain-enhancer of activated β cells) as well as causing endothelial dysfunction leading to oxidative stress, release of adhesion molecules, and decreased NOS [30]. Recently studies showed that inflammatory mediators such as TNF-α, IL-6, and IL-12 increased resistin mRNA secretion by increasing resistin gene expression [31]. Experimental studies showed TNF-α increased mRNA secretion from leukocytes [32].
A close relationship between CAE pathogenesis and chronic inflammation has been observed. Reilly et al. stated that higher serum resistin levels are predictive of coronary calcium score [33]. Butler et al. and Frankel et al. reported that higher serum resistin levels were associated with increased incidence of heart failure, and severity and risk of adverse events [34, 35]. Hussain et al. and Wang et al., in their study, found a significant correlation between the increase in serum resistin level and the severity and extent of CAD [36, 37]. Pourmoghaddas and Elahifar and Chu et al. reported that higher serum resistin levels were predictive of discrimination of acute coronary syndrome and stable CAD [38, 39]. According to Harsch et al. a strong correlation was found between serum IL-6, ICAM-1 levels and serum resistin in OSAS patients [40]. Güngör et al. found higher serum resistin levels in patients with atrial fibrillation after a bypass operation [41].
A close relationship has been noted between CAE pathogenesis and chronic inflammation. Histopathological studies have demonstrated that there is a large amount of inflammatory cells in the vascular media layer [1–3]. Li et al. reported that IL-6 and CRP, which are the most important markers of systemic inflammation, were found to be higher in the CAE group [42]. Other studies showed that TNF-α and C-reactive protein are higher in patients with CAE [43]. Yıldırım et al. found that the cellular adhesion molecules CD-16 and CD 45 increased in patients with CAE [44]. In a large meta-analysis published recently, hs-CRP, IL-6, TNF-α, and RDW levels were found to be high in the CAE group [45].
The present study has some limitations. The main limitations of the study are that it relied on a single center, with small samples. The diagnosis of coronary artery ectasia was based on contrast angiography, which may lead to missed cases. Modern imaging techniques which show subclinical atherosclerosis such as IVUS could not be used. We did not perform evaluation with other inflammatory markers such as CRP and fibrinogen. Another limitation of the study is that short- and long-term follow-up were not performed.
In conclusion, our study is the first to demonstrate that serum resistin level is an independent risk factor for CAE and correlates with CAE severity. We think that this result will help us understand the underlying pathophysiology of CAE and enable the development of new treatment strategies. Further prospective and large sample studies are warranted to gain a better understanding of the pathogenesis of CAE and the diagnostic and therapeutic value of serum resistin level in CAE patients.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Devabhaktuni S, Mercedes A, Diep J, et al. Coronary artery ectasia – a review of current literature. Curr Cardiol Rev 2016; 12: 318-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boles U, Rakhit R, Shiu M, et al. Coronary artery ectasia as a culprit for acute myocardial infarction: review of pathophysiology and management. Anadolu Kardiyol Derg 2013; 13: 695-701. [DOI] [PubMed] [Google Scholar]
- 3.Abou Sherif S, Ozden Tok O, Taşköylü Ö. Coronary artery aneurysms: a review of the epidemiology, pathophysiology, diagnosis, and treatment. Front Cardiovasc Med 2017; 4: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Wu C, Liu W. Coronary artery ectasia presenting with thrombus embolization and acute myocardial infarction. Medicine 2017; 96: e5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willner NA, Ehrenberg S, Musallam A. Coronary artery ectasia: prevalence, angiographic characteristics and clinical outcome. Open Heart 2020; 7: e001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashraf H, Soltani D, Sobh-Rakhshankhah A. Visfatin as marker of isolated coronary artery ectasia and its severity. Cytokine 2019; 113: 216-20. [DOI] [PubMed] [Google Scholar]
- 7.Acquarone E, Monacelli F, Borghi R. Resistin: a reappraisal. Mech Ageing Dev 2019; 178: 46-63. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb IN, Kabakoff RC, Chan B, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J 2000; 19: 4046-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HK, Kwak MK, Kim HJ, et al. Linking resistin, inflammation, and cardiometabolic diseases. Korean J Intern Med 2017; 32: 239-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bermúdez P, Palop EL, Martínez-Luengas RL, et al. Coronary ectasia: prevalence, and clinical and angiographic characteristics. Rev Esp Cardiol 2003; 56: 473-9. [DOI] [PubMed] [Google Scholar]
- 11.Saglam M. Identifying cardiovascular risk factors in a patient population with coronary artery ectasia. Angiology 2007; 58: 698-703. [DOI] [PubMed] [Google Scholar]
- 12.Gunes Y, Boztosun B, Yildiz A. Clinical profile and outcome of coronary artery ectasia. Heart 2006; 92: 1159-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999; 282: 2035-42. [DOI] [PubMed] [Google Scholar]
- 14.Singh PK, Marzo A, Howard B, et al. Effects of smoking and hypertension on wall shear stress and oscillatory shear index at the site of intracranial aneurysm formation. Clin Neurol Neurosurg 2010; 112: 306-13. [DOI] [PubMed] [Google Scholar]
- 15.Markis JE, Joffe CD. Clinical significance of coronary arterial ectasia. Am J Cardiol 1976; 37: 217-22. [DOI] [PubMed] [Google Scholar]
- 16.Qin Y, Tang C, Ma C. Risk factors for coronary artery ectasia and the relationship between hyperlipidemia and coronary artery ectasia. Coron Artery Dis 2019; 30: 211-5. [DOI] [PubMed] [Google Scholar]
- 17.Kundi H, Gök M, Topçuoglu C, Ornek E. Association of serglycin levels with isolated coronary artery ectasia. Kardiol Pol 2017; 75: 990-6. [DOI] [PubMed] [Google Scholar]
- 18.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014; 34: 509-15. [DOI] [PubMed] [Google Scholar]
- 19.Bergoeing MP, Arif B, Hackmann AE. Cigarette smoking increases aortic dilatation without affecting matrix metalloproteinase-9 and -12 expression in a modified mouse model of aneurysm formation. J Vasc Surg 2007; 45: 1217-27. [DOI] [PubMed] [Google Scholar]
- 20.Dastgir N, Masood A. Frequency of risk factors in patients of acute coronary syndrome due to coronary ectasia. Asian Cardiovasc Thorac Ann 2020; 28: 312-5. [DOI] [PubMed] [Google Scholar]
- 21.Fariba F, Moradi M. Prevalence of coronary artery ectasia with atherosclerosis and associated risk factors in the west of iran: a cross-sectional study. J Res Health Sci Winter 2016; 16: 22-5. [PMC free article] [PubMed] [Google Scholar]
- 22.Ozturk S, Yetkin E. Molecular and cellular insights into the pathogenesis of coronary artery ectasia Cardiovasc Pathol 2018; 35: 37-47. [DOI] [PubMed] [Google Scholar]
- 23.Antoniadis AP, Chatzizisis YS, Giannoglou GD. Pathogenetic mechanisms of coronary ectasia. Int J Cardiol 2008; 130: 335-43. [DOI] [PubMed] [Google Scholar]
- 24.Sudhir K, Ports T, Amidon TM. Increased prevalence of coronary ectasia in heterozygous familial hypercholesterolemia. Circulation 1995; 91: 1375-80. [DOI] [PubMed] [Google Scholar]
- 25.Fang CT, Fang YP, Huang YB, Kuo CC, Chen CY. Epidemiology and risk factors of coronary artery aneurysm in Taiwan: a population based case control study. BMJ Open 2017; 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baman TS, Cole JH, Devireddy CM, Sperling LS. Risk factors and outcomes in patients with coronary artery aneurysms. Am J Cardiol 2004; 93: 1549-51. [DOI] [PubMed] [Google Scholar]
- 27.Qin Y, Tang C. Risk factors for coronary artery ectasia and the relationship between hyperlipidemia and coronary artery ectasia. Coron Artery Dis 2019; 30: 211-5. [DOI] [PubMed] [Google Scholar]
- 28.Nyamu P, Ajit MS, Joseph PK, et al. The prevalence and clinical profile of angiographic coronary ectasia. Asian Cardiovasc Thorac Ann 2003; 11: 122-6. [DOI] [PubMed] [Google Scholar]
- 29.Acquarone E, Monacelli F, Borghi R. Resistin: a reappraisal. Mech Ageing Dev 2019; 178: 46-63. [DOI] [PubMed] [Google Scholar]
- 30.Tripathi D, Kant S, Pandey S. Resistin in metabolism, inflammation, and disease FEBS J 2020; 287: 3141-9. [DOI] [PubMed] [Google Scholar]
- 31.Ding Q, White SP, Ling C. Resistin and cardiovascular disease. Trends Cardiovasc Med 2011; 21: 20-7. [DOI] [PubMed] [Google Scholar]
- 32.Pang SS, Le YY. Role of resistin in inflammation and inflammation-related diseases. Cell Mol Immunol 2006; 3: 29-34. [PubMed] [Google Scholar]
- 33.Reilly M, Lehrke M. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005; 111: 932-9. [DOI] [PubMed] [Google Scholar]
- 34.Butler J, Kalogeropoulos A, Georgiopoulou V. Serum resistin concentrations and risk of new onset heart failure in older persons: the health, aging, and body composition (Health ABC) study. Arterioscler Thromb Vasc Biol 2009; 29: 1144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frankel DS, Vasan RS, D’Agostino RB S, et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol 2009; 53: 754-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hussain S, Bibi S, Javed Q. Heritability of genetic variants of resistin gene in patients with coronary artery disease: a family-based study. Clin Biochem 2011; 44: 618-22. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Chen DY, Cao J, He ZY, Zhu BP, Long M. High serum resistin level may be an indicator of the severity of coronary disease in acute coronary syndrome. Chin Med Sci J 2009; 24: 161-6. [DOI] [PubMed] [Google Scholar]
- 38.Pourmoghaddas A, Elahifar A. Resistin and prooxidant-antioxidant balance: markers to discriminate acute coronary syndrome from stable angina. ARYA Atheroscler 2020; 16: 46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu S, Ding W, Li K, et al. Plasma resistin associated with myocardium injury in patients with acute coronary syndrome. Circ J 2008; 72: 1249-53. [DOI] [PubMed] [Google Scholar]
- 40.Harsch IA, Koebnick C, Wallaschofski H, et al. Resistin levels in patients with obstructive sleep apnoea syndrome: the link to subclinical inflammation? Med Sci Monit 2004; 10: 510-5. [PubMed] [Google Scholar]
- 41.Gungor H, Ayik MF. Serum resistin level: as a predictor of atrial fibrillation after coronary artery bypass graft surgery. Coron Artery Dis 2011; 22: 484-90. [DOI] [PubMed] [Google Scholar]
- 42.Li JJ, Nie SP, Qian XW, Zeng HS, Zhang CY. Chronic inflammatory status in patients with coronary artery ectasia. Cytokine 2009; 46: 61-4. [DOI] [PubMed] [Google Scholar]
- 43.Aydin M, Tekin IO, Dogan SM, et al. The levels of tumor necrosis factor-alpha and interleukin-6 in patients with isolated coronary artery ectasia. Mediators Inflamm 2009; 2009: 106145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yildirim N, Tekin IO, Dogan SM. Expression of monocyte and lymphocyte adhesion molecules is increased in isolated coronary artery ectasia. Coron Artery Dis 2007; 18: 49-53. [DOI] [PubMed] [Google Scholar]
- 45.Vrachatis DA, Papathanasiou KA, Kazantzis D. Inflammatory biomarkers in coronary artery ectasia: a systematic review and meta-analysis. Diagnostics 2022; 12: 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]