Coronavirus disease 2019 (COVID-19), which emerged in Wuhan, Huabei Province, China, in December 2019, still remains a global pandemic, despite the significant improvement in the understanding of its pathophysiology and targeted anti-inflammatory agents, development of highly efficacious against severe disease vaccines and the recent introduction of antiviral drugs, such as molnupiravir.
By May 2022, more than 530 million people had been infected by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), while almost 6.3 million people had died during the disease course. Endothelial injury and dysfunction have now been recognized as a significant pathophysiologic mechanism underlying COVID-19 complications [1, 2]. Impairment of endothelial function results in the formation of thrombi through the induction of a pro-coagulant state, finally leading to decreased organ perfusion and multi-organ dysfunction [3]. Therefore, we sought to determine the effect of SARS-CoV-2 on endothelial function in adult subjects.
We searched the PubMed database, from inception to 1st June 2022, for observational studies enrolling adult patients with COVID-19, either hospitalized or outpatients, assessing brachial artery flow mediated dilation (FMD) in patients with documented SARS-CoV-2 infection compared to controls. We utilized data from published reports, also searching relevant supplementary appendices for any missing data of specific interest. We excluded case reports/case series, narrative reviews and commentaries (except for research letters). We did not apply any filter regarding study setting or publication language. Two independent reviewers (D.P. and A.D.) extracted the data from the eligible reports. We set as the primary efficacy outcome the difference in FMD between SARS-CoV-2 positive patients and healthy controls. Differences were calculated using the mean difference (MD), with 95% confidence interval (CI), after implementation of the Mantel-Haenszel (M-H) random effects formula. Statistical heterogeneity among studies was assessed by using I2 statistics. All analyses were performed at the 0.05 significance level.
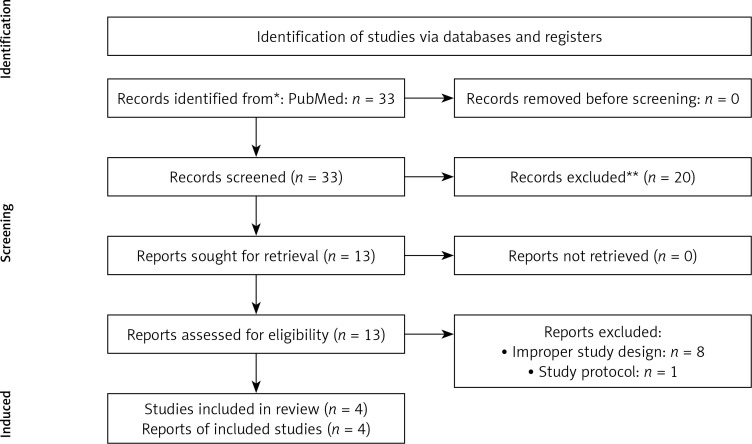
We finally pooled data from 4 observational studies in a total of 465 enrolled subjects. Notably, all studies were conducted in the post-acute setting [4–7]. A summary of participants’ baseline characteristics across the selected studies is provided in Table I. The study selection process is depicted in Figure 1.
Table I.
Summary of participants’ baseline characteristics across the selected studies
| Parameter | Ratchford et al. (2021) | Jud et al. (2021) | Ambrosino et al. (2021) | Lambadiari et al. (2021) |
|---|---|---|---|---|
| Number of enrolled subjects | SARS-CoV-2: 11 | SARS-CoV-2: 14 | SARS-CoV-2: 133 | SARS-CoV-2: 70 |
| Control: 20 | Controls: 14 | Control: 133 | Control: 70 | |
| Age [years] | SARS-CoV-2: 20.1 (1.1) | SARS-CoV-2: 68.7 (12) | SARS-CoV-2: 61.6 (10.6) | SARS-CoV-2: 54.5 (9) |
| Control: 23 (1.3) | Control: 30.7 (4.2) | Control: 60.4 (11.5) | Control: 54.8 (8.9) | |
| Male to female ratio | SARS-CoV-2: 4/7 | SARS-CoV-2: 7/7 | SARS-CoV-2: 108/25 | SARS-CoV-2: 44/26 |
| Control: 5/15 | Control: 7/7 | Control: 107/26 | Control: 44/26 | |
| Body mass index [kg/m2] | SARS-CoV-2: 23.5 (2.9) | SARS-CoV-2: 29.4 (8.3) | SARS-CoV-2: NR | SARS-CoV-2: NR |
| Control: 22.5 (2.2) | Control: 23.8 (3.2) | Control: NR | Control: NR | |
| Setting (acute or post-acute) | Post-acute | Post-acute | Post-acute | Post-acute |
| Hypertension | NR | SARS-CoV-2: 6/14 | SARS-CoV-2: 68/133 | None |
| Control: 0/14 | Control: 74/133 | |||
| Diabetes mellitus | NR | SARS-CoV-2: 0/14 | SARS-CoV-2: 21/133 | None |
| Control: 0/14 | Control: 23/133 | |||
| Dyslipidemia | NR | SARS-CoV-2: 8/14 | SARS-CoV-2: 12/133 | None |
| Control: 0/14 | Control: 14/133 | |||
| Current smoking | NR | SARS-CoV-2: 0/14 | SARS-CoV-2: 12/133 | SARS-CoV-2: 16/70 |
| Control: 4/14 | Control: 12/133 | Control: 21/70 |
Data are presented as mean (standard deviation) or absolute numbers, unless otherwise stated. †NR – not reported.
Figure 1.
Flow diagram depicting the study selection process. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only
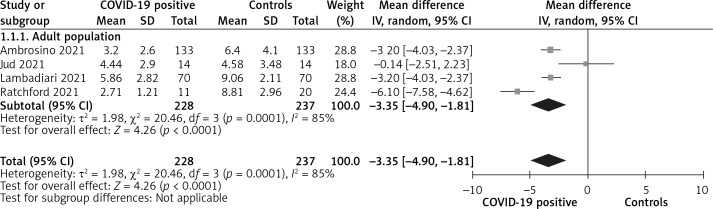
Overall, patients with a history of SARS-CoV-2 infection had significantly lower FMD values compared to healthy controls (MD = –3.35%, 95% CI: –4.90 to –1.81, I2 = 85%, p < 0.001), as shown in Figure 2, indicating significant endothelial dysfunction, even in the post-acute setting.
Figure 2.
Comparison of FMD values between patients with history of SARS-CoV-2 infection and healthy controls
This is the first relevant meta-analysis addressing the hot topic of endothelial dysfunction in COVID-19. Brachial artery FMD, an endothelium-dependent, largely nitric oxide (NO)-mediated dilatation of conduit arteries in response to an imposed increase in blood flow and shear stress, is an established marker of endothelial function in clinical practice, especially for the assessment of patients with prior cardiovascular disease or with multiple cardiovascular risk factors [8]. FMD has been shown to have a significant, positive correlation with pulmonary function, as assessed with forced expiratory volume in 1 s, forced vital capacity, and diffusing capacity for carbon monoxide [5]. Therefore, it may be an additional tool for the prediction of the risk for impaired lung function after a recent SARS-CoV-2 infection. In addition, it has been shown in another observational study that FMD with an optimal cut-off value of 3.43% might predict in-hospital mortality and duration of hospitalization [9]. Of note, an inverse correlation with inflammatory markers has also been demonstrated, confirming the strong association between low-grade inflammation and endothelial dysfunction, which might be predictive of future development of atherosclerosis [10]. However, no studies have assessed so far the association of post-COVID-19 endothelial function with future cardiovascular events.
Collectively, even post-COVID-19 patients present with significant endothelial dysfunction compared to healthy controls, highlighting the importance of the assessment of endothelial function in hospitalized patients, since it might predict adverse complications of SARS-CoV-2 infection. Long-term implications of this generalized endothelial dysfunction among post-COVID-19 subjects, however, remain unknown. Further, long-term observational studies will shed further light on this important and interesting research question.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Perico L, Benigni A, Casiraghi F, Ng LFP, Renia L, Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol 2021; 17: 46-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383: 120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nägele MP, Haubner B, Tanner FC, Ruschitzka F, Flammer AJ. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis 2020; 314: 58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jud P, Gressenberger P, Muster V, et al. Evaluation of Endothelial dysfunction and inflammatory vasculopathy after SARS-CoV-2 infection-a cross-sectional study. Front Cardiovasc Med 2021; 8: 750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosino P, Calcaterra I, Molino A, et al. Persistent endothelial dysfunction in post-acute COVID-19 syndrome: a case-control study. Biomedicines 2021; 9: 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambadiari V, Mitrakou A, Kountouri A, et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur J Heart Fail 2021; 23: 1916-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratchford SM, Stickford JL, Province VM, et al. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol 2020; 320: H404-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thijssen DHJ, Bruno RM, van Mil ACCM, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 2019; 40: 2534-47. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira MR, Back GD, da Luz Goulart C, Domingos BC, Arena R, Borghi-Silva A. Endothelial function provides early prognostic information in patients with COVID-19: a cohort study. Respir Med 2021; 185: 106469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao YP, Zhou W, Huang PN, et al. Persistent endothelial dysfunction in coronavirus disease-2019 survivors late after recovery. Front Med 2022; 9: 809033. [DOI] [PMC free article] [PubMed] [Google Scholar]