ABSTRACT
Objectives
To use ultrasound (US) imaging to determine the validity and reliability of needle placement of two dry needling (DN) protocols for the lumbar multifidus (LM) in individuals with a high body mass index (BMI).
Methods
Twenty-one participants with a BMI higher than 25 kg/m2 completed the study. A US scanner was used to determine the location of needle placement after a 100 mm long needle was inserted in the LM at L4 and L5 following two DN protocols for the deep LM muscle. US images were saved and viewed 6 months later to determine the intra-tester reliability.
Results
The probability of reaching the deep LM muscle was high (85–95%) at L4 and L5. Although the needle reached a bony landmark 85–100% of the time, it only reached the vertebra lamina as intended 70–75% of the time. The intra-tester reliability of needle placements based on analysis of real-time and recorded US images was poor-to-moderate.
Conclusions
Although the bony drop may not indicate that the needle has reached the vertebra lamina as the protocol intended, reaching a bony drop is still meaningful as it coincided with reaching the LM in the majority of participants.
KEYWORDS: Lumbar spine, vertebra lamina, manual therapy, acupuncture, ultrasonography
Introduction
The lumbar multifidus (LM) muscle, specifically the deep layer, has been of interest to researchers and clinicians, as altered muscle activity and morphology of the LM have been observed in patients with low back pain (LBP) [1–3]. A rehabilitation program for LBP often aims to improve this muscle’s function in order to reduce physical limitations. In recent years, dry needling (DN) has been used by orthopedic manual physical therapists (OMPTs) as an adjunct intervention along with exercise and other forms of manual therapy in the management of this patient population. The available evidence shows favorable outcomes of DN, particularly for pain reduction [4,5].
As DN has gained popularity among OMPTs, different needling methods have been suggested regarding the insertion site, needling direction, and needle length for reaching the LM muscle [6–8]. Of these needling approaches, one needling protocol has been used to reach the deep LM muscle [6,7]. This needling protocol requires inserting the needle approximately 1.0–2.0 cm lateral to the spinous process and angling the needle in a 20–40° inferior-medial direction. Another needling protocol, which was advocated for the needle to reach the deep LM muscle in an electromyographic study, requires inserting the needle about 4.0 cm lateral to the spinous process, and angling the needling in a 45° medial direction [1]. Although there are variations in the distance of the insertion site from the spinous process and the insertion angle, the endpoint of the needling is described consistently as reaching the vertebra lamina. Further, 50 or 60 mm is the recommended needle length for needling the LM.
Despite these specific recommendations for needle insertion sites and angles, clinicians may find them inadequate, as it is unknown if 50 mm or 60 mm needles are long enough to reach the LM muscle, specifically for the deep layer of the LM in individuals with a high body mass index (BMI). In addition, when bony drop is not felt, it is uncertain whether the needle has reached the vertebra lamina and deep LM as intended because the depth and shape of the vertebra lamina is unknown. Ultrasound imaging has been the modality of choice to confirm the needle placement for needling cervical multifidus [9], as well as for biopsy [10] and needle electromyographic studies [1,8] of the deep LM. In recent years, ultrasound-guided DN has been advocated for needling deep muscles in the treatment of various musculoskeletal disorders, such as supraspinatus tendinopathy [11], piriformis syndrome [12,13], and chronic neck pain [14]. The purpose of ultrasound-guided DN is for safety and for accuracy of needle placement. However, ultrasound-guided DN has not been examined for needling the deep LM muscle. If ultrasound-guided DN is necessary for needling the deep LM, OMPTs may be required to undergo ultrasound-guided DN training to become proficient in this technique.
Therefore, the primary purpose of this study was to validate needle placement in the LM muscle using real-time ultrasonographic imaging. Specifically, whether or not the needles would reach the targeted deep LM muscle and corresponding vertebral lamina following the two needling procedures was examined. The secondary purpose of the study was to determine intra-tester reliability of needle placement in the LM using ultrasonographic imaging.
Methods
Study design and participants
A laboratory descriptive study was conducted for the primary purpose and a methodological study was used to establish the intra-tester reliability of needle placements using ultrasound imaging. This study was approved by the Texas Woman’s University, Institutional Review Board – Dallas prior to the commencement of data collection. Convenience sampling was used to recruit participants from the investigators’ affiliated institutions and from local communities. The eligible participants were individuals who had a BMI equal to or larger than 25 kg/m2. Exclusion criteria included bleeding disorders, use of anti-coagulants, previous low back surgery, systemic joint disease (e.g. rheumatoid arthritis), active infection, diabetes, cancer of the lower quadrant, neurological disorders, allergic reaction to ultrasound gel, and inability to obtain the testing position (i.e. prone lying). Each eligible participant was informed of the risks and procedures of the study, and then signed a written informed consent form before data collection.
Procedure
A Sonosite M-Turbo ultrasound scanner (Sonosite, Inc., Bothell, WA, USA) and a curvilinear transducer (3–5 MHz) were used to capture ultrasound image of the LM muscles and needle placement during the needling procedures. Each participant was instructed to lie prone on an examination table with their arms at their sides. A pillow was placed under the participant’s abdomen to minimize the lumbar lordortic curve and an inclinometer was placed on the lumbosacral junction to ensure that the lumbar curve was less than 10° [15].
A 0.30 mm (diameter) × 100 mm (length) needle (Myotech Dry Needles, Red Coral, Australia) was used to needle the LM for all of the participant regardless of the size of the participants. A total of four needles were inserted on the right side of each participant using the two dry needling protocols at the L4 and L5 segments. Each of the four needles was inserted one at a time in order to visualize the needle placement during ultrasound imaging assessment. Universal precautions were followed during the needling procedure, including wearing latex-free gloves and washing hands before and after donning and removing the gloves. When there was a droplet of blood upon removal of needles, a sterile gauze pad was used to stop bleeding. In addition, needle insertion sites were cleaned using alcohol swabs before needling, and the used needles were disposed of in a puncture-resistant sharps container after being removed from the participants.
Two investigators (KE, APS) performed all of the ultrasonographic assessments, but alternated their role during the assessment. One investigator was responsible for the identification of bony landmarks and for needle insertion. Another investigator was responsible for holding a half-circle plastic goniometer (Devore™ Pocket Goniometer, North Coast Medical Inc., Morgan Hill, CA) during needle insertion, and capturing ultrasound images. Prior to data collection, the procedures of ultrasound assessment were standardized. Both investigators went through 20 hours of practice for the needling procedure and the ultrasound assessments under the instruction of the principal investigator (SWP), who had more than 12 years prior experience using ultrasound imaging for the LM muscle. In addition, the principal investigator (SWP) was present during needling to view needle placements for all ultrasound assessments.
The needling procedure was performed in the order of L4 and then L5. First, the spinous processes of the L1 – S2 were identified by palpation and marked with a skin marker. Next, the investigator marked the 4 needle insertion sites: 2.0 cm and 4.0 cm lateral to the L4 and L5 spinous processes, respectively, and then used the half-circle goniometer as a guide for the needling angle. For method 1, the needle was inserted 2.0 cm lateral to the spinous process in a posterior-to-anterior direction with a 20° medial angle toward the spinous process (Figure 1(a)). For method 2, the needle was inserted at 4.0 cm lateral to the spinous process in a posterior-to-anterior direction with a 45°medial angle toward the spinous process (Figure 1(b)).
Figure 1.
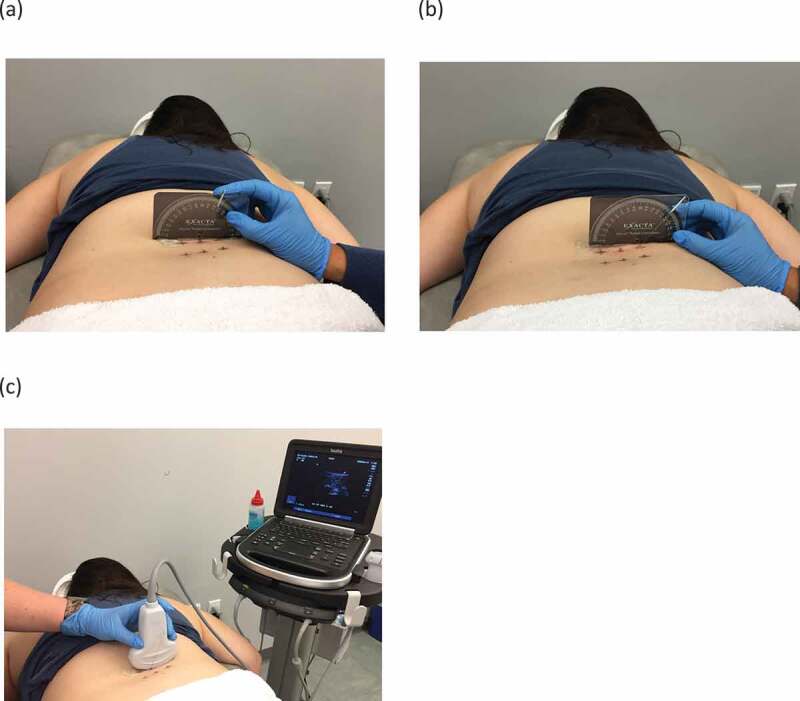
Two methods for needling deep lumbar multifidus muscles: (a) method 1, needle insertion at 2 cm lateral to spinous process in a 20° medial angle direction, (b) method 2, needle insertion at 4 cm lateral to spinous process in a 45° medial angle direction, (c) ultrasound transducer placement.
After the needle pierced the skin, the needle insertion continued until a bony drop was felt or when the entire shaft of the needle was completely subcutaneous. The needling investigator placed the ultrasound transducer cranially or caudally relative to the needle in a transverse direction (Figure 1(c)) so that the spinous process was viewed on the left side of the ultrasound image. The needle was then pulled in and out slightly within the muscle while the investigators observed the needle placement. Once the needle placement was observed, the investigators recorded the following for each of the four needling insertion sites: 1) whether or not a bony drop was felt, 2) whether or not the needle reached the vertebra lamina and which bony landmark that needle reached, and 3) whether or not the needle reached the deep LM muscle. In addition, the unused needle length (i.e. the needle part was not subcutaneous) was measured.
During observation of each needle placement, the non-needling investigator captured a still image and recorded a 3-second ultrasound clip, and then saved them for the offline analysis to determine the intra-tester reliability of the needle placements. The two investigators who were responsible for ultrasonographic assessment were blinded to the results of needle placements in real-time and viewed the saved still images and video clips 6 months later. To limit the investigators’ ability to recall the needle placement results on the ultrasonographic images, 3–4 months between the two assessments were indicated in a previous ultrasonographic study [16]. However, in this study, the second assessments were delayed to 6 months later due to the availability of the investigators. Whether or not the needle reached the vertebra lamina and deep LM, as described earlier, was recorded.
Data analysis
For the primary purpose of the study, four descriptive statistics were calculated: 1) frequency of lack of bony drop, 2) frequency of the needle reaching the lumbar vertebra lamina, 3) frequency of the needle reaching the deep LM muscle, and 4) needle length used to reach a bony drop or used completely. Cohen’s kappa (k) was used to determine the intra-tester reliability of needle placements recorded in real-time and 6 months later. Strength of reliability was interpreted as following: k > 0.80 indicating excellent agreement, k = 0.60–0.80 indicating substantial agreement, k = 0.40–0.60 indicating moderate agreement, and k < 0.40 indicating poor-to-fair agreement.
Results
Twenty-five individuals were screened for eligibility, and five did not meet the BMI criteria. Consequently, 20 participants completed the study: 8 males, 12 females, aged 29.1 ± 8.8 years (range: 23 − 54) with an average BMI of 29.0 ± 5.4 kg/m2 (range: 25.0–45.1). No adverse events from the needling procedure occurred except that 6 participants had a droplet of blood upon needle removal at one needle insertion site each.
Figure 2 displays ultrasound images of various needle placements. Table 1 lists the frequencies of the absence of bony drop, the needle reaching the lumbar vertebra lamina and other bony landmarks, and the needle reaching the deep or superficial LM muscle. Absence of bony drop occurred 5 times from 3 participants, once at L4 when using method 1, once at L5 when using method 1, and 3 times at L5 when using method 2. These 3 participants had a BMI > 30 kg/m2 (31.6–45.1). Only 70–75% of insertions reached the vertebra lamina as intended, but in the remainder of the instances when a bony drop was felt, the needle reached either the spinous process or the junction of lamina and spinous process. The probability that the needled reached the deep LM was high, ranging from 85% to 95%. On average, a needle of 53–67 mm was required to reach a bony drop (see Table 1). Table 2 lists k statistics for both needling methods at the L4 and L5 insertion sites, indicating poor-to-moderate intra-tester reliability of needle placements based on analysis of real-time and recorded US images.
Figure 2.
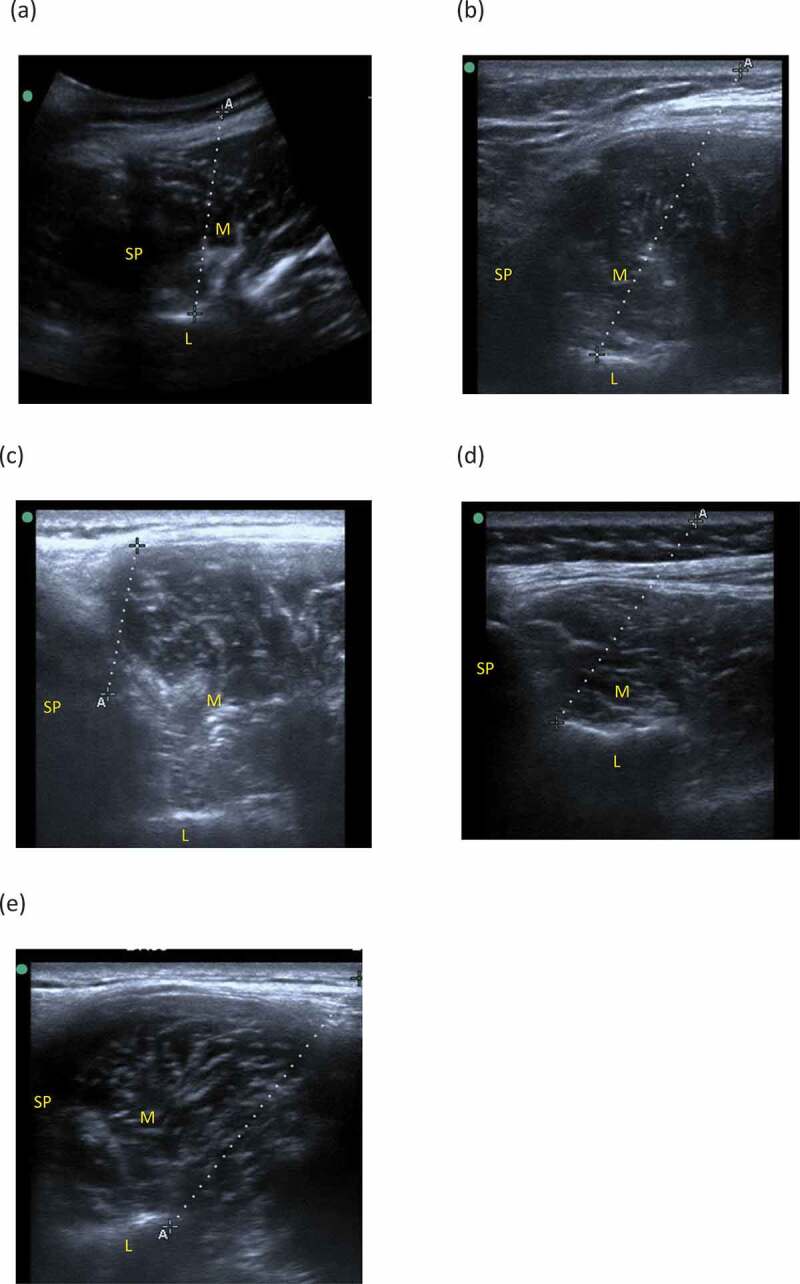
Ultrasound images of needle placement (the dotted line) in the lumbar multifidus muscle (M): (a) needle reached the lumbar lamina (L) using method 1, (b) needle reached the lumbar lamina (L) using method 2, (c) needle reached the spinous process (SP), (d) needle reached the junction of the spinous process (SP) of the lumbar lamina, (e) needle did not reach a bony landmark. ”A” and ”+” indicate the both ends of the subcutaneous part of the needle.
Table 1.
Frequencies of the needle reaching a bony landmark and lumbar multifidus (LM) muscle using ultrasound imaging (n = 20).
| Method 1 (2 cm from Spinous Process, 20° Angle) |
Method 2 (4 cm from Spinous Process, 45° Angle) |
|||
|---|---|---|---|---|
| at L4 | at L5 | at L4 | at L5 | |
| Reaching bony landmark, count (%) | ||||
| Absence of bony drop Lamina Junction of lamina and spinous process Spinous process |
1 (5%) 14 (70%) 3 (15%) 2 (10%) |
0 (0%) 14 (70%) 1 (5%) 5 (25%) |
1 (5%) 14 (70%) 1 (5%) 4 (20%) |
3 (15%) 15 (75%) 2 (10%) 0 (0%) |
| Research lumbar multifidus, count (%) | ||||
| Reaching deep LM Reaching superficial LM Did not reach |
19 (95%) 1 (5%) 0 (0%) |
19 (95%) 1 (5%) 0 (0%) |
17 (85%) 2 (10%) 1 (5%) |
19 (95%) 0 (0%) 1 (5%) |
| Length (mm) of needle subcutaneously, mean ± SD (range) | 53 ± 19 (11–100) |
58 ± 16 (33–100) |
55 ± 18 (24–88) |
67 ± 19 (45–100) |
Table 2.
Intra-rater reliability (kappa statistics ± 95% CI) of needle placements using ultrasound imaging (n = 20).
| |
Method 1 (2 cm from Spinous Process, 20° Angle) |
Method 2 (4 cm from Spinous Process, 450° Angle) |
| Reaching the vertebra lamina | ||
| L4 L5 |
0.571 (0.142, 1.000) 0.286 (−0.163, 0.735) |
0.211 (−0.244, 0.666) -0.091 (−0.248, 0.066) |
| Reaching the deep lumbar multifidus | ||
| L4 L5 |
0.459 (−0.139, 1.057) 0.273 (−0.164, 0.710) |
0.608 (0.114, 1.102) 0.459 (−0.139, 1.057) |
Discussion
Using either of the two common needling methods for the deep LM, the results showed that the occurrence of the needle reaching the LM and a bony drop was high in participants who were overweight (i.e. BMI = 25–30 kg/m2), although the needle may not necessarily have reached the intended bony landmark (i.e. vertebra lamina). In addition, there were five occurrences of absence of bony drop in the three obese participants (BMI > 30 kg/m2). Presently, when ultrasound imaging is not available, clinicians solely rely on a bony drop to determine whether the needle has reached the deep LM. Use of the vertebra lamina for the endpoint of needling the deep LM has been reported consistently in the literature [6,7,17], but none of these studies reported any occurrences of the absence of a bony drop. The vertebra lamina also is used for needling the thoracic multifidus [18] and cervical multifidi [9]. Although severe adverse effects, such as pneumothorax and cervical spinal cord injury have been reported from needling the thoracic and cervical multifidus muscles, serious complications rarely occur from needling the LM, partly because the lumbar lamina is broad and tall. Nevertheless, the specific needle insertion sites and angles used in this study may not apply sufficiently to all patients, as it is impossible to determine each individual’s size and depth of lumbar vertebra. Because using a single insertion angle for all patients may be inadequate for clinical practice, ultrasound imaging may assist clinicians in guiding the needle to the intended lumbar vertebra when needling the deep LM muscle.
Although the needle did not consistently reach the intended bony landmarks, the chance that the needle reached the LM was high. Therefore, reaching a bony drop is still meaningful, as it coincided with reaching the LM in the majority of participants. There were six occurrences out of a total of 80 needle insertions (from L4 and L5 of 20 participants using both of the two methods) of the needle not reaching the deep LM. Of these occurrences, the needle reached the superficial LM when the needle was stopped prematurely by the spinous process. This could have been due to anatomical variations of lumbar vertebra (e.g. shape of spinous process) or the investigator might have tilted the needle more than the specified angle during needle insertion. The other two occurrences in which the needle did not reach the deep LM because of insufficient needle length in two large participants with a concurrent absence of bony drop. Therefore, the use of a 50 mm or 60 mm long needle which has been recommended in common DN methods for deep LM may not be adequate, particularly for obese participants. Ultrasound-guided DN may be helpful when needling the deep LM in patients with a high BMI (>30 kg/m2).
The k statistics showed fair-to-moderate intra-tester reliability of ultrasonographic assessment of needle placements for the deep LM. The intra-tester reliability was poor-to-moderate when using ultrasound imaging to assess whether the needle reached the lumbar lamina. The inconsistency could be primarily due to the investigators’ inability to identify needle placement when viewing the still images and video clips. This inability to locate the needle accurately when viewing the recorded images was unlikely due to the quality of ultrasound images, but could have been a result of the investigator’s lack of perceptivity of the position of the hand and the needle in real time. In addition, the inability to distinguish between the lamina and the junction of the spinous process and the lamina could have further affected the inter-tester reliability. It is also possible that ultrasound images and video clips themselves may not provide an adequate visibility of the needle due to the small caliber size (0.30 mm in diameter) of the needle used in this study [19]. Nevertheless, to our knowledge, this study was the first to attempt to establish the intra-tester reliability of needle placement using saved ultrasound images and clips.
There were limitations of the study. Although a goniometer was used to guide needling angles, the needling angle could have changed after piercing through the skin due to a slight bend of the needle. The slight deviation of angle may have affected the needle path and the final placement. In addition, the ultrasound imaging used in this study only captures a 2-D image and the entire needle path may not be fully visualized in some occasions, and this could have impacted the reliability results. However, a 2-D ultrasound scanner is a feasible and affordable modality for clinicians as compared to 3-D ultrasound scanner [20] when verification of needle placement is necessitated. In addition, a slight muscle contraction could have occurred during ultrasound imaging and could have changed the needle path and needle placement. When muscle contraction was observed, the investigators would ask the participants to relax and re-image the needle placement. Lastly, the investigator who inserted the needle also interpreted the results of the ultrasound images. These non-independent assessments may be considered a threat to the validity of this study. However, due to the small size of the needle, it was important to move the needle to visualize the needle placement during the interpretation of ultrasound images.
Conclusion
In conclusion, the results of the study showed that the two most common protocols for needling deep LM are valid, although the bony drop may not always indicate the needle reaching the intended lumbar lamina. Ultrasound imaging may assist clinicians in guiding the needle to the intended anatomical targets, particularly for individuals with high BMI and for accommodating anatomical variations in the lumbar spine.
Biographies
SWP: Conceiving and designing the study, obtaining IRB approval, recruiting/enrolling participants, analyzing and interpreting the data, writing the manuscript in whole. KE and PS: Designing the study, recruiting/enrolling participants, collecting the data, analyzing the data, assisting in writing the methods section. KB: Designing the study, analyzing and interpreting the data, writing portions of the manuscript. JZ: Conceiving and designing the study, analyzing and interpreting the data, writing portions of the manuscript.
Sharon S. Wang-Price PT, PhD, OCS, FAAOMPT, is a Professor and serves as core faculty and the Coordinator of Professional Studies for the Doctor of Physical Therapy Program and PhD Program at Texas Woman’s University - Dallas. She completed her BS in Physical Therapy from National Taiwan University, MS in Health Related Professions, Sports Physical Therapy from University of Pittsburgh, and her PhD in Physical Therapy from Texas Woman’s University - Houston. She is a Board-certified Clinical Specialist in Orthopedic Physical Therapy and a Fellow in the American Academy of Orthopaedic Manual Physical Therapists. She received her manual therapy training with the North American Institute of Orthopedic Manual Therapy. Dr. Wang-Price has been a faculty member at TWU for 21 years and has 40+ peer-reviewed publications, over 70 presentations in musculoskeletal physical therapy related topics nationally and internationally. She received the 2021 AAOMPT Cardon Research Award for a research project in dry needling.
Kristen N. Etibo PT, DPT, currently is an orthopedic physical therapy resident at UT Southwestern Medical Center in Dallas, TX. She completed her BS in Pre-Health from University of Texas at Dallas, and her Doctor of Physical therapy from Texas Woman’s University – Dallas. This project was completed to fulfill her Critical Inquiry Project requirement for her DPT degree.
Alicia P. Short PT, DPT, currently is a physical therapist practicing at Therapy Partners of North Texas in Haslet, TX. She completed her BS in Kinesiology from Texas Woman’s University - Denton, and her Doctor of Physical therapy from Texas Woman’s University – Dallas. This project was completed to fulfill her Critical Inquiry Project requirement for her DPT degree.
Kelli Brizzolara PT, PhD, OCS, is an Associate Professor and serves as core faculty for the Doctor of Physical Therapy Program and PhD Program at Texas Woman’s University - Dallas. She completed her BS in Exercise Physiology from Texas A& M University, MS in Physical Therapy from Texas Woman’s University – Houston, and her PhD in Physical Therapy from Texas Woman’s University - Dallas. She is a Board Certified Board-certified Clinical Specialist in Orthopedic Physical Therapy. Dr. Brizzolara has been a faculty member at TWU for 8 years and has 15 peer-reviewed publications, over 25 presentations in musculoskeletal physical therapy related topics nationally and internationally.
Jason Zafereo PT PhD, OCS, FAAOMPT, is an Associate Professor and serves as core faculty and the Research Coordinator for the Doctor of Physical Therapy Program at UT Southwestern Medical Center. He completed his BA in Biology from Baylor University, Master of Physical Therapy from UT Southwestern Medical Center, and his PhD in Physical Therapy from Texas Woman’s University - Dallas. He is a Board-certified Clinical Specialist in Orthopedic Physical Therapy and a Fellow in the American Academy of Orthopaedic Manual Physical Therapists. He received his manual therapy training with the Manual Therapy Institute. Dr. Zafereo has been a faculty member at UTSW Medical Center for 14 years and has 22 peer-reviewed publications and over 20 presentations in musculoskeletal physical therapy related topics nationally and internationally. He was recently recognized as a Distinguished Teaching Professor by the Southwestern Academy of Teachers.
Funding Statement
The data that supports the findings of this study are available on request from the corresponding author, [SWP]. The data is not publicly available due to it containing information that could compromise the privacy of research participants.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Moseley GL, Hodges PW, Gandevia SC.. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine (Phila Pa 1976). 2002;27(2):E29–36. [DOI] [PubMed] [Google Scholar]
- [2].Wallwork TL, Stanton WR, Freke M, et al. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man Ther. 2009;14(5):496–500. [DOI] [PubMed] [Google Scholar]
- [3].Zhang S, Xu Y, Han X, et al. Functional and morphological changes in the deep lumbar multifidus using electromyography and ultrasound. Sci Rep. 2018;8(1):6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hu HT, Gao H, Ma RJ, et al. Is dry needling effective for low back pain?: a systematic review and PRISMA-compliant meta-analysis. Medicine (Baltimore). 2018;97(26):e11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Téllez-García M, De-la-llave-rincón AI, Salom-Moreno J, et al. Neuroscience education in addition to trigger point dry needling for the management of patients with mechanical chronic low back pain: a preliminary clinical trial. J Bodyw Mov Ther. 2015;19(3):464–472. [DOI] [PubMed] [Google Scholar]
- [6].Dar G, Hicks GE. The immediate effect of dry needling on multifidus muscles’ function in healthy individuals. J Back Musculoskelet Rehabil. 2016. Apr 27;29(2):273–278. [DOI] [PubMed] [Google Scholar]
- [7].Koppenhaver SL, Walker MJ, Smith RW, et al. Baseline examination factors associated with clinical improvement after dry needling in individuals with low back pain. J Orthop Sports Phys Ther. 2015;45(8):604–612. [DOI] [PubMed] [Google Scholar]
- [8].Okubo Y, Kaneoka K, Imai A, et al. Electromyographic analysis of transversus abdominis and lumbar multifidus using wire electrodes during lumbar stabilization exercises. J Orthop Sports Phys Ther. 2010;40(11):743–750. [DOI] [PubMed] [Google Scholar]
- [9].Fernández-de-las-peñas C, Mesa-Jiménez JA, Paredes-Mancilla JA, et al. Cadaveric and ultrasonographic validation of needling placement in the cervical multifidus muscle. J Manipulative Physiol Ther. 2017;40(5):365–370. [DOI] [PubMed] [Google Scholar]
- [10].Agten A, Verbrugghe J, Stevens S, et al. Feasibility, accuracy and safety of a percutaneous fine-needle biopsy technique to obtain qualitative muscle samples of the lumbar multifidus and erector spinae muscle in persons with low back pain. J Anat. 2018;233(4):542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Settergren R. Treatment of supraspinatus tendinopathy with ultrasound guided dry needling. J Chiropr Med. 2013;12(1):26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fusco P, Di Carlo S, Scimia P, et al. Ultrasound-guided dry needling treatment of myofascial trigger points for piriformis syndrome management: a case series. J Chiropr Med. 2018;17(3):198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tabatabaiee A, Takamjani IE, Sarrafzadeh J, et al. Ultrasound-guided dry needling decreases pain in patients with piriformis syndrome. Muscle Nerve. 2019;60(5):558–565. [DOI] [PubMed] [Google Scholar]
- [14].Zheng Y, Shi D, Wu X, et al. Ultrasound-guided miniscalpel-needle release versus dry needling for chronic neck pain: a randomized controlled trial. Evid Based Complement Alternat Med. 2014;2014:235817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kiesel KB, Uhl TL, Underwood FB, et al. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man Ther. 2007;12(2):161–166. [DOI] [PubMed] [Google Scholar]
- [16].Koski JM, Saarakkala S, Helle M, et al. Assessing the intra- and inter-reader reliability of dynamic ultrasound images in power Doppler ultrasonography. Ann Rheum Dis. 2006;65(12):1658–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Puentedura EJ, Buckingham SJ, Morton D, et al. Immediate changes in resting and contracted thickness of transversus abdominis after dry needling of lumbar multifidus in healthy participants: a randomized controlled crossover trial. J Manipulative Physiol Ther. 2017;40(8):615–623. [DOI] [PubMed] [Google Scholar]
- [18].Rock JM, Rainey CE. Treatment of nonspecific thoracic spine pain with trigger point dry needling and intramuscular electrical stimulation: a case series. Int J Sports Phys Ther. 2014;9(5):699–711. [PMC free article] [PubMed] [Google Scholar]
- [19].de Jong TL, van de Berg NJ, Tas L, et al. Needle placement errors: do we need steerable needles in interventional radiology? Med Devices. 2018;11:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu S, Kruecker J, Jiang H, et al. 3D ultrasound guidance system for needle placement procedures. proceedings volume 6918, medical imaging 2008: visualization, image-guided procedures, and modeling. 2008;69180H (17 March 2008). 10.1117/12.769853 [DOI] [Google Scholar]


