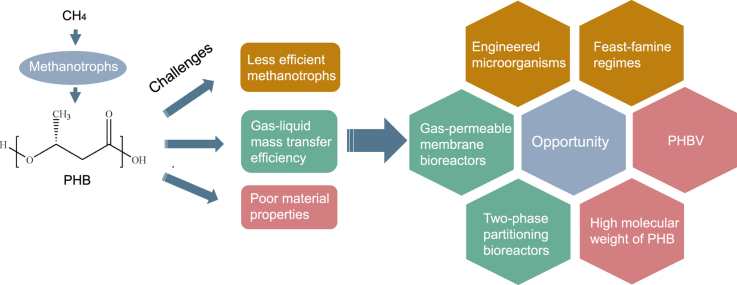
Abstract
Methane emissions and plastic pollution are critical global challenges. The biological conversion of methane to poly-β-hydroxybutyrate (PHB) not only mitigates methane emissions but also provides biodegradable polymer substitutes for petroleum-based materials used in plastics production. This work provides an early overview of the methane-based PHB advances and discusses challenges and related strategies. Recent advances of PHB, including PHB biosynthetic pathways, methanotrophs, bioreactors, and the performances of PHB materials are introduced. Major challenges of methane-based PHB production are discussed in detail; these include low efficiency of methanotrophs, low gas-liquid mass transfer efficiency, and poor material properties. To overcome these limitations, various approaches are also explored, such as feast-famine regimes, engineered microorganisms, gas-permeable membrane bioreactors, two-phase partitioning bioreactors, poly-β-hydroxybutyrate-co-hydroxyvalerate synthesis, and molecular weight manipulation.
Keywords: Polyhydroxyalkanoate, Methanotrophs, Methane, Biodegradable plastics, Greenhouse gas
Graphical abstract
Highlights
-
•
Methane-based PHB has the potential to mitigate methane emission and replace petroleum-based plastics.
-
•
Feast-famine strategy and engineered microorganisms are beneficial to obtain high-efficiency methanotrophs.
-
•
Gas permeable membrane bioreactor and two-phase partitioning bioreactor will be help for enhancing gas-liquid transfer rates.
-
•
High-performance PHB can be achieved by generating PHBV and manipulating molecular weight of PHB.
1. Introduction
Plastics have become indispensable in our daily lives and are widely used in packing materials, household appliances, transportation equipment, and electronic devices [1]. The estimated annual plastics production is 311 million tons [2], and is predicted to surpass 500 million metric tons by 2050 [3]. Plastics are non-degradable, which is the primary disadvantage [4] and has led to the accumulation of plastics in many environments [5]. Further, only a small portion of plastics can be effectively recycled. The cumulative quantity of plastic waste entering the oceans has been estimated at up to 12.7 million metric tons and is predicted to increase by an order of magnitude by 2025 in the absence of waste-management infrastructure enhancements [6]. More importantly, microplastics transfer pollutants via food chains [7] and have negative effects on human health due to their small size and wide distribution in water bodies [7,8]. Moreover, conventional plastics generate two greenhouse gases (GHGs), methane and ethylene, when exposed to ambient solar radiation [9]. Methane is the second-most prevalent GHG after carbon dioxide (CO2), contributing 18% of the total atmospheric radiation forcing [10]. The global warming potential of methane has increased from 25 to 34 times that of CO2 over the past 100 years [11], indicating that methane emissions have a significant environmental impact [12]. More than 63% of global methane emissions are anthropogenic [13] and are generated from fossil fuels, anaerobic wastewater treatment, landfilling, coal mining, and natural gas refineries [14]. Moreover, methane accounts for 90% of natural gas, worldwide demand for which is estimated to increase by 44.0% by 2040 [15,16].
Polyhydroxyalkanoates (PHAs) are a kind of polyester produced by microorganisms [17] with potential applications as a substitute material for conventional plastics [18]. Methanotrophs could accumulate PHB when their nutrient supply is imbalanced [19], and the theoretical yield of PHB synthesis is 67% [20]. Recently, based on its properties of biodegradability and biocompatibility [21,22], demand for PHB products has expanded from its initial applications in packaging materials [22,23] to industrial and agricultural applications [24] and to the biomedical and pharmaceutical sectors [5,25]. Employing PHB into commodities does not require new technological investments, as existing equipment originally developed for processing polyethylene and polypropylene can be used [26]. However, the high production costs of PHB, which are 3–4 times higher than those of conventional plastics and similar biopolymer plastics, limits its commercial utilization [[27], [28], [29]]. A major proportion of the total costs of PHB (30–40%) is attributed to feedstock [27,30], such as palm oil, glucose, sucrose, and corn starch [19,31,32]. The utilization of methane could reduce production costs by at least 30–35% and make the PHB production process more economically and environmentally friendly [33,34]. A study of the economic feasibility of methane-based PHB production showed that the biosynthesis cost was $8.5/kg PHB when produced at a relatively small scale (500 tons/a) [34]. If the scale of production would be expanded to 100,000 tons/a, costs would be reduced to $4.1–$6.8/kg PHB [33]. Methane emissions from landfills and anaerobic digesters could be used to synthesize PHB, which could theoretically replace 20–30% of the total annual plastics market [35,36]. Therefore, the introduction of methane-based PHB not only opens up a new path for PHB production, but also mitigates the issues related to non-degradable plastics and GHG emissions.
As methane-based PHB has economic and environmental advantages over petroleum-based plastics, this review explores PHB biosynthetic pathways, methanotrophs, bioreactors, and material performance of PHB with an emphasis on overcoming the challenges such as low efficiency of methanotrophs, low gas-liquid mass transfer efficiency, and poor material properties and strategies to overcome them. Further, development opportunities, including feast-famine regimes, engineered microorganisms, gas-permeable membrane bioreactors, two-phase partitioning bioreactors, poly-β-hydroxybutyrate-co-hydroxyvalerate, and high molecular weight of PHB are outlooked.
2. PHB biosynthetic pathway
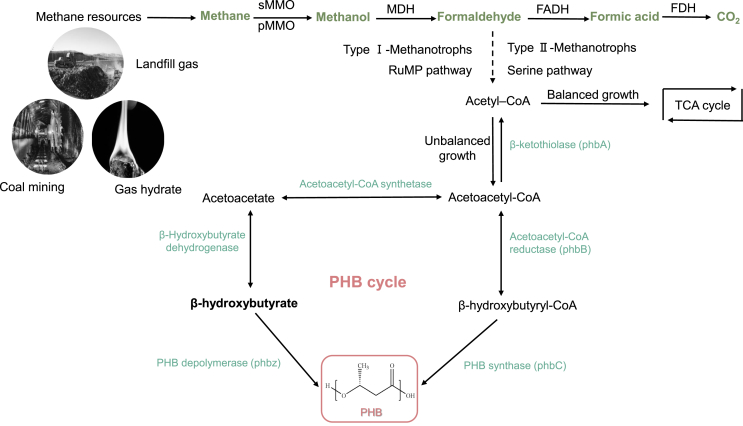
The oxidation of methane to methanol is catalyzed by methane monooxygenase (MMO) enzymes in both particulate and soluble forms (pMMO and sMMO, respectively) (Fig. 1) [39,40]. Methanol is oxidized to formaldehyde, and formaldehyde can either be converted into biomass or further oxidized to formic acid and CO2 [39]. Throughout these oxidation steps, cellular carbon and the energy required to continuously uptake methane are obtained [39].
Fig. 1.
Methane metabolism in methanotrophs. Adapted from Ref. [37,38].
Furthermore, PHB synthesis occurs in three sequential enzymatic steps [41]: two acetyl-CoA molecules condense in the presence of the enzyme β-ketothiolase (phbA) to form acetyl-CoA acetyl-CoA, which is then reduced by nicotinamide adenine dinucleotide (NADH)-dependent acetoacetyl-CoA reductase (phbB) [42]; finally, the polymerization of monomers into PHB polymers is catalyzed by PHB synthase (phbC) [43]. Serine is metabolized to acetyl-CoA, which is directed to the tricarboxylic acid cycle (TCA) cycle under nutrient-sufficient conditions or the PHB cycle under nutrient-deficient conditions [41]. Methane accumulates in the form of PHB by type II methanotrophs via the serine pathway, but ribulose monophosphate methanotrophs may be incapable of PHB synthesis. Type II genera were found to sequence phbC and accumulate PHB, whereas in type I genera, no phbC or measurable PHB was detected [44].
3. Methanotrophs for PHB production
3.1. Methanotrophs
Methanotrophs are classified into two major groups: Group I (α-Proteobacteria; referred to as Type II) [40] and Group II (γ-Proteobacteria; referred to as Type I and Type X) [45]. Type II methanotrophs are the most effective PHB producers and utilize the serine pathway [46]. Isolating efficient methanotrophs with high cell concentrations [20], rapid growth [47], and high PHB content [48] is crucial for improving PHB yields and reducing PHB production costs. Methanotrophic strains utilized in PHB synthesis mainly include Methylosinus trichosporium OB3b [49], Methylosinus trichosporium IMV3011 [38], and Methylocystis hirsute [46]. These methanotrophs are mainly sourced from the Culture Collections and contain PHB contents of 30%, 40%, and 42.5%, respectively. The CZ-2 was identified as Methylobacterium organophilum and PHB accumulation in the consortium and the isolate in a bioreactor reached 34% and 38% (w/w), respectively [50]. Notably, nitrogen limitation promoted PHB accumulation by up to 57% (w/w) in bioreactors [50]. Furthermore, a high-throughput screening technique combined with a Nile Red flow-cytometric assay dramatically reduced handling time for each experiment in the PHB accumulation studies, creating an integrated system to optimize the methanotrophic consortium [51]. Optimization of calcium and cooper levels significantly enhanced PHB production to 49.4% in pure cultures of Methylocystis parvus OBBP while decreasing doubling time from 10.6 h to 8.6 h [51]. These results indicate that the directional isolation of methanotrophs could promote higher PHB production capacities and that highly efficient methanotrophs are very scarce. To gain better insight into PHB processes, future investigations should attempt to isolate highly efficient methanotrophs, and pure-culture fundamental studies should be conducted.
3.2. Isolation of highly efficient methanotrophs
Feast-famine strategies have been proven to allow the most effective selection of PHB-accumulating organisms [52]. Providing sufficient carbon sources and nutrients during the feast stage is conducive to the rapid consumption of substrate and accelerates biomass growth, while the growth of biomass is limited by restricting the supply of nutrients in the famine stage, thus inducing carbon storage in the form of PHB [53,54]. During a longer famine stage, non-target microorganisms were selectively died out due to the specific culture conditions for methanotrophs [55,56]. Further, PHB storage capacity and PHB storage rates can be increased by repeated feast-famine cycles in a mixed microbial culture [57,58]; for example, a community of acetate-fed microorganisms rapidly produced up to 89% (w/w) PHA content under these conditions [58]. During the famine phase, PHB yields and PHB concentrations reached 30% and 0.6 g/L, respectively [38]. Adding 0.30 g/L citric acid as an inhibitor of the TCA cycle caused PHB content and concentrations to reach ∼40% and 0.26 g/L, respectively [38]. After combining high-throughput screening techniques with a Nile Red assay and optimizing calcium and copper concentrations, the PHB content increased from 18.1% to 49.4% by using Methylocystis parvus OBBP [51]. The combination of high-throughput screening technology and the feast-famine strategy may significantly improve the efficiency of isolating high-producing methanotrophs.
3.3. Engineered microorganisms
Methanotrophs could generate various potential target products through genetic engineering; for instance, Methylomicrobium buryatense has been genetically engineered to produce C-4 carboxylic acid [59], lactic acid [60] and 2,3-butanediol [61], demonstrating the potential of methanotrophs to produce methane-based PHB via this approach. Through the metabolic modification of microorganisms, regulation of gene expressions in metabolic pathways, and adjustment of cellular metabolic networks, PHB yields could be increased [62]. The overexpression of PHB synthesis pathways involving three genes (phbA, phbB, and phbC) could achieve increased PHB accumulation [63]. Thus, the simultaneous adjustment of the expression of three enzymes could directly optimize the accumulation rates, molecular weight, and performance of PHB [63]. Notably, PHB biosynthesis is actively connected to core metabolic networks and methods to improve metabolic flux in PHB synthesis have been extensively studied [48,64,65], including oxygen limitation [66,67], overexpression of NADH synthesis enzymes [68], substrate limitation [69], and overexpression of PHB synthesis operons [70]. PHB biosynthesis competes with several alternative metabolites and intermediates, making it vital to attenuate competitive pathways for PHB synthesis pathways [62] via, for example, reducing acetyl-CoA consumption during the TCA cycle [71] or preventing β-oxidation [72,73]. Meanwhile, the clustered regularly interspaced short palindromic repeats interference (CRISPRi) has been employed in PHA biosynthesis to induce direct metabolic flux [74,75]. The phbC activity can be regulated by designing CRISPRi binding sites using various sgRNA [62,74]. Interestingly, phbC activity was found to influence PHB content and molecular weights in direct and reverse proportions, respectively [76], allowing PHB contents to be controlled at 1.47–75.21% (w/w%) levels and molecular weights at 2–6 million Dalton (MDa) [74]. These results suggest the expanding potential of manipulating and optimizing methanotrophic PHB synthesis by engineered microorganisms.
4. Bioreactor configuration and operation
4.1. Bioreactors
Methane-based PHB production faces unique challenges due to the low solubility of methane and oxygen in aqueous solutions (22 mg/L and 9 mg/L at 20 °C and 1 atm, respectively) [77]. Low gas-liquid mass transfer rates result in low cell density and PHB synthesis rates, greatly limiting the large-scale production of PHB by methanotrophs [78]. [79] used pressure bioreactors to increase the mass transfer efficiency, and PHB content reached 51% [20]. reduced the liquid medium from 0.5 L to 0.1 L and enhanced the cell density of methanotrophs from 1.4 g/L to 5.0 g/L. Although high pressure would significantly increase the solubility of methane, creating such conditions can be expensive and detrimental for microorganisms [80]. In another study, vigorous agitation reduced the size of bubbles and achieved a large gas-liquid volumetric mass transfer coefficient (kLa) of 102.9 h−1 [77], but it increased the power consumption of the operation impeller and led to high shear stress that resulted in cell damage [81]. Biosynthesis of PHB by Methylocystis hirsuta from natural gas using bubble column bioreactors (BCBs) and vertical tubular loop bioreactors, resulted in PHB contents of 42.5% and 51.6%, respectively [46]. Under optimum conditions, the high cell density cultivation of Methylocystis hirsuta using natural gas in a BCB lead to PHB accumulation of up to 73.4% [82]. A 25% w/w PHB content was obtained under optimized operating conditions and engineering parameters in a BCB [83]. Suspended-growth BCBs can overcome mass transfer limitations through the use of ultrafine bubble diffusers with micropores size <0.5 μm [84].
4.2. Optimization of bioreactor design
4.2.1. Gas-permeable bioreactors
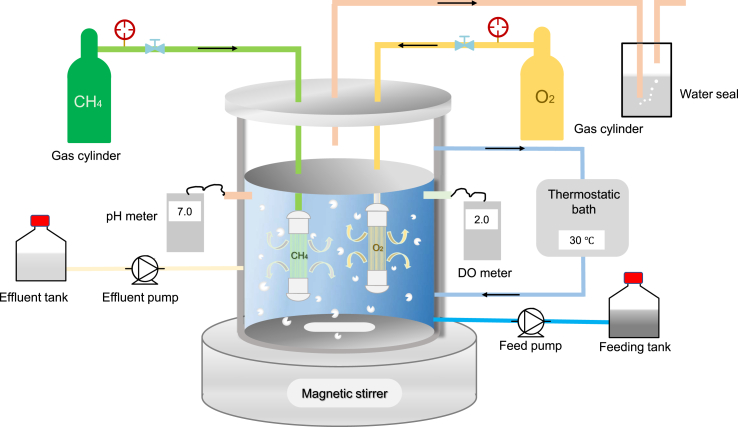
The low solubility of methane and oxygen limits the production efficiency of methane-based PHB to some extent. A novel membrane biofilm reactor application could deliver gaseous substrates (e.g. methane and oxygen) to microorganisms [85,86], similar to a H2-based membrane biofilm reactor. Gas-permeable hollow fibers could be used to efficiently deliver methane, which would enable the gas diffusion free of bubbling [87]. The nitrate removal rate was increased to ∼700 mg N/L/d when methane was employed as an electron donor through gas-permeable hollow fiber membranes [88,89]. Furthermore, hollow fiber membrane bioreactors are considered as an effective method to promote methane utilization, as they shorten the methane transfer pathway [90] and mitigate limitations associated with methane transfer [[91], [92], [93]]. Therefore, it seems feasible that the efficiency of the gas-liquid mass transfer of methane and oxygen could be significantly improved via gas permeable bioreactors [94]. used hollow fiber membranes to achieve a high kLa of methane (150.1 h−1) and cell growth rate for Methylosinus trichosporium OB3b was increased by 67.3%. Our laboratory has designed a double-membrane aerobic methanotrophs-culture device (ZL201821323775.2), shown in Fig. 2. The small pore size and high contact area of membranes would significantly enhance the mass transfer rate of methane and oxygen. Based on their high gas transfer efficiency and homogeneous diffusion, gas permeable membranes appear to be an advantageous option [95]. However, gas-permeable membranes also have certain drawbacks, such as membrane fouling and membrane resistance to mass transfer [96]. Apart from their physical characteristics, gas-permeable membrane bioreactors have potential to allow a safe and efficient gas-supply technology and establish an efficient PHB-synthesis process.
Fig. 2.
Schematic of a gas permeable membrane bioreactor.
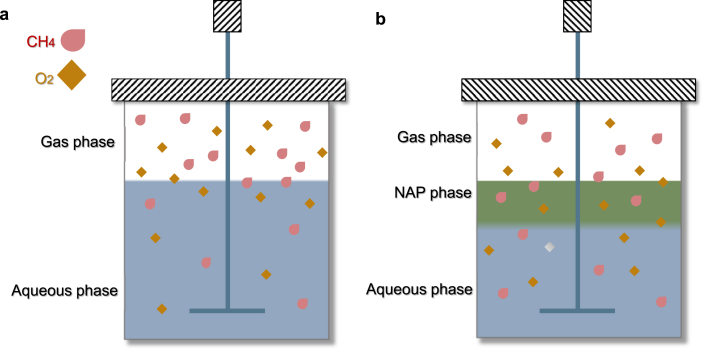
4.3. Two-phase partitioning bioreactors
Based on the addition of a non-aqueous phase (NAP) with a high affinity for poorly water-soluble compounds (e.g., methane and oxygen) [97,98], two-phase partitioning bioreactors (TPPBs) have a higher potential to drive mass transfer [99] (Fig. 3). Furthermore, most NAPs used in TPPBs have shown a high affinity for oxygen; thus, the mass transfer rate of oxygen is also improved [100,101]. The methane conversion efficiency can be artificially improved by adding a methane vector such as silicone oil or paraffin oil. Methane average volumetric elimination capacity increased by 131% up to 51 g m−3 h−1 with added 10% (v/v) silicone oil in TPPBs [102]. Under nitrogen-limited conditions, the CZ-2 achieved a PHB accumulation of 57% in TPPBs [50]. After the addition of 1-propanol, the cell growth rate and the maximum optical density of Methylosinus trichosporium OB3b enhanced by 700% and 730%, respectively [103]. TPPBs are also particularly suitable for the treatment of enhancing the kLa of methane and oxygen. The long-term stability of TPPBs is vital, because the NAP may be degraded or lost during continuous operation [104]. Moreover, the feasibility of the use of silicone oil as a NAP in TPPBs is limited due to its expense. However, the cost of industrial-grade silicone oil is ∼55 times lower than that of high-purity silicone oil [101], and the efficacy of industrial-grade silicone oil should be experimentally assessed. Further, electrolytes (e.g., KCl, NaCl, K2SO4, Na2SO4) can suppress the coalescence of methane bubbles, increasing the gas-liquid kLa of methane from 103 h−1 to 711 h−1 [78].
Fig. 3.
Mass transfer models in (a) conventional systems (b) two-phase partitioning bioreactors. NAP: non-aqueous phase.
5. Strategies for enhancing PHB-material performances
Current PHB applications are restricted due to PHB’s narrow melt processing window, thermal instability, and brittleness [105]. PHB quality has a decisive impact on the mechanical performance and processability of subsequent products [76]. Manipulation of the molecular weight (Mw) and the synthesis of poly-β-hydroxybutyrate-co-hydroxyvalerate (PHBV) seems to be effective methods to improve the material performance and explore its potential applications [25].
5.1. Manipulation of high molecular weight PHB
The Mw of PHB determines its thermodynamic properties and crystallization characteristics, which strongly influence its mechanical properties and processability [106]. Increasing the Mw of PHB can promote β-form crystallization, and in turn, enhance the mechanical properties of PHB [107]. The Mw of PHB (105–106 Da) depends on the PHB’s cultivation conditions and the host microorganism. Normal PHB is characterized by brittleness and a secondary crystallization phenomenon [108], while the tensile strength of PHB with an ultra-high Mw can reach 1320 MPa, which is higher than petroleum-based products [109]. Strategies for high Mw of PHB production are summarized in Table 1.
Table 1.
Strategies for high molecular weight PHB production.
| Microorganisms | Mw (MDa) | Reactor Type | Technology | PHB (%) | YPHB/CH4 (g g−1) | References |
|---|---|---|---|---|---|---|
| Methylocystic sp. GB 25 DSM 7674 | 3.10 | Pressure bioreactor | Potassium deficiency | 33.60 | 0.45 | [105] |
| Methylocystic sp. GB 25 DSM 7674 | 2.46 | Pressure bioreactor | Sulfur deficiency | 32.60 | 0.40 | [105] |
| Methylocystic sp. GB 25 DSM 7674 | 1.81 | Pressure bioreactor | Iron deficiency | 10.40 | 0.22 | [105] |
| Methylosinus trichospporium IMV3011 | 1.50 | Shake flasks | Citric acid +Methanol |
40.00 | – | [38] |
| Methylocystis sp. GB25 DSMZ 7674 | 2.50 | Pressure bioreactor | Ammonium deficiency | 51.30 | 0.52 | [110] |
| Methylocystis sp. GB25 DSMZ 7674 | 2.50 | Pressure bioreactor | Phosphorus deficiency | 46.80 | 0.55 | [110] |
| Methylocystis sp. GB25 DSMZ 7674 | 2.50 | Pressure bioreactor | Magnesium deficiency | 28.30 | 0.37 | [110] |
| Methylocystis sp. GB25 DSM 7674 | 2.30 | Pressure bioreactor | Nitrogen deficiency | 51.00 | 0.54 | [111] |
Under potassium-limited conditions, the production of PHB with an Mw up to 3.1 MDa was accumulated [105]. When sulfur or iron was limited, the Mw of PHB decreased by 20.6% and 41.6%, respectively [105], indicating PHB synthesis is hampered under iron-deficient conditions. The addition of citric acid and methanol (0.1%) increased PHB yields from 12% to 40% and increased PHB Mw up to 1.5 MDa [38]. The expression level or activity of phbC [76,112] and depolymerase [76] and the effectiveness of the chain transfer reactions [113] could also affect the Mw of PHB. To change the expression of PHB synthase, a rationally designed library of ribosomal binding sites with defined strengths for the PHB synthesis operon of phbA, phbB, and phbC were constructed in high or low copy-number plasmids in E. coli. High-quality PHA (Mw: 2.7–6.8 MDa) could be efficiently produced through the combination of a one-step library and proper screening [63].
5.2. PHBV synthesis
One strategy to enhance the processability of PHB is the introduction of 3-hydroxyvalerate (3HV) units into PHB polymers to produce PHBV. The melting point of PHBV lowers as the proportion of 3HV monomers increases, leading to a broader thermal processing window [114,115] and improved polymer ductility and flexibility [116]. Hence, PHBV has attracted increasing attention for integrated biomedical applications [117] and food-packing applications [118] due to its improved resilience, flexibility, and strength. PHBV synthesis strategies are summarized in Table 2.
Table 2.
Strategies for PHBV synthesis using methane as carbon substrate.
| Microorganism | HV (mol%) | PHAs content (w%) | Co-substrate | References |
|---|---|---|---|---|
| Methylocystis parvus OBBP | 22 | 54 | Valerate (100 mg/L) | [119] |
| Methylocystis parvus OBBP | 37 | – | Valerate (400 mg/L) | [119] |
| Methylocystis parvus OBBP | 8 | 32 | Propionate (100 mg/L) | [119] |
| Methylocystis sp. WRRC1 | 60 | 78 | Valerate | [120] |
| Methylocystis-dominated | 18–22 | 43–45 | Valerate (100 mg/L) | [115] |
| Methylocystis-dominated | 37–40 | 27–32 | Valerate (400 mg/L) | [115] |
| Methylobacterium organophilum CZ-2 | 35 | 82 | Citrate | [121] |
| Methylobacterium organophilum CZ-2 | 25 | 68 | Propionate | [121] |
As the HV content increased to 40%, the melting temperature of PHBV significantly decreased from 180 °C to 75 °C [122]. Only PHB, not PHBV, was synthesized when methane was used as the sole carbon substrate by Methylocystis-enriched bacteria [119]. PHBV was synthesized and the mol% of 3HV was increased with added valerate [115]. Adding propionate and valerate can modify the mol% 3HV composition, where valerate addition leads to higher PHB content and mol% 3HV [119]. High HV content (25–35%) was obtained in methane-propionate experiments by the CZ-2 [121]. The copolymer had a 3HB:3HV:3HO ratio of 55:35:10 when the feedstock consisted of citrate plus methane, and had an HB: HV ratio of 75:25 with propionate feeding plus methane [121]. Tailored PHB-copolymer composition could be generated through the addition of different co-substrates [115]. Under cobalt-deficient conditions, Methylobacterium extorquens AM1 was able to synthesize PHBV from methanol alone [123]. Although methanotrophs can produce PHBV, it is still necessary to provide co-substrates and carbon source supplements, which increases the cost of PHBV production.
6. Conclusions
Methane-based PHB production has an excellent potential to benefit the environment as a substitute for petroleum-based materials and to reduce GHG emissions. This review provided an analysis of PHB biosynthetic pathways and identified the challenges and opportunities associated with methanotrophic PHB production. Obtaining high-efficiency methanotrophs through a feast-famine strategy and microorganism engineering, coupled with gas-permeable membrane bioreactors and two-phase partitioning bioreactors could help enhance gas-liquid transfer efficiency. Further, manipulating the molecular weight of PHB and generating PHBV are necessary to achieve high-quality products. Further investigations of methane-based PHB would help support the complex needs of the bioplastics market.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Acknowledgements
The authors would like to thank the Natural Science Foundation of China (grant no. 51778173 and 51808167), Fundamental Research Funds for the Central Universities, the Fok Ying Tung Education Foundation, and State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (2018DX05) for supporting this study.
References
- 1.Reddy M.V., Mawatari Y., Onodera R., Nakamura Y., Yajima Y., Chang Y.C. Bacterial conversion of waste into polyhydroxybutyrate (PHB): a new approach of bio-circular economy for treating waste and energy generation. Bioresource Technology Reports. 2019;7:100246. [Google Scholar]
- 2.Bornscheuer U.T. Feeding on plastic. Science. 2016;351(6278):1154–1155. doi: 10.1126/science.aaf2853. [DOI] [PubMed] [Google Scholar]
- 3.Chen X., Yan N. A brief overview of renewable plastics. Materials Today Sustainability. 2020;7–8:100031. [Google Scholar]
- 4.Akaraonye E., Keshavarz T., Roy I. Production of polyhydroxyalkanoates: the future green materials of choice. J. Chem. Technol. Biotechnol. 2010;85(6):732–743. [Google Scholar]
- 5.Raza Z.A., Abid S., Banat I.M. Polyhydroxyalkanoates: characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018;126:45–56. [Google Scholar]
- 6.Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Law K.L. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 7.Laskar N., Kumar U. Plastics and microplastics: a threat to environment. Environmental Technology & Innovation. 2019;14:100352. [Google Scholar]
- 8.Backhaus T., Wagner M. 2018. Microplastics in the Environment: Much Ado about Nothing? A Debate. (Global Challenges) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royer S.J., Ferrón S., Wilson S., Karl D. Production of methane and ethylene from plastic in the environment. PloS One. 2018;13(8) doi: 10.1371/journal.pone.0200574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunfield P.F., Yuryev A., Senin P., Smirnova A.V., Stott M.B., Hou S., Ly B., Saw J.H., Zhou Z., Ren Y., Wang J., Mountain B.W., Crowe M.A., Weatherby T.M., Bodelier P.L.E., Liesack W., Feng L., Wang L., Alam M. Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia. Nature. 2007;450(7171):879–882. doi: 10.1038/nature06411. [DOI] [PubMed] [Google Scholar]
- 11.Cantera S., Lebrero R., Sadornil L., García-Encina P.A., Muñoz R. Valorization of CH4 emissions into high-added-value products: assessing the production of ectoine coupled with CH4 abatement. J. Environ. Manag. 2016;182:160–165. doi: 10.1016/j.jenvman.2016.07.064. [DOI] [PubMed] [Google Scholar]
- 12.Stocker T.F., Qin D., Plattner G.K., Tignor M., Allen S.K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P.M. 2013. Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 1535. [Google Scholar]
- 13.Strong P.J., Xie S., Clarke W.P. Methane as a resource: can the methanotrophs add value? Environ. Sci. Technol. 2015;49(7):4001–4018. doi: 10.1021/es504242n. [DOI] [PubMed] [Google Scholar]
- 14.La H., Hettiaratchi J.P.A., Achari G., Dunfield P.F. Biofiltration of methane. Bioresour. Technol. 2018;268:759–772. doi: 10.1016/j.biortech.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 15.Lee O.K., Hur D.H., Nguyen D.T.N., Lee E.Y. Metabolic engineering of methanotrophs and its application to production of chemicals and biofuels from methane. Biofuels, Bioproducts and Biorefining. 2016;10(6):848–863. [Google Scholar]
- 16.Yin Z., Linga P. Methane hydrates: a future clean energy resource. Chin. J. Chem. Eng. 2019;27(9):2026–2036. [Google Scholar]
- 17.Madison L.L., Huisman G.W. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. : MMBR (Microbiol. Mol. Biol. Rev.) 1999;63(1):21–53. doi: 10.1128/mmbr.63.1.21-53.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jong Min J., Brigham C., Kim Y.H., Kim H.j., Yi D.H., Kim H., Rha C., Sinskey A., Yang Y.H. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P(HB-co-HHx)) from butyrate using engineered Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2014;98(12):5461–5469. doi: 10.1007/s00253-014-5617-7. [DOI] [PubMed] [Google Scholar]
- 19.Chidambarampadmavathy K., Karthikeyan O.P., Heimann K. Sustainable bio-plastic production through landfill methane recycling. Renew. Sustain. Energy Rev. 2017;71:555–562. [Google Scholar]
- 20.Asenjo J.A., Suk J.S. Microbial Conversion of Methane into poly-β-hydroxybutyrate (PHB): growth and intracellular product accumulation in a type II methanotroph. J. Ferment. Technol. 1986;64(4):271–278. [Google Scholar]
- 21.Saratale G.D., Oh M.K. Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int. J. Biol. Macromol. 2015;80:627–635. doi: 10.1016/j.ijbiomac.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 22.Yeo J.C.C., Muiruri J.K., Thitsartarn W., Li Z., He C. Recent advances in the development of biodegradable PHB-based toughening materials: approaches, advantages and applications. Mater. Sci. Eng. C. 2018;92:1092–1116. doi: 10.1016/j.msec.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Liao Q., Tsui A., Billington S., Frank C.W. Extruded foams from microbial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and its blends with cellulose acetate butyrate. Polym. Eng. Sci. 2012;52(7):1495–1508. [Google Scholar]
- 24.Tsui A., Hu X., Kaplan D.L., Frank C.W. American Chemical Society; 2013. Green Polymer Chemistry: Biocatalysis and Materials II; pp. 251–279. [Google Scholar]
- 25.Peña C., Castillo T., García A., Millán M., Segura D. Biotechnological strategies to improve production of microbial poly-(3-hydroxybutyrate): a review of recent research work. Microbial Biotechnology. 2014;7(4):278–293. doi: 10.1111/1751-7915.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna S., Srivastava A.K. Computer simulated fed-batch cultivation for over production of PHB: a comparison of simultaneous and alternate feeding of carbon and nitrogen. Biochem. Eng. J. 2006;27(3):197–203. [Google Scholar]
- 27.Andler R., Vivod R., Steinbüchel A. Synthesis of polyhydroxyalkanoates through the biodegradation of poly(cis-1,4-isoprene) rubber. J. Biosci. Bioeng. 2019;127(3):360–365. doi: 10.1016/j.jbiosc.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Kourmentza C., Plácido J., Venetsaneas N., Burniol Figols A., Varrone C., Gavala H.N., Reis M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering. 2017;4(2):55. doi: 10.3390/bioengineering4020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh A.K., Srivastava J.K., Chandel A.K., Sharma L., Mallick N., Singh S.P. Biomedical applications of microbially engineered polyhydroxyalkanoates: an insight into recent advances, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2019;103(5):2007–2032. doi: 10.1007/s00253-018-09604-y. [DOI] [PubMed] [Google Scholar]
- 30.Butt F.I., Muhammad N., Hamid A., Moniruzzaman M., Sharif F. Recent progress in the utilization of biosynthesized polyhydroxyalkanoates for biomedical applications – Review. Int. J. Biol. Macromol. 2018;120:1294–1305. doi: 10.1016/j.ijbiomac.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Florez J.P., Fazeli M., Simão R.A. Preparation and characterization of thermoplastic starch composite reinforced by plasma-treated poly (hydroxybutyrate) PHB. Int. J. Biol. Macromol. 2019;123:609–621. doi: 10.1016/j.ijbiomac.2018.11.070. [DOI] [PubMed] [Google Scholar]
- 32.Harding K.G., Dennis J.S., von Blottnitz H., Harrison S.T.L. Environmental analysis of plastic production processes: comparing petroleum-based polypropylene and polyethylene with biologically-based poly-β-hydroxybutyric acid using life cycle analysis. J. Biotechnol. 2007;130(1):57–66. doi: 10.1016/j.jbiotec.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Levett I., Birkett G., Davies N., Bell A., Langford A., Laycock B., Lant P., Pratt S. Techno-economic assessment of poly-3-hydroxybutyrate (PHB) production from methane—the case for thermophilic bioprocessing. Journal of Environmental Chemical Engineering. 2016;4(4):3724–3733. [Google Scholar]
- 34.Listewnik H.F., Wendlandt K.D., Jechorek M., Mirschel G. Process design for the microbial synthesis of poly-β-hydroxybutyrate (PHB) from natural gas. Eng. Life Sci. 2007;7(3):278–282. [Google Scholar]
- 35.DiGregorio B.E. Biobased performance bioplastic: mirel. Chem. Biol. 2009;16(1):1–2. doi: 10.1016/j.chembiol.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Rostkowski K.H., Criddle C.S., Lepech M.D. Cradle-to-Gate life cycle assessment for a cradle-to-cradle cycle: biogas-to-bioplastic (and back) Environ. Sci. Technol. 2012;46(18):9822–9829. doi: 10.1021/es204541w. [DOI] [PubMed] [Google Scholar]
- 37.Hanson R., Hanson T. Methanotrophic bacteria. Microbiol. Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Xin J., Chen L., Song H., Xia C. Biosynthesis of poly-3-hydroxybutyrate with a high molecular weight by methanotroph from methane and methanol. J. Nat. Gas Chem. 2008;17(1):103–109. [Google Scholar]
- 39.Kalyuzhnaya M.G., Puri A.W., Lidstrom M.E. Metabolic engineering in methanotrophic bacteria. Metab. Eng. 2015;29:142–152. doi: 10.1016/j.ymben.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Semrau J.D., Dispirito A.A., Yoon S. Methanotrophs and copper. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 2010;34(4):496–531. doi: 10.1111/j.1574-6976.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- 41.Strong P.J., Laycock B., Mahamud S.N.S.M., Jensen P.D., Lant P.A., Tyson G., Pratt S. The opportunity for high-performance biomaterials from methane. Microorganisms. 2016;4(1):11. doi: 10.3390/microorganisms4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kavitha G., Rengasamy R., Inbakandan D. Polyhydroxybutyrate production from marine source and its application. Int. J. Biol. Macromol. 2018;111:102–108. doi: 10.1016/j.ijbiomac.2017.12.155. [DOI] [PubMed] [Google Scholar]
- 43.Khanna S., Srivastava A.K. Recent advances in microbial polyhydroxyalkanoates. Process Biochem. 2005;40(2):607–619. [Google Scholar]
- 44.Pieja A.J., Rostkowski K.H., Criddle C.S. Distribution and selection of poly-3-hydroxybutyrate production capacity in methanotrophic proteobacteria. Microb. Ecol. 2011;62(3):564–573. doi: 10.1007/s00248-011-9873-0. [DOI] [PubMed] [Google Scholar]
- 45.Dedysh S.N., Horz H.-P., Dunfield P.F., Liesack W. A novel pmoA lineage represented by the acidophilic methanotrophic bacterium Methylocapsa acidophila B2. Arch. Microbiol. 2001;177(1):117–121. doi: 10.1007/s00203-001-0362-6. [DOI] [PubMed] [Google Scholar]
- 46.Rahnama F., Vasheghani-Farahani E., Yazdian F., Shojaosadati S.A. PHB production by Methylocystis hirsuta from natural gas in a bubble column and a vertical loop bioreactor. Biochem. Eng. J. 2012;65:51–56. [Google Scholar]
- 47.Jiang X., Chen G. Morphology engineering of bacteria for bio-production. Biotechnol. Adv. 2016;34(4):435–440. doi: 10.1016/j.biotechadv.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Yin J., Chen G. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014;30:59–65. doi: 10.1016/j.copbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Doronina N.V., Ezhov V.A., Trotsenko Y.A. Growth of Methylosinus trichosporium OB3b on methane and poly-β-hydroxybutyrate biosynthesis. Appl. Biochem. Microbiol. 2008;44(2):182–185. [PubMed] [Google Scholar]
- 50.Zuniga C., Morales M., Le Borgne S., Revah S. Production of poly-β-hydroxybutyrate (PHB) by Methylobacterium organophilum isolated from a methanotrophic consortium in a two-phase partition bioreactor. J. Hazard Mater. 2011;190(1–3):876–882. doi: 10.1016/j.jhazmat.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Sundstrom E.R., Criddle C.S. Optimization of methanotrophic growth and production of poly(3-hydroxybutyrate) in a high throughput microbioreactor system. Appl. Environ. Microbiol. 2015;81(14):4767–4773. doi: 10.1128/AEM.00025-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dias J.M.L., Lemos P.C., Serafim L.S., Oliveira C., Eiroa M., Albuquerque M.G.E., Ramos A.M., Oliveira R., Reis M.A.M. Recent advances in polyhydroxyalkanoate production by mixed aerobic cultures: from the substrate to the final product. Macromol. Biosci. 2006;6(11):885–906. doi: 10.1002/mabi.200600112. [DOI] [PubMed] [Google Scholar]
- 53.Frigon D., Muyzer G., van Loosdrecht M., Raskin L. rRNA and poly-β-hydroxybutyrate dynamics in bioreactors subjected to feast and famine cycles. Appl. Environ. Microbiol. 2006;72(4):2322–2330. doi: 10.1128/AEM.72.4.2322-2330.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reis M.A.M., Serafim L.S., Lemos P.C., Ramos A.M., Aguiar F.R., Van Loosdrecht M.C.M. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioproc. Biosyst. Eng. 2003;25(6):377–385. doi: 10.1007/s00449-003-0322-4. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z., Zhao L., Ji Y., Wen Q., Long H. Reconsideration on the effect of nitrogen on mixed culture polyhydroxyalkanoate production toward high organic loading enrichment history. Front. Environ. Sci. Eng. 2019;13(4):54. [Google Scholar]
- 56.López J.C., Merchán L., Lebrero R., Muñoz R. Feast-famine biofilter operation for methane mitigation. J. Clean. Prod. 2018;170:108–118. [Google Scholar]
- 57.Dionisi D., Majone M., Papa V., Beccari M. Biodegradable polymers from organic acids by using activated sludge enriched by aerobic periodic feeding. Biotechnol. Bioeng. 2004;85(6):569–579. doi: 10.1002/bit.10910. [DOI] [PubMed] [Google Scholar]
- 58.Johnson K., Jiang Y., Kleerebezem R., Muyzer G., van Loosdrecht M. Enrichment of a mixed bacterial culture with a high polyhydroxyalkanoate storage capacity. Biomacromolecules. 2009;10(4):670–676. doi: 10.1021/bm8013796. [DOI] [PubMed] [Google Scholar]
- 59.Garg S., Wu H., Clomburg J.M., Bennett G.N. Bioconversion of methane to C-4 carboxylic acids using carbon flux through acetyl-CoA in engineered Methylomicrobium buryatense 5GB1C. Metab. Eng. 2018;48:175–183. doi: 10.1016/j.ymben.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Garg S., Clomburg J.M., Gonzalez R. A modular approach for high-flux lactic acid production from methane in an industrial medium using engineered Methylomicrobium buryatense 5GB1. J. Ind. Microbiol. Biotechnol. 2018;45(6):379–391. doi: 10.1007/s10295-018-2035-3. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen A.D., Hwang I.Y., Lee O.K., Kim D., Kalyuzhnaya M.G., Mariyana R., Hadiyati S., Kim M.S., Lee E.Y. Systematic metabolic engineering of Methylomicrobium alcaliphilum 20Z for 2,3-butanediol production from methane. Metab. Eng. 2018;47:323–333. doi: 10.1016/j.ymben.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 62.Chen G., Jiang X. Engineering microorganisms for improving polyhydroxyalkanoate biosynthesis. Curr. Opin. Biotechnol. 2018;53:20–25. doi: 10.1016/j.copbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Li T., Ye J., Shen R., Zong Y., Zhao X., Lou C., Chen G. Semirational approach for ultrahigh poly(3-hydroxybutyrate) accumulation in Escherichia coli by combining one-step library construction and high-throughput screening. ACS Synth. Biol. 2016;5(11):1308–1317. doi: 10.1021/acssynbio.6b00083. [DOI] [PubMed] [Google Scholar]
- 64.Choi J., Lee S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 1999;51(1):13–21. [Google Scholar]
- 65.Koutinas A., Vlysidis A., Pleissner D., Kopsahelis N., Garcia I., Kookos I., Papanikolaou S., Kwan T.H., Lin C. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014;43(8):2587–2627. doi: 10.1039/c3cs60293a. [DOI] [PubMed] [Google Scholar]
- 66.Carlson R., Wlaschin A., Srienc F. Kinetic studies and biochemical pathway analysis of anaerobic poly-(R)-3-Hydroxybutyric acid synthesis in Escherichia coli. Appl. Environ. Microbiol. 2005;71(2):713–720. doi: 10.1128/AEM.71.2.713-720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nikel P.I., Pettinari M.J., Galvagno M.A., Méndez B.S. Poly(3-hydroxybutyrate) synthesis from glycerol by a recombinant Escherichia coli arcA mutant in fed-batch microaerobic cultures. Appl. Microbiol. Biotechnol. 2008;77(6):1337–1343. doi: 10.1007/s00253-007-1255-7. [DOI] [PubMed] [Google Scholar]
- 68.Fu X., Tan D., Aibaidula G., Wu Q., Chen J., Chen G. Development of Halomonas TD01 as a host for open production of chemicals. Metab. Eng. 2014;23:78–91. doi: 10.1016/j.ymben.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 69.Lee S.H., Oh D.H., Ahn W.S., Lee Y., Choi J.i., Lee S.Y. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol. Bioeng. 2000;67(2):240–244. doi: 10.1002/(sici)1097-0290(20000120)67:2<240::aid-bit14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 70.Cavalheiro J., Raposo R., Almeida M.C., Cesário M., Sevrin C., Grandfils C., Fonseca M. Effect of cultivation parameters on the production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour. Technol. 2012;111:391–397. doi: 10.1016/j.biortech.2012.01.176. [DOI] [PubMed] [Google Scholar]
- 71.Tao W., Lv L., Chen G. Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi. Microb. Cell Factories. 2017;16(1):48. doi: 10.1186/s12934-017-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chung A.L., Jin H., Huang L., Ye H., Chen J., Wu Q., Chen G. Biosynthesis and characterization of poly (3-hydroxydodecanoate) by β-oxidation inhibited mutant of Pseudomonas entomophila L48. Biomacromolecules. 2011;12:3559–3566. doi: 10.1021/bm200770m. [DOI] [PubMed] [Google Scholar]
- 73.Zhuang Q., Wang Q., Liang Q., Qi Q. Synthesis of polyhydroxyalkanoates from glucose that contain medium-chain-length monomers via the reversed fatty acid β-oxidation cycle in Escherichia coli. Metab. Eng. 2014;24:78–86. doi: 10.1016/j.ymben.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Li D., Lv L., Chen J., Chen G. Controlling microbial PHB synthesis via CRISPRi. Appl. Microbiol. Biotechnol. 2017;101(14):5861–5867. doi: 10.1007/s00253-017-8374-6. [DOI] [PubMed] [Google Scholar]
- 75.Lv L., Ren Y., Chen J., Wu Q., Chen G. Application of CRISPRi for prokaryotic metabolic engineering involving multiple genes, a case study: controllable P(3HB-co-4HB) biosynthesis. Metab. Eng. 2015;29:160–168. doi: 10.1016/j.ymben.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Tsuge T. Fundamental factors determining the molecular weight of polyhydroxyalkanoate during biosynthesis. Polym. J. 2016;48(11):1051–1057. [Google Scholar]
- 77.Lee J., Yasin M., Park S., Chang I.S., Ha K.S., Lee E.Y., Lee J., Kim C. Gas-liquid mass transfer coefficient of methane in bubble column reactor. Kor. J. Chem. Eng. 2015;32(6):1060–1063. [Google Scholar]
- 78.Kim K., Lee J., Seo K., Kim M.G., Ha K.S., Kim C. Enhancement of methane–water volumetric mass transfer coefficient by inhibiting bubble coalescence with electrolyte. J. Ind. Eng. Chem. 2016;33:326–329. [Google Scholar]
- 79.Wendlandt K.D., Jechorek M., Brühl E. The influence of pressure on the growth of methanotrophic bacteria. Acta Biotechnol. 1993;13(2):111–115. [Google Scholar]
- 80.Duan Z., Mao S. A thermodynamic model for calculating methane solubility, density and gas phase composition of methane-bearing aqueous fluids from 273 to 523K and from 1 to 2000bar. Geochem. Cosmochim. Acta. 2006;70(13):3369–3386. [Google Scholar]
- 81.Chang I.S., Kim B.H., Lovitt R.W., Bang J.S. Effect of CO partial pressure on cell-recycled continuous CO fermentation by Eubacterium limosum KIST612. Process Biochem. 2001;37(4):411–421. [Google Scholar]
- 82.Ghoddosi F., Golzar H., Yazdian F., Khosravi Darani K., Vasheghani Farahani E. Effect of carbon sources for PHB production in bubble column bioreactor: emphasis on improvement of methane uptake. Journal of Environmental Chemical Engineering. 2019;7(2):102978. [Google Scholar]
- 83.Khosravi Darani K., Yazdian F., Babapour F., Amirsadeghi A.R. Poly(3-hydroxybutyrate) production from natural gas by a methanotroph native bacterium in a bubble column bioreactor. Chem. Biochem. Eng. Q. 2019;33:69–77. [Google Scholar]
- 84.García Pérez T., López J.C., Passos F., Lebrero R., Revah S., Muñoz R. Simultaneous methane abatement and PHB production by Methylocystis hirsuta in a novel gas-recycling bubble column bioreactor. Chem. Eng. J. 2018;334:691–697. [Google Scholar]
- 85.Lai C., Zhong L., Zhang Y., Chen J., Wen L., Shi L., Sun Y., Ma F., Rittmann B.E., Zhou C., Tang Y., Zheng P., Zhao H. Bioreduction of chromate in a methane-based membrane biofilm reactor. Environ. Sci. Technol. 2016;50(11):5832–5839. doi: 10.1021/acs.est.5b06177. [DOI] [PubMed] [Google Scholar]
- 86.Xie G., Liu T., Cai C., Hu S., Yuan Z. Achieving high-level nitrogen removal in mainstream by coupling anammox with denitrifying anaerobic methane oxidation in a membrane biofilm reactor. Water Res. 2018;131:196–204. doi: 10.1016/j.watres.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 87.Martin K.J., Nerenberg R. The membrane biofilm reactor (MBfR) for water and wastewater treatment: principles, applications, and recent developments. Bioresour. Technol. 2012;122:83–94. doi: 10.1016/j.biortech.2012.02.110. [DOI] [PubMed] [Google Scholar]
- 88.Cai C., Hu S., Guo J., Shi Y., Xie G., Yuan Z. Nitrate reduction by denitrifying anaerobic methane oxidizing microorganisms can reach a practically useful rate. Water Res. 2015;87:211–217. doi: 10.1016/j.watres.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 89.Shi Y., Hu S., Lou J., Lu P., Keller J., Yuan Z. Nitrogen removal from wastewater by coupling anammox and methane-dependent denitrification in a membrane biofilm reactor. Environ. Sci. Technol. 2013;47(20):11577–11583. doi: 10.1021/es402775z. [DOI] [PubMed] [Google Scholar]
- 90.Chen X., Guo J., Shi Y., Hu S., Yuan Z., Ni B.-J. Modeling of simultaneous anaerobic methane and ammonium oxidation in a membrane biofilm reactor. Environ. Sci. Technol. 2014;48(16):9540–9547. doi: 10.1021/es502608s. [DOI] [PubMed] [Google Scholar]
- 91.Fu L., Ding J., Lu Y., Ding Z., Bai Y., Zeng R.J. Hollow fiber membrane bioreactor affects microbial community and morphology of the DAMO and Anammox co-culture system. Bioresour. Technol. 2017;232:247–253. doi: 10.1016/j.biortech.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 92.Nie W., Xie G., Ding J., Lu Y., Liu B., Xing D., Wang Q., Han H., Yuan Z., Ren N. High performance nitrogen removal through integrating denitrifying anaerobic methane oxidation and Anammox: from enrichment to application. Environ. Int. 2019;132:105107. doi: 10.1016/j.envint.2019.105107. [DOI] [PubMed] [Google Scholar]
- 93.Xie G., Cai C., Hu S., Yuan Z. Complete nitrogen removal from synthetic anaerobic sludge digestion liquor through integrating anammox and denitrifying anaerobic methane oxidation in a membrane biofilm reactor. Environ. Sci. Technol. 2017;51(2):819–827. doi: 10.1021/acs.est.6b04500. [DOI] [PubMed] [Google Scholar]
- 94.Lee J., Jang N., Yasin M., Lee E.Y., Chang I.S., Kim C. Enhanced mass transfer rate of methane via hollow fiber membrane modules for Methylosinus trichosporium OB3b fermentation. J. Ind. Eng. Chem. 2016;39:149–152. [Google Scholar]
- 95.Li H., Zhang Z., Xu X., Liang J., Xia S. Bioreduction of para-chloronitrobenzene in a hydrogen-based hollow-fiber membrane biofilm reactor: effects of nitrate and sulfate. Biodegradation. 2014;25(2):205–215. doi: 10.1007/s10532-013-9652-3. [DOI] [PubMed] [Google Scholar]
- 96.Gupta V., Goel R. Managing dissolved methane gas in anaerobic effluents using microbial resource management-based strategies. Bioresour. Technol. 2019;289:121601. doi: 10.1016/j.biortech.2019.121601. [DOI] [PubMed] [Google Scholar]
- 97.Kennelly C., Clifford E., Gerrity S., Walsh R., Rodgers M., Collins G. A horizontal flow biofilm reactor (HFBR) technology for the removal of methane and hydrogen sulphide at low temperatures. Water Sci. Technol. 2012;66(9):1997–2006. doi: 10.2166/wst.2012.411. [DOI] [PubMed] [Google Scholar]
- 98.Ordaz A., López J.C., Figueroa González I., Muñoz R., Quijano G. Assessment of methane biodegradation kinetics in two-phase partitioning bioreactors by pulse respirometry. Water Res. 2014;67:46–54. doi: 10.1016/j.watres.2014.08.054. [DOI] [PubMed] [Google Scholar]
- 99.Clarke K.G., Williams P.C., Smit M.S., Harrison S.T.L. Enhancement and repression of the volumetric oxygen transfer coefficient through hydrocarbon addition and its influence on oxygen transfer rate in stirred tank bioreactors. Biochem. Eng. J. 2006;28(3):237–242. [Google Scholar]
- 100.Bailón L., Nikolausz M., Kästner M., Veiga M.C., Kennes C. Removal of dichloromethane from waste gases in one- and two-liquid-phase stirred tank bioreactors and biotrickling filters. Water Res. 2009;43(1):11–20. doi: 10.1016/j.watres.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 101.Muñoz R., Daugulis A.J., Hernández M., Quijano G. Recent advances in two-phase partitioning bioreactors for the treatment of volatile organic compounds. Biotechnol. Adv. 2012;30(6):1707–1720. doi: 10.1016/j.biotechadv.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 102.Rocha Rios J., Bordel S., Hernández S., Revah S. Methane degradation in two-phase partition bioreactors. Chem. Eng. J. 2009;152(1):289–292. [Google Scholar]
- 103.Kim K., Kim Y., Yang J., Ha K.S., Usta H., Lee J., Kim C. Enhanced mass transfer rate and solubility of methane via addition of alcohols for Methylosinus trichosporium OB3b fermentation. J. Ind. Eng. Chem. 2017;46:350–355. [Google Scholar]
- 104.Muñoz R., Villaverde S., Guieysse B., Revah S. Two-phase partitioning bioreactors for treatment of volatile organic compounds. Biotechnol. Adv. 2007;25(4):410–422. doi: 10.1016/j.biotechadv.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Helm J., Wendlandt K.D., Jechorek M., Stottmeister U. Potassium deficiency results in accumulation of ultra-high molecular weight poly-β-hydroxybutyrate in a methane-utilizing mixed culture. J. Appl. Microbiol. 2008;105(4):1054–1061. doi: 10.1111/j.1365-2672.2008.03831.x. [DOI] [PubMed] [Google Scholar]
- 106.Choi J., Lee S.Y. High level production of supra molecular weight poly (3-hydroxybutyrate) by metabolically engineered Escherichia coli. Biotechnol. Bioproc. Eng. 2004;9(3):196–200. [Google Scholar]
- 107.Kabe T., Tsuge T., Kasuya K.I., Takemura A., Hikima T., Takata M., Iwata T. Physical and structural effects of adding ultrahigh-molecular-weight poly[(R)-3-hydroxybutyrate] to wild-type poly[(R)-3-hydroxybutyrate] Macromolecules. 2012;45(4):1858–1865. [Google Scholar]
- 108.Hong S.G., Heng Wei H., Ye M.T. Thermal properties and applications of low molecular weight polyhydroxybutyrate. J. Therm. Anal. Calorim. 2012;111(2):1243–1250. [Google Scholar]
- 109.Iwata T. Strong fibers and films of microbial polyesters. Macromol. Biosci. 2005;5:689–701. doi: 10.1002/mabi.200500066. [DOI] [PubMed] [Google Scholar]
- 110.Wendlandt K.D., Jechorek M., Helm J., Stottmeister U. Producing poly-3-hydroxybutyrate with a high molecular mass from methane. J. Biotechnol. 2001;86(2):127–133. doi: 10.1016/s0168-1656(00)00408-9. [DOI] [PubMed] [Google Scholar]
- 111.Wendlandt K.D., Jechorek M., Helm J., Stottmeister U. Production of PHB with a high molecular mass from methane. Polym. Degrad. Stabil. 1998;59(1–3):191–194. [Google Scholar]
- 112.Sim S.J., Snell K.D., Hogan S.A., Stubbe J., Rha C., Sinskey A.J. PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nat. Biotechnol. 1997;15(1):63–67. doi: 10.1038/nbt0197-63. [DOI] [PubMed] [Google Scholar]
- 113.Hiroe A., Hyakutake M., Thomson N.M., Sivaniah E., Tsuge T. Endogenous ethanol affects biopolyester molecular weight in recombinant Escherichia coli. ACS Chem. Biol. 2013;8(11):2568–2576. doi: 10.1021/cb400465p. [DOI] [PubMed] [Google Scholar]
- 114.Kotnis M., O’Brien G., Willett J. Processing and mechanical properties of biodegradable Poly(hydroxybutyrate-co-valerate)-starch compositions. J. Environ. Polym. Degrad. 1995;3(2):97–105. [Google Scholar]
- 115.Myung J., Galega W.M., Van Nostrand J.D., Yuan T., Zhou J., Criddle C.S. Long-term cultivation of a stable Methylocystis-dominated methanotrophic enrichment enabling tailored production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Bioresour. Technol. 2015;198:811–818. doi: 10.1016/j.biortech.2015.09.094. [DOI] [PubMed] [Google Scholar]
- 116.Larsson M., Markbo O., Jannasch P. Melt processability and thermomechanical properties of blends based on polyhydroxyalkanoates and poly(butylene adipate-co-terephthalate) RSC Adv. 2016;6(50):44354–44363. [Google Scholar]
- 117.Tebaldi M.L., Maia A.L.C., Poletto F., de Andrade F.V., Soares D.C.F. Poly(-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): current advances in synthesis methodologies, antitumor applications and biocompatibility. J. Drug Deliv. Sci. Technol. 2019;51:115–126. [Google Scholar]
- 118.Zhao X., Ji K., Kurt K., Cornish K., Vodovotz Y. Optimal mechanical properties of biodegradable natural rubber-toughened PHBV bioplastics intended for food packaging applications. Food Packaging and Shelf Life. 2019;21:100348. [Google Scholar]
- 119.Myung J., Flanagan J.C.A., Waymouth R.M., Criddle C.S. Methane or methanol-oxidation dependent synthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by obligate type II methanotrophs. Process Biochem. 2016;51(5):561–567. [Google Scholar]
- 120.Cal A.J., Sikkema W.D., Ponce M.I., Franqui-Villanueva D., Riiff T.J., Orts W.J., Pieja A.J., Lee C.C. Methanotrophic production of polyhydroxybutyrate-co-hydroxyvalerate with high hydroxyvalerate content. Int. J. Biol. Macromol. 2016;87:302–307. doi: 10.1016/j.ijbiomac.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 121.Zuniga C., Morales M., Revah S. Polyhydroxyalkanoates accumulation by Methylobacterium organophilum CZ-2 during methane degradation using citrate or propionate as cosubstrates. Bioresour. Technol. 2013;129:686–689. doi: 10.1016/j.biortech.2012.11.120. [DOI] [PubMed] [Google Scholar]
- 122.Kunioka M., Tamaki A., Doi Y. Crystalline and thermal properties of bacterial copolyesters: poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Macromolecules. 1989;22(2):694–697. [Google Scholar]
- 123.Orita I., Nishikawa K., Nakamura S., Fukui T. Biosynthesis of polyhydroxyalkanoate copolymers from methanol by Methylobacterium extorquens AM1 and the engineered strains under cobalt-deficient conditions. Appl. Microbiol. Biotechnol. 2014;98(8):3715–3725. doi: 10.1007/s00253-013-5490-9. [DOI] [PubMed] [Google Scholar]