Abstract
The energy consumption in building ventilation, air, and heating conditioning systems, accounts for about 25% of the overall energy consumption in modern society. Therefore, cutting carbon emissions and reducing energy consumption is a growing priority in building construction. Electrochromic devices (ECDs) are considered to be a highly promising energy-saving technology, due to their simple structure, active control, and low energy input characteristics. At present, H+, OH- and Li+ are the main electrolyte ions used for ECDs. However, H+ and OH- based electrolytes have a high erosive effect on the material surface and have a relatively short lifetime. Li+-based electrolytes are limited due to their high cost and safety concerns. In this study, inspired by prior research on Ca2+ batteries and supercapacitors, CaF2 films were prepared by electron beam evaporation as a Ca2+-based electrolyte layer to construct ECDs. The structure, morphology, and optical properties of CaF2 films were characterized. ECDs with the structure of ITO (indium tin oxide) glass/WO3/CaF2/NiO/ITO show short switching times (22.8 s for the coloring process, 2.8 s for the bleaching process). Additionally, optical modulation of the ECDs is about 38.8% at 750 nm. These findings indicate that Ca2+ based ECDs have the potential to become a competitive and attractive choice for large-scale commercial smart windows.
Keywords: Electrochromic devices, Smart windows, Evaporation, Carbon emission
Graphical abstract
Highlights
-
•
The CaF2 film is prepared by electron beam evaporation.
-
•
The structure, morphology and optical properties of CaF2 films are characterized.
-
•
The ECDs with the structure of ITO glass/WO3/CaF2/NiO/ITO are assembled.
-
•
The ΔT of the ECD at wavelength of 750 nm is approximately 38.8%.
-
•
The tc and tb are 22.8 s and 2.8 s, respectively.
1. Introduction
The energy consumption in building ventilation, air, and heating conditioning systems accounts for about 25% of the total energy consumption in our society [[1], [2], [3]]. Among various technologies, smart windows provide an effective and feasible solution for improving a building's energy efficiency by altering the transmittance and absorbance of light [1,[3], [4], [5]]. The most promising candidates for smart windows are electrochromic devices (ECDs), which can reversibly change the optical property by applying voltage [6,7]. The properties of ECDs are determined by the insertion and extraction of electrolyte ions during electrochemical reaction processes [8,9]. Currently, Li+-based electrolytes are the most widely used and effective electrolytes in ECDs [[10], [11], [12], [13], [14]]. Despite the successful commercialization of Li+-based ECDs, safety concerns of Li-salts during preparation processing and limited Li resources are a challenge for widespread Li+-based ECD use in smart windows [6,[15], [16], [17]]. Therefore, it is important to explore a new generation of naturally abundant and low-cost electrolytes for ECDs.
Calcium is the fifth most abundant element in the Earth's crust and exhibits a high theoretical electrochemical capacity [18,19]. Compared to other multivalent ions, Ca2+ has a low polarization strength, similar to Li+, and therefore holds promise for fast reaction kinetics and good electrochemical performance [[20], [21], [22], [23]]. Moreover, thanks to their low cost and reduced safety concerns, compared to Li+-based systems, Ca2+-based electrochemical systems have been extensively investigated in batteries and supercapacitors [[20], [21], [22], [23], [24], [25]]. Following previous research on Ca2+ batteries and supercapacitors [[20], [21], [22], [23], [24], [25]], Tong et al. [26] assembled a novel and safe aqueous Ca2+ electrochromic battery, which demonstrated high stable, high-rate capability, a high energy density, and greenish-yellow to black electrochromism. However, to our knowledge, this is the first study on Ca2+-based electrochromism aimed at developing safe, low-cost, and green Ca2+-based ECDs.
CaF2 is a promising solid-state electrolyte, due to its superior chemical and physical stability [27,28], high ionic conductivity [29], and high transparency ranging from ∼150 nm to 12 μm [30,31]. Electron beam evaporation is a fast, environmentally friendly, and economical method to prepare high quality homogeneous thin films. In this paper, CaF2 films were prepared by electron beam evaporation as the Ca2+-based electrolyte layer to construct the ECDs with the structure of ITO glass/WO3/CaF2/NiO/ITO. Additionally, the structure, morphology, and optical properties of CaF2 films were characterized. The electrochromic (EC) performances of these devices were evaluated by coloring at -2.5 V for 30 s and bleaching at 2.5 V for 20 s via in situ transmittance recording. More importantly, the optical modulation (38.8% at 750 nm) is comparable to Ca2+-based liquid state ECDs [26], and the switching times (22.8 s for coloring and 2.8 s for the bleaching) are shorter than many of the previously reported Li+-based solid-state ECDs.
2. Materials and methods
2.1. Deposition of CaF2 films
The silica glasses substrates were ultrasonically cleaned in deionized water followed by alcohol, for 10 min each. The CaF2 films were prepared by electron beam evaporation using CaF2 particles with a diameter of ∼1–3 mm. The detailed conditions of CaF2 film preparation are provided in Table S1. After evaporation, the CaF2 films were annealed at 200 °C for 60 min in a muffle furnace to improve film adhesion.
2.2. Deposition of the ECD
ITO glass with an average transmittance of 80% and a sheet resistance of 10 Ω were used as the substrate. All layers of the ECDs were deposited by electron beam evaporation. The detailed parameters of WO3, NiO, and top ITO layers follow our previously reported method [10,12,15] and are given in Table S1. The deposition of each layer was performed continuously with pre-evaporation for 10 min before each deposition. After evaporation, the ECDs were annealed at 200 °C for 60 min in a muffle furnace to enhance the electrical and optical properties of the top ITO films.
2.3. Characterization
The surface and cross-section morphologies were characterized using scanning electron microscopy (SEM, Merlin Compact, Zeiss, Germany). The surface roughness of CaF2 film was characterized by Atomic Force Microscope (AFM, Dimension Fastscan, Bruker, Germany). The crystalline structure of the CaF2 film was characterized by X-ray diffraction (XRD, X'Pert, Panalytical, Netherlands) in the 2θ range of 10°–80°. The transmittance spectra of the CaF2 film were characterized by UV-Vis-NIR optical spectroscopy (Lambda 1050, PerkinElmer, USA). The EC performances of ECDs were investigated with a combination of a Vis-NIR fiber optic spectrometer (Maya 2000-Pro, Ocean Optics, USA) and an electrochemical station (CHI 660E, Chenhua, China).
3. Results and discussion
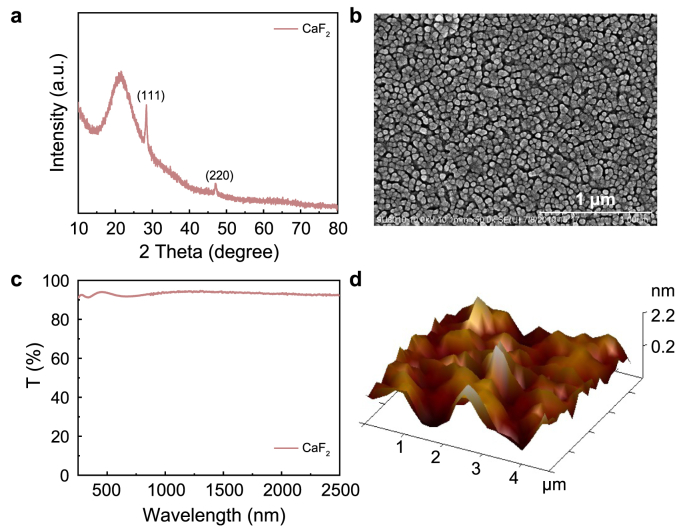
The performances of CaF2 films are strongly dependent on their structure, morphology, and optical properties. As shown in Fig. 1a, there is a broad peak and two diffraction peaks in the XRD pattern of the CaF2 film. The broad peak in the range of 15–25° can be assigned to the silica glass substrate [15]. Peaks located at 28.2° and 47.0° represent the (111) and (220) planes of CaF2 cubic structure (JCPDS Card (No. 35-0816)) [31]. The surface morphology of the CaF2 films, shown in Fig. 1b, are composed of particles with uniform size, which is favorable for ion transportation. As depicted in Fig. 1c, the CaF2 film shows a high transmittance, over 90%, from 250 to 2500 nm, which is necessary for the electrolyte layer [32]. The AFM image of the CaF2 film is presented in Fig. 1d. The root mean square surface roughness is approximately 0.6 nm and, thus, the CaF2 films can effectively avoid short circuits [33,34].
Fig. 1.
Characterization of CaF2 films: a, XRD pattern; b, SEM micrograph; c, Transmittance spectra; and d, AFM surface 3D image.
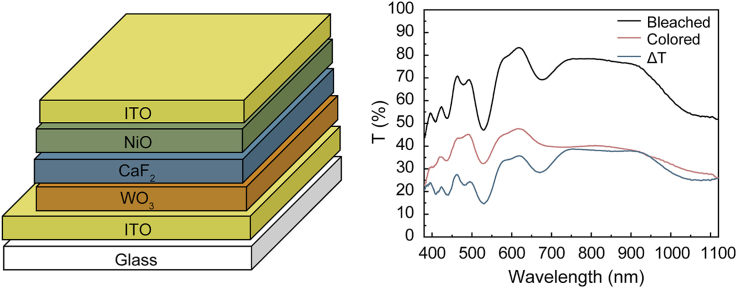
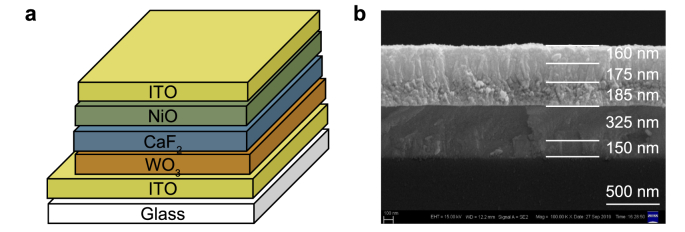
Fig. 2a shows the schematic diagram of the ECD, which is similar to our previously reported Li+- and Mg2+-based ECDs. The cross-sectional image, with thickness of each layer of the solid-state ECD, is shown in Fig. 2b. The device exhibits a five-layer structure and the thicknesses of WO3, CaF2, NiO and top ITO films consistent with the setting values, which are 325, 185, 175, and 160 nm, respectively. The identified interfaces between the CaF2 film and EC films are favorable for ion transportation and suggest these layers are physically stable with no interfacial reaction [10,15]. The vague interface between EC films and ITO films is mainly ascribed to the interdiffusion of their similar surface morphologies [15], which are favorable for charge transportation [35].
Fig. 2.
All solid-state ECD: a, Schematic diagram and b, Cross-sectional image.
As shown in Fig. S1, the ECDs are coloring at -2.5 V for 30 s and bleaching at 2.5 V for 20 s. Fig. 3a displays the transmittance spectra at the colored and bleached states of the all solid-state ECDs, with corresponding digital photos in Fig. S2. The ionic conductive mechanism of CaF2 in ECDs is similar to Li + -based ECDs [36]. The intercalation and extraction of Ca2+ ions and electrons between the WO3 film and NiO film can be described by:
| (1) |
| (2) |
Fig. 3.
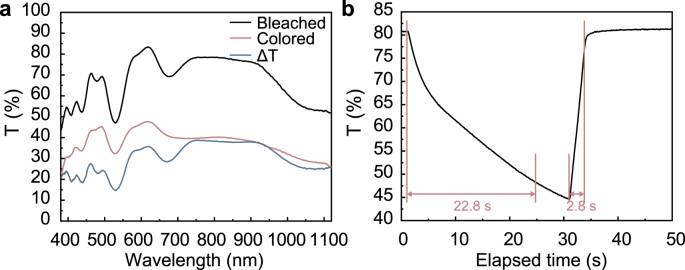
a, Transmittance spectra of the ECD at different states and optical modulation during initial cycles. b, In situ transmittance spectra at 600 nm (30 s for coloring and 20 s for bleaching).
When a negative voltage is applied to the ECD, the Ca2+ ions extract from NiO films and insert into WO3 films, and the ECD changes to the colored state. When applying a positive voltage, the Ca2+ ions extract from WO3 films and insert into NiO films, and the ECD changes to the bleached state. The ECD optical modulation (ΔT) is determined by:
| (3) |
where Tb and Tc are the transmittance of the ECD for the bleached state and colored state, respectively. As illustrated in Fig. 3a, the maximum ΔT is 38.8% at 750 nm, where the Tc and Tb are 39.6% and 78.4%, respectively. As depicted in Table S2, the ΔT in this study is comparable to Ca2+ based ECDs and less than most Li + -based ECDs. This is attributed to the higher valence of Ca2+ and the greater electrostatic force between Ca2+ and the frame.
Switching time, which is the time required to achieve 90% of total transmittance variation between two states [37,38], was examined to further evaluate the ECD performance. As shown in Fig. 3b, monitoring the transmittance change at 600 nm, the coloring and bleaching switching times of ECD were 22.8 s and 2.8 s, respectively. Notably, the coloring process takes longer than the bleaching process, which is caused by the greater electrostatic force between Ca2+ and EC layer [39] arising from the higher valence and larger ionic radius of Ca2+. Regardless, the switching times of the ECD are shorter than most other all solid-state inorganic ECDs (Table S2) and comparable to reported ECDs using gel electrolytes [40].
The in situ transmittance measurements of the ECD during multiple potentials at 600 nm are presented in Fig. S3. The transmittance of the bleached state fluctuates slightly and stabilizes around 81%. The transmittance of the colored state increases from 44% at the first cycle to 58% at the 125th cycle and then remained stable, which is the result of the different types of ions intercalation. According to a prior study [41], there are two different types of ion intercalation for WO3; shallow ones that are reversible and easy to insert and extract, and deep ones that are difficult to access and wherein ions become permanently trapped. The ionic radius of the Ca2+ (0.099 nm) is bigger than that of Li+ (0.06 nm), which can lead to much deep ion intercalation in WO3 and poor cyclic stability. The cyclic stability is closely related to the size of insertion ions and, the composition and microstructure of the EC materials. Therefore, WO3 can be doped with other elements (e.g. V, Ta, and Mo) or prepared with suitable porosity for future work aimed at enhancing the cyclic stability of ECDs [1].
The coloration efficiency (CE) is an important criterion to evaluate the performance of ECDs, representing the change in the optical density (ΔOD) per unit charge density (ΔQ). It can be calculated according to the following equations:
| (4) |
| (5) |
| (6) |
where Tb and Tc are the transmittances of the ECD in the bleached and colored states at 600 nm, ΔQ represents the intercalation charge density. As shown in Fig. S4, the calculated CE value of ECD is 52.4 cm2 C-1, which is larger than that of some reported Li+ and Mg2+ based all solid-state ECDs [15,42]. These results indicate that CaF2 films have great potential to be used as inorganic electrolytes for the next generation of all solid-state ECDs.
4. Conclusions
CaF2 films are successfully prepared by electron beam evaporation for use as the Ca2+-based electrolyte layer to construct ECDs. The structure, morphology, and optical properties of CaF2 films are characterized. The ECD shows a five-layer structure ITO/WO3/CaF2/NiO/ITO and short switching times (22.8 s for the coloring process and 2.8 s for the bleaching process). Additionally, the optical modulation of the ECDs is approximately 38.8% at 750 nm. This study demonstrates Ca2+ based electrolyte is a promising alternative for an easily produced and environmentally friendly electrolyte to be used in EC smart windows.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
National Natural Science Foundation of China, China (52002097).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2022.100164.
Contributor Information
Xiang Zhang, Email: zhangxhit@hit.edu.cn.
Yao Li, Email: yaoli@hit.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Huang Y., Wang B., Chen F., Han Y., Zhang W., Wu X., Li R., Jiang Q., Jia X., Zhang R. Electrochromic materials based on ions insertion and extraction. Adv. Opt. Mater. 2021 [Google Scholar]
- 2.Islam S.M., Hernandez T.S., McGehee M.D., Barile C.J. Hybrid dynamic windows using reversible metal electrodeposition and ion insertion. Nat. Energy. 2019;4:223–229. [Google Scholar]
- 3.Wang S., Jiang T., Meng Y., Yang R., Tan G., Long Y. Scalable thermochromic smart windows with passive radiative cooling regulation. Science. 2021;374:1501–1504. doi: 10.1126/science.abg0291. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary A., Pathak D.K., Tanwar M., Koch J., Pfnur H., Kumar R. Polythiophene-nanoWO3 bilayer as electrochromic infrared filter: a transparent heat shield. J. Mater. Chem. C. 2020;8:1773. [Google Scholar]
- 5.Ye R.I., Yong M.K., Lee Y., Choi W.Y., Hong C.M. Ultra-low power electrochromic heat shutters through tailoring diffusion-controlled behaviors. ACS Appl. Mater. Interfaces. 2020;12 doi: 10.1021/acsami.0c05918. 30635−30642. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S., Cao S., Zhang T., Fisher A., Lee J.Y. Al3+ intercalation/de-intercalation-enabled dual-band electrochromic smart windows with a high optical modulation, quick response and long cycle life. Energy Environ. Sci. 2018;11:2884–2892. [Google Scholar]
- 7.Ma D., Lee-Sie E.A., Cao S., Lee P.S., Wang J. Wide-spectrum modulated electrochromic smart windows based on MnO2/PB films. ACS Appl. Mater. Interfaces. 2022;14(1):1443–1451. doi: 10.1021/acsami.1c20011. [DOI] [PubMed] [Google Scholar]
- 8.Heo S., Kim J., Ong G.K., Milliron D.J. Template-free mesoporous electrochromic films on flexible substrates from tungsten oxide nanorods. Nano Lett. 2017;17:5756–5761. doi: 10.1021/acs.nanolett.7b02730. [DOI] [PubMed] [Google Scholar]
- 9.Azam A., Kim J., Park J., Novak T.G., Tiwari A.P., Song S.H., Kim B., Jeon S. Two-Dimensional WO3 nanosheets chemically converted from layered WS2 for High-Performance electrochromic devices. Nano Lett. 2018;18:5646–5651. doi: 10.1021/acs.nanolett.8b02150. [DOI] [PubMed] [Google Scholar]
- 10.Chen X., Dou S., Li W., Liu D., Zhang Y., Zhao Y., Li Y., Zhao J., Zhang X. All solid state electrochromic devices based on the LiF electrolyte. Chem. Commun. 2020;56:5018–5021. doi: 10.1039/d0cc00697a. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y., Zhang X., Chen X., Li W., Li Z., Chen M., Sun W., Zhao J., Li Y. All-solid-state electrochromic devices based on the LiAlSiO4 electrolyte. Mater. Lett. 2021;292 [Google Scholar]
- 12.Li W., Zhang X., Chen X., Zhao Y., Wang L., Liu D., Li X., Chen M., Zhao J., Li Y. Preparation and performance of fast-response ITO/Li-NiO/Li-WO3/ITO all-solid-state electrochromic devices by evaporation method. Mater. Lett. 2020;265 [Google Scholar]
- 13.Kim S.Y., Yun T.Y., Yu K.S., Hong C.M. Reliable, high-performance electrochromic supercapacitors based on metal-doped nickel oxide. ACS Appl. Mater. Interfaces. 2020;12 doi: 10.1021/acsami.0c15424. 51978−51986. [DOI] [PubMed] [Google Scholar]
- 14.Chen P.W., Chang C.T., Ko T.F., Hsu S.C., Wu J.Y. Fast response of complementary electrochromic device based on WO3/NiO electrodes. Sci. Rep. 2020;10:8430. doi: 10.1038/s41598-020-65191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X., Li W., Dou S., Wang L., Zhao Y., Zhang X., Li Y., Zhao J. MgF2 as abundant and environmentally friendly electrolytes for high performance electrochromic devices. J. Materiomics. 2021;7:1318–1323. [Google Scholar]
- 16.Li R., Li K., Wang G., Li L., Zhang Q., Yan J., Chen Y., Zhang Q., Hou C., Li Y., Wang H. Ion-transport design for high-performance Na+ based electrochromics. ACS Nano. 2018;12:3759–3768. doi: 10.1021/acsnano.8b00974. [DOI] [PubMed] [Google Scholar]
- 17.Tian Y., Zhang W., Cong S., Zheng Y., Geng F., Zhao Z. Unconventional aluminum ion Intercalation/Deintercalation for fast switching and highly stable electrochromism. Adv. Funct. Mater. 2015;25:5833–5839. [Google Scholar]
- 18.Gummow R.J., Vamvounis G., Kannan M.B., He Y. Calcium-Ion batteries: current State-of-the-Art and future perspectives. Adv. Mater. 2018;30 doi: 10.1002/adma.201801702. [DOI] [PubMed] [Google Scholar]
- 19.Ponrouch A., Palacin M.R. On the road toward calcium-based batteries. Curr. Opin. Electrochem. 2018;9:1–7. [Google Scholar]
- 20.He Y., Gu M., Xiao H., Luo L., Shao Y., Gao F., Du Y., Mao S.X., Wang C. Atomistic conversion reaction mechanism of WO3 in secondary ion batteries of Li, Na, and Ca. Angew Chem. Int. Ed. Engl. 2016;55:6244–6247. doi: 10.1002/anie.201601542. [DOI] [PubMed] [Google Scholar]
- 21.Padigi P., Goncher G., Evans D., Solanki R. Potassium barium hexacyanoferrate-a potential cathode material for rechargeable calcium ion batteries. J. Power Sources. 2015;273:460–464. [Google Scholar]
- 22.Murata Y., Takada S., Obata T., Tojo T., Inada R., Sakurai Y. Effect of water in electrolyte on the Ca2+ insertion/extraction properties of V2O5. Electrochim. Acta. 2019;294:210–216. [Google Scholar]
- 23.Tojo T., Tawa H., Oshida N., Inada R., Sakurai Y. Electrochemical characterization of a layered α-MoO3 as a new cathode material for calcium ion batteries. J. Electroanal. Chem. 2018;825:51–56. [Google Scholar]
- 24.Nolis G.M., Adil A., Yoo H.D., Hu L., Bayliss R.D., Lapidus S.H., Berkland L., Phillips P.J., Freeland J.W., Kim C., Klie R.F., Cabana J. Electrochemical reduction of a Spinel-Type manganese oxide cathode in aqueous electrolytes with Ca2+ or Zn2+ J. Phys. Chem. C. 2018;122:4182–4188. [Google Scholar]
- 25.Kuperman N., Padigi P., Goncher G., Evans D., Thiebes J., Solanki R. High performance Prussian Blue cathode for nonaqueous Ca-ion intercalation battery. J. Power Sources. 2017;342:414–418. [Google Scholar]
- 26.Tong Z., Kang T., Wan Y., Yang R., Wu Y., Shen D., Liu S., Tang Y., Lee C.S. A Ca-ion electrochromic battery via a water-in-salt electrolyte. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 27.Liu X., Liu J., Huang T., Yu A. CaF2-coated Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials for Li-ion batteries. Electrochim. Acta. 2013;109:52–58. [Google Scholar]
- 28.Shi S.J., Tu J.P., Mai Y.J., Zhang Y.Q., Tang Y.Y., Wang X.L. Structure and electrochemical performance of CaF2 coated LiMn1/3Ni1/3Co1/3O2 cathode material for Li-ion batteries. Electrochim. Acta. 2012;83:105–112. [Google Scholar]
- 29.Puin W., Rodewald S., Ramlau R., Heitjans P., Maier J. Local and overall ionic conductivity in nanocrystalline CaF2. Solid State Ionics. 2000;131:159–164. [Google Scholar]
- 30.Pilvi T., Arstila K., Leskela M., Ritala M. Novel ALD process for depositing CaF2 thin films. Chem. Mater. 2007;19:3387–3392. [Google Scholar]
- 31.Pandurangappa C., Lakshminarasappa B.N., Nagabhushana B.M. Synthesis and characterization of CaF2 nanocrystals. J. Alloy. Compd. 2010;489:592–595. [Google Scholar]
- 32.Xie L., Zhao S., Zhu Y., Zhang Q., Chang T., Huang A., Jin P., Ren S., Bao S. High performance and excellent stability of all-solid-state electrochromic devices based on a Li1.85AlOz ion conducting layer. ACS Sustain. Chem. Eng. 2019;7:17390–17396. [Google Scholar]
- 33.Dong D., Wang W., Rougier A., Dong G., Rocha M.D., Presmanes L., Zrikem K., Song G., Diao X., Barnabé A. Life-cycling and uncovering cation-trapping evidence of a monolithic inorganic electrochromic device: glass/ITO/WO3/LiTaO3/NiO/ITO. Nanoscale. 2018:16521–16530. doi: 10.1039/c8nr02267d. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Y., Dong G., Guo J., Liu Q., Huang Q., Zhang Q., Zhong X., Diao X. Thickness dependent surface roughness of sputtered Li2.5TaOx ion conductor and its effect on electro-optical performance of inorganic monolithic electrochromic device. Sol. Energy Mater. Sol. Cell. 2018;179:319–327. [Google Scholar]
- 35.Wang M., Liu Q., Dong G., He Y., Diao X. Influence of thickness on the structure, electrical, optical and electrochromic properties of AZO thin films and their inorganic all-solid-state devices. Electrochim. Acta. 2017;258:1336–1347. [Google Scholar]
- 36.Xiao Y., Zhong X., Guo J., Zhou C., Zuo H., Liu Q., Huang Q., Zhang Q., Diao X. The role of interface between LiPON solid electrolyte and electrode in inorganic monolithic electrochromic devices, Electrochim. Acta. 2018;260:254–263. [Google Scholar]
- 37.Dong D., Wang W., Dong G., Zhou Y., Wu Z., Wang M., Liu F., Diao X. Electrochromic properties of NiO:H films deposited by DC magnetron sputtering for ITO/NiO:H/ZrO2/WO3/ITO device. Appl. Surf. Sci. 2015;357:799–805. [Google Scholar]
- 38.Zhou Y., Diao X., Dong G., Wu Z., Dong D., Wang M. Enhanced transmittance modulation of ITO/NiOx/ZrO2:H/WO3/ITO electrochromic devices. Ionics. 2016;22:25–32. [Google Scholar]
- 39.Ju X., Yang F., Zhu X., Jia X. Zinc ion Intercalation/Deintercalation of metal organic Framework-Derived nanostructured NiO@c for Low-Transmittance and High-Performance electrochromism. ACS Sustain. Chem. Eng. 2020;8:12222–12229. [Google Scholar]
- 40.Cai G., Darmawan P., Cui M., Chen J., Wang X., Eh A.L., Magdassi S., Lee P.S. Inkjet-printed all solid-state electrochromic devices based on NiO/WO3 nanoparticle complementary electrodes. Nanoscale. 2016;8:348. doi: 10.1039/c5nr06995e. [DOI] [PubMed] [Google Scholar]
- 41.Wen R.T., Granqvist C.G., Niklasson G.A. Eliminating degradation and uncovering ion-trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 2015;14:996–1001. doi: 10.1038/nmat4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oukassi S., Giroud-Garampon C., Dubarry C., Ducros C., Salot R. All inorganic thin film electrochromic device using LiPON as the ion conductor. Sol. Energy Mater. Sol. Cells. 2016;145:2–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.