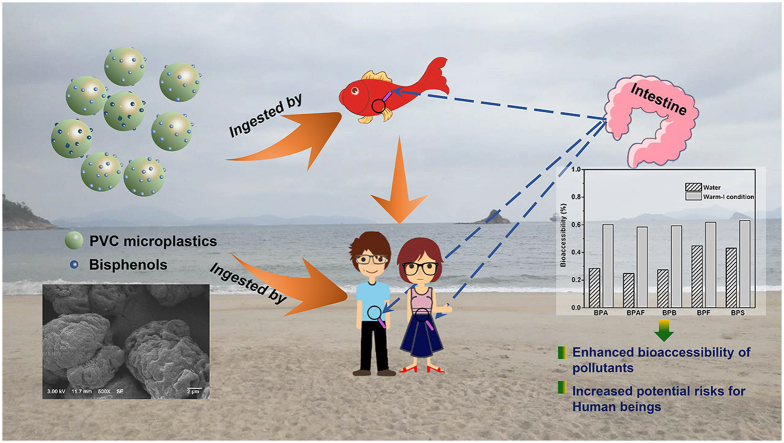
Abstract
The ever-increasing prevalence of microplastics and different bisphenols made the presence of bisphenol-attached microplastics a critical concern. In this study, experiments were performed to examine desorption behaviors and cytotoxicity performance of contaminated microplastics in aquatic surroundings and intestinal environment after ingestion by organisms (cold-/warm-blooded). The kinetic study shows that the rate of desorption for bisphenols can be enhanced threefold under simulated warm intestinal conditions. The Freundlich isotherms indicate multiple-layer desorption of the bisphenols on the heterogeneous surfaces of polyvinyl chloride (PVC) microplastics. Hysteresis was detected in the adsorption/desorption of bisphenols in a water environment, but no adsorption/desorption hysteresis was observed in the simulated intestinal conditions of warm-blooded organisms. Due to enhanced bioaccessibility, the desorption results imply that the environmental risk of contaminated PVC microplastics may be significantly increased after ingestion at a high bisphenols dosage. Although with different IC50, the five bisphenols released under the intestinal conditions of warm-blooded organisms can cause higher proliferation reduction in fish and human cell lines than the bisphenols released in water. This study helps elucidate the consequential fate and potential cytotoxicity of contaminated microplastics and the possible implications of the microplastics as a critical vector for bisphenols to increase the potential health risks.
Keywords: Microplastics, Bisphenols, Desorption behavior, Bioaccessibility, Cytotoxicity
Graphical abstract
Highlights
-
•
Simulated intestinal fluid can enhance bisphenol release from PVC microplastics.
-
•
Adsorption/desorption hysteresis of bisphenols were observed in aquatic conditions.
-
•
Released bisphenols play a crucial role in cytotoxicity and bioaccessibility study.
-
•
Microplastics incline to act as a vector to raise potential environmental risks.
1. Introduction
As the current global-scale exposure is hardly reversible, plastic litter materials have been reported to fulfill the essential conditions of planetary boundary threats [1,2]. Plastics may ultimately enter and persist in the aquatic environment and then undergo embrittlement, fragmentation and degradation caused by effects such as physical stress, ultraviolet radiation, temperature changes, oxidizing conditions, wave impacts, and biological processes [3]. Once these fragments reach a diameter of less than 5 mm, they are typically defined as microplastics by the National Oceanic and Atmospheric Administration [4]. For examples, it has been predicted that 45–129 thousand tonnes of plastics debris float inside the North Pacific Subtropical Gyre [5], while an approximately up to 2.55 × 104 items·m-3 of microplastics were found in the surface water of an inland water within Guangdong-Hong Kong-Macao Greater Bay Area [6]. These microplastics would contain a large specific surface area [7], and they may thus act as potential carriers or adsorbents for concentrating pollutants such as endocrine disrupting chemicals (EDCs) [1], which may give rise to further contamination of the aquatic ecosystem [5,8].
Currently, a series of conceptual frameworks have been discussed for the potential propagated effects of pristine and/or contaminated microplastics. For example, microplastics can interfere with algal feeding by causing a false feeling of satiation [9]. At a higher trophic level, hard biodegradable microplastics were found in a crab’s stomach upon exposure and were retained in the intestinal tract for almost 14 days [10]. Several previous studies have demonstrated that the association between microplastics and polychlorinated biphenyls (PCBs) could increase lipid accumulation and mortality, generating an increased public health impact [11,12]. On the other hand, Diepens and Kolemans [13] found that more ingestion of microplastics could decrease the toxicity of PCBs in the stimulated food web by weakening their bioaccessibility . Although these effects are still controversial, it is generally recognized that contaminated microplastics can generate toxicity for aquatic organisms by the following two pathways [[14], [15], [16]]: small microplastics can penetrate the cell membrane and directly induce great intracellular oxidative stress, and the attached pollutants can be released from the contaminated microplastics and generate further impacts.
Thus, the transfer and release behaviors of contaminated microplastics are of critical importance in the exploration of their role as culprits and/or vectors for the aforementioned toxicity. Therefore, systematic experiments were performed in this study to examine the desorption behaviors and cytotoxicity performance of contaminated microplastics. Desorption can occur in aquatic surroundings or in the intestinal environment after the microplastics are ingested by organisms (cold- or warm-blooded) [15,17]. Since polyvinyl chloride (PVC) microplastics are widely distributed along the water column [6,18,19] and are one of the most toxic plastics as described by Healthy Child Healthy World [20], they were selected as the representative microplastics in this study. In addition, bisphenols (e.g., bisphenol A, bisphenol AF, bisphenol B, bisphenol F, and bisphenol S) are widely applied as plasticizers in the production of PVC plastics [3,19]. Since they are identified as typical EDCs, bisphenols were further selected as the representative contaminants in our study. Bisphenols can disrupt hormone synthesis and induce hepatic stress in fish cells [21,22]. Moreover, BPA and its analogues were also found in the human urine [23], blood [24] and even breast milk [25] at a relatively high level (ng·mL−1), due to the long history of plastic products like the epoxy resins and polycarbonates plastics [24,25]. The toxicity of the bisphenols for organisms or human beings has been reported by a large volume of literature [23,26,27]. For example, the oxidative stress and damage in human cells have been discovered in human erythrocytes [23] with the exposure of bisphenols. It was also reported that bisphenol analogues contain the estrogenic/antiandrogenic potencies, which are similar as or greater than that of BPA at around dozens or hundreds of nanograms per milliliter [26,27]. Therefore, to further evaluate the cytotoxicity of bisphenol-attached microplastics, two model cell lines (the grass carp hepatocyte cell line, L8824; the human breast adenocarcinoma cells, MCF-7) commonly applied as the perfect model in hepatotoxicity [28] or estrogen toxicity [29] studies, were selected in this study to represent the cold- and warm-blooded organism cell lines, respectively.
This study first elucidated the desorption behaviors of bisphenols from bisphenol-attached PVC microplastics under different scenarios such as water and simulated intestinal conditions of cold- and warm-blooded organisms. Subsequently, cytotoxicity assays of pristine microplastics and bisphenols were performed on L8824 and MCF-7. Then, the potential impacts of the bisphenols released from the adsorbed microplastics were evaluated. By exploring the transfer behaviors and potential cytotoxicity of the contaminated microplastics, this study will contribute to a better understanding of their roles as sources or carriers for emerging pollutants, which may help to fill the knowledge gap in studies on the impacts of pollutant-associated microplastics.
2. Material and methods
2.1. Raw materials
PVC microplastics (d50 = 13.2 μm; ρ = 1.4 g cm−3) were obtained from Goodfellow Company (Huntington, UK), with Figure S1 of Supporting Information (SI) showing the surface morphology, X-ray diffraction pattern and zeta potential of the raw material. Bisphenols including BPA, BPS, BPF, BPB and BPAF, were purchased from Sigma−Aldrich (St. Louis, USA), with the corresponding octanol−water partition coefficients (log Dow) listed in Table S1 of the SI. Optimal-grade methanol was obtained from Fisher Scientific (Pittsburgh, USA). The sodium chloride (NaCl) was of analytical grade (Aladdin Chemistry Co., Shanghai, China), and the sodium carbonate (Na2CO3) and sodium azide (NaN3) were purchased from Fisher Scientific (Ottawa, Canada). Other detailed information regarding these raw materials is further provided in “Section 1.1” of the SI.
2.2. Adsorption and desorption of bisphenols from PVC microplastics
First, the adsorption of bisphenols onto PVC microplastics was examined according to the procedures reported by Wu et al. [3]. In summary, each bisphenol was dissolved in ethanol and then diluted in the glass bottle (1 L) with initial concentrations ranging from 0 to 1.0 mg L−1, representing the common and limiting conditions [24,30,31]. 1.5 g of PVC microplastics were then added and the containers were shaken with 200 rpm at room temperature for 48 h to reach adsorption equilibrium. Three replicates were performed for every adsorption experiment. Then, the amounts of adsorbed BPA, BPAF, BPB, BPF and BPS on the PVC were measured and were found to be 0.19, 0.24, 0.23, 0.16, and 0.15 mg g−1, respectively (as described in “Section 1.2” of the SI). According to the method reported in previous studies [17,31], desorption of the PVC microplastics attached by each bisphenol was transferred to three different conditions, including water and simulated intestinal environments of both cold- and warm-blooded organisms (simplified as “cold-I″ and “warm-I″, respectively). In brief, the simulated intestinal liquid was composed of sodium chloride (0.12 M), sodium carbohydrate (0.02 M), and sodium taurocholate (15.50 mM), with different pH values and temperatures [32,33] (cold-I: 7.5, 291K; warm-I: 6.5, 311K; aquatic: 7.0, 291K). “Section 1.3” of the SI provides a detailed description of the procedures used for the preparation of the three aforementioned types of desorption conditions.
The glass bottle (1 L) sealed with Teflon screw caps for desorption experiments were placed in an oil bath with rotary agitation at 200 rpm until desorption equilibrium was reached. The temperature was set at 291 K for the water system and the cold-blooded system and at 311 K for the warm-blooded system. Experiments for the desorption kinetics were carried out by measuring the concentrations of bisphenols after desorption at different time intervals from 0 to 75 h. Desorption isotherms were obtained for the bisphenol-attached PVC microplastics from the adsorption processes with initial bisphenol concentrations in the 0.10–1.00 mg·L−1 range. After filtering the supernatants of each series using a 0.45 μm glass microfiber filter (Whatman, UK), the concentrations of the bisphenols were measured according to the methods described in “Section 1.4” of the SI. Prior to the batch experiments, two series of control experiments were conducted, and their detailed parameters are summarized in Table S2 of the SI. The results of the control experiments show that no bisphenols were determined during the adsorption/desorption process with only PVC microplastics present, and the loss of each bisphenol was below 3% in the processes without the microplastics. With such low losses, each concentration of the desorbed bisphenol was almost equal to the detected value after desorption equilibrium. All of the experiments were conducted in triplicate.
2.3. Cell culture and cytotoxicity assay
The L8824 and MCF-7 cell lines were purchased from Wuhan Cell Institutional Repository (Wuhan, China) and American Type Culture Collection (ATCC NO.: HTB-22, Manassas, USA), respectively. The L8824 cells were cultured in minimum Eagle’s medium (MEM, Sigma, USA) with 10% of fetal calf serum added (FCS, Sijiqing, Hangzhou, China). Following the method described by Wei et al. [34], the MCF-7 cells were preserved in phenol red-free Dulbecco’s modified Eagles’s medium (DMEM, Thermo Fisher Scientific, USA) with 10% of fetal bovine serum (FBS, Thermo Fisher Scientific, USA). In the cultures for both L8824 and MCF-7, the concentrations of penicillin and streptomycin were 100 units·mL−1 and 100 μg·mL−1, respectively. The incubation was conducted in a humid atmosphere with 5% of CO2 at 291 K for L8824 and 311 K for MCF-7. The mediums were refreshed every 24 h, and subculturing was carried out after the cells were almost 80% confluent. The cytotoxicity assays were carried out by plating the two cell lines onto a 96-well plate. The two types of cells were maintained in their corresponding media for 48 h and then were harvested by 0.25% trypsin. Then, each type of cell was suspended again and transferred into a new dish to achieve approximately 105 cells per milliliter. Then, the above solution (0.10 mL) was seeded into each well and incubated for another 24 h.
Cytotoxicity assays were considered and determined from two aspects: pristine PVC microplastics and each bisphenol analogue. The pristine PVC microplastics (with the amount as 1.50 g·L−1) were first sonicated in MEM or DMEM for 10 min and were then immediately vortexed to obtain a stable solution prior to addition into the cells. Each bisphenol was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, USA) at a series of concentrations (0.10, 0.50, 1.00, 5.00, 10.00, 20.00, 50.00, 100.00, 150.00 and 200.00 μM) for cytotoxicity measurements. The medium in the wells was replaced with either the PVC microplastics or the serial bisphenols-containing medium. All of the exposed cells were cultured for another 72 h at 291 K for L8824 and at 311 K for MCF-7.3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) (10.00 μL, 5 g·L−1) in phosphate-buffered saline (PBS, Sigma-Aldrich, USA) was added to each well. After 4 h, the medium was removed, and DMSO (100 μL) was added to dissolve the purple formazan precipitate. Finally, the light absorbance at 490 nm was determined using a multilabel plate counter (VICTOR X3, PerkinElmer, Washington, USA). Each cytotoxicity experiment was conducted with six replicates. A control study was also performed on the cells by exposing the cells to 0.5% DMSO-containing medium without bisphenols.
3. Results and discussion
3.1. Transfer behavior of bisphenols from the bisphenol-attached microplastics
Our preliminary study found that a reversible process always accompanied the adsorption of bisphenols onto PVC microplastics [3]. A study was further conducted on the desorption of bisphenols from bisphenol-attached PVC microplastics to determine the desorption kinetics, desorption isotherm and adsorption/desorption hysteresis. The kinetics results were used to illustrate different desorption stages, while the isotherms reflected the equilibrium amount of each bisphenol released from the adsorbed microplastics.
3.1.1. Desorption kinetics
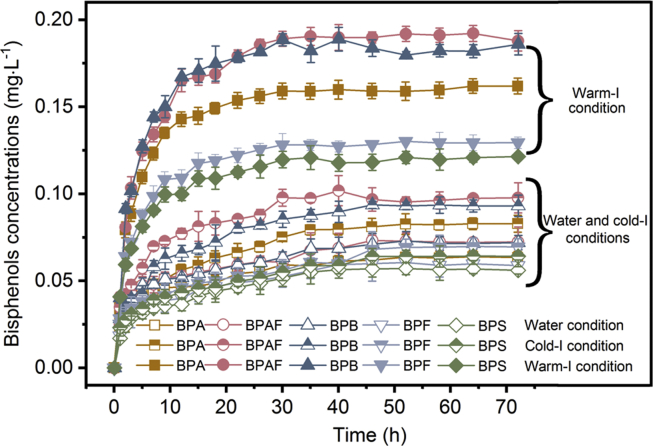
Kinetics studies were conducted to analyze the variation in bisphenol concentrations with prolonged desorption processes under the conditions of water and simulated intestinal environments of warm- and cold-blooded organisms. Fig. 1 summarizes the desorption kinetics of the five bisphenols in the three different environmental conditions, and the results show that the release of bisphenols increased substantially during the first 5 h and then began to slow until reaching the desorption equilibrium at approximately 35 h.
Fig. 1.
Concentrations of the five different bisphenols in the desorption liquids of the water, cold-I and warm-I conditions, representing the desorption amounts of bisphenols from the bisphenol-attached PVC microplastics at different times during the 75 h desorption process. Water condition: pH 7.0 & 291 K; Cold-I condition: pH 7.5 & 291 K; Warm-I condition: pH 6.5 & 311 K. Cold-I and warm-I conditions represent the simulated intestinal liquids of cold- and warm-blooded organisms, respectively (NaCl 0.12 M, Na2CO3 0.02 M, and C26H44NNaO7S 15.5 mM).
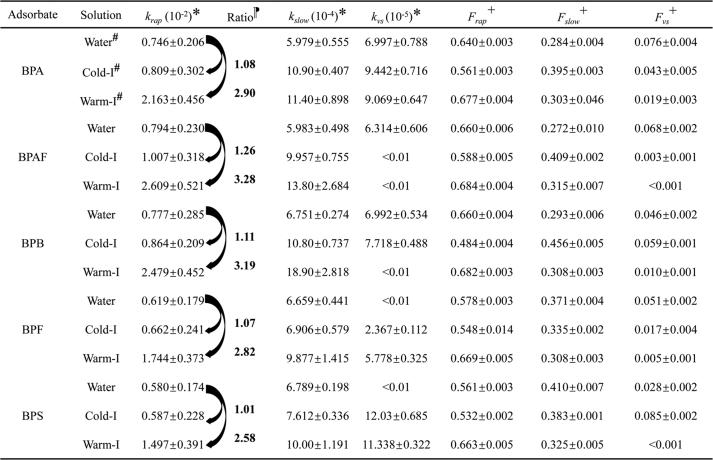
Based on these results, a first-order model (Eqs. S2-S6 of the SI) was applied to describe the desorption processes of the bisphenols, with the fitting results summarized in Table 1. The rapid fraction (Frap) of every bisphenol had the highest value in the warm-I environment relative to the corresponding values for the other two environments, suggesting that the highest desorption rate of bisphenols is found in the intestinal liquid of warm-blooded organisms. The slow fraction (Fslow) of BPA, BPAF and BPB was observed with the highest value in the cold-I environment, indicating that the highest proportion of the above three bisphenols is desorbed slowly in the intestinal environment of cold-blooded organisms compared to the case in the other two environments. Meanwhile, the highest Fslow of BPF and BPS found in the water environment shows that the largest fraction of each of these bisphenols was released slowly in the aquatic environment relative to the other bisphenols. In the very slow stage, a significant decrease in the Fvs was found in the warm-I environment comparing to the other two conditions. This phenomenon indicated that a certain amount of adsorbed bisphenols, which may release in the very slow stage, was transferred to the rapid or slow fractions with the enhancement of the warm-I environment.
Table 1.
First-order model fitting results for bisphenol desorption from bisphenol-attached PVC microplastics in the water, cold-I, and swarm-I conditions.
Since the Frap value is higher than the values of the other two fractions (Fslow and Fvs) for all of the bisphenols, the rapid stage can be regarded as the most crucial process that should be examined further. Therefore, the rapid rate constants (krap) of each bisphenol were selected for comparison among the different environments. Table 1 shows the calculated ratios of krap in the cold-I and warm-I environments to those in water. While the ratios of cold-I/water range from only 1.01 to 1.26, a much higher range (2.58–3.28) was observed for the corresponding warm-I/water ratios. The krap value for each bisphenol is very close in cold-I and water, indicating a similar rate of desorption in both environmental conditions. However, as reflected from the krap ratios of warm-I to water, the bisphenols desorption rate can be enhanced by ∼3 times in warm-I compared to those in cold-I and water environments, which may be due to deformation of the interstitial pore structure at lower pH values and the availability of additional energy at higher temperatures.
3.1.2. Desorption isotherms
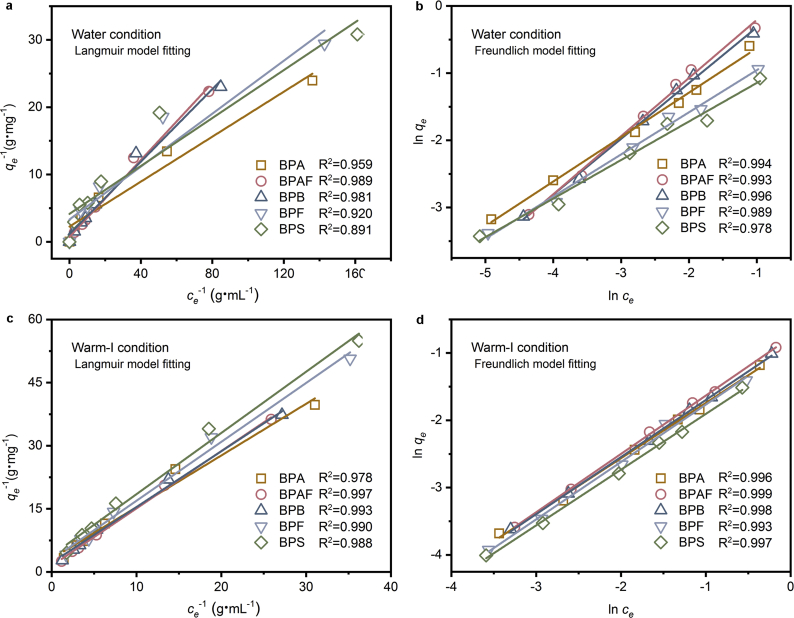
From the kinetic results above, a similar desorption behavior was observed under the water and cold-I conditions, while a significant difference was detected in the warm-I condition. Therefore, an isotherm study was then performed to compare the water and warm-I environments. Langmuir and Freundlich desorption isotherm models (Eqs. S7 and S8 of the SI) were employed to describe the relationships between the bisphenol amounts on PVC microplastics after desorption (qe) and the bisphenol concentrations in the solution at equilibrium (ce) [35], with the results summarized in Fig. 2 and Table S3 of the SI. The correlation coefficients (R2) of the Freundlich model range from 0.978 to 0.996 in water and from 0.993 to 0.999 in the warm-I condition for all of the bisphenols, and these values are higher than the corresponding values obtained by Langmuir modeling (0.891–0.989 in water and 0.978–0.997 in the warm-I condition). Better fitting of the experimental data was observed for the Freundlich model than for the Langmuir model, indicating a multiple-layer process of bisphenols desorption from the bisphenol-attached microplastics, which is in agreement with our previous adsorption results [3].
Fig. 2.
Desorption isotherms of the five bisphenols from the contaminated PVC microplastics fitted by Langmuir and Freundlich models under the water and warm-I conditions. The correlation coefficients obtained by Freundlich fitting (0.978–0.996 in water and 0.993–0.999 in the warm-I condition) are higher than those for the Langmuir equation (0.891–0.989 in water and 0.978–0.997 in the warm-I condition), indicating the better fitting by the Freundlich model. Water condition: pH 7.0 & 291 K. The warm-I condition (pH 6.5 & 311 K) represents the simulated intestinal liquids of warm-blooded organisms (NaCl 0.12 M, Na2CO3 0.02 M, and C26H44NNaO7S 15.5 mM).
According to the Freundlich model, the slope and intercept correspond to (1/n) and , respectively. The value of n > 1 for each bisphenol indicates that the desorption is nonlinear and favorable, implying that the desorption may be enhanced with increased amounts of bisphenols initially adsorbed onto the PVC microplastics [36]. Moreover, the value of (Table S3 of the SI) for each bisphenol in the water was larger than the corresponding value in the warm-I condition, which may be due to the higher affinity between the bisphenol and PVC microplastics in the water environment [37,38]. In other words, the desorption degree of bisphenols from PVC microplastics was relatively lower in the water condition. Moreover, the order of (water) > (water) > (warm-I) also suggested that the hysteresis may occur in the water condition rather than the warm-I condition. Thus, a part of the adsorbed bisphenols will be irreversibly retained in PVC microplastics in the water condition. Based on these results, the desorption routes of the bisphenols may be different under these two environmental conditions, requiring further examination.
3.2. Hysteresis analysis
Adsorption/desorption hysteresis, which is a consequence of the difference between the routes of the adsorption and desorption processes, was observed for the five bisphenols on PVC microplastics under the water and warm-I conditions. The parameters obtained from the isotherm measurements are summarized in Table 2, together with the adsorption data from our previous study [3] and the desorption data under both the water and warm-I conditions of this study. It was observed that there was no adsorption/desorption hysteresis in the simulated intestinal conditions of warm-blooded organisms, with the value of each bisphenol in the warm-I condition lower than the corresponding value of the bisphenol in the water. This phenomenon can be further explained by the fact that the desorption of bisphenols was promoted under conditions with a lower pH and higher temperature. However, clear adsorption/desorption hysteresis was observed for bisphenols in the water condition, demonstrating that the bisphenol adsorption by PVC microplastics may not be fully reversible.
Table 2.
Parameters from Freundlich modeling and hysteresis analysis for the adsorption/desorption of bisphenols on PVC microplastics.
| Adsorptiona (water) |
Desorption (water) |
Desorption (warm-Ic) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| b | nb | R2b | b | nb | R2b | HI⁋ | b | nb | R2b | HI⁋ | |
| BPA | 0.801 | 1.21 | 0.994 | 1.033 | 1.512 | 0.995 | 0.289 | 0.398 | 1.218 | 0.997 | <0 |
| BPAF | 1.458 | 1.14 | 0.993 | 1.933 | 1.155 | 0.994 | 0.326 | 0.465 | 1.154 | 0.999 | <0 |
| BPB | 1.182 | 1.16 | 0.993 | 1.542 | 1.237 | 0.996 | 0.304 | 0.428 | 1.179 | 0.999 | <0 |
| BPF | 0.575 | 1.13 | 0.993 | 0.720 | 1.594 | 0.991 | 0.252 | 0.388 | 1.186 | 0.993 | <0 |
| BPS | 0.503 | 1.15 | 0.995 | 0.602 | 1.649 | 0.971 | 0.197 | 0.341 | 1.207 | 0.998 | <0 |
⁋ HI is the hysteresis index.
Adsorption parameters were taken from Wu et al.3.
and (mg−1/n·L−1/n·g−1) are the Freundlich constants for adsorption and desorption, respectively. n is the surface heterogeneity constant. R2 is the correlation coefficient of the model.
Warm-I represents the simulated intestinal liquids of warm-blooded organisms.
Hysteresis results can be classified as either artifacts [39] or true hysteresis [40], with the artifacts mainly caused by adsorbate loss and incomplete desorption. Adsorbate loss occurs due to degradation, volatilization, and glass-wall adsorption [39]. However, in our case, degradation is unlikely to occur because NaN3 salt was added to all of the solutions to avoid any degradation. Moreover, control experiments showed that the mass losses of all bisphenols were less than 3%, implying that glass-wall adsorption can be neglected. In addition, adequate time (48 h for adsorption and 75 h for desorption) was provided in this study to reach adsorption/desorption equilibrium for the bisphenols in the water condition. Hence, it is unlikely that artifacts are the primary origin of the hysteresis phenomenon observed in this study. True hysteresis may be derived from two sources, namely, the formation of irreversible bonding between the sorbents and sorbates [38] and the entrapment of the sorbates by the intrinsic structure of the sorbents [41,42]. In our previous study [3], noncovalent bonding (hydrogen and halogen bonding) was reported to be involved in bisphenol adsorption onto PVC microplastics and may give rise to the adsorption/desorption hysteresis. The specific surface area of the pristine microplastics is 9.77 ± 0.28 m2·g−1, while that of the microplastics after desorption is 8.64 ± 0.51 (water) and 11.12 ± 0.32 (warm-I) m2·g−1, indicating different desorption behavior of bisphenols in water and warm-I conditions. In water, bisphenols can be entrapped in the dense and glassy structure [43,44] of the PVC microplastics, which therefore plays a substantial role in giving rise to the irreversible desorption. However, with larger porosity of the microplastics in warm-I conditions, the desorption rate of bisphenols can be enhanced.
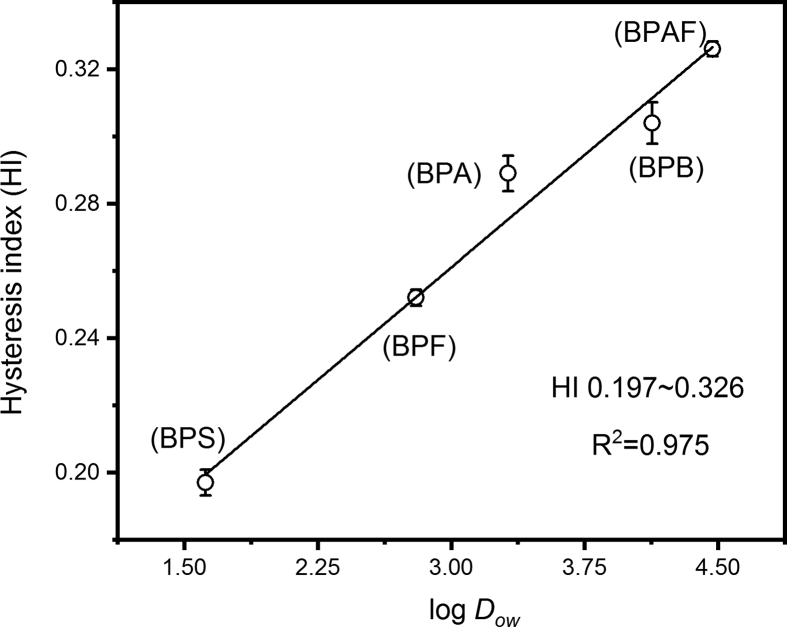
Furthermore, the hysteresis index (HI) of each bisphenol was calculated and is listed in Table 2, while the hysteresis loops were illustrated in Figure S2 of the SI. It was observed that the HI of the adsorption/desorption processes in water increased in the order of BPS < BPF < BPA < BPB < BPAF. Moreover, the correlation analysis presented in Fig. 3 further illustrates the excellent linear association (R2 = 0.975) between the HI and log Dow data sets, showing a positive correlation between the adsorption/desorption hysteresis and the hydrophobicity of the bisphenols. Moreover, more hydrophobic bisphenols (e.g., BPAF and BPB with higher log Dow value) can be more easily entrapped in the inner spaces of the glassy PVC microplastics due to micropore filling [42], but this is energetically unfavorable for their subsequent desorption into the bulk aqueous solution. However, the less-hydrophobic bisphenols (BPS and BPF) appear to be unlikely to enter the inner polymeric domain because of surface adsorption by PVC microplastics, as reported by Wu et al. [3]. As a result, the desorption of BPS and BPF from the contaminated PVC microplastics was more reversible than that of the other bisphenols.
Fig. 3.
Correlation between the octanol-water partition coefficient (log Dow) for the five bisphenols and the adsorption/desorption hysteresis index (HI) in the water condition. The correlation coefficient is 0.975, indicating the critical effect of hydrophobic interactions on the adsorption/desorption hysteresis of bisphenols from the bisphenol-attached PVC microplastics.
3.3. pH and temperature effect on the desorption of bisphenols
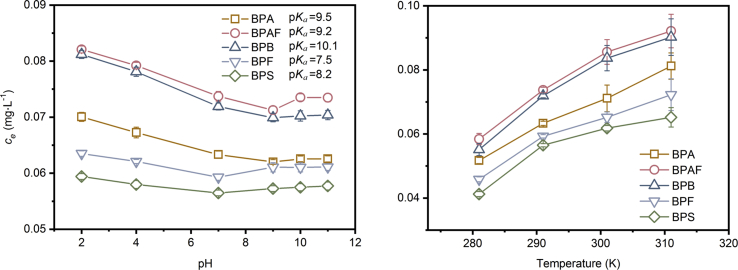
As shown in Fig. 4a, in solutions with pH lower than pKa, the desorption of bisphenols were decreased from 0.070 to 0.062 mg·L−1 for BPA, 0.082 to 0.071 mg·L−1 for BPAF, 0.081 to 0.070 mg·L−1 for BPB, 0.064 to 0.059 mg·L−1 for BPF, and 0.059 to 0.056 mg·L−1 for BPS, respectively with the increased pH value. However, at pH higher than each acid dissociation constant of BPA (pKa = 9.5), BPB (pKa = 10.1), BPAF (pKa = 8.2), BPF (pKa = 7.55), BPS (pKa = 9.2), the desorption amount was found to increase slightly with the further elevated pH value. The slight increase of desorption at higher pH range (8.0–11.0) is attributed to the electrical repulsion generated between PVC microplastics and bisphenols. The majority of the bisphenols would be deprotonated and exist as anions in conditions with pH higher than pKa [3], while the surface of PVC microplastics is negatively charged in the solution when pH value is higher than its zero-point charge (pHpzc 3.41) (Figure S1(b)). Therefore, the concentration of each chemical in the solution would be enhanced by electrostatic repulsion between PVC microplastics and the anionic fractions partially ionized from the bisphenols. However, the bisphenols mainly exist as molecules in condition with pH < pKa, and in this case the electrical repulsion is not predominant any more. However, more protons in the solution will attack or corrode the microstructure of the adsorbents, causing larger inner space [38,42], causing more bisphenols molecules released according to the micropore filling mechanism [42]. Therefore, in solutions with pH ≤ 7.0, the number of bisphenols was higher at lower pH value.
Fig. 4.
Desorption concentration of bisphenols in conditions with different (a) pH and (b) temperatures. The desorption experiments were carried out in solutions at 291 K, stirring at 200 rpm until reaching the equilibrium. The initial amounts of the five bisphenols adsorbed on PVC plastics were within the range of 0.787–1.050 mg·g−1. For the desorption experiment under different temperatures, the solution pH is 7.0.
Fig. 4b further demonstrates the desorption of bisphenols in solutions with different temperatures (281, 291, 301, 311K). The results show that the desorption amount was largely enhanced in the solutions with higher temperatures, with the value of 0.052–0.081, 0.058–0.092, 0.055–0.090, 0.045–0.072, and 0.041–0.065 mg·L−1 for BPA, BPB, BPAF, BPF, and BPS, respectively. The increase in bisphenol desorption with elevated temperatures can be explained by the thermodynamic behavior of the compounds [3]. The bisphenol molecules possessing polar groups are able to have electrostatic interaction with the surface of PVC microplastics. At higher temperatures, the equilibrium will be shifted to the reverse side, causing more molecules released from the adsorbed microplastics.
3.4. Cytotoxicity and potential health effects of bisphenol-attached PVC microplastics
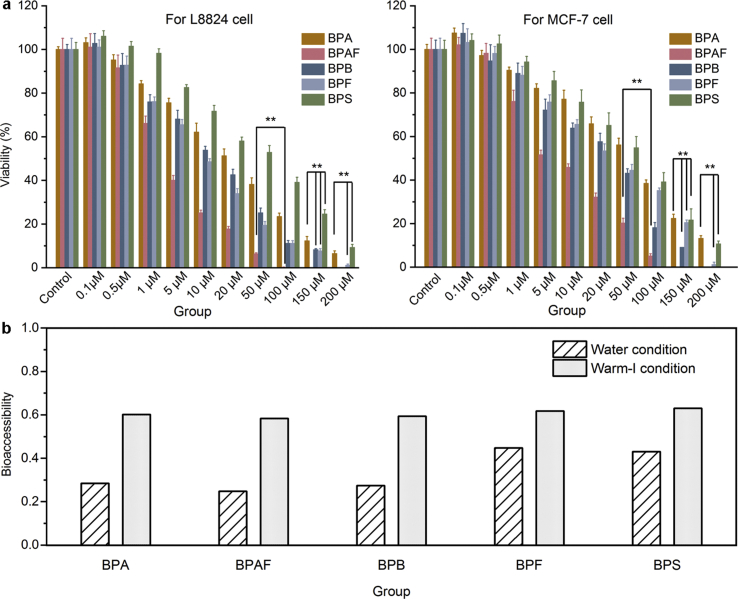
To evaluate the potential health effects of bisphenol-attached microplastics, the cytotoxicity of pristine PVC microplastics and bisphenols was tested on both grass carp hepatocyte (L8824) and breast adenocarcinoma (MCF-7) cell lines. The corresponding viability of both cell lines was measured via the MTT method, and the data in Fig. 5a and Figure S3 of the SI illustrate the effect of bisphenols and pristine PVC microplastics on the cell viability, respectively. An examination of the results shows that the pristine PVC microplastics (with a concentration of 1.5 g·L-1) did not display obvious cytotoxicity toward either L8824 or MCF-7 cells, which still maintained a log-growth phase. However, with an increased dosage of bisphenols, the cell viability was found to increase slightly and then decrease clearly (Fig. 5a). Table 3 lists the IC50 values of the bisphenols, and it was observed that the IC50 of each bisphenol for L8824 showed a slightly lower value than the corresponding value for MCF-7. The obvious impairment of both cell lines is clearly illustrated by the data presented in Fig. 5a, and the IC50 values (Table 3) for both cell lines follow the same order of BPAF < BPB < BPF < BPA < BPS. With an IC50 < 10 μM, BPAF exhibited the strongest ability to induce acute cytotoxicity effects [47] in both cell lines, while BPB expressed an acute cytotoxicity effect toward the L8824 cells. In addition, as the bisphenol with the lowest cytotoxicity in this study, BPS showed IC50 values for L8824 and MCF-7 that were 10.46 and 7.35 times higher than those for BPAF, respectively. The comparable IC50 values for MCF-7 were also reported in previous studies [45,46], as well as a similar cytotoxicity sequence of different bisphenol analogues [47,48]. The differences in the toxic sensitivities of the cells to the chemicals may originate from the physiochemical properties of the materials such as hydrophobicity, and solubility [[47], [48], [49]]. Generally, chemicals with a relatively low hydrophobicity always exhibit low toxicity [47,48], which is consistent with our findings that the IC50 values increase with decreasing hydrophobicity for most of the bisphenols (BPAF, BPB, BPA and BPS). However, different behavior is observed for BPF, which showed a higher toxic effect than that of BPA but had a lower log Dow (2.80 of BPF vs 3.32 of BPA). As reported in our previous study, BPAF, BPB, BPA and BPS are found mainly in the molecular form in neutral conditions, while the solubility of BPF can be enhanced by a higher degree of deprotonation. Therefore, higher cytotoxicity was observed for BPF than for BPA, which may be due to the increased combination of BPF with proteins [50]. In addition to the properties of the chemical itself, bioaccessibility has also been regarded as a critical feature in evaluating potential health risks [51]. In this study, the bioaccessibility of bisphenol-attached microplastics was characterized by the desorption performance of the bisphenol-attached PVC microplastics. After desorption in the water and warm-I conditions, the concentrations of the five bisphenols were measured, with the obtained results listed in Table 4. For a common scenario (initial bisphenols concentration as 0.1 mg·L−1), 1.8%–15.3% of bisphenols were desorbed from the PVC microplastics in water, but 43.7%–54.0% were desorbed in the warm-I conditions. Meanwhile, in the limiting scenario (initial bisphenols concentration as 2.0 mg·L−1), 24.9–43.1% of the bisphenols were desorbed from the PVC microplastics in water, but 58.4%–63.1% were desorbed in the warm-I condition (Fig. 5b). Such high bioaccessibility of the bisphenol-attached microplastics in the warm-I condition may be due to the lower pH and higher temperature, as discussed in the kinetics study section.
Fig. 5.
(a) Cell viability after incubation with bisphenols for a grass carp hepatocyte cell line (L8824) and breast adenocarcinoma cells (MCF-7). ∗∗: p < 0.01. The IC50 values of BPA, BPAF, BPB, BPF and BPS were 16.19 ± 3.22, 3.43 ± 0.54, 9.67 ± 0.92, 11.29 ± 2.12 and 35.89 ± 6.01 μM, respectively, for L8824, while the corresponding values of 36.88 ± 4.46, 7.87 ± 3.98, 21.65 ± 4.92, 31.89 ± 1.69 and 57.92 ± 7.76 μM, respectively, were obtained for MCF-7. (b) Bioaccessibility of the bisphenols from the bisphenol-attached PVC microplastics in the water (striped bars) and warm-I conditions (gray bars). The bioaccessibility was calculated as the percentage of desorbed bisphenols to the total adsorbed bisphenols on the PVC microplastics (with initial bisphenols concentration as 2.0 mg·L−1).
Table 3.
IC50 values (μM) of the bisphenols to L8824 and MCF-7 cell lines.
| L8824 (μM) | L8824 (mg·L−1) | MCF-7 (μM) | MCF-7 (mg·L−1) | |
|---|---|---|---|---|
| BPA | 16.19 ± 3.22a | 3.70 ± 0.73 | 36.88 ± 4.46 | 8.42 ± 1.02 |
| BPAF | 3.43 ± 0.54 | 1.15 ± 0.18 | 7.87 ± 3.98 | 2.65 ± 1.34 |
| BPB | 9.67 ± 0.92 | 2.34 ± 0.23 | 21.65 ± 4.92 | 5.25 ± 1.19 |
| BPF | 11.29 ± 2.12 | 2.26 ± 0.43 | 31.89 ± 1.69 | 6.39 ± 0.34 |
| BPS | 35.89 ± 6.01 | 8.98 ± 1.51 | 57.92 ± 7.76 | 14.50 ± 1.95 |
The data was reported as “mean ± standard error” based on six replicates.
Table 4.
The desorbed concentration of each bisphenol and its potential cytotoxicity for L8824 and MCF-7 in water and warm-I conditions.
| Bisphenosl | Water (μM) | Water (mg·L−1) | Cytotoxicity (L8824, %) | Cytotoxicity (MCF-7, %) | Warm-I (μM) | Warm-I (mg·L−1) | Cytotoxicity (L8824, %) | Cytotoxicity (MCF-7, %) | |
|---|---|---|---|---|---|---|---|---|---|
| Low concentration (0.1 mg L−1) | BPA | 0.031 | 0.007 | – | – | 0.140 | 0.032 | – | – |
| BPAF | 0.036 | 0.012 | – | – | 0.104 | 0.035 | – | – | |
| BPB | 0.041 | 0.010 | – | – | 0.140 | 0.034 | – | – | |
| BPF | 0.035 | 0.007 | – | – | 0.139 | 0.028 | – | – | |
| BPS | 0.039 | 0.001 | – | – | 0.108 | 0.027 | – | – | |
| High concentration (2 mg L−1) | BPA | 1.45 | 0.33 | 20.08 | 12.36 | 3.05 | 0.70 | 29.75 | 21.75 |
| BPAF | 1.07 | 0.36 | 32.70 | 24.19 | 2.50 | 0.84 | 46.31 | 36.87 | |
| BPB | 1.53 | 0.37 | 26.25 | 18.79 | 3.30 | 0.80 | 36.95 | 29.53 | |
| BPF | 1.87 | 0.38 | 27.15 | 20.83 | 2.95 | 0.59 | 33.24 | 26.54 | |
| BPS | 1.54 | 0.39 | 12.89 | 12.56 | 2.28 | 0.57 | 17.59 | 17.33 |
(with both units as μM and mg·L−1).
Accordingly, the proliferation reductions in L8824 and MCF-7 were calculated following the procedures described in detail in “Section 1.5” of the SI, and the obtained results are summarized in Table 4. The proliferation reduction values of BPA, BPAF, BPB, BPF and BPS in the water environment, were 20.08%, 32.70%, 26.25%, 27.15% and 12.89%, respectively, for L8824 and 12.36%, 24.19%, 18.79%, 20.83%, and 12.56%, respectively, for MCF-7, while they were 29.75%, 46.31%, 36.95%, 33.24% and 17.59%, respectively, for L8824 and 21.75%, 36.87%, 29.53%, 26.54% and 17.33%, respectively, for MCF-7 in the warm-I condition.
4. Conclusions
The conclusions of this study can be further emphasized, as described below, with respect to the transfer/release behaviors and potential toxicity of bisphenols-attached PVC microplastics. The release of bisphenols is much more serious in the simulated intestinal environment (warm-I conditions) than in the aquatic environment (water). The enhanced bioaccessibility indicates that the health risk for bisphenol-attached PVC microplastics may be significantly increased after ingestion. The released bisphenols play a crucial role in the potential toxicity, and microplastics acting as a vector for bisphenols and even other pollutants may provide a critical route for increasing the potential health risks.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China(NSFC) (41977329), and the State Environmental Protection Key Laboratory of Integrated Surface Water-Groundwater Pollution Control.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2020.100027.
Contributor Information
Yuanyuan Tang, Email: tangyy@sustech.edu.cn.
Zongwei Cai, Email: zwcai@hkbu.edu.hk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Galloway T.S., Lewis C.N. Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. U.S.A. 2016;113(9):2331–2333. doi: 10.1073/pnas.1600715113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galloway T.S., Cole M., Lewis C., Atkinson A., Allen J.I. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017;1:116. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- 3.Wu P., Cai Z., Jin H., Tang Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019;650(1):671–678. doi: 10.1016/j.scitotenv.2018.09.049. [DOI] [PubMed] [Google Scholar]
- 4.Arthur C., Baker J., Bamford H. Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris. NOAA Technical Memorandum NOS- OR & R-30. NOAA; Silver Spring, U.S.A: 2009. [Google Scholar]
- 5.Lebreton L.C.M., van der Zwet J., Damsteeg J.W., Slat B., Andrady A., Reisser J. River plastic emissions to the world’s oceans. Nat. Commun. 2017;8:15611. doi: 10.1038/ncomms15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P., Tang Y., Dang M., Wang S., Jin H., Liu Y., Jing H., Zheng C., Yi S., Cai Z. Spatial-temporal distribution of microplastics in surface water and sediments of maozhou river within Guangdong-Hong Kong-Macao greater Bay area. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2019.135187. [DOI] [PubMed] [Google Scholar]
- 7.Teuten E.L., Rowland S.J., Galloway T.S., Thompson R.C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007;41(22):7759–7764. doi: 10.1021/es071737s. [DOI] [PubMed] [Google Scholar]
- 8.Bradney L., Wijesekara H., Palansooriya K., Obadamudalige N., Bolan N., Ok Y., Rinklebe J., Kim K., Kirkham M. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019;131 doi: 10.1016/j.envint.2019.104937. [DOI] [PubMed] [Google Scholar]
- 9.Cole M., Lindeque P., Fileman E., Halsband C., Goodhead R., Moger J., Galloway T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013;47:6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- 10.Watts A.J.R., Lewis C., Goodhead R.M., Beckett S.J., Moger J., Tyler C.R., Galloway T.S. Uptake and retention of microplastics by the shore crab carcinus maenas. Environ. Sci. Technol. 2014;48(15):8823–8830. doi: 10.1021/es501090e. [DOI] [PubMed] [Google Scholar]
- 11.Besseling E., Wegner A., Foekema E.M., van den Heuvelgreve M.J., Koelmans A.A. Effects of microplastic on fitness and PCB bioaccumulation by the Lugworm Arenicola marina (L.) Environ. Sci. Technol. 2013;47(1):593–600. doi: 10.1021/es302763x. [DOI] [PubMed] [Google Scholar]
- 12.Carbery M., O’Connor W., Thavamani P. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018;115:400–409. doi: 10.1016/j.envint.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Diepens N., Koelmans A. Accumulation of plastic debris and associated contaminants in aquatic food webs. Environ. Sci. Technol. 2018;52:8510–8520. doi: 10.1021/acs.est.8b02515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakir A., O’Connor I.A., Rowland S.J., Hendriks A.J., Thompson R.C. Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environ. Pollut. 2016;219:56–65. doi: 10.1016/j.envpol.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Koelmans A.A., Bakir A., Burton G.A., Janssen C.R. Microplastic as a vector for chemicals in the aquatic environment: critical review and modelsupported reinterpretation of empirical studies. Environ. Sci. Technol. 2016;50:3315–3326. doi: 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X., Chen B., Li Q., Liu N., Xia B., Zhu L., Qu K. Toxicities of polystyrene nano- and microplastics toward marine bacterium Halomonas alkaliphile. Sci. Total Environ. 2018;642:1378–1385. doi: 10.1016/j.scitotenv.2018.06.141. [DOI] [PubMed] [Google Scholar]
- 17.Bakir A., Rowland S., Thompson R. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014;185:16–23. doi: 10.1016/j.envpol.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Avio C.G., Gorbi S., Regoli F. Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar. Environ. Res. 2016;128:1–10. doi: 10.1016/j.marenvres.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Duis K., Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016;28(1):2. doi: 10.1186/s12302-015-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavigan C., Streep M. Healthy Child Healthy World; New York, USA: 2008. Healthy Child Healthy World: Creating a Cleaner, Greener, Safer Home. [Google Scholar]
- 21.Regnault C., Usal M., Veyrenc S., Couturier K., Batandier C., Bulteau A., Lejon D., Sapin A., Combourieu B., Chetiveaux M., May C., Lafond T., Raveton M., Reynaud S. Unexpected metabolic disorders induced by endocrine disruptors in Xenopus tropicalis provide new lead for understanding amphibian decline. Proc. Natl. Acad. Sci. U.S.A. 2018;19(115):4416–4425. doi: 10.1073/pnas.1721267115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S., Kim C., Shin H., Kho Y., Choi K. Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish. Chemosphere. 2019;221:115–123. doi: 10.1016/j.chemosphere.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Maćczak A., Cyrkler M., Bukowska B., Michałowicz J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study) Toxicol. Vitro. 2017;41:143–149. doi: 10.1016/j.tiv.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Jin H., Zhu J., Chen Z., Hong Y., Cai Z. Occurrence and partitioning of bisphenol analogues in adults’ blood from China. Environ. Sci. Technol. 2018;52:812–820. doi: 10.1021/acs.est.7b03958. [DOI] [PubMed] [Google Scholar]
- 25.Wu L., Zhang X., Wang F., Gao C., Chen D., Palumbo J., Guo Y., Zeng E. Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci. Total Environ. 2018;615:87–98. doi: 10.1016/j.scitotenv.2017.09.194. [DOI] [PubMed] [Google Scholar]
- 26.Chen D., Kannan K., Tan H., Zheng Z., Feng Y., Wu Y., Widelka M. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-A Review. Environ. Sci. Technol. 2016;50:5438–5453. doi: 10.1021/acs.est.5b05387. [DOI] [PubMed] [Google Scholar]
- 27.Kinch C., Ibhazehiebo K., Jeong J., Habibi H., Kurrasch D. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc. Natl. Acad. Sci. U.S.A. 2015;112(5):1475–1480. doi: 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang S., Liang S., Yin N., Faiola F. Establishment of a human embryonic stem cell-based liver differentiation model for hepatotoxicity evaluations. Ecotoxicol. Environ. Saf. 2019;174:353–362. doi: 10.1016/j.ecoenv.2019.02.091. [DOI] [PubMed] [Google Scholar]
- 29.Lei B., Xu J., Peng W., Wen Y., Zeng X., Yu Z., Wang Y., Chen T. In vitro profiling of toxicity and endocrine disrupting effects of bisphenol analogues by employing MCF-7 cells and two-hybrid yeast bioassay. Environ. Toxicol. 2015;32:278–289. doi: 10.1002/tox.22234. [DOI] [PubMed] [Google Scholar]
- 30.Bohdziewicz J., Kaminska G. Kinetics and equilibrium of the sorption of bisphenol A by carbon nanotubes from wastewater. Water Sci. Technol. 2013;68:1306–1314. doi: 10.2166/wst.2013.373. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed Nor N., Koelmans A. Transfer of PCBs from microplastics under simulated gut fluid conditions is biphasic and reversible. Environ. Sci. Technol. 2018;53:1874–1883. doi: 10.1021/acs.est.8b05143. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z., Zhao J., Song L., Mashayekhi H., Chefetz B., Xing B. Adsorption and desorption of phenanthrene on carbon nanotubes in simulated gastrointestinal fluids. Environ. Sci. Technol. 2011;45(14):6018–6024. doi: 10.1021/es200790x. [DOI] [PubMed] [Google Scholar]
- 33.Moreno F., Quintanilla-Lopez J., Lebron-Aguilar R., Olano A., Sanz M. Mass spectrometric characterization of glycated p-Lactoglobulin peptides derived from galacto-oligosaccharides surviving the in vitro gastrointestinal digestion. J. Am. Soc. Mass Spectrom. 2008;19:927–937. doi: 10.1016/j.jasms.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Wei J., Xiang L., Yuan Z., Li S., Yang C., Liu H., Jiang Y., Cai Z. Metabolic profiling on the effect of 2,20,4,40-tetrabromodiphenyl ether (BDE-47) in MCF-7 cells. Chemosphere. 2018;192:297–304. doi: 10.1016/j.chemosphere.2017.10.170. [DOI] [PubMed] [Google Scholar]
- 35.Daneshvar E., Vazirzadeh A., Niazi A., Kousha M., Naushad M., Bhatnagar A. Desorption of methylene blue dye from brown macroalga: effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017;152(20):443–453. [Google Scholar]
- 36.Hameed B.H., Rahman A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard Mater. 2008;160(2−3):576–581. doi: 10.1016/j.jhazmat.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 37.Li Q., Han C., Horton S.R., Cabrera M.F., Sumpter B.G., Lu W., Bernholc J., Maksymovych P., Pan M. Supramolecular self-assembly of π-conjugated hydrocarbons via 2D cooperative CH/π interaction. ACS Nano. 2012;6(1):566–572. doi: 10.1021/nn203952e. [DOI] [PubMed] [Google Scholar]
- 38.Liu J., Ma Y., Zhu D., Xia T., Qi Y., Yao Y., Guo X., Ji R., Chen W. Polystyrene nanoplastics-enhanced contaminant transport: role of irreversible adsorption in glassy polymeric domain. Environ. Sci. Technol. 2018;52:2677–2685. doi: 10.1021/acs.est.7b05211. [DOI] [PubMed] [Google Scholar]
- 39.Ma X., Anand D., Zhang X., Talapatra S. Adsorption and desorption of chlorinated compounds from pristine and thermally treated multiwalled carbon nanotubes. J. Phys. Chem. C. 2011;115:4552–4557. [Google Scholar]
- 40.Pan B., Lin D., Mashayekhi H., Xing B. Adsorption and hysteresis of bisphenol A and 17α-ethinyl estradiol on carbon nanomaterials. Environ. Sci. Technol. 2008;42:5480–5485. doi: 10.1021/es8001184. [DOI] [PubMed] [Google Scholar]
- 41.Lian F., Yu W., Wang Z., Xing B. New insights into black carbon nanoparticle-induced dispersibility of goethite colloids and configuration-dependent sorption for phenanthrene. Environ. Sci. Technol. 2019;53(2):661–670. doi: 10.1021/acs.est.8b05066. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L., Wang L., Zhang P., Kan A.T., Chen W., Tomson M.B. Facilitated transport of 2,2’,5,5’-polychlorinated biphenyl and phenanthrene by fullerene nanoparticles through sandy soil columns. Environ. Sci. Technol. 2011;45(4):1341–1348. doi: 10.1021/es102316m. [DOI] [PubMed] [Google Scholar]
- 43.Guo X., Wang X., Zhou X., Kong X., Tao S., Xing B. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: role of chemical and physical composition. Environ. Sci. Technol. 2012;46:7252–7259. doi: 10.1021/es301386z. [DOI] [PubMed] [Google Scholar]
- 44.Bernard C., Bahlouli N., Wagner-Kocher C., Lin J., Ahzi S., Remond Y. Multiscale description and prediction of the thermomechanical behavior of multilayered plasticized PVC under a wide range of strain rate. J. Mater. Sci. 2018;53:14834–14849. [Google Scholar]
- 45.Russo G., Capuozzo A., Barbato F., Irace C., Santamaria R., Grumetto L. Cytotoxicity of seven bisphenol analogues compared to bisphenol A and relationships with membrane affinity data. Chemosphere. 2018;201:432–440. doi: 10.1016/j.chemosphere.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Lei B., Xu J., Peng W., Wen Y., Zeng X., Yu Z., Wang Y., Chen T. In vitro profiling of toxicity and endocrine disrupting effects of bisphenol analogues by employing MCF-7 cells and two-hybrid yeast bioassay. Environ. Toxicol. 2015;32:278–289. doi: 10.1002/tox.22234. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y., Liu D., Wang D., Wang Y., Fu Q., Fallon J., Yang X., He Z., Liu F. Combinational delivery of hydrophobic and hydrophilic anticancer drugs in single nanoemulsions to treat MDR in cancer. Mol. Pharm. 2014;11:2623–2630. doi: 10.1021/mp400778r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu X., Wu G., Xing Y., Wang C., Yuan X., Li B. Evaluation of single and combined toxicity of bisphenol A and its analogues using a highly-sensitive micro-biosensor. J. Hazard Mater. 2020;381 doi: 10.1016/j.jhazmat.2019.120908. [DOI] [PubMed] [Google Scholar]
- 49.Yin L.M., Edwards M.A., Li J., Yip C.M., Deber C.M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptidemembrane interactions. J. Biol. Chem. 2012;287:7738–7745. doi: 10.1074/jbc.M111.303602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Zhang X., Fei X., Wang S., Gao H. Binding of bisphenol A and acrylamide to BSA and DNA: insights into the comparative interactions of harmful chemicals with functional biomacromolecules. J. Hazard Mater. 2010;182(1–3):877–885. doi: 10.1016/j.jhazmat.2010.06.131. [DOI] [PubMed] [Google Scholar]
- 51.Li W., Zhao J., Zhao Q., Zheng H., Du P., Tao S., Xing B. Adsorption and bioaccessibility of phenanthrene on carbon nanotubes in the in vitro gastrointestinal system. Sci. Total Environ. 2016;566:50–56. doi: 10.1016/j.scitotenv.2016.04.204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.