Abstract
Lack of suitable electron donors or acceptors is in many cases the key reason for pollutants to persist in the environment. Externally supplementation of electron donors or acceptors is often difficult to control and/or involves chemical additions with limited lifespan, residue formation or other adverse side effects. Microbial electrochemistry has evolved very fast in the past years – this field relates to the study of electrochemical interactions between microorganisms and solid-state electron donors or acceptors. Current can be supplied in such so-called bioelectrochemical systems (BESs) at low voltage to provide or extract electrons in a very precise manner. A plethora of metabolisms can be linked to electrical current now, from metals reductions to denitrification and dechlorination. In this perspective, we provide an overview of the emerging applications of BES and derived technologies towards the bioremediation field and outline how this approach can be game changing.
1. Background
The release of potentially harmful pollutants in the environment has been a cause for concern for many years. Both inorganic and organic pollutants can be identified, and most of the pollutants can be chemically and/or biologically transformed into non-toxic or less toxic forms. Inorganic pollutants, for example, nitrate that is ubiquitously present above drinking water standards in groundwater can be reduced to nitrogen gas [1]; arsenite can be oxidized to arsenate which stably adsorbs onto metal-oxides [2]; and soluble heavy metals such as U(VI) and Cr(VI) can be reduced to insoluble U(IV) and Cr(III), then removed by precipitation [3,4]. In terms of organic pollutants, oil and fuel spills lead to hydrocarbons such as BTEX compounds (benzene, toluene, ethylbenzene, and xylenes) release, which can be fully degraded by oxidation [5]. Halogenated compounds (e.g., trichloroethylene (TCE), perchloroethylene (PCE) and polychlorinated biphenyl (PCB) can be reductively dechlorinated [6,7]. However, some organic pollutants are extremely persistent in the environment, such as amphiphilic per- and polyfluoroalkyl substances (PFAS), whose degradation need strong catalytic activity to cleave their chemical structures [8,9].
In most cases, the transformation of pollutants in nature is slow due to the lack of suitable electron donors or acceptors, a suitable driving force is ultimately needed to drive their biotic or abiotic reductions or oxidations, respectively. To accelerate pollutant transformation via natural processes, technologies were factitiously introduced consisting of precipitation, adsorption, electrodialysis, ion exchange, phytoremediation, microbiological methods, membrane filtration and nanotechnology [[10], [11], [12], [13], [14]]. Nevertheless, these conventional technologies are generally energy and chemical intensive, difficult to control and/or time consuming [11,[13], [14], [15]]. Moreover, the limitation of electron donor/acceptor availability, the lack of contact between the pollutants and amendments, the interference of other co-existing pollutants and the generation of toxic by-products have further restricted applications of those technologies [[16], [17], [18]].
In the past decade, approaches derived from microbial electrochemistry have been suggested as alternative strategies to overcome limitations of the conventional remediation technologies [19,20]. Here in this perspective we provide a brief overview of these alternative technologies especially from an application point of view. This allows putting the key advantages and challenges of these approaches into perspective.
2. What is microbial electrochemistry?
Microbial electrochemistry is a branch of bioelectrochemistry which analyzes and applies electron transfer reactions taking place between living microbial cells and electron conductors such as solid-state electrodes or naturally occurring minerals (e.g., iron-, and manganese-oxides, metallic particles) [21]. Although early studies on microbial electrochemistry date back more than a hundred years [22], only in the past 15 years this research area has been subject of systematic research. This has not only elucidated the fundamental aspects of microbial extracellular electron transfer (EET) and the diversity and metabolic capabilities of microorganisms involved, but also contributed to identifying key challenging aspects linked to technology development and in some cases game-changing, industrial and environmental applications [23].
Technology development in this area, referred to as “Microbial electrochemical technology” (MET; https://www.is-met.org/), implies that the microbial metabolism is deliberately linked to a solid-state electron donor or acceptor, which can be a mineral particle or an electrode. Where the electrode functions as electron acceptor, it is effectively an anode as opposed to a cathode where the electrode donates electrons.
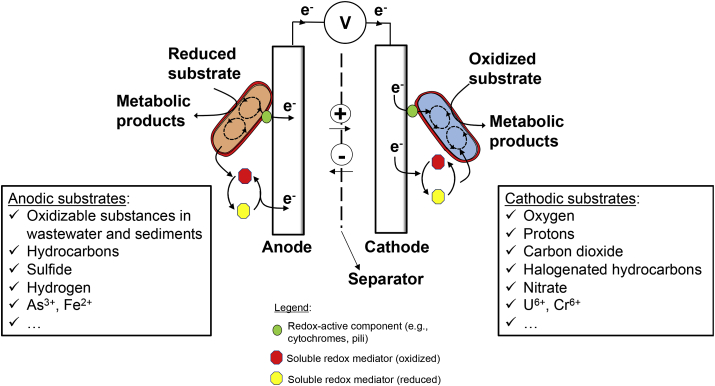
The “Microbial Fuel Cell” (MFC) is the archetype of MET and probably the most well-known example [24]. MFC is commonly applied for the bioremediation of organic pollutants [25,26]. Electroactive microorganisms oxidize substrates, such as organic acids or hydrocarbons, using the anode as electron acceptor via diverse metabolisms (Fig. 1) [27]. Electrons flow from the anode to the cathode where, in the presence of a suitable catalyst, higher potential electron acceptors are reduced. As this implies a current flow over a positive potential difference, net electrical energy is generated. The study of anodic EET has initially focused on two model microorganisms, Shewanella oneidensis MR-1 and Geobacter sulfurreducens PCA, both Gram-negative mesophilic bacteria that in their natural habitats use insoluble iron- or manganese-oxides as respiratory electron acceptors in their energy metabolism [27]. To date, almost 100 different isolated microorganisms from the bacterial and archaeal domain can generate various levels of electric currents [28,29]. Interestingly, many of them are not iron- or manganese-oxide reducers, thereby raising intriguing questions regarding the ecological and evolutionary basis of EET in these microorganisms.
Fig. 1.
Schematic overview of microbially-catalyzed reactions taking place at the anode and at the cathode of a microbial electrochemical systems.
In an MFC the electron flow is captured for electrical power generation. By contrast, in a “Microbial Electrolysis Cell” (MEC) additional energy is input to produce at the cathode a species which would not be feasible to be formed due to thermodynamic or kinetic constraints. Examples are the reduction of water to hydrogen, carbon dioxide (CO2) to methane (CH4), or Cr(VI) to Cr(III) [30]. In principle, certain reductions (e.g. N2 from NO3−) can occur either in MFC or MEC mode, depending on aspects such as the anode reaction or the current density [15,31,32]. In most cases a small amount of electrical energy (0.5–1.0 V), needs to be added to the circuit in order to drive the otherwise energetically unfavorable cathodic reactions and/or to overcome reaction overpotentials. While initially abiotic catalysts were almost exclusively considered to drive cathodic reactions (primarily hydrogen evolution) at sustainable rates. In recent years there has been an ever-increasing interest in microbially catalyzed cathodic reactions which are, in turn, opening new sustainable bioprocessing possibilities for MEC and microbial electrochemical technologies in general [33].
3. From microbial electrochemistry to bioremediation processes
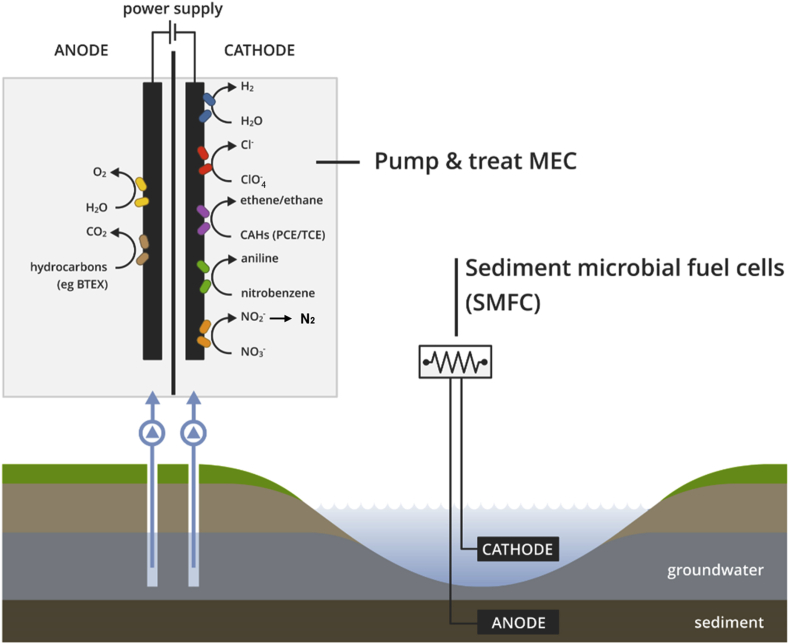
In a bioremediation context, many oxidized and reduced species require removal which opens up opportunities for METs (Fig. 2). In the following section, we highlight some of the key studied electricity driven processes for pollutant removal.
Fig. 2.
Schematic overview of microbial electrochemical bioremediation methods.
3.1. Denitrification
Full denitrification to nitrogen gas driven by a cathode was first demonstrated by Ref. [34] using a mixed population, whereas a pure culture of Geobacter metallireducens was reported earlier to reduce nitrate to nitrite [4]. Since then, this process has been extensively studied. MFCs were able to remove nitrate at the cathode by feeding acetate at the bioanode as electron donor [34,35], while MECs showed higher denitrification rates due to the external energy input [36]. Several studies have proven that the cathode potential applied controls the electron availability and drives the presence of intermediates [[36], [37], [38], [39], [40]]. Indeed, the cathode potential influences the removal rate, the presence of undesirable intermediates (nitrite and nitrous oxide) and the stability of the biocathode [41]. Operational conditions including nitrate load, hydraulic retention time (HRT) and process configuration also impact the denitrification rate and the energy consumption [36]. A recent review [42] evaluated the energy consumption of treating nitrate-contaminated groundwater by BES, leading to the conclusion that in situ MFCs consumed the least energy (0.34 kWh kg NO3−-N−1), while ex situ MFCs required more energy inputs (1.6 kWh kg NO3−-N−1) as the groundwater should be pumped out. Much higher energy costs (19 kWh kg NO3−-N−1 in situ and 10 kWh kg NO3−-N−1 ex situ, respectively) were reported for the MECs due to external power supply.
3.2. Perchlorate reduction
Perchlorate is often a co-contaminant of nitrate, due to the common use of nitrogen in the production of explosives. Nitrate (NO3−/N2 E° = 0.75 V vs. SHE) and perchlorate (ClO4−/Cl− E° = 0.87 V vs. SHE) have high reduction potential and are considered as ideal electron acceptors for microbial reduction [11]. [43] demonstrated the microbial reduction of perchlorate in a biocathode (containing Dechloromonas ans Azospira species) with 2,6-anthraquinone disulfonate as a mediator. This result opened the door to further studies using bioelectrical reduction for perchlorate removal to overcome several issues (i.e. inhibition to oxygen and to some extent nitrate) associated with bioreactor-based processes [44].
3.3. Reductive dechlorination
Biological reductive dechlorination is catalyzed by several groups of bacteria. Some of them (i.e. Dehalococcoides mccartyi and Dehalobacter species) are specialized for organochlorine respiration and are restricted, in their natural environments, to the use of H2 as electron donor [45,46]. Once an enriched dechlorinating consortia is established at the biocathode, it enables the reduction of various refractory organic pollutants including TCE, halogenated aromatics, halogenated phenols, nitroaromatics and others (D [[47], [48], [49], [50], [51], [52]]. The rate and extent are typically influenced by several parameters such as the set cathode potential. Three times higher dechlorination rates of 2,4,6-trichlorophenol via electro-stimulation were reported (cathode potential of −0.36 V vs. SHE) as compared to non-electro conditions, and the complete dechlorination with phenol as the end dechlorination product was achieved [50]. Another study observed 3.7 times higher TCE dechlorination rate by decreasing the cathode potential from −0.25 V to −0.45 V (vs. SHE). However, at this lower potential (−0.45 V) competitive reactions can occur leading to a consumption of over 60% of the electric current for other microbial metabolisms, such as methane generation [53]. Similarly [54], observed the highest TCE dechlorination rate (1 mmol/L d) at −0.26 V, while higher (−0.06 V) or lower potentials (−0.46/-0.66 V) (vs. SHE) resulted in 7–49% lower dechlorination rates. The removal efficiencies and rates can be reinforced via a sequential anaerobic/aerobic treatment, which can be realized in a flow-through bioelectrochemical reactor. Under anaerobic conditions, many microorganisms can transform highly chlorinated parent chlorinated aliphatic hydrocarbons (CAHs) into their less-chlorinated (yet still toxic) daughter CAHs via reductive dichlorination. Then, they are fully biodegraded in the following aerobic anodic chamber, whereby electrolytic oxygen evolution from water occurs [55].
3.4. Hydrocarbon oxidation
Mono aromatic hydrocarbons such as benzene, toluene, ethylbenzene and the three xylene isomers (i.e., BTEX) are frequently found at sites polluted by petroleum refining and petrochemical industries. BTEX removal has been studied quite extensively at anodes with modest energy consumption and at high levels of removal [16] (e.g., pump & treat MECs as shown in Fig. 2). Toluene is the easiest degradable component of BTEX, with >90% removal reported in different studies by using mixed cultures [[56], [57], [58]]. [59] applied a recently developed reactor configuration (referred to as “bioelectric well”) for the treatment of a synthetic groundwater containing a mixture of BTEX. The rate and extent of removal was higher for toluene (up to 70 mg/L d−1) and substantially lower for benzene, ethylbenzene and xylenes, probably due to the fact that the latter compounds were present in the groundwater at lower concentrations.
3.5. PAH removal
Polycyclic aromatic hydrocarbons (PAHs) are a class of persistent organic pollutants which are carcinogenic, mutagenic, and/or teratogenic. They are preliminary found in soils (coal and tar deposits) and produced by the thermal decomposition of organic matter, and other activities. Microbial co-metabolism is an important pathway for the degradation of these refractory organic pollutants [60,61]. Sediment/soil based MFCs (shown in Fig. 2) were deployed by Ref. [62] to assess the degradation of PAHs polluted soils. They found that the system increased the removal rates of anthracene, phenanthrene, and pyrene meanwhile producing electricity (12 mW/m2). The electricity production is only a minor component of the technology but is mostly a good real-time indicator of the level of the degradation activity. Another study [63] also showed bioremediation capabilities of sediment MFCs on the removal of naphthalene, acenaphthene and phenanthrene.
3.6. Mineralization of herbicides and antibiotics in soil
Herbicides like atrazine or isoproturon have been extensively used for treating weeds in soil leading to the pollution of soils. Interestingly, the use of electrodes polarized at potentials as high as +600 mV (vs. Ag/AgCl) was proved to boost microbial mineralization of 14C-labelled atrazine and isoproturon in soil by 20-fold comparing to electrode-free controls [64,65]. The same strategy was applied on manure bioremediation, resulting in 10-fold enhancement of sulfamethazine (antibiotics frequently used in veterinary medicine) mineralization at a reducing potential of −400 mV (vs. Ag/AgCl) [66]. Additionally, this electrochemical bioremediation strategy can, in principle, drastically reduce the ecotoxicity associated to the treated soil.
3.7. An unusual configuration: bioelectrochemical wetland systems
Constructed wetlands are now well established for wastewater treatment. The so-called METland® (Fig. 3) is a MET-based application that integrates the use of electro-conductive granular material in constructed wetlands to effectively perform bioremediation in diverse contexts. Originally, METland® was designed to operate under flooded conditions and short-circuit modes as “snorkel” electrodes [67]. The natural redox gradient between the bottom of the system and the naturally oxygenated surface greatly enhanced microbial oxidative metabolism for removing organic pollutants [67]. The electron flow along the METland® bed was elegantly demonstrated by measuring the profile of electric potential along distances larger than 40 cm [68,69]. Full scale METland® units have been constructed with different electroconductive granular material like electroconductive coke [67] or more sustainable materials like electroconductive biochar (ec-biochar) obtained after wood pyrolysis at high temperature [70]. Although most of the MET-based applications are classically operated under anoxic conditions to avoid oxygen competition with anodic reactions, METland® has been recently proved to be effective even under down-flow aerated mode [71]. Electroactive bacteria from the genus Geobacter have been found to outcompete in such redox mixed environments and, interestingly, such aerobic conditions favoured nitrification so METland® can be operated to remove COD as well as nutrients [71]. Indeed, last generation of METland® was operated under hybrid operation combining aerated aerobic downflow with anoxic flooded mode in order to clean-up 25m3/day urban wastewater in less than 0.5 m2 per person equivalent (www.imetland.eu). METland® is especially effective for removing recalcitrant pollutants like pharmaceuticals present in urban wastewater. As reported by a recent study, seven pharmaceutical compounds (sulfamethoxazole, paraxanthine, carbamazepine, caffeine, ampyrone, atenolol, and naproxen) in emerging contaminants were removed over 95% in a horizontal subsurface flow electroconductive filter similar to METland® configuration [72].
Fig. 3.
METland® unit (20m2) for treating wastewater from 50pe at Carrion de los Céspedes wastewater treatment plant (Spain).
4. Why microbial electrochemistry may be the game changer?
Without generalizing too much, it can be stated that most pollutants are not, or are only slowly, degraded or removed in the environment due to the lack of a suitable electron donor or acceptor. In some cases, there is also a lack of suitable (bio)catalyst. Current bioremediation strategies thus focus on provision of this donor/acceptor which tends to involve the addition of chemicals to the polluted matrix. Microbial electrochemical processes deal with this issue by directly providing or extracting electrons via an electrode or a solid state electron donor/acceptor such as zerovalent iron [73]. A direct and quite specific interaction can thus be set up between the donor/acceptor and the biocatalyst.
A key advantage of METs is that there is no chemical addition leading to no major alterations of the quality of the environment over time. Particularly in cases where water streams are treated towards human consumption or use, this lack of chemical addition is highly attractive. In the application of water softening, calcium and magnesium can be precipitated by the alkaline effluent generated in the cathode of MEC, instead of adding external alkaline compounds [74]. For groundwater remediation with low nitrate and COD concentrations, the autotrophic denitrifiers in the microbial electrochemical process reduce the carbon requirements needed for conventional heterotrophic denitrification [75].
Some bioremediation processes through microbial electrochemistry require no energy input whatsoever, for example the sediment MFCs and METland® technologies mentioned above. The energy requirement can be maintained at very low levels where electricity consumption is inevitable. For instance, the specific power consumption (energy needed to treat per gram of pollutant) of nitrate removal by a denitrifying MEC designed by Pous et al. (2015) was 6.8 × 10−3 kWh/(g NO3−-N removed) which was much lower as compared to other denitrification processes (e.g., membrane bioreactor 2.04 × 10−2 kWh/(g NO3−-N removed) [76].
Conventional bioremediation methods requiring chemical replenishment also have limited controllability. For example, injected organic electron donors can lead to competition by undesired processes for this substrate. METs can operate over long time periods, as current is continuously replenished and is easy to monitor and control. Indeed, current is straightforward to monitor without risks for sensor bias and signals can be transmitted easily. Response to changes can be immediate, as current can be corrected within very short timeframes. This enables dynamic control over the bioremediation process. The overall process can be driven by (renewable) electricity which in most cases avoids transports and makes use of a locally available resource.
5. From challenge to opportunity
Despite the increasingly recognized potential for the application of microbial electrochemistry, today it is not a process put to practice. Several hurdles exist, the first being the lack of knowledge on the electroactive microorganisms and their remediation capabilities, particularly from a reductive perspective. Given the recent development of this field, this is not unexpected and will be resolved in the years to come. The outcome of this should be a better control of microbial activity and the means to cultivate isolates to be introduced with the technology at the onset of the remediation process. Second, there is a technological challenge. METs require the introduction of electrochemical technology or at least solid state electron donors/acceptors into settings for which electrochemical systems are not designed. This requires novel designs, often accompanied by low cost materials, or even new concepts to bring the pollutants to this surface-based technology. The latter is key, in some instances where a groundwater flow exists a spontaneous transport towards the electrodes occurs but typical sediments do not have this option. In the latter case forced flow can be organized from sediments, such as done for sulfidic systems [16,77]. A pump and treat approach can be implemented, as the process is in most cases anaerobic the water loop can be closed leading to limited energy requirements to overcome hydrostatic pressure differences. Such an approach also allows intensifying the technology e.g. by using capacitive fluidized bed systems [78]. In some cases metallic or metal oxide particles can be injected into the subsurface distributing a solid electron donor/acceptor to drive activity over longer time periods [79]. Third, the control of the system requires a dynamic adaptation for which algorithms are yet to be defined and which responds to fluctuations in temperature, organic matter flux and pollutant concentrations [80]. Finally, bioremediation is a costly undertaking, hence the introduction of new technologies tends to be slow due to the financial constraints often existing. Despite this, it appears that microbial electrochemistry is here to stay, with deployments to be expected in the years to come.
Acknowledgements
This work was funded through the European Union’s Horizon 2020 project ELECTRA under grant agreement No. 826244 and National Natural Science Foundation of China (NSFC) (No. 31861133001, 31861133002, 31861133003). S.P is a Serra Húnter Fellow (UdG-AG-575) and acknowledges the funding from the ICREA Acadèmia award. LEQUiA has been recognized as consolidated research group by the Catalan Government with code 2017-SGR-1552. KR is supported by the Ghent University special research fund under grant No. BOF19/GOA/026.
References
- 1.Ghafari S., Hasan M., Aroua M.K. Bio-electrochemical removal of nitrate from water and wastewater—a review. Bioresour. Technol. 2008;99:3965–3974. doi: 10.1016/J.BIORTECH.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Pous N., Casentini B., Rossetti S., Fazi S., Puig S., Aulenta F. Anaerobic arsenite oxidation with an electrode serving as the sole electron acceptor: a novel approach to the bioremediation of arsenic-polluted groundwater. J. Hazard Mater. 2015;283:617–622. doi: 10.1016/J.JHAZMAT.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Abbas S.Z., Rafatullah M., Ismail N., Syakir M.I. A review on sediment microbial fuel cells as a new source of sustainable energy and heavy metal remediation: mechanisms and future prospective. Int. J. Energy Res. 2017;41:1242–1264. doi: 10.1002/er.3706. [DOI] [Google Scholar]
- 4.Gregory K.B., Lovley D.R. Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ. Sci. Technol. 2005;39:8943–8947. doi: 10.1021/ES050457E. [DOI] [PubMed] [Google Scholar]
- 5.Daghio M., Espinoza Tofalos A., Leoni B., Cristiani P., Papacchini M., Jalilnejad E., Bestetti G., Franzetti A. Bioelectrochemical BTEX removal at different voltages: assessment of the degradation and characterization of the microbial communities. J. Hazard Mater. 2018;341:120–127. doi: 10.1016/J.JHAZMAT.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Farrell J., Melitas N., Kason M., Li T. Electrochemical and column investigation of iron-mediated reductive dechlorination of trichloroethylene and perchloroethylene. Environ. Sci. Technol. 2000;34:2549–2556. doi: 10.1021/ES991135B. [DOI] [Google Scholar]
- 7.Yu H., Feng C., Liu X., Yi X., Ren Y., Wei C. Enhanced anaerobic dechlorination of polychlorinated biphenyl in sediments by bioanode stimulation. Environ. Pollut. 2016;211:81–89. doi: 10.1016/j.envpol.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Kucharzyk K.H., Darlington R., Benotti M., Deeb R., Hawley E. Novel treatment technologies for PFAS compounds: a critical review. J. Environ. Manag. 2017;204:757–764. doi: 10.1016/j.jenvman.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Liu J., Van Hoomissen D.J., Liu T., Maizel A., Huo X., Fernández S.R., Ren C., Xiao X., Fang Y., Schaefer C.E., Higgins C.P., Vyas S., Strathmann T.J. Reductive defluorination of branched per- and polyfluoroalkyl substances with cobalt complex catalysts. Environ. Sci. Technol. Lett. 2018;5:289–294. doi: 10.1021/acs.estlett.8b00122. [DOI] [Google Scholar]
- 10.Bolong N., Ismail A.F., Salim M.R., Matsuura T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination. 2009;239:229–246. doi: 10.1016/J.DESAL.2008.03.020. [DOI] [Google Scholar]
- 11.Sevda S., Sreekishnan T.R., Pous N., Puig S., Pant D. Bioelectroremediation of perchlorate and nitrate contaminated water: a review. Bioresour. Technol. 2018;255:331–339. doi: 10.1016/J.BIORTECH.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Tijani J.O., Fatoba O.O., Madzivire G., Petrik L.F. A review of combined advanced oxidation technologies for the removal of organic pollutants from water. Water, Air, Soil Pollut. 2014;225:2102. doi: 10.1007/s11270-014-2102-y. [DOI] [Google Scholar]
- 13.Wang H., Ren Z.J. Bioelectrochemical metal recovery from wastewater: a review. Water Res. 2014;66:219–232. doi: 10.1016/J.WATRES.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Zou Y., Wang Xiangxue, Khan A., Wang P., Liu Y., Alsaedi A., Hayat T., Wang Xiangke. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review. Environ. Sci. Technol. 2016;50:7290–7304. doi: 10.1021/acs.est.6b01897. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez Arredondo M., Kuntke P., Jeremiasse A.W., Sleutels T.H.J.A., Buisman C.J.N., Ter Heijne A. Bioelectrochemical systems for nitrogen removal and recovery from wastewater. Environ. Sci. Water Res. Technol. 2015;1:22–33. doi: 10.1039/c4ew00066h. [DOI] [Google Scholar]
- 16.Daghio M., Aulenta F., Vaiopoulou E., Franzetti A., Arends J.B.A., Sherry A., Su Arez-Su Arez A., Head I.M., Bestetti G., Rabaey K. 2017. Electrobioremediation of Oil Spills. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y.-J., Liu P.-W.G., Hsu Y.-S., Whang L.-M., Lin T.-F., Hung W.-N., Cho K.-C. Application of molecular biological tools for monitoring efficiency of trichloroethylene remediation. Chemosphere. 2019;233:697–704. doi: 10.1016/J.CHEMOSPHERE.2019.05.203. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S., You J., Kennes C., Cheng Z., Ye J., Chen D., Chen J., Wang L. Current advances of VOCs degradation by bioelectrochemical systems: a review. Chem. Eng. J. 2018;334:2625–2637. doi: 10.1016/J.CEJ.2017.11.014. [DOI] [Google Scholar]
- 19.Wang H., Luo H., Fallgren P.H., Jin S., Ren Z.J. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015;33:317–334. doi: 10.1016/J.BIOTECHADV.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Ren Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013;31:1796–1807. doi: 10.1016/J.BIOTECHADV.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Schröder U., Harnisch F., Angenent L.T. Microbial electrochemistry and technology: terminology and classification. Energy Environ. Sci. 2015;8:513–519. doi: 10.1039/C4EE03359K. [DOI] [Google Scholar]
- 22.Potter M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. B Biol. Sci. 1911;84:260–276. doi: 10.1098/rspb.1911.0073. [DOI] [Google Scholar]
- 23.Arends J.B.A., Verstraete W. 100 years of microbial electricity production: three concepts for the future. Microb. Biotechnol. 2012;5:333–346. doi: 10.1111/j.1751-7915.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logan B.E., Rabaey K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science (80-. ) 2012;337:686–690. doi: 10.1126/science.1217412. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Ge Z., He Z. A fluidized bed membrane bioelectrochemical reactor for energy-efficient wastewater treatment. Bioresour. Technol. 2014;167:310–315. doi: 10.1016/j.biortech.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 26.Ren L., Ahn Y., Logan B.E. A two-stage microbial fuel cell and anaerobic fluidized bed membrane bioreactor (MFC-AFMBR) system for effective domestic wastewater treatment. Environ. Sci. Technol. 2014;48:4199–4206. doi: 10.1021/es500737m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovley D.R. Extracellular electron transfer: wires, capacitors, iron lungs, and more. Geobiology. 2008;6:225–231. doi: 10.1111/j.1472-4669.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 28.Doyle L.E., Marsili E. Weak electricigens: a new avenue for bioelectrochemical research. Bioresour. Technol. 2018;258:354–364. doi: 10.1016/j.biortech.2018.02.073. [DOI] [PubMed] [Google Scholar]
- 29.Logan B.E., Rossi R., Ragab A., Saikaly P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019;17:307–319. doi: 10.1038/s41579-019-0173-x. [DOI] [PubMed] [Google Scholar]
- 30.Logan B.E., Call D., Cheng S., Hamelers H.V.M., Sleutels T.H.J.A., Jeremiasse A.W., Rozendal R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008;42:8630–8640. doi: 10.1021/es801553z. [DOI] [PubMed] [Google Scholar]
- 31.Puig S., Coma M., Desloover J., Boon N., Colprim J., Balaguer M.D. Autotrophic denitrification in microbial fuel cells treating low ionic strength waters. Environ. Sci. Technol. 2012;46:2309–2315. doi: 10.1021/es2030609. [DOI] [PubMed] [Google Scholar]
- 32.Vijay A., Vaishnava M., Chhabra M. Microbial fuel cell assisted nitrate nitrogen removal using cow manure and soil. Environ. Sci. Pollut. Res. 2016;23:7744–7756. doi: 10.1007/s11356-015-5934-0. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbaum M., Aulenta F., Villano M., Angenent L.T. Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011;102:324–333. doi: 10.1016/j.biortech.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Clauwaert P., Rabaey K., Aelterman P., De Schamphelaire L., Pham T.H., Boeckx P., Boon N., Verstraete W. Biological denitrification in microbial fuel cells. envi. 2007;41:3354–3360. doi: 10.1021/ES062580R. [DOI] [PubMed] [Google Scholar]
- 35.Pous N., Puig S., Coma M., Balaguer M.D., Colprim J. Bioremediation of nitrate-polluted groundwater in a microbial fuel cell. J. Chem. Technol. Biotechnol. 2013;88:1690–1696. doi: 10.1002/jctb.4020. [DOI] [Google Scholar]
- 36.Cecconet D., Devecseri M., Callegari A., Capodaglio A.G. Effects of process operating conditions on the autotrophic denitrification of nitrate-contaminated groundwater using bioelectrochemical systems. Sci. Total Environ. 2018;613–614:663–671. doi: 10.1016/j.scitotenv.2017.09.149. [DOI] [PubMed] [Google Scholar]
- 37.Clauwaert P., Desloover J., Shea C., Nerenberg R., Boon N., Verstraete W. Enhanced nitrogen removal in bio-electrochemical systems by pH control. Biotechnol. Lett. 2009;31:1537–1543. doi: 10.1007/s10529-009-0048-8. [DOI] [PubMed] [Google Scholar]
- 38.Desloover J., Puig S., Virdis B., Clauwaert P., Boeckx P., Verstraete W., Boon N. Biocathodic nitrous oxide removal in bioelectrochemical systems. Environ. Sci. Technol. 2011;45:10557–10566. doi: 10.1021/es202047x. [DOI] [PubMed] [Google Scholar]
- 39.Puig S., Serra M., Vilar-Sanz A., Cabré M., Bañeras L., Colprim J., Balaguer M.D. Autotrophic nitrite removal in the cathode of microbial fuel cells. Bioresour. Technol. 2011;102:4462–4467. doi: 10.1016/J.BIORTECH.2010.12.100. [DOI] [PubMed] [Google Scholar]
- 40.Virdis B., Rabaey K., Yuan Z., Rozendal R.A., Keller J. Electron fluxes in a microbial fuel cell performing carbon and nitrogen removal. Environ. Sci. Technol. 2009;43:5144. doi: 10.1021/es8036302. [DOI] [PubMed] [Google Scholar]
- 41.Pous N., Puig S., Dolors Balaguer M., Colprim J. Cathode potential and anode electron donor evaluation for a suitable treatment of nitrate-contaminated groundwater in bioelectrochemical systems. Chem. Eng. J. 2015;263:151–159. doi: 10.1016/j.cej.2014.11.002. [DOI] [Google Scholar]
- 42.Cecconet Daniele, Zou S., Capodaglio A.G., He Z. Evaluation of energy consumption of treating nitrate-contaminated groundwater by bioelectrochemical systems. Sci. Total Environ. 2018;636:881–890. doi: 10.1016/J.SCITOTENV.2018.04.336. [DOI] [PubMed] [Google Scholar]
- 43.Thrash J.C., Van Trump J.I., Weber K.A., Miller E., Achenbach L.A., Coates J.D. Electrochemical stimulation of microbial perchlorate reduction. Environ. Sci. Technol. 2007;41:1740–1746. doi: 10.1021/ES062772M. [DOI] [PubMed] [Google Scholar]
- 44.Clark I.C., Carlson H.K., Iavarone A.T., Coates J.D. Bioelectrical redox cycling of anthraquinone-2,6-disulfonate coupled to perchlorate reduction. Energy Environ. Sci. 2012;5:7970. doi: 10.1039/c2ee21594b. [DOI] [Google Scholar]
- 45.Grostern A., Edwards E.A. Growth of Dehalobacter and Dehalococcoides spp. during degradation of chlorinated ethanes. Appl. Environ. Microbiol. 2006;72:428–436. doi: 10.1128/AEM.72.1.428-436.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai Y., Becker J.G. Compounded effects of chlorinated ethene inhibition on ecological interactions and population abundance in a dehalococcoides - dehalobacter coculture. Environ. Sci. Technol. 2013;47 doi: 10.1021/es3034582. 130124125717007. [DOI] [PubMed] [Google Scholar]
- 47.Chen D., Shen J., Jiang X., Su G., Han W., Sun X., Li J., Mu Y., Wang L. Simultaneous debromination and mineralization of bromophenol in an up-flow electricity-stimulated anaerobic system. Water Res. 2019;157:8–18. doi: 10.1016/J.WATRES.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 48.Jiang X., Shen J., Han Y., Lou S., Han W., Sun X., Li J., Mu Y., Wang L. Efficient nitro reduction and dechlorination of 2,4-dinitrochlorobenzene through the integration of bioelectrochemical system into upflow anaerobic sludge blanket: a comprehensive study. Water Res. 2016;88:257–265. doi: 10.1016/J.WATRES.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Liang B., Ma J., Cai W., Li Z., Liu W., Qi M., Zhao Y., Ma X., Deng Y., Wang A., Zhou J. Response of chloramphenicol-reducing biocathode resistome to continuous electrical stimulation. Water Res. 2019;148:398–406. doi: 10.1016/J.WATRES.2018.10.073. [DOI] [PubMed] [Google Scholar]
- 50.Lin X.-Q., Li Z.-L., Liang B., Zhai H.-L., Cai W.-W., Nan J., Wang A.-J. Accelerated microbial reductive dechlorination of 2,4,6-trichlorophenol by weak electrical stimulation. Water Res. 2019;162:236–245. doi: 10.1016/J.WATRES.2019.06.068. [DOI] [PubMed] [Google Scholar]
- 51.Sun Q., Li Z., Wang Y., Cui D., Liang B., Thangavel S., Chung J.S., Wang A. A horizontal plug-flow baffled bioelectrocatalyzed reactor for the reductive decolorization of Alizarin. Yellow R. Bioresour. Technol. 2015;195:73–77. doi: 10.1016/J.BIORTECH.2015.06.086. [DOI] [PubMed] [Google Scholar]
- 52.Wang A.-J., Cheng H.-Y., Liang B., Ren N.-Q., Cui D., Lin N., Hong Kim B., Rabaey K. Efficient reduction of nitrobenzene to aniline with a biocatalyzed cathode. Environ. Sci. Technol. 2011;45:35. doi: 10.1021/es202356w. [DOI] [PubMed] [Google Scholar]
- 53.Aulenta F., Tocca L., Verdini R., Reale P., Majone M. Dechlorination of trichloroethene in a continuous-flow bioelectrochemical reactor: effect of cathode potential on rate, selectivity, and electron transfer mechanisms. Environ. Sci. Technol. 2011;45:8444–8451. doi: 10.1021/es202262y. [DOI] [PubMed] [Google Scholar]
- 54.Chen F., Li Z.-L., Liang B., Yang J.-Q., Cheng H.-Y., Huang C., Nan J., Wang A.-J. Electrostimulated bio-dechlorination of trichloroethene by potential regulation: kinetics, microbial community structure and function. Chem. Eng. J. 2019;357:633–640. doi: 10.1016/J.CEJ.2018.09.191. [DOI] [Google Scholar]
- 55.Lai A., Aulenta F., Mingazzini M., Palumbo M.T., Papini M.P., Verdini R., Majone M. Bioelectrochemical approach for reductive and oxidative dechlorination of chlorinated aliphatic hydrocarbons (CAHs) Chemosphere. 2017;169:351–360. doi: 10.1016/J.CHEMOSPHERE.2016.11.072. [DOI] [PubMed] [Google Scholar]
- 56.Lin C.-W., Wu C.-H., Chiu Y.-H., Tsai S.-L. Effects of different mediators on electricity generation and microbial structure of a toluene powered microbial fuel cell. Fuel. 2014;125:30–35. doi: 10.1016/J.FUEL.2014.02.018. [DOI] [Google Scholar]
- 57.Venkidusamy K., Megharaj M., Marzorati M., Lockington R., Naidu R. Enhanced removal of petroleum hydrocarbons using a bioelectrochemical remediation system with pre-cultured anodes. Sci. Total Environ. 2016;539:61–69. doi: 10.1016/J.SCITOTENV.2015.08.098. [DOI] [PubMed] [Google Scholar]
- 58.Zhang T., Gannon S.M., Nevin K.P., Franks A.E., Lovley D.R. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ. Microbiol. 2010;12:1011–1020. doi: 10.1111/j.1462-2920.2009.02145.x. [DOI] [PubMed] [Google Scholar]
- 59.Palma E., Espinoza Tofalos A., Daghio M., Franzetti A., Tsiota P., Cruz Viggi C., Papini M.P., Aulenta F. Bioelectrochemical treatment of groundwater containing BTEX in a continuous-flow system: substrate interactions, microbial community analysis, and impact of sulfate as a co-contaminant. N. Biotech. 2019;53:41–48. doi: 10.1016/J.NBT.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Nzila A. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons under anaerobic conditions: overview of studies, proposed pathways and future perspectives. Environ. Pollut. 2018;239:788–802. doi: 10.1016/J.ENVPOL.2018.04.074. [DOI] [PubMed] [Google Scholar]
- 61.Nzila A. Update on the cometabolism of organic pollutants by bacteria. Environ. Pollut. 2013;178:474–482. doi: 10.1016/J.ENVPOL.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 62.Yu B., Tian J., Feng L. Remediation of PAH polluted soils using a soil microbial fuel cell: influence of electrode interval and role of microbial community. J. Hazard Mater. 2017;336:110–118. doi: 10.1016/J.JHAZMAT.2017.04.066. [DOI] [PubMed] [Google Scholar]
- 63.Sherafatmand M., Ng H.Y. Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs) Bioresour. Technol. 2015;195:122–130. doi: 10.1016/J.BIORTECH.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Domínguez-Garay A., Quejigo J.R., Dörfler U., Schroll R., Esteve-Núñez A. Bioelectroventing: an electrochemical-assisted bioremediation strategy for cleaning-up atrazine-polluted soils. Microb. Biotechnol. 2018;11:50–62. doi: 10.1111/1751-7915.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quejigo J.R., Dörfler U., Schroll R., Esteve-Núñez A. Stimulating soil microorganisms for mineralizing the herbicide isoproturon by means of microbial electroremediating cells. Microb. Biotechnol. 2016;9:369–380. doi: 10.1111/1751-7915.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quejigo J.R., Tejedor-Sanz S., Schroll R., Esteve-Núñez A. Electrodes boost microbial metabolism to mineralize antibiotics in manure. Bioelectrochemistry. 2019;128:283–290. doi: 10.1016/J.BIOELECHEM.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 67.Aguirre-Sierra A., Bacchetti-De Gregoris T., Berná A., Salas J.J., Aragón C., Esteve-Núñez A. Microbial electrochemical systems outperform fixed-bed biofilters in cleaning up urban wastewater. Environ. Sci. Water Res. Technol. 2016;2:984–993. doi: 10.1039/C6EW00172F. [DOI] [Google Scholar]
- 68.Ramírez-Vargas C.A., Arias C.A., Carvalho P., Zhang L., Esteve-Núñez A., Brix H. Electroactive biofilm-based constructed wetland (EABB-CW): a mesocosm-scale test of an innovative setup for wastewater treatment. Sci. Total Environ. 2019;659:796–806. doi: 10.1016/J.SCITOTENV.2018.12.432. [DOI] [PubMed] [Google Scholar]
- 69.Ramírez-Vargas C.A., Prado A., Arias C.A., Carvalho P.N., Esteve-Núñez A., Brix H. Microbial electrochemical technologies for wastewater treatment: principles and evolution from microbial fuel cells to bioelectrochemical-based constructed wetlands. Water (Switzerland) 2018;10:1–29. doi: 10.3390/w10091128. [DOI] [Google Scholar]
- 70.Prado A., Berenguer R., Esteve-Núñez A. Electroactive biochar outperforms highly conductive carbon materials for biodegrading pollutants by enhancing microbial extracellular electron transfer. Carbon N. Y. 2019;146:597–609. doi: 10.1016/J.CARBON.2019.02.038. [DOI] [Google Scholar]
- 71.Aguirre-Sierra A., Baccheti T., Salas J., Deus A., Esteve-Núñez A. A new concept in constructed wetlands: assessment of aerobic electroconductive biofilters. Environ. Sci. Water Res. Technol. 2019 doi: 10.1039/c9ew00696f. [DOI] [Google Scholar]
- 72.Pun Á., Boltes K., Letón P., Esteve-Nuñez A. Detoxification of wastewater containing pharmaceuticals using horizontal flow bioelectrochemical filter. Bioresour. Technol. Reports. 2019;7:100296. doi: 10.1016/J.BITEB.2019.100296. [DOI] [Google Scholar]
- 73.Xing W., Li D., Li J., Hu Q., Deng S. Nitrate removal and microbial analysis by combined micro-electrolysis and autotrophic denitrification. Bioresour. Technol. 2016;211:240–247. doi: 10.1016/j.biortech.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 74.Clauwaert P., De Paepe J., Jiang F., Alonso-Fariñas B., Vaiopoulou E., Verliefde A., Rabaey K. Electrochemical tap water softening: a zero chemical input approach. Water Res. 2020;169:115263. doi: 10.1016/J.WATRES.2019.115263. [DOI] [PubMed] [Google Scholar]
- 75.Virdis B., Rabaey K., Yuan Z., Keller J. Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res. 2008;42:3013–3024. doi: 10.1016/J.WATRES.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 76.McAdam E.J., Judd S.J. Immersed membrane bioreactors for nitrate removal from drinking water: cost and feasibility. Desalination. 2008;231:52–60. doi: 10.1016/J.DESAL.2007.11.038. [DOI] [Google Scholar]
- 77.Nielsen M.E., Reimers C.E., Stecher H.A. 2007. Enhanced Power from Chambered Benthic Microbial Fuel Cells. [DOI] [PubMed] [Google Scholar]
- 78.Deeke A., Sleutels T.H.J.A., Donkers T.F.W., Hamelers H.V.M., Buisman C.J.N., Ter Heijne A. Fluidized capacitive bioanode as a novel reactor concept for the microbial fuel cell. Environ. Sci. Technol. 2015;49:1929–1935. doi: 10.1021/es503063n. [DOI] [PubMed] [Google Scholar]
- 79.Montalvo D., Vanderschueren R., Fritzsche A., Meckenstock R.U., Smolders E. Efficient removal of arsenate from oxic contaminated water by colloidal humic acid-coated goethite: batch and column experiments. J. Clean. Prod. 2018;189:510–518. doi: 10.1016/J.JCLEPRO.2018.04.055. [DOI] [Google Scholar]
- 80.Molderez T.R., de Wit B., Rabaey K., Verhelst M. IECON 2017-43rd Annual Conference of the IEEE Industrial Electronics Society. IEEE; 2017. Successive parabolic interpolation as extremum seeking control for microbial fuel & electrolysis cells; pp. 3128–3133. [DOI] [Google Scholar]