Abstract
Sex differences in the neurobiological mechanisms involved in fear conditioning and extinction have been suggested to contribute to differential vulnerability for the development of posttraumatic stress disorder (PTSD) in women compared with men. Reproductive hormones, such as estradiol, have been shown to facilitate fear conditioning and extinction learning and may explain some of these differences. However, the effect of commonly used hormonal contraceptives on the neurobiological mechanisms of fear conditioning and extinction is poorly understood. A laboratory study was conducted in trauma-exposed men and women with and without full or partial PTSD to examine effects of sex and use of hormonal birth control on fear conditioning, fear extinction learning, and extinction retention. Participants underwent fear conditioning with stimuli that were paired (CS+) or unpaired (CS−) with shock. Extinction learning occurred 72 h later, and extinction retention was tested 1 wk after extinction. Women on hormonal contraceptives (HCs) demonstrated enhanced acquisition of fear conditioning and enhanced extinction of fear as compared with women off hormonal birth control and men. While clinical implications have yet to be determined, these results suggest that hormonal contraceptives may facilitate learning during both fear acquisition and extinction. Understanding the impact of sex and hormones on fear conditioning and extinction processes may lead to new insights into the pathophysiology of PTSD and result in advancements in treatment that may vary by sex.
It is widely recognized that posttraumatic stress disorder (PTSD) is a common consequence of trauma exposure, and women are at particularly high risk, with some but not all studies finding that women develop PTSD at twice the rate of men, despite greater trauma exposure in men (Breslau et al. 1998; Tanielian et al. 2008). Although some have suggested that greater exposure to interpersonal violence may contribute to higher rates of PTSD in women, other evidence implicates sex differences in the neurobiological mechanisms that are involved in fear conditioning and extinction. Enhanced fear conditioning and diminished extinction of conditioned fear have been associated with higher levels of endogenous estrogen in women, as well as the development and maintenance of PTSD in both sexes (Orr et al. 2000; Milad et al. 2009b; Glover et al. 2012). Furthermore, as one of the most empirically supported treatments for PTSD is prolonged exposure therapy, which is largely based on fear extinction principles and the success of extinction learning (Rothbaum and Davis 2003), a clear understanding of the individual factors impacting fear conditioning and extinction is critical.
Although some of the studies examining sex differences in fear conditioning are inconsistent (Guimaraes et al. 1991; Zorawski et al. 2005; Milad et al. 2006), an increasing body of evidence from rodent and human studies supports the existence of sex differences in fear extinction learning and recall (Maren et al. 1994; Pryce et al. 1999; Milad et al. 2009a; Merz et al. 2013). One possible explanation for the lack of consistent findings in the fear conditioning literature may be related to potential floor effects associated with subclinical impairment of nonclinical samples, as most laboratory studies examining sex differences were conducted in healthy humans. Another explanation that is gaining substantial support is the impact of hormones that differ between the sexes, among individuals, and even within individuals across time (Quirk and Mueller 2008; Lebron-Milad and Milad 2012; Arevalo et al. 2015; Hwang et al. 2015; Herrera et al. 2017; Maeng et al. 2017; Antov and Stockhorst 2018).
The available literature indicates that estradiol, the primary estrogen in women during the childbearing years, is also present at overall lower concentrations in males and plays a large role in the fear conditioning and extinction differences observed between men and women (Gupta et al. 2001; Jasnow et al. 2006; Chang et al. 2009; Milad et al. 2009a, 2010; Zeidan et al. 2011; Maddox et al. 2018; Matsumoto et al. 2018; Carvalho et al. 2021). In both sexes, estradiol plays a variety of important functions in the brain, including the regulation of oxidative stress, inflammation, and gene expression, as well as in cognitive functions such as learning and memory (Hammoud et al. 2020). Estrogen receptors are found throughout brain regions that are important for fear conditioning and extinction processes (e.g., the amygdala, ventromedial prefrontal cortex, and hippocampus), likely via enhancements to learning and memory (Milad et al. 2008; Quirk and Mueller 2008; Lebron-Milad and Milad 2012). In women, as peripheral levels of estradiol vary over the course of the menstrual cycle, so do levels of estradiol in the brain (Arevalo et al. 2015). Estradiol has been shown to enhance memory consolidation across stages of fear conditioning, extinction, and retention (Lebron-Milad and Milad 2012). Most relevant to learning in PTSD, women with high levels of estradiol demonstrate enhanced memory formation in the presence of stress exposure (Herrera et al. 2017; Antov and Stockhorst 2018). In animal and human models, higher estradiol levels appear to facilitate acquisition of fear conditioning and extinction (Maeng et al. 2017). For example, women with high endogenous estradiol levels have enhanced responses in fear circuitry during fear conditioning, extinction, and recall as compared with men (Hwang et al. 2015). When phase of menstrual cycle has been taken into account, differences have been observed in conditioned fear responses and severity of PTSD symptoms. Specifically, when women are in the midluteal phase of the menstrual cycle (higher endogenous estradiol), they demonstrate a stronger positive relationship between SCR during fear conditioning and PTSD symptoms than women in the early follicular phase of menstruation (lower endogenous estradiol) (Carpenter et al. 2022). In the complimentary literature on the startle response, low-estradiol women demonstrated reduced discrimination between CS+ and CS− during fear conditioning and reduced inhibition of fear-potentiated startle during extinction and extinction recall, indicating less successful learning than their high-estradiol counterparts (Glover et al. 2012, 2013; Armbruster et al. 2018). This literature suggests that higher levels of estradiol relate to enhanced acquisition of associations between an unconditioned stimulus (UCS) and a conditioned stimulus (CS) during fear conditioning and enhanced extinction of this association during the extinction phase due to greater memory consolidation.
A variety of factors can account for hormone differences in women, including menstrual phase, age, and use of hormonal birth control. Approximately 11%–20% of women aged 20–39 yr use oral contraceptives (OCs) (Daniels and Abma 2020). Commonly used OCs directly affect estradiol levels and hormonal fluctuation associated with the menstrual cycle. However, hormonal contraceptives (HCs) have received little attention in the fear conditioning literature. At the time of writing, we were unable to locate studies examining the impact of other HCs on fear conditioning circuitry, although these forms of birth control are increasingly popular among women, and evidence suggests that a variety of HCs has impacts on brain structure, function, and cognitive processes (Brønnick et al. 2020). Literature suggests that estradiol levels in women on HCs are typically low, similar to those of women in the early follicular phase of menstruation (Brynhildsen 2014). Although Hwang et al. (2015) did not demonstrate an effect of HCs on fear conditioning, many HCs contain ethinyl estradiol, which is synthetic estrogen that binds to estrogen receptors at high levels. Further research is needed to determine whether synthetic estrogen present in HCs impacts fear conditioning and fear extinction and contributes to associated sex differences, particularly in a highly sensitized population such as those with PTSD. In the current study, differences in fear conditioning, extinction, and retention were examined in women on hormonal birth control and in the early follicular phase of the menstrual cycle as compared with men to determine whether the synthetic hormones present in HCs confer any enhancement to these processes over and above women off of birth control with theoretically low levels of endogenous estradiol.
Fear conditioning is measured by assessing the differential SCR to a conditioned stimulus (CS+) paired with an unconditioned stimulus (shock; UCS) and a stimulus unpaired with a shock (CS−). Greater acquisition of fear conditioning is evidenced by greater SCR response to the CS+ when compared with the CS−. Fear extinction refers to repeated exposure to the CS in the absence of the US, which results in diminishing reactivity to previously conditioned stimuli due to an inhibitory neural link that is formed (Myers and Davis 2007). Extinction is therefore operationalized by reduced discrimination of responding to the CS+ and CS− over time, and its retention is evidenced by a maintenance of low differential SCR in response to presentation of CS+ and CS− cues at follow-up.
In previous work, our group examined sex differences in skin conductance responses to a fear conditioning paradigm in men and women with PTSD (Inslicht et al. 2013). In that sample, women were premenopausal and underwent conditioning during the follicular phase of the menstrual cycle. We found that women had greater differential fear acquisition compared with men. Other work has indicated that the effects of endogenous gonadal hormones on fear extinction are moderated by PTSD diagnosis, such that women with PTSD demonstrated impaired fear extinction during the midluteal phase of menstruation but not during the early follicular phase (Pineles et al. 2016b). The current study examines the effect of sex, HC use, and PTSD severity on fear conditioning, fear extinction, and extinction retention in medically healthy trauma-exposed premenopausal women on or off HCs and age-matched men across a range of PTSD symptom severity. We used a validated laboratory conditioning paradigm (Inslicht et al. 2021) that occurred over several days in which fear acquisition was separated from extinction by 72 h to avoid influencing consolidation of fear conditioning, and extinction retention was evaluated 1 wk after the fear extinction session to provide a test of durability of extinction over time.
Given evidence for impaired fear extinction in PTSD, we hypothesized that participants high in PTSD (men and women combined) would have decreased fear extinction learning and extinction retention compared with those with low PTSD symptom scores. As estradiol appears to enhance learning during stress in women, we predicted that women on HCs would demonstrate higher differential SCR during acquisition and lower differential SCR during extinction and extinction retention than naturally cycling women in the early follicular phase of menstruation and men. Finally, we predicted a PTSD × sex interaction effect for extinction learning and retention; women on HCs with high levels of current PTSD would have enhanced acquisition but decreased extinction learning and retention compared with women with low levels of current PTSD and men.
Results
Sample characteristics
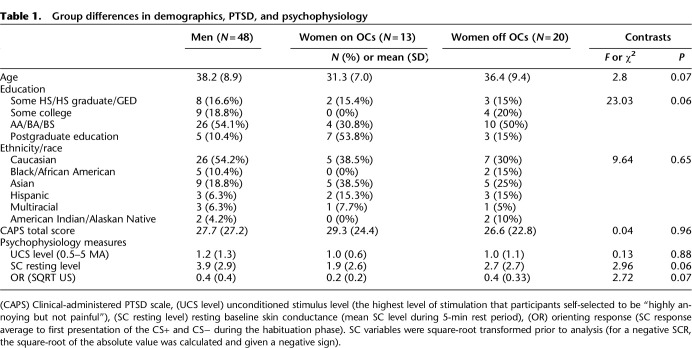
Means, standard deviations, and results of ANOVA and χ2 comparisons between men, women on HCs, and women off HCs for demographics and clinical characteristics are presented in Table 1. There were no significant differences between groups for age, ethnicity/race, highest level of education, PTSD symptom severity, or use of psychiatric medications. CAPS scores ranged from 0 to 81. Out of 81 participants, 42 met criteria for PTSD. Most participants (70 out of 81) correctly identified the color that was paired with the shock, indicating explicit awareness of the CS–UCS contingency; there were no group differences. Although not significant, trend level differences in age and education were observed between sex groups (see Table 1). These variables were not found to be associated significantly with SCR nor were they found to significantly alter the results presented below when included as a covariate in the models of interest, and so they were not included in final reported results.
Table 1.
Group differences in demographics, PTSD, and psychophysiology
Baseline skin conductance and shock levels
As presented in Table 1, mean shock level, mean prestimulus SCL during habituation, and mean SC-orienting response during the habituation phase did not differ between groups (Ps > 0.11), nor were they associated with differential SCR (Ps > 0.22). Thus, differences between groups during extinction learning and retention are not likely to be attributable to differences in shock level selected, SC-orienting response magnitude, or baseline resting SCL.
Habituation and fear conditioning
Habituation and conditioning phases were first analyzed using a group (men, women on HC, women off HC) × CS condition × CAPS score × trials (five) random intercept model in order to test the differential effects of group, condition, and PTSD severity on SCR. These analyses were followed by a group (men, women on HC, women off HC) × trials (five) × CAPS score random intercept mixed model with SCR difference score as the dependent variable in order to test the impact of group and PTSD severity on the change in SCR difference score over trials. The first CS+ and CS− trials of conditioning were dropped from analyses because the UCS presentation occurred at the offset of CS+, so no conditioning could be observed until the second trial.
Although we did not expect group differences or PTSD effects during habituation, we tested these effects to confirm that there were no differences. For habituation, there were no significant sex or PTSD effects or any significant interactions involving these factors (all Ps > 0.25). There was a significant CS condition × trial effect (b = −0.04, CI −0.08 and −0.00, P = 0.05), such that SCR to CS+ decreased significantly more than CS− over trials, indicating successful habituation to the CS stimuli.
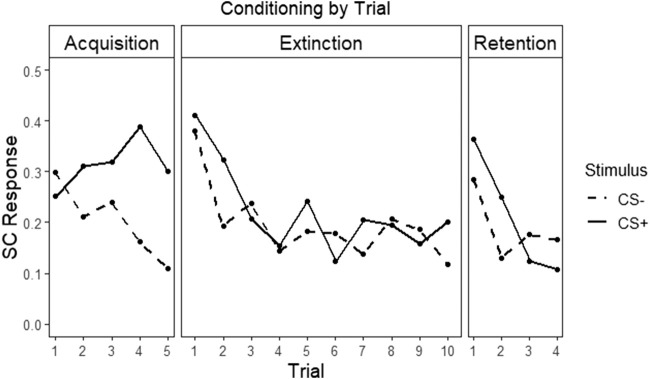
Effects on mean SCR during acquisition were examined first (see Fig. 1). During fear conditioning, there was a significant effect of CS+ versus CS− [χ2(1) = 24.05, P < 0.001], indicating successful acquisition of fear responding. There were no significant effects of PTSD severity or its interaction with CS condition on SCR (Ps > 0.25). There was no main effect of sex or its interaction with CS condition on SCR (Ps > 0.25).
Figure 1.
Differential SCR response across phases of the experiment. Mean skin conductance response scores to CS+ and CS− for each trial during each phase of the experiment (acquisition, extinction, and retention).
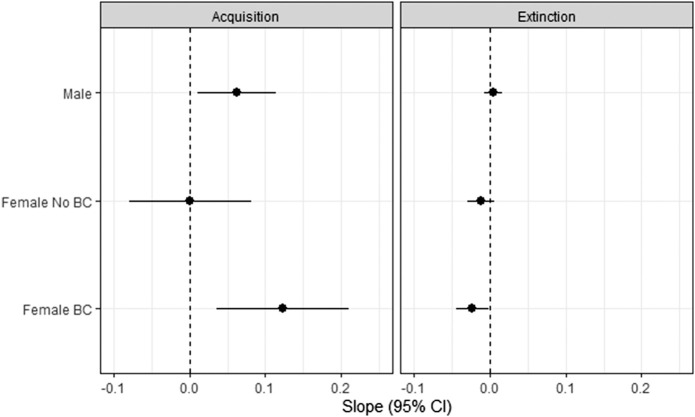
Effects on change in differential (SCR during CS− subtracted from SCR during CS+) over the course of acquisition trials (slope) were examined second (see Fig. 2). Women off HCs showed a lower differential SCR over the course of acquisition trials as compared with women on HCs (b = −0.24, CI −0.41 and −0.09, P = 0.002) and men (b = −0.13, CI −0.26 and −0.005, P = 0.04), indicating that women on HCs demonstrated enhanced acquisition of the association between CS and UCS over the course of acquisition trials (b = 0.14, CI 0.02 and 0.26, P = 0.02).
Figure 2.
Differential SCR by sex and hormonal contraceptive status during acquisition and extinction. The standardized regression coefficient, representing the change in differential SCR over trials, is presented for each sex and hormonal contraceptive group for acquisition and extinction phases. Error bars represent 95% confidence intervals.
Extinction learning effects
Extinction trials were analyzed using random intercept mixed models similar to that for habituation and fear conditioning. Effects on mean SCR during extinction learning were examined first. A main effect of CS condition on SCR over trials was not found [χ2(1) = 2.80, P = 0.09] (see Fig. 1). There were no significant main effects of PTSD severity (P = 0.82) or its interaction with CS condition on SCR (P = 0.32). There was an effect of sex on SCR, such that women off HCs demonstrated lower overall SCR than men (z = −2.11, P = 0.03). The interaction effect of sex and CS condition on SCR was not found to be significant (P = 0.65).
Effects on change in differential (SCR during CS− subtracted from SCR during CS+) over the course of extinction trials (slope) were examined second (see Fig. 2). There was a significant main effect of sex on SCR difference score such that women on HCs demonstrated a more negative slope over the course of extinction learning trials (b = −0.03, CI −0.06 and −0.00, P = 0.05) than men, indicating enhanced extinction learning. No difference between women off HCs and women on HCs (P = 0.50) or men (P = 0.14) was observed.
Extinction retention effects
Extinction retention was measured by the differential SCR (CS+ vs. CS−) over the first four trials of the extinction retention phase and analyzed using a sex group × CAPS score × CS-type mixed model with random intercepts. As shown in Figure 1, extinction learning was retained, as evidenced by a lack of significant difference between CS conditions on SCR [χ2(1) = 0.82, P = 0.36]. There was a main effect of sex, such that women off HCs demonstrated lower overall SCR than men (z = 2.31, P = 0.02). No other main effects or interactions reached statistical significance.
Discussion
The results of this study offer support for the impact of synthetic hormones on fear conditioning and fear extinction learning for trauma-exposed women in the early follicular phase. In our sample, women on HCs demonstrated a more positive change in SCR during fear conditioning, indicating that they learned the differential association between CS+ and CS− more quickly than women off HCs in the early follicular phase of menstruation and men. Additionally, women on HCs demonstrated a more negative change in SCR during extinction, indicating that they acquired the inhibitory association between CS+ and shock more quickly than men, but not women off HCs. Given that women on birth control and women in the early follicular phase of menstruation are believed to have similar levels of endogenous estradiol, these data suggest that synthetic hormones present in HCs offer enhancements in the learning and extinction of conditioned fear. As previous research has been inconclusive as to the impact of HCs on fear learning, these data offer an important addition to the literature and suggest that hormonal birth control should be included as a consideration in any future analysis of sex differences in fear conditioning.
Studies of the effects of sex on fear conditioning and extinction have been historically mixed (for review, see Peyrot et al. 2020), with some finding differences in SCR during fear conditioning between male and female participants and others finding no such difference. Current results, in combination with the existing literature, suggest that these mixed findings could be due in part to hormonal fluctuations in women associated with menstruation and phase of life, as women with higher levels of estradiol demonstrate enhanced fear learning when compared with low-estradiol women and men (for review, see Stevens et al. 2018). Few studies of the role of HCs in fear conditioning among women have been conducted, and to our knowledge there is a paucity of data on the difference in fear learning between women on HCs and women with low endogenous estradiol (i.e., in the early follicular phase of menstruation). Both groups have been previously shown to have low endogenous estradiol (Hwang et al. 2015), but women on HCs have higher levels of synthetic estrogen. Our data suggest that the synthetic hormones found in HCs have a significant impact on fear acquisition and extinction, with women on birth control showing faster and higher elevations in overall levels of SCR during acquisition of fear learning as well as faster overall reduction in SCR during extinction learning than early follicular phase women and men. These findings mirror previous findings associated with endogenous estradiol in women during fear conditioning and extinction paradigms (Arevalo et al. 2015; Herrera et al. 2017; Maeng et al. 2017; Antov and Stockhorst 2018).
However, our findings contrast those of Graham and Milad (2013), who found decreased extinction recall in women using oral contraceptives compared with women with high levels of endogenous estradiol, but no differences in fear acquisition and fear extinction learning. However, one possible reason for these differences is that in the Graham and Milad (2013) study, women were tested in a 2-d paradigm starting at ∼5 d after the onset of menstruation and divided according to endogenous estradiol or oral contraceptive usage, whereas the present study used a multiday paradigm in which women started the conditioning session earlier in the follicular phase at days 1–3 after menses, when estradiol is known to be lower, and extinction learning at days 4–7, and completed retention at days 11–14 at the late follicular phase. A second difference is that their study included only healthy women, whereas this sample included all trauma-exposed participants with varying degrees of PTSD symptomatology. Both the follicular phase and PTSD status have been associated with impaired fear inhibition and greater difficulties differentiating fear from safety signals (Glover et al. 2013). Our findings suggest that HCs may rescue these possible impairments associated with the follicular phase or in those with trauma exposure.
Fear extinction is believed to be a core mechanism of prolonged exposure therapy, an evidence-based treatment for PTSD that shares many similarities with classic fear extinction paradigms (Foa 2000). Although we did not find significant effects of PTSD or an interaction with sex group on fear learning in this sample, the implications for treatment of PTSD in women remain. These data suggest that women with low endogenous estradiol may face greater challenges in extinction learning than their high-estradiol counterparts. This is in line with previous work that suggests that success of fear conditioning increases during menstruation phases marked by high endogenous estrogen, which subsequently increases PTSD symptoms (Nillni et al. 2015). Importantly, given that the primary mechanism of this increase is learning and memory consolidation, fear extinction success increases during this period as well (Nillni et al. 2015). In a whole-health approach to PTSD treatment, it follows that monitoring the phase of menstruation, or even providing estrogen as a pharmacological adjunct, may be helpful in increasing the success of a treatment that is largely contingent on the success of learning (Glover et al. 2015; Hammoud et al. 2020).
Limitations
One consideration in the interpretation of the data presented is reliance on self-reporting for assessment of phase of menstruation and HC status to make assumptions about the level of endogenous estradiol of each of the sex groups. It is likely that variation in endogenous estradiol exists within women in the early follicular phase of menstruation as well as women on hormonal birth control. Furthermore, there is a possibility that errors in self-reporting either phase of menstruation or adherence to hormonal birth control occurred. To minimize this risk, female participants were questioned regarding the first day of their most recent menstrual period and average length of cycle or their birth control status at each study visit, and these data were checked for consistency.
Additionally, among cycling women, only those who completed fear conditioning during the early follicular phase of menstruation were included. This restricted our ability to compare fear learning observed in our sample versus women with theoretically higher levels of endogenous estradiol at different phases of the menstrual cycle. However, the selection of these groups was intentional, as it allowed us to test the effect of birth control on fear conditioning and extinction and to determine whether women on HCs differ from other theoretically low-estradiol female groups. Future work should include women across phases of menstruation and on and off birth control in order to further assess the impact of endogenous and exogenous estrogen on fear learning in women. A wealth of evidence suggests that estradiol plays a principal role in supporting fear learning. However, other hormones, such as progesterone, may also contribute to fear learning and merit further investigation. Findings related to progesterone have been limited and mixed, with some studies reporting no significant effect of progesterone (Milad et al. 2010; Zeidan et al. 2011) and others finding that progesterone levels impact fear learning (as assessed via prepulse inhibition) in women with PTSD (Pineles et al. 2016a,b). Additionally, the types of HCs used by the women in our sample varied naturally. It is possible that different kinds of HCs have different impacts on hormones both in the periphery and in the brain (Brønnick et al. 2020) and thus different impacts on fear conditioning and extinction learning; however, our sample was not well powered to detect these effects if they exist. Future research evaluating the impacts of different types of HCs on the hormonal and neurobiological mechanisms supporting fear conditioning and extinction and confirming blood hormone levels to more directly assess the role of individual estradiol level in fear conditioning and its relationship to birth control use in a sample of trauma-exposed individuals would be informative.
Although we have found effects of sex and birth control on differential SCR during fear conditioning and extinction in our sample, the effects are relatively small and likely limited by a number of factors. First, we were unable to examine third-order interaction effects of PTSD and sex group on differential SCR due to a substantially larger sample size needed for adequate power to detect potential effects. Additionally, the use of habituation trials prior to acquisition may have impacted the robustness of fear conditioning and the manipulation we were testing. Inclusion of habituation trials in a psychophysiological study of fear conditioning is especially important, as anticipatory anxiety and orienting responses to novel stimuli could confound the ability to detect the associative learning specific to the CS+ association. Including a habituation phase reduces a potential impact of anticipatory anxiety and the orienting response to the initial presentations of visual stimuli on the fear conditioning phase. Indeed, the differential effect observed in this sample is similar to effects seen in other work from our group (Inslicht et al. 2013, 2021).
Finally, an important question is whether groups may have differed in UCR response as a result of some participants choosing a lower level of shock during the UCS calibration procedure in which participants self-selected a level that they experienced as “highly annoying but not painful.” Women have been shown to have a lower pain threshold and higher ratings of pain intensity and unpleasantness to experimental pain tasks compared with men (Fillingim et al. 2009); therefore, individualized shock level is important for both detecting an effect and retaining participants. To examine the potential confound, we examined shock level and determined that this did not differ significantly between groups and fell within a limited range (Table 1). However, future studies using standardized nonpainful stimuli such as sound or airpuff would provide additional confirmation that differences in fear conditioning and extinction are not affected by possible differences in pain sensitivity.
Overall, the current study suggests that hormonal birth control enhances fear conditioning and extinction learning in women when compared with women in the follicular phase of menstruation. These data strongly suggest that birth control status should be considered in future studies of fear learning and support a potential role of estradiol in fear learning and extinction learning rates. These data have implications for hormonal considerations in evidence-based treatment of PTSD. Future work will seek to further examine the hormonal millieu associated with birth control use and phase of menstruation in the context of fear learning and PTSD.
Materials and Methods
Participants
Medically healthy male and female (off birth control = 13, on birth control = 20) individuals, aged 22–51 yr, were recruited for participation (N = 81). Following informed consent, individuals were evaluated for PTSD symptomatology using the CAPS-IV (Blake et al. 1995) and screened for other current and lifetime axis I disorders using the Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) (First et al. 2002). Women were premenopausal as determined by screening of participants for their history of menstrual bleeding patterns and defined by consistent menstrual period in the past 3 mo with no change in regularity in the past 12 mo as recommended by the Stages of Reproductive Aging Workshop (Soules et al. 2001).
Individuals were excluded if they had current or recent trauma in the previous 3 mo or active suicidality or met lifetime criteria for schizophrenia, schizoaffective disorder, bipolar disorder, obsessive-compulsive disorder, specific phobias, substance abuse or dependence, or alcohol dependence within the previous 3 mo. Individuals who met criteria for full lifetime PTSD as defined above for any prior period but did not meet criteria for current partial or full DSM-IV criteria for PTSD were excluded from the control group. Medical exclusions included a history of seizure disorders; neurological disorder; mild, moderate, or severe traumatic brain injury (head injury with loss of consciousness for >10 min or ongoing symptoms of TBI); current infectious illness; systemic illness affecting CNS function; or medically unstable injuries. Participants who in the 2 mo prior to study participation took psychotropic medications, including SSRIs, alpha- and beta-adrenergic agents, antipsychotics, benzodiazepines, mood stabilizers, anticonvulsants, antihypertensives, sympathomimetics, steroids, or any other general medications that could influence psychophysiology or hormone levels (other than birth control), were excluded. Additional exclusions for women included markedly irregular menstrual periods, bilateral oophorectomy, pregnancy, or lactation. We required that participants not consume heavy amounts of alcohol (more than seven drinks for women, >14 drinks for men) or use illicit drugs for 1 wk prior to participation. On the days of testing, participants were asked to abstain from caffeine, smoking, and eating for 1 h prior to the session, and these variables were assessed via self-reporting during their study visit.
Measures
Clinical measures
The following scales were used:
Background: Age, ethnicity, income, education, marital status, and military service details (e.g., years of service, location) were obtained.
Health: Health-related variables included day of menstrual cycle for cycling women, smoking, medication history, neurobehavioral screen for TBI, and body mass index (BMI: weight in kilograms/[height in meters]2).
Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) (First et al. 2002): The SCID-I/NP is a structured diagnostic interview protocol for the determination of DSM-IV diagnoses. The SCID-I/NP was used to assess current comorbid psychiatric diagnoses and prior diagnoses.
Clinician-administered PTSD scale (CAPS) (Blake et al. 1995): The CAPS provides information on current and lifetime PTSD symptoms and status, providing a diagnosis, frequency, and intensity of symptoms.
Psychophysiological measures
Skin conductance level (SCL) was measured by Biopac MP150 for Windows (Biopac Systems, Inc.) using a constant 0.5 V through 8-mm (sensor diameter) Ag/AgCl electrodes that were placed along the hypothenar surface (the fleshy portion of the palm between the wrist and little finger) of the participant's nondominant hand (Fowles et al. 1981). The SCL analog signal was acquired at a sampling rate of 1 kHz, amplified, and digitized using the Biopac system.
Procedures
Procedures occurred over four study visits. During the initial visit, study procedures were fully explained, and written informed consent was obtained for the University of California at San Francisco Institutional Review Board.
Eligibility was determined by structured diagnostic interview for psychiatric and medical history, and blood and urine samples were collected to assess exclusionary conditions and use of medications or drugs as well as to test for pregnancy in women. Eligible participants then set the shock level that would serve as the UCS (at a level they found to be “highly annoying but not painful”) in subsequent sessions to avoid any possible influence of initial shock exposures on fear conditioning.
Upon determination of eligibility, participants were scheduled to complete the classical aversive conditioning paradigm over three subsequent study visits. The first session for fear conditioning was scheduled during the early follicular phase for women (day 2 or 3 following the start of menses) and on a random day for men. All sessions were scheduled between 1:00 p.m. and 4:00 p.m. to minimize potential circadian effects.
Experimental paradigm testing fear conditioning
All psychophysiology testing sessions took place in a dedicated psychophysiology laboratory. The experimental apparatus was located in the adjoining room and participants were monitored by an unobtrusive video camera during the experiment. Procedures and participant instructions, adapted from Orr et al. (2000) and previously described (Inslicht et al. 2013), were identical across sessions. Electrodes for SCL recording and those for administering the UCS were attached. Experimental stimuli were presented using SuperLab 5.0 for Windows (Cedrus, Inc.). The conditioned stimuli (CS+ [paired with the UCS] and CS− [not paired with the UCS]) were two different colored computer-generated 15.2-cm diameter circles, randomly selected for each participant and presented for 8 sec on a 28.5-cm × 21.5-cm monitor positioned 1 m in front of the participant, with an intertrial interval of 20 sec ± 5 sec.
The UCS was a 500-msec electric pulse, which ranged from 0.5 to 5.0 mA, as previously determined by the participant to be “highly annoying but not painful,” generated by a Biopac programmable stimulator module (STM100C) and delivered through electrodes attached to the second and third fingers of the dominant hand. Participants were told that they “may or may not receive any electrical stimulation prior to each session,” including at the fear conditioning, extinction learning, and retention sessions, in order to elicit threat-enhanced conditions. Each session started with a 5-min baseline recording period, during which SCL was sampled at 1000 Hz.
Session 1: habituation and fear conditioning
During session 1 and following a 5-min baseline recording, participants were presented with five of each of the colored circles (to be CS+ and to be CS−) in the absence of the shock (UCS) and with no more than two consecutive presentations of the same stimulus type, constituting the habituation phase. This was followed by the fear acquisition phase, in which each presentation of the CS+ was followed by a 500-msec shock and the CS– was not. The CS+ and CS− were each presented five times in random order.
Session 2: fear extinction
Participants were provided with identical instructions and set up as during fear conditioning. Following the 5-min baseline recording, participants were presented with 10 nonreinforced presentations each of the CS+ and CS−.
Session 3: extinction retention
One week following extinction, participants underwent an identical setup, instructions, and baseline recording. They were then shown four nonreinforced presentations each of the CS+ and CS−.
Contingency awareness was determined after each session, when participants were asked whether they could predict when the shock would occur, to identify the color of the CS+, and to rate their level of “annoyance” with the shock on a five-point Likert-type scale. Upon completion of the final session, participants were debriefed, thanked, and reimbursed.
Psychophysiological response scores
The SC response (SCR) scores for CS and UCS intervals were calculated as reported previously (Orr et al. 2000; Inslicht et al. 2013, 2021). The SCR score for each CS interval was calculated by subtracting the mean SCL for the 2 sec preceding CS onset from the peak during the 8-sec CS interval. The UCR was calculated by subtracting the average SCL within 6–8 sec following CS onset, from the maximum increase in SC level during the 0.5- to 6.5-sec interval following CS offset (corresponding to the onset of the 0.5-sec US). The SCR scores were normalized by sign-square-root transformation due to a skewed distribution.
Resting level skin conductance was mean SC during a 5-min rest period.
Orienting response (OR) was SC response average to first CS+ and CS− during habituation.
Unconditioned response was averaged UR for CS+ trials during the acquisition phase.
Differential SC responses were average CS interval response to the CS+ trials minus averaged CS interval response to the CS− trials for habituation, fear conditioning, extinction, and retention phases.
Statistical analyses
Hypotheses for aim 1 were tested using a random intercepts mixed model analysis of differential SC responses (the primary dependent measure) over trials during habituation, acquisition, extinction, and retention phases of the experiment. Sex group and continuous PTSD score were fixed factors, trials were within-subjects repeated factors, and subject was a random factor. Hypothesis 1—that greater PTSD symptomatology is related to decreased fear extinction—was tested by the PTSD effect during the extinction phase. Hypothesis 2—that women on HCs will demonstrate greater fear learning than women off HCs during the early follicular phase of menstruation and men—was tested using planned contrasts for sex group following the mixed model during the acquisition, extinction, and retention phases. Hypothesis 3—that PTSD severity moderates the relationship between sex group and fear conditioning such that women off HCs with elevated PTSD will demonstrate the highest level of fear conditioning during acquisition and retention, as well as reduced extinction learning—was tested using the sex group × PTSD interaction in the mixed model during acquisition, extinction, and retention phases.
Statistical analyses were conducted using linear mixed-effects modeling in Stata 16 (https://www.stata.com). Separate analyses were conducted for each phase; namely, fear conditioning, extinction learning, and extinction retention. The dependent measure used in all analyses was the SCR difference score. Eleven outlier trials, defined as z-scores greater than two standard deviations from the mean, were winsorized (i.e., replaced with ±2). Following visual inspection of the standardized responses, two standard deviations was chosen so as to balance the minimization of data loss with the prevention of extreme outliers’ undue influence on results. Each model included random intercepts for subjects and fixed effects for group and trials (5, habituation; 5, conditioning; 10, extinction learning; 4, extinction retention), and their interactions. Trials were modeled in three ways: as a continuous fixed effect, as a continuous random effect (i.e., with subject-specific slopes), and as a fixed categorical variable. The best-fitting model was selected on the basis of likelihood tests or AIC fit criteria before conducting any statistical inferences.
Competing interest statement
T.C.N. has provided consultation to Jazz Pharmaceuticals and Eisai, Inc. S.S.I. has provided consultation to Eisai, Inc. M.E.B., V.R., T.J.M., J.G., M.T., C.F., and S.L.L. declare no competing interests.
Acknowledgments
We thank Mohammed Milad, PhD, Scott Orr, PhD, Suzanne Pineles, PhD, and Alexandra Cowden Hindash, PhD, for their valuable scientific contributions to the design of this study; Callan Lujan, Maureen Barrientos, PhD, Cynthia Villaneuva, Tierney Baum, Sarah Wagner, and Meghan Howard for study coordination, recruitment, and data collection; Kristin Samuelson, PhD, Christi Zenteno, PhD, and Nazneen Bharassa, PhD, for overseeing diagnostic assessments; and Gary Tarasovsky and Olga Mayzel for their assistance with data management. This research was supported by grant funding from the Department of Veteran Affairs Clinical Science Research and Development (1I01CX000720-01A2), a Discovery Award by the Department of Defense (PR192475), and the Heart and Armor Foundation to S.S.I. Resources and the use of facilities were provided by the Mental Illness Research and Education Clinical Center (MIRECC) of the US Veterans Health Administration, Veterans Administration Medical Center, San Francisco, California, and the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health, through a University of California at San Francisco-Clinical and Translational Science Institute grant (KL2 RR024130). The sponsors had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the sponsors.
Author contributions: S.S.I. designed the study and wrote the protocol. S.S.I. and M.E.B. wrote the first draft of the manuscript. M.E.B., V.R., and T.J.M. undertook the statistical analysis and interpretation of findings and contributed to the discussion. J.G. and S.L.L. assisted with data collection, provided technical assistance, and contributed to the interpretation of findings. T.C.N. assisted with the design of the study, interpretation of findings, and all drafts of the manuscript. All authors contributed to and approved the final manuscript.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053597.122.
Freely available online through the Learning & Memory Open access option.
References
- Antov MI, Stockhorst U. 2018. Women with high estradiol status are protected against declarative memory impairment by pre-learning stress. Neurobiol Learn Mem 155: 403–411. 10.1016/j.nlm.2018.08.018 [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Azcoitia I, Garcia-Segura LM. 2015. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci 16: 17–29. 10.1038/nrn3856 [DOI] [PubMed] [Google Scholar]
- Armbruster D, Grage T, Kirschbaum C, Strobel A. 2018. Processing emotions: effects of menstrual cycle phase and premenstrual symptoms on the startle reflex, facial EMG and heart rate. Behav Brain Res 351: 178–187. 10.1016/j.bbr.2018.05.030 [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. 1995. The development of a clinician-administered PTSD scale. J Trauma Stress 8: 75–90. 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. 1998. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit area survey of trauma. Arch Gen Psychiatry 55: 626–632. 10.1001/archpsyc.55.7.626 [DOI] [PubMed] [Google Scholar]
- Brønnick MK, Økland I, Graugaard C, Brønnick KK. 2020. The effects of hormonal contraceptives on the brain: a systematic review of neuroimaging studies. Front Psychol 11: 556577. 10.3389/fpsyg.2020.556577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynhildsen J. 2014. Combined hormonal contraceptives: prescribing patterns, compliance, and benefits versus risks. Ther Adv Drug Saf 5: 201–213. 10.1177/2042098614548857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JK, Bragdon L, Pineles SL. 2022. Conditioned physiological reactivity and PTSD symptoms across the menstrual cycle: anxiety sensitivity as a moderator. Psychol Trauma 14: 453–461. 10.1037/tra0001129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho MC, Genaro K, Leite-Panissi CRA, Lovick TA. 2021. Influence of estrous cycle stage on acquisition and expression of fear conditioning in female rats. Physiol Behav 234: 113372. 10.1016/j.physbeh.2021.113372 [DOI] [PubMed] [Google Scholar]
- Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. 2009. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus 19: 1142–1150. 10.1002/hipo.20581 [DOI] [PubMed] [Google Scholar]
- Daniels K, Abma JC. 2020. Current contraceptive status among women aged 15–49: United States, 2017–2019. NCHS Data Brief, no. 388. National Center for Health Statistics, Hyattsville, MD. https://www.cdc.gov/nchs/products/databriefs/db388.htm [PubMed] [Google Scholar]
- Fillingim R, King C, Ribeiro-Dasilva M, Rahim-Williams B, Riley J III. 2009. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10: 447–485. 10.1016/j.jpain.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon ML, Williams JBW. 2002. Structured clinical interview for DSM-IV axis I disorders, research version, non-patient edition. (SCID-I/NP). Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Foa EB. 2000. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry 61: 43–48. [PubMed] [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. 1981. Committee report. Publication recommendations for electrodermal measurements. Psychophysiology 18: 232–239. 10.1111/j.1469-8986.1981.tb03024.x [DOI] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. 2012. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry 72: 19–24. 10.1016/j.biopsych.2012.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, Ressler KJ, Jovanovic T. 2013. Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci 38: 341–348. 10.1503/jpn.120129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Norrholm SD. 2015. Estrogen and extinction of fear memories: implications for posttraumatic stress disorder treatment. Biol Psychiatry 78: 178–185. 10.1016/j.biopsych.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. 2013. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry 73: 371–378. 10.1016/j.biopsych.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes FS, Hellewell J, Hensman R, Wang M, Deakin JF. 1991. Characterization of a psychophysiological model of classical fear conditioning in healthy volunteers: influence of gender, instruction, personality and placebo. Psychopharmacology 104: 231–236. 10.1007/BF02244184 [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. 2001. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1). Brain Res 888: 356–365. 10.1016/S0006-8993(00)03116-4 [DOI] [PubMed] [Google Scholar]
- Hammoud MZ, Foa EB, Milad MR. 2020. Oestradiol, threat conditioning and extinction, post-traumatic stress disorder, and prolonged exposure therapy: a common link. J Neuroendocrinol 32: e12800. 10.1111/jne.12800 [DOI] [PubMed] [Google Scholar]
- Herrera AY, Hodis HN, Mack WJ, Mather M. 2017. Estradiol therapy after menopause mitigates effects of stress on cortisol and working memory. J Clin Endocrinol Metab 102: 4457–4466. 10.1210/jc.2017-00825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang MJ, Zsido RG, Song H, Pace-Schott EF, Miller KK, Lebron-Milad K, Marin MF, Milad MR. 2015. Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry 15: 295. 10.1186/s12888-015-0673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, Marmar CR, Neylan TC. 2013. Sex differences in fear conditioning in posttraumatic stress disorder. J Psychiatr Res 47: 64–71. 10.1016/j.jpsychires.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Niles AN, Metzler TJ, Lipshitz SL, Otte C, Milad MR, Orr SP, Marmar CR, Neylan TC. 2021. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology 10.1038/s41386-021-01222-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. 2006. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav 49: 197–205. 10.1016/j.yhbeh.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. 2012. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord 2: 3. 10.1186/2045-5380-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Kilaru V, Shin J, Jovanovic T, Almli LM, Dias BG, Norrholm SD, Fani N, Michopoulos V, Ding Z, et al. 2018. Estrogen-dependent association of HDAC4 with fear in female mice and women with PTSD. Mol Psychiatry 23: 658–665. 10.1038/mp.2016.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Cover KK, Taha MB, Landau AJ, Milad MR, Lebron-Milad K. 2017. Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J Neurosci Res 95: 163–175. 10.1002/jnr.23826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. 1994. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res 661: 25–34. 10.1016/0006-8993(94)91176-2 [DOI] [PubMed] [Google Scholar]
- Matsumoto YK, Kasai M, Tomihara K. 2018. The enhancement effect of estradiol on contextual fear conditioning in female mice. PLoS ONE 13: e0197441. 10.1371/journal.pone.0197441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, Schweckendiek J, Klucken T, Vaitl D, Stark R. 2013. Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology 38: 2529–2541. 10.1016/j.psyneuen.2013.05.015 [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. 2006. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav Neurosci 120: 1196–1203. 10.1037/0735-7044.120.5.1196 [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. 2008. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 42: 515–520. 10.1016/j.jpsychires.2008.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. 2009a. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164: 887–895. 10.1016/j.neuroscience.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. 2009b. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: 1075–1082. 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. 2010. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience 168: 652–658. 10.1016/j.neuroscience.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. 2007. Mechanisms of fear extinction. Mol Psychiatry 12: 120–150. 10.1038/sj.mp.4001939 [DOI] [PubMed] [Google Scholar]
- Nillni YI, Pineles SL, Patton SC, Rouse MH, Sawyer AT, Rasmusson AM. 2015. Menstrual cycle effects on psychological symptoms in women with PTSD. J Trauma Stress 28: 1–7. 10.1002/jts.21984 [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. 2000. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol 109: 290–298. 10.1037/0021-843X.109.2.290 [DOI] [PubMed] [Google Scholar]
- Peyrot C, Brouillard A, Morand-Beaulieu S, Marin MF. 2020. A review on how stress modulates fear conditioning: let's not forget the role of sex and sex hormones. Behav Res Ther 129: 103615. 10.1016/j.brat.2020.103615 [DOI] [PubMed] [Google Scholar]
- Pineles SL, Blumenthal TD, Curreri AJ, Nillni YI, Putnam KM, Resick PA, Rasmusson AM, Orr SP. 2016a. Prepulse inhibition deficits in women with PTSD. Psychophysiology 53: 1377–1385. 10.1111/psyp.12679 [DOI] [PubMed] [Google Scholar]
- Pineles SL, Nillni YI, King MW, Patton SC, Bauer MR, Mostoufi SM, Gerber MR, Hauger R, Resick PA, Rasmusson AM, et al. 2016b. Extinction retention and the menstrual cycle: different associations for women with posttraumatic stress disorder. J Abnorm Psychol 125: 349–355. 10.1037/abn0000138 [DOI] [PubMed] [Google Scholar]
- Pryce CR, Lehmann J, Feldon J. 1999. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacol Biochem Behav 64: 753–759. 10.1016/S0091-3057(99)00147-1 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. 2008. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72. 10.1038/sj.npp.1301555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. 2003. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci 1008: 112–121. 10.1196/annals.1301.012 [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. 2001. Executive summary: stages of reproductive aging workshop (STRAW). Climacteric 4: 267–272. 10.1080/cmt.4.4.267.272 [DOI] [PubMed] [Google Scholar]
- Stevens JS, van Rooij SJH, Jovanovic T. 2018. Developmental contributors to trauma response: the importance of sensitive periods, early environment, and sex differences. Curr Top Behav Neurosci 38: 1–22. 10.1007/7854_2016_38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanielian T, Jaycox LH, Schell TL, Marshall GN, Vaiana ME. 2008. Treating the invisible wounds of war: conclusions and recommendations. In Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery (ed. Tanielian T, Jaycox L), pp. 431–453. RAND Corporation, Santa Monica, CA. [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. 2011. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 70: 920–927. 10.1016/j.biopsych.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorawski M, Cook CA, Kuhn CM, LaBar KS. 2005. Sex, stress, and fear: individual differences in conditioned learning. Cogn Affect Behav Neurosci 5: 191–201. 10.3758/cabn.5.2.191 [DOI] [PubMed] [Google Scholar]