Abstract
Wastewater treatment is essential to guarantee human health and ecological security. Catalytic ozonation with nanocatalysts is a widely studied and efficient treatment technology. However, this method has always been limited by nanocatalysts disadvantages such as easily loss, difficult to separate and reuse, and catalytic ability decay caused by aggregation, which could cause severe resources waste and potential risk to human health and ecosystem. To remedy these challenges, a magnetic-void-porous MnFe2O4/carbon microsphere shell nanocatalyst (CMS-MnFe2O4) was successfully synthesized using renewable natural microalgae. The separation test showed CMS-MnFe2O4 was rapidly separated within 2 min under an external magnetic field. In catalytic ozonation of oxalic acid (OA), CMS-MnFe2O4 showed efficient and stable catalytic efficiency, reaching a maximum total organic carbon removal efficiency of 96.59 % and maintained a 93.88 % efficiency after 4 cycles. The stable catalytic efficiency was due to the supporting effects of the carbon microsphere shell, which significantly enhanced CMS-MnFe2O4 chemical stability and reduced the metal ions leaching to 10–20 % of MnFe2O4 through electron transfer. To explore the catalytic mechanism, radical experiments were conducted and a new degradation pathway of OA involving superoxide anions rather than hydroxyl radicals was proposed. Consequently, this study suggests that an efficient, recyclable, stable, and durable catalyst for catalytic ozonation could be prepared.
Keywords: Catalytic ozonation, Magnetic nanocatalyst, Separation recycling, Catalytic mechanism, Metal leaching
Graphical abstract
Highlights
-
•
A magnetic-void-porous MnFe2O4/carbon microsphere shell is developed.
-
•
The catalyst can be separated within 2 min by an outer magnet.
-
•
The carbon shells prevent the metal ions leaching from MnFe2O4.
-
•
A new catalytic mechanism of oxalic acid is proposed.
1. Introduction
Wastewater treatment is essential to guarantee human health and ecological security [1]. Heterogeneous catalytic ozonation, as an efficient wastewater treatment technology, has extensively attracted the attention of global researchers over the years [2]. As the key to catalytic ozonation, catalysts with various materials, such as activated carbon [3,4], graphene [5], metals [6] and metal oxides [7,8], have been successfully developed. Studies have revealed that nanocatalysts usually possess excellent and unique catalytic activity due to their vast specific surface area, unique dimensional properties, abundant active sites, and many grain interfaces [9,10]. Therefore, an increasing number of researchers have focused on nanomaterials and various new nanocatalysts with high catalytic abilities have been developed [[11], [12], [13]].
However, most of these reported nanocatalysts are still in the early stages of laboratory research, which is due to the following unavoidable disadvantages of nanomaterials [14]. The nanoscale size makes it difficult to separate the nanocatalysts from the treated wastewater for reuse [15]. Nanomaterials are usually high in production costs. If efficient separation and reuse could not be achieved, it would cause severe economic loss and resource wastes [16]. The vast specific surface area and high surface atomic ratio not only gives the nanocatalyst its high catalytic activity but also makes them prone to particle aggregation during application, resulting in severe activity loss and pressure drop [17]. Finally, some studies reported that if the nanomaterials enter into the aquatic system after wastewater treatment, which might cause adverse effects on the natural ecosystem and human health, which is against the cleaner production principles [18]. Unfortunately, there are only a few studies aimed at solving these problems. To promote the practical application of nanocatalysts and meet the requirements of cleaner production, developing advanced materials or methods to solve these problems are necessary.
Recently, using magnetic materials as ozonation catalysts has gradually attracted researchers' attention. Ferroferric oxide (Fe3O4) [15], copper ferrite spinel (CuFe2O4) [19], nickel ferrite spinel (NiFe2O4) [20] and bismuth copper oxide (Bi2CuO4) [21] have been proven to be efficient in catalytic ozonation for refractory organic pollutant degradation. Although the magnetic response makes it convenient for magnetic nanocatalysts to be separated and reused, the aggregation of magnetic nanoparticles still occurs and is even more severe. Loading nanocatalysts on other materials, such as ceramic membrane [22], carbon aerogel [23] and activated carbon [24], has been proven to be a possible method to avoid this issue. The structure, properties, and size of the support material will affect the catalyst activity [25]. Hence, finding the proper carriers is not only helpful to maintain high catalytic activity but also prevents the loss and aggregation of the nanocatalysts.
Microalgae is an inexpensive, abundant, and renewable natural biological material that could be a desired and excellent carrier after simple treatment [[26], [27], [28]]. For example, Bi and Pan (2014) used Chlorella pyrenoidosa (C. pyrenoidosa) and ferric ammonium citrate as precursors to synthesize rattle-type multiple magnetite core microspheres (RMMCMs) through hydrothermal treatment and found that RMMCMs showed excellent drug delivery ability [29]. These excellent properties are mainly due to the unique hollow porous microsphere shell structures of RMMCMs which include abundant pores that ensure the free diffusion of the solution and prevent the loss, collision and aggregation of supported nanoparticles. The shell structure also possesses a double specific surface area of volume compared with the same volume sphere, which could provide more reaction zones [30]. Hence, we hypothesize that the hollow porous microsphere shells prepared by microalgae have the potential the be carriers for catalytic ozonation. Currently, there are only a few studies that have applied this unique carrier in catalytic ozonation.
It can be observed that an efficient, easily separated and recycled, durable nanocatalyst is helpful to solve the disadvantages of nanocatalysts and promote their field application in wastewater treatment. Hence, a novel magnetic-void-porous CMS-MnFe2O4 nanocatalyst was synthesized by integrating magnetic manganese ferrite spinel (MnFe2O4) and CMS prepared with C. pyrenoidosa into a unique supported nanocomposite. The structure-to-performance relationships of CMS-MnFe2O4 were analyzed and discussed based on structure/morphology characterizations and catalytic ozonation process. Oxalic acid (OA) was selected as the target pollutant since it is usually applied to measure the final mineralization ability of catalysts [31]. Nano titanium dioxide (nano-TiO2), a widely reported catalyst in the literature, was studied for comparison. Recyclability and stability of nanocatalysts and metal ions leaching related to secondary pollution and environmental risks were also studied and discussed.
2. Materials and methods
2.1. Preparation and characterization of catalyst
The CMS-MnFe2O4 magnetic nanocomposite was prepared with C. pyrenoidosa (Guangyu Biological Technology Co., Ltd, Shanghai, China), which has a thick cell wall, manganese(II) chloride (MnCl2), and iron nitrate nonahydrate (FeNO3·9H2O) through calcination and hydrothermal treatment (Fig. S1 in the Supplementary Material). The detailed synthetic process of the catalysts is described in Text S1 in the Supplementary Material.
The physicochemical properties of the synthesized CMSs-MnFe2O4 were characterized by the following techniques. The catalyst structure and morphology were characterized using high-resolution transmission electron microscopy (HRTEM, JEM-2100, Tecnai, U.S.A.) and scanning electron microscopy (SEM, JSM-6460LV, Japan). with an energy-dispersive spectrometer (EDS). The surface chemical compositions were determined using an X-ray photoelectron spectroscopy (XPS, PHI Quantera, Japan). The metal crystallographic forms were acquired using X-ray diffraction meter (XRD, Rigaku D/max, Japan). The average size of the shell pores was measured by a surface area and porosity analyzer (ASAP2460, Micromeritics, U.S.A.). A vibrating sample magnetometer (VSM, SQUID-VSM, Quantum Design, U.S.A.) was used to measure the magnetic property of the catalyst. The zeta potential of the catalyst was acquired using a Zetasizer (Nano ZS, Malvern Co., UK).
2.2. Catalytic ozonation process
The ozonation experiments were carried out in a laboratory-scale reactor (ca. 1.5 L) equipped with agitation. Ozone was produced using pure oxygen in an ozone generator (3S-A3, Tonglin, China) monitored with an ozone analyzer (3S-J5000, Tonglin, China), and continuously bubbled into the OA solution through a porous titanium alloy aerator. Ozone leaving the reactor was removed with an ozone destructor.
In each ozonation experiment, the reactor was filled with 1 L of 100 mg/L OA solution at a natural pH (approximately 3). For catalytic ozonation experiments, 3 g of CMS-MnFe2O4 (containing 0.5 g MnFe2O4), 0.5 g of MnFe2O4, and 0.5 g of nano-TiO2 (Text S2) were introduced into the reactor. The ozone flow rate was 100 mL/min and the concentration was maintained at 50 mg/L (optimum ozone concentration was decided by a pre-experiment (Fig. S2)). The stirring speed was 120 rpm to keep the reactor contents perfectly mixed. In cyclic experiments, the same procedure was followed and the treated solution was separated under an outer magnet. Then, the recycled CMS-MnFe2O4 sample was used directly in another run after each experiment. Other experimental factors, such as pH, anions, and dissolved organic matter (DOM), were observed. The detailed experimental conditions are described in Text S3. In the radical quenching experiments, 100 mg of p-benzoquinone (p-BQ) and 100 mg of tert-butanol (TBA) were added to the solution (1 L). For comparative purposes, adsorption of OA on the catalyst (aerated with O2 rather than O3) and ozonation alone were conducted under identical experimental conditions. All experiments were performed in triplicate at ambient pressure and temperature.
2.3. Sampling and analysis
To monitor OA degradation, a 30 mL aliquot was taken through the sample tap at 0, 10, 15, 20, 30, 40, and 70 min. Then, the solution was transferred into a beaker and placed on a magnet. The supernatant was filtered with a membrane with a 0.45 μm pore size to collect the water samples. The total organic carbon (TOC) and inorganic carbon (IC) were analyzed using an organic carbon analyzer (TOC-Lcph, Shimadzu, Japan), and the OA concentration was measured by ion chromatography (ICS-1000, Dionex, U.S.A.). The calculation of TOC and OA removal efficiency are described in Text S4. To detect the radical species, coumarin and 4-chloro-7-nitro-1,2,3-benzoxadiazole (NBD-Cl) were used as the probe molecules. Electron paramagnetic resonance (EPR) was also applied to detect radical species by using dimethylpyridine N-oxide (DMPO) as a sensitive spin-trapping agent (detailed procedures are described in Text S5).
3. Results and discussion
3.1. Characterization of catalysts
C. pyrenoidosa cells, with evenly distributed particle sizes (10–20 μm), could be viewed as a kind of core/shell material. The cell wall is constructed primarily of cellulose and hemicellulose, which is considerably hard and infusible. The core is mainly composed of proteins, amino acids, vitamins, nucleic acids DNA and RNA, which easily decompose under high temperatures [32]. Proper calcination treatment could prepare a unique CMS structure. As shown in the SEM images (Fig. 1(b)), an intact CMS structure was obtained after calcination and some micropores appeared compared with the relatively smooth surface of untreated C. pyrenoidosa cells (Fig. 1(a)). Wang et al. (2005) used a similar method to treat B. oleracea pollen grains and obtained similar CMS structures. Data calculated using the Barrett–Joyner–Halenda (BJH) method revealed that the average size of the shell pores was approximately 11.27 nm (Table S1) [32]. Pore formation might be due to the different thermal decomposition resistances of different components in the cell wall [29,32]. After hydrothermal treatment, Fig. 1(c) shows that nanoparticles were distributed on the CMS surface. The TEM image (Fig. 1(d) and (e)) revealed that the nanoparticles, with sizes of approximately 20–40 nm, were evenly distributed in the CMS wall and interior. The elemental mapping characterization (Fig. 1(c1) and Fig. 1(c2)) also indicated that Mn and Fe were evenly distributed on the CMS. The HRTEM image (Fig. 1(f)) showed that these nanocrystals had distinct 0.255 nm fringe spacings, corresponding to the (311) planes of cubic MnFe2O4.
Fig. 1.
Microstructure and morphology of C. pyrenoidosa cells, CMS and CMS-MnFe2O4: (a) SEM images of C. pyrenoidosa; (b) SEM images of CMS; (c), (c1), and (c2) SEM images of CMS-MnFe2O4 and elemental maps of Mn and Fe; (d) and (e) TEM images of CMS-MnFe2O4; (f) HRTEM images of CMS-MnFe2O4.
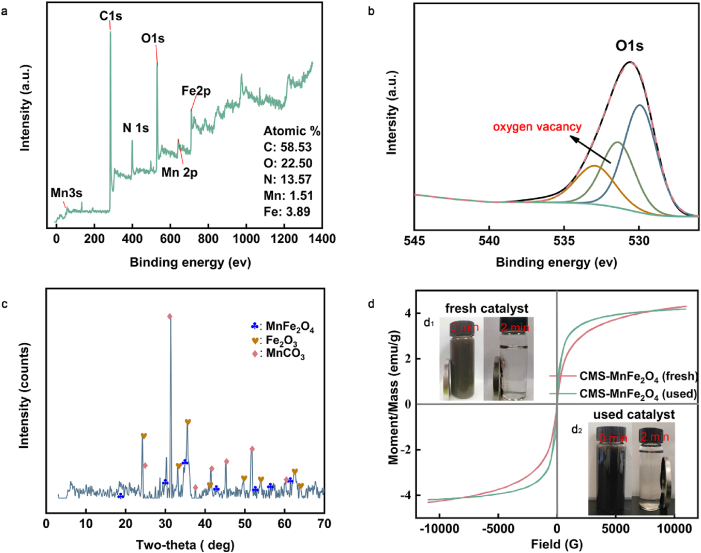
To further explore the characterization of the nanoparticles, XPS was applied to analyze the elemental composition on the catalyst surface. As shown in Fig. 2(a), C, O, Mn, N, and Fe signals were observed, and the atomic ratios (AR) were 58.53 (C): 22.50 (O): N (13.57): 1.51 (Mn): 3.89 (Fe). The higher AR of O compared with the AR of MnFe2O4 indicated that the CMS retained oxygen-containing functional groups or organic components. The AR of Mn with Fe was lower than 1:2, which meant that Mn and Fe might not completely be transformed into MnFe2O4. This speculation was coincident with the XRD results (Fig. 2(c)), where MnFe2O4, Fe2O3, and MnCO3 were synthesized after hydrothermal treatment. Similar to previous studies, unexpected diffraction peaks of α-Fe2O3 were detected. This observation might be due to the imbalance of hydrated cations (Mn2+, Fe3+) at the microzone in the solution [33]. The appearance of MnCO3 might be due to some organic components in CMS decomposing under hydrothermal treatment and producing carbonic acid that can react with Mn2+ to form MnCO3. VSM was introduced to analyze the CMS-MnFe2O4 magnetic properties, and the hysteresis loops are shown in Fig. 2(d). The CMS-MnFe2O4 saturation magnetization (Ms) was 4.31 emu/g, which ensured that the dispersed catalyst particles could be separated from the solution within 2 min (Fig. 2(d1)). This encouraging result revealed magnetic separation has a promising application potential in the rapid efficient separation and reuse of nanocatalysts in the field application of wastewater treatment.
Fig. 2.
The physical and chemical properties characterization of CMS-MnFe2O4 and MnFe2O4: (a) survey XPS spectrogram of CMS-MnFe2O4; (b) high-resolution XPS O1s spectra of CMS-MnFe2O4;(c) XRD spectrogram of CMS-MnFe2O4; (d), (d1) and (d2) VSM curves and magnetic separation of (inset) for the CMS-MnFe2O4 (fresh and used).
3.2. Catalytic ozonation of oxalic acid
In the catalytic ozonation experiment, OA was selected as the target pollutant to test the catalytic ability. In addition, the anion concentration [34], pH, and humic acid (HA) concentration [35], which usually affect the catalytic ozonation process, were also studied.
3.2.1. The removal of oxalic acid
As shown in Fig. 3(a), MnFe2O4 and CMS-MnFe2O4 both showed an excellent adsorption capacity for OA, and a maximum TOC removal efficiency of 34.45 % and 34.88 %, respectively, were reached within 1 min. However, CMS and TiO2 hardly had any adsorption on OA. The absorption capacity might be related to the surface electrical properties of CMS-MnFe2O4 (Fig. S3) because CMS-MnFe2O4 particles were positively charged at pH 3 and could adsorb oxalate ions by electrostatic attraction. At 10 min, ozone was introduced and the final TOC removal efficiencies were 16.29 %, 38.39 %, 70.28 %, 96.59 %, and 92.12 % after 1 h for ozonation alone and ozonation with CMS, nano-TiO2, CMS-MnFe2O4, and MnFe2O4, respectively. CMS-MnFe2O4 showed excellent catalytic activity for OA degradation, which was better than CMS, MnFe2O4, nano-TiO2, and almost 5–6 times that of ozonation alone. The XPS results revealed that N (13.57 %) existed in CMS-MnFe2O4 and the high-resolution XPS N1s spectra (Fig. S4) showed that N mainly consisted of Graphitic N (34.25 %), Pyrrolic N (25.00 %), and Pyridinic N (34.25 %) of which pyridinic N has been proven to be the active site in catalytic ozonation. Hence, the improved performance of CMS-MnFe2O4 relative to MnFe2O4 might be due to the catalytic activity of CMS [5]. The concentration of OA was also monitored during catalytic ozonation (Fig. 3(b)). The degradation tendency of OA was similar to that of TOC removal, which indicated that no stable intermediate products existed during the catalytic ozonation of OA in the presence of CMS-MnFe2O4.
Fig. 3.
The catalytic performance of CMS, MnFe2O4, nano-TiO2, CMS-MnFe2O4 in ozonation degradation of OA: (a) and (b) TOC and OA removal efficiency with ozonation alone and catalytic ozonation in the presence of nano-TiO2, CMS-MnFe2O4 and MnFe2O4 (fresh); the effects of pH (c), HA (d), Cl− (e) and SO42− (f) on the catalytic ozonation of OA with CMS-MnFe2O4 (reaction conditions: temperature, 295 K; ozone concentration in the gas, 50 mg/L; gas flow, 100 mL/min; OA concentration, ∼100 mg/L; concentration of CMS-MnFe2O4 and MnFe2O4 used in the catalytic ozonation, 3 g/L and 0.5 g/L, respectively; natural pH, ∼3).
The above results revealed that CMS-MnFe2O4 had excellent catalytic ability in OA ozonation. Compared with the nanopowder of MnFe2O4, the loading process of active components did not reduce the catalytic activity, which indicated that the CMS prepared by C. pyrenoidosa was an excellent carrier.
3.2.2. The effect of pH, anions and humic acid
As shown in Fig. 3(c), the TOC removal efficiency reached a maximum at pH 2.96 and dramatically decreased with increasing pH. When the pH was higher than the point of zero charge (PZC) (approximately pH 4) of CMS-MnFe2O4, the surface electrical properties became negatively charged, which led to a decrease in OA adsorption. The ozone decomposition results at different pH values (Fig. S5) showed that ozone decomposed completely within 30 s under different pH conditions, which revealed that CMS-MnFe2O4 possessed stable ozone decomposition ability under a broad pH condition. Hence, we concluded that pH affected the catalytic efficiency of OA mainly by changing the OA adsorption on CMS-MnFe2O4.
DOM, which exists widely in natural water and wastewater, usually has a significant effect on catalytic ozonation. HA, a vital component of DOM, was applied to explore the impact on OA catalytic ozonation with CMS-MnFe2O4 [35]. Fig. 3(d) shows that the degradation efficiency of OA slightly decreased as the HA dose increased from 0 to 200 mg/L. The HA in bulk water hardly showed a competitive relation with OA decomposition. Hence, combined with the results of the OA adsorption experiment, this trend indicated that the decomposition of OA occurred on the surface of CMS-MnFe2O4 but not in bulk water. Although HA might consume part of the ozone, this effect was insignificant compared with the high efficiency of catalytic ozonation.
In addition to pH and DOM, the effect of anions in solution has been widely reported in the literature [34]. Cl− and SO42− are the common anions in wastewater that were selected to be studied. Fig. 3(e) shows that the adsorption of OA decreased from 34.87 % to 21.82 % in the presence of 1 mM of Cl− due to the competitive adsorption between OA and Cl−. Interestingly, the decrease in OA adsorption did not cause a change in the final TOC removal efficiency. Meanwhile, as the Cl− concentration increased from 1 to 10 mM, the final TOC removal efficiencies hardly changed. A similar phenomenon was observed when SO42− was added (Fig. 3(f)). These results indicated that CMS-MnFe2O4 was resistant to anions during the catalytic ozonation of OA.
3.3. The catalytic activity identification and radical experiment
To explore the catalytic mechanism of OA in the presence of CMS-MnFe2O4, the catalytic activity identification of CMS-MnFe2O4 was examined in detail. Radical detection and quenching experiments were also conducted.
3.3.1. The catalytic activity identification
The absorption and decomposition of O3 is critical to the catalytic process. Previous studies revealed that the coordination of O and metal atoms on the metal oxide surface is usually not saturated [36]. This unsaturated coordination would generate oxygen vacancies [11] and Lewis acid sites [37] on the catalysts surface. Oxygen vacancies showed excellent absorption capacity with O3. The XPS results in Fig. 2(b) revealed that CMS-MnFe2O4 possessed abundant oxygen vacancies. In detail, the high-resolution XPS O1s spectra of CMS-MnFe2O4 clearly showed three peaks. The peak at 529.91 eV was due to oxygen atoms bound to metals, the peak at 532.75 eV was related to hydroxy species of surface-adsorbed water molecules, and the peak at 531.38 eV was attributed to defect sites with low oxygen coordination [38]. The Lewis acid site could usually absorb the Lewis base (anion) or be hydrated and generate surface hydroxyl groups. The OA anion could be absorbed on the Lewis acid site through electrostatic interactions, which contributed to the degradation of OA with CMS-MnFe2O4.
In addition to O3 adsorption, the decomposition of O3 is related to the production of reactive oxygen species (ROSs; e.g, •OH (hydroxyl radical) or •O2− (superoxide anion)). Studies have revealed that the electron transfer between metal ion redox couples (Men−1+/Men+) contributes to the formation of ROSs in the catalytic process [[39], [40], [41]]. The XPS results of CMS-MnFe2O4 are shown in Fig. 4. In Fig. 4(a), the Fe 2p spectra showed two main peaks at 711.15 (Fe 2p3/2) and 724.68 eV (Fe 2p1/2) while the peaks at binding energies of 711.65 and 725.1 eV indicated the presence of Fe3+ and the peaks at 709.85 and 723.12 eV indicated the presence of Fe2+ [42]. In Fig. 4(b), the Mn 2p region could also be fitted into two components. Peaks at 640.2 eV and 652.0 eV could be attributed to Mn2+, while those at 641.9 eV and 653.7 eV corresponded to Mn3+ [43]. The above results revealed that Fe2+/Fe3+ and Mn2+/Mn3+ redox couples existed at the CMS-MnFe2O4 surface, which indicated that CMS-MnFe2O4 had a promising ability to catalyze ozone decomposition and produce active radicals.
Fig. 4.
XPS spectra of MnFe2O4 and CMS-MnFe2O4: Fe 2p (a) and Mn 2p (b) before and after the reaction of CMS-MnFe2O4; Fe 2p (c) and Mn 2p (d) before and after the reaction of MnFe2O4.
3.3.2. Radical detection
Fluorescence microscopy image analysis and EPR, as well-known methods to detect radical species, were applied in this study [44]. Coumarin and NBD-Cl were applied as probe molecules. •OH attack coumarin via hydroxylation, forming the stable compound 7-hydroxycoumarin (7-HC), which exhibits good fluorescence sensitivity [44]. NBD-Cl reacts with •O2− and generates a fluorescent product [45]. Fig. 5(a) and (b) reveals that CMS-MnFe2O4 and the solution near the catalyst both showed distinct fluorescence in the presence of coumarin and NBD-Cl. The fluorescence results were in agreement with EPR. As shown in Fig. 5(c), a clear DMPO-OOH peak (aN = 1.42 mT; aHβ = 1.14 mT; aHγ1 = 0.12 mT), correlating with •O2−, was observed in the DMSO matrix. For •OH, a distinct signal of DMPO-OOH and a weaker signal of DMPO-OH (hyperfine peak splitting parameter: AN = AH = 1.48 mT) were both observed, which revealed that the main produced radical species was •O2− during catalytic ozonation in the presence of CMS-MnFe2O4 [7]. Then, •O2− transformed into •OH through a series of radical chain reactions [46]. The aforementioned results showed that •O2− and •OH were both generated in the catalytic process, which indicated that CMS-MnFe2O4 had the potential to be an excellent catalyst in wastewater treatment.
Fig. 5.
The fluorescence microscopy image in the presence of coumarin (a) and NBD-Cl (b); (c) EPR identification of hydroxyl radicals and superoxide radicals (using dimethyl sulfoxide (DMSO) as the solvent); (d) the effect of TBA and p-benzoquinone on OA removal efficiency in the presence of CMS-MnFe2O4 (used).
3.3.3. Radical quenching experiment
•OH is an important active species involved in catalytic ozonation. TBA (a well-known •OH quencher) was added to the solution to verify whether •OH contributed to OA degradation [47]. Compared with catalytic ozonation with CMS-MnFe2O4, the OA degradation rate was significantly improved, and the final OA removal efficiency was also increased by 5.21 % with TBA and CMS-MnFe2O4 (Fig. 5(d)). Interestingly, TBA showed a positive effect instead of a negative effect, which was different from the widely reported studies [48,49]. Orge et al. (2012) observed a similar result in OA catalytic ozonation using manganese oxide–carbon composites. In their study, OA adsorption on Mn–O was also faster, reaching a 25 % maximum removal rate within just a few minutes. They proposed that OA was absorbed on the surface of manganese oxide–carbon composites and then directly oxidized by ozone, and similar mechanisms were reported for catalytic ozonation of phenol [50] or azo dyes [51] with brucite.
In contrast to comprehensive reports of •OH, the contribution of •O2− to catalytic ozonation began to be reported recently [11,52]. The p-BQ, a well-known quencher of •O2−, was applied to determine whether •O2− is involved in OA degradation [53]. Fig. 5(d) shows that the OA removal efficiencies were 47.32 % and 91.02 % with and without p-BQ, respectively, indicating that •O2− had a significant contribution to OA degradation. The OA removal efficiency by the absorption process decreased from 35.86 % to 23.29 % after adding p-BQ. Hence, it is proposed that there are two pathways for the hindrance of p-BQ: (Ⅰ) p-BQ and OA are competitively adsorbed, resulting in a decrease in OA absorption and (Ⅱ) p-BQ rapidly consumes •O2− and impedes the reaction between OA (absorbed) and •O2−.
3.4. The mechanism of OA degradation
Based on the above results, a new detailed degradation mechanism of OA involving •O2− rather than •OH with CMS-MnFe2O4 (Fig. 6) is proposed. To easily illustrate the catalytic process it was divided into 4 steps:
-
(I)
OA anions and O3 were absorbed on the catalyst surface. At the initial reaction stage (Fig. 6(a)), OA (ka1 = 5.9∗10−2, ka2 = 6.4∗10−5) was ionized into HC2O4− at pH 3, which is a Lewis base and absorbed on the MnFe2O4 surface () (Eq. (1), Eqs. Eq. 2, Eq. 3)) (Orge et al., 2012). Meanwhile, O3 was absorbed on the oxygen vacancy (Wang et al., 2019).
-
(II)
O3 was decomposed and generated •O2− (Eq. (4)). Ozone was absorbed on the oxygen vacancy, then the surface Mn2+/Fe2+ (≡Mn2+/Fe2+) lost an e- and generated Mn3+/Fe3+(≡Mn3+/Fe3+), while O3 obtained an e- and generated O and •O2− [54].
-
(Ⅲ)
OA was decomposed under the attack of •O2−. Carbon atoms in OA are electropositive (0.43 V). Furthermore, the electronic absorption ability of ≡Mn3+/Fe3+ is stronger than ≡Mn2+/Fe2+, which caused the electron cloud of the absorbed HC2O4− to deflect to ≡Mn3+/Fe3+ and further increased the electropositivity of the carbon atoms near the surface. Hence, •O2−, possessing nucleophilic addition ability, attacked the C–C and generated ≡S–CO2–O2 and •CHO2 (Eq. 5) [55]. Then, ≡S–CO2–O2 underwent electron rearrangement, desorption, and generated CO2 and O2 (Eq. (6)). Meanwhile, ≡Mn3+/Fe3+ acquired an e- and formed ≡Mn2+/Fe2+, resulting in the desorption of O. •CHO2 reacted with O or O3 and generated H2CO3 (O2) (Eq. (7)).
-
(IV)
The absorbed OA and O were consumed and desorbed, and then O3 and OA in the solution were reabsorbed on the surface of recovered MnFe2O4. The above steps were repeated.
Fig. 6.
The proposed reaction mechanism of OA catalytic ozonation with CMS-MnFe2O4: (a) initial reaction stage; (b) anaphase of reaction.
Along with the degradation of OA, the pH of the solution increased, and part of OA was ionized into C2O42− (Fig. S6). Steps (Ⅰ) and (Ⅲ) had a minor change. C2O42− was absorbed on the catalyst surface and formed ≡S–C2O4–S≡ (Eq. (8)). Monomolecular ≡S–C2O4–S≡ reacted with •O2− and generated two molecules, ≡S–CO2–O2 (Eq. (9)), which were decomposed into O2 and CO2 without H2CO3. By monitoring the inorganic carbon concentration of the solution (Fig. S5), it was observed that the IC concentration increased with time in the early reaction, reached a maximum, and then decreased with time. This interesting phenomenon corresponded with the mechanism described in Fig. 6(b).
| Eq. 1 |
| Eq. 2 |
| Eq. 3 |
| Eq. 4 |
| Eq.5 |
| Eq. 6 |
| Eq. 7 |
| Eq. 8 |
| Eq. 9 |
When adding p-BQ, •O2− is quenched, and Eqs. (5) and (9) are blocked, resulting in a decrease in OA removal efficiency. The above radical experiments revealed that •OH was not involved in OA degradation. Previous studies showed that surface hydroxyl groups could catalyze O3 to generate •OH, and the excess •OH could react with •O2− [46] and O3 [2] following Eqs. (10)–(14), resulting in the consumption of •O2− and O3 and adversely affecting Eqs. (4) and (5). When adding TBA, •OH is consumed by TBA, and the following reactions (Eqs. (10)–(14)) are hindered, which explains the improvement in OA degradation after adding TBA. Cl− and SO42−, also having quenching effects on •OH, plays a similar stimulative role with TBA, which might balance the side effects of the decrease in OA adsorption when adding anions [34].
| Eq. 10 |
| Eq.11 |
| Eq. 12 |
| Eq. 13 |
| Eq. 14 |
As a common target pollutant, previous studies have conducted experiments on OA's decomposition mechanisms. However, due to the sample molecular structure, OA could not form a stable intermediate that can be detected. Existing studies divided and summarized the mechanisms into decomposition involving •OH in bulk and OA (adsorbed) directedly attacked by ozone molecules based on the radical experiment results [31,56]. Based on existing mechanisms, a brand new and detailed OA decomposition mechanism involving OA adsorption is proposed through •O2− attack and electron transfer in this study. This mechanism has guiding significance for the further exploration of the catalytic degradation mechanism of small molecule acids and the search for new and efficient catalysts for application in wastewater treatment.
3.5. The CMS function
For catalytic ozonation, the separation, recyclability, and stability of nanocatalysts are regarded as the most critical factors affecting the potential for field applications. In this study, CMS was expected to increase the catalyst stability. Hence, recycling experiments of MnFe2O4 and CMS-MnFe2O4 for OA ozonation degradation were conducted. The catalytic ability, magnetic properties, chemical compositions, and metal leaching were monitored and discussed in this section.
3.5.1. Durability of catalytic ability
With nanocatalyst recycling, their catalytic ability is usually weakened due to the increase in nanoparticle size caused by collisions between particles. Zhu et al. (2017) found that the degradation of quinoline decreased from 100 % to 93.45 % when the average size (d0.5) of the fresh MgO catalyst increased from 17.1 nm to 36.9 nm [17]. Wang et al. (2019) also reported that the TOC removal efficiency decreased from 95 % to 72 % after 4 cycles with Mn2O3/LaMnO3-δ [11]. As shown in Fig. 7(b), MnFe2O4 showed a similar result where the maximum TOC removal efficiency decreased from 92.12 % to 85.46 % after 4 cycles as the size of MnFe2O4 increased from 121.2 nm to 189.6 nm. However, compared with MnFe2O4, CMS-MnFe2O4 showed an excellent and stable catalytic efficiency and the TOC removal efficiency was maintained at 93.88 % after 4 cycles. The SEM images (Fig. 7(a)) revealed that CMS-MnFe2O4 maintained an intact structure after 4 cycles. The CMS could fix nanosized MnFe2O4 particles, as shown in Fig. 1 (d) and 1(e). For the nanoparticles inside the different CMSs, CMS also acted as insulation, reducing particle collisions and aggregation. Hence, CMS efficiently mitigated the side effects caused by the increase in nanoparticle size.
Fig. 7.
Size and morphology changes of MnFe2O4 and CMS-MnFe2O4 before and after catalytic ozonation (a); cycle test of CMS-MnFe2O4 and MnFe2O4 on OA catalytic ozonation (b).
3.5.2. Improvement of catalyst stability
Excellent chemical stability is important to ensure the successful application of catalysts. The XRD results in Fig. 8 show that the main crystal type of synthesized MnFe2O4 and CMS-MnFe2O4 remained magnetic MnFe2O4 after 4 cycles. This trend revealed that MnFe2O4 possessed excellent chemical resistance towards ozone. The stable crystal structure endowed CMS-MnFe2O4 with an excellent magnetic response, which ensured the efficient separation after application and avoiding CMS-MnFe2O4 being discharged into natural aquatic systems causing potential environmental risks [57]. Fig. 2(d) shows that the magnetic saturation hardly changed (from 4.31 to 4.18 emu/g) after 4 cycles.
Fig. 8.
Crystal shape change of catalysts: XRD spectra of fresh (a) and used (b) CMS-MnFe2O4, fresh (c) and used (d) MnFe2O4.
In addition to the crystal structure stability, XPS was applied to observe the surface valence changes of MnFe2O4 and CMS-MnFe2O4 because catalytic ability degradation due to valence changes of metal ions is always an important issue when using metal or metal oxide catalysts [5]. Wang et al. (2019) applied Mn2O3/LaMnO3-δ in the catalytic ozonation of OA and found that the amount of surface Mn4+ increased from 28.2 % to 34.3 %, while that of Mn3+ decreased from 61.6 % to 53.1 % as the TOC removal efficiency decreased from 99 % to 72 % after 4 runs [11]. Similarly, as shown in Fig. 4 (c) and (d), the proportion of Fe2+ and Mn2+ for MnFe2O4 decreased from 44.07 % and 61.33 % to34.11 % and 44.88 % after 4 cycles, respectively. However, the proportions of Mn2+/Mn3+ and Fe2+/Fe3+ for CMS-MnFe2O4 hardly changed after 4 cycles (Fig. 4 (a) and (b)). According to the mechanism diagram in Fig. 6(b), when the electron donor (≡S–CO2–O2) could not meet the requirement for the reduction of Mn3+ and Fe3+ along with the decrease of OA, CMS could act as the electron donor and restore the active component to its original chemical state. The electron-donor capacity of carbon material carriers was also reported in previous studies [30,52]. The C1s XPS result (Fig. 9 (b)) showed that the ratios of C–O–C/C–OH, –C O and O–C O increased from 21.63 %, 11.93 %, and 5.43 % to 26.30 %, 14.49 % and 10.52 %, respectively, after catalytic ozonation, which revealed that electrons indeed were transferred from CMS to MnFe2O4 during catalytic ozonation. Compared with MnFe2O4, CMS could obviously improve the chemical stability and ensure the catalytic ability of CMS-MnFe2O4.
Fig. 9.
Schematic diagram of leaching Mn and Fe ions of CMS-MnFe2O4 and MnFe2O4 at the reaction anaphase (a); the C1s XPS spectra before and after the reaction of CMS-MnFe2O4 (b).
The aforementioned results indicated that CMS-MnFe2O4, with high chemical stability and catalytic ability, possessed the immense potential to become an efficient catalyst in wastewater treatment. The intact magnetic saturation ensures the catalyst efficient separation and reuse and prevents the economic loss and potential environmental risk caused by the potential loss of nanoparticles during the catalytic process.
3.5.3. Mitigation of metal leaching
Secondary pollution brought by excessive metal leaching has always been an issue for metal-based catalyst applications [58]. If the leaching metal ions enter into natural aquatic system, they can usually cause new environmental risks to human health and the aquatic ecosystem [59]. Hence, we measured the concentrations of Mn and Fe ions in the solution after each run. The leaching values of Fe and Mn for MnFe2O4 were 2.67 and 0.45 mg/L for the 1st cycle and notably increased to 3.87 and 1.19 mg/L after the 4th cycle. For CMS-MnFe2O4, the leaching values of Fe and Mn were only 0.34 and 0.28 mg/L (1st cycle) and remained at much lower levels of 0.65 and 0.16 mg/L after 4 cycles, respectively. The results revealed that CMS could effectively reduce the leaching of metal ions to 10–20 % of the initial MnFe2O4 concentration. Nawaz et al. (2015) found a similar resistance to metal leaching when they applied active carbon to support α-MnO2 in the catalytic ozonation of 4-nitrophenol. However, they did not provide a reasonable and detailed explanation of this observation. We thought that the electron donor function of CMS contributed to mitigating Mn- and Fe-ion leaching. According to the electroneutrality principle of Linus Pauling, stable molecules and crystals are electrically neutral [60]. Hence, when the oxidization of Mn3+/Fe3+ by O3 could not be reduced by acquiring enough e- from ≡S–CO2–O2 at the anaphase of the reaction, the Mn3+/Fe3+ would accumulate (Fig. 9(a)). Excess Mn3+/Fe3+ would upset the charge balance and stabilization of the MnFe2O4 crystal. Then, the Mn and Fe ions would run off from the lattice to restore the electrical neutrality of the MnFe2O4 crystal. For CMS-MnFe2O4, the electron-rich CMS could act as electron donors, which efficiently mitigated the accumulation of Mn3+/Fe3+ and decreased the leaching of Mn and Fe ions (Fig. 9(a)).
The above results showed that CMS has positive effects on improving catalyst stability and avoiding the environmental risks caused by excessive metal ions in the effluent. This method has important guiding significance for the further exploration of metal ion leaching mechanisms during catalytic ozonation and provides a potential strategy to mitigate the side effects of metal-based catalysts causing by metal ion leaching in wastewater treatment processes.
4. Conclusions
Addressing the main challenges of nanocatalysts, including easily lost, difficult to separate and reuse, and decrease of catalytic ability caused by aggregation, this work provided novel concepts and practical solutions to remedy these unavoidable challenges. By integrating magnetic MnFe2O4 and CMS prepared with microalgae, a magnetic-void-porous CMS-MnFe2O4 nanocatalyst was developed. A thorough investigation of this catalyst was conducted. The magnetic separation and recycling tests revealed that CMS-MnFe2O4 was an efficient recyclable and durable nanocatalyst. CMS not only efficiently mitigated the catalytic ability decay caused by nanocatalyst aggregation, but also significantly enhanced the chemical stability of CMS-MnFe2O4 and reduced metal ions leaching through electron transfer. Lastly, a new detailed catalytic ozonation mechanism involving OA adsorption, •O2− attack and electron transfer was proposed. Consequently, CMS-MnFe2O4 is a novel, efficient, and recyclable nanocatalyst, and also has significantly positive effects on the promotion of nanocatalyst application in wastewater treatment.
Acknowledgements
This work is financially supported by the China special S&T project on treatment and control of water pollution (2017ZX07402002). Lei Bi and Jun Chen are acknowledged for their valuable assistance with conducting experiments and writing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2021.100110.
Contributor Information
Changyong Wu, Email: changyongwu@126.com.
Yuexi Zhou, Email: zhouyuexi@263.net.
Jiane Zuo, Email: jiane.zuo@tsinghua.edu.cn.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Grant S.B., Saphores J.D., Feldman D.L., Hamilton A.J., Fletcher T.D., Cook P.L., Stewardson M., Sanders B.F., Levin L.A., Ambrose R.F., Deletic A., Brown R., Jiang S.C., Rosso D., Cooper W.J., Marusic I. Taking the "waste" out of "wastewater" for human water security and ecosystem sustainability. Science. 2012;337:681–686. doi: 10.1126/science.1216852. [DOI] [PubMed] [Google Scholar]
- 2.Kasprzyk-Hordern B., Ziółek M., Nawrocki J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal., B. 2003;46:639–669. [Google Scholar]
- 3.Xiong W., Cui W., Li R., Feng C., Liu Y., Ma N., Deng J., Xing L., Gao Y., Chen N. Mineralization of phenol by ozone combined with activated carbon: performance and mechanism under different pH levels. Environmental Science and Ecotechnology. 2020;1:100005. [Google Scholar]
- 4.Tian S.-Q., Qi J.-Y., Wang Y.-P., Liu Y.-L., Wang L., Ma J. Heterogeneous catalytic ozonation of atrazine with Mn-loaded and Fe-loaded biochar. Water Res. 2021;193:116860. doi: 10.1016/j.watres.2021.116860. [DOI] [PubMed] [Google Scholar]
- 5.Song Z., Wang M., Wang Z., Wang Y., Li R., Zhang Y., Liu C., Liu Y., Xu B., Qi F. Insights into heteroatom-doped graphene for catalytic ozonation: active centers, reactive oxygen species evolution, and catalytic mechanism. Environ. Sci. Technol. 2019;53:5337–5348. doi: 10.1021/acs.est.9b01361. [DOI] [PubMed] [Google Scholar]
- 6.Lin X., Li X., Fei J., Ma L., Huang Y. In-situ growing protective Cr-substituted goethite film on iron shavings (Fe0) as efficient catalytic ozonation catalysts: a comparative study. J. Clean. Prod. 2021;288:125653. [Google Scholar]
- 7.Wei K., Cao X., Gu W., Liang P., Huang X., Zhang X. Ni-induced C-Al2O3-framework (NiCAF) supported core–multishell catalysts for efficient catalytic ozonation: a structure-to-performance study. Environ. Sci. Technol. 2019;53:6917–6926. doi: 10.1021/acs.est.8b07132. [DOI] [PubMed] [Google Scholar]
- 8.He Y., Wang L., Chen Z., Shen B., Wei J., Zeng P., Wen X. Catalytic ozonation for metoprolol and ibuprofen removal over different MnO2 nanocrystals: efficiency, transformation and mechanism. Sci. Total Environ. 2021;785:147328. doi: 10.1016/j.scitotenv.2021.147328. [DOI] [PubMed] [Google Scholar]
- 9.Tesh S.J., Scott T.B. Nano-composites for water remediation: a review. Adv. Mater. 2014;26:6056–6068. doi: 10.1002/adma.201401376. [DOI] [PubMed] [Google Scholar]
- 10.Bharath G., Rambabu K., Hai A., Banat F., Taher H., Schmidt J.E., Show P.L. Catalytic hydrodeoxygenation of biomass-derived pyrolysis oil over alloyed bimetallic Ni3Fe nanocatalyst for high-grade biofuel production. Energy Convers. Manag. 2020;213:112859. [Google Scholar]
- 11.Wang Y., Chen L., Cao H., Chi Z., Chen C., Duan X., Xie Y., Qi F., Song W., Liu J., Wang S. Role of oxygen vacancies and Mn sites in hierarchical Mn2O3/LaMnO3-δ perovskite composites for aqueous organic pollutants decontamination. Appl. Catal., B. 2019;245:546–554. [Google Scholar]
- 12.Zhang S., Quan X., Wang D. Catalytic ozonation in arrayed zinc oxide nanotubes as highly efficient mini-column catalyst reactors (MCRs): augmentation of hydroxyl radical exposure. Environ. Sci. Technol. 2018;52:8701–8711. doi: 10.1021/acs.est.8b02103. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Xin B., Shan C., Zhang W., Dionysiou D.D., Pan B. Roles of oxygen-containing functional groups of O-doped g-C3N4 in catalytic ozonation: quantitative relationship and first-principles investigation. Appl. Catal., B. 2021;292:120155. [Google Scholar]
- 14.Zhang Y., Wu B., Xu H., Liu H., Wang M., He Y., Pan B. Nanomaterials-enabled water and wastewater treatment. NanoImpact. 2016;3–4:22–39. [Google Scholar]
- 15.Kermani M., Kakavandi B., Farzadkia M., Esrafili A., Jokandan S.F., Shahsavani A. Catalytic ozonation of high concentrations of catechol over TiO2@Fe3O4 magnetic core-shell nanocatalyst: optimization, toxicity and degradation pathway studies. J. Clean. Prod. 2018;192:597–607. [Google Scholar]
- 16.Al-Hamadani Y.A.J., Chu K.H., Son A., Heo J., Her N., Jang M., Park C.M., Yoon Y. Stabilization and dispersion of carbon nanomaterials in aqueous solutions: a review. Separ. Purif. Technol. 2015;156:861–874. [Google Scholar]
- 17.Zhu H., Ma W., Han H., Han Y., Ma W. Catalytic ozonation of quinoline using nano-MgO: efficacy, pathways, mechanisms and its application to real biologically pretreated coal gasification wastewater. Chem. Eng. J. 2017;327:91–99. [Google Scholar]
- 18.Rana A., Yadav K., Jagadevan S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: mechanism, application and toxicity. J. Clean. Prod. 2020;272:122880. [Google Scholar]
- 19.Qi F., Chu W., Xu B. Ozonation of phenacetin in associated with a magnetic catalyst CuFe2O4: the reaction and transformation. Chem. Eng. J. 2015;262:552–562. [Google Scholar]
- 20.Zhao H., Dong Y., Jiang P., Wang G., Zhang J., Li K. An insight into the kinetics and interface sensitivity for catalytic ozonation: the case of nano-sized NiFe2O4. Catal. Sci. Technol. 2014;4:494–501. [Google Scholar]
- 21.Anandan S., Lee G.-J., Yang C.-K., Ashokkumar M., Wu J.J. Sonochemical synthesis of Bi2CuO4 nanoparticles for catalytic degradation of nonylphenol ethoxylate. Chem. Eng. J. 2012;183:46–52. [Google Scholar]
- 22.Guo Y., Xu B., Qi F. A novel ceramic membrane coated with MnO2–Co3O4 nanoparticles catalytic ozonation for benzophenone-3 degradation in aqueous solution: fabrication, characterization and performance. Chem. Eng. J. 2016;287:381–389. [Google Scholar]
- 23.Hu E., Wu X., Shang S., Tao X.-m., Jiang S.-x., Gan L. Catalytic ozonation of simulated textile dyeing wastewater using mesoporous carbon aerogel supported copper oxide catalyst. J. Clean. Prod. 2016;112:4710–4718. [Google Scholar]
- 24.Ferreiro C., Villota N., Lombraña J.I., Rivero M.J. An efficient catalytic process for the treatment of genotoxic aniline wastewater using a new granular activated carbon-supported titanium dioxide composite. J. Clean. Prod. 2019;228:1282–1295. [Google Scholar]
- 25.Ghuge S.P., Saroha A.K. Catalytic ozonation for the treatment of synthetic and industrial effluents - application of mesoporous materials: a review. J. Environ. Manag. 2018;211:83–102. doi: 10.1016/j.jenvman.2018.01.052. [DOI] [PubMed] [Google Scholar]
- 26.Georgianna D.R., Mayfield S.P. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature. 2012;488:329–335. doi: 10.1038/nature11479. [DOI] [PubMed] [Google Scholar]
- 27.Taghizadeh S.-M., Berenjian A., Chew K.W., Show P.L., Mohd Zaid H.F., Ramezani H., Ghasemi Y., Raee M.J., Ebrahiminezhad A. Impact of magnetic immobilization on the cell physiology of green unicellular algae Chlorella vulgaris. Bioengineered. 2020;11:141–153. doi: 10.1080/21655979.2020.1718477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J., Kim J., Jung D., Phiri I., Bae H.-S., Hong J., Kim S., Lee Y.-G., Ryou M.-H., Lee K. Microalgae-templated spray drying for hierarchical and porous Fe3O4/C composite microspheres as Li-ion battery anode materials. Nanomaterials. 2020;10:2074. doi: 10.3390/nano10102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi L., Pan G. Facile and green fabrication of multiple magnetite nano-cores@void@porous shell microspheres for delivery vehicles. J. Mater. Chem. A. 2014;2:3715–3718. [Google Scholar]
- 30.Xia Y., Xiao Z., Dou X., Huang H., Lu X., Yan R., Gan Y., Zhu W., Tu J., Zhang W., Tao X. Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano. 2013;7:7083–7092. doi: 10.1021/nn4023894. [DOI] [PubMed] [Google Scholar]
- 31.Orge C.A., Órfão J.J.M., Pereira M.F.R. Composites of manganese oxide with carbon materials as catalysts for the ozonation of oxalic acid. J. Hazard Mater. 2012;213–214:133–139. doi: 10.1016/j.jhazmat.2012.01.066. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Liu Z., Han B., Huang Y., Yang G. Carbon microspheres with supported silver nanoparticles prepared from pollen grains. Langmuir. 2005;21:10846–10849. doi: 10.1021/la051790z. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B.B., Xu J.C., Xin P.H., Han Y.B., Hong B., Jin H.X., Jin D.F., Peng X.L., Li J., Gong J., Ge H.L., Zhu Z.W., Wang X.Q. Magnetic properties and adsorptive performance of manganese–zinc ferrites/activated carbon nanocomposites. J. Solid State Chem. 2015;221:302–305. [Google Scholar]
- 34.Sun X., Wu C., Zhou Y., Han W. Using DOM fraction method to investigate the mechanism of catalytic ozonation for real wastewater. Chem. Eng. J. 2019;369:100–108. [Google Scholar]
- 35.Ikhlaq A., Brown D.R., Kasprzyk-Hordern B. Catalytic ozonation for the removal of organic contaminants in water on alumina. Appl. Catal., B. 2015;165:408–418. [Google Scholar]
- 36.Zhang X., Li X., Qin W. Investigation of the catalytic activity for ozonation on the surface of NiO nanoparticles. Chem. Phys. Lett. 2009;479:310–315. [Google Scholar]
- 37.Fei Q., Chen Z., Xu B., Shen J., Ma J., Joll C., Heitz A. Influence of surface texture and acid-base properties on ozone decomposition catalyzed by aluminium (hydroxyl) oxides. Appl. Catal., B. 2008;84:684–690. [Google Scholar]
- 38.Zhang B., Wang L., Zhang Y., Ding Y., Bi Y. Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanodes for efficient water oxidation. Angew. Chem. Int. Ed. 2018;57:2248–2252. doi: 10.1002/anie.201712499. [DOI] [PubMed] [Google Scholar]
- 39.Lai C., Huang F., Zeng G., Huang D., Qin L., Cheng M., Zhang C., Li B., Yi H., Liu S., Li L., Chen L. Fabrication of novel magnetic MnFe2O4/bio-char composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere. 2019;224:910–921. doi: 10.1016/j.chemosphere.2019.02.193. [DOI] [PubMed] [Google Scholar]
- 40.Orge C.A., Órfão J.J.M., Pereira M.F.R., Duarte de Farias A.M., Neto R.C.R., Fraga M.A. Ozonation of model organic compounds catalysed by nanostructured cerium oxides. Appl. Catal., B. 2011;103:190–199. [Google Scholar]
- 41.Yu Y., An H., Zhao Y., Feng J., Wei T., Yu S., Ren Y., Chen Y. MnFe2O4 decorated graphene as a heterogeneous catalyst for efficient degradation of di-n-butyl phthalate using catalytic ozonation in water. Separ. Purif. Technol. 2021;259:118097. [Google Scholar]
- 42.Leem G., Zhang S., Jamison A.C., Galstyan E., Rusakova I., Lorenz B., Litvinov D., Lee T.R. Light-induced covalent immobilization of monolayers of magnetic nanoparticles on hydrogen-terminated silicon. ACS Appl. Mater. Interfaces. 2010;2:2789–2796. doi: 10.1021/am100457v. [DOI] [PubMed] [Google Scholar]
- 43.Wang D., Xu H., Ma J., Lu X., Qi J., Song S. Morphology control studies of MnTiO3 nanostructures with exposed {0001} facets as a high-performance catalyst for water purification. ACS Appl. Mater. Interfaces. 2018;10:31631–31640. doi: 10.1021/acsami.8b11132. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S., Quan X., Zheng J.-F., Wang D. Probing the interphase “HO zone” originated by carbon nanotube during catalytic ozonation. Water Res. 2017;122:86–95. doi: 10.1016/j.watres.2017.05.063. [DOI] [PubMed] [Google Scholar]
- 45.Ikhlaq A., Brown D.R., Kasprzyk-Hordern B. Mechanisms of catalytic ozonation: an investigation into superoxide ion radical and hydrogen peroxide formation during catalytic ozonation on alumina and zeolites in water. Appl. Catal., B. 2013;129:437–449. [Google Scholar]
- 46.Zhang Y., Li Q., Long Y., Zou J., Song Z., Liu C., Liu L., Qi F., Xu B., Chen Z. Catalytic ozonation benefit from the enhancement of electron transfer by the coupling of g-C3N4 and LaCoO3: discussion on catalyst fabrication and electron transfer pathway. Appl. Catal., B. 2019;254:569–579. [Google Scholar]
- 47.Pines D.S., Reckhow D.A. Effect of dissolved cobalt(II) on the ozonation of oxalic acid. Environ. Sci. Technol. 2002;36:4046–4051. doi: 10.1021/es011230w. [DOI] [PubMed] [Google Scholar]
- 48.Nawrocki J. Catalytic ozonation in water: controversies and questions. Discussion paper. Appl. Catal., B. 2013;142–143:465–471. [Google Scholar]
- 49.Orge C.A., Órfão J.J.M., Pereira M.F.R. Catalytic ozonation of organic pollutants in the presence of cerium oxide–carbon composites. Appl. Catal., B. 2011;102:539–546. [Google Scholar]
- 50.He K., Dong Y.M., Li Z., Yin L., Zhang A.M., Zheng Y.C. Catalytic ozonation of phenol in water with natural brucite and magnesia. J. Hazard Mater. 2008;159:587–592. doi: 10.1016/j.jhazmat.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 51.Dong Y., He K., Zhao B., Yin Y., Yin L., Zhang A. Catalytic ozonation of azo dye active brilliant red X-3B in water with natural mineral brucite. Catal. Commun. 2007;8:1599–1603. [Google Scholar]
- 52.Wang Y., Xie Y., Sun H., Xiao J., Cao H., Wang S. 2D/2D nano-hybrids of γ-MnO2 on reduced graphene oxide for catalytic ozonation and coupling peroxymonosulfate activation. J. Hazard Mater. 2016;301:56–64. doi: 10.1016/j.jhazmat.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 53.Samoilova R.I., Crofts A.R., Dikanov S.A. Reaction of superoxide radical with quinone molecules. J. Phys. Chem. A. 2011;115:11589–11593. doi: 10.1021/jp204891n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qi F., Xu B., Chu W. Heterogeneous catalytic ozonation of phenacetin in water using magnetic spinel ferrite as catalyst: comparison of surface property and efficiency. J. Mol. Catal. Chem. 2015;396:164–173. [Google Scholar]
- 55.Villamena F.A., Xia S., Merle J.K., Lauricella R., Tuccio B., Hadad C.M., Zweier J.L. Reactivity of superoxide radical anion with cyclic Nitrones: role of intramolecular H-bond and electrostatic effects. J. Am. Chem. Soc. 2007;129:8177–8191. doi: 10.1021/ja0702622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sui M., Sheng L., Lu K., Tian F. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity. Appl. Catal., B. 2010;96:94–100. [Google Scholar]
- 57.Dale A.L., Casman E.A., Lowry G.V., Lead J.R., Viparelli E., Baalousha M. Modeling nanomaterial environmental fate in aquatic systems. Environ. Sci. Technol. 2015;49:2587–2593. doi: 10.1021/es505076w. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y., Xie Y., Sun H., Xiao J., Cao H., Wang S. Efficient catalytic ozonation over reduced graphene oxide for p-hydroxylbenzoic acid (PHBA) destruction: active site and mechanism. ACS Appl. Mater. Interfaces. 2016;8:9710–9720. doi: 10.1021/acsami.6b01175. [DOI] [PubMed] [Google Scholar]
- 59.Chai W.S., Cheun J.Y., Kumar P.S., Mubashir M., Majeed Z., Banat F., Ho S.-H., Show P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021;296:126589. [Google Scholar]
- 60.Pauling L. The modern theory of valency. J. Chem. Soc. 1948:1461–1467. doi: 10.1039/jr9480001461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.