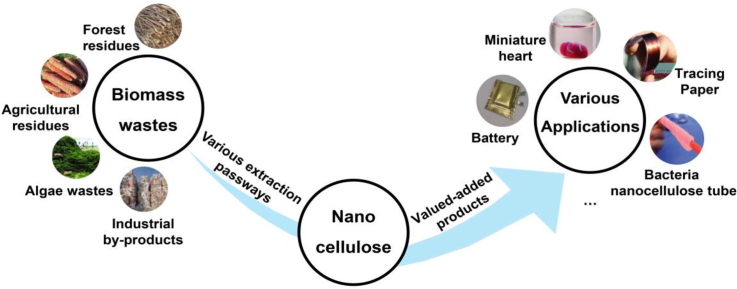
Abstract
Biomass waste comes from a wide range of sources, such as forest, agricultural, algae wastes, as well as other relevant industrial by-products. It is an important alternative energy source as well as a unique source for various bioproducts applied in many fields. For the past two decades, how to reuse, recycle and best recover various biomass wastes for high value-added bioproducts has received significant attention, which has not only come from various academia communities but also from many civil and medical industries. To summarize one of the cutting-edge technologies applied with nanocellulose biomaterials, this review focused on various preparation methods and strategies to make nanocellulose from diverse biomass wastes and their potential applications in biomedical areas and other promising new fields.
Keywords: Biomass waste, Nanocellulose, Biomedical application, Environmentally-functional materials
Graphical abstract
Highlights
-
•
Biomass wastes represent cost-effective renewable energy and biopolymers sources.
-
•
Strategies for nanocellulose extraction from biomass waste were extensively reviewed.
-
•
Nanocellulose-based value-added products can be recovered from various biomass wastes.
-
•
Nanocellulose extraction from biomass wastes may solve the intractable eco-problems.
Abbreviation list
- BC
Bacterial Cellulose
- BNC
Bacterial nanocellulose
- BNCs
Bionanocomposites
- CCNF
Coconut Coir Nanocellulose
- CFs
Cellulose Fibers
- CPF
Citrus Pulp of Floater
- CNCs
Cellulose Nanocrystals
- CNFs/NFCs
Cellulose Nanofibers
- CNWs
Nanocellulose Whiskers
- ECM
Extracellular Matrices
- MFC
Microfibrillated Cellulose
- NCCs
Nanocrystalline Celluloses
- PEDOT
Poly(3,4-Ethylenedioxythiophene)
- PSS
Poly(4-Styrene Sulfonate)
- PVA
Poly Vinyl Alcohol
- TEMPO
2,2,6,6-Tetramethylpiperidine-1-Oxyl
1. Introduction
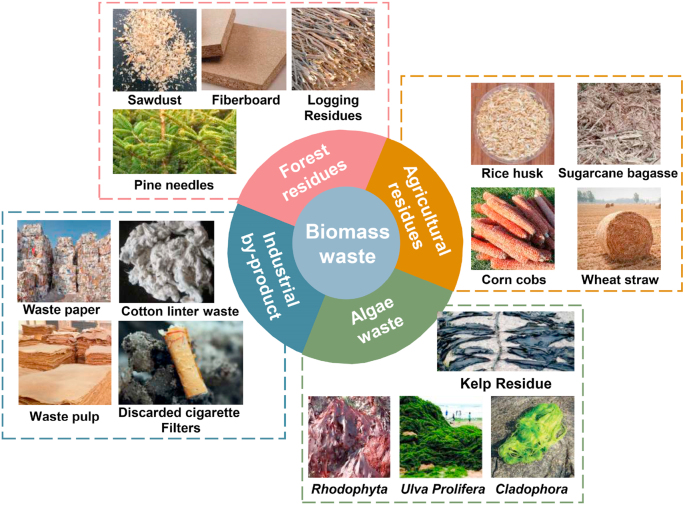
Biomass is commonly referred to various organisms that comes from plants, animals, and microorganisms [1]. In other words, biomass may refer to all non-fossil organic matter that can participate in carbon cycling and regeneration. In addition, biomass waste can also be produced in a process of using biomass that has been abandoned or given up by humans, where it is mostly generated as a by-product of other activities (e.g., shavings and crop residues). The sources of biomass waste includes (Fig. 1), but are not limited to, agriculture, forestry, and the biodegradable fraction produced from various industrial wastes and municipal biosolids [2].
Fig. 1.
The various sources of biomass waste, adapted with a permission from Refs. [[14], [15], [16], [17], [18], [19]].
In line with an emerging concern on both the depletion of non-renewable energy and the rise in the global temperature, biomass waste as a renewable source in large quantities on earth, has no doubt received a great deal of attention over the past decade as an alternative energy source [[3], [4], [5], [6], [7]]. However, currently most of the utilization of biomass waste, after a recycling or recovery processing, is largely limited to a few low value-added products. For example, bagasse, one of the common agricultural residues, is commonly to be used in produce steam and electric power via direct combustion by the sugar industry [8]. In addition, home cooking or heating via the direct burning of wood or agricultural residues is a common recycling application in most developing countries for waste biomass recycling. Although these applications can utilize a portion of the biomass waste for recycling, their utilization values are typically recognized in low, and, in many circumstances, it may also produce secondary pollution for our environment during recycling processing (e.g., air pollution from field biomass combustion) [9].
As a major contributor to renewable energy in the world (about 50% of total energy in 2018), biomass is a versatile energy producing material [10]. It can store and be transformed into almost any other form of energy and can also be used as a biomaterial [11]. Therefore, how to effectively and sustainably use biomass waste has become an important and popular research topic. A variety of different biomass have been utilized as raw materials for biodiesel and bioethanol production [12]. Pileidis et al. has directly extracted levulinic acid from lignocellulose, which can be used as a biofuel additive and a substitute for petroleum derivatives [13]. Through the development of such high value-added products, it will not only reduce the loss of non-renewable energy and protect the environment, but also promote an economic development for a region where the biomass is generated.
Cellulose is the major component of biomass wastes and the efficient utilization of this component in a high value-added way may open a new window in a bright future for biomass waste utilization. Nanocellulose is referred to cellulosic material with one dimension in the nanometer range, and its main sources includes plants, animals and bacteria. Increasing attention has been paid to the extraction of nanocellulose from different biomass sources for applications in a variety of fields (Fig. 3). Nanocellulose mainly includes three subcategories, (1) cellulose nanocrystals (CNCs), with the synonyms of nanocrystalline celluloses (NCCs), nanocrystalline cellulose, nanocellulose whiskers (CNWs), rodlike cellulose microcrystals, which are mainly prepared by acid hydrolysis of lignocellulosic materials to remove amorphous cellulose and leaving the rod-like cellulose nanocrystals. (2) cellulose nanofibers (CNFs/NFCs), with other names such as microfibrillated cellulose (MFC), nanofibrils and microfibrils, which are prepared by mechanical processing (or combined with chemical or enzymatic pretreatments) for defibrillation of cellulosic fibers. (3) bacterial cellulose (BC), also defined as bacterial nanocellulose (BNC), microbial cellulose, biocellulose, which are mainly synthesized by several species of the Acetobacteraceae [20]. As an abundant and biobased material, nanocellulose can be prepared from various plant biomass, bacteria, algae, as well as some marine invertebrate animals (tunicates) using different methods [21]. Nanocellulose share the inherent chemical structure of cellulose with abundant hydroxy groups and a certain amount of aldehyde groups and carboxy groups for further functionalization. Owing to the nanostructure of the material, nanocellulose shows significant high specific surface area, active functionalization groups, mechanical strength, and crystallinity, which provides a broad spectrum of applications in different areas [[22], [23], [24], [25]].
Fig. 3.
Schematic diagram of biomass wastes-based processing for nanocellulose preparation [52,53]. TEM images of nanocellulose extracted with chemical (a), biological (b), mechanical (c) and combined approach (d) were reproduced with permission from early reports [[54], [55], [56]].
According to the “Global Nanocellulose Fiber Market Report " released by Research Insights in 2020, the global market value of nanocellulose is expected to reach as high as $2.712 billion by 2025, and the annual growth rate during the forecasted period from 2018 to 2025 is predicted to be 18.80% [26]. However, in order to achieve this significance and economic growth of nanocellulose-based materials, challenges lie in the production (e.g., techniques evolution, environment concern), marketing (e.g., public acceptance, marker penetration, cost control), and safety issues (e.g., in food and biomedical applications) still need to be addressed [27]. Currently, only a few companies have successfully commercialized nanocellulose and its high value-added products. The Swedish company CELLINK life sciences has successfully mixed nanocellulose with alginate to formulate a series of bioprinting bioinks at a price of €251.20 for 9 mL, which shows a significantly higher added value when compare with to raw lignocellulose materials (Fig. 2). Similarly, GrowInk® and GrowDex®, launched by the Finish company UPM, mainly consists of nanocellulose and have shown high added value as well. For example, a 10 mL GrowInk® and GrowDex® have a sale price of €450.0 and €290.0 (Fig. 2), respectively.
Fig. 2.
The market value of products from biomass waste, adapted from the CELLINK® and UPM®.
Currently, most of the nanocellulose used commercially is prepared from high-quality fiber materials such as wood pulp, dissolving pulp, and cotton. However, more and more reports and efforts have confirmed that it is also feasible to use biomass waste as a feedstock to prepare nanocellulose suitable for commercial purposes. These findings have paved a new future for biomass waste utilization at a high-value added product. Currently, various types of biomass wastes have been used to prepare fertilizer, decorations, and aquaculture feed. In general, the market competitiveness of these products is not strong. Clearly, the aim of the market is not accurately identified for a product potential from various biomass wastes. This trend is largely because it is still in a low-value added product development stage. In many cases, inappropriate processing from biomass wastes may also cause a potentially secondary pollution problem. However, as a representative of high value-added products, nanocellulose has presented its promising functions with some unique and competitive properties, which demonstrates a broad spectrum of potential application areas, such as composite materials, fine chemicals and biomedical fields. It is expected that the current markets may ask that a variety of suitable materials be developed from biomass, while high quality biomedical materials are expected to be far from being able to meet the current standards. This further supports the need for the high value-added products to be developed from various biomass wastes. Generally, nanocellulose preparation methods can be divided into four categories: physical methods, chemical methods, biological methods, and combined methods. However, due to the raw material source variations, the same preparation method may also yield nanocellulose with different structural and chemical properties. Therefore, the current paper summarized the extraction methods, properties, and respective application of the nanocellulose based on the sources of the biomass waste.
Therefore, the current overview will primarily focus on discussing the sources of biomass waste materials, nanocellulose extraction methods and strategies from these waste sources, as well as their industrial potentials for the development of high value-added products for use in different fields from nanocellulose.
2. Sources of biomass wastes and their nanocellulose preparation
2.1. Forest residues
It is well known that humans produce a lot of residue in the process of using wood, such as logging residues, sawdust, plywood waste, and shavings (Fig. 1). In 2018, there were about 1.1977 billion tons of forest residues produced worldwide. The recovery rate of logging residues and sawdust waste depends on the type of wood and its origins. In densely populated locations, the deforestation rates can reach up to 66%, of which 34% are residues which included branches and leaves. In places where people are sparsely populated, logging residues are likely to be abandoned and left to rot in the forest [28]. Sawdust, plywood waste, and shavings are all a result of the processing of wood. During the processing of logs, due to the difference in technology and tree species, as well as shavings and modifications, the loss of logs may account for about 20%–66% of the total wood processed [28]. In addition, the trees themselves produce a certain amount of waste during the growth process due to defoliation, pruning or replanting (e.g., branches, leaves, and shrubs). Taking a Cunninghamia lanceolata forest as an example, the amount of waste produced by pruning and replanting is approximately 15.1 t/hm2 per year [29]. Simply combustion of this part of waste only provides an economic benefit at €9.53/hm2.
The examples mentioned above show that forest residues are an important source of biomass wastes. In general, wood residues are often used to produce a low value-added product, such as particles and agglomerates (bioenergy), firewood, charcoal (adsorbents), fillers, and wood boards (furniture and building materials) [30]. But currently, the government’s policy from China regarding biomass reuse is constantly strengthening on a high value-added product, which has inevitably become an important and popular area of research both in academia and in some relevant industries.
Due to the unique biocompatibility and mechanical properties, nanocellulose as a promising candidate for developing high value-added products, has certainly attracted a lot of attention across the world. In general, the extraction methods of nanocellulose from waste wood material can be classified into four different categories (Fig. 3): physical, chemical, biological, and a combined method from any of two methods together for a complementary advantage, where a very few efforts are made on biological methods to extract nanocellulose from forest waste due to an economic or efficient concern in processing.
Chemical methods are the most widely used extraction methods to obtain nanocellulose from forest residues (Fig. 3). Moriana et al. obtained the CNCs from logging residues by alkaline treatment (4.5 wt% NaOH, 80 °C 2h), bleaching treatment, and acid hydrolysis (65 wt% sulfuric acid, 45 °C 40 min). The CNCs present at a high aspect ratio (>10) and crystallinity, hold the greatest potential for application in the reinforcement of composite materials [31]. However, this method has disadvantages of long reaction time, low yield and high energy and corrosive chemicals consumption.
Physical methods are also commonly used for obtaining nanocellulose from forest residues with significantly higher yield of nanocellulose (Fig. 3). Phanthong et al. used a planetary ball mill to obtain nanocellulose with a yield of 93.1%. The obtained nanocellulose had a 10–25 nm diameter and a higher thermal stability [32]. Veigel et al. pulped the beech waste and then fibrillated the pulp. Finally, the nanocellulose was obtained by a high-pressure homogenizer treatment. The resulted nanocellulose presented a high yield, which can be potentially applied as a binder for particle board and oriented particle board [33]. However, these physical methods would generally consume more energy in processing.
As a novel strategy proposed to deal with the advantages or disadvantages of each approach mentioned above, a combination of physical and chemical methods has been proposed and currently, it has become the most widely utilized strategy in the laboratory for the preparation of nanocellulose from forest residues.
One of the most promising directions reported in the recent literature is to pretreat the wood residue first to remove lignin and hemicellulose, and then to the isolated the cellulose component which will be oxidized by the TEMPO-mediated oxidation system under alkaline conditions. In the next step, a gentle homogenization process is designed as a physical treatment to obtain nanocellulose. Vallejos et al. successfully obtained nanocellulose from eucalyptus sawdust by this method. The specific surface area and average diameter of the CNFs was 60 m2 g−1 and 41.0 nm, respectively (Table 1) [30]. Carvalho et al. also used birch and spruce sawdust to prepare CNFs (Table 1) [34]. The lignin was removed first using sodium hydroxide, followed by Soxhlet extraction, acetic acid, sodium acetate and sodium chlorite treatment. The resulted cellulose suspension was defibrillated by a micro fluidizer in a five-part sequence. In this study, the CNFs obtained from the spruce samples showed a higher thermal stability relative to birch samples. The resulted nanopaper also had a better tensile strength (80–200 MPa) and Young’s modulus (4.8–8.5 GPa) [8].
Table 1.
Sources, extracted methods, properties and applications of nanocellulose.
| Sources | Extraction methods | Properties | Applications | References | ||
|---|---|---|---|---|---|---|
| Forest residues | Eucalyptus sawdust | TEMPO oxidation | Surface area: 60 m2 g−1; Average diameter: 41.0 nm. |
Papermaking | [30] | |
| Birch and Spruce sawdust | Sodium hydroxide and Soxhlet extraction followed by acetic acid, sodium acetate and sodium chlorite treatment. | Tensile strength:80–200 MPa; Young’s modulus: 4.8–8.5 GPa. |
Papermaking | [8] | ||
| Medium density fiberboard | Soxhlet extraction, sodium hydroxide and repeated bleaching. | Length: 164.7 nm; Width: 6.7 nm; Crystallinity: 71%. |
Nanocomposites or papermaking | [38] | ||
| Beech wastes pulp | Fibrilization | Diameters: 20–65 nm. Average: 35 nm |
Adhesive | [33] | ||
| Cordia goeldiana veneer wastes | Alkali treatment, bleaching, homogenization and casting. | Maximum processing temperatures: 300 °C. | E-papers, organic electronic devices and transparent solar cells | [35] | ||
| Pinecone biomass | Acidification, alkali treatment, mechanical grinding. | Tensile strength: 273 MPa; Elastic modulus: 17 GPa; Crystallinity: 70%; Diameter: 5–20 nm. |
Bionanocomposites | [36] | ||
| Logging residues | Alkaline treatment, bleaching treatment and acid hydrolysis. | High aspect ratio:>10; Good thermal stability. |
Reinforcing agents | [31] | ||
| Pine needles | Chemical pretreatments followed by ultrasonic treatments | Narrow diameter: 30–70 nm; Cellulose I type; Crystallinity: 66.19%; Highly flexible, highly ultralight and good thermal properties. |
Thermoplastic composites | [37] | ||
| Bamboo log chips | Glycerol pretreatment, extrusion treatment and mechanical refining using 0.15% sulfuric acid (conc.) as a catalyst. | Diameter: 20–80 nm; Crystallinity: 52.7%. | / | [39] | ||
| Agricultural residues | Raw rice husk | Chemical pretreatment, homogenization and high-intensity ultrasonication processes (500 W,40min). | Diameters: 6–20 nm; High aspect ratio (177); Crystallinity: 65%; Good thermal stability. |
Green nanocomposites, filtration media, tissue, engineering. | [15] | |
| Waste sugarcane bagasse | Alkali hydrolysis; bleaching treatment; acid hydrolysis and ultrasonic treatment. | Cellulose II; Diameters:18.17–32.84 nm; Crystallinity: 93%. |
Food packaging | [77] | ||
| Corn cobs | One-step mechanochemical esterification | Diameter: 1.5–2.8 nm; Transparency:89% (550 nm); Young’s modulus: 5.5 GPa; Tensile strength: 110–125 MPa. |
Advanced materials in electronics and other application | [49] | ||
| Jute dried stalks | Alkali treatment followed by steam explosion; sodium chlorite bleaching and oxalic acid treatment followed by steam explosion. | Average diameter: 50 nm; Higher crystallinity; Young’s modulus: 138 GPa; Good reinforcing properties. |
Reinforcing agent | [78] | ||
| Wheat straw | Bleaching treatment, pressure sieve and high-pressure homogenization; | Good adsorption capacity | Nanosorbent | [47] | ||
| Coconut husk | Ultrasonic-assisted solvent immersion, alkaline treatment, bleaching treatment, milder TEMPO -mediated oxidation (TEMPO/NaClO/NaClO2, pH = 4.8) | Average diameter: 5.6 ± 1.5 nm; Length: 150–350 nm; Good mechanical properties and thermal stability. |
PVA composite strength enhancer | [51] | ||
| Kenaf bast fiber | Alkali pulping process; bleaching process and mechanical treatment. | Diameter: 1.2–34 nm; Crystallinity: 82.52%; Yield: 60.25%. |
Reinforcement material | [79] | ||
| Citrus waste | Bleaching treatment, acid hydrolysis and ultrasonic assisted treatment. | Higher crystallinity; Average diameter: 9.7 nm. |
Manufacturing necessities industry | [80] | ||
| Branch-barks of mulberry | Alkali treatment, bleaching treatment, acid hydrolysis and ultrasonic treatment. | Diameter: 25–30 nm; Length: 400–500 nm; Crystallinity: 73.4%. |
/ | [81] | ||
| Pea hull | Acid hydrolysis | Length: 400-240 nm; Diameter: 7–12 nm; Good UV absorption, transparency, tensile strength, elongation at break and water resistance. |
Nanocomposites | [45] | ||
| Pineapple leaves | Steam explosion and acid hydrolysis | Diameter: 5–40 nm; Large surface area and specific properties of nanotechnology. |
Polymer reinforcing agent | [48] | ||
| Raw apple stem | Acid hydrolysis | Yield: 5.2%; Higher crystallinity; Good thermal stability. |
/ | [46] | ||
| Algae waste | Cladophorales | Acid hydrolysis | Width: 20–30 nm; Crystallinity: 100%; High specific surface area and rheological properties. |
Biological field | [18,61] | |
| Red algae | Acid hydrolysis | Length: 432 nm; Good thermal degradation resistance. |
/ | [64] | ||
| Industrial kelp (Laminaria japonica) waste | Acid hydrolysis | Yield: 52.3%; Crystalline form: cellulose I; Crystallinity: 69.4%; Shape: rod-like; Poor thermostability. |
/ | [60] | ||
| Ulva lactuca | Methanol decolorization, bleaching and acid hydrolysis. | Good absorption | Adsorbents | [62] | ||
| Dealginate kelp residue | 2 wt% Na2CO3 swelling treatment, 2 wt% NaOH extraction of residual sodium alginate, ultrasonic smashing, 0.7 wt% NaClO2 buffer solution bleaching treatment, delignification and sulfuric acid hydrolysis. | High aspect ratio: 30–70; Crystallinity: 74.55%. |
/ | [65] | ||
| Cystoseria myricaas algae | Soxhlet pretreatment, 3% NaOH treatment, NaClO2 bleaching treatment and acid hydrolysis. | Average crystallite’s grain size (Fe3O4-Nanocellulose): 21 nm. | Heavy metal mercury adsorbent | [82] | ||
| Gelidium elegansred | Alkali treatment, bleaching treatment and acid hydrolysis. | Average diameter: 21.8 ± 11.1 nm; Length: 547.3 ± 23.7 nm; Good thermal stability. |
Nanocomposites | [83] | ||
| Gelidium sesquipedale | Soxhlet extraction, bleaching treatment, 5% KOH solution treatment and acid hydrolysis. | Crystallinity: 69.8%; Diameter: 6–40 nm; Length: 80–450 nm; High aspect ratio: 40. |
Food packaging industry | [84] | ||
| Chaetomorpha antennina | Bleaching treatment, acid hydrolysis, and ultrasonic assisted treatment. | Yield: 34 ± 0.9%; Crystallinity:85.02%; Good thermal stability and tensile strength. |
Environmentally friendly products | [63] | ||
| Industrial by-product | Sweet lime pulp waste | Komagataeibacter europaeus SGP37 incubation under static intermittent fed-batch cultivation | Yield: 27.0–38 g/L. | Bacterial nanocellulose | [74] | |
| Waste paper | Aqueous NaOH/thiourea | Crystallinity: 48.85%; Average size: 50 nm; Good thermal stability. |
Transistors and batteries | [71] | ||
| Cotton linter waste | Acid hydrolysis | Length: 177 nm; Width: 12 nm; Crystallinity: 90.45%; High hydrophilicity. |
Hydrophilic nanocomposites | [85] | ||
| Discarded cigarette filters | Ethanol extraction, bleaching, alkali treatment and acid hydrolysis | Length: 143 nm; Width: 8 nm; Crystallinity: 96.77%. |
Biomedical composites | [75] | ||
| Beer industrial residuals | Acid hydrolysis and ultrasound assisted techniques. | Average diameter: 73–145 nm; Crystallinity:79%–89%; Yield: 25.8%; Good thermal stability. |
Packaging, coatings, pharmaceuticals, cosmetics and defense | [69] | ||
| Cassava peel | Alkali treatment followed by bleaching process. | Yield: 17.8%; Crystallinity: 51.2%; Length: 100–300 nm; Width: 3–8 nm. |
/ | [86] | ||
| Pulp and paper mills | Ammonium persulphate | Length:150–500 nm; Width:10–20 nm. |
Antimicrobial, photocatalytic, textile finishing and water treatment. | [72] | ||
| Wood furniture industry waste (Pinus elliotii) | Steam explosion and acid hydrolysis process | Diameter: 18.0–40.5 nm. | Oil absorption | [70] | ||
| Olive industry solid waste | Pulping, bleaching and sulfuric acid hydrolysis. | Free-flowing porous | Wastewater treatment | [73] | ||
| Lime residues | Autoclaving, high-shear and high-pressure homogenization | Diameter: 5–28 nm; Crystallinity: 44–46%; High aspect ratio; Good water redispersibility. |
Packaging film | [76] | ||
| Recycled Tetra Pak Food Packaging Wastes | Alkaline purification, bleaching treatment and acid hydrolysis. | Length: 258 ± 54 nm; Aspect ratios: >10. |
Bionanocomposites. | [14] | ||
| Citrus Pulp of Floater (CPF) | Enzymatic hydrolysis | Crystallinity: 60%; Purity: 98%. |
/ | [87] | ||
This combined strategy between chemical and physical methods can be used not only to produce cellulose particles, but also to prepare nanocellulose films for various purposes. Bufalino et al. found that nanocellulose films from Amazon forest wood wastes had a competitive structural and thermal properties. The nanocellulose film can be fabricated by a chemical modification via bleaching, followed by a casting method. After an alkali treatment and bleaching processing, the crystallization index of the nanocellulose film would increase with a better thermal stability [35]. Rambabu et al. hydrolyzed pinecone powder with 0.05 M HCl for 2 h at 80 °C. The pH was then adjusted to about 9 at room temperature with a dilute ammonium hydroxide solution. The generated sample was then subjected to an alkali treatment and stirred overnight. The pretreated cellulose pulp was continuously subjected to fiber removal and separation of the nanofibers as a colloid. Finally, the nanocellulose was obtained by a mechanical grinding process. As a high value-added novel material, it can be potentially used to make bio-nanocomposites in different industries [36].
In addition to the physical homogenization and grinding methods being combined with a chemical method, ultrasound and screw extruding methods are also recommended to be combined with chemical treatment. Xiao et al. prepared pine needle nanofibers through an acidified sodium chlorite treatment (75 °C 1h), followed by an alkaline treatment (KOH), acid hydrolysis (HCl, 80 °C 2h), and eventually combined with an ultrasonic treatment (60 KHz, 30min). The generated nanofibers presented a width of 30–70 nm with a crystallinity at 66.19%, as well as a good thermoplastic property, which no doubt demonstrated a promising application in the field of thermoplastic composites [37]. Couret et al. extracted cellulose from medium density fiberboard by Soxhlet extraction (toluene: ethanol = 2:1 mixture), sodium hydroxide treatment, and ultrasonic treatment for CNCs preparation (Table 1). The obtained CNCs has some similar morphological and physical properties to the CNCs obtained from wood, where its length, width and crystallinity evaluated on the generated CNCs can reach 164.7 nm, 6.7 nm, and 71%, respectively [38]. Lu et al. demonstrated the possibility of nanocellulose preparation from bamboo tablets using glycerin pretreatment at a mass/liquid ratio of 1:8 (120 °C, 2 h), followed by screw extruding and mechanically refining with an acid (0.15% H2SO4, room temperature, 5 min) as a catalyst. The resulted nanofiber showed a diameter of 20–80 nm and the length of a few microns, as well as a crystallinity at 52.7% [39].
Generally, the combined methods are relatively effective than pure chemical or physical approaches. But chemical reagents such as acids or alkalis are usually used in the preparation process, which is easy to produce a waste liquid. Therefore, it is necessary to explore a new and more environmentally friendly and effective methods for preparing nanocellulose from wood wastes. Biological methods may be effective approaches, but there are very few reports on the extraction of nanocellulose from forest residues, which suggests there is a need to shift more efforts towards improving our current methods.
2.2. Agricultural residues
Chemical composition of agricultural residues primarily consists of cellulose, lignin, hemicellulose, pectin and other minor compounds, which are very similar to those forest residues in their components mentioned above, but they may present some different physical and chemical properties due to a different living environments and growth cycles and characteristics [40]. In general, the cellulose content of agricultural residues is slightly lower than that of forest residues, but their lignin composition is significantly higher than that of most forest wastes [41,42]. To be precise, agricultural cellulosic residues refers to all plant parts except harvested crops or fruits, such as rice straws, wheat straws, sugarcane bagasse, corn stover, rice husk, and corn cobs (Fig. 1) [43]. There are approximately 998 million tons of various types of agricultural residues yearly produced across the world, which includes, but not limited to, main crop residuals, such as rice/wheat straw, corn stover, as well as a variety of other economic crop residuals [44]. Currently, the use of residual wastes from agricultural processing remains at its early stage, which are mainly characterized by low value-added utilizations, such as a direct residual combustion in producing heat or electronic production, or usage as a fertilizer/compost conversion applied in agriculture. However, from the perspectives of economic values or environmental benefits, these utilizations have not yet accessed its true potentials or values from a material conversion point of view. As a matter of fact, lignocellulosic agricultural residues can be used to generate different types of nanocellulose (CNCs, CNFs) that can be widely applied in various material development in the fields of cosmetics, pharmaceuticals, biomedical, or many other industries. Clearly, agricultural waste can also be considered as a suitable source of sustainable and renewable cellulose [2]. To date, the above mentioned four preparation methods can also be utilized to extract nanocellulose from agricultural waste (Fig. 3).
The first chemical preparation method of nanocellulose evaluated has many processing steps, but in general, the properties of the obtained nanocellulose presents excellent physicochemical performance (Fig. 3). Chen et al. prepared nanowhiskers by acid hydrolysis from Pea hull fibers, where its length and diameter were measured as 400-240 nm and 12-7 nm, respectively (Table 1). As a new exploration for composite material, the nanowhiskers from pea hull fibers and pea starch can be incorporated into a novel nanocomposite material that would present some unique or improved properties, such as a good UV absorption, an enhanced transparency rate, an improved tensile strength and elongation capacity at break, and decent water resistance [45]. Phantong et al. tried to first remove the lignin from a raw apple stem via a specific buffer solution (acetic acid/sodium chlorite/distilled water) (Table 1). The hemicellulose was then targeted for removal by an alkali treatment to isolate the cellulose. Lastly, the nanocellulose was successfully extracted by an acid processing step at a yield of about 5.2%, where the nanocellulose was characterized with a diameter of 10–20 nm, a high degree of crystallinity, and also exhibited a good thermal stability under a high temperature stress [46].
The second pathway is a physical method, which is usually characterized with a physical processing tool/equipment, such as high-pressure homogenization, mechanical grinding, and ultrasonic treatment (Fig. 3). Suopajrvi et al. used a bleached wheat straw pulp as a raw material for nanofibrillated cellulose gel preparation through a high-pressure homogenization (Table 1). Sulfonated nanosorbent were synthesized by a consecutive periodate oxidation and sodium metabisulfite sulfonation using the prepared nanofibrillar cellulose gel. This sulfonated nanosorbent showed a competitive adsorption capacity for lead (1.2 mmol/g at pH 5) [47]. Abraham et al. extracted cellulose from pineapple leaves and jute fibers for nanocellulose preparation by steam explosion (Table 1). The obtained nanocellulose had a diameter of 5–40 nm with a large surface area, which can be used as a polymer reinforcing agent [48]. In general, the physical method was characterized as a simple treatment to operate, but required a special tool/equipment to operate at a high-energy input, where the converted nanocellulose in diameter is relatively wide. Therefore, a physical tool is often used or recommended to combine with other approaches to extract the high quality of nanocellulose.
The third pathway focused on a biological method as a biological processing option for nanocellulose extraction by a microorganism involvement or a specific enzyme processing as a catalyst on the cellulose substrates, such as a bacterial degradation processing (Acetobacter exylinum, Rhizobium leguminosarum), or a specific cellulolytic enzyme decomposition (cellulase) (Fig. 3) [40]. The advantage of a biological degradation reaction on cellulose is that the structural characteristics of nanocellulose can accurately controlled and easy to obtain a stable and high quality nanocellulose. The downside of this method is the relatively long reaction time and high cost with a low yield [41,42]. Currently, there are only few published reports on the use of biological methods to extract nanocellulose from agricultural residues due to its efficacy and cost limitations.
To enhance the conversion efficacy and its quality in the production of nanocellulose products from agricultural residues, a combination pathway that may incorporate one approach to another for complementary advantages was recently proposed (Fig. 3). This combined pathway has become one of the most commonly recommended preparation methods. But it may be inevitably led to more processing steps.
Kang et al. has successfully extracted nanocellulose by a mechanochemical esterification from corn cob cellulose, where the obtained nanocellulose had a particle diameter in the range of 1.5–2.8 nm. The nanopaper made from this nanocellulose product has a greater optical property with a transparency rate of up to 89% at 550 nm, where the Young’s modulus and tensile strength of the nanopaper were recorded as high as 5.5 GPa and 110–125 MPa, respectively. These improved properties of nanopaper can be potentially applied to enhance a designed material in its electronics and other special parameters [49].
It is worthwhile to mention that Slavutsky et al. obtained cellulose fibers (CF) from sugarcane bagasse waste by combining both alkali hydrolysis and bleaching processing together. Following these steps, the CNCs product can be then obtained by a consecutive two step processing approach that combines acid-hydrolysis and ultrasonic treatment [50]. As a result, with this novel combination approach, the CNCs presented a similar chemical structure to a true synthetic polymer matrix due to its strong hydrogen bonds in binding. Thus, the CNCs material can be potentially applied as a reinforcing additive to improve nanocomposites that will indicate a great application potential in the field of food packaging.
Another combination approach was successfully designed recently where a mild TEMPO-mediated oxidation processing was proposed in combination with an ultrasonic treatment to extract a nanocellulose product from waste coconut coir fibers [51]. The crystallinity of the coconut coir nanocellulose (CCNF) was recorded as high as 56.3%, where the diameters and lengths of nanofibrils were in the range of 2–10 nm and 150–350 nm, respectively. The composites of the CCNF/poly vinyl alcohol (PVA) demonstrated some competitive mechanical properties and thermal stability, suggesting a great application potential in the PVA film strength enhancing.
Clearly, the combination approach for extracting nanocellulose from agricultural wastes are indeed more effective and competitive due to its conversion efficacy, product quality, as well as its versatility for a variety of composite materials applied in different areas.
2.3. Algae wastes
Algae, including pluricellular macroalgae and unicellular microalgae, represents another group of abundant aquatic biomass which have been used for centuries as fertilizer, food and feed (Fig. 1). However, increasing attention has been given to the value-added products development from those non-edible algae species, invasive species, as well as traditional algae processing residue. Due to the global warming and water eutrophication potential, the outbreaks from some invasive algae species will not only lead to a significant negative impact on the landscape but also poses a serious hazard to the aquatic ecosystem. Taking China as an example, the report from the 2019 Bulletin of China Marine Disaster reveal that the area of algae outbreaks in coastal areas is as high as tens of thousands of square kilometers, plaguing people in coastal areas for many years [57].
However, in another aspect, algae waste is actually presented as a very valuable biomass or a unique biological resource due to its rich proteins and polysaccharides contents. Currently, how to convert these algae waste biomass as a high value-added product has become one of the hot spots both in academia and industry.
In recent years, the use of algae or algal waste as feedstocks to extract nanocellulose has become more of a focus to use for the development of high value-added products. Some non-edible green algae species or invasive green algae species (e.g., Cladophorales, Ulva, Chlorella, Spirogyra and Chaetomorph) that caused algae bloom have been identified as an excellent source for nanocellulose preparation [[58], [59], [60]]. Similar to the method of extracting nanocellulose from forest residue and agricultural waste, algae nanocellulose preparation mainly adopts chemical methods and combination methods (pairwise combination between physical, chemical or biological methods). Generally, nanocellulose prepared from algae shows a higher cellulose crystallinity than prepared from forest wastes and agricultural residues. The Cladophorales nanocellulose with a width of 20–30 nm has been extracted by acid hydrolysis, giving a cellulose crystallinity of up to 100% and high specific surface area (Table 1) [18,61]. Ulva lactuca nanocellulose crystalline has been extracted by methanol decolorization, bleaching and acid hydrolysis (Table 1). The resultant nanocellulose with a high specific surface area showed a high adsorption capacity of antibiotics, which may have an application in drug delivery [62]. Similarly, Bhutiya et al. extracted nanocellulose from Chaetomorpha antennina by a bleaching treatment, acid hydrolysis, and ultrasonic assisted treatment with a yield of 34% and cellulose crystallinity at 85% (Table 1). After a combination with Cu2O, the resultant composites showed a good thermal stability, tensile strength, and a certain antibacterial ability, suggesting its great application potential in environmentally friendly products [63].
Red and brown algae waste or residue, such as Gelidium elegans, Gelidium sesquipedale, Laminaria japonica, have also be used to prepare nanocellulose with an acid hydrolysis approach (Table 1). However, a series of pretreatments, such as Soxhlet extraction, alkali treatment, and bleaching treatment need to be applied to extract lipids, fatty acids, and polysaccharides (e.g., agar and alginate). Nanocellulose extracted from red algae Gelidium elegans with a diameter of 6–40 nm and length of 80–547 nm exhibited a sound thermal stability and high aspect ratio, showing its potential application value in the field of nanocomposites (Table 1) [64]. While, nanocellulose prepared from brown algae waste, Laminaria japonica, using a similar extraction approach, presented a poor thermal stability and dispersed size [60,65]. The residue alginate in the nanocellulose could be the reason for this. Interestingly, such natural alginate-nanocellulose composite with a unique potential to form capsulate under the CaCl2 crosslinking may find its additional value as bioinks in bioprinting [66].
Generally, methods for extracting nanocellulose from algae residues are relatively simple relative to forest wastes and agriculture residues due to the noncoherent cellular structure and the absence of lignin. However, the algae nanocellulose often demonstrate a higher degree of crystallinity and specific surface area, and excellent rheological and mechanical properties, which will endow a broader spectrum in its applications.
2.4. Industrial by-products
Industrial by-products or residues produced from food processing, furniture manufacture, pulping and papermaking, printing and packaging, such as bagasse, sweet lime pulp waste, and recycle paper, are also referred to as another type of important recyclable biomass resource for nanocellulose extraction (Fig. 3). As a matter of fact, only the food processing industry will produce over 142 million tons of sugarcane bagasse residue in each year in Brazil, leaving a significant amount of lignocellulosic waste for its potential applications at a high value-added product [67].
The extraction approach of nanocellulose from industrial residues is variable and highly dependent on the residue type due to the variations in the chemical and structural composition of the feedstock. Sugarcane bagasse, characterized with a high lignocellulose content and loose structure, can be directly subjected to acid hydrolysis to extract nanocellulose [68,69]. But pretreatment with pulping and bleaching would need to be applied in the case of extracting nanocellulose from the olive industry solid waste or recycled paper and packaging waste prior to the acid hydrolysis or other chemical extraction process (Table 1). De Oliveira et al. proposed the steam explosion pretreatment and acid hydrolysis process to extract nanocellulose from the furniture industry processing residues (Pinus elliottii waste). The resultant nanocellulose with a diameter of 18–40 nm was subjected to surface-modification and freeze-drying to prepare hydrophobic aerogel for potential oil absorption [70]. For another type of industrial residues, waste paper was purified and converted into spherical nanocellulose with a crystallinity index of 48.85% and an average size of 50 nm using a NaOH/Thiourea system (Table 1) [71]. Similarly, Gibril et al. used ammonium persulfate under oxidizing conditions to convert fibers in pulp and paper mills sludge into CNCs particles with a width of 10–20 nm and length of 150–500 nm (Table 1) [72]. Inorganic nanoparticles (ZnO, Ag, and HP) were composited with the prepared CNCs using a sol-gel process for antimicrobial and photocatalytic applications in paper mills or as an additive in textile finishing and water treatment [72]. As a unique approach, enzymatic hydrolysis can be applied to assist the nanocellulose extraction from juicing industrial residues that presents a soft and loose cellular structure, such as the juicing industrial residue (citrus pulp) [14,73].
In addition to the extraction of nanocellulose from industrial solid biomass residues, bacterial nanocellulose cultivation using nutrient rich industrial processing water is another promising approach for nanocellulose production from low value biomass. Dubey et al. converted the sweet lime pulp waste into high quality bacterial nanocellulose using the Komagataeibacter europaeus SGP37 under a static intermittent fed-batch cultivation, providing a new way to convert waste into high value-added products (Table 1) [74]. In addition, wastes from other industries and households are also potential sources for nanocellulose extraction. Ogundare et al. extracted nanocellulose crystals from discarded cigarette filters by ethanol extraction, bleaching, alkali treatment and acid hydrolysis (Table 1). The average length, width and crystallinity of the extracted nanocellulose crystals was 143 nm, 8 nm and 96.77%, respectively. The nanocellulose at a high purity may have some potential applications in the field of biomedical composites [75]. Jongaroontaprangsee et al. extracted CNFs from lime residues using autoclaving, high-shear and high-pressure homogenization. The yielded CNFs with width of 5–28 nm and crystallinity of 44–46%, which exhibited a superior water redispersibility, showing a potential application in the field of packaging (Table 1) [76].
3. Application areas and their potential values
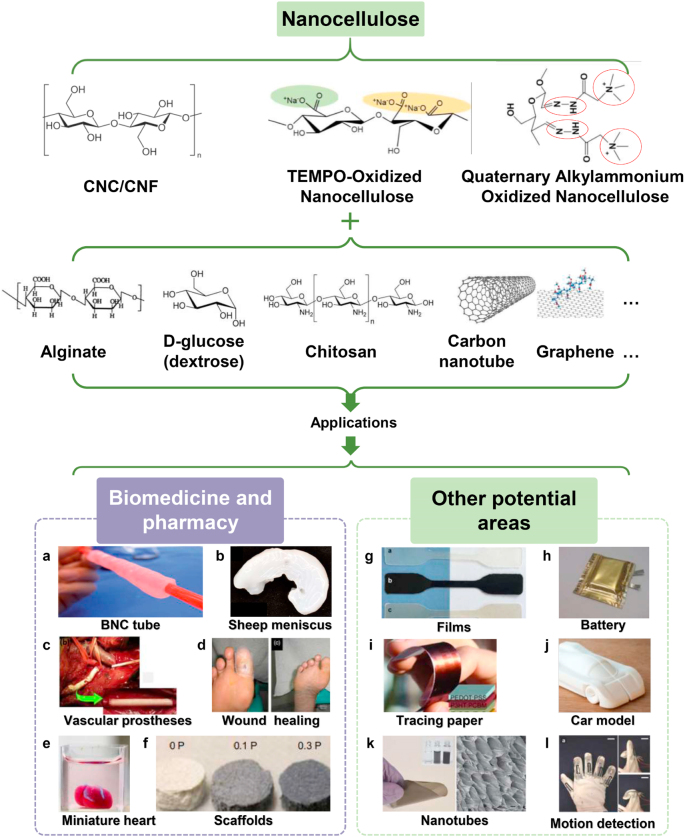
Owing to a variety of unique merits, such as non-toxicity, biocompatibility, high water absorption and retention ability, as well as its excellent mechanical properties, nanocellulose holds a huge potential to be widely used in biomedicine and pharmacy, functional food and feed, cosmetics, packaging, electronics and optoelectronic devices (Fig. 4) [37,88,89]. In particular, nanocellulose isolated from various biomass wastes also shows its cost-effective benefits at a wider range of raw material sources. Therefore, the development of high value-added products using nanocellulose that is extracted from biomass wastes not only promotes economic development, but also play a key role in environmental protection. In this section, the discussion on nanocellulose will be focused on its potential applications in biomedical, pharmaceutical, as well as other potential applications presented with various composite materials.
Fig. 4.
Applications of nanocellulose or its composite in biomedical, pharmaceutical, and other emerging fields. “P” denoted in Fig. 4f represents poly (3,4-ethylenedioxythiophene) poly (4-styrene sulfonate) (PEDOT:PSS). Figures were reproduced with permission from publishers [103,[110], [111], [112], [113], [114], [115]].
3.1. Biomedicine and pharmacy
Owing to the inherently low cytotoxicity, tunable biological activities, favorable rheological properties, and structural similarity to extracellular matrices (ECM), nanocellulose can provide an ideal living environment for cells, and thereby promoting cell proliferation and differentiation [90,91]. Therefore, it is widely utilized in many biomedical applications, such as wound healing, drug delivery, bionanocomposites (BNCs) tube and tissue engineering (include sheep meniscus, vascular grafts, skin, neuron, bone, heart and liver) (Fig. 4a–e) [92,93]. For example, a 3D scaffold with tunable mechanical strength was printed by using a TEMPO-medicated oxidized CNFs for 3D cell culture of fibroblasts for potential wound healing application (Fig. 4d) [90]. The highly hydrated nanocellulose and alginate are combined to mimic the natural ECM environment for human nasal chondrocytes survival and differentiation, showing a potential to be used to treat patients with acquired or congenital auricular defects [94]. It has been reported that nanocellulose-based particles, nanopaper, and nanofoam share unique colloidal properties, high specific surface area, good rheological properties, non-toxicity, and biodegradability of nanocellulose and can be used as a universal excipient for the delivery of poorly soluble drugs [95]. By selecting a suitable nanocellulose as a matrix material, drug release at specific sites and prolonged drug release profile can be achieved, suggesting the promising application for customized drug development and therapy [96].
Bacterial nanocellulose has traditionally been utilized as cosmetic mask and wound dressing materials due to its fine and uniform fibrils, high purity and crystallinity, as well as a high water retention ability [97]. It has been reported that the repair of abdominal wall defects using a bacterial nanocellulose membrane synthesized by Мedusomyces gisev Sa-12 symbiotic culture was significant and functional. More experiments showed that the body was able to form a stable connective tissue after 60 days of implantation. Moreover, the density of fibroblasts and fibrin is lower than that of polypropylene mesh-based grafts [98]. Generally, nanocellulose itself does not present the ability to regenerate and heal tissue. But, it can be combined with other materials (such as collagen, growth factors, hyaluronic acid, etc.) to provide a platform for tissue regeneration and repair self-healing [99,100]. For instance, nanocellulose crystals are combined with polyethylene glycol and polylactic acid, followed by electrospinning to prepare nanocomposite scaffold. The resultant scaffold exhibits a competitive biocompatibility to human mesenchymal stem cells, providing potential applications in bone tissue engineering scaffolds (Fig. 4f). Similarly, nanocellulose crystals are combined with injectable hyaluronic acid hydrogels to form nanocomposites. This composite material exhibits a good proliferative capacity for human adipose derived stem cells, which indeed expands its potential applications in biomedical field [101].
3.2. Other applications for composite material
The nanocellulose materials obtained by the aforementioned methods have been recognized as carbon neutral, sustainable, recyclable, and non-toxic [102]. Thus, it is expected these materials will become a green nanomaterial with its excellent performance in the composite materials formulation. By combining this nanomaterial with other organic or inorganic materials, the obtained composite materials can be widely used in the fields of packaging, models, electronics, human motion detection and optoelectronic devices etc (Fig. 4a-l) [103]. For example, nanocellulose films impregnated with a phenolic resin to prepare a composite material with a higher average shear strength (∼9.6 MPa) compared with that of non-nonocellulose Cu–Cu joints (∼4.7 MPa) [104,105]. The bacterial nanocellulose composite film showed significantly high Young’s modulus (28 GPa) relative to other composites based on fibrillated kraft pulp due to the higher crystallinity, purity, aspect ratio and uniform size [106]. Similarly, Jeevahan et al. reported that the reinforcement of edible films with nanocellulose which has the potential to overcome environmental pollution and oil consumption problems related with plastic packaging [107]. In addition, Leal et al. prepared a cellulose nanocrystals composite film by a formulation with tapioca starch and glycerin, where the resultant composite films were found to have a decent oxidation barrier and mechanical properties. The water vapor permeability was 2.5 times smaller but maximum stress was approximately 2 times greater than starch film without nanocellulose. This composite film showed an application potential in the field of food packaging [108]. A supercapacitor consists of carbon nanotubes and nanocellulose was reported to have a capacitance of 14.9–16.5 mF and equivalent series resistance of 74–155 Ω at the area of 1.8 cm2, presenting a much higher energy storage capacity than that of carbon nanotube films (10 mF and 30Ω) at a high power density and a long cycle life (Fig. 4k). This low-cost energy storage device has obvious applications in autonomous intelligence products [109]. Interestingly, nanocellulose-based composites have also been developed for those applications in electrochemical cells, binders for wastewater treatment, and automotive or aerospace models [102,110].
4. Challenges and outlook
Extraction of nanocellulose from various biomass wastes for further functional materials preparation supports the novel approach potentials towards some high value-added products and applications. However, technical challenges and cost issues still need to be addressed before large scale production and application is possible [116]. High cost challenges mainly range from the biomass waste collection, transportation, and storage, the expensive chemicals or enzymes, as well as the extensive energy consumption requirements during processing. In order to achieve commercial scale production, it is necessary to find efficient methods for producing a large number of cellulose nanofibers with excellent properties and uniform size at low cost (low energy, inexpensive reagents and effective enzymes) [30].
Technical challenges mainly include the development of efficient pretreatments and precise structural and functional modification. Efficient pretreatments to remove different impurities but leave cellulose in intact is the prerequisite for preparation of nanocellulose with high quality, especially those for biomedical applications. Due to the inherent properties of cellulose, such as bioinert, hydrophilicity, and thermal defects, precise functionalization are necessary to expand or endow novel functionalities for target application [117]. In addition, decisions need to be made to balance the benefits and drawbacks of nanocellulose utilization. For example, the addition of CNFs to the papermaking process can help to improve the mechanical strength, but it can reduce the drainage capacity of the slurry, reducing the production efficiency [30]. Although there are some problems in the application of nanocellulose, its excellent mechanical properties, high specific surface area, natural richness, environmental friendliness, biodegradability, recyclability, and non-toxicity are attracting increasing researchers to develop high value-added end products and applications [[118], [119], [120], [121]].
5. Conclusion
From the recycling point of view, biomass wastes represent an unignorable biopolymer resource. Therefore, the current work summarizes the potential sources of biomass waste as raw materials for nanocellulose preparation and their high potential value-added applications, mainly based on works over the past five years. Nanocellulose with its unique properties can be extracted from forest and agricultural residues, algae waste, or industrial by-products using different approaches, which will support, particularly when developed as a composite format, some promising applications in biomedical, pharmaceutical, and other new emerging areas [92]. In general, the excellent mechanical, optical, and thermal properties of nanocellulose endow a broad application in all aspects of our life. However, technical challenges and cost issues still need to be addressed in order to achieve a mass production application. From the perspective of energy and the environment, nanocellulose is a renewable energy source with a wide range of sources and abundant content. Development of nanocellulose-based high value-added products can not only save energy but also solves some intractable environmental problems, while also at same time promoting economic development.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the National Key R&D Program of China (Grant No. 2018YFE0107100), Jiangsu Province Natural Science Foundation (BK20190842), and the Start-up Fund for Introduced Scholar of Jiangsu University (4111370004), the foundation of Key Laboratory of Pulp and Paper Science and Technology of Ministry of Education of China.
Contributor Information
Jianzhong Sun, Email: jzsun1002@ujs.edu.cn.
Jun Liu, Email: Junliu115142@hotmail.com.
References
- 1.Agency I.E. IEA Bioenergy 28th update[J]. Biomass and Bioenergy. 2007;31(5):I–XI. [Google Scholar]
- 2.García A., et al. Industrial and crop wastes: a new source for nanocellulose biorefinery[J] Ind. Crop. Prod. 2016;93:26–38. [Google Scholar]
- 3.Kocar G. Anaerobic digesters: from Waste to energy Crops as an alternative energy source. Energy sources, Part A: recovery, utilization. and Environmental Effects. 2008;30(7):660–669. [Google Scholar]
- 4.Questell-Santiago Y.M., et al. Stabilization strategies in biomass depolymerization using chemical functionalization. Nature Reviews Chemistry. 2020;4(6):311–330. doi: 10.1038/s41570-020-0187-y. [DOI] [PubMed] [Google Scholar]
- 5.Montella S., et al. Discovery of genes coding for carbohydrate-active enzyme by metagenomic analysis of lignocellulosic biomasses. Sci. Rep. 2017;7:42623. doi: 10.1038/srep42623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao D., et al. Hemicellulases and auxiliary enzymes for improved conversion of lignocellulosic biomass to monosaccharides. Biotechnol. Biofuels. 2011;4:1–11. doi: 10.1186/1754-6834-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso D.M., et al. Increasing the revenue from lignocellulosic biomass: maximizing feedstock utilization. Sci. Adv. 2017;3:1–7. doi: 10.1126/sciadv.1603301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Achaby M., et al. Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse. Int. J. Biol. Macromol. 2017;96:340–352. doi: 10.1016/j.ijbiomac.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T., Katai S., Namiki T. Safety of smoke generated by Japanese moxa upon combustion[J] European Journal of Integrative Medicine. 2016;8(4):414–422. [Google Scholar]
- 10.Christ D., et al. Energy from Organic Materials. Biomass; 2019. Thermochemical conversion of solid biofuels: processes and techniques[J] pp. 393–413. [Google Scholar]
- 11.Cigolotti V. Fuel Cells in the Waste-To-Energy Chain. 2012. Biomass and waste as sustainable resources; pp. 23–44. [Google Scholar]
- 12.Corma A., et al. Production of high-quality diesel from biomass waste products[J] Angew Chem. Int. Ed. Engl. 2011;50(10):2375–2378. doi: 10.1002/anie.201007508. [DOI] [PubMed] [Google Scholar]
- 13.Pileidis F.D., Titirici M.-M. Levulinic acid biorefineries: new challenges for efficient utilization of biomass. ChemSusChem. 2016;9(6):562–582. doi: 10.1002/cssc.201501405. [DOI] [PubMed] [Google Scholar]
- 14.Diop C.I.K., Lavoie J.-M. Isolation of nanocrystalline cellulose: a technological route for valorizing recycled tetra pak aseptic multilayered food packaging wastes. Waste and Biomass Valorization. 2016;8(1):41–56. [Google Scholar]
- 15.Dilamian M., Noroozi B. A combined homogenization-high intensity ultrasonication process for individualization of cellulose micro-nano fibers from rice straw. Cellulose. 2019;26(10):5831–5849. [Google Scholar]
- 16.Lee H.V., Hamid S.B., Zain S.K. Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. ScientificWorldJournal. 2014;2014:631013. doi: 10.1155/2014/631013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johar N., Ahmad I., Dufresne A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012;37(1):93–99. [Google Scholar]
- 18.Mihranyan A. Cellulose from cladophorales green algae: from environmental problem to high-tech composite materials. J. Appl. Polym. Sci. 2011;119(4):2449–2460. [Google Scholar]
- 19.Alila S., et al. Non-woody plants as raw materials for production of microfibrillated cellulose (MFC): a comparative study. Ind. Crop. Prod. 2013;41:250–259. [Google Scholar]
- 20.Lin N., Dufresne A. Nanocellulose in biomedicine: current status and future prospect. Eur. Polym. J. 2014;59:302–325. [Google Scholar]
- 21.Li M., et al. Effect of surface wettability on the antibacterial activity of nanocellulose-based material with quaternary ammonium groups[ J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2018;554:122–128. [Google Scholar]
- 22.Mondal S. Review on nanocellulose polymer nanocomposites[J] Polym. Plast. Technol. Eng. 2017;57(13):1377–1391. [Google Scholar]
- 23.Helenius G., et al. In vivo biocompatibility of bacterial cellulose[J] J. Biomed. Mater. Res. 2006;76(2):431–438. doi: 10.1002/jbm.a.30570. [DOI] [PubMed] [Google Scholar]
- 24.Halib N., et al. Potential applications of nanocellulose-containing materials in the biomedical field[J] Materials. 2017;10(8) doi: 10.3390/ma10080977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao P., et al. Recent advances in materials for extended-release antibiotic delivery system[J] J. Antibiot. (Tokyo) 2011;64(9):625–634. doi: 10.1038/ja.2011.58. [DOI] [PubMed] [Google Scholar]
- 26.LIMITED C.M.A.M. 2019. Nanocellulose Market Worth; p. 66. [Google Scholar]
- 27.Chauve∗ G.e., Bras J. Industrial point of view of nanocellulose materials. Materials and Energy. 2014;14:233–252. [Google Scholar]
- 28.Koppejan A.K.a.J. 1997. Agricultural and Forest Residues Generation, Utilization and Availability; pp. 1–23. [Google Scholar]
- 29.Tang W. Development and utilization of forest energy. JournaI of Anhui AgricuIIuraI Sciences. 2002;30(4):600–602. [Google Scholar]
- 30.Vallejos M.E., et al. Nanofibrillated cellulose (CNF) from eucalyptus sawdust as a dry strength agent of unrefined eucalyptus handsheets. Carbohydr. Polym. 2016;139:99–105. doi: 10.1016/j.carbpol.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Moriana R., Vilaplana F., Ek M. Cellulose nanocrystals from forest residues as reinforcing agents for composites: a study from macro- to nano-dimensions. Carbohydr. Polym. 2016;139:139–149. doi: 10.1016/j.carbpol.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Phanthong P., et al. A facile one-step way for extraction of nanocellulose with high yield by ball milling with ionic liquid. Cellulose. 2017;24(5):2083–2093. [Google Scholar]
- 33.Veigel S., et al. Particle board and oriented strand board prepared with nanocellulose-reinforced adhesive. J. Nanomater. 2012;2012:1–8. [Google Scholar]
- 34.Carvalho D.M.d., et al. Assessment of chemical transformations in eucalyptus, sugarcane bagasse and straw during hydrothermal, dilute acid, and alkaline pretreatments. Ind. Crop. Prod. 2015;73:118–126. [Google Scholar]
- 35.Bufalino L., et al. Nanocellulose films from Amazon forest wood wastes: structural and thermal properties. Key Eng. Mater. 2015;668:110–117. [Google Scholar]
- 36.Rambabu N., et al. Production of nanocellulose fibers from pinecone biomass: evaluation and optimization of chemical and mechanical treatment conditions on mechanical properties of nanocellulose films. Ind. Crop. Prod. 2016;83:746–754. [Google Scholar]
- 37.Xiao S., et al. Fabrication and characterization of nanofibrillated cellulose and its aerogels from natural pine needles. Carbohydr. Polym. 2015;119:202–209. doi: 10.1016/j.carbpol.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 38.Couret L., et al. Extraction and characterization of cellulose nanocrystals from post-consumer wood fiberboard waste. Cellulose. 2017;24(5):2125–2137. [Google Scholar]
- 39.Lu H., et al. A novel method to prepare lignocellulose nanofibrils directly from bamboo chips. Cellulose. 2018;25(12):7043–7051. [Google Scholar]
- 40.Ibrahim M.M., et al. Synthesis of tosylated and trimethylsilylated methyl cellulose as pH-sensitive carrier matrix[J] Life Sci. J. 2015;12(1):29–37. [Google Scholar]
- 41.Ximenes F.A., Gardner W.D., Kathuria A. Proportion of above-ground biomass in commercial logs and residues following the harvest of five commercial forest species in Australia. For. Ecol. Manag. 2008;256(3):335–346. [Google Scholar]
- 42.Ibrahim M.M., Fahmy T.Y.A. Synthesis of tosylated and trimethylsilylated methyl cellulose as pH-sensitive carrier matrix. Life Sci. J. 2015;12(1):29–37. [Google Scholar]
- 43.Kätterer T., et al. Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric. Ecosyst. Environ. 2011;141(1–2):184–192. [Google Scholar]
- 44.Obi F.O., Ugwuishiwu B.O., Nwakaire J.N. Agricultural waste concept, generation, utilization and management. Nigerian Journal of Technology. 2016;35(4) [Google Scholar]
- 45.Chen Y., et al. Bionanocomposites based on pea starch and cellulose nanowhiskers hydrolyzed from pea hull fibre: effect of hydrolysis time. Carbohydr. Polym. 2009;76(4):607–615. [Google Scholar]
- 46.PHANTHONG P., MA Y., GUAN G., ABUDULA A. Extraction of nanocellulose from raw apple stem[J] J. Jpn. Inst. Energy. 2015;94(8):787–793. [Google Scholar]
- 47.Suopajärvi T., et al. Lead adsorption with sulfonated wheat pulp nanocelluloses. Journal of Water Process Engineering. 2015;5:136–142. [Google Scholar]
- 48.Abraham E., et al. Extraction of nanocellulose fibrils from lignocellulosic fibres: a novel approach. Carbohydr. Polym. 2011;86(4):1468–1475. [Google Scholar]
- 49.Kang X., et al. Thin cellulose nanofiber from corncob cellulose and its performance in transparent nanopaper. ACS Sustain. Chem. Eng. 2017;5(3):2529–2534. [Google Scholar]
- 50.Slavutsky A.M., Bertuzzi M.A. Water barrier properties of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydr. Polym. 2014;110:53–61. doi: 10.1016/j.carbpol.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 51.Wu J., et al. Preparation and characterization of cellulose nanofibrils from coconut coir fibers and their reinforcements in biodegradable composite films. Carbohydr. Polym. 2019;211:49–56. doi: 10.1016/j.carbpol.2019.01.093. [DOI] [PubMed] [Google Scholar]
- 52.Jun-hua Z., Hai-nong S., Lu L. Effects of high-pressure homogenization treatment on the preparation of microfibrillated cellulose. Journal of Cellulose Science and Technology. 2009;17 [Google Scholar]
- 53.Yuanyuan L., Hongqi D. Preparation of nano-crystalline cellulose by chemical methods. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2012;36 [Google Scholar]
- 54.Kaushika A., Singhb M., Vermaa G. Green nanocomposites based on thermoplastic starch and steam exploded cellulose nanofibrils from wheat straw. Carbohydr. Polym. 2010;82:337–345. [Google Scholar]
- 55.Zhao J., et al. Extraction of cellulose nanofibrils from dry softwood pulp using high shear homogenization. Carbohydr. Polym. 2013;97(2):695–702. doi: 10.1016/j.carbpol.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 56.Dima S.O., et al. Bacterial nanocellulose from side-streams of kombucha beverages production: preparation and physical-chemical properties. Polymers. 2017;9(8) doi: 10.3390/polym9080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ministry of Natural Resources of the People’s Republic of China . 2019. Bulletin of China Marine Disaster. N, 2020. [Google Scholar]
- 58.Herth W. Arrays of plasma-membrane "rosettes" involved in cellulose microfibril formation of Spirogyra[J] Microfibril formation of Spirogyra. 1983;159:347–356. doi: 10.1007/BF00393174. [DOI] [PubMed] [Google Scholar]
- 59.Gouveia L., et al. Chlorella vulgaris biomass used as colouring source in traditional butter cookies[J] Innovat. Food Sci. Emerg. Technol. 2007;8(3):433–436. [Google Scholar]
- 60.Liu Z., et al. Extraction, isolation and characterization of nanocrystalline cellulose from industrial kelp (Laminaria japonica) waste. Carbohydr. Polym. 2017;173:353–359. doi: 10.1016/j.carbpol.2017.05.079. [DOI] [PubMed] [Google Scholar]
- 61.Hua K., et al. Nanocellulose from green algae modulates the in vitro inflammatory response of monocytes/macrophages. Cellulose. 2015;22(6):3673–3688. [Google Scholar]
- 62.Rathod M., Haldar S., Basha S. Nanocrystalline cellulose for removal of tetracycline hydrochloride from water via biosorption: equilibrium, kinetic and thermodynamic studies. Ecol. Eng. 2015;84:240–249. [Google Scholar]
- 63.Bhutiya P.L., et al. Nested seaweed cellulose fiber deposited with cuprous oxide nanorods for antimicrobial activity. Int. J. Biol. Macromol. 2018;117:435–444. doi: 10.1016/j.ijbiomac.2018.05.210. [DOI] [PubMed] [Google Scholar]
- 64.Van Hai L., Son H.N., Seo Y.B. Physical and bio-composite properties of nanocrystalline cellulose from wood, cotton linters, cattail, and red algae. Cellulose. 2015;22(3):1789–1798. [Google Scholar]
- 65.Feng X., et al. Extraction and preparation of cellulose nanocrystals from dealginate kelp residue: structures and morphological characterization. Cellulose. 2015;22(3):1763–1772. [Google Scholar]
- 66.Lemahieu L., et al. Extrusion of nanocellulose-reinforced nanocomposites using the dispersed nano-objects protective encapsulation (DOPE) process. Macromol. Mater. Eng. 2011;296(11):984–991. [Google Scholar]
- 67.Alves J.O., et al. Characterization of nanomaterials produced from sugarcane bagasse. Journal of Materials Research and Technology. 2012;1(1):31–34. [Google Scholar]
- 68.Mandal A., Chakrabarty D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011;86(3):1291–1299. [Google Scholar]
- 69.Shahabi-Ghahafarrokhi I., et al. Preparation and characterization of nanocellulose from beer industrial residues using acid hydrolysis/ultrasound. Fibers Polym. 2015;16(3):529–536. [Google Scholar]
- 70.de Oliveira P.B., Godinho M., Zattera A.J. Oils sorption on hydrophobic nanocellulose aerogel obtained from the wood furniture industry waste. Cellulose. 2018;25(5):3105–3119. [Google Scholar]
- 71.Zhang S., et al. Preparation of spherical nanocellulose from waste paper by aqueous NaOH/thiourea. Cellulose. 2019;26(8):5177–5185. [Google Scholar]
- 72.Gibril M.E., et al. Beneficiation of pulp and paper mill sludge: production and characterisation of functionalised crystalline nanocellulose[J] Clean Technol. Environ. Policy. 2018;20(8):1835–1845. [Google Scholar]
- 73.Jodeh S., et al. Magnetic nanocellulose from olive industry solid waste for the effective removal of methylene blue from wastewater. Environ. Sci. Pollut. Res. Int. 2018;25(22):22060–22074. doi: 10.1007/s11356-018-2107-y. [DOI] [PubMed] [Google Scholar]
- 74.Dubey S., Singh J., Singh R.P. Biotransformation of sweet lime pulp waste into high-quality nanocellulose with an excellent productivity using Komagataeibacter europaeus SGP37 under static intermittent fed-batch cultivation. Bioresour. Technol. 2018;247:73–80. doi: 10.1016/j.biortech.2017.09.089. [DOI] [PubMed] [Google Scholar]
- 75.Ogundare S.A., Moodley V., van Zyl W.E. Nanocrystalline cellulose isolated from discarded cigarette filters. Carbohydr. Polym. 2017;175:273–281. doi: 10.1016/j.carbpol.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Jongaroontaprangsee S., Chiewchan N., Devahastin S. Production of nanofibrillated cellulose with superior water redispersibility from lime residues via a chemical-free process. Carbohydr. Polym. 2018;193:249–258. doi: 10.1016/j.carbpol.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 77.Sirvio J.A., et al. Biocomposite cellulose-alginate films: promising packaging materials. Food Chem. 2014;151:343–351. doi: 10.1016/j.foodchem.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 78.Thomas M.G., et al. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: preparation and characterization. Int. J. Biol. Macromol. 2015;81:768–777. doi: 10.1016/j.ijbiomac.2015.08.053. [DOI] [PubMed] [Google Scholar]
- 79.Karimi S., et al. Kenaf bast cellulosic fibers hierarchy: a comprehensive approach from micro to nano. Carbohydr. Polym. 2014;101:878–885. doi: 10.1016/j.carbpol.2013.09.106. [DOI] [PubMed] [Google Scholar]
- 80.Naz S., et al. Management of citrus waste by switching in the production of nanocellulose. IET Nanobiotechnol. 2016;10(6):395–399. doi: 10.1049/iet-nbt.2015.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li R., et al. Cellulose whiskers extracted from mulberry: a novel biomass production. Carbohydr. Polym. 2009;76(1):94–99. [Google Scholar]
- 82.Zarei S., Niad M., Raanaei H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: synthesis, characterizations and DFT studies. J. Hazard Mater. 2018;344:258–273. doi: 10.1016/j.jhazmat.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y.W., et al. Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 2016;151:1210–1219. doi: 10.1016/j.carbpol.2016.06.083. [DOI] [PubMed] [Google Scholar]
- 84.de Oliveira J.P., et al. Development of food packaging bioactive aerogels through the valorization of Gelidium sesquipedale seaweed. Food Hydrocolloids. 2019;89:337–350. [Google Scholar]
- 85.Morais J.P., et al. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013;91(1):229–235. doi: 10.1016/j.carbpol.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 86.Widiarto S., et al. Preparation and characterization of cellulose and nanocellulose from agro-industrial waste - cassava peel. IOP Conf. Ser. Mater. Sci. Eng. 2017:176. [Google Scholar]
- 87.Cypriano D.Z., da Silva L.L., Tasic L. High value-added products from the orange juice industry waste. Waste Manag. 2018;79:71–78. doi: 10.1016/j.wasman.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 88.Ge W., et al. Rapid self-healing, stretchable, moldable, antioxidant and antibacterial tannic acid-cellulose nanofibril composite hydrogels. Carbohydr. Polym. 2019;224:115147. doi: 10.1016/j.carbpol.2019.115147. [DOI] [PubMed] [Google Scholar]
- 89.Luo H., et al. Advances in tissue engineering of nanocellulose-based scaffolds: a review. Carbohydr. Polym. 2019;224:115144. doi: 10.1016/j.carbpol.2019.115144. [DOI] [PubMed] [Google Scholar]
- 90.Xu W., et al. On low-concentration inks formulated by nanocellulose assisted with gelatin methacrylate (GelMA) for 3D printing toward wound healing application. ACS Appl. Mater. Interfaces. 2019;11(9):8838–8848. doi: 10.1021/acsami.8b21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roman M. Toxicity of cellulose nanocrystals: a review[J] Ind. Biotechnol. 2015;11(1):25–33. [Google Scholar]
- 92.Lin N., Dufresne A. Nanocellulose in biomedicine: current status and future prospect[J] Eur. Polym. J. 2014;59:302–325. [Google Scholar]
- 93.Markstedt K., et al. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications[J] Biomacromolecules. 2015;16(5):1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 94.Martínez Ávila H., et al. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting. 2016;1–2:22–35. [Google Scholar]
- 95.Lobmann K., Svagan A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017;533(1):285–297. doi: 10.1016/j.ijpharm.2017.09.064. [DOI] [PubMed] [Google Scholar]
- 96.Löbmann K., et al. Cellulose nanopaper and nanofoam for patient-tailored drug delivery. Advanced Materials Interfaces. 2017;4(9) [Google Scholar]
- 97.Horbert V., et al. In vitro analysis of the potential cartilage implant bacterial nanocellulose using the bovine cartilage punch model[J] Cellulose. 2019;26(1):631–645. [Google Scholar]
- 98.Zharikov A.N., et al. Early morphological changes in tissues when replacing abdominal wall defects by bacterial nanocellulose in experimental trials. J. Mater. Sci. Mater. Med. 2018;29(7):95. doi: 10.1007/s10856-018-6111-z. [DOI] [PubMed] [Google Scholar]
- 99.Abitbol T., et al. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016;39:76–88. doi: 10.1016/j.copbio.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Millon L.E., Wan W.K. The polyvinyl alcohol-bacterial cellulose system as a new nanocomposite for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2006;79(2):245–253. doi: 10.1002/jbm.b.30535. [DOI] [PubMed] [Google Scholar]
- 101.Domingues R.M., et al. Development of injectable hyaluronic acid/cellulose nanocrystals bionanocomposite hydrogels for tissue engineering applications[J] Bioconjugate Chem. 2015;26(8):1571–1581. doi: 10.1021/acs.bioconjchem.5b00209. [DOI] [PubMed] [Google Scholar]
- 102.Nelson K., et al. 2016. American Process: Production Of Low Cost Nanocellulose For Renewable, Advanced Materials Applications, in Materials Research For Manufacturing; pp. 267–302. [Google Scholar]
- 103.Kim J.-H., et al. Review of nanocellulose for sustainable future materials. International Journal of Precision Engineering and Manufacturing-Green Technology. 2015;2(2):197–213. [Google Scholar]
- 104.João C.F.C., et al. Fibrous and Textile Materials for Composite Applications. 2016. Natural nanofibres for composite applications; pp. 261–299. [Google Scholar]
- 105.Gao R., et al. Effects of phenolic resin addition on the electrical conductivity and mechanical strength of nano-copper paste formed Cu-Cu joints. J. Electron. Mater. 2017;46(11):6388–6394. [Google Scholar]
- 106.Nakagaito A.N., Iwamoto S., Yano H. Bacterial cellulose: the ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A. 2005;80(1):93–97. [Google Scholar]
- 107.Jeevahan J., Chandrasekaran M. Nanoedible films for food packaging: a review. J. Mater. Sci. 2019;54(19):12290–12318. [Google Scholar]
- 108.Leal I.L., et al. Development and application starch films: PBAT with additives for evaluating the shelf life of Tommy Atkins mango in the fresh-cut state. J. Appl. Polym. Sci. 2019;136(43) [Google Scholar]
- 109.Tuukkanen S., et al. Proceedings of the 5th Electronics System-Integration Technology Conference. ESTC; 2014. Printable and disposable supercapacitor from nanocellulose and carbon nanotubes[J] pp. 1–6. [Google Scholar]
- 110.Muhd Julkapli N., Bagheri S. Nanocellulose as a green and sustainable emerging material in energy applications: a review. Polym. Adv. Technol. 2017;28(12):1583–1594. [Google Scholar]
- 111.Markstedt K., et al. 3D bioprinting human chondrocytes with Nanocellulose−Alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16:1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 112.Shen X., et al. MMA-enabled ultraclean graphene transfer for fast-response graphene/GaN ultraviolet photodetectors. Carbon. 2020;169:92–98. [Google Scholar]
- 113.Baimova J.A., et al. Mechanical properties of crumpled graphene under hydrostatic and uniaxial compression. J. Phys. Appl. Phys. 2015;48(9) [Google Scholar]
- 114.shahini A., Yazdimamaghani M., Walker K.J. 3D conductive nanocomposite scaffold for bone tissue engineering. Int. J. Nanomed. 2014;9:167–181. doi: 10.2147/IJN.S54668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yan C., et al. Highly stretchable piezoresistive graphene-nanocellulose nanopaper for strain sensors. Adv. Mater. 2014;26(13):2022–2027. doi: 10.1002/adma.201304742. [DOI] [PubMed] [Google Scholar]
- 116.Ngwabebhoh F.A., Yildiz U. Nature-derived fibrous nanomaterial toward biomedicine and environmental remediation: today’s state and future prospects[J] J. Appl. Polym. Sci. 2019;136(35) [Google Scholar]
- 117.Clemons C. Nanocellulose in spun continuous fibers: a review and future outlook[J] Journal of Renewable Materials. 2016;4(5):327–339. [Google Scholar]
- 118.Wang J., et al. Preparation of nanocellulose and its potential in reinforced composites: a review. J. Biomater. Sci. Polym. Ed. 2019;30(11):919–946. doi: 10.1080/09205063.2019.1612726. [DOI] [PubMed] [Google Scholar]
- 119.Phanthong P., et al. Nanocellulose: extraction and application. Carbon Resources Conversion. 2018;1(1):32–43. [Google Scholar]
- 120.Kim J.H., et al. Nanocellulose for energy storage systems: beyond the limits of synthetic materials. Adv. Mater. 2019;31(20) doi: 10.1002/adma.201804826. [DOI] [PubMed] [Google Scholar]
- 121.Börjesson M., et al. Increased thermal stability of nanocellulose composites by functionalization of the sulfate groups on cellulose nanocrystals with azetidinium ions. J. Appl. Polym. Sci. 2018;135(10) [Google Scholar]