Abstract
Formation of acid mine drainage (AMD) is a widespread environmental issue that has not subsided throughout decades of continuing research. Highly acidic and highly concentrated metallic streams are characteristics of such streams. Humans, plants and surrounding ecosystems that are in proximity to AMD producing sites face immediate threats. Remediation options include active and passive biological treatments which are markedly different in many aspects. Sulfate reducing bacteria (SRB) remove sulfate and heavy metals to generate non-toxic streams. Passive systems are inexpensive to operate but entail fundamental drawbacks such as large land requirements and prolonged treatment period. Active bioreactors offer greater operational predictability and quicker treatment time but require higher investment costs and wide scale usage is limited by lack of expertise. Recent advancements include the use of renewable raw materials for AMD clean up purposes, which will likely achieve much greener mitigation solutions.
Keywords: Biological remediation, Acid Mine, Drainage, Environmental, Optimization
Graphical abstract
Highlights
-
•
Various bio-remediation techniques available for AMD treatment are reviewed.
-
•
SRB based acidic effluent treatment with necessary information on their metabolism and process parameters are described.
-
•
Current trends in active and passive biological treatment methods with recent innovations in the field are presented.
-
•
Future outlook and potential research gaps in bio-remediation of AMD treatment are discussed.
1. Introduction
In recent times, acid mine drainage (AMD) has become a serious global issue owing to its hazardous impact on the environment and living organisms causing most dangerous effects [1]. AMD is mostly generated as a by-product of various industrial processes of mining and other related industries. A detailed description of AMD origin and different routes which contribute to AMD generation have been reported in the literature [2,3]. AMD generation is very acute in inactive and abandoned mining sites associated with various minerals and metallurgical extraction. In brief, accelerated oxidation of sulfidic minerals (especially iron pyrites and other heavy metal pyrites) due to their exposure to water and oxygen is the major reason for AMD generation. Also, generation of acidic streams with high sulfate content is commonly caused by exposure and oxidation of sulfide-bound minerals or rocks. AMD or acid rock drainage (ARD) formation is common in many mining and mineral refining industries. Chemical reactions that underlie AMD generation is simple and well-researched (outlined in Sec 2.1), but drainage composition can differ drastically from one region to another due to local geology, microclimate, group of microorganisms and source of water [4].
High concentrations of dissolved metalloids and metals, extremely acidic pH and large amounts of sulfate in the AMD pose a serious threat to underground and surface water contamination leading to lethal effects. Extended impacts include biodiversity loss and deterioration of aquatic ecosystems [5,6]. In order to prevent the damages of AMD and to enhance ecological sustainability, a proper treatment and management system for AMD is required. Economically-effective remediation technologies have been proposed to counteract the effects of AMD repercussions. Specifically, biological treatments generate great interest among researchers for its promising ability for AMD clean-up [[7], [8], [9], [10]]. Biological treatments of AMD, also known as bio-remediation, has been attractive compared to other chemical based treatments of AMD with respect to low operational and labor costs, easy process design and control, and with better sulfate and metal recovery [11,12].
In principle, bioremediation of AMD involves the usage of sulfate reducing bacteria (SRB) to microbially recover metals and sulfates present in AMD as metal sulfides [13]. The metabolism of SRB reduces the excessive sulfate present in AMD to hydrogen sulfide (H2S). The biogenic H2S easily binds with the metals in the AMD stream to precipitate as metal sulfides which are very stable and can be easily recovered and recycled. The overall biochemical reactions associated with the SRB reduction of metals and sulfides in AMD are shown in Eqs. (1), (2), (3)).
| SO42− + 4H2 + H+ → HS− + 4H2O | (1) |
| Organic matter (C, O and H) + SO42− → HS− + HCO3− | (2) |
| M2+ + HS− → MS (↓) + H+ (M2+ - Metal cation) | (3) |
Further, SRB metabolism also helps in acidity neutralization of AMD due to the alkalinity resulting from its biochemical reactions. This aids in the easy filtration of the metal sulfides from the liquid phase of the reaction system.
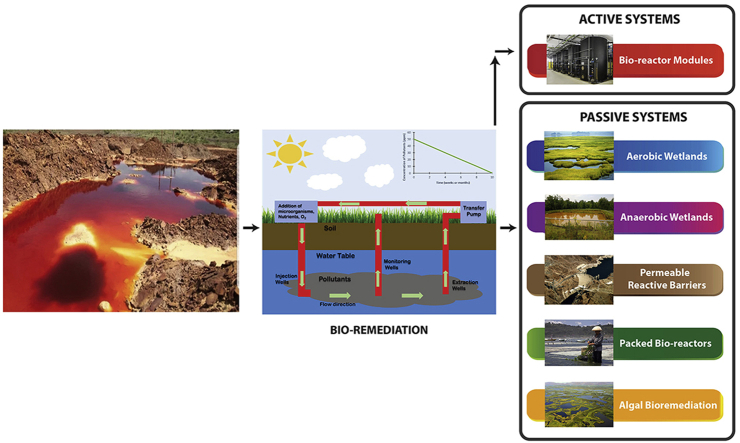
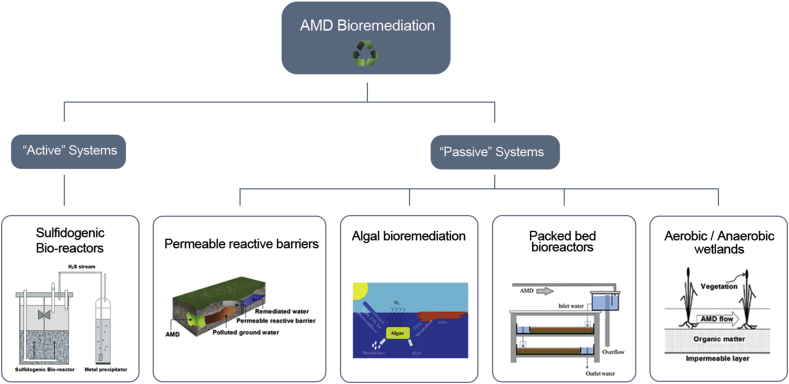
A range of biological methods falls under the spectrum of bio-remediation of AMD which can be grouped into active and passive treatment methods. Fig. 1 presents a simple classification of these bio-remediation techniques. A biologically active treatment system is usually a continuous process which requires constant resource input and immobilizes the metal contaminants by generation of sufficient alkalinity. These types of treatments provides better process control and ability for functional modifications in the reaction system [[14], [15], [16]]. Passive systems require relatively low resource input and are quite advantageous from the perspective of operational costs but more expensive to set-up.
Fig. 1.
Bioremediation methods for AMD treatment.
This paper reviews the current understanding of AMD treatment methods, with emphasis on biologically-based treatment options. It describes, in sequence, occurrence of AMD, role of sulfate reducing bacteria in AMD treatment, passive and active biological treatments, and finally, highlights the current biological processes based technology trends for AMD treatment. It should be noted that the term AMD has seemingly been used interchangeably with ARD but we will use AMD here to emphasize the anthropogenic-induced problems which are more frequent and of greater concern than that of natural acidic drainage.
2. Acid mine drainage
2.1. Overview of AMD
Generation of waste streams rich with sulfate and metallic ions is not limited to mining and mineral processing alone. Other industrial activities such as flue-gas scrubbing, pulp and paper milling streams, chemical manufacture etc. produce effluents which contain similar characteristics of AMD [17,18]. Acid mine drainage (AMD) is initiated by the hydro-geochemical weathering of sulfide bearing rocks (marcasite, arsenopyrite and pyrite) which are in contact with oxygen and water. The reaction is catalyzed by sulfur oxidizing microorganisms and Iron (Fe). For instance, coal mine wastes in addition to artificial irrigation leached acid drainages contains high concentrations of Pb, Fe, Cr and SO42− etc. which reflects a typical acid mine drainage.
Some of the metal sulfides and their chemical formula are shown in Table 1. Additionally, AMD is prominent in both operating and abandoned mines and open pit sites. Its damage may not be apparent while mines are in full operation, when constant pumping limits the rise of water table.
Table 1.
Predominant metal sulfides for acid drainage production.
| Metal Sulfide | Chemical Formula |
|---|---|
| Pyrite | FeS2 |
| Covelite | CuS |
| Chalcopyrite | Cu2S |
| Galena | PbS |
| Sphalerite | ZnS |
| Millerite | NiS |
| Molybdenite | MoS2 |
| Pyrrhotite | Fe(1-x)S |
| Marcasite | FeS2 |
| Arsenopyrite | FeAsS |
Air enters the rock mass creating iron sulfate salts that can dissolve in groundwater, thus contaminating it. The resulting water is low in organic materials but high in dissolved iron salts and often contains free sulfuric acid. The pH may drop below 2 and if there are iron-stone beds present in the strata, the iron level may reach 2000 mg/L.
Pyrite and marcasite are two most common sulfide minerals which are abundantly available [19]. The oxidation of pyrite proceeds through several pathways that involve surface interactions of dissolved oxygen and Fe3+ ions which forms dissolved iron, sulfate and hydrogen (Eq. (4)). Yet, rate of oxidation and acid production is highly dependent on minerals variability, microbial activity and oxygen and water availability [20].
| 2FeS2 + 7O2 + 2H2O → 2Fe2+ + 4SO42− + 4H+ | (4) |
It is also suggested that presence of sulfate in mine waste drainage is typically a preliminary indication of sulfide mineral exposure and oxidation [20]. Under oxidizing environments (with pH greater than 3.5 and presence of microbes), ferrous iron released in Eq. (4) could be further oxidized to ferric iron according to Eq. (5).
| 4Fe2+ + O2 + 4H+ ↔ 4Fe3+ + 2H2O | (5) |
However, in under oxygen-deprived conditions, Eq. (5) will not proceed until pH rises to 8.5. This indicates Eq. (5) is often the rate-limiting step in pyrite oxidation as conversion of ferrous to ferric is sluggish at pH 5 and below [21]. Furthermore, at pH 2.3 to 3.5, ferric iron formed in Eq. (5) may precipitate as Fe(OH)3, reducing amount of Fe3+ in original pool and continuously decrease the existing pH.
| Fe3+ + 3H2O ↔ Fe(OH)3 + 3H+ | (6) |
Under extremely acidic conditions (pH < 2), the hydrolysis product Fe(OH)3 from Eq. (6) is thermodynamically unstable and may not form. In turn, remaining Fe3+ in solution is utilized for oxidation of additional pyrite materials (Eq. (7)). This, in turn, plunges pH of AMD into ultra-acidic state [22].
| FeS2 + 14Fe3+ + 8H2O → 15Fe2+ + 2SO42− + 16H+ | (7) |
2.2. Effects of AMD
As a consequence of highly acidic conditions created by excess H+ and sulfuric acid, a vast array of metals and mineral are dissolved by AMD, and an acidic and metalliferous toxic flow results. This lethal stream taints groundwater, rivers and other water bodies that are in proximity. AMD destroys ecosystems, corrodes infrastructure and contaminates water supplies, often in regions where freshwater is scarce [23].
Table 2 summarizes potential effects of various heavy metals toxicity to human health and plant physiology. The pH of AMD is another toxic feature that further threatens plants. Highly acidic solutions induce release of more H+ ions that proliferates bioavailable metallic species. Surge in uptake of metal ions such as aluminum (Al3+) binds to cell membrane and inhibits rhizosphere function [24,25]. It has been suggested that fungal and bacterial activity within soils could encourage soil acidification and plant root dysfunction [26]. Thus, the mobility and permeability of AMD are affected by several factors including soil conditions, metal species, solubility of ions and related microorganisms [9].
Table 2.
Main effects of heavy metal on human health and plant physiology [9,[27], [28], [29], [30], [31], [32]].
| Heavy Metals | Main Effects |
|
|---|---|---|
| Potential repercussion on human health | Potential plant physiological responses | |
| Arsenic | Bronchitis, Skin and bladder cancer, Kidney failure, haemolysis, bone marrow depression | Growth inhibition, Loss of yield and fruit production, Food chain poisoning |
| Cadmium | Renal dysfunction, lung disease, lung cancer | Decreases seed germination and lipid content |
| Lead | Mental retardation in children, developmental delay | Reduces chlorophyll production and plant growth |
| Manganese | Damage of central nervous system | – |
| Mercury | Impaired neurodevelopment, decrease in memory | Decreases photosynthetic activity, water uptake and antioxidant |
| Nickel | Allergic contact dermatitis, chronic bronchitis, Lung and nasal cancer | Decreases seed germination, protein and enzyme production |
| Zinc | Damage to nervous membrane | Reduces Ni toxicity, promotes plant growth |
| Chromium | Liver/kidney necrosis, skin ulcers | Decreases plant growth through membrane damage, chlorosis and root damage |
| Copper | Anemia, liver and kidney damage | Inhibits photosynthesis and reproductive process |
2.3. Remediation options
Techniques for AMD treatment have been established through years of research into source control and mitigation approaches [33]. Source controls are technically much more demanding to implement as the working principle involves the removal of oxygen and water to eliminate oxidation process of pyritic materials. Also, most mining facilities ceased operation before the discovery of the hazards of AMD, and retrofitting is not always achievable [34]. Mitigation control is directed at treatment of drainage given proper evaluation of local land formation and water table levels. In general, the mitigation control approach is generally based on the neutralization of its pH and the precipitation of metals [35]. Two categories apply to mitigation techniques, namely active and passive treatment. However, advances in research have reclassified techniques into abiotic and biological treatment with sub-classes of active and passive systems.
Biological treatments offer several advantages which include permanent removal of sulfate and metals, generation of less-hazardous water and greater ability to recover the valuable metals. The goal of these treatments is accelerated conversion of the pollutants to an acceptable form or conditions in which they have least or null environmental impact through biological actions [36]. Such systems generally engage sulfate or sulfur reducing bacteria as main working agents, introduced into wetlands or injected as substrate barriers to control pH and metal leachate. The following describes more of these biological systems.
3. Sulfate reducing bacteria
3.1. Sulfate reduction and bacterial types
Sulfate reduction by specific anaerobic bacteria groups occur through either assimilatory or dissimilatory pathways [37]. Assimilatory reduction generates reduced sulfur compounds for biosynthesis of proteins and amino acids, dispelling any direct excretion of sulfide. Sulfate (or sulfur), undergoing dissimilatory pathway, is reduced by obligatory anaerobic sulfate (or sulfur) reducing bacteria (Eq. (8)).
| SO42− + 8e− + 4H2O → S2− + 8OH− | (8) |
Hydrogen sulfide (H2S), a highly toxic, corrosive and flammable gas with noticeable odor is evolved from sulfate reduction. This gas is found in varying concentrations in natural gas from oil reservoirs, biogas from renewable wastes, and pulp and paper industry exhausts. Due to its corrosive nature, removal of hydrogen sulfide prior to transportation and distribution is required for control of subsequent acidic deposition. There are a known few commercial physicochemical processes for treating sulfide-laden streams, which include Claus, Alkanolamine and Holmes-Stretford. Although effective for removal, these are highly energy-demanding options with costly chemicals involved. Additionally, physicochemical processes are designed to achieve process and economic feasibility, given that the treatment stream is high in volume and sulfide-rich.
Contrastingly, biological treatments are less costly to operate and do not require high concentration of sulfide in targeted stream to function. These treatments are conducted by the well-studied group of sulfate reducing bacteria (SRB). Phylogenetically, SRB exist on three major branches: (i) δ-subclass of proteobacteria, (ii) gram positive bacteria (Desulfotomaculum, Desulfosporosinus) and (iii) branches formed by Thermodesulfobacterium and Thermodesulfovibrio. The latter group is thermophilic while the former two groups consist of psychrophilic, mesophilic and thermophilic species.
Energy and carbon sources for SRB are of large variety, including hydrogen, formate, acetate, lactate, butyrate, malate, succinate, methanol, ethanol, glycerol and acetaldehyde. Amino acids, methylated compounds, aromatic and saturated hydrocarbons are among other compounds utilized by SRB. To reduce cost, inexpensive carbon sources such as hay, wood chips, manure, sludge and compost have also been used to sustain SRB growth within bioreactor. Integration of sulfate reduction process by SRB along with biosorption of metals producing a synergetic effect for AMD treatment has also been reported [38].
3.2. Metabolism of SRB for sulfate reduction
The complete metabolism of SRB is too complex as it involves many enzyme catalyzed bio-chemical reactions such as oxidation of alcohols, aldehydes and ketones, degradation of aliphatic and aromatic hydrocarbons, decomposition of arylnitro and arylamino compounds, demethylation reactions and reduction of sulfate and metals [39]. Sulfate is a thermodynamically stable form of sulfur making it unsuitable as a direct electron donor for the metabolism of SRB. The redox potential for the sulfate-sulfite copulation is −516 mV, which is quite high for the intracellular electron mediators nicotinamide adenine dinucleotide phosphate (NADH) or ferredoxin [40]. Sulfate reduction in SRB is initiated with the conversion sulfate to adenosine-phosphosulfate (APS) through adenosine phosphates. For this, sulfate is actively transported across the cytoplasmic membrane of SRB and is activated by adenosine monophosphate (AMP) and adenosine triphosphate (ATP) sulfurylase to yield APS and pyrophosphates. This reduces the redox potential of sulfate-sulfite copulation to −60 mV which is biochemically achievable [40]. The obtained APS is metabolically reduced to biogenic-sulfite and then to biogenic-sulfide by intracellular electron mediators. The necessary reactions for the sulfate reduction by SRB metabolism are presented in Eqs. (9), (10), (11)) [39].
| SO42− + AMP4− + H+ → APS2− + HP2O73− | (9) |
| APS2− + H+ + 2e2− → HSO3− + AMP2− | (10) |
| HSO3− + 6H+ + 6e2− → HS− + H2O (M2+ - Metal cation) | (11) |
The bio-genic sulfide thus obtained would undergo reaction with the metal cations present in the AMD stream to precipitate the metals and metalloids as metallic sulfides as illustrated in Eq. (3). Addition of carbon substrate becomes essential for the APS synthesis due to the endergonic nature of the reaction. Reduction of APS to biogenic-sulfite is facilitated by APS reductase [41]. Reduction of sulfite to sulfide can be explained through a two different mechanisms. The trithionate and thiosulfate pathway explains this reduction using three two-electron reduction steps while a direct six-electron reduction step is also quite inevitable.
3.3. SRB activity range
SRB are known to thrive in pH ranges of 5.0 to 9.0 and present reduced activity rates at other pH levels. However, some strains of SRB have been found to survive in low pH environments such as Desulfotomaculum genus. Furthermore, mixed cultured SRB are more tolerant of extreme conditions than their pure counterparts. Significant portions of SRB are strictly thermophiles that do not tolerate high temperature conditions. It has been reported that an increase in temperature of up to 40 °C led to decrease in bacterial activity [42]. Conversely, acclimatizing to low temperatures of 1–8 °C allowed SRB to assume normal activity rates [43].
Anaerobic degradation by SRB features several important drawbacks such as (a) Toxicity of hydrogen sulfide produced is major concern during metabolic process since an additional oxidizing stage is required for H2S to be converted to elemental sulfur; (b) Concentration of microorganism group in bioreactor due to formation of symbiotic relationships, for instance SRB forms microbiological consortia with metanogenic archaea; (c) Sensitivity towards changes in pH, temperature, oscillation of hydraulic and substrate loading; (d) Duration for complete mineralization of organic compounds is much longer than aerobic processes due to multistage biodegradation process.
Studies have suggested metal redox state reduction contributes to lower mobility and toxicity of contaminated streams [7,44]. Higher metal redox states allow precipitation of metallic sulfides from solution, facilitated by SRB communities. Fig. 2 illustrates sulfide speciation across the pH range. This coincides with selective formation of H2S by SRB in AMD treatment systems that are acidic by nature. Geochemically, sulfur and sulfate reducing bacteria hold major roles of precipitating toxic metals. For instance, the precipitation of U (VI) and Cr (VI) by a process mediated by multiheme cytochrome c proteins [8]. Section 5 of this review presents more information on selective precipitation.
Fig. 2.
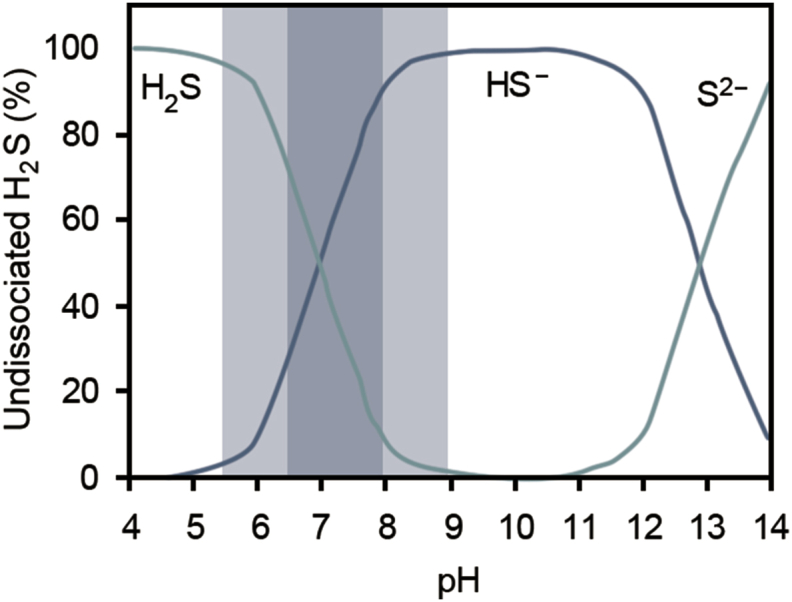
Sulfide speciation as a function of pH at 25 °C. Optimum ranges of SRB are shown in light and dark grey areas [47].
SRB metabolism leads to the precipitation of biogenic pyrite nanoparticles efficient at co-precipitating and sorbing arsenic [45,46]. The experimental field was an industrial site where shallow groundwater in an unrestricted sandy aquifer was contaminated by arsenic. Therefore, biodegradable organic carbon, sulfate, ferrous iron and fertilizer were injected into groundwater and SRB metabolism began about 1 week later. Specifically, molasses (27.2 kg), ferrous sulfate (2.5–5 kg), and agricultural-grade fertilizer (0.9) kg in 1,000 gallons of water were introduced into the sites. The main sequestration stage, with total arsenic removal rate of more than 90%, lasted for at least 6 months until the arrival and mixing of untreated groundwater from up-gradient. FeSO4 amendments increased the concentrations of Fe2+ and SO42− in the ground water from 0.47 mg/L and 16 mg/L (pre-injection groundwater concentrations) to 40 mg/L and 60 mg/L, respectively. Arsenic levels decreased from an initial level of 0.35 mg/L to a very low level of 0.01 mg/L which surpassed the cleanup goal of 0.05 mg/L. Treated groundwater with the most active bacterial sulfate reduction became enriched in heavy 34S (range from 2.02 to 4.00%) compared to unaffected well water (0.40–0.61%).
4. Passive biological treatment
Apart from neutralizing acidic tailings from mine drainage, passive biological treatment systems take advantage of naturally occurring geochemical processes and microbial activity for improving the quality of influent water. These systems have minimal operation and maintenance requirements, although local abiotic factors (oxygen, water and soil texture) may change throughout the application period and dictate the rate of reaction [48]. Key parameters such as pH, temperature, salinity, metal concentrations etc. play a crucial role in determining the effectiveness of these methods for AMD treatment [49].
Passive treatment based on SRB for groundwater applications include enhancement of beneath-ground microbial activity through concentrated substrate injection or permeable reactive barriers (Fig. 3A and B). For surface flow of AMD, infiltration beds, anoxic ponds and modified wetland systems are used (Fig. 3C, D and 3E).
Fig. 3.
Passive biological treatment applications for AMD affected ground and surface waters. a) Substrate injection; b) Reactive permeable barrier; c) Infiltration bed; d) anoxic pond; e) anaerobic wetland (adapted from Ref. [47,48]).
4.1. Organic substrate injection
One technique for in situ remediation is by injecting rich organic substrates into boreholes or mineshafts that penetrate to reach AMD occurring depths. These organic substrates could be ammonium phosphates or acetate-bearing compounds that provide energy for SRB underground [50]. Research has reported high removal efficiencies for Al, Cd, Co and Zn metals and pH increment of mine water flowing through organic substrate enriched area. However, such effects were counteracted during high flow rate seasons whereby precipitated metals are resolubilised [51].
4.2. Permeable reactive barriers
Permeable reactive barrier (PRB) is another in situ remediation technique that installs a reactive media perpendicular to the plume of contaminated waterbody which often requires accurate flow trajectory estimation [52]. Due to natural hydraulic gradient, AMD will passively migrate through the reactive barrier, undergoing neutralization and precipitation of metals. Substrates that can be embedded within a PRB are presented in Table 3. In addition, reactive barriers often rely on natural flow for transportation of AMD through designed treatment zones where processing time is significantly prolonged and less tractable. Depletion of substrates and clogging of precipitated minerals can affect system efficiency [53].
Table 3.
Reactive media and targeted contaminants.
| Reactive media | Contaminant | Reference |
|---|---|---|
| Zero valent iron | Copper, arsenic compounds, molybdenum | [54] |
| Activated carbons | Most heavy metals | [55] |
| Zeolites | Lead, copper | [56] |
| Lime (alkaline minerals) | Most heavy metals | [34] |
| Transformed red mud | Iron, copper, zinc, nickel and lead | [57] |
| Oxides | Arsenic compounds | [58] |
| Bio-barriers | Aerobic degradation – Calcium peroxide, magnesium peroxide and hydrogen peroxide | [59] |
| Anaerobic degradation – Peat, sewage sludge, manure, sawdust, SRB | [60] |
4.3. Infiltration beds
An extension of the concept of permeable barriers is the use of infiltration beds that target surface and shallow sub-surface of AMD to a depth of 50 cm. Trenches are filled with organic materials that promote proliferation and activity of SRB. An impermeable liner is used to cover reactive substrates, rendering a blanketing effect for development of anaerobic conditions [47]. Maintenance of constant hydraulic conductivity of the infiltration media and anti-clogging designs are essential for prevention of metal dissolution [48].
4.4. Anoxic ponds
Anoxic ponds may be used upstream of more sensitive treatment systems to reduce dissolved oxygen and ferric ions (Fe3+) a priori. Plastic lining placed beneath a gravel layer acts as a gas barrier while preventing leakage of metals and acidic streams. The system is designed to minimize atmospheric oxygen inputs while accumulating CO2 production. Generally, the effluent pH and high metal concentration will be improved through this technique [61].
4.5. Wetlands
Wetlands are the most common treatment system and have been recognized as a low-cost remediation solution for AMD and have been implemented at full-scale [62]. Wetlands are highly complex ecosystems due to interacting effects of physical, chemical and biological processes that influence output water quality. Broadly, aerobic and anaerobic wetlands are two types of treatment systems available. Aerobic wetlands targets net alkaline waters; whereas the anaerobic wetland involves submerging rich organic substrates, limestone and SRB inoculum for ameliorating acidic-metalliferous waters, allowing the reduction of iron and sulfate compounds to be achieved. The planting of vegetation on submerged substrate is an over-arching issue with uncertain effects. Surface vegetation is recommended as cover and energy for underlying microbial communities [47]. It has also been reported that surface plants adversely affect the performance of SRB [48]. Table 4 consolidates the main features from both perspectives. Notably, growth of dense surface vegetation should be avoided to reduce sublayer compaction and prevent oxidizing conditions. Buoyant species may be planted sparingly for erosion control. Apart from these issues, wetland treatment may not be desirable in arid or mid-arid climate regions. Surging and contracting water levels throughout different seasons could force metal-sulfide sediments to be re-oxidized, re-dissolved and re-acidify treatment areas [63].
Table 4.
Main aspects of presence or absence of surface vegetation.
| Surface vegetation present | No surface vegetation |
|---|---|
| Continuous carbon and energy supply provided to underlying microbial communities | Root penetration alters favorable anaerobic conditions |
| Protection against wind and rain erosion | No protection from erosion sources |
| Preferential flow (or by-passing) of untreated stream due to dense plant growth and wildlife burrowing | Reduces natural aesthetic and denies original ecosystem function |
| Compaction of substrate layers which reduces system performance | Loose layering of substrates which provides good conductivity and connectivity |
| Densely vegetated wetlands require maintenance to ensure proper functioning | No maintenance with regard to vegetation |
Wetlands are classified hydrologically as surface flow (SF), subsurface flow (SSF) and hybrid systems [64]. Most of the natural wetlands fall under the category of SF where the wastewater has a shallow flow over the substrate. In case of SSF, the AMD flows through the substrate either in a horizontal or vertical direction [65]. Oxygen deficiency in the horizontal flow SSF promotes the anaerobic bacterial decomposition and denitrification of the contaminants [66]. On the other hand, the intermittent bed draining caused by the longitudinal flow of wastewater in the vertical flow SSF results in better air circulation and enhances the oxygen transfer rate leading to substantial nitrification effects [67]. Hybrid variants of wetlands are aimed at efficient removal of contaminants, especially for nitrogen based pollutants [68]. Added substrates forms an indispensable part of the wetlands and plays a crucial role in the effectiveness of the contaminants removal. In addition to the support nature for the vegetation, the substrates also influence the biological reactions and the metabolic pathways for the contaminant reduction [67].
Long-term performance and operational sustainability are the major challenges faced by wetlands. While the substrate and plant species type influence the wetlands performance, operational parameters such as load, hydraulic retention time, flow mode, water depth and design seriously affects the sustainability of the wetlands [65,69]. In addition, a variety of biogenic pollutant removal processes such as plant uptake, adsorption, volatilization, precipitation, filtration, sedimentation etc. are greatly influenced by the in-situ and ex-situ environmental conditions of the wetlands [70,71]. All these factors in turn affect the contaminant removal efficiency and causes vigorous variation in the performance of the wetlands [72].
Despite its potential as being a cost-effective measure for AMD treatment, passive wetlands interfere with hydrogeological patterns [73], geochemical processes [74] and ecosystem function and diversity [75]. Following such disturbances, groundwater quality and movement must be monitored to ensure proper functioning of wetland and to ensure that no further harm is generated due to its implementation. Environmental tracers such as oxygen and hydrogen isotopes provide information of direction and velocity of water flow, allowing better understanding of local water cycling [76]. Usage of natural tracers of δ18O and δ2H for characterizing mine tailings have been reported [77]. These tracers have helped to lead to the conclusion that ion content is mainly driven by dissolution of rock minerals. However, the water chemical composition is controlled by the microorganisms present [78].
5. Active biological treatment
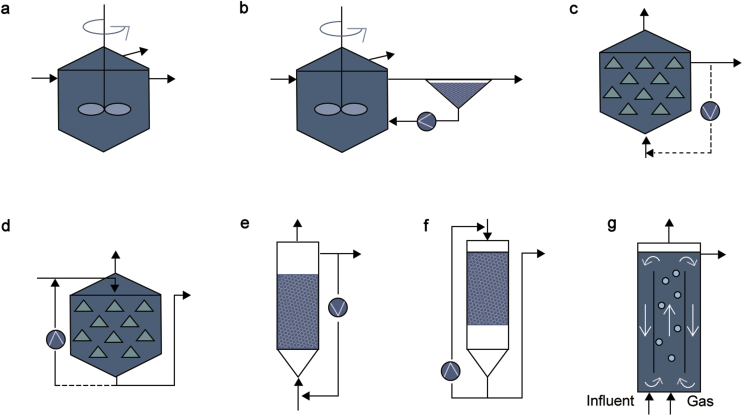
Extensive research into AMD active treatment with biological reactors has been conducted. Active bioreactors are markedly different from passive treatment systems as they do not operate in concert with the surrounding environment, creating an industrialized setting. Biological sulfate reduction has been studied using various reactor designs such as continuously stirred tank reactors, sequencing batch reactors, gas lift reactors, fluidized-bed reactors, anaerobic filters/packed bed reactors, anaerobic hybrid reactors, anaerobic sludge blanket reactors and membrane bioreactors [79]. The most significant bioreactor designs for biological sulfate reduction process have been reported in Fig. 4. Some of the notable variants of continuous flow reactors are (i) continuously stirred tank reactor (Fig. 4A), (ii) Anaerobic contact process reactor (Fig. 4B), (iii) Upflow anaerobic filter reactor (Fig. 4C), (iv) Downflow anaerobic filter reactor (Fig. 4D), (v) Fluidized bed reactor (Fig. 4E), (vi) Downflow fluidized bed reactor (Fig. 4F) and (vii) Gas lift reactor (Fig. 4G). Active bioreactors require installation of a plant with agitating reactors, precipitators and clarifiers. In addition, robust pumping systems with durable materials and precise piping networks are vital for operational feat. Capital and operating cost for such systems remain high for decades as AMD from mining facilities are continuously produced after decommissioning [48]. This inherent cost negates development of such systems on many sites, ergo redirecting interest in employing passive treatment options over bioreactors. However, constant advancement in microbial technology has gradually paved an economical path for active bioreactors to be implemented. Table 5 compares characteristics of these treatment methods.
Fig. 4.
Continuous flow reactors used in anaerobic water treatment – (a) Continuously stirred tank reactor; (b) Anaerobic contact process; (c) Upflow anaerobic filter reactor (UAFR); (d) Downflow anerobic filter reactor; (e) Fluidized bed reactor; (f) Downflow fluidzed bed reactor; (g) Gas-lift reactor (Extracted from Ref. [47]).
Table 5.
Categorical comparison of characteristics between passive and active biological treatments (adapted from Ref. [47]).
| Characteristics | Passive Treatment | Active Treatment |
|---|---|---|
| Cost of operation | Relatively Low ($0.3–$0.4/kg of metal removed)∗ | Relatively high (($0.7–$1/kg of metal removed)∗ |
| Labor requirement | Less | More |
| Area of Treatment | Large (0.1–2 m2/kg of metal removed)+ | Small (0.01–0.2 m2/kg of metal removed)+ |
| Recovery of metals | Difficult | Easy |
| System control | Poor | Good |
| Effluent predictability | Poor | Good |
∗ cost typically accounts for capital and operational expenses. Maintenance cost for active treatment is 5–10 times higher than the passive treatment.
∗,+ Actual values can vary depending on the geography, AMD volume, specific composition of the AMD and its pollutant(s), climatic conditions and other influencing factors.
Sulfate reduction processes utilize sulfate in AMD solution as an electron acceptor during anaerobic digestion of organic matter initiated by SRB. Studies that engage bioreactors for sulfate reduction treatment have realized that a low breakthrough capacity limits solution sulfate loadings to around 2.0 kg SO42−/m3 [80]. Achievable loading rates of this process are dependent on biomass retention in the reactor and illustrates the needs for process design for consistent, long-term performance. Fig. 4 depicts types of bioreactor process configuration for AMD treatment. Sulfate reduction (SR) reaction and metal precipitation (MP) reaction are two main steps of biogenic H2S-based process ventured by SRB.
5.1. Single-stage processes
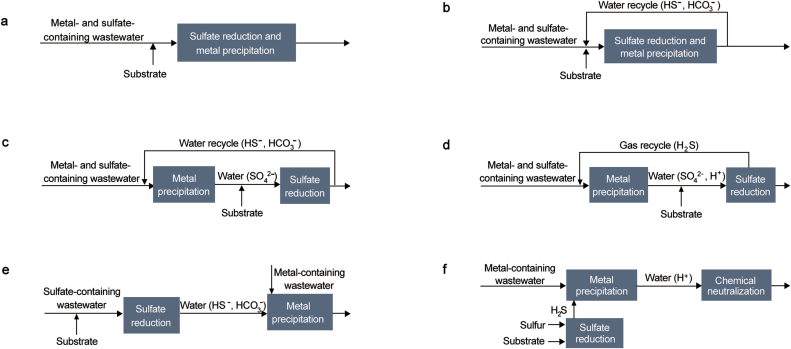
Biological sulfate reduction and metal precipitation using biogenic H2S can be operated in a single stage approach (Fig. 5A). Generally, an anaerobic filter reactor (ARB) or up flow aerobic sludge-blanket reactor (UASB) can be used for AMD treatment. Pre-filtration of suspended solids may be required to protect filter or sludge contents. Alkalinity is generated by SRB through production of H2S, which in turn enhances precipitation of metals. Excess sulfide is carried to a separate oxidizing reactor to yield elemental sulfur [81]. Although single-stage processes are relatively inexpensive, highly acidic or concentrated streams may not be appropriately treated with their use. Alkaline materials are doped in organic substrates to maintain performance. Furthermore, recycling of treated effluent can be used to dilute inlet streams (Fig. 5B), albeit at the cost of extra piping and pumping.
Fig. 5.
Plausible reactor configurations for active biological treatment of AM.
5.2. Metal precipitation before sulfate reduction
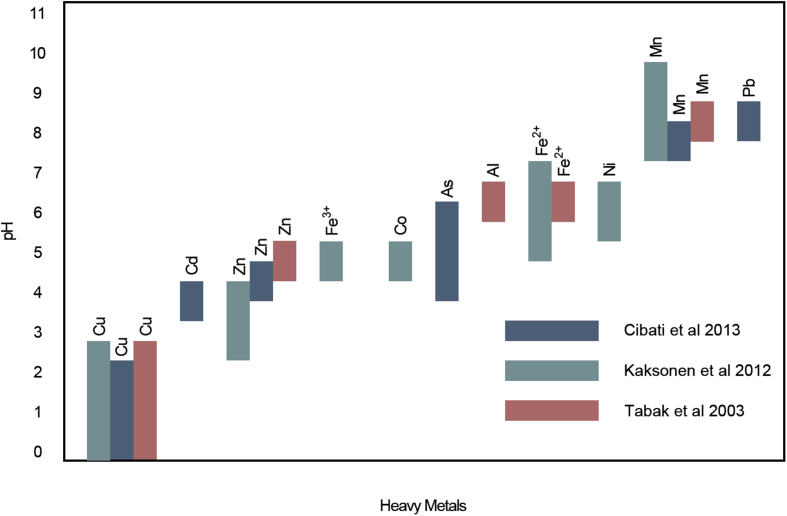
Chemically precipitating metals from AMD streams can be instituted by having separate (in series) reactors as shown in Fig. 5C. Sulfide-containing effluent from biological sulfate reduction reactor can be recycled upstream for two purposes. Firstly, SRB in downstream bioreactor is protected from concentrated sulfide waters as recycled streams are not introduced directly. High dissolved sulfide content is likely toxic to SRB, thus, hindering microbial activity as seen in single-stage processes. Secondly, significant pH drop (pH < 3) introduced by recycling streams selectively precipitates certain metal species (such as copper and zinc) within MP reactor (Fig. 6). Alternatively, biogenic H2S formed may be reintroduced to MP reactor (Fig. 5D) for low pH selective precipitation. Carbonate materials or bioreactor effluent may be incorporated with the highly acidic H2S stream to stipulate for specific metal precipitation.
Fig. 6.
Recommended pH ranges for selective precipitation of metals as sulfides or hydroxides (consolidated from Ref. [63,85,86]).
Different type of mechanisms explains the complexation and precipitation of the metal sulfides in the sulfate reduction reactor. In practice, the co-precipitation of several other products present in the AMD such as micronutrients, phosphates, complexing agents and other polymeric substances makes the theory of metal precipitation difficult for interpretation [82]. The most widely accepted mechanism for metal precipitation is the sorption mechanism which is explained through ion exchange on the biofilm surface or surface precipitation technique. Sorption mechanism involves the formation of strong ligand complexes through surface –OH groups on bacterially produced metal sulfides. Hydration of these bacterially produced metal sulfides produces surface hydroxyl groups which complexes itself as a ligand using the metal and results in the inner-sphere metal complexes formation which settles down. Metal precipitation in a sulfate reducing bioreactor happens with the aid of several functional groups such as carbonates, hydroxides and phosphates present in the AMD [83]. These functional groups are also produced due to the metabolism of the microbial agent of the reactor. Metal sulfide separation through precipitation technique is well pronounced in passive treatment systems of AMD with the pH of the system playing a critical role on the precipitation technique.
5.3. Combined treatment of multiple streams
Fig. 5E presents configuration of metal and sulfate removal from different fractions of water. Bioreactor liquid effluent that is sulfide-rich and relatively less acidic is applied to MP reactor for precipitation reaction and elemental sulfur production. Conversely, sulfate-containing effluent is recycled to sulfate reduction reactor for continuous input supply [84].
5.4. External H2S source
In rare cases where treatment of metal contaminated waters do not contain sulfate compounds; sulfur reducing bacteria is used to reduce elemental sulfur as an alternate electron acceptor. This external supply of H2S from sulfur reduction is done on an offline bioreactor, where SRB generated H2S gas is directed into metal precipitation units on main process line (Fig. 5F). Since biogenic alkalinity is absent from treatment system, pH adjustment of water may also be required after metal recovery.
In addition, potential toxicity of SRB caused by biogenic sulfide is diminished. Selective precipitation of metals is still possible by controlling pH of solution through H2S dosing. This configuration escalates investment and operational costs by adding more complex units to process line. However, biomass accumulation in metal sulfide sludge is comparatively reduced, simplifying downstream processes altogether [87].
There are limitations of active bioreactor treatments that still require further scientific development prior to full-scale industrial application. Process effectiveness for sulfate removal is well-recorded [88,89], but residual effluent chemical organic demand (COD) is high due to acetate concentration generated from SRB. Reactor residence time is limited by sulfate reduction (rate limiting reaction) which may take up to 24 h [90]. For most AMD treatments, preliminary pH increment is required to protect influent receiving SRB community against acidity; thereby, further adding to total cost involved.
6. Current trends
Progressing from well-established SRB treatment systems, AMD mitigation options have moved towards decreasing reliance on SRB community itself, emphasizing more on usage of second-generation raw materials from other industrial processes. Such developments suppress inflating costs needed for conventional methods and recapitalize on minimizing industrial waste products.
In light of expanding the applicable areas of treatment system, research efforts have targeted the alleviation of sulfate-laden or metal-contaminated streams that are not specifically derived from AMD sites and tailings. Technologies presented in Table 6 are efforts to hedge against increasing environmental pollution and harm.
Table 6.
Current trends for AMD treatment.
| Technology | Objective | Base Materials | Main Features |
|---|---|---|---|
| Fresh Water microalgae [91] | Clean up and biofuel production | AMD as flocculating agent |
|
| Organic carbon amendment [92] | Clean up | Primary treated AMD and rice bran |
|
| Metallurgical slags [88] | Clean up | Metallurgical slags |
|
| Permeable reactive kiddle [93] | Clean up | Permeable reactive kiddle |
|
| Bioelectrochemical process [94] | Clean up | AMD sludge |
|
The core sustainability dynamics and environmental footprint of active and passive treatments should be assessed in a systematic and quantitative manner. Life Cycle Assessment (LCA) of current AMD mitigation technologies have been conducted [95]. Active treatments, in general, demonstrated higher LCA impacts than of passive treatments; lime slaking resulted in overall increase of footprint regardless of treatment type. Material transportation was also noted to be a significant contributing factor for environmental and social impacts.
Anoxic limestone drains (ALDs) treat the water filled with acidic and potential metals, sending them through an underground passage that is filled with crushed limestone. ALDs typically enter a settled pond or wetland to provide an opportunity for metals to precipitate and settle [96]. The problem with ALDs is that they often experience arming - described by adhesion under stagnant conditions-that leads to limestone accumulation and possibly acts as a drain cover [96,97]. To effectively establish ALD, many suggest that dissolved oxygen, Fe3+, and Al3+ concentrations be less than 1 mg/L; Some authors have suggested that Fe3+ and Al3+ concentrations may be higher between 1 and 5 mg/L. In any case, this is a very low value when dealing with the drainage of the mine. Attempts using natural alkaline material instead of limestone have also been reported [98].
6.1. Bioreactors
Inactive bioreactors occur in lined trenches or pits that may contain a wide variety of materials, most commonly a mixture of cobbles, compost, other organic materials and/or an alkaline agent. Sometimes ground tanks and other strainer filter type materials with any type of material mentioned above are used in the bio-treatment of municipal wastewaters to precipitate metals and establish suitable microorganisms to adjust the pH, have been used as “bioreactors”. Tank types of bioreactors are not discussed in this paper, although they have been used to treat acid mine drainage. They are both valid in using the term “bioreactor”, as they are using biological reactions for the treatment of water. The term “bioreactor” would also include permeable reactive barriers, successive alkalinity producing aystems (discussed below), and Wetlands but here we are referring specifically to the bioreactors referenced here.
6.2. Successive Alkalinity Producing Systems
Successive alkalinity production systems (SAPS) have the following basic elements: organic wetting layer, limestone layer, and a drainage system - must also include a flushing system. This technique was created by Kepler and McClary [61]. The idea is that the mine cell forms a top layer of water that prevents oxygen infiltration into the bottom layers (water is also used in tails holding dams in this way). The organic layer serves to remove dissolved oxygen from the water, supporting the establishment of sulfate reducing bacteria away from anaerobic conditions. The anaerobic environment is a reducing environment that changes Fe3+ to Fe2+ thereby reducing the possibility of iron hydroxide precipitation (Eq. (6)). Since these units would encourage reducing conditions and the establishment of SRB as a major contributor to water treatment, these units are sometimes referred to as RAPS - Reducing and Alkalinity Producing Systems [99]. Finally, water enters the limestone zone, essentially devoid of oxygen to block the limestone. Water leaving the SAPS is usually directed to an aerobic settling pond or wetland to allow metals to precipitate and spark further water [100].
6.3. Permeable reactive barriers
Permeable reactive barriers (PRBs) are barriers that react with specific chemicals of concern that are placed in the path of groundwater flow that allows water to flow easily [101]. PRBs designed to treat acid mine drainage (AMD) with metal contamination typically consist of solid organic materials, such as municipal compost, leaf manure and wood chips/sawdust [102]. Organic matter encourages the proliferation of sulfate reducing bacteria that will reduce sulfate. Research has been conducted to evaluate the efficiency of using PRBs to remove uranium contamination at abandoned mine sites including other possible reactive materials such as zero-valent iron, bone char phosphate, and amorphous ferric oxyhydroxide [103].
6.4. Bio-solids
Bio-solids are treated municipal sewage sludge. In accordance to Environmental Protection Agency (EPA), bio-solids are defined as nutrient-rich organic matter resulting from the treatment of sewage sludge. These bio solids can be safely recycled and be used as fertilizer to stimulate growth in plants and improve the soil quality. The application of bio-solids does not reduce the number of metals present in the soil. The sites treated with bio-solids do not experience a decrease in total metals, but rather the availability of metals is reduced [104]. Metals are stabilized by carbonates, phosphates, sulfides, silicates, and organic materials through melting and precipitation in the form of hydroxides [105]. In some cases vegetation may be responsible for stabilizing metals, or may also remove metals from the soil, also known as phytoextraction.
6.5. Phytoremediation
Phytoremediation suggests the in situ use of plants for the treatment or removal of contamination. The technique assures to provide sustainable solution for the AMD treatment along with the biofuel production scope [106]. The method includes the usage of green plants and their associated microbiota, soil amendment, and agronomic techniques for AMD treatment. Phytoremediation mainly involves two main mechanisms: phytoextraction and phytostabilization. In case of phytoextraction, the plants uptake the contaminant metals present in the soil through their metabolism and accumulate them as a part of their biomass. On the other hand, phytostabilization involves the immobilization of the metals within the plant rhizosphere and thus reducing the metal availability [107]. Phytoremediation differs from constructed wetlands from the virtue of their in situ nature (direct use on the site) to contain, degrade or remove the contaminants while the later passive methodology is an artificially engineered technique [62]. Also, this advanced technique depends on the natural ability of the plants (hyperaccumulators) to bio-accumulate or immobilize the metal contaminants unlike wetlands which depends on the functions of soil, vegetation and organisms to treat AMD. Additionally, phytoremediation does not rely on any substrate while the wetlands performance for AMD treatment are highly determined by these supplementary substrates [108]. The process is beneficial if the contaminants of concern will be converted into less-toxic forms and for the complete elimination of the contaminants from the infested soil. Disadvantages are uncertainty about metabolites, accumulation of unhealthy plants, and uncertainty about other components at the site, namely, where there is a form of contamination there may be many more and one must understand how they react with the vegetation and the soil.
6.6. Membrane technology
Membrane technology is seen as a most recent alternative for AMD treatment due to very low chemical consumption, less sludge formation and small scale of the operations [109]. One of the important membrane technologies for metals and sulfate rejection are conventional reverse osmosis (RO) and nano-filtration (NF). In most cases of treatment of monovalents ions, a nano-filtration membrane is adopted for the waste water treatment. A better rejection efficiency of 93% to 98% is observed when NF membranes were applied for salts separation. If higher rejection of monovalents needs to be attained, a RO membrane is usually employed to produce a rejection efficiency as high as 99%. Studies have showed that a sulfate content as low as 10 mg/L has been achieved by membrane based treatment, either by single or two stage filtration [110]. Recently, membrane distillation based approach for treatment of AMD has also been reported [111]. Also, the pH, feed concentration, permeate flux and temperature determine the salt and metal rejection efficiency of the membrane based AMD treatment.
6.7. Engineered SRB consortium
Engineered microbial consortia is a promising methodology to enhance metal and sulfate elimination in industrial bioreactors. These microbial conglomeration paves way to culture most of the microbes present in the AMD streams with a chance to understand their characteristics and functionalities. This kind of engineered microbial consortia technique plays an important role in accelerated bioremediation of acidic drains susceptible to environmental changes and streams formed in hostile environments [112]. This biomass exhibits symbiotic nature which results in the syntrophic decomposition of complex substances present in the acidic streams. Simultaneous removal of sulfates and salts at rapid rate can be obtained through selective combination of the microbial species. These kind of consortia provides vital genetic information to create modified microbiomes that can separate metals and sulfates more effectively with a resistance mechanism that enhances the remediation [113]. They can also result in increased microbial diversity even in low pH nature of the incipient soils [114]. Additionally, metal additives (especially iron and iron oxide) included SRB systems proves to be more efficient for AMD treatment owing to the bacterial activity enhancement by these incorporated metallic compounds [115].
6.8. Algae based bioremediation
Bioremediation using algae strains is a recent and attractive biological method of AMD treatment owing to its cost effectiveness and high efficiency of metal and sulfates removal. Various algal strains such as Anabaena, Chlamydomonas, Chlorella, Cladophora, Oscillatoria, Phaeodactylum, Scenedesmus, Spirulina sp., etc. have been studied for the bioremediation of acidic rich streams [116,117]. These algal strains acts as “hyper-sorbents” and “hyper-accumulators” with a high degree of selectivity for various metals and elements. Also, the metabolism of the algal biomass produces high alkalinity neutralizing the acidic nature of the drain stream and also aiding the easy precipitation of metals. However the variation of pH, oxygen concentration and temperature of the acidic streams greatly influence the treatment effectiveness of this method because of which the algae based bioremediation is always used in conjunction with other treatment techniques. Recent studies on usage of macroalgaes as potential bioindicators for the pollutant detection and dispersal have been reported [118].
7. Future perspective and research potential
The fact that there is no single reliable method for AMD treatment has triggered researchers all over the globe to develop effective and efficient techniques for handling the acidic mine effluents. The primary step to AMD treatment is to avoid the generation of these acidic effluents to the possible extent. In particular, steps have to be taken to avoid surface water, underground water and rain water coming in contact with these sites. Prevention of AMD from contaminating the potential water sources for domestic and industrial purposes could be seen as a secondary measure of control while the treatment and management systems from the tertiary strategy of abating the problems caused by acidic drains. Recent researches on AMD treatment shows that biological treatment processes are very promising as these techniques avoid the problems of huge operational costs and sludge disposal associated with chemical treatment methods.
Bioremediation techniques are quite easy and feasible for implementation for a wide range of acidic effluents with varying concentrations and other aqueous parameters. While SRB based biological techniques have shown commercial potential, limitations of these processes such as low biomass concentrations, product inhibition by unionized biogenic-H2S, substrate transfer limitations when H2 is the electron donor, and low gas production causing insufficient mixing has prompted research on advanced biological systems for acidic mine effluent treatments [119]. Recent research using permeable reactive barriers, engineered SRB consortium and algae based bioremediation have resulted in new directions and scope for the biological processes of AMD treatment. These techniques have also facilitated in the better understanding of microbial biomass characteristics and their metabolic interactions with the various constituents of the acidic streams (based on reactions described in Eqs. (4), (5), (6), (7), (8))). Future research scope lies in development of predictive models for mapping the microbial characteristics with the chemical composition of mining sites and development of mineral specific AMD treatment systems. Also, the effective recovery of the metal from the bio-sorbed or precipitated metal sulfides is additional research which has to be explored with the application of modern biological methodologies. Development and analysis of the characteristics and metabolic interactions along with the metal and sulfates reduction mechanisms of newer microbial eco-systems comprising of heterogeneous biomass is also an added venue of research for the bioremediation of AMD.
8. Conclusions
Active and passive biological remediation techniques devised for controlling AMD have been widely developed to constrain the deleterious effects. Major factors involved are cost for transportation of liming materials and modification chemicals; available land size and topography; sludge disposal or waste streams generation if ill-managed; and maintenance and labor costs. Critically, factors listed above should be evaluated as a function of one another and not singled out for individual assessment. As seen from current evolving trends, optimization of high efficiency bioreactors requires much less area which allows reduction of land requirements. Focus should be more on enhancing overall process design that takes life cycle assessment into account. Additionally, AMD remediation may be perceived as capitalizing on generation of renewable raw materials as metal recovery from bio treatment systems may provide a good return of revenue.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Fundamental Research Grant Scheme, Malaysia [FRGS/1/2019/STG05/UNIM/02/2].
References
- 1.Kefeni K.K., Msagati T.A.M., Mamba B.B. Acid mine drainage: prevention, treatment options, and resource recovery: a review. J. Clean. Prod. 2017 doi: 10.1016/j.jclepro.2017.03.082. [DOI] [Google Scholar]
- 2.Johnson D.B. Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines. Water Air Soil Pollut. Focus. 2003 doi: 10.1023/A:1022107520836. [DOI] [Google Scholar]
- 3.Johnson D.B., Hallberg K.B. Acid mine drainage remediation options: a review. Sci. Total Environ. 2005 doi: 10.1016/j.scitotenv.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Simate G.S., Ndlovu S. Acid mine drainage: challenges and opportunities. J. Environ. Chem. Eng. 2014 doi: 10.1016/j.jece.2014.07.021. [DOI] [Google Scholar]
- 5.Ngure V., Davies T., Kinuthia G., Sitati N., Shisia S., Oyoo-Okoth E. Concentration levels of potentially harmful elements from gold mining in Lake Victoria Region, Kenya: environmental and health implications. J. Geochem. Explor. 2014 doi: 10.1016/j.gexplo.2014.04.004. [DOI] [Google Scholar]
- 6.Mulopo J. Continuous pilot scale assessment of the alkaline barium calcium desalination process for acid mine drainage treatment. J. Environ. Chem. Eng. 2015 doi: 10.1016/j.jece.2014.12.001. [DOI] [Google Scholar]
- 7.Finneran K.T., Housewright M.E., Lovley D.R. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 2002 doi: 10.1046/j.1462-2920.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- 8.Gadd G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma. 2004 doi: 10.1016/j.geoderma.2004.01.002. [DOI] [Google Scholar]
- 9.Gardea-Torresdey J.L., Peralta-Videa J.R., De La Rosa G., Parsons J.G. Phytoremediation of heavy metals and study of the metal coordination by X-ray absorption spectroscopy. Coord. Chem. Rev. 2005 doi: 10.1016/j.ccr.2005.01.001. [DOI] [Google Scholar]
- 10.McCullough C.D., Lund M.A. Bioremediation of Acidic and Metalliferous Drainage (AMD) through organic carbon amendment by municipal sewage and green waste. J. Environ. Manag. 2011 doi: 10.1016/j.jenvman.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Jamil I.N., Clarke W.P. Bioremediation for acid mine drainage: organic solid waste as carbon sources for sulfate-reducing bacteria: a review. J. Mech. Eng. Sci. 2013 doi: 10.15282/jmes.5.2013.3.0054. [DOI] [Google Scholar]
- 12.Fernandez-Rojo L., Héry M., Le Pape P., Braungardt C., Desoeuvre A., Torres E., Tardy V., Resongles E., Laroche E., Delpoux S., Joulian C., Battaglia-Brunet F., Boisson J., Grapin G., Morin G., Casiot C. Biological attenuation of arsenic and iron in a continuous flow bioreactor treating acid mine drainage (AMD) Water Res. 2017 doi: 10.1016/j.watres.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 13.Luptakova A., Kusnierova M. Bioremediation of acid mine drainage contaminated by SRB. Hydrometallurgy. 2005 doi: 10.1016/j.hydromet.2004.10.019. [DOI] [Google Scholar]
- 14.Santos A.L., Johnson D.B. Design and application of a low pH upflow biofilm sulfidogenic bioreactor for recovering transition metals from synthetic waste water at a Brazilian copper mine. Front. Microbiol. 2018 doi: 10.3389/fmicb.2018.02051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nancucheo I., Bitencourt J.A.P., Sahoo P.K., Alves J.O., Siqueira J.O., Oliveira G. Recent developments for remediating acidic mine waters using sulfidogenic bacteria. BioMed Res. Int. 2017 doi: 10.1155/2017/7256582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopi Kiran M., Pakshirajan K., Das G. An overview of sulfidogenic biological reactors for the simultaneous treatment of sulfate and heavy metal rich wastewater. Chem. Eng. Sci. 2017 doi: 10.1016/j.ces.2016.11.002. [DOI] [Google Scholar]
- 17.Gontia P., Janssen M. Life cycle assessment of bio-based sodium polyacrylate production from pulp mill side streams: case study of thermo-mechanical and sulfite pulp mills. J. Clean. Prod. 2016 doi: 10.1016/j.jclepro.2016.04.155. [DOI] [Google Scholar]
- 18.Vaiopoulou E., Provijn T., Prévoteau A., Pikaar I., Rabaey K. Electrochemical sulfide removal and caustic recovery from spent caustic streams. Water Res. 2016 doi: 10.1016/j.watres.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 19.Verburg R., Bezuidenhout N., Chatwin T., Ferguson K. The global acid rock drainage guide (GARD Guide) Mine Water Environ. 2009 doi: 10.1007/s10230-009-0078-4. [DOI] [Google Scholar]
- 20.Lapakko K. 2002. Metal Mine Rock and Waste Characterization Tools: an Overview. [Google Scholar]
- 21.Qiu G., Luo Y., Chen C., Lv Q., Tan W., Liu F., Liu C. Influence factors for the oxidation of pyrite by oxygen and birnessite in aqueous systems. J. Environ. Sci. (China) 2015 doi: 10.1016/j.jes.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Kocaman A.T., Cemek M., Edwards K.J. Kinetics of pyrite, pyrrhotite, and chalcopyrite dissolution by Acidithiobacillus ferrooxidans. Can. J. Microbiol. 2016 doi: 10.1139/cjm-2016-0085. [DOI] [PubMed] [Google Scholar]
- 23.Ambiado K., Bustos C., Schwarz A., Bórquez R. Membrane technology applied to acid mine drainage from copper mining. Water Sci. Technol. 2017 doi: 10.2166/wst.2016.556. [DOI] [PubMed] [Google Scholar]
- 24.Ma J.F., Ryan P.R., Delhaize E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001 doi: 10.1016/S1360-1385(01)01961-6. [DOI] [PubMed] [Google Scholar]
- 25.Fernández F., Hoeft R. Agron. Handbook. 24th Ed. Univ; 2009. Managing soil pH and crop nutrients. [Google Scholar]
- 26.Rousk J., Brookes P.C., Bååth E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009 doi: 10.1128/AEM.02775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon F. Impacts of copper on aquatic ecosystems and human health. Mining. Com. Mag. 2009 [Google Scholar]
- 28.Yadav S.K. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afr. J. Bot. 2010 doi: 10.1016/j.sajb.2009.10.007. [DOI] [Google Scholar]
- 29.Yang H., Wang P., Peng Q., Rong R., Liu C., Lereclus D., Zhang J., Song F., Huang D. Weak transcription of the cry1ac gene in nonsporulating bacillus thuringiensis cells. Appl. Environ. Microbiol. 2012 doi: 10.1128/AEM.01229-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng H., Hu Y., Luo J., Xu B., Zhao J. Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J. Hazard Mater. 2009 doi: 10.1016/j.jhazmat.2008.10.070. [DOI] [PubMed] [Google Scholar]
- 31.Finnegan P.M., Chen W. Arsenic toxicity: the effects on plant metabolism. Front. Physiol. 2012 doi: 10.3389/fphys.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaumlöffel D. Nickel species: analysis and toxic effects. J. Trace Elem. Med. Biol. 2012 doi: 10.1016/j.jtemb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Akcil A., Koldas S. Acid Mine Drainage (AMD): causes, treatment and case studies. J. Clean. Prod. 2006 doi: 10.1016/j.jclepro.2004.09.006. [DOI] [Google Scholar]
- 34.Skousen J.G., Ziemkiewicz P.F., McDonald L.M. Acid mine drainage formation, control and treatment: approaches and strategies. Extr. Ind. Soc. 2019 doi: 10.1016/j.exis.2018.09.008. [DOI] [Google Scholar]
- 35.Yilmaz T., Yucel A., Cakmak Y., Uyanik S., Yurtsever A., Ucar D. Treatment of acidic mine drainage in up-flow sulfidogenic reactor: metal recovery and the pH neutralization. J. Water Process Eng. 2019 doi: 10.1016/j.jwpe.2019.100916. [DOI] [Google Scholar]
- 36.Villegas-Plazas M., Sanabria J., Junca H. A composite taxonomical and functional framework of microbiomes under acid mine drainage bioremediation systems. J. Environ. Manag. 2019 doi: 10.1016/j.jenvman.2019.109581. [DOI] [PubMed] [Google Scholar]
- 37.Tang K., Baskaran V., Nemati M. Bacteria of the sulfur cycle: an overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochem. Eng. J. 2009 doi: 10.1016/j.bej.2008.12.011. [DOI] [Google Scholar]
- 38.Hurtado C., Viedma P., Cotoras D. Design of a bioprocess for metal and sulfate removal from acid mine drainage. Hydrometallurgy. 2018 doi: 10.1016/j.hydromet.2018.07.006. [DOI] [Google Scholar]
- 39.Li X., ming Lan S., ping Zhu Z., Zhang C., ming Zeng G., guo Liu Y., cheng Cao W., Song B., Yang H., fan Wang S., hua Wu S. The bioenergetics mechanisms and applications of sulfate-reducing bacteria in remediation of pollutants in drainage: a review. Ecotoxicol. Environ. Saf. 2018 doi: 10.1016/j.ecoenv.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Muyzer G., Stams A.J.M. The ecology and biotechnology of sulfate-reducing bacteria. Nat. Rev. Microbiol. 2008 doi: 10.1038/nrmicro1892. [DOI] [PubMed] [Google Scholar]
- 41.Broco M., Rousset M., Oliveira S., Rodrigues-Pousada C. Deletion of flavoredoxin gene in Desulfovibrio gigas reveals its participation in thiosulfate reduction. FEBS Lett. 2005 doi: 10.1016/j.febslet.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 42.Moosa S., Nemati M., Harrison S.T.L. A kinetic study on anaerobic reduction of sulfate, part II: incorporation of temperature effects in the kinetic model. Chem. Eng. Sci. 2005 doi: 10.1016/j.ces.2004.11.036. [DOI] [Google Scholar]
- 43.Kuyucak N., Chabot F., Martschuk J. 7th Int. Conf. Acid Rock Drain. 2006, ICARD - Also Serves as 23rd Annu. Meet. Am. Soc. Min. Reclam. 2006. Successful implementation and operation of a passive treatment system in an extremely cold climate, Northern Quebec, Canada. [DOI] [Google Scholar]
- 44.Lovley D.R. Anaerobes to the rescue. Science. 2001;80 doi: 10.1126/science.1063294. [DOI] [PubMed] [Google Scholar]
- 45.Lee M.K., Saunders J.A., Wilson T., Levitt E., Saffari Ghandehari S., Dhakal P., Redwine J., Marks J., Billor Z.M., Miller B., Han D., Wang L. Field-scale bioremediation of arsenic-contaminated groundwater using sulfate-reducing bacteria and biogenic pyrite. Bioremediat. J. 2019 doi: 10.1080/10889868.2018.1516617. [DOI] [Google Scholar]
- 46.Alam R., McPhedran K. Applications of biological sulfate reduction for remediation of arsenic – a review. Chemosphere. 2019 doi: 10.1016/j.chemosphere.2019.01.194. [DOI] [Google Scholar]
- 47.Kaksonen A.H., Puhakka J.A. Sulfate reduction based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Eng. Life Sci. 2007 doi: 10.1002/elsc.200720216. [DOI] [Google Scholar]
- 48.Gazea B., Adam K., Kontopoulos A. A review of passive systems for the treatment of acid mine drainage. Miner. Eng. 1996 doi: 10.1016/0892-6875(95)00129-8. [DOI] [Google Scholar]
- 49.Ben Ali H.E., Neculita C.M., Molson J.W., Maqsoud A., Zagury G.J. Efficiency of batch biochemical reactors for mine drainage treatment at low temperature and high salinity. Appl. Geochem. 2019 doi: 10.1016/j.apgeochem.2019.01.014. [DOI] [Google Scholar]
- 50.Jeen S.W., Mattson B. Evaluation of layered and mixed passive treatment systems for acid mine drainage. Environ. Technol. 2016 doi: 10.1080/09593330.2016.1167249. [DOI] [PubMed] [Google Scholar]
- 51.Canty M., Hiebert R., Harrington-Baker M.A., Bless D. Int. Contain. Remediat. Technol. Conf. Exhib. 2001. Innovative, in situ use of sulfate reducing bacteria to remove heavy metals from acid mine drainage. [Google Scholar]
- 52.Obiri-Nyarko F., Grajales-Mesa S.J., Malina G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere. 2014 doi: 10.1016/j.chemosphere.2014.03.112. [DOI] [PubMed] [Google Scholar]
- 53.Skinner S.J.W., Schutte C.F. The feasibility of a permeable reactive barrier to treat acidic sulfate- and nitrate-contaminated groundwater. WaterSA. 2006 doi: 10.4314/wsa.v32i2.5253. [DOI] [Google Scholar]
- 54.Wilkin R.T., Puls R.W., Sewell G.W. Long-term performance of permeable reactive barriers using zero-valent iron: an evaluation at two sites. USEPA Environ. Res. Br. 2000 [Google Scholar]
- 55.Thiruvenkatachari R., Vigneswaran S., Naidu R. Permeable reactive barrier for groundwater remediation. J. Ind. Eng. Chem. 2008 doi: 10.1016/j.jiec.2007.10.001. [DOI] [Google Scholar]
- 56.Qi G., Lei X., Li L., Sun Y., Yuan C., Wang B., Yin L., Xu H., Wang Y. Coal fly ash-derived mesoporous calcium-silicate material (MCSM) for the efficient removal of Cd(II), Cr(III), Ni(II) and Pb(II) from acidic solutions. Procedia Environ. Sci. 2016 doi: 10.1016/j.proenv.2016.02.088. [DOI] [Google Scholar]
- 57.Lapointe F., Fytas K., McConchie D. Efficiency of BauxsolTM in permeable reactive barriers to treat acid rock drainage. Mine Water Environ. 2006 doi: 10.1007/s10230-006-0106-6. [DOI] [Google Scholar]
- 58.Giménez J., Martínez M., de Pablo J., Rovira M., Duro L. Arsenic sorption onto natural hematite, magnetite, and goethite. J. Hazard Mater. 2007 doi: 10.1016/j.jhazmat.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 59.Scherer M.M., Richter S., Valentine R.L., Alvarez P.J.J. Chemistry and microbiology of permeable reactive barriers for in situ groundwater clean up. Crit. Rev. Microbiol. 2000 doi: 10.1080/10408410091154237. [DOI] [PubMed] [Google Scholar]
- 60.Schipper L.A., McGill A. Nitrogen transformation in a denitrification layer irrigated with dairy factory effluent. Water Res. 2008 doi: 10.1016/j.watres.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 61.Skousen J., Zipper C.E., Rose A., Ziemkiewicz P.F., Nairn R., McDonald L.M., Kleinmann R.L. Review of passive systems for acid mine drainage treatment. Mine Water Environ. 2017 doi: 10.1007/s10230-016-0417-1. [DOI] [Google Scholar]
- 62.RoyChowdhury A., Sarkar D., Datta R. Remediation of acid mine drainage-impacted water. Curr. Pollut. Reports. 2015 doi: 10.1007/s40726-015-0011-3. [DOI] [Google Scholar]
- 63.Kaksonen A.H., Sahinkaya E. Review of sulfate reduction based bioprocesses for acid mine drainage treatment and metals recovery. Annu. Conf. - Int. Mine Water Assoc. 2012:207–214. [Google Scholar]
- 64.Tilley E., Ulrich L., Luethi C., Reymond P., Zurburegg C., Lüthi C., Morel A., Zurbrügg C., Schertenleib R. Compendium of sanitation systems and technologies. Development. 2014 [Google Scholar]
- 65.Wu H., Zhang J., Ngo H.H., Guo W., Hu Z., Liang S., Fan J., Liu H. A review on the sustainability of constructed wetlands for wastewater treatment: design and operation. Bioresour. Technol. 2015 doi: 10.1016/j.biortech.2014.10.068. [DOI] [PubMed] [Google Scholar]
- 66.Vymazal J. Constructed wetlands for treatment of industrial wastewaters: a review. Ecol. Eng. 2014 doi: 10.1016/j.ecoleng.2014.09.034. [DOI] [Google Scholar]
- 67.Wang Y., Cai Z., Sheng S., Pan F., Chen F., Fu J. Comprehensive evaluation of substrate materials for contaminants removal in constructed wetlands. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2019.134736. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann H., Platzer C., Winker M., Von Münch E. Technology review of constructed wetlands. Sustain. Sanit. Ecosan. 2011 [Google Scholar]
- 69.Wu S., Kuschk P., Brix H., Vymazal J., Dong R. Development of constructed wetlands inperformance intensifications for wastewater treatment: a nitrogen and organic matter targeted review. Water Res. 2014 doi: 10.1016/j.watres.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y., Wen Y., Cheng J., Xue C.H., Yang D., Zhou Q. Effects of dissolved oxygen on extracellular enzymes activities and transformation of carbon sources from plant biomass: implications for denitrification in constructed wetlands. Bioresour. Technol. 2011 doi: 10.1016/j.biortech.2010.10.122. [DOI] [PubMed] [Google Scholar]
- 71.Saeed T., Sun G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012 doi: 10.1016/j.jenvman.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 72.Meng P., Pei H., Hu W., Shao Y., Li Z. How to increase microbial degradation in constructed wetlands: influencing factors and improvement measures. Bioresour. Technol. 2014 doi: 10.1016/j.biortech.2014.01.095. [DOI] [PubMed] [Google Scholar]
- 73.Bethune J., Randell J., Runkel R.L., Singha K. Non-invasive flow path characterization in a mining-impacted wetland. J. Contam. Hydrol. 2015 doi: 10.1016/j.jconhyd.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Reid R.J., Mosley L.M. Comparative contributions of solution geochemistry, microbial metabolism and aquatic photosynthesis to the development of high pH in ephemeral wetlands in South East Australia. Sci. Total Environ. 2016 doi: 10.1016/j.scitotenv.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 75.Wentzell B.M., Boylen C.W., Nierzwicki-Bauer S.A. Wetland ecosystem comparison using a suite of plant assessment measures. Ecol. Indicat. 2016 doi: 10.1016/j.ecolind.2016.02.056. [DOI] [Google Scholar]
- 76.Gammons C.H., Poulson S.R., Pellicori D.A., Reed P.J., Roesler A.J., Petrescu E.M. The hydrogen and oxygen isotopic composition of precipitation, evaporated mine water, and river water in Montana, USA. J. Hydrol. 2006 doi: 10.1016/j.jhydrol.2005.12.005. [DOI] [Google Scholar]
- 77.Cozma A.I., Baciu C., Moldovan M., Pop I.-C. Using natural tracers to track the groundwater flow in a mining area. Procedia Environ. Sci. 2016 doi: 10.1016/j.proenv.2016.03.026. [DOI] [Google Scholar]
- 78.Yan N., Marschner P., Cao W., Zuo C., Qin W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015 doi: 10.1016/j.iswcr.2015.11.003. [DOI] [Google Scholar]
- 79.Yildiz M., Yilmaz T., Arzum C.S., Yurtsever A., Kaksonen A.H., Ucar D. Sulfate reduction in acetate- and ethanol-fed bioreactors: acidic mine drainage treatment and selective metal recovery. Miner. Eng. 2019 doi: 10.1016/j.mineng.2019.01.007. [DOI] [Google Scholar]
- 80.Kaksonen A.H., Franzmann P.D., Puhakka J.A. Performance and ethanol oxidation kinetics of a sulfate-reducing fluidized-bed reactor treating acidic metal-containing wastewater. Biodegradation. 2003 doi: 10.1023/A:1024262607099. [DOI] [PubMed] [Google Scholar]
- 81.Bekmezci O.K., Ucar D., Kaksonen A.H., Sahinkaya E. Sulfidogenic biotreatment of synthetic acid mine drainage and sulfide oxidation in anaerobic baffled reactor. J. Hazard Mater. 2011 doi: 10.1016/j.jhazmat.2011.01.087. [DOI] [PubMed] [Google Scholar]
- 82.Gomez D.V. 2013. Simultaneous Sulfate Reduction and Metal Precipitation in an Inverse Fluidized Bed Reactor. [Google Scholar]
- 83.Neculita C.M., Zagury G.J., Bussière B. Effectiveness of sulfate-reducing passive bioreactors for treating highly contaminated acid mine drainage: II. Metal removal mechanisms and potential mobility. Appl. Geochem. 2008 doi: 10.1016/j.apgeochem.2008.08.014. [DOI] [Google Scholar]
- 84.Damianovic M.H.R.Z., de Godoi L.A.G., Saia F.T., Foresti E. Horizontal-flow anaerobic immobilized biomass (HAIB) reactor for organic matter and sulfate removal from paper recycling plant wastewater with simultaneous conversion of sulfide into elemental sulfur. J. Environ. Chem. Eng. 2018 doi: 10.1016/j.jece.2018.01.024. [DOI] [Google Scholar]
- 85.Cibati A., Cheng K.Y., Morris C., Ginige M.P., Sahinkaya E., Pagnanelli F., Kaksonen A.H. Selective precipitation of metals from synthetic spent refinery catalyst leach liquor with biogenic H2S produced in a lactate-fed anaerobic baffled reactor. Hydrometallurgy. 2013 doi: 10.1016/j.hydromet.2013.01.022. [DOI] [Google Scholar]
- 86.Tabak H.H., Scharp R., Burckle J., Kawahara F.K., Govind R. Advances in biotreatment of acid mine drainage and biorecovery of metals: 1. Metal precipitation for recovery and recycle. Biodegradation. 2003 doi: 10.1023/A:1027332902740. [DOI] [PubMed] [Google Scholar]
- 87.Mara D., Horan N. Handbook of water and wastewater microbiology. 2003. [DOI]
- 88.Name T., Sheridan C. Remediation of acid mine drainage using metallurgical slags. Miner. Eng. 2014 doi: 10.1016/j.mineng.2014.03.024. [DOI] [Google Scholar]
- 89.Lefticariu L., Walters E.R., Pugh C.W., Bender K.S. Sulfate reducing bioreactor dependence on organic substrates for remediation of coal-generated acid mine drainage: field experiments. Appl. Geochem. 2015 doi: 10.1016/j.apgeochem.2015.08.002. [DOI] [Google Scholar]
- 90.Bertolino S.M., Rodrigues I.C.B., Guerra-Sá R., Aquino S.F., Leão V.A. Implications of volatile fatty acid profile on the metabolic pathway during continuous sulfate reduction. J. Environ. Manag. 2012 doi: 10.1016/j.jenvman.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 91.Salama E.S., Jeon B.H., Kurade M.B., Abou-Shanab R.A.I., Govindwar S.P., Lee S.H., Yang I.S., Lee D.S. Harvesting of freshwater microalgae Scenedesmus obliquus and Chlorella vulgaris using acid mine drainage as a cost effective flocculant for biofuel production. Energy Convers. Manag. 2016 doi: 10.1016/j.enconman.2016.05.020. [DOI] [Google Scholar]
- 92.Sato Y., Hamai T., Hori T., Habe H., Kobayashi M., Sakata T. Year-round performance of a passive sulfate-reducing bioreactor that uses rice bran as an organic carbon source to treat acid mine drainage. Mine Water Environ. 2018 doi: 10.1007/s10230-017-0489-6. [DOI] [Google Scholar]
- 93.Lee W.C., Lee S.W., Yun S.T., Lee P.K., Hwang Y.S., Kim S.O. A novel method of utilizing permeable reactive kiddle (PRK) for the remediation of acid mine drainage. J. Hazard Mater. 2016 doi: 10.1016/j.jhazmat.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 94.Pozo G., Pongy S., Keller J., Ledezma P., Freguia S. A novel bioelectrochemical system for chemical-free permanent treatment of acid mine drainage. Water Res. 2017 doi: 10.1016/j.watres.2017.09.058. [DOI] [PubMed] [Google Scholar]
- 95.Hengen T.J., Squillace M.K., O'Sullivan A.D., Stone J.J. Life cycle assessment analysis of active and passive acid mine drainage treatment technologies. Resour. Conserv. Recycl. 2014 doi: 10.1016/j.resconrec.2014.01.003. [DOI] [Google Scholar]
- 96.Cravotta C.A. Size and performance of anoxic limestone drains to neutralize acdic mine drainagei. J. Environ. Qual. 2003 doi: 10.2134/jeq2004.1164a. [DOI] [PubMed] [Google Scholar]
- 97.Brodie G.A., Britt C.R., Tomaszewski T.M., Taylor H.N. Use of passive anoxic limestone drains to enhance performance of acid drainage treatment wetlands. J. Am. Soc. Min. Reclam. 1991 doi: 10.21000/jasmr91010211. [DOI] [Google Scholar]
- 98.García-Valero A., Martínez-Martínez S., Faz A., Rivera J., Acosta J.A. Environmentally sustainable acid mine drainage remediation: use of natural alkaline material. J. Water Process Eng. 2020 doi: 10.1016/j.jwpe.2019.101064. [DOI] [Google Scholar]
- 99.Jarvis A., England A. Operational and treatment performance of an unique Reducing and Alkalinity Producing System (RAPS) for acidic leachate remediation in Lancashire, UK. Uranium Aquat. Environ. 2002 doi: 10.1007/978-3-642-55668-5_118. [DOI] [Google Scholar]
- 100.Jage C.R., Zipper C.E., Noble R. Factors affecting alkalinity generation by successive alkalinity-producing systems: regression analysis. J. Environ. Qual. 2001 doi: 10.2134/jeq2001.3031015x. [DOI] [PubMed] [Google Scholar]
- 101.Lapointe F., Fytas K., McConchie D. Using permeable reactive barriers for the treatment of acid rock drainage. Int. J. Surf. Min. Reclamat. Environ. 2005 doi: 10.1080/13895260500045241. [DOI] [Google Scholar]
- 102.Sasaki K., Blowes D.W., Ptacek C.J., Gould W.D. Immobilization of Se(VI) in mine drainage by permeable reactive barriers: column performance. Appl. Geochem. 2008 doi: 10.1016/j.apgeochem.2007.08.007. [DOI] [Google Scholar]
- 103.Kornilovych B., Wireman M., Ubaldini S., Guglietta D., Koshik Y., Caruso B., Kovalchuk I. Uranium removal from groundwater by permeable reactive barrier with zero-valent iron and organic carbon mixtures: laboratory and field studies. Metals. 2018 doi: 10.3390/met8060408. [DOI] [Google Scholar]
- 104.Toffey W.E. Clean. Up Contam. Prop. Reuse Revital. Eff. Tech. Approaches Tools. 2003. Twenty-five years of mine reclamation with biosolids in Pennsylvania. [Google Scholar]
- 105.Wijesekara H., Bolan N.S., Kumarathilaka P., Geekiyanage N., Kunhikrishnan A., Seshadri B., Saint C., Surapaneni A., Vithanage M. Environ. Mater. Waste Resour. Recover. Pollut. Prev. 2016. Biosolids enhance mine site rehabilitation and revegetation. [DOI] [Google Scholar]
- 106.Punia A. Innovative and sustainable approach for phytoremediation of mine tailings: a review. Waste Dispos. Sustain. Energy. 2019 doi: 10.1007/s42768-019-00022-y. [DOI] [Google Scholar]
- 107.Mendez M.O., Maier R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Biotechnol. 2008 doi: 10.1007/s11157-007-9125-4. [DOI] [Google Scholar]
- 108.Wang L., Ji B., Hu Y., Liu R., Sun W. A review on in situ phytoremediation of mine tailings. Chemosphere. 2017 doi: 10.1016/j.chemosphere.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 109.Al-Zoubi H., Rieger A., Steinberger P., Pelz W., Haseneder R., Härtel G. Optimization study for treatment of acid mine drainage using membrane technology. Separ. Sci. Technol. 2010 doi: 10.1080/01496395.2010.480963. [DOI] [Google Scholar]
- 110.Kinnunen P., Kyllönen H., Kaartinen T., Mäkinen J., Heikkinen J., Miettinen V. Sulfate removal from mine water with chemical, biological and membrane technologies. Water Sci. Technol. 2018 doi: 10.2166/wst.2018.102. [DOI] [PubMed] [Google Scholar]
- 111.Foureaux A.F.S., Moreira V.R., Lebron Y.A.R., Santos L.V.S., Amaral M.C.S. Direct contact membrane distillation as an alternative to the conventional methods for value-added compounds recovery from acidic effluents: a review. Separ. Purif. Technol. 2020 doi: 10.1016/j.seppur.2019.116251. [DOI] [Google Scholar]
- 112.Brune K.D., Bayer T.S. Engineering microbial consortia to enhance biomining and bioremediation. Front. Microbiol. 2012 doi: 10.3389/fmicb.2012.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dunbar W.S. Biotechnology and the mine of tomorrow. Trends Biotechnol. 2017 doi: 10.1016/j.tibtech.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 114.Rojas C., Gutierrez R.M., Bruns M.A. Bacterial and eukaryal diversity in soils forming from acid mine drainage precipitates under reclaimed vegetation and biological crusts. Appl. Soil Ecol. 2016 doi: 10.1016/j.apsoil.2016.03.012. [DOI] [Google Scholar]
- 115.Fernando W.A.M., Ilankoon I.M.S.K., Syed T.H., Yellishetty M. Challenges and opportunities in the removal of sulfate ions in contaminated mine water: a review. Miner. Eng. 2018 doi: 10.1016/j.mineng.2017.12.004. [DOI] [Google Scholar]
- 116.Bwapwa J.K., Jaiyeola A.T., Chetty R. Bioremediation of acid mine drainage using algae strains: a review. S. Afr. J. Chem. Eng. 2017 doi: 10.1016/j.sajce.2017.06.005. [DOI] [Google Scholar]
- 117.Dean A.P., Hartley A., McIntosh O.A., Smith A., Feord H.K., Holmberg N.H., King T., Yardley E., White K.N., Pittman J.K. Metabolic adaptation of a Chlamydomonas acidophila strain isolated from acid mine drainage ponds with low eukaryotic diversity. Sci. Total Environ. 2019 doi: 10.1016/j.scitotenv.2018.07.445. [DOI] [PubMed] [Google Scholar]
- 118.Chalkley R., Child F., Al-Thaqafi K., Dean A.P., White K.N., Pittman J.K. Macroalgae as spatial and temporal bioindicators of coastal metal pollution following remediation and diversion of acid mine drainage. Ecotoxicol. Environ. Saf. 2019 doi: 10.1016/j.ecoenv.2019.109458. [DOI] [PubMed] [Google Scholar]
- 119.Kumar M., Sinharoy A., Pakshirajan K. Process integration for biological sulfate reduction in a carbon monoxide fed packed bed reactor. J. Environ. Manag. 2018 doi: 10.1016/j.jenvman.2018.04.033. [DOI] [PubMed] [Google Scholar]