Abstract
Greenhouse gas emissions from wetlands are significantly promoted by global nitrogen input for changing the rate of soil carbon and nitrogen cycling, and are substantially affected by soil labile carbon and nitrogen conversely. However, the driving mechanism by which soil labile carbon and nitrogen affect greenhouse gas emissions from wetland ecosystems under global nitrogen input is not well understood. Working out the driving factor of nitrogen input on greenhouse gas emissions from wetlands is critical to reducing global warming from nitrogen input. Thus, we synthesized 72 published studies (2144 paired observations) of greenhouse gas fluxes and soil labile compounds of carbon and nitrogen (ammonium, nitrate, dissolved organic carbon, soil microbial biomass nitrogen and carbon), to understand the effects of labile carbon and nitrogen on greenhouse gas emissions under global nitrogen input. Across the data set, nitrogen input significantly promoted carbon dioxide, methane and nitrous oxide emissions from wetlands. In particular, at lower nitrogen rates (<100 kg ha−1·yr−1) and with added ammonium compounds, freshwater wetland significantly promoted carbon dioxide and methane emissions. Peatland was the largest nitrous oxide source under these conditions. This meta-analysis also revealed that nitrogen input stimulated dissolved organic carbon, ammonium, nitrate, microbial biomass carbon and microbial biomass nitrogen accumulation in the wetland ecosystem. The variation-partitioning analysis and structural equation model were used to analyze the relationship between the greenhouse gas and labile carbon and nitrogen further. These results revealed that dissolved organic carbon (DOC) is the primary factor driving greenhouse gas emission from wetlands under global nitrogen input, whereas microbial biomass carbon (MBC) more directly affects greenhouse gas emission than other labile carbon and nitrogen.
Keywords: Wetland, Greenhouse gas, Nitrogen deposition, Fertilization, Soil labile compounds
Graphical abstract
Highlights
-
•
GHG emissions from wetlands is mainly driven by DOC under global nitrogen input.
-
•
GHG emissions from wetlands is directly driven by MBC under global nitrogen input.
-
•
Adding NH4+-N lower than 100 kg ha−1·yr−1 greatly promote GHG emissions from wetland.
-
•
Freshwater wetlands are the largest CO2 and CH4 source under global nitrogen input.
-
•
Nitrogen input to peatland greatly and significantly promotes N2O emission.
Acronyms and symbols
- DOC
dissolved organic carbon
- SMD
the standardized mean difference
- MBC
microbial biomass carbon
- CIs
the confidence intervals
- CNKI
China National Knowledge Infrastructure
- kg
kilogram
- N2O
nitrous oxide
- ha
Hectares
- CH4
methane
- yr
year
- CO2
carbon dioxide
- NH4+/NH4+–N
ammonium
- cm
centimeter
- NO3−/NO3−–N
nitrate
- C
the control treatment
- NH4NO3
ammonium nitrate
- E
the experimental treatment
- QM
the between-group heterogeneity
- d
the effect size
- N
the number of observations
- v
variance
- SEM
The structural equation model
- XC
the mean values of an index in the control treatment
- X2
Chi-Square value
- XE
the mean values of an index in the experimental treatment
- df
degree of freedom
- NC
the sample size of an index in the control treatment
- GFI
high goodness-of-fit index
- NE
the sample size of an index in the experimental treatment
- CFI
the comparative fit index
- SC
the standard deviation of an index in the control treatment
- RMSEA
the low root means square errors of approximation
- SE
the standard deviation of an index in the experimental treatment
- MBN
microbial biomass carbon
- wi
weight factor
1. Introduction
In recent decades, nitrogen input into ecosystems has substantially increased at the global scale due to atmospheric deposition, agricultural input, fossil fuel combustion, and other anthropogenic activities [[1], [2], [3]]. Not only can nitrogen be a limiting nutrient [4], but it can also be a pollutant in many terrestrial ecosystems [[5], [6], [7]]. The wetland ecosystem is the key ecotone between terrestrial and aquatic ecosystems, and nitrogen can move from wetland ecosystems to rivers or lakes, leading to water eutrophication [5,8,9]. The nitrogen trapped by wetlands could also impact element cycling by changing the soil physicochemical properties [10,11] and microbial communities [12,13]. The soil physicochemical properties (e.g. temperature, moisture content, electrical conductivity and so on) and microbial communities usually determine the availability of soil carbon and nitrogen [14], thereby determining the biomass of vegetation communities [15]. Therefore, soil carbon and nitrogen availability play key roles in the substance cycling of wetland ecosystems. Nitrogen input typically alters the soil nitrogen and carbon availability by affecting labile carbon and nitrogen, including ammonium, nitrate [16,17], dissolved organic carbon [2], soil microbial biomass carbon and nitrogen [18]. However, how the availability of soil carbon and nitrogen in wetlands responds to nitrogen input is often controversial [[19], [20], [21]]. For example, Song et al. [21] pointed that DOC content reduced and ammonium augmented with the increase of nitrogen input rate, but Cui et al. [19] revealed an opposite trend in the peatlands of Northeast China. Song et al. [22] found that nitrogen addition increased nitrate content and MBC, which results contrast with the study of Kastovska et al. [20] and Song et al. [2]. Therefore, exploring the influence of nitrogen enrichment on the availability of soil labile carbon and nitrogen is critically important to understanding substance cycling in wetland ecosystems on a global scale.
Although previous studies have verified that terrestrial ecosystems act a sink/source of greenhouse gases and have quantitatively analyzed the effect of nitrogen input on greenhouse gas emissions [[23], [24], [25]], these results might not accurately describe the effect of wetlands on greenhouse gas emissions because the wetland ecosystem is very different from other types of terrestrial ecosystems [9]. The wetland ecosystem is located at the junction of the terrestrial-aquatic interlaced zone, and has some special characteristics, including frequent changes in water level, great redox fluctuation from highly anaerobic to highly aerobic conditions, and interception of partial nitrogen runoff [[26], [27], [28]]. These characteristics lead to the distinct greenhouse gas emission regulars from wetlands compared to other types of terrestrial ecosystems. Although wetlands occupy only 6%–8% of the earth’s land surface, they are an important sink/source of greenhouse gas [29,30]. For example, the IPCC [31] reported that methane emissions from wetlands account for an estimated 63% of all natural methane emissions. Thus, understanding how nitrogen input affects greenhouse gas emissions from wetlands is critically important when attempting to understand the future global climate.
Nitrogen input could not only influence greenhouse gas emissions from wetland ecosystems by altering the soil nitrogen and carbon cycling [23,32], but could also affect soil microbes due to its influences on soil nitrogen and carbon availability, thereby affecting greenhouse gas emissions [[33], [34], [35]]. Thus, revealing the interactions between soil labile carbon and nitrogen and greenhouse gases will contribute to understanding the mechanism of greenhouse gas emissions from wetland ecosystems to the atmosphere, resulting in elucidating the contribution of wetland ecosystems to the global greenhouse effect. However, previous studies found that soil labile carbon and nitrogen showed both positive and negative effects on greenhouse gas emissions from wetland ecosystems under global nitrogen input [[36], [37], [38], [39]]. The wetland types and climates also affected the relationships between soil labile carbon and nitrogen and greenhouse gas under nitrogen input. Therefore, the mechanism of soil labile carbon and nitrogen on greenhouse gas emissions from wetland ecosystems is complicated and currently not well understood under nitrogen input at global scales. Thus, there is a desperate need to clarify the driving mechanism of labile carbon and nitrogen on greenhouse gas emission under global nitrogen input by combining the conclusions from various studies using a meta-analysis.
To untangle these controversial and uncertain issues, we used a meta-analysis to analyze studies on nitrogen input experiments published prior to September 2019. We used soil carbon dioxide emissions, methane emissions, nitrous oxide emissions, soil labile carbon and nitrogen, and soil microbial biomass to address the following questions: (i) How do the soil greenhouse gas emissions from wetland ecosystems fluctuate as a result of varying nitrogen input in terms of rates, compounds and environmental factors? (ii) What key factors affect soil greenhouse gas emissions as a response to nitrogen input? (iii) What is the major effect of labile compounds on greenhouse gas emissions from wetland ecosystems under global nitrogen input?
2. Materials and method
2.1. Meta data collection
The IPCC [40] indicated that the increase of nitrogen deposition could promote greenhouse gas emissions into the atmosphere, and Liu et al. [41] and Deng et al. [23] utilized meta-analyses to reveal the effects of nitrogen input on soil greenhouse gas from terrestrial systems. However, these studies had some limitations and constraints. First, these studies covered a variety of terrestrial ecosystems. However, the wetland ecosystem is the key ecotone between terrestrial and aquatic ecosystems, and therefore its response to nitrogen input is greatly different from that of other types of terrestrial ecosystems. A new meta-analytical study needs to consider the particular pattern of greenhouse gas emissions in wetland ecosystems. Second, Liu et al. [41] and Tian et al. [64] utilized global models to determine the emission patterns and effects of different factor prior to 2009. Deng et al. [23] focused on the relationships between greenhouse gas emissions and carbon pools. Thus, we conducted a literature search in September 2019 for all papers published over the past decade on greenhouse gases, nitrogen input and wetland ecosystems. This literature search used the Web of Science, ScienceDirect, Google Scholar, and CNKI. The keywords for the online search were: (wetland OR peatland OR marsh OR bog OR fen) AND (nitrogen input OR nitrogen addition OR nitrogen enrichment OR nitrogen deposition OR nitrogen fertilizer) AND (greenhouse gas OR nitrous oxide OR N2O OR methane OR CH4 OR carbon dioxide OR CO2). The selected studies satisfied the following criteria: (a) the control experiment was defined by no nitrogen input or atmospheric nitrogen deposition; (b) non-repetitive experimental studies were excluded; (c) non-experimental studies (such as modeling, meta-analyses, and reviews) were excluded.
Based on these criteria, approximately 2144 paired observations (Fig. 1) from 72 papers published from 2009 to 2019 on greenhouse gas emissions (including CO2, CH4 and N2O) and labile carbon or nitrogen (mainly including dissolved organic carbon, ammonium, nitrate, microbial biomass carbon and nitrogen) under global nitrogen input were selected for data collection. Data sources included tables, text, figures and supplementary files. The data in figures were collected using the GetData 2.25 software (http://getdata-graph-digitizer.com/). If the key data was not directly acquired, we obtained the data from the authors. The rate of nitrogen input, types of nitrogen input, climate, nitrogen compounds, and types of wetlands were collected. The labile compounds were also collected, including ammonium, nitrate, dissolved organic carbon, soil microbial biomass carbon, and soil microbial biomass nitrogen from the surface soil (the depth ranges from 0 to 20 cm). We summarized the latitude and longitude of each site from the published papers, or we extracted these data online (http://www.worldclim.org/; Table. A1).
Fig. 1.
Geographical distribution of the study sites.
2.2. Meta data analysis
The effect size was calculated using Hedges’ d, which is a measurement of the unbiased standardized mean difference between the control (C) and experimental (E) means [42,43]. The equations for the effect size (d) and variance (v) are listed as in the follows:
| (1) |
| (2) |
| (3) |
XC and XE represent the mean values of an index in the control and experimental treatment, respectively. NC and NE represent the sample size of an index in the control and experimental treatment, respectively. SC and SE represent the standard deviation of an index in control and the experimental treatment, respectively.
The weight factor (wi) was determined as follow:
| (4) |
The d of the control and nitrogen input treatments were used to calculate the weighted standardized mean difference (SMD):
| (5) |
where n refers to the number of observations, wi and di represent the weight factor and effect size of observation i, respectively.
The calculated mean effect size considered the confidence intervals (Bootstrap CIs, bootstrapping by 4999 iterations). If the Bootstrap CIs had nonzero overlap, the nitrogen input significantly influenced the greenhouse gas emissions [44,45]. Negative Hedges’d values indicated that the nitrogen input decreased the greenhouse gas emissions. Positive Hedges’d values indicated that nitrogen input increased the greenhouse gas emissions [46].
To test the effects of nitrogen input on greenhouse gas emissions, we categorized the nitrogen input treatments into four groups: nitrogen input rates (0–50, 50–100, 100–200, 200–300, >300 kg ha−1·yr−1), climate (alpine climate, temperate continental climate, temperate marine climate, monsoon climate of medium latitudes, subtropical monsoon climate, subtropical humid climate), nitrogen compounds (NH4+, NO3−, NH4NO3, organic nitrogen fertilizer) and type of wetland (freshwater marsh, alpine wetland, estuary wetland, peatland, salt marsh). The data were analyzed using a mixed-effects model [47]. There are random variations in effect sizes among all the observations, whereas each individual observation is weighted by the reciprocal of the mixed-model variance [48,49]. If the between-group heterogeneity (QM) test was smaller than 0.05, it indicates that significant differences exist among the different groups. We tested the QM of CO2, CH4 and N2O and the results are shown in Table .1. Meanwhile, we also tested the QM of ammonium, nitrate, soil microbial biomass nitrogen, dissolved organic carbon and soil microbial biomass carbon (Table. B1).
Table 1.
Results of statistical comparisons among groups for greenhouse gas.
| Item | CO2 |
CH4 |
N2O |
|||
|---|---|---|---|---|---|---|
| QM | p-value | QM | p-value | QM | p-value | |
| Rate of nitrogen input | 24.03 | <0.001 | 13.94 | <0.001 | 28.21 | <0.001 |
| Climate | 37.52 | <0.001 | 28.52 | <0.001 | 31.18 | <0.001 |
| Nitrogen compounds | 9.81 | <0.05 | 30.29 | <0.001 | 38.09 | <0.001 |
| Types of wetlands | 29.00 | <0.001 | 24.35 | <0.001 | 43.01 | <0.001 |
Notes: All data were grouped into five nitrogen input rates (0–50, 50–100, 100–200, 200–300, >300 kg ha−1·yr−1), six climate types (alpine climate, temperate continental climate, temperate marine climate, monsoon climate of medium latitudes, subtropical monsoon climate, subtropical humid climate), four nitrogen compounds types (NH4+, NO3−, NH4NO3, organic nitrogen fertilizer) and five wetlands types (freshwater marsh, alpine wetland, estuary wetland, peatland, salt marsh). CO2 is carbon dioxide, CH4 is methane, and N2O is nitrous oxide. QM: heterogeneity in group cumulative effect sizes.
2.3. Publication bias
Publication bias means there is a higher possibility of publishing highly positive or negative results or not reporting non-significant effects [43]. We tested the publication bias for greenhouse gas emissions using weighted histograms and a fail-safe number. Weighted histograms consist of the effect sizes and weight of data (Eq. (4)), rather than the frequency of effect size [50]. The fail-safe number is substantially larger than 5 N + 10 (N represent the number of observations in this study), where 5 N + 10 was defined using the acceptable threshold in the literature. The results indicate that the observations from this study can be treated as a reliable estimate of the true effect [43,51]. Therefore, the results shown in Fig. B1 indicate that there were no biases in the selected publications.
2.4. Statistic analysis
All of the standardized mean differences, the between-group heterogeneity, Bootstrap CIs and fail-safe number were counted using MetaWin 2.1.3 software (http://www.metawinsoft.com/, Sinauer Associates Inc., Sunderland, MA, USA). The figures were constructed using OriginPro 2017 and R (3.6.1) software. The variation-partitioning analysis was conducted using R (3.6.1) software for the effects of soil labile carbon and nitrogen on greenhouse gas emissions. The Pearson correlation analysis was performed using SPSS 20.0 (IBM Corporation, Armonk, NY, USA) for indicating the relationships between greenhouse gas and labile carbon and nitrogen, where p values smaller than 0.05 are considered statistically significant. The regression analysis was conducted using the OriginPro 2017 software for the effect size of greenhouse gas emission and mean annual precipitation, mean annual temperature at the global level.
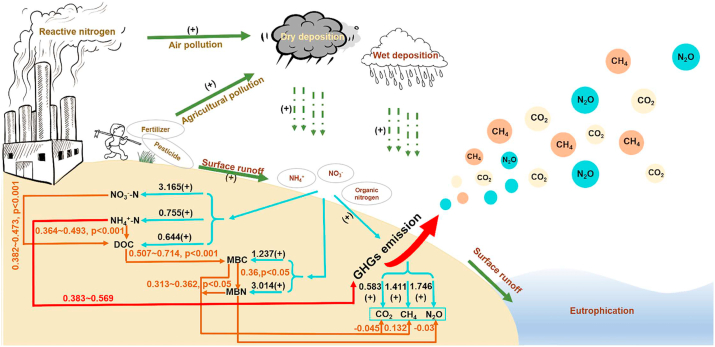
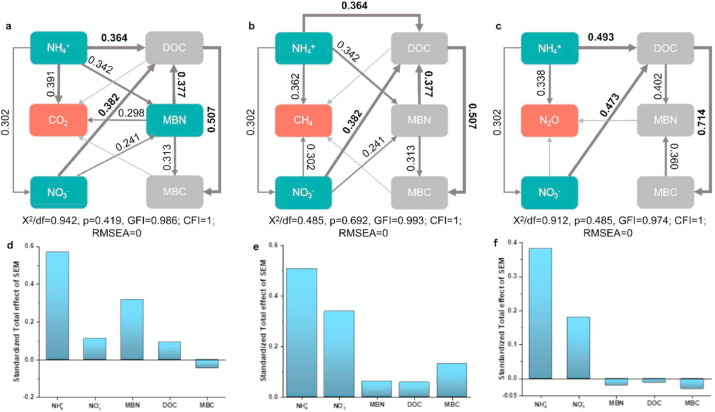
The structural equation model (SEM) could reveal the driving factors and impacts of greenhouse gas emissions under nitrogen input and be constructed using Amos (Version 21). Several tests were used to determine the adequacy of model fitting, including the X2 test (0.05 < p < 1.00, 0 ≤ X2/df ≤ 2), high goodness-of-fit index (GFI, 0.9 < GFI < 1.0), the comparative fit index (CFI, 0.9 < CFI < 1.0), and the low root means square errors of approximation (RMSEA, < 0.05). The effect value and pathway of the model were obtained after the model was constructed. The obtained test results, including X2/df < 2, p > 0.05, GFI and CFI close to1, and RMSEA <0.05, for the SEM (Fig. 7) indicated that the SEM could be considered to be a perfect fit.
Fig. 7.
The driving mechanisms of labile carbon and nitrogen on greenhouse gas emissions from wetland ecosystems under nitrogen input. The black numbers represent the effect size of various parameters, the red numbers represent the total effects of ammonium on greenhouse gas emissions, the brown numbers represent the direct effects of labile carbon and nitrogen on greenhouse gas emissions and the direct effects between various labile carbon and nitrogen.
3. Results
3.1. Greenhouse gas emissions from wetland ecosystems under nitrogen input
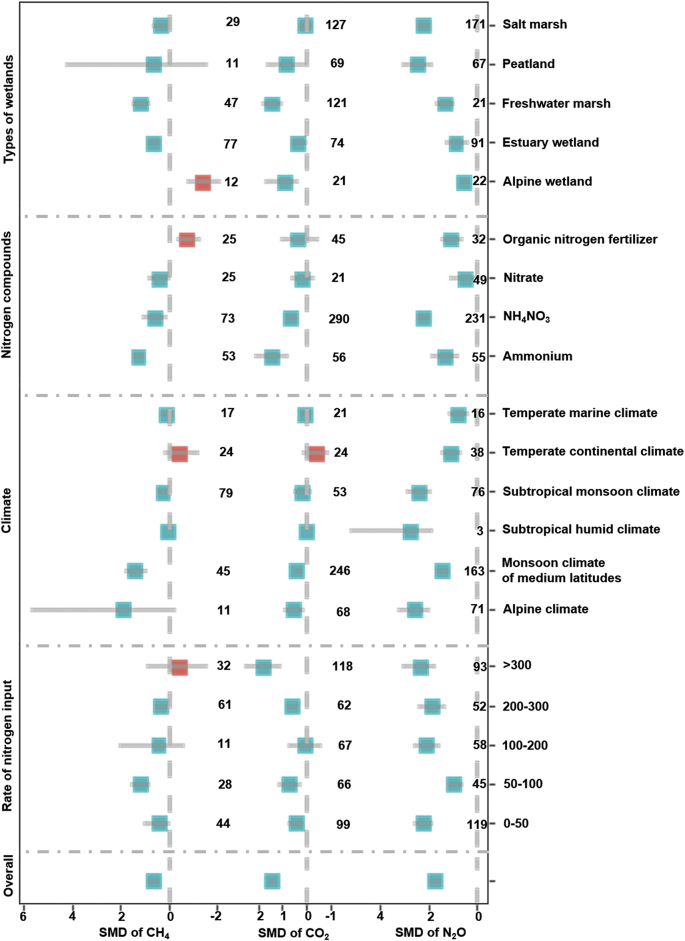
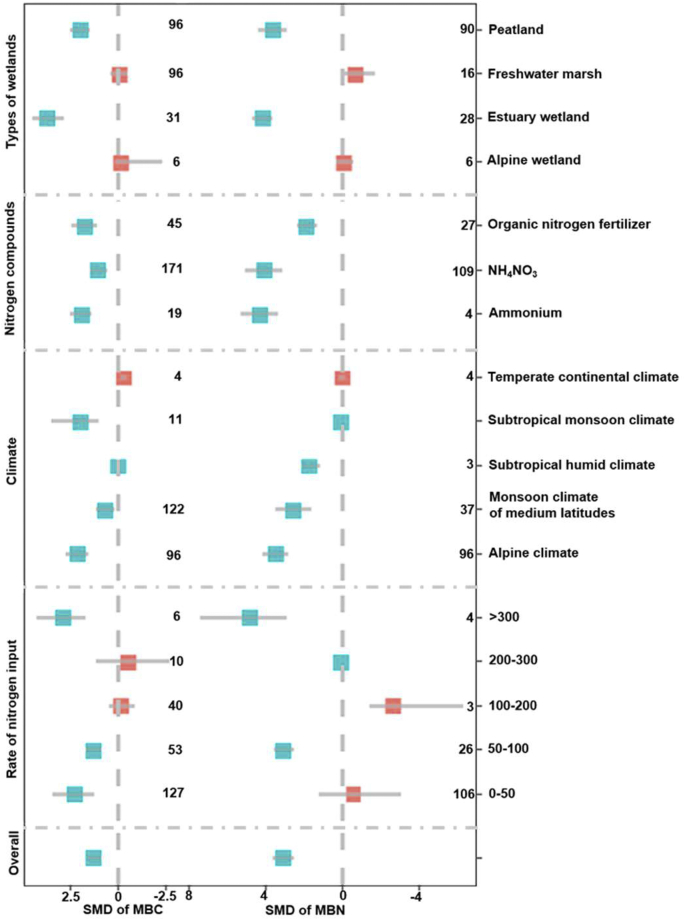
Across all observations, the overall standardized mean difference (SMD) of carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) were 1.41, 0.58 and 1.74, respectively (Fig. 2; Bootstrap CIs of 1.24–1.57, 0.31 to 0.85, and 1.52 to 1.97, respectively), and presented a significantly positive effect because the Bootstrap CIs had nonzero overlap. Nitrogen input increased greenhouse gas emissions for all types of wetlands, except for the Alpine wetland, which had significantly decreased methane emission (SMD = −1.41, Bootstrap CIs = −2.14 to −0.74). Compared to CO2 and CH4, all types of climate significantly and positively promoted N2O emissions. Specially, the SMD of CO2 and CH4 were negative under temperate continental climate. This suggested that nitrogen input under temperate continental climate decreased CO2 and CH4 emissions in comparison to that no nitrogen addition in the wetland.
Fig. 2.
Standardized mean difference for greenhouse gases emissions from different wetland environments under nitrogen inputs. The numbers in the figure represent the number of case studies. A standardized mean difference >0 reveals a positive effect on greenhouse gas emissions, whereas values < 0 reveal negative effects. Error bars are the bootstrap confidence intervals (CIs). CIs that do not include 0 and do not overlap indicate a significant effect on greenhouse gas emissions and significant differences among groups, respectively. SMD represents the standardized mean difference, which is a type of effect size. The unit of nitrogen input rate is kg·ha−1·yr−1.
Nitrogen input via NH4+ and NH4NO3 significantly promoted greenhouse gas emissions (Fig. 2). However, organic nitrogen fertilizer significantly reduced CH4 emissions (SMD = −0.76, Bootstrap CIs = −1.36 to −0.30). We also found that different nitrogen input rates had a positive effect on the CO2 and N2O emissions in wetlands ecosystem (SMD = 0.069 to 2.32 in Bootstrap CIs). Specially, the nitrogen input rate of 50–100 kg ha−1·yr−1 had the largest impact on CO2 and N2O emissions among all nitrogen input rates. Meanwhile, nitrogen input rate of 0–50 kg ha−1·yr−1 (SMD = 1.16 in Bootstrap CIs) had the largest effect on CH4 emissions among all nitrogen input rates. This suggested that lower nitrogen input rates (<100 kg ha−1·yr−1) significantly promoted greenhouse gas emissions.
3.2. Changes in soil labile carbon and nitrogen under nitrogen input in wetland ecosystems
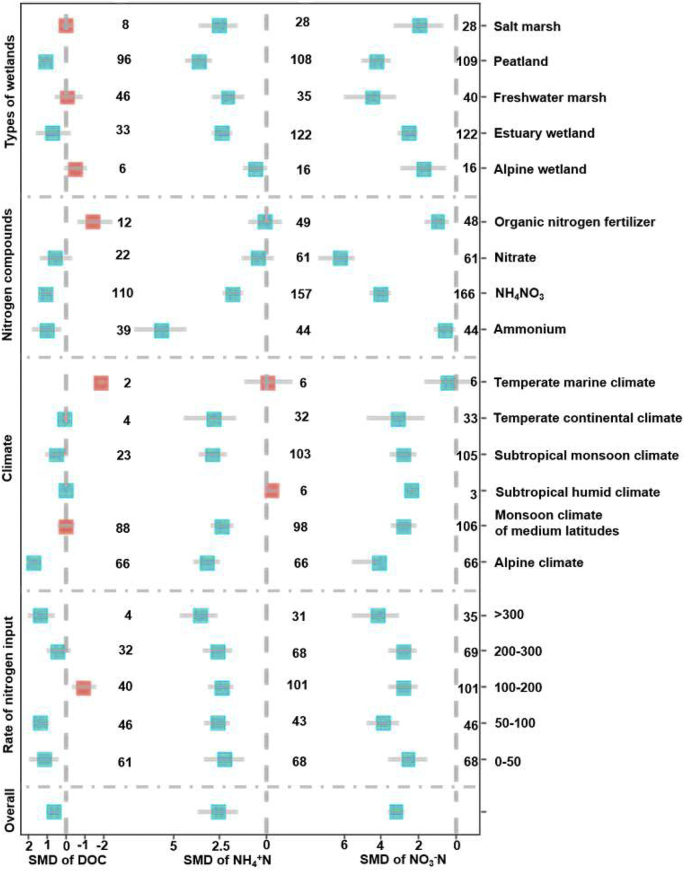
As illustrated in Fig. 3, the overall SMD of dissolved organic carbon (DOC), ammonium (NH4+–N), and nitrate (NO3−–N) range from 0.64 to 3.58 indicated that the nitrogen input augmented soil labile carbon and nitrogen contents in wetland ecosystems. For all wetland types, nitrogen input dwindled the soil NH4+–N and NO3−–N contents. For peatland, the soil DOC was significantly increased by nitrogen input, whereas the soil DOC was reduced by nitrogen input for freshwater marshes, alpine wetlands and salt marshes. Fig. 3 shows that for all types of climates except the temperate marine climate, the nitrogen input significantly augmented the soil NO3−–N contents. The nitrogen input significantly increased the soil NH4+–N content for the alpine climate, temperate continental climate, monsoon climate of medium latitudes and subtropical monsoon climate. The soil DOC was significantly added by nitrogen input for the alpine climate (SMD = 1.72, Bootstrap CIs = 1.36 to 2.11), whereas the nitrogen input under the temperate marine climate diminished the soil DOC content.
Fig. 3.
Standardized mean difference for DOC, NH4+–N, NO3−–N from different wetland environments under nitrogen inputs. The numbers in the figure represent the number of case studies. For details on effect size interpretation, refer to Fig. 2.
For all types of nitrogen compounds, nitrogen input added the soil NH4+–N and NO3−–N (Fig. 3). The effect of nitrogen input in terms of NH4+, NH4NO3 and NO3− on the soil NH4+–N and NO3−–N content was larger than that of organic nitrogen fertilizer. This means that inorganic nitrogen input significantly and directly promotes soil NH4+–N and NO3−–N formation. Similarly, the soil DOC content was increased by adding inorganic nitrogen, and significantly lessened by adding organic nitrogen fertilizer. We also revealed that all nitrogen input rates promoted NH4+–N and NO3−–N formation. However, nitrogen input rate of 0–50 and 50–100 kg ha−1·yr−1 had larger impacts on the soil NH4+–N and NO3−–N formation than other input rates. It signifies that lower nitrogen input rates significantly expanded the soil nitrogen availability. Similarly, lower nitrogen input rates showed significant and positive effects on the soil DOC contents. In contrast, a nitrogen input rate of 100–200 kg ha−1·yr−1 (SMD = −0.96, Bootstrap CIs = −1.66 to −0.35) significantly decreased the soil DOC content.
3.3. Changes in soil microbial biomass under nitrogen input in wetland ecosystems
Fig. 4 shows that the overall SMD of soil microbial biomass carbon (MBC) and nitrogen (MBN) were 1.24 and 3.01 (Bootstrap CIs of 0.87–1.61 and 2.47 to 3.56, respectively), and presented positive effects of nitrogen input on MBC and MBN significantly. The results showed that for estuary wetlands and peatlands, nitrogen input significantly added the MBC (means of SMD = 3.54 to 4.33 in Bootstrap CIs) and the MBN (means of SMD = 1.91 to 3.65 in Bootstrap CIs). For freshwater marsh and alpine wetland, nitrogen input reduced the MBC and MBN. Compared to other climates, the alpine climate had the largest SMD of MBC (SMD = 2.10, Bootstrap CIs = 1.51 to 2.73) and MBN (SMD = 3.44, Bootstrap CIs = 2.80 to 4.14 in). It suggests that for the alpine climate, nitrogen input significantly multiplied the activity of soil microbes. Similarly, the effect of nitrogen deposition on soil microbial biomass was greater than that of fertilization.
Fig. 4.
Mean effect size for MBC and MBN from different wetland environments under nitrogen inputs. The numbers in the figure represent the number of case studies. For details on effect size interpretation, refer to Fig. 2.
As shown in Fig. 4, inorganic nitrogen input significantly increased the MBC (SMD range from 1.79 to 4.27 in Bootstrap CIs) and MBN (means of SMD range from 1.00 to 1.88 in Bootstrap CIs). Specially, the SMD was the largest for nitrogen input as NH4+ compared to the other compounds. This means that NH4+ input could significantly multiply soil microbial biomass formation. As seen from Fig. 4, nitrogen input rates of 0–100 kg ha−1·yr−1 significantly added the MBC (means of SMD = 1.28, 2.86 in Bootstrap CIs) and MBN (means of SMD = 3.01, 4.79 in Bootstrap CIs). In contrast, nitrogen input rates of more than 100 kg ha−1·yr−1 dwindled the MBC and MBN, except for the case of nitrogen input rates of more than 300 kg ha−1·yr−1. This indicated that lower nitrogen input rates significantly increased the soil microbial biomass contents.
4. Discussion
4.1. Impact of soil labile carbon and nitrogen compounds on greenhouse gas emissions
4.1.1. The effect of soil labile carbon and nitrogen on greenhouse gas emissions
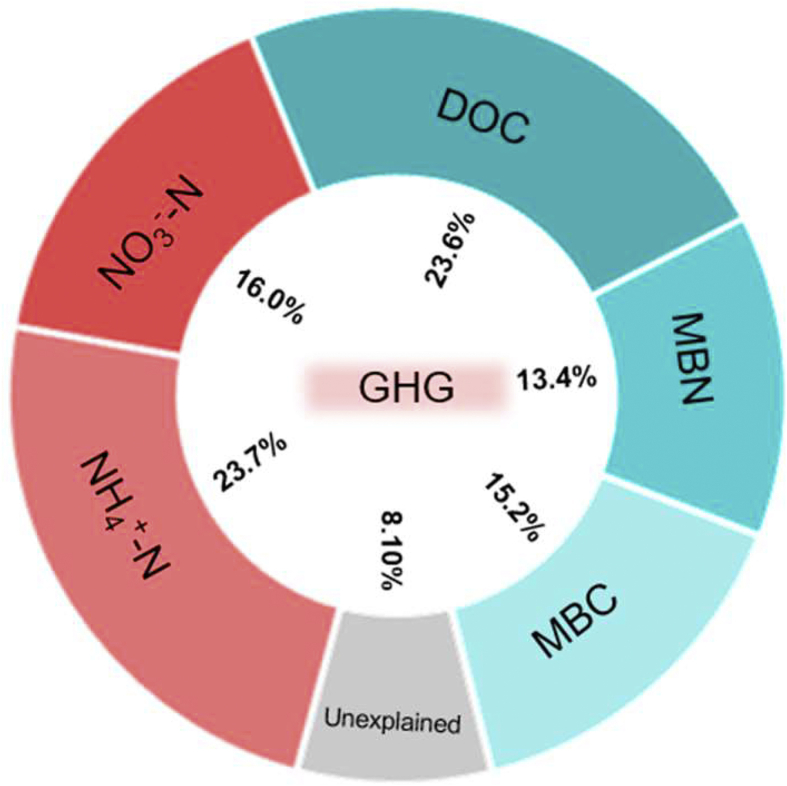
The meta-analysis indicated that the nitrogen input significantly augmented the soil labile carbon and nitrogen content at the global scale (Fig. 3). The increase of soil labile carbon and nitrogen maybe because the nitrogen input changed the stability of soil aggregates and promoted the leaching of DOC, NH4+–N and NO3−–N from soil [39,52]. The meta-analysis also revealed that nitrogen input significantly multiplied decomposition of organic matter and subsequent gas formation (Fig. 2, Fig. 3). However, nitrogen input influenced the activity of soil microbe by altering the ratio of available carbon to nitrogen [53]. This study clarified that NH4+–N and DOC play leading roles in greenhouse gas emissions according to a variation-partitioning analysis (Fig. 5). Higher NH4+–N and DOC promoted CH4 emission due to the increase in carbon availability, which resulted in more substrate being available for methanogens [54]. However, excessive NH4+-N competitively inhibited CH4 oxidation [55,56]. Meanwhile, ammonium oxidation produced toxic byproducts that noncompetitively inhibit CH4 oxidation [57]. Additionally, the DOC could regulate carbon availability, thereby affecting the soil microbial activity [14,58]. The increase of DOC also altered the content of the soil inorganic nitrogen under the rewetting system because changing carbon availability would affect organic nitrogen mineralization and inorganic nitrogen assimilation [59]. Therefore, the effect of DOC on the CH4 and CO2 emissions is more important than NH4+–N, and higher DOC content could stimulate bacteria that are responsible for organic matter decomposition and methanogenesis [54], leading to promote CH4 and CO2 emissions.
Fig. 5.
Variation-partitioning analysis of the effects of soil labile carbon and nitrogen on greenhouse gas emissions. NH4+-N, ammonium; NO3−-N, nitrate; DOC, dissolved organic carbon; MBN, microbial biomass nitrogen; MBC, microbial biomass carbon.
It is known that N2O is mainly produced during nitrification but some N2O can also be formed during denitrification, which is affected by nitrogen availability [60]. However, with the increase of anthropogenic activities, nitrogen input disrupted the balance of soil elemental stoichiometry, thereby affecting nitrogen availability [61,62]. The soil elemental stoichiometry determines the concentration and fractions of soil carbon and nitrogen [63,64]. A Pearson correlation analysis revealed the effect of soil labile nitrogen and carbon on N2O emissions. The results showed that DOC had a significant and positive effect on N2O emissions (Table .2) because higher DOC increased the nitrogen utilization rate and microbial activity [14]. Meanwhile, NH4+–N also showed a significant and positive effect on N2O emissions (Table .2) because soil microbes utilize NH4+–N at a lower energy cost than NO3−–N [14,65].
Table 2.
The Pearson relationships between greenhouse gas and labile carbon and nitrogen.
| Parameters | NH4+–N | NO3−–N | DOC | MBN | MBC | |
|---|---|---|---|---|---|---|
| CO2 | Pearson Correlation | 0.567 | 0.381 | 0.53 | 0.508 | 0.428 |
| p-value | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | |
| CH4 | Pearson Correlation | 0.512 | 0.459 | 0.501 | 0.394 | 0.431 |
| p-value | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | |
| N2O | Pearson Correlation | 0.383 | 0.282 | 0.43 | 0.175 | 0.277 |
| p-value | 0.001 | 0.022 | <0.001 | 0.159 | 0.024 | |
To elucidate which soil labile carbon and nitrogen are the main drivers of greenhouse gas emissions from wetland ecosystems under global nitrogen input, a structural equation model was established. Structural equation models are often utilized to investigate “latent” effects among various measured variables [9,66]. This research indicated that NH4+–N showed a positive and significant direct effect on CO2 emissions based on the structural equation model (Fig. 6, Fig. 7), because higher NH4+-N could increase the availability of carbon [67,68]. In addition, higher soil NH4+-N could also promote plant photosynthesis and increase plant biomass, leading to an increase in the autotrophic respiration of plants [41]. However, the study revealed that NH4+–N significantly and indirectly affected CH4 and N2O emissions by affecting the DOC (Table .3, Fig. 6). Additionally, the total effect of NO3−–N (0.112–0.339) on a single greenhouse gas emission was larger than that of DOC (−0.02 to 0.093) according to Fig. 6(d, e, f) and Fig. 7. The effect of NO3−–N on greenhouse gas emissions was significant and was indirectly shown by its effect on DOC (Table .3). These results reveal that DOC was the most important factor for greenhouse gas emission. The results also suggested that DOC affected microbial activity more directly than NH4+–N and NO3−–N. The soil DOC is an organic carbon source directly utilized by microbes [69,70], and it is the main substrate and energy source for microbial metabolism [71]. Thus, DOC concentrations directly determine greenhouse gas emissions by regulating microbial metabolism in comparison to soil ammonium and nitrate [[72], [73], [74]].
Fig. 6.
Structural equation model (SEM) evaluating the direct effects on greenhouse gases (a–c) and the standardized total effect (direct plus indirect effects) derived from the SEM (d–f) on a global scale. The number represents the direct effects on greenhouse gas emissions. The various widths of the gray lines represent p < 0.001, p < 0.005, p<0.01 and p>0.01.
Table 3.
The indirect effect of labile carbon and nitrogen to greenhouse gas emissions from wetland ecosystem under nitrogen input.
| Parameters | NH4+-N | NO3−-N | MBN | DOC | MBC |
|---|---|---|---|---|---|
| CO2 | 0.177 | 0.112 | 0.021 | −0.023 | 0 |
| CH4 | 0.145 | 0.037 | 0.063 | 0.067 | 0 |
| N2O | 0.045 | −0.009 | −0.02 | −0.011 | 0 |
4.1.2. The effect of soil microbial biomass on greenhouse gas emission
Although soil microbial biomass only accounts for 1%–5% of soil organic matter, it plays an important role in promoting material transformation and energy flow in the soil [18,75]. This research showed that the global nitrogen input significantly added the soil microbial biomass carbon and nitrogen in wetland ecosystems (Fig. 4, Fig. 7). It might be that nitrogen input multiplies nitrogen sources for microbial metabolism [76]. Nitrogen input could also change greenhouse gas production and emission by increased decomposition rates [77,78]. Thus, based on the Pearson correlation analysis (Table .2), the MBC and MBN showed a significant relationship with CH4 and N2O (p < 0.01). The soil microbial biomass is a sensitive measure of microbial activity [79]. Soil microbial biomass is more easily utilized by the microorganism for mineralization and assimilation than other fractions of soil organic matter [80]. Therefore, soil microbial biomass influenced greenhouse gas production and emission by changing the substrate content for organic matter mineralization and methanogenesis.
To clearly elucidate the effect of soil microbial biomass carbon and nitrogen on greenhouse gas emission, this research utilized the structural equation model to determine that MBN significantly and indirectly influenced the CH4 and CO2 emission by affecting MBC (Fig. 7, Table .3). The total effect of MBC on greenhouse gas showed that MBC negatively affected CO2 and N2O emissions and positively affected CH4 emissions. Nitrogen input typically accelerated the anaerobic decomposition of MBC [14], and greater MBC provided more biologic residues as substrates for methanogens, which promoted the CH4 generation [21,81]. This SEM also illustrated that MBC is the main pathway by which DOC affects greenhouse gas emissions (Figs. 6 and 0.507 to 0.714, p < 0.001). Soil microbial biomass carbon is the labile fraction of soil organic carbon, and has some particular characteristics including poor stability, fast turnover rate, easy mineralization and decomposition [82,83]. MBC can act as metabolism substrate for soil microbes and can sensitively affect the activity of functional microorganisms, resulting to promote greenhouse gas production and emission. By combining a variation-partitioning analysis and a structural equation model, this study inferred that MBC was the most direct indicator of the response of greenhouse gas emissions to nitrogen input in wetlands ecosystems.
4.2. Impact of environmental factors on greenhouse gas emissions
4.2.1. The effect of wetland type and climates on greenhouse gas emissions
Greenhouse gas emission is affected by the different physicochemical properties (including plant types, water table, saline level and so on) of wetlands [[84], [85], [86]]. As seen in Fig. 2, for freshwater wetlands, nitrogen input significantly promoted CH4 and CO2 emissions compared to other types of wetlands. This may be because freshwater wetlands could decrease the effect of osmotic stress due to their lower saline level, leading to multiplying microbial activity and the decomposition rate of organic matter [30,87]. These results also revealed that for peatland, nitrogen input significantly promoted N2O emission compared to other wetlands (Fig. 2). Although peatlands cover only 3% of the Earth’s surface, they store one-third of the global organic carbon pool and conserve the higher nitrogen stocks [88,89]. Previous studies indicated natural peatlands display negligible N2O emissions and can even act as net sinks for N2O [89]. The external nitrogen enhanced microbial activity and triggered a priming effect that further facilitated the release of available nitrogen [105]. Thus, nitrogen input has a greater effect on N2O emission from peatlands than other wetlands.
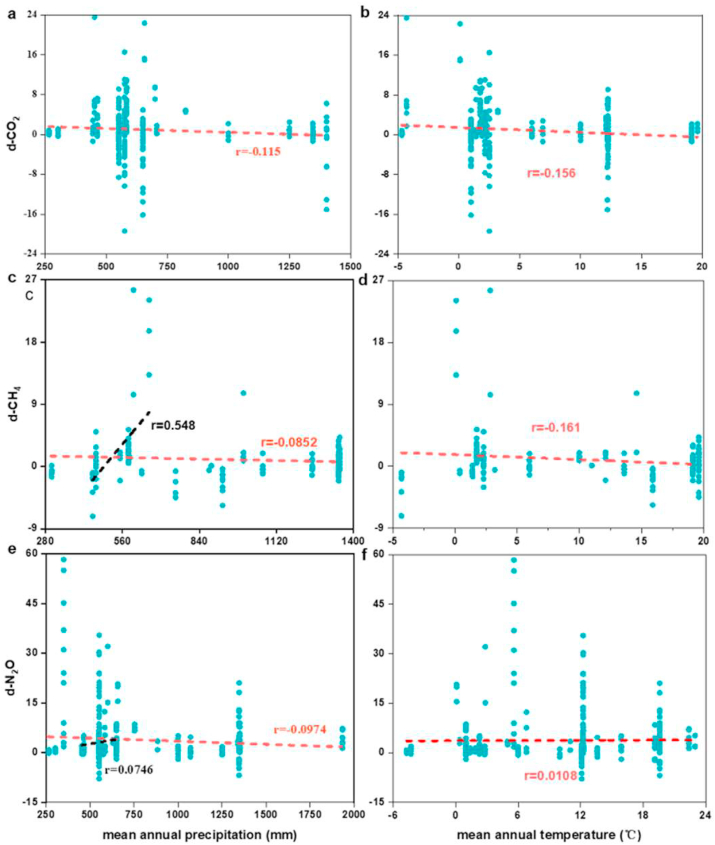
Climate can influence greenhouse gas emissions from wetlands by changing rainfall and temperature. A regression analysis revealed that greenhouse gas emission reduced as the mean annual precipitation (MAP) increased (Fig. 8 a, c, e). Fig. 2 shows that for temperate continental climate, nitrogen input dwindled CH4 and CO2 emissions. Although nitrogen input promoted N2O emissions for all types of climate, it was significantly lower for temperate continental climate than other types of climates. These results indicated that nitrogen input promoted greenhouse gas emissions the least for the temperate continental climate. In particular, the regression analysis of the effect size and MAP as it ranged from 400 mm to 700 mm (typical for the temperate continental climate) indicated that the CH4 and N2O emissions increased with the increase of mean annual precipitation (Fig. 8). Therefore, lower greenhouse gas emissions in the temperate continental climate were likely due to soil drought and osmotic stress caused by lower MAP [90], which would destroy the microbial community and restrain the microbial activity in the wetland ecosystem [91].
Fig. 8.
Regression analysis for the effect size of greenhouse gas emission and mean annual precipitation, mean annual temperature at the global level. The red short dash represents the regression analysis of the effect size and mean annual precipitation and mean annual temperature among all studies. The black short dash represents the regression analysis of the effect size and mean annual precipitation in the range from 400 to 700 mm. d-CO2 is the effect size of carbon dioxide, d-CH4 is the effect size of methane, d-N2O is the effect size of nitrous oxide.
The regression analysis revealed that greenhouse gas emissions reduced with the increase of mean annual temperature (MAT), except for N2O (Fig. 8 b, d, f). As we know, temperature could influence the soil microbial activity and thereby affect N2O emissions. It is likely that higher temperatures altered the content of the soil oxygen and available carbon, thus producing anoxic conditions for denitrifying bacteria [92,93]. In addition, when the soil temperature ranged from 10 to 35 °C, the denitrification activity increased with the increase in environmental temperature [94,95]. Contrary to N2O emissions, the CO2 and CH4 emissions decreased as the environment warming. It is likely that the interaction of warming and nitrogen input increased the content of soil available nitrogen and carbon and decreased the soil pore water, leading to promote the activity of methanotrophs higher than methanogens [36]. Warming increased the soil carbon mineralization and nitrogen turnover rate, whereas nitrogen input promoted the assimilation of labile carbon by soil microbes and led to carbon sequestration and lower rates of nitrogen cycling [96,97]. It was probably that the soil carbon sequestration was greater than mineralization under the interaction of warming and nitrogen input, leading to a decrease in CO2 emissions.
4.2.2. The effect of nitrogen input on greenhouse gas emission
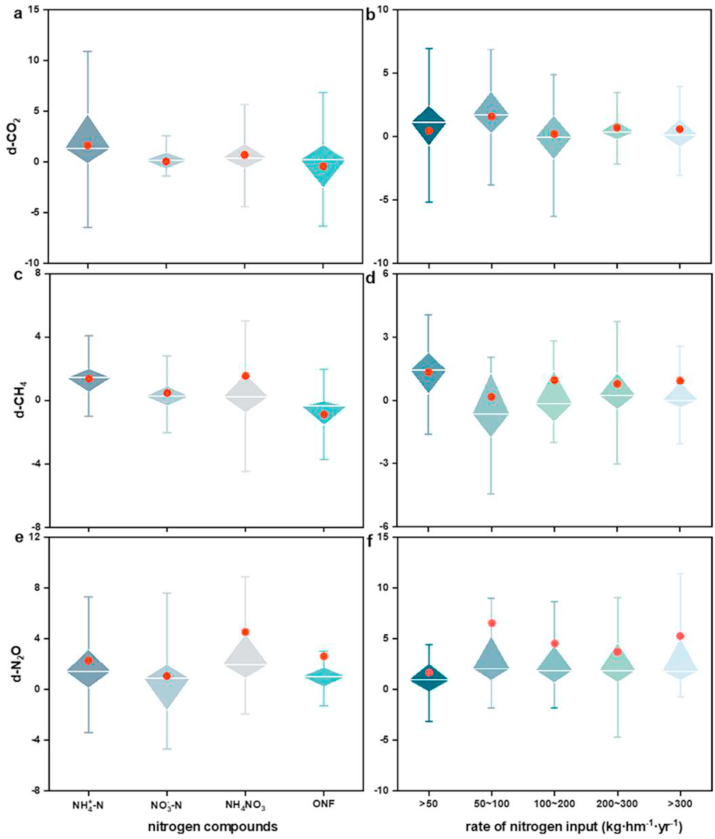
The continual increase of anthropogenic nitrogen inputs has already altered the rates of nitrogen cycling and nitrogen availability [[19], [98], [105]], which are affected by different nitrogen compounds and nitrogen input rates [[99], [100], [101]]. For different nitrogen compounds (Fig. 9 a, c, e), greenhouse gas emissions are promoted by nitrogen input as inorganic nitrogen (NH4+-N, NO3−-N and NH4NO3). Nitrogen input as organic nitrogen fertilizer suppresses CH4 and CO2 emissions and promotes N2O emissions. This may be because soil microorganisms have different capacities to use the various nitrogen compounds, leading to the differences in greenhouse gas emissions [47]. Compared to other nitrogen compounds, nitrogen input as NH4+-N and NH4NO3 largely promoted greenhouse gas emissions (Fig. 9 a, c, e). The microbial utilization of ammonium is preferred over nitrate due to the low energy cost, implying that soil ammonium oxidation and organic matter decomposition were stimulated with the input of ammonium [102]; Tao et al., 2018). Notably, nitrogen input rates of 0–50 kg ha−1·yr−1 significantly promoted CH4 emissions than other nitrogen input rates (Figs. 2 and 9 d). The CO2 and N2O emissions for nitrogen input rates of 50–100 kg ha−1·yr−1 were larger than for the other nitrogen input rates (Fig. 9 b, f). These results illustrated that lower nitrogen input significantly promoted greenhouse gas emissions from wetland ecosystems. This arose mainly because higher nitrogen input reduced the microbial biomass and activity by increasing the effect of osmotic stress and electrical conductivity [30,87]. Additionally, continuous and massive nitrogen input led to soil acidification, thereby directly or indirectly affecting the composition of the soil microbial diversity and community [103,104].
Fig. 9.
The effect size of greenhouse gas emission among different nitrogen compounds and the nitrogen input rate. d-CO2 is the effect size of carbon dioxide, d-CH4 is the effect size of methane, and d-N2O is the effect size of nitrous oxide. NH4+-N represents ammonium, NO3−-N represents nitrate, NH4NO3 represents ammonium nitrate, ONF represents organic nitrogen fertilizer. The red point represents the mean effect size of greenhouse gas emission under different nitrogen inputs and nitrogen input rates.
5. Conclusions
This meta-analysis found that nitrogen input significantly promoted greenhouse gas emissions from wetlands on a global scale. The driving effect of soil labile carbon and nitrogen and nitrogen inputs on greenhouse gas emissions from wetlands ecosystems are summarized as follows:
-
(1)
DOC is the most important driving factor for greenhouse gas emissions from wetlands under global nitrogen input.
-
(2)
MBC is the most direct driving factor for greenhouse gas emissions from wetlands under global nitrogen input.
-
(3)
Nitrogen input to freshwater wetlands shows the most significant and positive effects on CH4 and CO2 emissions from wetlands under global nitrogen input, whereas nitrogen input to peatland largely and significantly promotes N2O emissions compared to other wetlands.
-
(4)
Nitrogen input as ammonium compounds and at lower rates show the most significant and positive effects on greenhouse gas emissions from wetlands under global nitrogen input.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 51978099) and Chongqing Talents Plan for Young Talents (CQY201905062). Thanks for Gao Han from northwest agriculture and forestry university teaching me how to use software for meta-analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2020.100063.
Contributor Information
Mengli Chen, Email: abelardlewis@163.com.
Lian Chang, Email: changliancq@126.com.
Junmao Zhang, Email: zjmaooo@cqu.edu.cn.
Fucheng Guo, Email: fcguo@cqu.edu.cn.
Jan Vymazal, Email: vymazal@yahoo.com.
Qiang He, Email: heqiang@cqu.edu.cn.
Yi Chen, Email: chenyi8574@cqu.edu.cn.
Author contributions
Mengli Chen and Yi Chen designed the study. Mengli Chen, Lian Chang, Junmao Zhang and Fucheng Guo collected references and got data. Mengli Chen wrote and modified this article. Yi Chen, Jan Vymazal and Qiang He reviewed this article.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Galloway J.N., Dentener F.J., Capone D.G., Boyer E.W., Howarth R.W., Seitzinger S.P., Asner G.P., Cleveland C.C., Green P.A., Holland E.A., Karl D.M., Michaels A.F., Porter J.H., Townsend A.R., Vorosmarty C.J. Nitrogen cycles: past, present, and future. Biogeochemistry. 2004;70(2):153–226. doi: 10.1007/s10533-004-0370-0. [DOI] [Google Scholar]
- 2.Song C., Liu D., Song Y., Mao R. Effect of nitrogen addition on soil organic carbon in freshwater marsh of Northeast China. Environ. Earth Sci. 2013;70(4):1653–1659. doi: 10.1007/s12665-013-2252-z. [DOI] [Google Scholar]
- 3.Yamamoto A., Akiyama H., Nakajima Y., Hoshino Y.T. Estimate of bacterial and fungal N2O production processes after crop residue input and fertilizer application to an agricultural field by N-15 isotopomer analysis. Soil Biol. Biochem. 2017;108:9–16. doi: 10.1016/j.soilbio.2017.01.015. [DOI] [Google Scholar]
- 4.Tao B., Wang Y., Yu Y., Li Q., Luo C., Zhang B. Interactive effects of nitrogen forms and temperature on soil organic carbon decomposition in the coastal wetland of the Yellow River Delta, China. Catena. 2018;165:408–413. doi: 10.1016/j.catena.2018.02.025. [DOI] [Google Scholar]
- 5.Johnson D.S., Warren R.S., Deegan L.A., Mozdzer T.J. Saltmarsh plant responses to eutrophication. Ecol. Appl. 2016;26(8):2647–2659. doi: 10.1002/eap.1402. [DOI] [PubMed] [Google Scholar]
- 6.Li Q.W., Zhang X.Y., Gao J.Q., Song M.H., Liang J.F., Yue Y. Effects of N Addition frequency and quantity on Hydrocotyle vulgaris growth and greenhouse gas emissions from wetland microcosms. Sustainability. 2019;11(6) doi: 10.3390/su11061520. art1520. [DOI] [Google Scholar]
- 7.Zhao S., Su X., Wang Y., Yang X., Bi M., He Q., Chen Y. Copper oxide nanoparticles inhibited denitrifying enzymes and electron transport system activities to influence soil denitrification and N2O emission. Chemosphere. 2020;245 doi: 10.1016/j.chemosphere.2019.125394. art125394. [DOI] [PubMed] [Google Scholar]
- 8.Guo F., Zhang J., Yang X., He Q., Ao L., Chen Y. Impact of biochar on greenhouse gas emissions from constructed wetlands under various influent chemical oxygen demand to nitrogen ratios. Bioresour. Technol. 2020;303 doi: 10.1016/j.biortech.2020.122908. [DOI] [PubMed] [Google Scholar]
- 9.Su X., Chen Y., Wang Y., Yang X., He Q. Impacts of chlorothalonil on denitrification and N2O emission in riparian sediments: microbial metabolism mechanism. Water Res. 2019;148:188–197. doi: 10.1016/j.watres.2018.10.052. [DOI] [PubMed] [Google Scholar]
- 10.Doroski A.A., Helton A.M., Vadas T.M. Greenhouse gas fluxes from coastal wetlands at the intersection of urban pollution and saltwater intrusion: a soil core experiment. Soil Biol. Biochem. 2019;131:44–53. doi: 10.1016/j.soilbio.2018.12.023. [DOI] [Google Scholar]
- 11.Keller J.K., Bridgham S.D., Chapin C.T., Iversen C.M. Limited effects of six years of fertilization on carbon mineralization dynamics in a Minnesota fen. Soil Biol. Biochem. 2005;37(6):1197–1204. doi: 10.1016/j.soilbio.2004.11.018. [DOI] [Google Scholar]
- 12.Banger K., Tian H.Q., Lu C.Q. Do nitrogen fertilizers stimulate or inhibit methane emissions from rice fields? Global Change Biol. 2012;18(10):3259–3267. doi: 10.1111/j.1365-2486.2012.02762.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang X., He Q., Guo F., Sun X., Zhang J., Chen Y. Impacts of carbon-based nanomaterials on nutrient removal in constructed wetlands: microbial community structure, enzyme activities, and metabolism process. J. Hazard Mater. 2021;401:art123270. doi: 10.1016/j.jhazmat.2020.123270. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Liu J., He L., Dou J., Zhao H. Responses of carbon dynamics to nitrogen deposition in typical freshwater wetland of sanjiang plain. J. Chem. 2014;1–9 doi: 10.1155/2014/603948. [DOI] [Google Scholar]
- 15.Luan J.W., Wu J.H., Liu S.R., Roulet N., Wang M. Soil nitrogen determines greenhouse gas emissions from northern peatlands under concurrent warming and vegetation shifting. Commun. Biol. 2019;2:1–10. doi: 10.1038/s42003-019-0370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao W., Cheng S., Fang H., Chen Y., Yu G., Zhou M., Zhang P., Xu M. Effects of simulated atmospheric nitrogen deposition on inorganic nitrogen content and acidification in a cold-temperate coniferous forest soil. Acta Ecol. Sin. 2013;33(2):114–121. doi: 10.1016/j.chnaes.2013.01.008. [DOI] [Google Scholar]
- 17.Min K., Kang H., Lee D. Effects of ammonium and nitrate additions on carbon mineralization in wetland soils. Soil Biol. Biochem. 2011;43(12):2461–2469. doi: 10.1016/j.soilbio.2011.08.019. [DOI] [Google Scholar]
- 18.Ren F., Sun N., Xu M., Zhang X., Wu L., Xu M. vol. 194. Soil Tillage Res.; 2019. (Changes in Soil Microbial Biomass with Manure Application in Cropping Systems: A Meta-Analysis). [DOI] [Google Scholar]
- 19.Cui Q., Song C., Wang X., Shi F., Wang L., Guo Y. Rapid N2O fluxes at high level of nitrate nitrogen addition during freeze-thaw events in boreal peatlands of Northeast China. Atmos. Environ. 2016;135:1–8. doi: 10.1016/j.atmosenv.2016.03.053. [DOI] [Google Scholar]
- 20.Kastovska E., Picek T., Barta J., Mach J., Cajthaml T., Edwards K. Nutrient addition retards decomposition and C immobilization in two wet grasslands. Hydrobiologia. 2012;692(1):67–81. doi: 10.1007/s10750-012-1017-0. [DOI] [Google Scholar]
- 21.Song Y., Song C., Meng H., Swarzenski C.M., Wang X., Tan W. Nitrogen additions affect litter quality and soil biochemical properties in a peatland of Northeast China. Ecol. Eng. 2017;100:175–185. doi: 10.1016/j.ecoleng.2016.12.025. [DOI] [Google Scholar]
- 22.Song Y., Song C., Li Y., Hou C., Yang G., Zhu X. Short-term effects of nitrogen addition and vegetation removal on soil chemical and biological properties in a freshwater marsh in Sanjiang Plain, Northeast China. Catena. 2013;104:265–271. doi: 10.1016/j.catena.2012.12.008. [DOI] [Google Scholar]
- 23.Deng L., Huang C., Dong-Gill K., Shangguan Z., Wang K., Song X., Peng C. Soil GHG fluxes are altered by N deposition: new data indicate lower N stimulation of the N2O flux and greater stimulation of the calculated C pools. Glob. change biol. 2020;26(4):2613–2629. doi: 10.1111/gcb.14970. [DOI] [PubMed] [Google Scholar]
- 24.Juutinen S., Moore T.R., Bubier J.L., Arnkil S., Humphreys E., Marincak B., Roy C., Larmola T. Long-term nutrient addition increased CH4 emission from a bog through direct and indirect effects. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-22210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou F., Shang Z., Zeng Z., Piao S., Ciais P., Raymond P.A., Wang X., Wang R., Chen M., Yang C., Tao S., Zhao Y., Meng Q., Gao S., Mao Q. New model for capturing the variations of fertilizer-induced emission factors of N2O. Global Biogeochem. Cycles. 2015;29(6):885–897. doi: 10.1002/2014GB005046. [DOI] [Google Scholar]
- 26.Kim H., Bae H.-S., Reddy K.R., Ogram A. Distributions, abundances and activities of microbes associated with the nitrogen cycle in riparian and stream sediments of a river tributary. Water Res. 2016;106:51–61. doi: 10.1016/j.watres.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 27.Yang X., Chen Y., Guo F., Liu X., Su X., He Q. Metagenomic analysis of the biotoxicity of titanium dioxide nanoparticles to microbial nitrogen transformation in constructed wetlands. J. Hazard Mater. 2020;384 doi: 10.1016/j.jhazmat.2019.121376. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L., Shao H., Wang B., Zhang L., Qin X. Effects of nitrogen and phosphorus on the production of carbon dioxide and nitrous oxide in salt-affected soils under different vegetation communities. Atmos. Environ. 2019;204:78–88. doi: 10.1016/j.atmosenv.2019.02.024. [DOI] [Google Scholar]
- 29.Finlayson C.M., Van der Valk A.G. Wetland classification and inventory: a summary. Vegetatio. 1995;118(1):185–192. doi: 10.1007/BF00045199. [DOI] [Google Scholar]
- 30.Hu M., Penuelas J., Sardans J., Huang J., Li D., Tong C. Effects of nitrogen loading on emission of carbon gases from estuarine tidal marshes with varying salinity. Sci. Total Environ. 2019;667:648–657. doi: 10.1016/j.scitotenv.2019.02.429. [DOI] [PubMed] [Google Scholar]
- 31.IPCC . Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2013. Climate Change. The Physical Science Basis. [Google Scholar]
- 32.Wang C., Liu D., Bai E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018;120:126–133. doi: 10.1016/j.soilbio.2018.02.003. [DOI] [Google Scholar]
- 33.Berthrong S.T., Yeager C.M., Gallegos-Graves L., Steven B., Eichorst S.A., Jackson R.B., Kuske C.R. Nitrogen fertilization has a stronger effect on soil nitrogen-fixing bacterial communities than elevated atmospheric CO2. Appl. Environ. Microbiol. 2014;80(10):3103–3112. doi: 10.1128/AEM.04034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng L., Peng C.H., Zhu G.Y., Chen L., Liu Y.L., Shangguan Z.P. Positive responses of belowground C dynamics to nitrogen enrichment in China. Sci. Total Environ. 2018;616:1035–1044. doi: 10.1016/j.scitotenv.2017.10.215. [DOI] [PubMed] [Google Scholar]
- 35.Treseder K.K. Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol. Lett. 2008;11(10):1111–1120. doi: 10.1111/j.1461-0248.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen X., Wang G., Zhang T., Mao T., Wei D., Song C., Hu Z., Huang K. Effects of warming and nitrogen fertilization on GHG flux in an alpine swamp meadow of a permafrost region. Sci. Total Environ. 2017;601:1389–1399. doi: 10.1016/j.scitotenv.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Hu M., Wilson B.J., Sun Z., Ren P., Tong C. Effects of the addition of nitrogen and sulfate on CH4 and CO2 emissions, soil, and pore water chemistry in a high marsh of the Min River estuary in southeastern China. Sci. Total Environ. 2017;579:292–304. doi: 10.1016/j.scitotenv.2016.11.103. [DOI] [PubMed] [Google Scholar]
- 38.Lu M., Yang Y., Luo Y., Fang C., Zhou X., Chen J., Yang X., Li B. Responses of ecosystem nitrogen cycle to nitrogen addition: a meta-analysis. New Phytol. 2011;189(4):1040–1050. doi: 10.1111/j.1469-8137.2010.03563.x. [DOI] [PubMed] [Google Scholar]
- 39.Song Y., Song C., Tao B., Wang J., Zhu X., Wang X. Short-term responses of soil enzyme activities and carbon mineralization to added nitrogen and litter in a freshwater marsh of Northeast China. Eur. J. Soil Biol. 2014;61:72–79. doi: 10.1016/j.ejsobi.2014.02.001. [DOI] [Google Scholar]
- 40.IPCC . Cambridge University Press; Cambridge, UK and New York, NY: 2007. Climate Change 2007: the Physical Science Basis. [Google Scholar]
- 41.Liu L., Greaver T.L. A review of nitrogen enrichment effects on three biogenic GHGs: the CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol. Lett. 2009;12(10):1103–1117. doi: 10.1111/j.1461-0248.2009.01351.x. [DOI] [PubMed] [Google Scholar]
- 42.Hedges L.V., Olkin I. Statistical Methods for Meta-Analysis. 1985;1984(24):25–42. [Google Scholar]
- 43.Rosenberg M.S., Adams D.C., Gurevitch J. Sinauer Associates; Sunderland, MA: 2000. MetaWin: Statistical Software for Meta-Analysis. Version 2.0. [Google Scholar]
- 44.Aguilar R., Jacob Cristobal-Perez E., Balvino-Olvera F.J., Aguilar-Aguilar M.d.J., Aguirre-Acosta N., Ashworth L., Lobo J.A., Marten-Rodriguez S., Fuchs E.J., Sanchez-Montoya G., Bernardello G., Quesada M. Habitat fragmentation reduces plant progeny quality: a global synthesis. Ecol. Lett. 2019;22(7):1163–1173. doi: 10.1111/ele.13272. [DOI] [PubMed] [Google Scholar]
- 45.Xia L., Lam S.K., Chen D., Wang J., Tang Q., Yan X. Can knowledge-based N management produce more staple grain with lower greenhouse gas emission and reactive nitrogen pollution? A meta-analysis. Global Change Biol. 2017;23(5):1917–1925. doi: 10.1111/gcb.13455. [DOI] [PubMed] [Google Scholar]
- 46.Li L., Zheng Z., Wang W., Biederman J.A., Xu X., Ran Q., Qian R., Xu C., Zhang B., Wang F., Zhou S., Cui L., Che R., Hao Y., Cui X., Xu Z., Wang Y. Terrestrial N2 O emissions and related functional genes under climate change: a global meta-analysis. Glob. change biol. 2019;26(2):931–943. doi: 10.1111/gcb.14847. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y., Cheng H., Gao H., An S. Response and driving factors of soil microbial diversity related to global nitrogen addition. Land Degrad. Dev. 2019;31(2):190–204. doi: 10.1002/ldr.3439. [DOI] [Google Scholar]
- 48.Lajeunesse M.J. On the meta-analysis of response ratios for studies with correlated and multi-group designs. Ecology. 2011;92(11):2049–2055. doi: 10.1890/11-0423.1. [DOI] [PubMed] [Google Scholar]
- 49.Limpens J., Granath G., Gunnarsson U., Aerts R., Bayley S., Bragazza L., Bubier J., Buttler A., van den Berg L.J.L., Francez A.J., Gerdol R., Grosvernier P., Heijmans M.M.P.D., Hoosbeek M.R., Hotes S., Ilomets M., Leith I., Mitchell E.A.D., Moore T., Nilsson M.B., Nordbakken J.F., Rochefort L., Rydin H., Sheppard L.J., Thormann M., Wiedermann M.M., Williams B.L., Xu B. Climatic modifiers of the response to nitrogen deposition in peat-forming Sphagnum mosses: a meta-analysis. New Phytol. 2011;191(2):496–507. doi: 10.1111/j.1469-8137.2011.03680.x. [DOI] [PubMed] [Google Scholar]
- 50.Loydi A., Eckstein R.L., Otte A., Donath T.W. Effects of litter on seedling establishment in natural and semi-natural grasslands: a meta-analysis. J. Ecol. 2013;101(2):454–464. doi: 10.1111/1365-2745.12033. [DOI] [Google Scholar]
- 51.Zhou Y., Staver A.C. Enhanced activity of soil nutrient-releasing enzymes after plant invasion: a meta-analysis. Ecology. 2019;28:191–197. doi: 10.1002/ecy.2830. [DOI] [PubMed] [Google Scholar]
- 52.Wang F.L., Bettany J.R. Influence of freeze-thaw and flooding on the loss of soluble organic-carbon and carbon-dioxide from soil. J. Environ. Qual. 1993;22(4):709–714. [Google Scholar]
- 53.Janssens I.A., Dieleman W., Luyssaert S., Subke J.A., Reichstein M., Ceulemans R., Ciais P., Dolman A.J., Grace J., Matteucci G., Papale D., Piao S.L., Schulze E.D., Tang J., Law B.E. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010;3(5):315–322. doi: 10.1038/ngeo844. [DOI] [Google Scholar]
- 54.Bodelier P.L.E., Roslev P., Henckel T., Frenzel P.J.N. Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature. 2000;403:421–424. doi: 10.1038/35000193. [DOI] [PubMed] [Google Scholar]
- 55.Hu A., Lu Y. The differential effects of ammonium and nitrate on methanotrophs in rice field soil. Soil Biol. Biochem. 2015;85:31–38. doi: 10.1016/j.soilbio.2015.02.033. [DOI] [Google Scholar]
- 56.Wang Z.P., Ineson P. Methane oxidation in a temperate coniferous forest soil: effects of inorganic N. Soil Biol. Biochem. 2003;35(3):427–433. doi: 10.1016/S0038-0717(02)00294-8. [DOI] [Google Scholar]
- 57.Yang N., Lü F., He P., Shao L. Response of methanotrophs and methane oxidation on ammonium application in landfill soils. Appl. Microbiol. Biotechnol. 2011;92(5):1073–1082. doi: 10.1007/s00253-011-3389-x. [DOI] [PubMed] [Google Scholar]
- 58.Boyer J.N., Groffman P.M. Bioavailability of water extractable organic carbon fractions in forest and agricultural soil profiles. Soil Biol. Biochem. 1996;28(6) doi: 10.1016/0038-0717(96)00015-6. 0-790. [DOI] [Google Scholar]
- 59.Morillas L., Duran J., Rodriguez A., Roales J., Gallardo A., Lovett G.M., Groffman P.M. Nitrogen supply modulates the effect of changes in drying-rewetting frequency on soil C and N cycling and greenhouse gas exchange. Global Change Biol. 2015;21(10):3854–3863. doi: 10.1111/gcb.12956. [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto K., Takusagawa F., Suzuki H., Horiuchi K. Water-soluble organic nitrogen in the aerosols and rainwater at an urban site in Japan: implications for the nitrogen composition in the atmospheric deposition. Atmos. Environ. 2018;191:267–272. doi: 10.1016/j.atmosenv.2018.07.056. [DOI] [Google Scholar]
- 61.Li Y., Niu S., Yu G. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: a meta-analysis. Global Change Biol. 2016;22(2):934–943. doi: 10.1111/gcb.13125. [DOI] [PubMed] [Google Scholar]
- 62.Yang D., Song L., Jin G. The soil C:N:P stoichiometry is more sensitive than the leaf C:N:P stoichiometry to nitrogen addition: a four-year nitrogen addition experiment in a Pinus koraiensis plantation. Plant Soil. 2019;442(1–2):183–198. doi: 10.1007/s11104-019-04165-z. [DOI] [Google Scholar]
- 63.Cleveland C.C., Liptzin D. C : N : P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry. 2007;85(3):235–252. doi: 10.1007/s10533-007-9132-0. [DOI] [Google Scholar]
- 64.Tian H., Chen G., Zhang C., Melillo J.M., Hall C.A.S. Pattern and variation of C:N:P ratios in China’s soils: a synthesis of observational data. Biogeochemistry. 2010;98(1–3):139–151. doi: 10.1007/s10533-009-9382-0. [DOI] [Google Scholar]
- 65.Puri G., Ashman M.R. Microbial immobilization of 15N-labelled ammonium and nitrate in a temperate woodland soil. Soil Biol. Biochem. 1999;31(6):929–931. doi: 10.1016/S0038-0717(98)00172-2. [DOI] [Google Scholar]
- 66.Chen Y., Wen Y., Zhou J., Zhou Q., Vymazal J., Kuschk P. Transformation of chloroform in model treatment wetlands: from mass balance to microbial analysis. Environ. Sci. Technol. 2015;49(10):6198–6205. doi: 10.1021/es506357e. [DOI] [PubMed] [Google Scholar]
- 67.Leroy F., Gogo S., Guimbaud C., Francez A.-J., Zocatelli R., Defarge C., Bernard-Jannin L., Hu Z., Laggoun-Defarge F. Response of C and N cycles to N fertilization in Sphagnum and Molinia-dominated peat mesocosms. J. Environ. Sci. 2019;77:264–272. doi: 10.1016/j.jes.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Troelstra S.R., Wagenaar R., Smant W. Nitrogen-utilization by plant-species from acid heathland soils .1. Comparison between nitrate and ammonium nutrition at constant low ph. J. Exp. Bot. 1995;46(290):1103–1112. doi: 10.1093/jxb/46.9.1103. [DOI] [Google Scholar]
- 69.Guggenberger G., Kaiser K. Dissolved organic matter in soil: challenging the paradigm of sorptive preservation. Geoderma. 2003;113(3–4):293–310. doi: 10.1016/S0016-7061(02)00366-X. [DOI] [Google Scholar]
- 70.James J.N., Gross C.D., Dwivedi P., Myers T., Santos F., Bernardi R., de Faria M.F., Guerrini I.A., Harrison R., Butman D. Land use change alters the radiocarbon age and composition of soil and water-soluble organic matter in the Brazilian Cerrado. Geoderma. 2019;345:38–50. doi: 10.1016/j.geoderma.2019.03.019. [DOI] [Google Scholar]
- 71.Tian J., Lu S., Fan M., Li X., Kuzyakov Y. Labile soil organic matter fractions as influenced by non-flooded mulching cultivation and cropping season in rice-wheat rotation. Eur. J. Soil Biol. 2013;56:19–25. doi: 10.1016/j.ejsobi.2013.02.001. [DOI] [Google Scholar]
- 72.Lan Z.M., Chen C.R., Rashti M.R., Yang H., Zhang D.K. Stoichiometric ratio of dissolved organic carbon to nitrate regulates nitrous oxide emission from the biochar-amended soils. Sci. Total Environ. 2017;576:559–571. doi: 10.1016/j.scitotenv.2016.10.119. [DOI] [PubMed] [Google Scholar]
- 73.Li D., Liu M., Cheng Y., Wang D., Qin J., Jiao J., Li H., Hu F. Methane emissions from double-rice cropping system under conventional and no tillage in southeast China. Soil Tillage Res. 2011;113(2):77–81. doi: 10.1016/j.still.2011.02.006. [DOI] [Google Scholar]
- 74.Magill A.H., Aber J.D. Variation in soil net mineralization rates with dissolved organic carbon additions. Soil Biol. Biochem. 2000;32(5):597–601. doi: 10.1016/S0038-0717(99)00186-8. [DOI] [Google Scholar]
- 75.Manzoni S., Porporato A. Soil carbon and nitrogen mineralization: theory and models across scales. Soil Biol. Biochem. 2009;41(7):1355–1379. doi: 10.1016/j.soilbio.2009.02.031. [DOI] [Google Scholar]
- 76.Gougoulias C., Clark J.M., Shaw L.J. The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014;94(12):2362–2371. doi: 10.1002/jsfa.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han M.G., Zhu B. Changes in soil greenhouse gas fluxes by land use change from primary forest. Global Change Biol. 2020;26(4):2656–2667. doi: 10.1111/gcb.14993. [DOI] [PubMed] [Google Scholar]
- 78.Livesley S.J., Kiese R., Miehle P., Weston C.J., Butterbach-Bahl K., Arndt S.K. Soil-atmosphere exchange of greenhouse gases in a Eucalyptus marginata woodland, a clover-grass pasture, and Pinus radiata and Eucalyptus globulus plantations. Global Change Biol. 2009;15(2):425–440. doi: 10.1111/j.1365-2486.2008.01759.x. [DOI] [Google Scholar]
- 79.Deng S.P., Parham J.A., Hattey J.A., Babu D. Animal manure and anhydrous ammonia amendment alter microbial carbon use efficiency, microbial biomass, and activities of dehydrogenase and amidohydrolasess in semiarid agroecosystems. Appl. Soil Ecol. 2006;33(3):258–268. doi: 10.1016/j.apsoil.2005.10.004. [DOI] [Google Scholar]
- 80.Marks B.M., Chambers L., White J.R. Effect of fluctuating salinity on potential denitrification in coastal wetland soil and sediments. Soil Sci. Soc. Am. J. 2016;80(2):516–526. doi: 10.2136/sssaj2015.07.0265. [DOI] [Google Scholar]
- 81.Guan B., Xie B., Yang S., Hou A., Chen M., Han G. Effects of five years’ nitrogen deposition on soil properties and plant growth in a salinized reed wetland of the Yellow River Delta. Ecol. Eng. 2019;136:160–166. doi: 10.1016/j.ecoleng.2019.06.016. [DOI] [Google Scholar]
- 82.Huo L.L., Zou Y.C., Lyu X.G., Zhang Z.S., Wang X.H., An Y. Effect of wetland reclamation on soil organic carbon stability in peat mire soil around xingkai lake in Northeast China. Chin. Geogr. Sci. 2018;28(2):325–336. doi: 10.1007/s11769-018-0939-5. [DOI] [Google Scholar]
- 83.Plante A.F., Fernandez J.M., Haddix M.L., Steinweg J.M., Conant R.T. Biological, chemical and thermal indices of soil organic matter stability in four grassland soils. Soil Biol. Biochem. 2011;43(5):1051–1058. doi: 10.1016/j.soilbio.2011.01.024. [DOI] [Google Scholar]
- 84.Hatala J.A., Detto M., Sonnentag O., Deverel S.J., Verfaillie J., Baldocchi D.D. Greenhouse gas (CO2, CH4, N2O) fluxes from drained and flooded agricultural peatlands in the Sacramento-San Joaquin Delta. Agric. Ecosyst. Environ. 2012;150:1–18. doi: 10.1016/j.agee.2012.01.009. [DOI] [Google Scholar]
- 85.Knox S.H., Sturtevant C., Matthes J.H., Koteen L., Verfaillie J., Baldocchi D. Agricultural peatland restoration: effects of land-use change on greenhouse gas (CO2 and CH4) fluxes in the Sacramento-San Joaquin Delta. Global Change Biol. 2015;21(2):750–765. doi: 10.1111/gcb.12745. [DOI] [PubMed] [Google Scholar]
- 86.Krauss K.W., Holm G.O., Jr., Perez B.C., McWhorter D.E., Cormier N., Moss R.F., Johnson D.J., Neubauer S.C., Raynie R.C. Component greenhouse gas fluxes and radiative balance from two deltaic marshes in Louisiana: pairing chamber techniques and eddy covariance. J. Geophys. Res.-Biogeo. 2016;121(6):1503–1521. doi: 10.1002/2015JG003224. [DOI] [Google Scholar]
- 87.Chowdhury N., Marschner P., Burns R.G. Soil microbial activity and community composition: impact of changes in matric and osmotic potential. Soil Biol. Biochem. 2011;43(6):1229–1236. doi: 10.1016/j.soilbio.2011.02.012. [DOI] [Google Scholar]
- 88.Köchy M., Hiederer R., Freibauer A. Global distribution of soil organic carbon – Part 1: masses and frequency distributions of SOC stocks for the tropics, permafrost regions, wetlands, and the world. Soils. 2015;1:351–365. doi: 10.5194/soil-1-351-2015. [DOI] [Google Scholar]
- 89.Liimatainen m., Voigt C., Martikainen P.J., Hytonen J., Regina K., Oskarsson H., Maljanen M. Factors controlling nitrous oxide emissions from managed northern peat soils with low carbon to nitrogen ratio. Soil Biol. Biochem. 2018;122:186–195. doi: 10.1016/j.soilbio.2018.04.006. [DOI] [Google Scholar]
- 90.Hu Y., Wang L., Fu X., Yan J., Wu J., Tsang Y., Le Y., Sun Y. Salinity and nutrient contents of tidal water affects soil respiration and carbon sequestration of high and low tidal flats of Jiuduansha wetlands in different ways. Sci. Total Environ. 2016;565:637–648. doi: 10.1016/j.scitotenv.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 91.Rath K.M., Rousk J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: a review. Soil Biol. Biochem. 2015;81:108–123. doi: 10.1016/j.soilbio.2014.11.001. [DOI] [Google Scholar]
- 92.Butterbach-Bahl K., Dannenmann M. Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr. Opin. in Environ. Sustain. 2011;3(5):389–395. doi: 10.1016/j.cosust.2011.08.004. [DOI] [Google Scholar]
- 93.Castaldi S. Responses of nitrous oxide, dinitrogen and carbon dioxide production and oxygen consumption to temperature in forest and agricultural light-textured soils determined by model experiment. Biol. Fertil. Soils. 2000;32(1):67–72. doi: 10.1007/s003740000218. [DOI] [Google Scholar]
- 94.Braker G., Schwarz J., Conrad R. Influence of temperature on the composition and activity of denitrifying soil communities. FEMS Microbiol. Ecol. 2010;73(1):134–148. doi: 10.1111/j.1574-6941.2010.00884.x. [DOI] [PubMed] [Google Scholar]
- 95.Knowles R. Denitrification. Microbiol. Rev. 1982;46(1):43–70. doi: 10.1128/MMBR.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Contosta A.R., Frey S.D., Cooper A.B. Seasonal dynamics of soil respiration and N mineralization in chronically warmed and fertilized soils. Ecosphere. 2011;2(3) doi: 10.1890/ES10-00133.1. art36. [DOI] [Google Scholar]
- 97.Zhu X., Luo C., Wang S., Zhang Z., Cui S., Bao X., Jiang L., Li Y., Li X., Wang Q., Zhou Y. Effects of warming, grazing/cutting and nitrogen fertilization on greenhouse gas fluxes during growing seasons in an alpine meadow on the Tibetan Plateau. Agric. For. Meteorol. 2015;214:506–514. doi: 10.1016/j.agrformet.2015.09.008. [DOI] [Google Scholar]
- 98.Gong Y., Wu J., Vogt J., Le T.B. Warming reduces the increase in N2O emission under nitrogen fertilization in a boreal peatland. Sci. Total Environ. 2019;664:72–78. doi: 10.1016/j.scitotenv.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 99.He C.-E., Wang X., Liu X., Fangmeier A., Christie P., Zhang F. Nitrogen deposition and its contribution to nutrient inputs to intensively managed agricultural ecosystems. Ecol. Appl. 2010;20(1):80–90. doi: 10.1890/08-0582.1. [DOI] [PubMed] [Google Scholar]
- 100.Yang X., Wang C., Xu K. Response of soil CH4 fluxes to stimulated nitrogen deposition in a temperate deciduous forest in northern China: a 5-year nitrogen addition experiment. Eur. J. Soil Biol. 2017;82:43–49. doi: 10.1016/j.ejsobi.2017.08.004. [DOI] [Google Scholar]
- 101.Zhou X., Lv X., Tao Y., Wu L., Havrilla C.A., Zhang Y. Divergent responses of nitrous oxide, methane and carbon dioxide exchange to pulses of nitrogen addition in a desert in Central Asia. Catena. 2019;173:29–37. doi: 10.1016/j.catena.2018.09.048. [DOI] [Google Scholar]
- 102.Reay M.K., Charteris A.F., Jones D.L., Evershed R.P. N-15-amino sugar stable isotope probing (N-15-SIP) to trace the assimilation of fertiliser-N by soil bacterial and fungal communities. Soil Biol. Biochem. 2019;138 doi: 10.1016/j.soilbio.2019.107599. [DOI] [Google Scholar]
- 103.Zhang F.G., Che Y.Y., Xiao Y. Effects of rice straw incorporation and N fertilizer on ryegrass yield, soil quality, and greenhouse gas emissions from paddy soil. J. Soils Sediments. 2019;19(3):1053–1063. doi: 10.1007/s11368-018-2105-1. [DOI] [Google Scholar]
- 104.Zhang T.A., Chen H.Y.H., Ruan H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018;12(7):1817–1825. doi: 10.1038/s41396-018-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang N., Guo R., Song P., Guo J., Gao Y. Effects of warming and nitrogen deposition on the coupling mechanism between soil nitrogen and phosphorus in Songnen Meadow Steppe, northeastern China. Soil Biol. Biochem. 2013;65:96–104. doi: 10.1016/j.soilbio.2013.05.015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.