Abstract
Increasing global population and decreasing arable land pose tremendous pressures to agricultural production. The application of conventional chemical fertilizers improves agricultural production, but causes serious environmental problems and significant economic burdens. Biochar gains increasing interest as a soil amendment. Recently, more and more attentions have been paid to biochar-based slow-release of fertilizers (SRFs) due to the unique properties of biochar. This review summarizes recent advances in the development, synthesis, application, and tentative mechanism of biochar-based SRFs. The development mainly undergoes three stages: (i) soil amendment using biochar, (ii) interactions between nutrients and biochar, and (iii) biochar-based SRFs. Various methods are proposed to improve the fertilizer efficiency of biochar, majorly including in-situ pyrolysis, co-pyrolysis, impregnation, encapsulation, and granulation. Considering the distinct features of different methods, the integrated methods are promising for fabricating effective biochar-based SRFs. The in-depth understanding of the mechanism of nutrient loading and slow release is discussed based on current knowledge. Additionally, the perspectives and challenges of the potential application of biochar-based SRFs are described. Knowledge surveyed from this review indicates that applying biochar-based SRFs is a viable way of promoting sustainable agriculture.
Keywords: Biochar, Slow-release fertilizer, Sustainability, Soil amendment, Agricultural production
Graphical abstract
Highlights
-
•
Enhancing nutrient utilization in fertilizers is an urgent demand.
-
•
The development of biochar-based slow-release fertilizers is briefly summarized.
-
•
Various methods proposed for biochar-based SRFs synthesis are reviewed.
-
•
Biochar-based slow-release fertilizers are viable for promoting sustainable agriculture.
1. Introduction
Based on the prediction of the FAO, the global population will be up to 9.7 billion in 2050 [1]. To meet the rapid population growth, the world demand for food production increases sharply. Agriculture is currently the dominant channel of food supply. The contradiction between increasing global population and decreasing arable land poses tremendous pressures to agricultural production. In the past decades, the application of chemical fertilizers to agricultural production dramatically increases food production, contributing to about 50% extra crop yield [2]. With the wide utilization of chemical fertilizers, the negative impacts on the environment receive increasing attention worldwide. The over-use or suboptimal utilization of chemical fertilizers causes the decline of fertilizer efficiency and serious environmental problems. Nitrogen and phosphorus are essential elements required for crop growth, and a great quantity of nitrogen and phosphorus fertilizers are applied to agricultural production. The global demand for fertilizers, including nitrogen, phosphorus, and potassium, was 185.06 million tons in 2016, and it was predicted to be 200.92 in 2022 [3].
Nitrogen is a limiting nutrient for crop growth, and nitrogen fertilizer is the most consumed fertilizer. The global demand for nitrogen fertilizer is expected to be 111.59 million tons by 2022 [3]. Nitrogen fertilizers undergo ammonia (NH3) volatilization, nitrate (NO3−) leaching, and nitrous oxide (N2O) release, causing the serious pollution of surface water, groundwater, and atmosphere [4]. Only 20–30% N in fertilizers is taken up by crops and plants [5]. Phosphorus plays an important role in crop growth in agriculture production. Phosphorus fertilizers are rapidly consumed [6]. The global demand for phosphorus fertilizers will reach 49.10 million tons by 2022 [3]. The non-renewable phosphorus rock is depleting. The global reserves of high-quality phosphate resources will be depleted within 50–400 years, relying on phosphorus supply and demand dynamics [7]. Phosphorus fertilizers suffer from low availability of soluble phosphorus for plant uptake. Phosphorus has poor solubility, and its availability for plant uptake only occurs in the inorganic form as HPO42- or H2PO4− and H3PO4. Phosphorus is highly immobile due to soil interactions such as adsorption and precipitation, as well as the transformation into the organic form. Additionally, excessive phosphorus fertilization significantly enlarges the risk of phosphorus loss through leaching, runoff, and erosion [8]. The phosphorus loss into surface water is the major reason for eutrophication, and the accelerated eutrophication on a worldwide scale severely damages aquatic ecosystems [9].
The soluble chemical fertilizers are easily leached, runoff, and washed away. The estimated fertilizer loss in the environment includes 40–70% N, 80–90% P, and 50–70% K of fertilizers [10]. The phenomena not only cause serious environmental pollution, challenge global sustainability, but also result in significant financial loss. Moreover, excessive application of chemical fertilizers severely undermines the quality of farming lands via soil salinization and depleting organic matters [11]. To cope with the drawbacks of conventional fertilizers, slow-release fertilizers (SRFs) are purposefully designed and gain increasing global attention. SRFs are defined as fertilizers that delay their nutrient availability for plant uptake after application [12]. Compared to conventional fertilizers, SRFs have a low rate of releasing nutrients, prolonging nutrient availability for plant uptake, and diminishing nutrient loss into the environment.
Biochar is a carbon-rich solid material derived from biomass, generally produced through thermochemical the transformation of biomass feedstocks. The thermochemical processes include pyrolysis, gasification, hydrothermal carbonization, etc. (Fig. S1) [13,14]). The plant- or animal-derived biomass feedstocks are widely used for biochar fabrication. Biochar displays outstanding features for the potential application, such as large surface area, porous micro-morphology, graphitic structure, and abundant surface groups [15,16]. Due to its unique properties, biochar has been investigated for diverse applications, such as soil amendment [17], adsorption [18], waste management [19], catalysis [20,21], energy storage [22], and carbon sequestration [23]. The potential application of biochar in different fields is still in its infancy. Compared to other applications, biochar is favorable for soil amendment due to unnecessary precise regulation of molecular structure and morphology. Previous researches have proven that the soil application of biochar can promote nutrient supply and crop yield [24], ameliorate soil texture and properties [25], reduce greenhouse gas (GHG) emissions [26], and immobilize heavy metals and organic contaminants [[27], [28], [29]]. Biochar can decline the nutrients loss in soil and prolong fertilizer efficiency for plant uptake [30]. The pristine biochar has limited nutrients, and loading nutrients onto biochar is imperative to improve the fertilizer. Recently, researches on biochar-based SRFs have been reported [[31], [32], [33]]. As shown in Fig. S2, the published papers and citations notably increased in the past years, suggesting the growing research interest. With significant research advances, the summary of current knowledge about biochar-based SRFs is imperative. To the best of our knowledge, the systematic review of biochar-based SRFs has been rarely reported. In this work, the research advances on biochar-based SRFs are summarized based on the latest literature.
2. Research evolution of biochar-based SRFs
According to IBI (2012), biochar is defined as ‘‘a solid material obtained from the thermochemical conversion of biomass in an oxygen-limited environment’‘. It has attracted much attention in various fields such as agriculture, environment, and energy owing to its excellent properties. Biochar has a long history for soil application, dating back to ancient Amerindian Populations (Terra Preta de Indio), where dark earth formed through slash-and-char practice [34]. Studies on Terra Preta soils in the Amazon region verify the positive effects of biochar for soil fertility and sustainability. Numerous researches have been conducted to reveal the hiding secret of biochar for soil fertility.
Biochar manifests the properties of carbon content (23.6–87.5%), pH (5.2–10.3), surface area (0–642 m2 g−1), and cation exchange capacity (10–69 cmolc kg−1) [35]. The addition of biochar into soil produces multiple profits, including the amelioration of physicochemical and biological properties [36], the promotion of soil fertility and crop yield [37], the control of plant diseases [38], and immobilization of toxic metals and organic pollutants [15]. Also, biochar increases soil nutrient availability, cation exchange capacity and water holding capacity, and improves soil microbial community and microbe activity [4,39]. A decline of nutrient loss in soil associated with biochar addition was reported by Xiao et al. [40] Biochar promoted seed germination, crop growth, and crop production [41]. The microbe population and activity in soil were significantly improved after biochar regulation [42]. Besides nutrient retention, biochar can improve water retention in soil. Soil water holding capacity correlates to surface hydrophobicity, surface area, porosity, and surface groups of biochar [43]. Amoakwah et al. [44] found a significant increase in water retention in soil after the application of corn cob. The above studies verify the excellent benefits of biochar, suggesting that it is promising for soil application.
Nitrogen and phosphorus are essential nutrients for plant growth. The effects of biochar on nitrogen and phosphorus change in water and soil have been extensively investigated. Adsorption of nitrogen and phosphorus using biochar was significantly influenced by pyrolysis temperature and biomass feedstocks [45]. Mg-loaded biochar prepared by different biomass feedstocks was proved to be effective for nitrogen and phosphorus adsorption from aqueous solution, with a maximum adsorption capacity of 24.04 mg g−1 (NH4+-N) and 31.15 mg g−1 (TP) [46]. Iron-loaded biochar was verified to be an effective adsorbent for nitrogen and phosphorus removal from nutrient-rich wastewater [47]. Simultaneously adsorption of ammonium and phosphate from the water was obtained using biochar derived from Eupatorium adenophorum [48]. The factors influencing nitrogen and phosphorus adsorption include biochar amount, solution pH, nitrogen or phosphorus concentration, adsorption time, and temperature [49]. The adsorption mechanism involves ion exchange, hydrophobic interaction, hydrogen bonds, electronic interaction, and physical adsorption [50].
Inspired by effective N and P adsorption from water, the effects of biochar are extensively investigated on nitrogen and phosphorus in soil. Biochar was identified as an amendment to improve nitrogen removal in constructed wetland mesocosms [51]. Chicken litter biochar increased the pH of acid soil and pH buffering capacity, displayed a high affinity for N than P and K, and effectively controlled soil N availability [52]. Biochar manifested increased NH4+-N adsorption and low affinity for P in two soils [53]. Biochar has strong adsorption performance, immobilizes nitrogen and phosphorus in soil, and improves the nutrient availability for crop growth [54]. A meta-analysis for evaluating the effects of biochar on N and P availability in agricultural ecosystems manifested that biochar addition remarkably increased soil available P by 45%, but decreased NO3−-N by 12% and NH4+-N by 11% [55]. Biochar capture promoted the slow release of N in biochar amended soils and composts [56]. Simultaneous application of biochar with organic or inorganic fertilizers enhances fertilizer efficiency and crop yields. A 3-year fixed-site experiment comparing different treatments confirmed that mixing biochar with bare urea and controlled-release urea increased fertilization efficiency and rice yield [57]. Biochar had high sorption capacity to different fertilizers, and the biochar with attached nutrients can be directly used as SRFs in agricultural fields.
Due to the limited nutrients, a great amount of biochar is required as fertilizer. The storage, transportation, and application of massive biochar are highly challenging owing to the wide distribution of particle size, fragile feature, low density. During soil application, a high loss of 25% by wind-blown and 20–53% loss caused by surface runoff from intense rain events were reported [58]. Additionally, soil application of approximately 20–50 tons ha−1 biochar is questionable from the aspect of economic feasibility [59]. Because of unique features such as massive pore structure, large surface area, and abundant surface groups, biochar is promising as fertilizer carriers. To improve fertilizer efficiency, biochar-based fertilizers are fabricated by combining biochar with inorganic fertilizers through various physical or chemical techniques, such as impregnation, pelletizing, and encapsulation [31,60,61]. Biochar-based SRFs can provide nutrients for crop growth for the whole season via a single application, and reduce the stress and toxicity derived from excessive nutrients in the root zones [62]. The soil application of biochar-based SRFs displays simultaneous benefits of soil improvement, crop yields, nutrient retention, and carbon sequestration [15].
Based on the above discussion, the research evolution of biochar-based SRFs can be summarized as Fig. 1. Originated from ancient dark earth formed through slash-and-char practice, extensive researches on the hiding secret reveal the important role of biochar in soil. Biochar has a high affinity toward nutrients, including N and P in water and soil. Soil application of biochar enhances the slow-release effect of nutrients, providing an attractive strategy for reducing the economic and environmental burden of excessive conventional fertilizers. Biochar-based SRFs receive more and more global attention for sustainable agriculture.
Fig. 1.
Schematic of the research evolution of biochar-based SRFs.
3. Synthesis strategies of biochar-based SRFs
The pristine biochar obtained from direct thermochemical treatment of biomass feedstocks is generally deficient in nutrients as fertilizer. It is imperative to modify biochar to improve fertilizer efficiency. The excellent properties of biochar provide a sound platform for synthesizing biochar-based SRFs. To fabricate effective biochar-based SRFs, various methods have been proposed, such as impregnation [63], encapsulation [64], granulation [65], co-pyrolysis [66], etc. (Fig. 2). The slow-release performance of biochar-based SRFs fabricated by different methods is listed in Table 1.
Fig. 2.
Various methods for the synthesis of biochar-based SRFs.
Table 1.
Biochar-based SRFs fabricated by different methods and slow-release performance.
| Fertilizers | Preparation methods | Fabrication processes | Nutrients | Slow-release performance | References |
|---|---|---|---|---|---|
| Biochar-based P SRF | Impregnation | Corn stover was pyrolyzed at 500 °C for 30 min under limited oxygen, and the obtained biochar was immersed in KH2PO4 solution at a solid: solution ratio of 1:40 for 48 h, and then filtrated, washed, and dried at 60 °C. | 22 mg g−1 P | Bioavailable P release 40% after 5 d | [32] |
| Biochar-based P fertilizers | Co-pyrolysis | Coffee husk and poultry litter were mixed with concentrated phosphoric acid and magnesium oxide (P:Mg molar ratio 1:1). After thorough mixing, the samples were moistened by adding water and then left to rest for 16 h. After drying at 60 °C, pyrolysis was performed at 500 °C for 2 h, and samples were marked CHB (coffee husk) and PLB (poultry litter). | 201 mg g−1 P in CHB, and 178% P in PLB | Total P release 6.47% (CHB) and 8.99% (PLB) within 1 h | [67] |
| Biochar-based controlled release N fertilizer | Impregnation Granulation Encapsulation |
Biochar was produced from pyrolysis of corn stover. Biochar was added in ammonium sulfate solution (mass ratio of biochar to ammonium sulfate 1:1) and continually stirred for 20 min, and then kaolin clay was added and stirred for 20 min. The mixture was dried for 5 h at 65 °C, and then blended with methylcellulose and deionized water and pelletized into particles. The pellets were submerged in polylactic acid solution for 10 s and then air-dried at room temperature for 8 h. | – | N release 70% over 12 d in water and 25 d in soil. | [31] |
| Mg-enriched biochar fertilizer | Co-pyrolysis Impregnation |
Corn stalk was dispersed MgCl2·solution for 12 h, and the resulting mixture was completely dried at 80 °C. The dried mixture was pyrolyzed at 500 °C for 1 h under N2. The obtained biochar was added into the mixture of biogas effluent and KH2PO4 solution and stirred at 25 °C for 24 h | 200 mg g−1 N and 381 mg g−1 P | N release 18% and P release 16% at 48 h | [50] |
| Biochar-based P SRFs | Impregnation Co-pyrolysis Encapsulation |
MSRFs: Cotton straw powers were mixed with of MgCl2 solution for 2 h. After drying, the Mg-loaded biochar was prepared in a furnace under N2 at 550 °C for 2 h. Mg-loaded biochar was added into KH2PO4 solutions in a mechanical shaker at 120 rpm min−1 for 24 h at room temperature. BSRFs: 55% of cotton straw, 15% of Mg3(PO4)2 and 30% of bentonite were dispersed in distilled water and stirred at a speed of 650 r min−1 for 45 min. The dried samples were placed at 550 °C for 2 h under N2. CSRFs: BSRFs samples were thoroughly mixed with solution containing NaAlg and bentonite, and the resulting samples were gradually dripped into CaCl2 solution to generate crosslinks for wrapping BSRFs. PSRFs: starch, acrylamide, PVP, BSRFs, and N-MBA were added into boiling water, and then polymerization was performed by microwave irradiation at 300 W and stirred at a speed of 200 rpm min−1. After maintaining for 10 min, N2 was bubbled for 10 min, and then KPS was added to initiate the polymerization process |
– | P utilization efficiency 53.52% (MSRFs), 65.27% (BSRFs), 74.32% (CSRFs), and 75.83% (PSRFs) after 60 d | [68] |
| Biochar-based N/P/K fertilizers | Co-pyrolysis | Residues from biogas production collected from the anaerobic fermentation were air-dried and was ground, and then was pyrolyzed at a temperature of 500 °C for 120 min under nitrogen. Biogas residue-based biochar was mixed commercial compound fertilizer (N: P2O5: K2O = 4:3:3) with a mass ratio of 1:2. Pelletization was conducted using a universal material testing machine, and self-made cylindrical forming molds with different apertures were connected to the universal material testing machine. The pelletized RBF was dried at 25, 65, 105, 145, and 185 °C for 24 h, referred to as RBF-T (RBF-25, RBF-65, RBF-105, RBF-145, and RBF-185. | – | The cumulative N release of RBF-25, RBF-65, RBF-105, RBF-145, and RBF-185 reached 88.68%, 75.35%, 64.89%, 54.39%, and 41.71% after 413 h; the P release of RBF-25, RBF-65, RBF-105, RBF-145, and RBF-185 was 72.07%, 58.75%, 44.12%, 27.48%, and 23.93% after 413 h | [69] |
| Biochar scaffolded N SRF | Encapsulation Granulation |
Autothermal fast pyrolysis of pine biomass at 500 °C was conducted to prepare biochar. Chemical activation of the biochar was accomplished in 5% and 15% H3PO4 solution associated with heat-treated at 465 °C for 2 h under N2. The activated biochar was mixed with dry urea with ratios of 1:2, 1:3, 1:4, and 1:6. The resultant mixtures were ground finely using a mortar and then thermally (137–138 °C) treated for 75 min to melt and infuse the urea into the biochar pores. A 5% Ca-LS aqueous solution and 10% liquefied paraffin wax were added to the urea infused biochar and ground again to prepare the final composite. After manual pelletization of the urea-infused biochar fertilizer, the pellets were dried at 50–55 °C for 48 h. | N content 26.74–34.32% | Urea released in aqueous medium was 61–90% in 4320 min | [70] |
| Biochar and biofilm composite towards P SRF | Impregnation Encapsulation |
Compound biochar was obtained by pyrolysis of cotton stalk and montmorillonite at 400 °C. Adsorption of PO43- was conducted onto the composite biochar. Cotton straw, polyethylene glycol-400, and glycerin were mixed, and then concentrated sulfuric acid was added to react for 90 min at 160 °C to obtain liquefied cotton stalk-based polyol, which was further reacted with toluene diisocyanate at 75 °C for 20 min to obtain a bio-based membrane material. The toluene diisocyanate and the liquefied cotton stalk-based polyol were stirred at room temperature, and then the mixed liquid was coated on the surface of the composite biochar phosphate fertilizer with a brush. The coated biochar phosphate fertilizer was heated at 75 °C for 20 min. | PO43- 129.92 mg g−1 | The cumulative PO43- release after 30 d was 76%, 65%, 60%, and 53.5% (corresponding the membrane material 0%, 2%–4% and 6%). | [71] |
| Biochar-based N SRF | Impregnation Granulation Encapsulation |
Corn straw was soaked in MgCl2 for 2 h in 1:3 ratios. After drying, the sample was pyrolyzed at 500 °C for 3 h. The modified biochar was soaked in 3 M ammonium nitrate solution for 24 h and then air-dried. Polyvinyl alcohol and corn starch were used as the coating material. The biochar and coating material were mixed at a 1:5 ratio. The biochar was granulated, and 5% solution of coating material was sprayed onto the granules. | Total nitrogen 22.3% | The cumulative nitrate concentration was 886.4 mg L−1 after 24 d; the cumulative ammonium concentration was 1395.5 mg L−1 after 28 d. | [72] |
| Biochar embedded-semi-IPN based SRF | Encapsulation | Cotton stalks were heated to 600 °C for 1 h under N2 to prepare biochar. NaAlg, bentonite, biochar, NH4Cl, and K3PO4 were added in distilled water and stirred at 40 °C by mechanical stirring for 45 min. Then, AA, PVP, N-MBA, and APS solution were added into above solution. The polymerization was performed by microwave irradiation at 300 W for 4.5 min under N2. | – | The release ratios of N, P and K within 30 d were less than 80.0% | [73] |
| Bentonite modified biochar P SRF | Impregnation | Cotton straw was mixed with H-bentonite, N-bentonite, and OH-bentonite with a ratio of 1:9 and added in deionized water by mechanical stirring at a speed of 750 r min−1 for 45 min. After drying, crushing, and sieving, the samples were pyrolyzed at 600 °C for 2 h under N2. BSRFs was added into KH2PO4 aqueous solution and shaken at 120 rpm min−1 for 24 h at room temperature. | Phosphate was 245.56 mg g−1. | P release within 15 d was 72.6% | [63] |
| Biochar-coated urea | Granulation Encapsulation | (1) Urea was added to the coating machine at a speed of 80 rpm, and oxidized starch was sprayed into the machine. (2) biochar was sprinkled in after the urea particles had been uniformly coated with the oxidized starch, and the coating machine continued rotating for 5–10 min to ensure that the coated layer was tight, and then oxidized starch was sprayed again. (3) Step 2 was repeated until all the biochar had been coated onto the particles. (4) Resin was dripped in batches into a plastic container containing BCU from step 3 and embedded in a spiral oscillator to prepare BCU with protective layer I; paraffin was heated until melted and coated on BCU from step 3 with a high-pressure spray gun to prepare BCU with protective layer II. | N content 26.89–34.36% | Cumulative nitrogen release was 72.75–85.71% after 28 d | [74] |
| Biochar composite P fertilizer | Granulation Encapsulation | Wood chips were pyrolyzed at 350 °C for 1 h, and the obtained biochar was ground and sieved. Biochar fertilizers were obtained by blending (B) or coating (C) superphosphate with biochar in three proportions (5, 15 and 25% of biochar), and granulating using a rotating granulator. | Total P was 15.4–19.9% | Total P release within 1.5 h was 82% and 36% for blended biochar and coated biochar. | [75] |
| Biochar-based N/P fertilizer | Impregnation | The impregnation was done with an NPK fertilizer: oil palm kernel shell biochar was mixed NH4NO3 and KH2PO4 for 21 h at room temperature and then oven-dried at 105 °C until constant weight. | – | NO3− release was 52.9%, 77.4% for NH3 and 55.2% for PO43- after 1 h. | [76] |
| Carbon-based SRF treated by biooil coating | Granulation Encapsulation | The dried wheat straw was pyrolyzed at 400 °C for 2 h under CO2, N2 and H2O(g) atmosphere of to prepare the initial biochar. The biochar and KH2PO4 and KNO3 solution were mixed with a ratio of 2.7. After stirring and mixing, the mixture was maintained for 24 h, and then heated at a low temperature to remove the solution. The biochar was placed on the porous bottom plate of the fluidized spraying device, and the biochar particles were uniformly coated with a layer of bio-oil film, and then a desiccant was added and dried to obtain the coated carbon-based slow-release fertilizer. | – | the cumulative release of N, P, and K reached about 45% after 7 d. | [58] |
| Biochar/struvite composites as N and P fertilizer | Impregnation | A 1:1:1 M ratio of Mg2+/PO43−/NH4+ was stirred for 30 min and mixed with 10% biochar. After stirring for 30 min and aging for 24 h at room temperature, it was filtered, washed, and dried at 100 °C to obtain biochar/struvite composites. | N 4.93% and P2O5 22.19% | The accumulative release of N and P after 84 d was 10.62% and 6.84% in distilled water, and 59.32% and 59.12% in citric acid solution. | [77] |
| P-enriched biochar fertilizer | In-situ pyrolysis | E. coli fermentation waste was collected from the bio-fermentation corporation and dried in an oven at 60 °C for 2 d. Wet feedstock and dried feedstock were used for biochar fabrication. Pyrolysis was carried out in a muffle furnace at 600 °C under N2. | P content 84.7 mg g−1 | The extractable P in water and citric acid reached 52% and 61% after 5 d | [62] |
| Blended biochar pellet | Granulation | Biochar from rice hull was produced from a pyrolysis system at 400–500 °C for 4 h under O-free atmosphere. Biochar was blended with pig manure compost as a binder, mixed using an agitator for 5 min, sprayed with deionized water for 10 min, and then pelletized through the pellet machine. | – | Total water-soluble accumulative NH4+-N and PO43--P were 13.8 mg g−1 and 44.6 mg g−1 after 84 d | [78] |
| Biochar-based SRF encapsulated by waterborne copolymers | Encapsulation | Maize straw, rice straw and forest litter were dried, ground, and then heated at 500 °C for 2 h in the muffle furnace. Distilled water and PVA were mixed and was slowly raised to 90 °C with continuously stirring, and then PVP and butanol were added and continuously stirred for 2 h at 60 °C. Then, biochar was added and mixed for 1 h at 60 °C to prepare biochar-based copolymer film. Urea were in a sugar-coating machine for 0.5 h. Using a side-spray nozzle under 0.7 MPa pressure, the urea preheated granules were coated with the mixture solution of biochar and copolymer for 0.5 h. | – | Accumulative nitrogen leaching was 65.28% after 22 d. | [2] |
| Biochar-based P SRF | Co-Pyrolysis | The biomass (pine tree sawdust and switchgrass) and fertilizer (TSP and BM) were completely mixed, and pyrolyzed at 500 °C for 2 h under N2. | P content was 4.82–5.82%. | P release from TSP- and BM-composite biochar within 120 h were about 20.0 mg g−1 and 0.30 mg g−1 | [79] |
| Biochar pellets embedded with fertilizers | Granulation | Air-dried switchgrass was pyrolyzed at 525 °C for 90 s under N2 using a continuous dual auger pyrolysis process. The biochar was blended with different percentages of lignin as a binder using a mixer, and liquid fertilizer (12:4:8 = N:P2O5:K2O) was added. The biochar mixtures were pelletized using a pellet mill. The pelletized biochar was then processed at 105 or 180 °C for 24 h to dry in the oven. | K content 2.85–8.62 mg g−1, and P content 0.72–2.25 mg g−1 | K and P release was 53–62% and 49–62% within 24 h, and 78–87% and 73–78% after 18 d. | [35] |
3.1. In-situ pyrolysis
Rapid depletion of non-renewable resources such as phosphate rocks and sylvinite minerals requires recovery of nutrient elements from wastes. Biomass wastes rich in nutrients are highly attractive for fabricating biochar-based fertilizers. In some cases, the feedstocks of biomass have abundant nutrients and can be directly converted into biochar-based fertilizers. Banana peduncle [80], mushroom compost [81], and seaweeds [82] were reported to prepare K rich biochar. A few researches investigated P rich biochar from poultry litter [83], cattle carcasses [84], human manure [85], sewage sludge [86], bone waste [87], and bio-fermentation waste [62]. The phosphorus content in biochar derived from different biomass are listed in Table S1, and it ranges from 16.8 mg g−1 to 84.7 mg g−1. High P content in biochar implies the potential use as a fertilizer. K-rich banana peduncle and P-rich wastes sludge were co-pyrolyzed or treated by plasma for biochar production [88]. The biochar obtained by pyrolysis manifested a higher yield and total carbon content compared with plasma processing, but plasma processing resulted in higher nutrient content. Bioavailable P of biochar obtained by thermal plasma and slow pyrolysis was 4500–6000 and 5600 mg kg−1, respectively.
In the fermentation industry, a great number of bacterial wastes from Escherichia coli are produced, which contain high P content. Pyrolysis was performed in a muffle furnace at 600 °C under a N2 atmosphere to obtain P-enriched biochar [62]. The biochar was rich in P (84.7 mg g−1) and did not contain toxic elements out of limits. The biomass underwent dehydration, the vaporization of organic matter, and the formation of char during pyrolysis. Surface groups of N–H, C O, C–O, and PO43- were identified by FTIR spectra, and SEM and EDS showed the uniform distribution of P in macropore biochar. The P-enriched biochar was evaluated as potential P fertilizer, and promotion of the early growth of Lactuca sativa was observed. Thermal conversion of bacterial wastes into P-enriched biochar offers a practicable strategy for recycling biomass wastes derived from the fermentation industry. There are limitations for using P-enriched biochar from feedstocks such as sewage sludge and animal manures as potential fertilizer: (i) insufficient nutrient supply to soil and crops, and (ii) the presence of toxic elements [62].
3.2. Co-pyrolysis
Co-pyrolysis of biomass with P sources is an alternative to produce biochar-based SRFs. Co-pyrolysis enables the P immobilization in biochar by adsorption and chemical bonds. Biochar-based SRFs produced through co-pyrolysis typically manifest notable advantages, such as easy synthesis, high P loading, and impressive slow-release performance [68]. Zhao et al. [89] conducted co-pyrolysis of wheat straw with different P sources (H3PO4, phosphate rock, and triple superphosphate), and found reduced carbon loss during pyrolysis, lowered microbial mineralization, and improved biochar stability. Zhao and colleagues [79] studied the co-pyrolysis of biomass (pine sawdust or switchgrass) and fertilizer (triple superphosphate or bone meal) to produce biochar-based SRFs. The formation of C-O-PO3 or C–P resulted from the reaction between Ca(H2PO4)2 and biomass carbon occurred during pyrolysis, inducing slower P release.
Premixing biomass with an acid phosphate source leads to the low pH of biochar, and constraints its soil application. The addition of alkaline sources such as MgO prior to pyrolysis can regulate biochar pH and increase the nutrients of phosphate and magnesium. Lustosa Filho et al. [90] impregnated poultry litter with triple superphosphate and phosphoric acid in the presence or absence of MgO for preparing biochar-based SRFs. The prepared SRFs lowered P release, increased the soil pH, and promoted P uptake and maize growth [91]. Compared to triple superphosphate, the biochar-based SRFs promoted P use efficiency in tropical soil and promoted Marandu grass growth [92].
Montmorillonite catalyzed the conversion of cellulose in bamboo biomass to biochar under hydrothermal conditions [93]. A few researches reported biochar/clay mineral composites, such as bentonite/biochar and calcite/biochar [94], biochar/bentonite [95], montmorillonite/biochar [96], attapulgite/biochar [97]. Co-pyrolysis of montmorillonite and bamboo powder was conducted to prepare biochar composite [39]. Montmorillonite could catalyze bamboo pyrolysis and reduce the pyrolysis temperature for biochar formation. Biochar and montmorillonite in the composite offered surface and adsorptive sites for nutrients. The adsorption capacity reached 12.52 mg g−1 for NH4+ and 105.28 mg g−1 for PO43-. The release of NH4+ and PO43- from nutrient-laden biochar composite was 0.30–4.92% and 2.63–5.09%, displaying a good slow-release effect of N and P. Microwave-assisted co-pyrolysis of biomass, nutrient, and bentonite was proposed for the synthesis of slow-release fertilizer [66]. Cotton straw was mixed with K3PO4 and bentonite in solution, and then pyrolysis was conducted under microwave irradiation with limited oxygen (Fig. 3a). The heating behavior was enhanced in the presence of bentonite and K3PO4 (Fig. 3b). SEM image exhibited biochar with intensive pore structure (Fig. 3c), and EDX analysis confirmed P and K loaded on biochar (Fig. 3d). XPS spectra of P 2p identified the presence of HPO42−, PO43−, and H2PO4− (Fig. 3e–g). FTIR indicated that surface groups notably weakened since K3PO4 or bentonite accelerated the transformation of biomass components into aromatic carbon (Fig. 3h). XRD analysis revealed the presence of Mg3(PO4)2 and Ca(H2PO4)2 (Fig. 3i). The improved slow-release effect of P was verified by the increased utilization efficiency and diminished leaching loss (Fig. 3j). Pot experiments showed the improved growth of pepper seedlings, which might be ascribed to the slow-release performance of nutrients.
Fig. 3.
a. Schematic of BSRFs synthesis via co-pyrolysis; b. microwave-assisted heating behavior; c. SEM image, and d. EDX analysis; e-g. XPS P 2p spectra; h. FTIR spectra; i. XRD spectra; j. P leaching loss and the utilization efficiency. Reprinted (adapted) with permission from [66]. Copyright 2020 American Chemical Society.
3.3. Impregnation
Due to the low content of nutrients, the impregnation of pristine biochar in the nutrient solution effectively increases nutrient content. The porous structure and large surface area offer vessels and sites for nutrient storage, which prolongs the nutrient availability in biochar for the plant. The impregnation method displays easy operation and low cost. Biochar impregnated with ammonium salts [98], phosphates [99], and nitrates [100] was reported for preparing slow-release fertilizer to diminish nutrient loss and accelerate crop growth.
The typical impregnation process can be introduced based on the study reported by Khan et al [11]. Biochar derived from wheat was produced by conventional slow pyrolysis performed at a temperature of 300 °C or 350 °C. Nutrient-loading biochar was obtained by mixing biochar and salt solutions containing nutrients (P, K, Ca, and N), stirring for 3–4 h for adsorbing nutrients, then filtered and dried (Fig. S3a). The biochar was examined by multiple characterizations (Figs. S3b–g). Micro-morphology of biochar was mainly derived from wheat biomass, and loading nutrients after impregnation was proved by EDX. FTIR analysis revealed the presence of surface groups and the incorporation of nutrients on biochar surface. TGA examination identified the stable nature of the biochar. Obvious improvement of salt index, water retention, and slow-release nutrients was confirmed after impregnating biochar with nutrients.
Similarly, corncob biochar was impregnated with macro-nutrients (N, Ca, P, K, Mg) and micro-nutrients (Na, Zn, Fe), and the improved water retention and prolonged nutrient release were confirmed [101]. The influence of pristine and P-laden biochar (saturated with KH2PO4) was researched on soil P availability and fractions. Pristine biochar decreased P availability while P-laden biochar maintained high P availability in soil available P during the incubation, verifying P-laden biochar as SRF to promote soil P efficiency [8]. The impregnation of biochar derived from oil palm kernel shell with NH4NO3 and KH2PO4 was conducted, and the impregnated biochar displayed high nutrient retention (NO3− 52.9%, NH3 77.4%, and PO43- 55.2%) [76]. The N release performance of urea-impregnated biochar, hydrochar, zeolite, and hydroxyapatite was compared. The difference in the N release was seen, and the slow N release potentially reduced N loss and increased the fertilizer efficiency [102]. Biochar obtained from pyrolyzing cow dung was used for phosphorus adsorption from an aqueous solution. The P adsorption capacity was up to 345 mg g−1 [9]. XRD and XPS analysis proved P adsorption via precipitation mechanism, and P-laden biochar promoted seed germination and lettuce growth.
The impregnation method is significantly limited by the low adsorption capacity of pristine biochar toward nutrients. To satisfy the practical application, biochar modifications using various materials, such as MgO, K2O, bentonite, and coal gangue, have been developed to enhance adsorption capacity [63,103,104]. Biochar prepared from Mg-enriched tomato tissues effectively reclaimed phosphate from an aqueous solution with a maximum adsorption capacity of above 100 mg g−1 [105]. MgO modification of biochar significantly enhanced phosphate and ammonium adsorption from wastewater [106]. Mg-enriched biochar derived from corn straw showed excellent performance for reclaiming nutrients from biogas effluent [50]. The accumulative release ratio of N and P from Mg-enriched biochar was 7 times and 6 times lower than that of chemical fertilizer. The application strikingly promoted nutrient uptake and improved corn growth, implying the promising potential in agriculture application.
The composites of α-FeOOH-biochar synthesized by co-precipitation were used for phosphate adsorption from urine [57]. Phosphate adsorption was controlled by chemisorption and monolayer adsorption. The maximum adsorption capacity of phosphate was 57.4 mg g−1, while the extractable phosphate was 967.5 mg kg−1, indicating good bioavailability and a promising alternative as a slow-release fertilizer. Biochar composites were fabricated by direct pyrolysis of feedstocks and coal gangue [107]. The maximum P adsorption was 7.9 mg g−1, 4.6 times that of pristine biochar. The P-laden biochar was used as a slow-release fertilizer to improve bean seed germination and growth. Similarly, bentonite modified biochar by co-pyrolysis of cotton straw and bentonite was examined for phosphate adsorption from aqueous solution (Fig. S4a) [63]. SEM images displayed the typical structural characteristics of biochar (Figs. S4b–d). The high-resolution TEM pictures exhibited the lattice fringes assigned to MgO and CaO in biochar (Figs. S4e–g). XRD analysis (Fig. S4h) and XPS examination (Fig. S4i) also proved the presence of CaO and MgO.
In some cases, wastewater derived from different sources contains a large number of nutrients such as N and P. Biochar, after concentrating and adsorbing the nutrients from the wastewater, can be used as a soil amendment or fertilizer. Recovery of nutrients using biochar from the wastewater favors providing biochar-based SRFs for soil application and diminishing environmental problems. Previous reports manifested that biochar adsorbed N, P, and K from different sources, including urine, anaerobic digestate, landfill leachates, and livestock wastewater [108,109]. Soil improvement with the addition of digestate-enriched biochar was identified by increasing macronutrients (110–230%) and organic matter (232–514%) [110].
Interestingly, biochar/struvite composites were synthesized through crystallization-adsorption [77]. Biochar was mixed with Mg2+, PO43-, NH4+ solution (molar ratio 1:1:1), and the formation of biochar/struvite occurred. The high recovery of 99.02% N, 97.23% P and 95.22% Mg from aqueous solution formed 10% biochar/struvite. XRD patterns manifested a crystalline structure of struvite (MgNH4PO4·6H2O). SEM images showed a porous and coarse surface of biochar and the smooth and nonporous surface of struvite attached to biochar. The elements determined by EDS proved the presence of struvite. Biochar/struvite composite, as a potential slow-release fertilizer, demonstrated superior nutrient release to traditional chemical fertilizers. Wastewater containing abundant N and P can be a potential source for struvite synthesis, such as swine wastewater, calf manure wastewater, leather tanning wastewater, sewage sludge, dairy wastewater, and semiconductor wastewater [77].
Impregnation has unique advantages of easy operation and low cost, but the adsorption capacity of biochar limits the fertilizer efficiency of SRFs. Biochar modification can increase adsorption capacity toward nutrients, and the cost of the modification process should be considered. It is highly attractive to reclaim nutrients from wastewater through biochar impregnation. This strategy not only reduces the environmental pollution of wastewater such as eutrophication, but also makes full use of discharged nutrients. The limited nutrient uptake in biochar and co-existed toxic substances should be evaluated for real application.
3.4. Encapsulation
Encapsulation is a widely used method for fabrication SRFs, which coats fertilizers onto inorganic or organic substrates to reduce nutrient loss and increase nutrient utilization efficiency. Biochar-based SRFs prepared through encapsulation have been reported by many studies, such as biochar-biodegradable polymers-coated urea [111], biochar-waterborne copolymers-coated urea [2], and biochar-coated urea [112]. Biochar was co-composted with feedstocks of poultry manures and green waste, and organic coating enriched in nutrients of N and K formed on biochar surface and inner pore surface [113]. A slow-release fertilizer with rapeseed meal biochar and sodium alginate was synthesized by the graft-copolymerization process, and the water retention, nitrogen content, and its slow release of the graft copolymers were significantly improved [114]. The modified starch derived from cassava or corn as coating materials was used to prepare granular SRFs [115]. Khajavi-Shojaei et al. [72] reported granular MgCl2-modified biochar coated by polyvinyl alcohol and corn starch. Compared with uncoated MgCl2-modified biochar and chemical fertilizer, the granular coated biochar exhibited the slowest N release, the highest N content in soil and corn plant, and N utilization efficiency. A microwave-assisted method was conducted to prepare biochar-hydrogel composites [116]. Microwave irradiation accelerated structural alteration and cross-linkage reaction. The composite improved water absorbency and nitrogen release. Jia and colleagues fabricated biochar-coated urea using oxidized starch [74]. Biochar coated by urea displayed good slow-release N fertilizer for declining N loss and improving N utilization efficiency.
There exists a difference in fertilizer efficiency of biochar-coated fertilizer, which can be ascribed to biochar properties, coating materials, and encapsulation processes. Biochar with a large surface area was favorable for controlling nutrient release due to strong adsorption [117]. The alkaline groups and inorganic salts in biochar were reported to increase the loss of NH3 via volatilization [118]. The surface groups of biochar influenced the molding of granular fertilizer [119]. Various coating materials are used for the preparation of SRFs, including organic polymers and inorganic minerals [120]. Polymers, such as polyolefin, copolymers, polyvinylidene chloride, wax, and paraffin, are costly and nondegradable. Additionally, the accumulation of coating polymers deteriorates soil structure, suppresses soil nutrient cycling, and restrains microbial activity [115]. The coating SRFs tends to employ naturally biodegradable materials, including waste palm oil [121], starch [122], and chitosan and poly (vinyl alcohol) blend [123]. Green coating materials such as cotton stalk-based polyol [71], wood vinegar [124], sodium alginate, cellulose, starch, chitosan, and maltodextrin [125], were reported for the preparation of biochar-based SRFs. Biochar-based SRF was prepared using waterborne copolymers as coating materials. Biochar declined water absorbency of copolymer with an improved degradability, and favored the slower lease of coated urea [2]. Bio-oil derived from biomass pyrolysis was used for coating biochar loaded with N, P and K nutrients, and bio-oil coating effectively reinforced the slow-release capacity of nutrients [58].
Encapsulation is an important method for manufacturing SRFs with coating materials as a diffusion barrier to control nutrient release. Generally, the encapsulation process is complicated, involving the impregnation of biochar with nutrients, mixing different components (biochar, minerals, adhesive, binders, etc.), and pelletization. The complex and expensive encapsulation process notably limits the use of coated SRFs in agriculture. Coating materials have intrinsic features from agronomic, environmental, and economic perspectives. After nutrient release, the coating materials remain in soil due to poor degradability, and their accumulation over time induces potential environmental threats. Coating materials may add toxic organic matters, reduce soil permeability and microbial activity [126]. Green, bio-degradable and cheap coating materials are future directions.
3.5. Granulation
Currently, granulation or pelletization is a common method for fertilizer production. Due to brittleness, low density, and small particles, the storage and transport of biochar for soil application is highly challenging. High loss of biochar is caused by wind-blown or surface runoff from intense rain events, and the fine biochar induces the irritation of the eyes, skin, and respiratory system of humans [58,127]. In this regard, granulation is an effective option to decrease biochar loss and handling costs for soil application, and realizes practical large-scale application in agricultural practices.
Granulation of the mixtures of biochar and nutrients effectively lowers nutrient release by increasing diffusion resistance. As to biochar-based SRFs, granulation can be conducted in two ways: (i) carbonization of pelletized biomass containing nutrients and (ii) pelletization of biochar/fertilizer mixtures. For the former, impregnating the pelletized biochar with additional nutrients is necessary owing to the low nutrients derived from biomass. Poultry litter and switchgrass were blended, pelletized, and pyrolyzed at different temperatures to fabricate biochar pellets [83]. As to the latter method, after sufficient mixing biochar and fertilizer particles with uniform size, granulation is performed by disc granulation or mechanical extrusion, and binders are commonly used in the process. The biochar derived from switchgrass were mixed with liquid fertilizer and lignin, and the mixtures were granulated using a pellet mill [35]. The pellets produced by mixing biochar and manure compost was effective for NH4+-N adsorption, and the N-laden biochar pellets promoted plant growth [78].
The wheat straw biochar was mixed with diammonium phosphate, potassium chloride, and bentonite, and then a revolving drum granulation device was used for granulation with steam vapor as a binder [65]. The granulated biochar fertilizer had a total of 37% nutrients (18% N, 9% P2O5, and 10% K2O). A field experiment indicated that the granular fertilizer increased grain yield by 10.7% and maize production by 46.2%. The benefits were ascribed to increase P supply, slow nutrient release, and moisture regime for maize growth.
After impregnation with N–P–K nutrient solution prepared by dissolving ammonium nitrate, potassium dibasic phosphate, and single super phosphate, biochar was mixed with a binder of starch/PVA and granulated by extrusion [128]. The granulated fertilizer exhibited lower nutrient release and higher water retention of the amended sandy soil than conventional chemical fertilizer. The effect of granulating biochar produced by blending or coating triple superphosphate was studied on P release kinetics and plant growth [75]. Compared with triple superphosphate, the granulating biochar had lower P release, and the coating was more effective than blending for decreasing P release. Granular biochar was prepared by uniformly mixing biochar, clay minerals (bentonite and sepiolite) and urea, and subsequent mechanical pelletizing using wood vinegar as a binder [129]. Attaching urea to the particle surface of biochar and clay minerals was characterized in the composite. Leaching experiments suggested that the cumulative N release and the dissolved organic carbon of the granular biochar were remarkably decreased by above 70% and 8% compared to urea. The pot experiment revealed that the fresh maize shoot and root were increased by 14% and 25% with the application of the biochar fertilizer. The rice husk biochar was first impregnated with urea–hydrogen peroxide, and then pelletized in a disc pelletizer using kaolin as a binder [127]. The pelletization process was optimized using response surface methodology for lowering N release. Attachment of urea–hydrogen peroxide in biochar pores was proved, and the minimum cumulative N release of the granular fertilizer was 17.63%. Pot experiments showed improved cabbage growth.
The pellet properties are dependent on biomass characteristics and operating conditions [130]. [35] reported that the mechanical properties and nutrient release of granular fertilizer improved with increasing drying temperature. After mixing biogas residue biochar with commercial fertilizer, the binder-free granular fertilizer was synthesized using a compression mold [69]. Response surface methodology was used for the optimization of the granulation process. The physical properties of the pellets were the best under optimal conditions of diameter 7 mm, moisture content 7.84%, and compression speed 49.54 mm min−1. The excellent sustained nutrient release of the granular fertilizer was proved, and the slow-release effect of nutrients obeyed P>N>K. Although water or steam can be used for granulation, the binder-free granular biochar has poor mechanical performance for the application. Binders are used to improve the mechanical strength of biochar-based SRFs, and they include clay, sepiolite, bentonite, starch, etc. However, many organic binders may induce environmental damage owing to poor degradability. Moreover, the high cost and complex preparation limit the practical application of granular biochar-based SRFs [126].
3.6. Integrated methods
Besides the methods mentioned above, innovative approaches are proposed to synthesize biochar-based SRFs, especially for integrated methods. Biochar derived from rice husk was first mixed with phosphoric acid, then reacted with ammonia gas, and finally granulated using a small stainless-steel drum [131]. The formation of ammonium phosphate in pores of biochar was clarified. The discharge of the available nitrogen (NH4+-N and NO3−-N) from the granular fertilizers was slower than that of ammonium phosphate. Pot experiments demonstrated high N content and improved plant growth with the addition of granular biochar-ammonium phosphate. This work provides a method of manufacturing uncoated slow-release fertilizer.
An et al. [73] developed microwave-assisted co-polymerization for SRFs synthesis, and biochar was embedded into semi-interpenetrating polymer networks (Fig. S5a). SEM images displayed uniform and small pore networks of the polymer structure (Figs. S5b–d). TEM images manifested that the tiny particles assigned to K3PO4 and NH4H2PO4 were encapsulated inside the pore network (Figs. S5e–h). The stronger hydrophilicity was verified by the contact angle measurement (Fig. S5i). XRD patterns proved the presence of KH2PO4 and (NH4)2HPO4 (Fig. S5j). The presence of biochar in semi-interpenetrating polymer networks enhanced water retention, reinforced nutrient slow-release, improved the degradability of SRFs. Better growth of pepper seedlings was confirmed by pot experiments after the addition of biochar-based SRF.
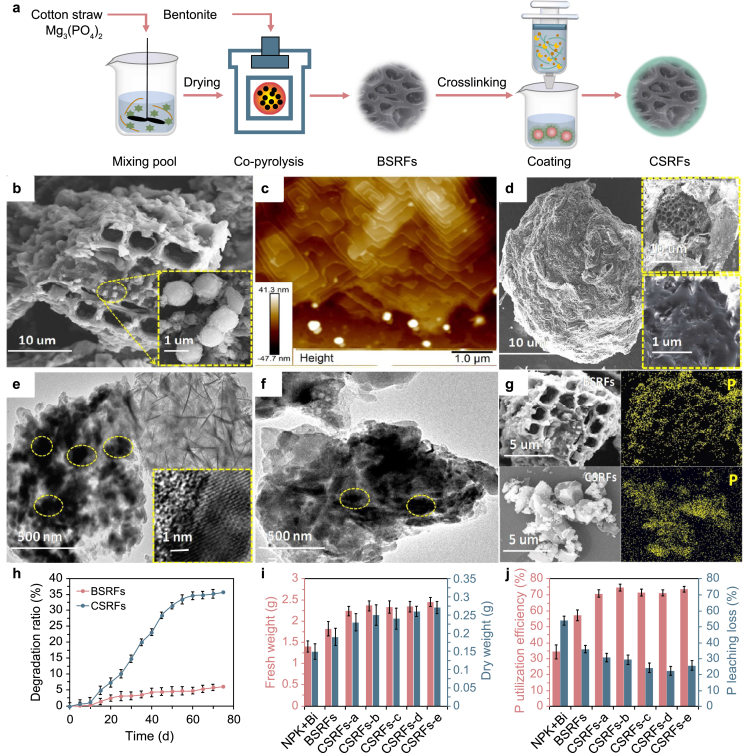
The intergradation of co-pyrolysis and encapsulation was conducted to synthesize coated biochar SRFs (Fig. 4a) [125]. SEM image showed a porous structure with randomly loaded bentonite and Mg3(PO4)2 (Fig. 4b, d). Highly ordered stripes were seen from the AFM image (Fig. 4c). TEM verified abundant fertilizer particles and irregular coating, and the dominant nutrient of P was proved by EDX analysis (Fig. 4e–g). Compared to the co-pyrolyzed SRFs, the coated SRFs had lower P release and superior degradability, and significantly improved plant growth (Fig. 4h–j). The research group further compared the performance of SRFs derived from phosphorus impregnated biochar, pyrolysis of biomass/nutrients/bentonite (BSRFs), the coated BSRFs, and co-polymerized BSRFs [68]. The co-polymerized BSRFs displayed the highest water retention and excellent P slow-release performance comparable to coated BSRFs. The good biodegradability and highest P utilization efficiency of co-polymerized BSRFs were verified.
Fig. 4.
a. Schematic of CSRFs preparation via integrating co-pyrolysis and encapsulation; b., d. SEM images; c. AFM image; e., f. TEM images; g. elemental mapping of P; h. degradation behavior of BSRFs and CSRFs; i. fresh weight and dry weight of plant, and j. P leaching loss and utilization efficiency. Reprinted (adapted) with permission from [125]. Copyright 2021 Elsevier.
An infiltration process with molten urea was developed to fabricate biochar-based fertilizer [126]. An infiltration device consisting of two glass tubes was used (Fig. S6a). The rice husk was added to the lower glass tube, the molten urea obtained at 140 °C was poured into the upper glass tube at a designed temperature in a drying oven. After the infiltration process, biochar granulation was conducted by the extrusion of the precursor. FTIR, TGA, and SEM characterizations suggested that small urea particles were evenly distributed on biochar surface with maximum N content of 29.19% (Figs. S6b–d). However, the N release and bioavailability were not investigated in this work.
A biochar-based slow-release fertilizer was fabricated by an integrated process (Fig. 5a) [70]. Pine biochar obtained by fast pyrolysis at 500 °C was mixed with the H3PO4 solutions. After shaking for 24 h and fully drying at 85 °C, the mixture was carbonized at 465 °C for 2 h under N2 atmosphere. The mixture of activated biochar and urea was ground and heated at 137 °C to infuse the urea into the pores of biochar. The granulation of the urea-infused biochar was conducted using calcium lignosulfonate solution and liquefied paraffin wax as binders. SEM-EDS, XRD, and FTIR analysis manifested urea grafting onto biochar through urea amine N and carbonyl C O bonds (Fig. 5b–d). The excellent slow-release effect of the biochar-based fertilizer was proved by slow urea release (61–90% in 4320 min), low N loss (68–71% after 41 leaching), and minor NH3–N volatilization (0.2–0.9%) (Fig. 5e–g). The developed fertilizer effectively diminished N loss from soil and provided long-term NH4+-N supply for plants.
Fig. 5.
a. Schematic of the synthesis of urea-infused biochar; b. FTIR of the slow-release fertilizers; c. XRD patterns of slow-release fertilizers prepared from H3PO4 activated biochar; d. SEM image of slow-release fertilizer; e. urea release, f. NH4+-N availability, and g. cumulative N leaching. Reprinted (adapted) with permission from [70]. Copyright 2021 American Chemical Society.
Microwave-assisted preparation of a biochar-based fertilizer containing nitrogen was proposed to enhance slow-release nitrogen and water retention [4]. Biochar adsorbed with NH4+ was uniformly mixed with acrylic acid, 2-acrylamide-2-methylpropanesulfonic acid, bentonite, cotton stalk, and potassium persulfate, and underwent complete polymerization under microwave irradiation. The biochar-based fertilizer reduced the N release (69.8% after 30 days), lowered N leaching (10.3%), and increased N use efficiency (64.27%). The fertilizer had a high water-retention capacity and improved cotton plant growth. Additionally, the good degradability was verified by the soil burial degradation test. A complex process, involving impregnation, granulation, and coating, was developed to prepare biochar-based SRFs (Fig. 6) [71]. A biofilm-derived liquefied cotton stalk was synthesized and used as coating material. SEM images manifested the porous structure of biochar and the successful loading of phosphorus nutrients. The improved growth of corn maize was observed with the addition of biochar-based SRFs, and the fertilizer with 2% biofilm showed the best efficiency.
Fig. 6.
a. Schematic of the synthesis of biofilm coated biochar-based SRFs; b. SEM images; c. P leaching, d. cumulative P release loss; e. fresh weight, f. dry weight, and g. pictures of maize growth. Reprinted (adapted) with permission from [71]. Copyright 2021 Elsevier.
Each method has distinct features for fabricating biochar-based SRFs (Fig. 7). In-situ pyrolysis enables the one-step synthesis of biochar-based SRFs, but it is limited by deficient nutrients in biomass feedstocks and the potential presence of toxic elements. Co-pyrolysis is easy to control the nutrients loading, but it is highly dependent on the N and P sources. Impregnation can be easily performed, and it is promising considering recovery nutrients from wastewater. For the impregnation method, modifications of pristine biochar are commonly necessary due to low adsorption capacity toward nutrients, which increases the process cost. Encapsulation enables coating nutrients onto biochar and is highly favorable to the slow-release of nutrients. The complex and expensive encapsulation process limits the application of coated SRFs, and the potential threat of coating materials requires special attention. Granulation is effective in reducing biochar loss and handling costs for real agricultural practices, and it is commonly integrated with other methods for the fabrication of effective biochar-based SRFs. Considering the multiple requirements of low cost, easy operation, high efficiency, and environmental friendliness, it is difficult to find a versatile process that meets all applications. Currently, the integrated methods gain more and more research interests.
Fig. 7.
The advantages and disadvantages of method for fabricating biochar-based SRFs.
4. Nutrients loading mechanism
SRFs enable gradual nutrient release for long-term supply of the nutrients and avoid leaching loss, improving fertilizer efficiency, and diminishing potential threats to the environment [35]. As a nutrient carrier, biochar potential declines the release rate of nutrient and improve nutrient utilization efficiency owing to high surface area and porous microstructure, favorable surface charge, abundant surface groups, and active carbon [59]. The performance of biochar-based SRFs can be improved by exploring the basic properties and the mechanism of nutrient loading. Extensive studies provide an in-depth understanding of biochar-based SRFs. A better understanding of nutrients loading mechanism helps discover effective biochar-based SRFs and evaluating fertilizer fate in the environment.
Adsorption of pollutants using biochar has been widely investigated, and in-depth discussion of the adsorption mechanism has been reported [132,133]. The adsorption of pollutants by biochar is closely related to the properties of pollutants and biochar. In the case of metal ions, the possible mechanism includes ion exchange, surface complexation, electrostatic attraction, physical adsorption, and surface precipitation [133]. For organic contaminants, the mechanism involves hydrogen bond, pore-filling, electrostatic interaction, hydrophobic interaction, and surface complexation (Fig. S7). Most adsorption mechanisms depend on biochar properties, such as surface area, porous structure, surface groups, and mineral components. The potential adsorption effect favors attaching nutrients onto biochar and fabricating SRFs.
Biochar is an excellent carrier of nutrients such as N, P, K, attributing to the large load ability of the porous structure and strong adsorption ability toward nitrate, ammonium, phosphate, etc. Ye et al. [58] found that biochar prepared under CO2 or H2O(g) atmosphere contained more surface groups for K adsorption, and active groups on biochar surface displayed good N and P adsorption. Reclaiming phosphate from water was controlled by the adsorption mechanism of P precipitation and deposition on Mg particles in biochar [105]. Phosphorus adsorption onto MgO modified magnetic biochar was ascribed to precipitation, electrostatic attraction, ligand exchange, and metallic complexation between surface groups and metals [134]. Chen et al. [9] reported that P adsorption onto cow dung biochar was dominated by surface precipitation. Vikrant et al. [135] summarized phosphate adsorption by various biochar (Fig. 8a). Nutrient uptake onto biochar/montmorillonite composites indicated that NH4+ adsorption was mainly ascribed to surface adsorption associated with ion exchange, while PO43- adsorption was controlled by the electrostatic attraction or ionic bonding between PO43- and metal cations [39]. N adsorption onto biochar was reported through ion exchange, surface groups, and electrostatic interaction [49]. Wang et al. [107] reported the dominant mechanism of electrostatic adsorption, ligand exchange, and surface precipitation for phosphate adsorption onto coal gangue modified biochar. Zhang' group [57] conducted phosphate recovery from synthetic urine using goethite/biochar composite, and found phosphate adsorption through the Fe–OH bonds and the formed inner-sphere complexes (Fe–O–P). Luo and colleagues [50] reported the effective reclaiming of N and P nutrients from biogas effluent by Mg-enriched biochar (Fig. 8b). P release was attributed to Mg–P precipitates formed on the biochar surface and the ‘P-trap’ effect of MgO through the PO43- precipitation process. N release was dominated by the hydrogen bonds and pore-filling for N-containing organic matter, and the incarceration effect and electrostatic attraction for NH4+.
Fig. 8.
a. Phosphate adsorption on various biochar materials. Adapted with permission from [135]. Copyright 2018 Elsevier. b. Schematic of nutrient attachment onto biochar-based SRFs. Adapted with permission from [50]. Copyright 2021 Elsevier.
As discussed above, various methods such as impregnation, encapsulation, granulation, and co-pyrolysis are developed to prepare biochar-based SRFs. Based on the synthesis processes, there is a difference in of nutrient loading mechanism. Impregnation is highly dependent on the properties of biochar. The unique properties, including large surface area, porous structure, and abundant surface groups, provide favorable sites for attaching nutrients onto biochar by adsorption. Granulation mainly aims at increasing diffusion resistance to reduce nutrient leaching loss. As to encapsulation, nutrients are dominantly embedded in the internal sites of SRFs via different interactions.
Shi et al. [129] fabricated granular biochar/mineral/urea composites as potential SRFs. Urea was bound to the surface of biochar and minerals in the composites, and this occurrence enhanced the N retention. Zhao's group [79] proposed a co-pyrolysis of biomass and P fertilizers to synthesize biochar-based SRFs. The lower P release was attributed to creating stable C–O–P or C–P bonds. Increasing temperature enhanced the interactions between biochar and nutrients in the SRFs, but requiring more energy consumption and high production cost. An and colleagues conducted co-pyrolysis of bentonite and bamboo to obtain the modified biochar [63]. The enhanced P adsorption and release performance of bentonite modified biochar were attributed to (i) the creation of porous structure, (ii) the reduction of surface negative charge, and (iii) the formation of precipitation due to the presence of Ca and Mg [63]. The P-release kinetics could be effectively regulated by changing the bentonite amount.
As to coated SRFs, the coating materials on fertilizer surface are barriers that prohibit nutrient diffusion [125]. Generally, the nutrient release from coated SRFs involves: (i) transferring water into the interior of fertilizer, (ii) the dissolution of nutrients, and (iii) the nutrient release through the coating [136]. The formation of a nutrient-rich organic coating was identified [56]. The coating material attached to the inner and outer surface of co-composted biochar, and increased the water and nutrient retention through increasing hydrophilicity, mesoporosity, and redox-active moieties. The blended and coated SRFs were compared relating to nutrient release. The blended biochar/superphosphate fertilizer demonstrated relatively poor P slow release, which was restricted by the diffusion and swelling/relaxation. The coated biochar/superphosphate fertilizer displayed superior P retention controlled by the swelling/relaxation [75].
The presence of biochar was favorable for the generation of porous structure and abundant cross-linking points in biochar embedded-semi-interpenetrating polymer networks based SRF, and biochar also improved the water retention and degradability of the Semi-IPN based SRF [73]. The infiltration of molten urea onto biochar as SRFs was proposed [126]. It was found the uniform distribution of molten urea infiltrated into biochar pores. The generation of different amides occurred by the reactions between molten urea and surface groups.
As discussed above, a better understanding of the nutrient loading mechanism is obtained, providing insights into the design and fabrication of biochar-based SRFs. Currently, the slow-release mechanism is tentatively understood based on nutrient loading onto biochar. Multiple characterizations, such as X-ray diffraction, infrared spectroscopy, and X-ray photoelectron spectroscopy, can be employed to reveal the elemental variation of biochar-based SRFs. The release behavior of nutrients is commonly determined in water or solution mediums, as well as column leaching in soil or sand [11,50,71]. The schematic diagram of the column leaching device is shown in Fig. S8. Release kinetics under different conditions provides insights into nutrient release from biochar-based SRFs. Owing to the difference in methods, processes, and devices, the results reported from different researches are difficult in comparison. The elemental transformation of nutrients is still poorly investigated after applying to soil. Especially, the effect of soil environments on nutrient release is rarely reported for biochar-based SRFs.
5. Potential agricultural applications
To evaluate the fertilizer efficiency of biochar-based SRFs, plant growth experiments are imperative. The effect of biochar-based SRFs on plant growth is commonly investigated through pot experiments at a bench scale. Reports on plant growth through pot experiments are listed in Table S2. Plants of pepper, corn, mung bean, oilseed rape, and Spinacia oleracea were used, and the growth period ranged from 10 days to 6 months. Plant height, root length, biomass weight, and chlorophyll content were used as plant growth indicators. The improvement of plant growth associated with biochar-based SRFs is verified by pot experiments.
Field experiment for evaluating biochar-based SRFs is imperative before widespread application for agricultural production. Recently, field studies on the application of biochar-based SRFs to agricultural production were reported. Field experiments reported by [137] exhibited that the low amount (less than 0.1%) of biochar-based compound fertilizer induced the improvement of maize yield and soil fertility and the significant decline of Cd contents in maize grains. Yan and colleagues conducted a field experiment in a tea orchard to examine the impact of biochar-based fertilizer on the yield and quality of tea and microbial community in soil [138]. Biochar-based fertilizer improved soil properties and altered bacterial and fungal diversity and community, and its application significantly enhanced the yield and quality of tea, which were positively related to some plant beneficial microbes. Experiments in field vegetable production indicated that biochar-based fertilizer treatment manifested higher yield, plant N uptake, and nitrogen use efficiency [139]. A 3-year field experiment in Northeast China was conducted to examine the impacts of biochar and controlled-release urea on rice yield and nitrogen-use efficiency [124]. Mixed application of bare urea, controlled-release urea, and biochar effectively improved rice yield and fertilization efficiency for sustainable agricultural production. Additionally, field studies were reported on the alternative benefits of biochar-based fertilizers [26]. conducted field experiments in a rice paddy field to reveal the mitigation of CH4 emission in a rice paddy field after the addition of biochar-based SRF [138]. performed field experiment in a karst mountainous area, and found that biochar-based fertilizer was effective for soil restoration by improving the complexity of microbial-related networks and soil nutrients. Similarly, Gao et al. [140] reported the improved bacterial communities of brown earth soil by biochar-based fertilizer. A field study in a subtropical Moso bamboo plantation indicated that the application of biochar-based fertilizer was effective for the mitigation of N2O emissions from subtropical plantation soils [33].
6. Perspectives and challenges
Numerous related researches have been reported, but the development of biochar-based SRFs for real application is still in its infancy. Currently, there are challenges from the aspects of (i) the synthesis of effective biochar-based SRFs, (ii) the evaluation of nutrient release and bioavailability, and (iii) the long-term assessment of a real application. The research perspectives are also discussed in terms of the above aspects.
The development of biochar-based SRFs aims to improve fertilizer utilization efficiency and diminish nutrient loss into the environment. Pristine biochar is not favorable as fertilizer due to the low content of nutrients, and the loading nutrients is limited to the poor adsorption and stabilization effects onto biochar. To improve the nutrient content, modifications are commonly conducted to increase the adsorption capacity or nutrient attachment of biochar. Phosphorus is a limiting nutrient for crop growth, but the non-renewable P resources are being depleted with a high P fertilizer consumption of above 20 Tg year−1 [6]. Recycling P from P-rich sources is a promising alternative to mitigate phosphate rock depletion. Biomass feedstocks, for example, fermentation wastes containing abundant phosphorus (7445 mg kg−1), manure and sludge contain high P, and be used to produce P-enrich biochar [62]. For wastewater, human urine accounts for below 1% of the total volume but contains above 50% of total phosphorus in wastewater [141]. Phosphorus is an indicator of surface water quality. Excessive phosphorus causes serious eutrophication, which threats the aquatic ecosystems and increases the economic burden. Effective reclaiming phosphate from P-enriched wastewater biochar benefits wastewater treatment and fertilizer production. Additionally, co-composting biochar with compost feedstocks containing abundant nutrients and organic carbon effectively strengthens the agronomic performance of biochar as a soil amendment [113].
Due to brittleness, low density, and small particles, high loss of biochar is caused by wind-blown or surface runoff from intense rain events. Moreover, the storage and transport of biochar for soil application are highly challenging. Granulation is an effective option to decrease biochar loss and handling costs for large-scale applications in agricultural practices. Encapsulation is highly efficient to enhance nutrient release, but the degradability of coating materials should be fully evaluated. Various methods developed for biochar-based SRFs have distinct features, and it is difficult to find a versatile process meeting all applications. According to previous reports, more and more complex processes are used to fabricate efficient SRFs. Additionally, researches currently focus on lowering nutrient release, but the precise control of nutrient release meeting the requirement of crop growth requires more effort in future work. Biochar-based SRFs require these features: (i) low production cost, (ii) simple fabricating process, (iii) environmental friendliness, (iv) excellent slow-release performance for nutrients, and (v) high bioavailability for crop growth. Therefore, finding a strategy for synthesizing biochar-based SRFs meeting these requirements is still imperative in future studies.
An in-depth understanding of nutrient slow release is imperative for fertilizer synthesis and evaluation of a real application. Although research advances on the mechanism of nutrient loading have been obtained, an in-depth understanding of the slow-release mechanism of nutrients from biochar-based SRFs is still insufficient [56]. Nutrient adsorption onto biochar via multiple interactions plays an important role in loading nutrients in biochar-based SRFs. Nutrient barrier or chemical reactions are also involved in nutrient uptake onto biochar. The nutrient release from biochar-based SRFs should be quantitatively evaluated. The nutrient release can be determined in water or solution mediums, and column leaching in soil or sand is also employed to examine the leaching loss of nutrients. The results obtained from different studies cannot be compared due to the difference in methods, processes, and devices. Proposing standard methods for determining the nutrient release is helpful to evaluate the slow-release performance of as-prepared biochar-based SRFs. Release kinetics under different conditions provides insights into nutrient capture and the trade-off between leaching loss and bioavailability. In addition, the revelation of interactions between nutrients and soil environments is of high importance to understand the slow-release mechanism of nutrients from biochar-based SRFs.
There are debates about the “net absolute benefits” of biochar application [110]. Some researchers argue that massive application biochar may induce soil toxicity from heavy metals [142,143]. Others claim that the high adsorption capacity of biochar restraints nutrient supply for plant uptake due to strong binding vital nutrients [144]. Additionally, biochar addition to soils does not lead to consistent crop yield improvement, and plant growth responded to biochar varies significantly. Therefore, the mechanism behind the capture and subsequent release of nutrients from biochar-based SRFs is not sufficiently understood.
The application of biochar-based SRFs can be an applicable strategy to alleviate soil deterioration and climate change by GHG emission reduction, carbon sequestration, and soil nutrient retention improvement. Zhang et al. reported biochar-based SRFs applied in rainfed maize cropland in China increased crop production by above 20% and reduced N2O emission by more than 30% over conventional fertilizer [145]. It was reported that carbon sequestration was up to 2.3 tons ha−1 in corn fields after applying biochar and cow manure compost, and the mitigation of equivalent CO2 emission was estimated to be 7.3–8.4 tons ha−1 [146]. Fidel et al. evaluated the potential reduction of GHG emissions from biochar-amended soils [147]. Biochar declined 27% soil CO2 emissions for the entire season of maize growing, and the NO2 reduction was observed in continuous cropping systems [147]. Dong and colleagues found that the addition of biochar-based SRF significantly reduced CH4 emissions by 33.4% during the rice-growing season, which was ascribed to the different species of N in slow-release fertilizer and available potassium in soil [26]. The long-term evaluation of GHG emissions associated with biochar-based SRFs application should be further examined.
Besides fertilizer efficiency, biochar has additional benefits, including increased soil texture, water retention, cation exchange capacity, and microbial diversity [110]. Biochar amendment can reduce the availability of potentially toxic metals while remediating contaminated soils due to the high adsorption capacity. Biochar-based fertilizer increased the microbial biomass of nutrients, changed the microbial community, and improved bacterial community network complexity [148]. Liao et al. also reported the improved microbial activity, stimulated soil nitrification, and reduced denitrification by shifting the microbial communities toward the nitrifying bacteria after applying biochar-based N fertilizer [149].
Water supply is a critical factor in agriculture production. The fertilizers with high water retention are preferable, especially in arid and semiarid regions. Superabsorbent polymers (SAPs) with excellent water absorption capacity are commonly used to improve the water retention of soil. The incorporation of nutrients into SAPs can be a promising strategy to improve the water retention of SRFs [150]. It was found that SAPs-based SRFs were highly appropriate for the application in drought land due to long-term retention of soil moisture [151]. Kenawy et al. synthesized sugarcane bagasse-g-poly (acrylamide)/attapulgite composites with excellent swelling capacity, and pointed out that the composites as SRFs can be regulated for nutrient release ratio by changing the attapulgite amount in the copolymerization process [152]. Cotton stalks and bentonite were proved to increase water retention, reduce nutrient leaching loss, and decline the degradability of biochar-based SRFs [4]. Biochar-based SRFs prepared by co-polymerization manifested better water retention, P slow-release, and biodegradability compared to the fertilizer fabricated by co-pyrolysis [68]. The application of biochar-based SRFs is promising for climate change migration, soil amendment, and crop production in future agricultural practice. A better understanding of the effects of the biochar-based SRFs influence on soil properties, GHG emission, and carbon sequestration still needs extensive researches, especially for long-term field studies. The additional benefits of biochar-based SRFs enhance sustainable agriculture in future applications.
7. Conclusions
Biochar application in soil originates from dark earth formed through slash-and-char practice. Researches reveal the beneficial role of biochar in soil amendment. Unique properties of biochar provide a platform for loading nutrients to improve fertilizer efficiency. Conventional chemical fertilizers suffer from the drawbacks of rapid nutrient leaching, serious environmental pollution, and significant economic burden, and biochar-based SRFs receive increasing global attention. Currently, most studies focus on the synthesis of effective biochar-based SRFs. Various methods, including in-situ pyrolysis, co-pyrolysis, impregnation, encapsulation, and granulation, have been proposed, and each method manifests distinct features. It is difficult to find a versatile method to meet all the requirements. Integrated processes are increasingly employed for the synthesis of biochar-based SRFs. Understanding of nutrient loading mechanism is discussed based on recent researches. The development of biochar-based SRFs for real application is still in its infancy. There are challenges from the aspects of (i) the synthesis of effective SRFs, (ii) the evaluation of nutrient release and bioavailability, and (iii) the long-term assessment of a real application. Nonetheless, current knowledge manifests that applying biochar-based SRFs is a viable way of promoting sustainable agriculture.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key Research and Development Project (2020YFC1908802), China; the National Natural Science Foundation of China (U1704252 and 51804276), China; and the Young Elite Scientist Sponsorship Program by CAST (2019QNRC001), China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2022.100167.
Abbreviations
- FAO
the Food and Agriculture Organization
- AAPFCO
he Association of American Plant Food Control Officials
- IBI
the International Biochar Initiative;
- GHG
greenhouse gas
- SAPs
superabsorbent polymers
- SRFs
slow-release fertilizers
- TG-DTA
thermogravimetric-differential thermal analysis
- FTIR
Fourier transform infrared spectroscopy
- SEM
scanning electron microscope
- EDS/EDX
energy dispersive x-ray spectroscopy
- XRD
X-ray Powder Diffraction
- XPS
X-ray photoelectron spectroscopy
- TGA
thermal gravimetric analysis
- TEM
transmission electron microscope
- AFM
atomic force microscope
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.FAO . 2017. World Fertilizer Trends and Outlook to 2020: Summary Report. [Google Scholar]
- 2.Chen S., Yang M., Ba C., Yu S., Jiang Y., Zou H., Zhang Y. Preparation and characterization of slow-release fertilizer encapsulated by biochar-based waterborne copolymers. Sci. Total Environ. 2018;615:431–437. doi: 10.1016/j.scitotenv.2017.09.209. [DOI] [PubMed] [Google Scholar]
- 3.FAO . 2019. World Fertilizer Trends and Outlook to 2022: Summary Report. [Google Scholar]
- 4.Wen P., Wu Z., Han Y., Cravotto G., Wang J., Ye B.C. Microwave-assisted synthesis of a novel biochar-based slow-release nitrogen fertilizer with enhanced water-retention capacity. ACS Sustain. Chem. Eng. 2017;5(8):7374–7382. [Google Scholar]
- 5.Naz M.Y., Sulaiman S.A. Slow release coating remedy for nitrogen loss from conventional urea: a review. J. Contr. Release. 2016;225:109–120. doi: 10.1016/j.jconrel.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Reijnders L. Phosphorus resources, their depletion and conservation, a review. Resour. Conserv. Recycl. 2014;93:32–49. [Google Scholar]
- 7.van Dijk K.C., Lesschen J.P., Oenema O. Phosphorus flows and balances of the European union member states. Sci. Total Environ. 2016;542:1078–1093. doi: 10.1016/j.scitotenv.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Li Y., Xu Y., Lu X. Biochar phosphorus fertilizer effects on soil phosphorus availability. Chemosphere. 2020;244:125471. doi: 10.1016/j.chemosphere.2019.125471. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q., Qin J., Sun P., Cheng Z., Shen G. Cow dung-derived engineered biochar for reclaiming phosphate from aqueous solution and its validation as slow-release fertilizer in soil-crop system. J. Clean. Prod. 2018;172:2009–2018. [Google Scholar]
- 10.Duhan J.S., Kumar R., Kumar N., Kaur P., Nehra K., Duhan S. Nanotechnology: the new perspective in precision agriculture. Biotechnol. Rep. 2017;15:11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan H.A., Naqvi S.R., Mehran M.T., Khoja A.H., Niazi M.B.K., Juchelková D., Atabani A. A performance evaluation study of nano-biochar as a potential slow-release nano-fertilizer from wheat straw residue for sustainable agriculture. Chemosphere. 2021;285:131382. doi: 10.1016/j.chemosphere.2021.131382. [DOI] [PubMed] [Google Scholar]
- 12.Association of American Plant Food Control Officials (AAPFCO) Vol. 48. 1995. Official Publication. [Google Scholar]
- 13.Shaheen S.M., Niazi N.K., Hassan N.E., Bibi I., Wang H., Tsang D.C., et al. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: a critical review. Int. Mater. Rev. 2019;64(4):216–247. [Google Scholar]
- 14.Zhang X., Huang R., Cao Y., Wang C. Rapid conversion of red mud into soil matrix by co-hydrothermal carbonization with biomass wastes. J. Environ. Chem. Eng. 2021;9(5):106039. [Google Scholar]
- 15.Ahmad M., Rajapaksha A.U., Lim J.E., Zhang M., Bolan N., Mohan D., et al. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014;99:19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- 16.Wang C., Sun R., Huang R. Highly dispersed iron-doped biochar derived from sawdust for Fenton-like degradation of toxic dyes. J. Clean. Prod. 2021;297:126681. [Google Scholar]
- 17.O'Connor J., Hoang S.A., Bradney L., Dutta S., Xiong X., Tsang D.C., et al. A review on the valorisation of food waste as a nutrient source and soil amendment. Environ. Pollut. 2021;272:115985. doi: 10.1016/j.envpol.2020.115985. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed M.J., Hameed B.H. Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: a review. J. Clean. Prod. 2020;265:121762. [Google Scholar]
- 19.Wang C., Zhang X., Sun R., Cao Y. Neutralization of red mud using bio-acid generated by hydrothermal carbonization of waste biomass for potential soil application. J. Clean. Prod. 2020;271:122525. [Google Scholar]
- 20.Sun R., Zhang X., Wang C., Cao Y. Co-carbonization of red mud and waste sawdust for functional application as Fenton catalyst: evaluation of catalytic activity and mechanism. J. Environ. Chem. Eng. 2021;9(4):105368. [Google Scholar]
- 21.Wang C., Huang R., Sun R., Yang J., Sillanpää M. A review on persulfates activation by functional biochar for organic contaminants removal: synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021;9(5):106267. [Google Scholar]
- 22.Liu W.J., Jiang H., Yu H.Q. Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ. Sci. 2019;12(6):1751–1779. [Google Scholar]
- 23.Feng D., Wang S., Zhang Y., Zhao Y., Sun S., Chang G., et al. Review of carbon fixation evaluation and emission reduction effectiveness for biochar in China. Energy Fuel. 2020;34(9):10583–10606. [Google Scholar]
- 24.Melo T.M., Bottlinger M., Schulz E., Leandro W.M., de Oliveira S.B., de Aguiar Filho A.M., et al. Management of biosolids-derived hydrochar (Sewchar): effect on plant germination, and farmers' acceptance. J. Environ. Manag. 2019;237:200–214. doi: 10.1016/j.jenvman.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Yu L., Lu X., He Y., Brookes P.C., Liao H., Xu J. Combined biochar and nitrogen fertilizer reduces soil acidity and promotes nutrient use efficiency by soybean crop. J. Soils Sediments. 2017;17(3):599–610. [Google Scholar]
- 26.Dong D., Li J., Ying S., Wu J., Han X., Teng Y., et al. Science of The Total Environment; 2021. Mitigation of Methane Emission in a Rice Paddy Field Amended with Biochar-Based Slow-Release Fertilizer; p. 148460. [DOI] [PubMed] [Google Scholar]
- 27.Qin P., Wang H., Yang X., He L., Müller K., Shaheen S.M., et al. Bamboo-and pig-derived biochars reduce leaching losses of dibutyl phthalate, cadmium, and lead from co-contaminated soils. Chemosphere. 2018;198:450–459. doi: 10.1016/j.chemosphere.2018.01.162. [DOI] [PubMed] [Google Scholar]
- 28.Qian Z.Y., Xue S.G., Cui M.Q., Wu C., Li W.C. Arsenic availability and transportation in soil-rice system affected by iron-modified biochar. J. Cent. S. Univ. 2021;28(6):1901–1918. [Google Scholar]
- 29.Wang C., Wang H., Cao Y. Pb (II) sorption by biochar derived from Cinnamomum camphora and its improvement with ultrasound-assisted alkali activation. Colloids Surf. A Physicochem. Eng. Asp. 2018;556:177–184. [Google Scholar]
- 30.Kim J., Yoo G., Kim D., Ding W., Kang H. Combined application of biochar and slow-release fertilizer reduces methane emission but enhances rice yield by different mechanisms. Appl. Soil Ecol. 2017;117:57–62. [Google Scholar]
- 31.Cen Z., Wei L., Muthukumarappan K., Sobhan A., McDaniel R. Assessment of a biochar-based controlled release nitrogen fertilizer coated with polylactic acid. J. Soil Sci. Plant Nutr. 2021:1–13. [Google Scholar]
- 32.Sepúlveda-Cadavid C., Romero J.H., Torres M., Becerra-Agudelo E., López J.E. Evaluation of a biochar-based slow-release P fertilizer to improve Spinacia oleracea P use, yield, and nutritional quality. J. Soil Sci. Plant Nutr. 2021;21(4):2980–2992. [Google Scholar]
- 33.Zhou J., Qu T., Li Y., Van Zwieten L., Wang H., Chen J., et al. Biochar-based fertilizer decreased while chemical fertilizer increased soil N2O emissions in a subtropical Moso bamboo plantation. Catena. 2021;202:105257. [Google Scholar]
- 34.Lehmann J. Amazonian Dark Earths: Wim Sombroek's Vision. Springer; Dordrecht: 2009. Terra preta Nova–where to from here? pp. 473–486. [Google Scholar]
- 35.Kim P., Hensley D., Labbé N. Nutrient release from switchgrass-derived biochar pellets embedded with fertilizers. Geoderma. 2014;232:341–351. [Google Scholar]
- 36.He K., He G., Wang C., Zhang H., Xu Y., Wang S., et al. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2020;155:103674. [Google Scholar]
- 37.Ding Y., Liu Y., Liu S., Li Z., Tan X., Huang X., et al. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016;36(2):1–18. [Google Scholar]
- 38.de Medeiros E.V., Lima N.T., de Sousa Lima J.R., Pinto K.M.S., da Costa D.P., Junior C.L.F., et al. Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: a critical review. Phytoparasitica. 2021:1–14. [Google Scholar]
- 39.Chen L., Chen X.L., Zhou C.H., Yang H.M., Ji S.F., Tong D.S., et al. Environmental-friendly montmorillonite-biochar composites: facile production and tunable adsorption-release of ammonium and phosphate. J. Clean. Prod. 2017;156:648–659. [Google Scholar]
- 40.Xiao L., Meng F. Evaluating the effect of biochar on salt leaching and nutrient retention of Yellow River Delta soil. Soil Use Manag. 2020;36(4):740–750. [Google Scholar]
- 41.Jeffery S., Abalos D., Prodana M., Bastos A.C., Van Groenigen J.W., Hungate B.A., Verheijen F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017;12(5) [Google Scholar]
- 42.Liang C., Zhu X., Fu S., Méndez A., Gascó G., Paz-Ferreiro J. Biochar alters the resistance and resilience to drought in a tropical soil. Environ. Res. Lett. 2014;9(6) [Google Scholar]
- 43.Batista E.M., Shultz J., Matos T.T., Fornari M.R., Ferreira T.M., Szpoganicz B., et al. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-28794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amoakwah E., Frimpong K.A., Okae-Anti D., Arthur E. Soil water retention, air flow and pore structure characteristics after corn cob biochar application to a tropical sandy loam. Geoderma. 2017;307:189–197. [Google Scholar]
- 45.Li S., Barreto V., Li R., Chen G., Hsieh Y.P. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrol. 2018;133:136–146. [Google Scholar]
- 46.Jiang Y.H., Li A.Y., Deng H., Ye C.H., Wu Y.Q., Linmu Y.D., Hang H.L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019;276:183–189. doi: 10.1016/j.biortech.2018.12.079. [DOI] [PubMed] [Google Scholar]
- 47.Min L., Zhongsheng Z., Zhe L., Haitao W. Removal of nitrogen and phosphorus pollutants from water by FeCl3-impregnated biochar. Ecol. Eng. 2020;149:105792. [Google Scholar]
- 48.Cheng N., Wang B., Feng Q., Zhang X., Chen M. Co-adsorption performance and mechanism of nitrogen and phosphorus onto eupatorium adenophorum biochar in water. Bioresour. Technol. 2021;340:125696. doi: 10.1016/j.biortech.2021.125696. [DOI] [PubMed] [Google Scholar]
- 49.Dai Y., Wang W., Lu L., Yan L., Yu D. Utilization of biochar for the removal of nitrogen and phosphorus. J. Clean. Prod. 2020;257:120573. [Google Scholar]
- 50.Luo W., Qian L., Liu W., Zhang X., Wang Q., Jiang H., et al. A potential Mg-enriched biochar fertilizer: excellent slow-release performance and release mechanism of nutrients. Sci. Total Environ. 2021;768:144454. doi: 10.1016/j.scitotenv.2020.144454. [DOI] [PubMed] [Google Scholar]
- 51.De Rozari P., Greenway M., El Hanandeh A. Nitrogen removal from sewage and septage in constructed wetland mesocosms using sand media amended with biochar. Ecol. Eng. 2018;111:1–10. doi: 10.1016/j.scitotenv.2016.06.096. [DOI] [PubMed] [Google Scholar]
- 52.Palanivell P., Ahmed O.H., Latifah O., Abdul Majid N.M. Adsorption and desorption of nitrogen, phosphorus, potassium, and soil buffering capacity following application of chicken litter biochar to an acid soil. Appl. Sci. 2020;10(1):295. [Google Scholar]
- 53.Rens H., Bera T., Alva A.K. Effects of biochar and biosolid on adsorption of nitrogen, phosphorus, and potassium in two soils. Water, Air, Soil Pollut. 2018;229(8):1–13. [Google Scholar]
- 54.Huang L.Q., Fu C., Li T.Z., Yan B., Wu Y., Zhang L., et al. Advances in research on effects of biochar on soil nitrogen and phosphorus. IOP Conf. Ser. Earth Environ. Sci. 2020;424(No. 1) 012015. IOP Publishing. [Google Scholar]
- 55.Gao S., DeLuca T.H., Cleveland C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: a meta-analysis. Sci. Total Environ. 2019;654:463–472. doi: 10.1016/j.scitotenv.2018.11.124. [DOI] [PubMed] [Google Scholar]
- 56.Hagemann N., Kammann C.I., Schmidt H.P., Kappler A., Behrens S. Nitrate capture and slow release in biochar amended compost and soil. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X., Gang D.D., Sun P., Lian Q., Yao H. Goethite dispersed corn straw-derived biochar for phosphate recovery from synthetic urine and its potential as a slow-release fertilizer. Chemosphere. 2021;262:127861. doi: 10.1016/j.chemosphere.2020.127861. [DOI] [PubMed] [Google Scholar]
- 58.Ye Z., Zhang L., Huang Q., Tan Z. Development of a carbon-based slow release fertilizer treated by bio-oil coating and study on its feedback effect on farmland application. J. Clean. Prod. 2019;239:118085. [Google Scholar]
- 59.Jia Y., Hu Z., Ba Y., Qi W. Application of biochar-coated urea controlled loss of fertilizer nitrogen and increased nitrogen use efficiency. Chem. Biol. Technol. Agri. 2021;8(1):1–11. [Google Scholar]
- 60.Barbosa C.F., Correa D.A., Carneiro J.S.D.S., Melo L.C.A. Biochar phosphate fertilizer loaded with urea preserves available nitrogen longer than conventional urea. Sustainability. 2022;14(2):686. [Google Scholar]
- 61.Das S.K., Ghosh G.K. Development and evaluation of biochar-based secondary and micronutrient enriched slow release nano-fertilizer for reduced nutrient losses. Biomass Convers. Biorefin. 2021:1–12. [Google Scholar]
- 62.Kim J., Vijayaraghavan K., Reddy D., Yun Y. A phosphorus-enriched biochar fertilizer from bio-fermentation waste: a potential alternative source for phosphorus fertilizers. J. Clean. Prod. 2018;196:163–171. [Google Scholar]
- 63.An X., Wu Z., Yu J., Ge L., Li T., Liu X., Yu B. High-efficiency reclaiming phosphate from an aqueous solution by bentonite modified biochars: a slow release fertilizer with a precise rate regulation. ACS Sustain. Chem. Eng. 2020;8(15):6090–6099. [Google Scholar]
- 64.Sim D.H.H., Tan I.A.W., Lim L.L.P., Hameed B.H. Encapsulated biochar-based sustained release fertilizer for precision agriculture: a review. J. Clean. Prod. 2021:127018. [Google Scholar]
- 65.Zheng J., Han J., Liu Z., Xia W., Zhang X., Li L., et al. Biochar compound fertilizer increases nitrogen productivity and economic benefits but decreases carbon emission of maize production. Agric. Ecosyst. Environ. 2017;241:70–78. [Google Scholar]
- 66.An X., Wu Z., Yu J., Cravotto G., Liu X., Li Q., Yu B. Copyrolysis of biomass, bentonite, and nutrients as a new strategy for the synthesis of improved biochar-based slow-release fertilizers. ACS Sustain. Chem. Eng. 2020;8(8):3181–3190. [Google Scholar]
- 67.da Silva Carneiro J.S., Ribeiro I.C.A., Nardis B.O., Barbosa C.F., Lustosa Filho J.F., Melo L.C.A. Long-term effect of biochar-based fertilizers application in tropical soil: agronomic efficiency and phosphorus availability. Sci. Total Environ. 2021;760:143955. doi: 10.1016/j.scitotenv.2020.143955. [DOI] [PubMed] [Google Scholar]
- 68.An X., Wu Z., Liu X., Shi W., Tian F., Yu B. A new class of biochar-based slow-release phosphorus fertilizers with high water retention based on integrated co-pyrolysis and co-polymerization. Chemosphere. 2021;285:131481. doi: 10.1016/j.chemosphere.2021.131481. [DOI] [PubMed] [Google Scholar]
- 69.Yu Z., Zhao J., Hua Y., Li X., Chen Q., Shen G. Optimization of granulation process for binder-free biochar-based fertilizer from digestate and its slow-release performance. Sustainability. 2021;13(15):8573. [Google Scholar]
- 70.Bakshi S., Banik C., Laird D.A., Smith R., Brown R.C. ACS Sustainable Chemistry & Engineering; 2021. Enhancing Biochar as Scaffolding for Slow Release of Nitrogen Fertilizer. [Google Scholar]
- 71.An T., Cheng H., Qin Y., Su W., Deng H., Wu J., et al. The dual mechanisms of composite biochar and biofilm towards sustainable nutrient release control of phosphate fertilizer: effect on phosphorus utilization and crop growth. J. Clean. Prod. 2021;311:127329. [Google Scholar]
- 72.Khajavi-Shojaei S., Moezzi A., Masir M.N., Taghavi M. Biomass Conversion and Biorefinery; 2020. Synthesis Modified Biochar-Based Slow-Release Nitrogen Fertilizer Increases Nitrogen Use Efficiency and Corn (Zea mays L.) Growth; pp. 1–9. [Google Scholar]
- 73.An X., Yu J., Yu J., Tahmasebi A., Wu Z., Liu X., Yu B. Incorporation of biochar into semi-interpenetrating polymer networks through graft co-polymerization for the synthesis of new slow-release fertilizers. J. Clean. Prod. 2020;272:122731. [Google Scholar]
- 74.Jia Y., Hu Z., Mu J., Zhang W., Xie Z., Wang G. Preparation of biochar as a coating material for biochar-coated urea. Sci. Total Environ. 2020;731:139063. doi: 10.1016/j.scitotenv.2020.139063. [DOI] [PubMed] [Google Scholar]
- 75.Pogorzelski D., Lustosa Filho J.F., Matias P.C., Santos W.O., Vergütz L., Melo L.C.A. Biochar as composite of phosphate fertilizer: characterization and agronomic effectiveness. Sci. Total Environ. 2020;743:140604. doi: 10.1016/j.scitotenv.2020.140604. [DOI] [PubMed] [Google Scholar]
- 76.Dominguez E.L., Uttran A., Loh S.K., Manero M.H., Upperton R., Tanimu M.I., Bachmann R.T. Characterisation of industrially produced oil palm kernel shell biochar and its potential as slow release nitrogen-phosphate fertilizer and carbon sink. Mater. Today Proc. 2020;31:221–227. [Google Scholar]
- 77.Hu P., Zhang Y., Liu L., Wang X., Luan X., Ma X., et al. Biochar/struvite composite as a novel potential material for slow release of N and P. Environ. Sci. Pollut. Control Ser. 2019;26(17):17152–17162. doi: 10.1007/s11356-019-04458-x. [DOI] [PubMed] [Google Scholar]
- 78.Shin J., Park S. Optimization of blended biochar pellet by the use of nutrient releasing model. Appl. Sci. 2018;8(11):2274. [Google Scholar]
- 79.Zhao L., Cao X., Zheng W., Scott J.W., Sharma B.K., Chen X. Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain. Chem. Eng. 2016;4(3):1630–1636. [Google Scholar]
- 80.Karim A.A., Kumar M., Mohapatra S., Singh S.K., Panda C.R. Co-plasma processing of banana peduncle with phosphogypsum waste for production of lesser toxic potassium–sulfur rich biochar. J. Mater. Cycles Waste Manag. 2019;21(1):107–115. [Google Scholar]
- 81.Lou Z., Sun Y., Bian S., Baig S.A., Hu B., Xu X. Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar. Chemosphere. 2017;169:23–31. doi: 10.1016/j.chemosphere.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 82.Roberts D.A., Paul N.A., Dworjanyn S.A., Bird M.I., de Nys R. Biochar from commercially cultivated seaweed for soil amelioration. Sci. Rep. 2015;5(1):1–6. doi: 10.1038/srep09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cantrell K.B., Martin J.H. American Society of Agricultural and Biological Engineers; Dallas, Texas: 2012. Poultry Litter and Switchgrass Blending and Pelletizing Characteristics for Biochar Production. 2012. July 29-August 1, 2012. [Google Scholar]
- 84.Ma Y.L., Matsunaka T. Biochar derived from dairy cattle carcasses as an alternative source of phosphorus and amendment for soil acidity. Soil Sci. Plant Nutr. 2013;59(4):628–641. [Google Scholar]
- 85.Liu X., Li Z., Zhang Y., Feng R., Mahmood I.B. Characterization of human manure-derived biochar and energy-balance analysis of slow pyrolysis process. Waste Manag. 2014;34(9):1619–1626. doi: 10.1016/j.wasman.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 86.Yuan H., Lu T., Huang H., Zhao D., Kobayashi N., Chen Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrol. 2015;112:284–289. [Google Scholar]
- 87.Zwetsloot M.J., Lehmann J., Bauerle T., Vanek S., Hestrin R., Nigussie A. Phosphorus availability from bone char in a P-fixing soil influenced by root-mycorrhizae-biochar interactions. Plant Soil. 2016;408(1):95–105. [Google Scholar]
- 88.Karim A.A., Kumar M., Mohapatra S., Singh S.K. Nutrient rich biomass and effluent sludge wastes co-utilization for production of biochar fertilizer through different thermal treatments. J. Clean. Prod. 2019;228:570–579. [Google Scholar]
- 89.Zhao L., Cao X., Zheng W., Kan Y. Phosphorus-assisted biomass thermal conversion: reducing carbon loss and improving biochar stability. PLoS One. 2014;9(12):e115373. doi: 10.1371/journal.pone.0115373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lustosa Filho J.F., Penido E.S., Castro P.P., Silva C.A., Melo L.C. Co-pyrolysis of poultry litter and phosphate and magnesium generates alternative slow-release fertilizer suitable for tropical soils. ACS Sustain. Chem. Eng. 2017;5(10):9043–9052. [Google Scholar]
- 91.Lustosa Filho J.F., Barbosa C.F., da Silva Carneiro J.S., Melo L.C.A. Diffusion and phosphorus solubility of biochar-based fertilizer: visualization, chemical assessment and availability to plants. Soil Tillage Res. 2019;194:104298. [Google Scholar]
- 92.Lustosa Filho J.F., da Silva Carneiro J.S., Barbosa C.F., de Lima K.P., do Amaral Leite A., Melo L.C.A. Aging of biochar-based fertilizers in soil: effects on phosphorus pools and availability to Urochloa brizantha grass. Sci. Total Environ. 2020;709:136028. doi: 10.1016/j.scitotenv.2019.136028. [DOI] [PubMed] [Google Scholar]
- 93.Wu L.M., Tong D.S., Li C.S., Ji S.F., Lin C.X., Yang H.M., et al. Insight into formation of montmorillonite-hydrochar nanocomposite under hydrothermal conditions. Appl. Clay Sci. 2016;119:116–125. [Google Scholar]
- 94.Ramola S., Belwal T., Li C.J., Wang Y.Y., Lu H.H., Yang S.M., Zhou C.H. Improved lead removal from aqueous solution using novel porous bentonite-and calcite-biochar composite. Sci. Total Environ. 2020;709:136171. doi: 10.1016/j.scitotenv.2019.136171. [DOI] [PubMed] [Google Scholar]
- 95.Ashiq A., Adassooriya N.M., Sarkar B., Rajapaksha A.U., Ok Y.S., Vithanage M. Municipal solid waste biochar-bentonite composite for the removal of antibiotic ciprofloxacin from aqueous media. J. Environ. Manag. 2019;236:428–435. doi: 10.1016/j.jenvman.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 96.Premarathna K.S.D., Rajapaksha A.U., Adassoriya N., Sarkar B., Sirimuthu N.M., Cooray A., et al. Clay-biochar composites for sorptive removal of tetracycline antibiotic in aqueous media. J. Environ. Manag. 2019;238:315–322. doi: 10.1016/j.jenvman.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 97.Wang Z., Yang X., Qin T., Liang G., Li Y., Xie X. Efficient removal of oxytetracycline from aqueous solution by a novel magnetic clay–biochar composite using natural attapulgite and cauliflower leaves. Environ. Sci. Pollut. Control Ser. 2019;26(8):7463–7475. doi: 10.1007/s11356-019-04172-8. [DOI] [PubMed] [Google Scholar]
- 98.Spokas K.A., Novak J.M., Venterea R.T. Biochar's role as an alternative N-fertilizer: ammonia capture. Plant Soil. 2012;350(1):35–42. [Google Scholar]
- 99.Xu G., Sun J., Shao H., Chang S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014;62:54–60. [Google Scholar]
- 100.Hale S.E., Alling V., Martinsen V., Mulder J., Breedveld G.D., Cornelissen G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere. 2013;91(11):1612–1619. doi: 10.1016/j.chemosphere.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 101.Lateef A., Nazir R., Jamil N., Alam S., Shah R., Khan M.N., Saleem M. Synthesis and characterization of environmental friendly corncob biochar based nano-composite–A potential slow release nano-fertilizer for sustainable agriculture. Environ. Nanotechnol., Monitor. Manage. 2019;11:100212. [Google Scholar]
- 102.Maghsoodi M.R., Najafi N., Reyhanitabar A., Oustan S. Hydroxyapatite nanorods, hydrochar, biochar, and zeolite for controlled-release urea fertilizers. Geoderma. 2020;379:114644. [Google Scholar]
- 103.Chandra S., Medha I., Bhattacharya J. Potassium-iron rice straw biochar composite for sorption of nitrate, phosphate, and ammonium ions in soil for timely and controlled release. Sci. Total Environ. 2020;712:136337. doi: 10.1016/j.scitotenv.2019.136337. [DOI] [PubMed] [Google Scholar]
- 104.Wu L., Wei C., Zhang S., Wang Y., Kuzyakov Y., Ding X. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J. Clean. Prod. 2019;235:901–909. [Google Scholar]
- 105.Yao Y., Gao B., Chen J., Yang L. Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ. Scie. Technol. 2013;47(15):8700–8708. doi: 10.1021/es4012977. [DOI] [PubMed] [Google Scholar]
- 106.Li R., Wang J.J., Zhou B., Zhang Z., Liu S., Lei S., Xiao R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 2017;147:96–107. [Google Scholar]
- 107.Wang B., Ma Y., Lee X., Wu P., Liu F., Zhang X., et al. Environmental-friendly coal gangue-biochar composites reclaiming phosphate from water as a slow-release fertilizer. Sci. Total Environ. 2021;758:143664. doi: 10.1016/j.scitotenv.2020.143664. [DOI] [PubMed] [Google Scholar]
- 108.Kizito S., Wu S., Kirui W.K., Lei M., Lu Q., Bah H., Dong R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015;505:102–112. doi: 10.1016/j.scitotenv.2014.09.096. [DOI] [PubMed] [Google Scholar]
- 109.Takaya C.A., Fletcher L.A., Singh S., Anyikude K.U., Ross A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere. 2016;145:518–527. doi: 10.1016/j.chemosphere.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 110.Kizito S., Luo H., Lu J., Bah H., Dong R., Wu S. Role of nutrient-enriched biochar as a soil amendment during maize growth: exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability. 2019;11(11):3211. [Google Scholar]
- 111.González M.E., Cea M., Medina J., González A., Diez M.C., Cartes P., et al. Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Sci. Total Environ. 2015;505:446–453. doi: 10.1016/j.scitotenv.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 112.Puga A.P., Queiroz M.C.D.A., Ligo M.A.V., Carvalho C.S., Pires A.M.M., Marcatto J.D.O.S., Andrade C.A.D. Nitrogen availability and ammonia volatilization in biochar-based fertilizers. Arch. Agron Soil Sci. 2020;66(7):992–1004. [Google Scholar]
- 113.Hagemann N., Joseph S., Schmidt H.P., Kammann C.I., Harter J., Borch T., et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017;8(1):1–11. doi: 10.1038/s41467-017-01123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baki M., Abedi-Koupai J. Preparation and characterization of a superabsorbent slow-release fertilizer with sodium alginate and biochar. J. Appl. Polym. Sci. 2018;135(10):45966. [Google Scholar]
- 115.Dong D., Wang C., Van Zwieten L., Wang H., Jiang P., Zhou M., Wu W. An effective biochar-based slow-release fertilizer for reducing nitrogen loss in paddy fields. J. Soils Sediments. 2020;20(8):3027–3040. [Google Scholar]
- 116.Wu Y., Brickler C., Li S., Chen G. Synthesis of microwave-mediated biochar-hydrogel composites for enhanced water absorbency and nitrogen release. Polym. Test. 2021;93:106996. [Google Scholar]
- 117.Sha Z., Li Q., Lv T., Misselbrook T., Liu X. Response of ammonia volatilization to biochar addition: a meta-analysis. Sci. Total Environ. 2019;655:1387–1396. doi: 10.1016/j.scitotenv.2018.11.316. [DOI] [PubMed] [Google Scholar]
- 118.Fidel R.B., Laird D.A., Thompson M.L., Lawrinenko M. Characterization and quantification of biochar alkalinity. Chemosphere. 2017;167:367–373. doi: 10.1016/j.chemosphere.2016.09.151. [DOI] [PubMed] [Google Scholar]
- 119.Wei H., Wang H., Chu H., Li J. Preparation and characterization of slow-release and water-retention fertilizer based on starch and halloysite. Int. J. Biol. Macromol. 2019;133:1210–1218. doi: 10.1016/j.ijbiomac.2019.04.183. [DOI] [PubMed] [Google Scholar]
- 120.Lawrencia D., Wong S.K., Low D.Y.S., Goh B.H., Goh J.K., Ruktanonchai U.R., et al. Controlled release fertilizers: a review on coating materials and mechanism of release. Plants. 2021;10(2):238. doi: 10.3390/plants10020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X., Yang Y., Gao B., Li Y., Wan Y. Environmentally friendly slow-release urea fertilizers based on waste frying oil for sustained nutrient release. ACS Sustain. Chem. Eng. 2017;5(7):6036–6045. [Google Scholar]
- 122.Tian H., Liu Z., Zhang M., Guo Y., Zheng L., Li Y.C. Biobased polyurethane, epoxy resin, and polyolefin wax composite coating for controlled-release fertilizer. ACS Appl. Mater. Interfaces. 2019;11(5):5380–5392. doi: 10.1021/acsami.8b16030. [DOI] [PubMed] [Google Scholar]
- 123.Vo P.T., Nguyen H.T., Trinh H.T., Nguyen V.M., Le A., Tran H.Q., Nguyen T.T.T. The nitrogen slow-release fertilizer based on urea incorporating chitosan and poly(vinyl alcohol) blend. Environ. Technol. Innovat. 2021;22:101528. [Google Scholar]
- 124.Zheng Y., Han X., Li Y., Liu S., Ji J., Tong Y. Effects of mixed controlled release nitrogen fertilizer with rice straw biochar on rice yield and nitrogen balance in northeast China. Sci. Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-66300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.An X., Wu Z., Qin H., Liu X., He Y., Xu X., et al. Integrated co-pyrolysis and coating for the synthesis of a new coated biochar-based fertilizer with enhanced slow-release performance. J. Clean. Prod. 2021;283:124642. [Google Scholar]
- 126.Xiang A., Qi R., Wang M., Zhang K., Jiang E., Ren Y., Hu Z. Study on the infiltration mechanism of molten urea and biochar for a novel fertilizer preparation. Ind. Crop. Prod. 2020;153:112558. [Google Scholar]
- 127.Chen L., Chen Q., Rao P., Yan L., Shakib A., Shen G. Formulating and optimizing a novel biochar-based fertilizer for simultaneous slow-release of nitrogen and immobilization of cadmium. Sustainability. 2018;10(8):2740. [Google Scholar]
- 128.Gwenzi W., Nyambishi T.J., Chaukura N., Mapope N. Synthesis and nutrient release patterns of a biochar-based N–P–K slow-release fertilizer. Int. J. Environ. Sci. Technol. 2018;15(2):405–414. [Google Scholar]
- 129.Shi W., Ju Y., Bian R., Li L., Joseph S., Mitchell D.R., et al. Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci. Total Environ. 2020;701:134424. doi: 10.1016/j.scitotenv.2019.134424. [DOI] [PubMed] [Google Scholar]
- 130.Mostafa M.E., Hu S., Wang Y., Su S., Hu X., Elsayed S.A., Xiang J. The significance of pelletization operating conditions: an analysis of physical and mechanical characteristics as well as energy consumption of biomass pellets. Renew. Sustain. Energy Rev. 2019;105:332–348. [Google Scholar]
- 131.El Sharkawi H.M., Tojo S., Chosa T., Malhat F.M., Youssef A.M. Biochar-ammonium phosphate as an uncoated-slow release fertilizer in sandy soil. Biomass Bioenergy. 2018;117:154–160. [Google Scholar]
- 132.Dai Y., Zhang N., Xing C., Cui Q., Sun Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: a review. Chemosphere. 2019;223:12–27. doi: 10.1016/j.chemosphere.2019.01.161. [DOI] [PubMed] [Google Scholar]
- 133.Tan X., Liu Y., Zeng G., Wang X., Hu X., Gu Y., Yang Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere. 2015;125:70–85. doi: 10.1016/j.chemosphere.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 134.Li R., Wang J.J., Zhou B., Awasthi M.K., Ali A., Zhang Z., et al. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresour. Technol. 2016;215:209–214. doi: 10.1016/j.biortech.2016.02.125. [DOI] [PubMed] [Google Scholar]
- 135.Vikrant K., Kim K.H., Ok Y.S., Tsang D.C., Tsang Y.F., Giri B.S., Singh R.S. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018;616:1242–1260. doi: 10.1016/j.scitotenv.2017.10.193. [DOI] [PubMed] [Google Scholar]
- 136.Mikula K., Izydorczyk G., Skrzypczak D., Mironiuk M., Moustakas K., Witek-Krowiak A., Chojnacka K. Controlled release micronutrient fertilizers for precision agriculture–A review. Sci. Total Environ. 2020;712:136365. doi: 10.1016/j.scitotenv.2019.136365. [DOI] [PubMed] [Google Scholar]
- 137.Chen Z., Pei J., Wei Z., Ruan X., Hua Y., Xu W., et al. A novel maize biochar-based compound fertilizer for immobilizing cadmium and improving soil quality and maize growth. Environ. Pollut. 2021;277:116455. doi: 10.1016/j.envpol.2021.116455. [DOI] [PubMed] [Google Scholar]
- 138.Yan T., Xue J., Zhou Z., Wu Y. Effects of biochar-based fertilizer on soil bacterial network structure in a karst mountainous area. Catena. 2021;206:105535. [Google Scholar]
- 139.Zhou M., Ying S., Chen J., Jiang P., Teng Y. Effects of biochar-based fertilizer on nitrogen use efficiency and nitrogen losses via leaching and ammonia volatilization from an open vegetable field. Environ. Sci. Pollut. Control Ser. 2021;28(46):65188–65199. doi: 10.1007/s11356-021-15210-9. [DOI] [PubMed] [Google Scholar]
- 140.Gao M., Yang J., Liu C., Gu B., Han M., Li J., et al. Effects of long-term biochar and biochar-based fertilizer application on brown earth soil bacterial communities. Agric. Ecosyst. Environ. 2021;309:107285. [Google Scholar]
- 141.Sun P., Li Y., Meng T., Zhang R., Song M., Ren J. Removal of sulfonamide antibiotics and human metabolite by biochar and biochar/H2O2 in synthetic urine. Water Res. 2018;147:91–100. doi: 10.1016/j.watres.2018.09.051. [DOI] [PubMed] [Google Scholar]
- 142.Hale S.E., Lehmann J., Rutherford D., Zimmerman A.R., Bachmann R.T., Shitumbanuma V., et al. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Scie. Technol. 2012;46(5):2830–2838. doi: 10.1021/es203984k. [DOI] [PubMed] [Google Scholar]
- 143.Oleszczuk P., Jośko I., Kuśmierz M., Futa B., Wielgosz E., Ligęza S., Pranagal J. Microbiological, biochemical and ecotoxicological evaluation of soils in the area of biochar production in relation to polycyclic aromatic hydrocarbon content. Geoderma. 2014;213:502–511. [Google Scholar]
- 144.Kasozi G.N., Zimmerman A.R., Nkedi-Kizza P., Gao B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars) Environ. Scie. Technol. 2010;44(16):6189–6195. doi: 10.1021/es1014423. [DOI] [PubMed] [Google Scholar]
- 145.Zhang D., Pan G., Wu G., Kibue G.W., Li L., Zhang X., et al. Biochar helps enhance maize productivity and reduce greenhouse gas emissions under balanced fertilization in a rainfed low fertility inceptisol. Chemosphere. 2016;142:106–113. doi: 10.1016/j.chemosphere.2015.04.088. [DOI] [PubMed] [Google Scholar]
- 146.Shin J., Hong S.G., Lee S., Hong S., Lee J. Estimation of soil carbon sequestration and profit analysis on mitigation of CO 2-eq. emission in cropland cooperated with compost and biochar. App. Biol. Chem. 2017;60(4):467–472. [Google Scholar]
- 147.Fidel R.B., Laird D.A., Parkin T.B. Effect of biochar on soil greenhouse gas emissions at the laboratory and field scales. Soil Sys. 2019;3(1):8. [Google Scholar]
- 148.Zhou Z., Gao T., Zhu Q., Yan T., Li D., Xue J., Wu Y. Increases in bacterial community network complexity induced by biochar-based fertilizer amendments to karst calcareous soil. Geoderma. 2019;337:691–700. [Google Scholar]
- 149.Liao J., Liu X., Hu A., Song H., Chen X., Zhang Z. Effects of biochar-based controlled release nitrogen fertilizer on nitrogen-use efficiency of oilseed rape (Brassica napus L.) Sci. Rep. 2020;10(1):1–14. doi: 10.1038/s41598-020-67528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Song J., Zhao H., Zhao G., Xiang Y., Liu Y. Novel semi-IPN nanocomposites with functions of both nutrient slow-release and water retention. 1. Microscopic structure, water absorbency, and degradation performance. J. Agric. Food Chem. 2019;67(27):7587–7597. doi: 10.1021/acs.jafc.9b00888. [DOI] [PubMed] [Google Scholar]
- 151.Cheng D., Liu Y., Yang G., Zhang A. Water-and fertilizer-integrated hydrogel derived from the polymerization of acrylic acid and urea as a slow-release N fertilizer and water retention in agriculture. J. Agric. Food Chem. 2018;66(23):5762–5769. doi: 10.1021/acs.jafc.8b00872. [DOI] [PubMed] [Google Scholar]
- 152.Kenawy E.R., Seggiani M., Cinelli P., Elnaby H.M.H., Azaam M.M. Swelling capacity of sugarcane bagasse-g-poly (acrylamide)/attapulgite superabsorbent composites and their application as slow release fertilizer. Eur. Polym. J. 2020;133:109769. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.