Abstract
Prior to coronavirus disease 2019 (COVID-19), tuberculosis (TB) was the worst killer among infectious diseases. The union of these two obnoxious respiratory diseases can be devastating, with severe public health implications. The COVID-19 pandemic has affected all TB-elimination programmes due to the severe burden on healthcare systems and the diversion of funds and attention towards controlling the pandemic. The emerging data show that the COVID-19 pandemic caused a marked decrease in case notifications and bacille Calmette–Guérin immunisations, ultimately promoting disease transmission and increasing the susceptible population. The similarity between the clinical characteristics of TB and COVID-19 adds to the public health complications, with evidence of immune dysregulation in both cases leading to severe consequences. Clinical evidence suggests that severe acute respiratory syndrome coronavirus 2 infection predisposes patients to TB infection or may lead to reactivation of latent disease. Similarly, underlying TB disease can worsen COVID-19. Treatment options are limited in COVID-19; therefore, using immunosuppressive and immunomodulatory regimens that can modulate the concomitant bacterial infection and interaction with anti-TB drugs requires caution. Thus, considering the synergistic impact of these two respiratory diseases, it is crucial to manage both diseases to combat the syndemic of TB and COVID-19.
Short abstract
M. tuberculosis is a deadly human pathogen, and the emergence of COVID-19 has hampered TB control measures. Better co-infection management can prevent disease-related complications and produce favourable outcomes. https://bit.ly/3LiiV57
Introduction
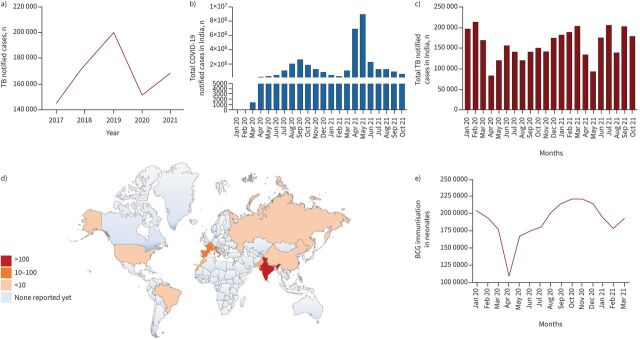
Mycobacterium tuberculosis (M. tb) is the tuberculosis (TB) pathogen that causes many deaths worldwide [1, 2]. One-third of the world's population is believed to harbour latent TB, which can be activated into active TB in immune-compromised people with comorbidities [3]. Recent evidence suggests that the suppression of the cell-mediated immunity caused by coronavirus disease 2019 (COVID-19) induces the activation of latent TB, imposing a severe impediment to eradicating TB by 2035 [4]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged as a more devastating infectious pathogen than M. tb, overtaking the TB death toll, threatening socio–economic development and causing the collapse of public health systems worldwide [5, 6]. The evolution and genetic diversity of emerging variants has fuelled a calamitous surge in cases, with devastating outcomes in most countries [7, 8]. The surge of SARS-CoV-2 in TB endemic regions shifted the unilateral focus from TB to COVID-19. This shift was not just in terms of the economic priorities of public health agencies but also in the reallocation of funds, healthcare staff and medical facilities [9]. These factors resulted in delayed turnaround times for TB diagnosis, a major hurdle in eradicating TB [10]. The most disturbing aspect was the global reduction in TB patients reporting to clinics since the emergence of COVID-19. A 25–30% reduction in reported TB cases was observed in endemic countries, including India, China, Indonesia and the Philippines (figure 1a–c), in the first half of 2020 [11]. The reduction in reported TB cases can be attributed to the hysteria around COVID-19 and the closure of outpatient departments due to lockdown. This led to delayed diagnosis of active TB patients, thus increasing the risk of TB transmission to household contacts and fuelling mortality. It has been reported that TB treatment dropped by more than one million worldwide, setting the fight against TB back by a decade. Almost 0.5 million more people are estimated to have died in 2020 than reported [12]. Of note, while COVID-19 caused more deaths in 2020, TB is still the biggest killer in low- and middle-income countries. Various mathematical models concluded that the COVID-19 pandemic would increase TB incidence and mortality by 5–10% in the coming 5 years [13–15]. The United States Agency for International Development estimated that over 1 million fewer cases of TB were reported in 2020 because of the COVID-19 pandemic in 24 high TB-burden countries. Recent modelling analysis has suggested that the lockdown-mediated disruption in TB services will likely translate into 6.3 million additional cases and 1.4 million deaths globally in the next 5 years. Therefore, concerted efforts and funding resources are required to mitigate the COVID-19 calamity and improve TB disease management in TB-endemic nations.
FIGURE 1.
a) Data from the Nikshay tuberculosis (TB) notification system reported in India from 2017 to 2021. Cases notified are average cases notified per month calculated based on annual notified cases. Source: Nikshay, Central TB Division, National TB Elimination Programme. b) The number of coronavirus disease 2019 (COVID-19)-infected patients reported in India from January 2020 to October 2021. Source: worldometers.info. c) Number of TB notified cases monthly in India from January 2020 to October 2021. Source: Nikshay, Central TB Division, National TB Elimination Programme. d) Global map showing countries that reported different TB/COVID-19 coinfection numbers. e) Routine bacillus Calmette–Guérin (BCG) immunisation data in neonates from India's National Health Mission's Health Management Information System from January 2020 to March 2021. Source: National Health Mission's Health Management Information System.
SARS-CoV-2 and M. tb co-infection
The eruption of the SARS-CoV-2 pandemic was itself catastrophic, but reports of M. tb/SARS-CoV-2 co-infection resulted in a double whammy (figure 1d). The additional burdens on healthcare systems caused by COVID-19 have hampered TB elimination goals. The severe economic burden on the already constrained healthcare systems in TB-endemic countries, as well as overworked healthcare workers, could further worsen the control measures against TB [16]. At the outset of the syndemic, a global cohort study of 49 patients reported concomitant infection of M. tb and SARS-CoV-2. A very high mortality rate (12.3%) was observed in the co-infected patients [17]. Meta-analysis of SARS-CoV-2 and M. tb co-infection showed a twofold increase in the mortality of co-infected patients, with multiple case-control studies corroborating findings that validate the altered disease pathology in co-infections [18]. Apart from M. tb, COVID-19 predisposes patients to multiple other infections that can modulate disease pathophysiology. A recent study in India showed a 47.1% co-infection rate among hospitalised COVID-19 patients [19]. However, a higher co-infection rate (94.2%) in COVID-19 patients was earlier observed in China [20]. SARS-CoV-2 co-infections with bacterial and viral pathogens significantly contributed to increased morbidity and mortality of COVID-19 patients [21]. Thus, the characterisation of co-infections, particularly M. tb, is necessary to save lives, with subsequent monitoring of the development of antimicrobial resistance due to the high antibiotic usage in co-infections [22]. The adversity of co-infection increases when we consider triple infection of HIV/M. tb/SARS-CoV-2. Although HIV co-infection with SARS-CoV-2 is not associated with increased disease severity and mortality [23], the existence of HIV co-infection with M. tb can worsen the pathogenesis of M. tb, which in turn can affect the mortality rate of COVID-19/TB co-infected patients. We are yet to fully delineate the possible biological interaction between these two respiratory pathogens, which represent a new cursed duet, as reported earlier for M. tb and HIV.
The clinical aspects of M. tb/SARS-CoV-2 co-infection
Many similarities exist between COVID-19 and TB pathogenesis and their clinical outcomes. The lung is the primary site of infection of SARS-CoV and M. tb; however, both pathogens can invade cells within multiple organs. Like M. tb, SARS-CoV-2 infects and replicates inside the ciliated mucus-secreting cells of bronchial epithelium, type-II alveolar/pneumocyte cells and macrophages in the lungs. Upon entry into the host cells, both pathogens induce pro-inflammatory cytokines that, once dysregulated, cause a cytokine storm. In turn, heightened levels of pro-inflammatory cytokines lead to the infiltration of neutrophils into the site of infection, thereby inducing acute damage to lung tissue [24]. The uncontrolled production of pro-inflammatory cytokines results in a severely compromised immune response that generates opportunities for co-infection, including by M. tb. The virus-mediated pyroptosis and associated vascular leakage trigger a subsequent T-helper cell 1 (Th1) response, leading to the pulmonary infiltration of immune cells to clear the infection.
In some cases, the ensuing dysfunctional immune response can cause severe lung and systemic pathology, as evident in severe COVID-19 [25]. The inflammatory monocytes (cluster of differentiation (CD)14+, CD16+) are major drivers of cytokine storms and exist in a significantly higher percentage in severe diseases than in milder cases of COVID-19. Unrestrained infiltration leads to excessive reactive oxygen species production and protease secretion, culminating in pulmonary oedema, limiting oxygen intake capacity and making lungs vulnerable to secondary infections. Besides lung cells, SARS-CoV-2 can infect other immune cells, akin to M. tb, leading to aberrant cytokine production and switching to an M2 transcriptional programme that induces host immunoparalysis, further benefiting the progression of COVID-19 [26]. In turn, both these pathogens have evolved discreet mechanisms to subdue host responses and escape the ensuing immunity that includes antagonising interferon responses, regulating downstream cytokine signalling, hampering antigen presentation and modulating cell-death pathways [27, 28].
The lymphopaenia observed in patients suggests the movement of T-cells to the site of infection to control viral replication. However, there is ample evidence to suggest that a Th1-biased T-cell response is responsible for protection against SARS-CoV-2, but the same subset seems to be an aggravating factor for pathogenesis [29, 30]. These observations and prior experience with vaccine formulations against SARS-CoV suggest a delicate balance between detrimental and protective T-cell responses. These inadequate T-cell responses impacted the current generation of vaccines as all efforts to develop vaccines against SARS-CoV-2 are biased towards optimal humoral immunity. Even though the antibody repertoire involved in protection is undefined, evoking neutralising antibodies against spikes is a consensus approach for antibody-based vaccine development. This approach is limited mainly by the advent of mutant strains that could render vaccination ineffective [31]. Despite mass immunisation campaigns, we are facing a surge of new variants of concern (B.1.617.2/AY.4.2), including the latest Omicron variant [32–34]. Several recent studies revealed that the Omicron variant manifests striking antibody evasion of most existing SARS-CoV-2 neutralising antibodies [35, 36]. Interestingly, it has been revealed that Omicron is less effective in replication and inducing pathogenesis in cell culture, hamster and mouse models [37, 38].
Despite T-cell-mediated immunity being a critical factor in controlling viral progression, COVID-19 effectively induces CD4+ T-cell depletion [4]. The severe decrease in CD4+ T-cells results in a remarkable decrease in effector cytokines, including interleukin (IL)-2, IL-4, IL-5 and IL-13, leading to latent TB progression into active disease [39, 40]. A recent study from Wuhan, China, showed that 76% of 522 COVID-19 patients had significantly reduced T-cell lymphocytes, including CD4+ and CD8+ cells, and surviving T-cells showed functional exhaustion [4, 41]. Severe depletion and dysfunction of T-cells are hypothesised to promote the development of active TB in patients with COVID-19 disease [4]. Interestingly, M. tb infection increases the expression of angiotensin-converting enzyme 2, the entry receptor for SARS-CoV-2 [42]. Given that more than 2 billion people have latent TB worldwide, it could predispose a significant proportion of the population to COVID-19 or alter the pathophysiology of the disease. There is an urgent need to address the co-infection conundrum in order to delineate the interactions and employ mitigating factors to prevent a possible catastrophe.
The effect of bacillus Calmette–Guérin (BCG) vaccination on COVID-19
Further, despite the deadly combination of COVID-19 and TB, the non-pathogenic mycobacterial species BCG vaccine was reported to possess a protective effect against COVID-19 [43, 44]. The BCG vaccine has been the cornerstone of vaccination programmes in most countries and has effectively controlled childhood TB, despite variability in the protection accorded to adults. Recent developments in vaccination strategies and heterologous protective effects of this vaccine against various diseases have reignited interest in the utility of this vaccine [45]. Interestingly, various reports observed that COVID-19 mortality and incidence are significantly lower in countries with mandatory BCG vaccination programmes [46]. These prophylactic and therapeutic effects have been attributed to the “trained immunity” imparted by BCG vaccines that protect against nonspecific infections [47]. Though attractive, the dramatically opposite action of two mycobacterial species on COVID-19 needs to be treated with caution as confounding variables could alter the outcome, and nothing substantial has emerged from the large-scale clinical studies. Intriguingly, we also observed one of the distressing effects of the pandemic on BCG immunisation in neonates. The immunisation strategy experienced a steep decline after the COVID-19 outbreak in March 2020 (figure 1e). The sudden fall in immunisation will undoubtedly affect the morbidity and mortality caused by TB in neonates.
SARS-CoV-2 treatment and possible interactions with TB co-infection/latent TB
TB infection is a predisposing factor for developing severe COVID-19; it is expected that M. tb/SARS-CoV-2 co-infection may lead to exacerbated disease. Lung damage caused by severe COVID-19 and subsequent vulnerability to TB is also a significant concern [48]. As therapeutic options are limited, the treatment of patients with severe COVID-19 is still challenging. Immunosuppressive drugs like corticosteroids and cytokine blockers have shown promising results in treating severe COVID-19 cases [49]. Nevertheless, immunosuppressive treatments pose a risk of acquiring opportunistic infections, including bacterial, fungal and viral infections. The immunosuppressive regimen against SARS-CoV-2 could potentially lead to the reactivation of latent M. tb, which can be detrimental in TB-endemic areas. Therefore, it is essential to rule out respiratory diseases such as TB, which have overlapping symptomatology to SARS-CoV-2 infection. Pertinently, the World Health Organization (WHO) reported that TB-associated deaths would increase by 0.2–0.4 million in 2021 due to poor disease management and follow-up, lack of proper diagnosis, incomplete treatment and the socio–economic conditions associated with COVID-19 disease. The effect would be more pronounced in high TB-endemic regions with limited resources and a shortage of skilled healthcare workers. The WHO reported a rise in TB deaths in 2020, which had been on a consistent decline since 2005. This implies a reversal of years of global progress in eradicating TB, with the reduction in TB deaths being a meagre 9.2% compared to a targeted reduction of 35% [1].
Further, in patients with severe COVID-19 and latent TB, it is essential to prioritise therapeutic modalities and consider prophylactic treatment using frontline anti-TB drugs with corticosteroid therapy [50]. The strategy would be beneficial in preventing the activation of latent TB into active disease while having the beneficial effects of corticosteroids in managing severe COVID-19. It is crucial to study the feasibility of TB treatment in co-infection conditions and its effect on disease outcomes.
COVID-19 vaccines
Despite pertinent challenges, we witnessed an unprecedented effort from the scientific community to develop vaccines against COVID-19 that was in stark contrast to TB research. Vaccine development for TB has been ongoing for decades without much progress. This could be attributed to the comparatively simpler genome of SARS-CoV-2 compared to M. tb. The evolutionary advantage of M. tb to hamper host cellular processes using many virulence factors hinders the scientific pursuit of suitable vaccines. Nevertheless, the decades of experience in characterising and validating vaccines to curb infectious diseases like TB have helped develop vaccines against COVID-19. One of the critical differences is that TB vaccines are majorly biased towards evoking T-cell immunity, with COVID-19 vaccines focused on B-cell immunity. The experience of producing optimal CD4+ and CD8+ effector and memory response by various anti-TB vaccines can effectively be translated to COVID-19 vaccines. The rapid progress in developing and administering COVID-19 vaccines should encourage us to strategise TB vaccine development. This would include generous funding from stakeholders, political will and commitment to fast-track the process. We need to assess the use of new platforms like mRNA vaccines, advanced computation and structure prediction, well-organised clinical trials, and extraordinary resource mobilisation to curb infectious diseases like TB, and the roadmap has already been drawn by the COVID-19 pandemic.
Moreover, the incidence of antibody-dependant enhancement cannot be completely ruled out by the sole focus on antibody-based immunity. Though the current generation of vaccines still relies on antibody-based protection, we propose shifting the paradigm to T-cell-based immunity [51]. The emerging evidence corroborates that SARS-CoV-2 effectively blunts the generation of long-lived antibody responses [52]. Interestingly, SARS-CoV-2-specific memory T-cells were observed in most convalescent individuals and asymptomatic cases with undetectable antibody responses. It is tempting to speculate that memory CD4+ and CD8+ could protect against SARS-CoV-2, and concerted efforts are required to develop new vaccines to generate memory T-cells and long-lived antibody responses [39].
Diagnosis of M. tb/SARS-CoV-2
Both M. tb and SARS-CoV-2 are respiratory pathogens, and accurate differential diagnosis is a stumbling block in proper management due to overlapping symptoms. There is an urgent need to devise strategies to screen, prevent and treat M. tb in patients with COVID-19. Real-time PCR and Xpert/MTB RIF assay should be performed on all samples collected in TB-endemic areas. There is a pressing need to reimagine TB diagnosis, and the COVID-19 blueprint should be applied to TB. TB surveillance and case notifications should be digitised with real-time data aggregation for COVID-19 to effectively direct public health responses. Artificial intelligence innovations could be directed towards remote TB diagnosis by automated interpretation of chest X-ray images. Cough analysers and digital stethoscopes developed for COVID-19 could be re-engineered for TB diagnosis to extend the reach of TB specialists to remote areas [53].
Points for clinical practice
Controlling COVID-19 has required exemplary medical innovations and their application at an unprecedented pace. The multi-sectoral collaboration between researchers, financiers, industries and policy makers was successful in controlling the pandemic and minimizing the loss of lives. This is starkly opposite to the political failure to control other pandemics of concern, such as TB. Furthermore, the syndemic of these two respiratory pathogens is currently plaguing overburdened healthcare systems and there is an urgent need to address this for universal health coverage. Co-infection by these respiratory pathogens is challenging to clinicians and researchers as well. COVID-19 diagnosis is rapid and requires immediate intervention, whereas TB diagnosis is still based on sputum smear staining and culture in endemic settings. The radiological evidence of COVID-19 even masks the detection of TB. Considering the enhanced mortality in cases of co-infections, there is a need to identify patients at risk. Diagnosis of TB infections needs to be prioritized, followed by a treatment algorithm to cater for both infections. The use of immunosuppressive therapy for COVID-19 requires reconsideration in patients at risk of TB infection. Drug–drug interaction data and adverse drug reactions need to be reported to streamline the treatment options. An effective policy for priority vaccination of TB-endemic populations is required, but marred by the vaccine inequity witnessed in poorer economies. Extra care and control measures may be required for populations at risk to limit the incidence of co-infections. The current global focus on COVID-19 pandemic control has revamped diagnostic capabilities everywhere, and TB-endemic countries should adapt these measures for early TB detection, continued surveillance and application of advances in digital healthcare to curb the spread of TB infections. The use of multiplex technologies for bilateral detection of COVID-19 and TB, more so in vulnerable populations with co-morbidities such as HIV and diabetes, need to be prioritised. Given that both these respiratory infections feed on social inequality, generous funding support by developed countries could enhance healthcare access and social security in affected economies to achieve universal health coverage.
Conclusions and perspectives
Interestingly, it has been predicted that social distancing, increased hygiene, use of masks and heightened awareness could play positive roles in containing the spread of M. tb, but it is too early to report the outcomes. Therefore, it is necessary to better understand the inter-relations and outcomes to devise a strategy to tilt the balance against TB. Past experiences suggest that the outbreak of HIV caused an unprecedented increase in M. tb infection and the emergence of multi-drug resistant TB cases [54, 55]. Available data regarding COVID-19 and M. tb co-infection are insufficient to derive a logical conclusion, thus warranting further prospective studies.
Acknowledgements
M. Shariq acknowledges the ICMR (Indian Council of Medical Research) for providing a Senior Research Associate Fellow at the National Institute of Pathology (NIOP), New Delhi. J.A. Sheikh is funded by the Start-up Research Grant from UGC and DST-SERB. N. Quadir is a DHR Young Scientist supported by the Department of Health Research, Ministry of Health and Family Welfare, Government of India (GoI). N. Sharma acknowledges the Senior Research Fellowship support from NIOP, New Delhi. S.E. Hasnain is a National Science Chair, Science and Engineering Research Board (SERB), Department of Science and Technology (DST), GoI and Robert Koch Fellow, Robert Koch Institute, Germany.
Provenance: Submitted article, peer reviewed.
Author contributions: N.Z. Ehtesham, S.E. Hasnain, M. Shariq and J.A. Sheikh: conceptualised the project M. Shariq, J.A. Sheikh, N. Quadir, N. Sharma, N.Z. Ehtesham, and S.E. Hasnain: wrote the manuscript. N. Quadir and M. Shariq: helped with schematic representations. N.Z. Ehtesham and S.E. Hasnain: project administration and supervision. All the authors read and approved the final version of the manuscript.
Conflict of interest: M. Shariq has nothing to disclose.
Conflict of interest: J.A. Sheikh has nothing to disclose.
Conflict of interest: N. Quadir has nothing to disclose.
Conflict of interest: N. Sharma has nothing to disclose.
Conflict of interest: S.E. Hasnain has nothing to disclose.
Conflict of interest: N.Z. Ehtesham has nothing to disclose.
Support statement: S.E. Hasnain and N.Z. Ehtesham are supported by DBT North-East Grants BT/PR23099/NER/95/632/2017 and BT/PR23155/NER/95/634/2017 by the Department of Biotechnology, Ministry of Science and Technology (MoS&T), Government of India (GoI). S.E. Hasnain is supported by the SERB, DST, MoS&T, GoI. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.World Health Organization . Global Tuberculosis Report 2021. Geneva, WHO, 2021. [Google Scholar]
- 2.Chakaya J, Khan M, Ntoumi F, et al. Global Tuberculosis Report 2020 – Reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis 2021; 113: S7–S12. doi: 10.1016/j.ijid.2021.02.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016; 13: e1002152. doi: 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khayat M, Fan H, Vali Y. COVID-19 promoting the development of active tuberculosis in a patient with latent tuberculosis infection: A case report. Respir Med Case Rep 2021; 32: 101344. doi: 10.1016/j.rmcr.2021.101344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sironi M, Hasnain SE, Rosenthal B, et al. SARS-CoV-2 and COVID-19: A genetic, epidemiological, and evolutionary perspective. Infect Genet Evol 2020; 84: 104384. doi: 10.1016/j.meegid.2020.104384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh H, Singh J, Khubaib M, et al. Mapping the genomic landscape and diversity of COVID-19 based on >3950 clinical isolates of SARS-CoV-2: Likely origin and transmission dynamics of isolates sequenced in India. Indian J Med Res 2020; 151: 474–478. doi: 10.4103/ijmr.IJMR_1253_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormick KD, Jacobs JL, Mellors JW. The emerging plasticity of SARS-CoV-2. Science 2021; 371: 1306–1308. doi: 10.1126/science.abg4493 [DOI] [PubMed] [Google Scholar]
- 8.Sheikh JA, Singh J, Singh H, et al. Emerging genetic diversity among clinical isolates of SARS-CoV-2: Lessons for today. Infect Genet Evol 2020; 84: 104330. doi: 10.1016/j.meegid.2020.104330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh J, Alam A, Samal J, et al. Role of multiple factors likely contributing to severity-mortality of COVID-19. Infect Genet Evol 2021; 96: 105101. doi: 10.1016/j.meegid.2021.105101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wingfield T, Karmadwala F, MacPherson P, et al. Challenges and opportunities to end tuberculosis in the COVID-19 era. Lancet Respir Med 2021; 9: 556–558. doi: 10.1016/S2213-2600(21)00161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Zhang K. Insight into the impact of the COVID-19 epidemic on tuberculosis burden in China. Eur Respir J 2020; 56: 2001948. doi: 10.1183/13993003.01948-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts L. How COVID hurt the fight against other dangerous diseases. Nature 2021; 592: 502–504. doi: 10.1038/d41586-021-01022-x [DOI] [PubMed] [Google Scholar]
- 13.McQuaid CF, McCreesh N, Read JM, et al. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J 2020; 56: 2001718. doi: 10.1183/13993003.01718-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cilloni L, Fu H, Vesga JF, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine 2020; 28: 100603. doi: 10.1016/j.eclinm.2020.100603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zumla A, Marais BJ, McHugh TD, et al. COVID-19 and tuberculosis—threats and opportunities. Int J Tuberc Lung Dis 2020; 24: 757–760. doi: 10.5588/ijtld.20.0387. [DOI] [PubMed] [Google Scholar]
- 16.Singh J, Ehtesham NZ, Hasnain SE. Two parallel pandemics: the challenges faced by countries with COVID-19 and TB. Int J Tuberc Lung Dis 2020; 24: 1319–1320. doi: 10.5588/ijtld.20.0592 [DOI] [PubMed] [Google Scholar]
- 17.Tadolini M, Codecasa LR, García-García J-M, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J 2020; 56: 2001398. doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar S, Khanna P, Singh AK. Impact of COVID-19 in patients with concurrent co-infections: A systematic review and meta-analyses. J Med Virol 2021; 93: 2385–2395. doi: 10.1002/jmv.26740 [DOI] [PubMed] [Google Scholar]
- 19.Sreenath K, Batra P, Vinayaraj EV, et al. Coinfections with other respiratory pathogens among patients with COVID-19. Microbiol Spectr 2021; 9: e0016321. doi: 10.1128/Spectrum.00163-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai CC, Wang CY, Hsueh PR. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect 2020; 53: 505–512. doi: 10.1016/j.jmii.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharifipour E, Shams S, Esmkhani M, et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis 2020; 20: 646. doi: 10.1186/s12879-020-05374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakaya JM, Marais B, du Cros P, et al. Programmatic versus personalised approaches to managing the global epidemic of multidrug-resistant tuberculosis. Lancet Respir Med 2020; 8: 334–335. doi: 10.1016/S2213-2600(20)30104-1 [DOI] [PubMed] [Google Scholar]
- 23.Gervasoni C, Meraviglia P, Riva A, et al. Clinical features and outcomes of patients with human immunodeficiency virus with COVID-19. Clin Infect Dis 2020; 71: 2276–2278. doi: 10.1093/cid/ciaa579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol 2020; 11: 1446. doi: 10.3389/fimmu.2020.01446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegler CGK, Miao VN, Owings AH, et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell 2021; 184: 4713–4733.e22. doi: 10.1016/j.cell.2021.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boumaza A, Gay L, Mezouar S, et al. Monocytes and macrophages, targets of severe acute respiratory syndrome coronavirus 2: the clue for coronavirus disease 2019 immunoparalysis. J Infect Dis 2021; 224: 395–406. doi: 10.1093/infdis/jiab044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai W, Wu F, Zhang Y, et al. The immune escape mechanisms of Mycobacterium tuberculosis. Int J Mol Sci 2019; 20: 340. doi: 10.3390/ijms20020340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callaway E. Fast-spreading COVID variant can elude immune responses. Nature 2021; 589: 500–501. doi: 10.1038/d41586-021-00121-z [DOI] [PubMed] [Google Scholar]
- 29.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021; 590: 630–634. doi: 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584: 457–462. doi: 10.1038/s41586-020-2550-z [DOI] [PubMed] [Google Scholar]
- 31.Noh JY, Jeong HW, Kim JH, et al. T cell-oriented strategies for controlling the COVID-19 pandemic. Nat Rev Immunol 2021; 21: 687–688. doi: 10.1038/s41577-021-00625-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh J, Rahman SA, Ehtesham NZ, et al. SARS-CoV-2 variants of concern are emerging in India. Nat Med 2021; 27: 1131–1133. doi: 10.1038/s41591-021-01397-4. [DOI] [PubMed] [Google Scholar]
- 33.Callaway E, Ledford H. How bad is Omicron? What scientists know so far. Nature 2021; 600: 197–199. doi: 10.1038/d41586-021-03614-z. [DOI] [PubMed] [Google Scholar]
- 34.Singh J, Samal J, Kumar V, et al. Structure-function analyses of new SARS-CoV-2 variants B.1.1.7, B.1.351 and B.1.1.28.1: clinical, diagnostic, therapeutic and public health implications. Viruses 2021; 13: 439. doi: 10.3390/v13030439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature 2021; 602: 676–681. doi: 10.1038/s41586-021-04388-0 [DOI] [PubMed] [Google Scholar]
- 36.Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2021; 602: 657–663. doi: 10.1038/s41586-021-04385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shuai H, Chan JF, Hu B, et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 2022; 603: 693–699. [DOI] [PubMed] [Google Scholar]
- 38.Halfmann PJ, Iida S, Iwatsuki-Horimoto K, et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 2022; 603: 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sewell HF, Agius RM, Kendrick D, et al. Covid-19 vaccines: delivering protective immunity. BMJ 2020; 371: m4838. doi: 10.1136/bmj.m4838 [DOI] [PubMed] [Google Scholar]
- 40.Amelio P, Portevin D, Hella J, et al. HIV infection functionally impairs Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses. J Virol 2019; 93: e01728-18. doi: 10.1128/JVI.01728-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 2020; 11: 827. doi: 10.3389/fimmu.2020.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020; 181: 1016–1035.e19. doi: 10.1016/j.cell.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redelman-Sidi G. Could BCG be used to protect against COVID-19? Nat Rev Urol 2020; 17: 316–317. doi: 10.1038/s41585-020-0325-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehtesham NZ, Samal J, Ahmad F, et al. Will bacille Calmette-Guerin immunization arrest the COVID-19 pandemic? Indian J Med Res 2020; 152: 16–20. doi: 10.4103/ijmr.IJMR_1563_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheikh JA, Ehtesham NZ, Hasnain SE. Revisiting BCG to control tuberculosis: mucosal delivery and delipidation? Lancet Infect Dis 2020; 20: 272–273. doi: 10.1016/S1473-3099(19)30702-9 [DOI] [PubMed] [Google Scholar]
- 46.Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci USA 2020; 117: 17720–17726. doi: 10.1073/pnas.2008410117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtis N, Sparrow A, Ghebreyesus TA, et al. Considering BCG vaccination to reduce the impact of COVID-19. Lancet 2020; 395: 1545–1546. doi: 10.1016/S0140-6736(20)31025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mousquer GT, Peres A, Fiegenbaum M. Pathology of TB/COVID-19 co-Infection: The phantom menace. Tuberculosis 2021; 126: 102020. doi: 10.1016/j.tube.2020.102020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324: 1330–1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gopalaswamy R, Subbian S. Corticosteroids for COVID-19 therapy: potential implications on tuberculosis. Int J Mol Sci 2021; 22: 3773. doi: 10.3390/ijms22073773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King A. Vaccines beyond antibodies. EMBO Rep 2021; 22: e54073. doi: 10.15252/embr.202154073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cañete PF, Vinuesa CG. COVID-19 makes B cells forget, but T cells remember. Cell 2020; 183: 13–15. doi: 10.1016/j.cell.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruhwald M, Carmona S, Pai M. Learning from COVID-19 to reimagine tuberculosis diagnosis. Lancet Microbe 2021; 2: e169–e170. doi: 10.1016/S2666-5247(21)00057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marais BJ, Chakaya J, Swaminathan S, et al. Tackling long-term morbidity and mortality after successful tuberculosis treatment. Lancet Infect Dis 2020; 20: 641–642. doi: 10.1016/S1473-3099(20)30167-5 [DOI] [PubMed] [Google Scholar]
- 55.Dean AS, Zignol M, Falzon D, et al. HIV and multidrug-resistant tuberculosis: overlapping epidemics. Eur Respir J 2014; 44: 251–254. doi: 10.1183/09031936.00205413 [DOI] [PubMed] [Google Scholar]