Abstract
Idiopathic pulmonary fibrosis (IPF) is characterised by the progressive deposition of excessive extracellular matrix proteins within the lung parenchyma and represents the most rapidly progressive and fatal of all fibrotic conditions. Current anti-fibrotic drugs approved for the treatment of IPF fail to halt disease progression and have significant side-effect profiles. Therefore, there remains a pressing need to develop novel therapeutic strategies for IPF. Mammalian target of rapamycin (mTOR) forms the catalytic subunit of two complexes, mTORC1 and mTORC2. mTORC1 acts as critical cellular sensor which integrates intracellular and extracellular signals to reciprocally regulate a variety of anabolic and catabolic processes. The emerging evidence for a critical role for mTORC1 in influencing extracellular matrix production, metabolism, autophagy and senescence in the setting of IPF highlights this axis as a novel therapeutic target with the potential to impact multiple IPF pathomechanisms.
Short abstract
Current evidence supports the scientific rationale for targeting the mTOR pathway in idiopathic pulmonary fibrosis https://bit.ly/33OQiYf
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most rapidly progressive and fatal of all fibrotic conditions. Current anti-fibrotic drugs approved for the treatment of IPF fail to halt disease progression and have significant side-effect profiles. Therefore, there is an urgent need to develop novel therapeutic strategies for IPF and other fibrotic conditions. The emerging evidence for a critical role for the mammalian target of rapamycin (mTOR) signalling hub during fibrogenesis highlights several potential novel anti-fibrotic approaches. In this mini-review, we discuss the role and therapeutic potential of targeting mTOR in the context of IPF.
Idiopathic pulmonary fibrosis
IPF represents the most rapidly progressive and lethal form of all fibrotic diseases and is associated with a dismal median survival of 3.5 years from diagnosis. The approval of pirfenidone and nintedanib for the treatment of IPF was a major landmark for the development of anti-fibrotic therapeutics. Although these agents slow disease progression, mortality remains largely unchanged so that there remains a pressing need to identify novel anti-fibrotic therapeutic approaches [1]. Myofibroblasts are the key effector cells responsible for the synthesis and deposition of a collagen-rich extracellular matrix (ECM) during normal wound healing [2]. These cells can be derived from the differentiation of multiple cell types, including tissue resident fibroblasts, and are characterised by high ECM synthesis rates and the de novo expression and assembly of α-smooth muscle actin into contractile stress fibres. The excessive accumulation and persistence of myofibroblasts, as a result of a dysregulated wound healing response in the presence of a network of pro-fibrotic cytokines and growth factors within a homeostatically dysregulated tissue microenvironment, represents a key common mechanism underlying the development of pathological fibrosis in multiple conditions, including IPF.
In terms of the cytokine and growth factor network implicated in pathological fibrosis, including IPF, there is overwhelming evidence for a pivotal role for the pleiotropic cytokine transforming growth factor (TGF)-β (in particular the TGFβ1 isoform) in promoting the differentiation of fibroblasts into highly ECM synthetic myofibroblasts. TGFβ1 signalling is principally mediated via the high-affinity TGFβ receptors (TGFβR) I and II, which function as serine–threonine kinases. TGFβ1 binding to TGFβRII leads to the phosphorylation of TGFβRI and downstream signalling via the canonical SMAD pathway and several non-canonical pathways, including the mTOR pathway [3, 4].
The mTOR signalling hub
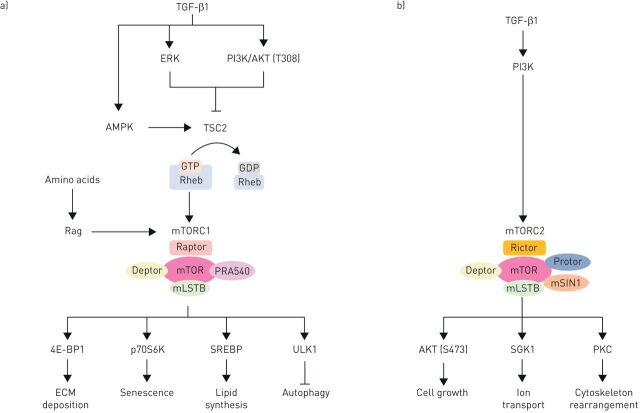
The mTOR signalling hub integrates intracellular and extracellular signals and serves as a central regulator of cell metabolism, growth, proliferation and survival. mTOR forms the catalytic subunit of two complexes, mTOR complex (mTORC)1 and mTORC2, which have different effector proteins, are activated by a variety of upstream inputs and elicit distinct downstream cellular responses (figure 1a). mTORC1 comprises mTOR, as well as raptor (regulatory associated protein of mTOR), PRAS40 (proline-rich AKT substrate 40 kDa) and mLST8 (mammalian lethal with sec-13), while mTORC2 is composed of mTOR, mLST8, rictor (raptor independent companion of mTOR), mSIN1 (mammalian stress-activated protein kinase interacting protein 1) and Protor-1 (protein observed with rictor-1) [5]. mTORC1 activity is highly dependent on the small GTPase RAS homologue enriched in brain (Rheb), which is under negative regulatory control by the tuberous sclerosis complex (TSC), comprising TSC1 and TSC2. TSC1/2, through its GTPase-activating protein activity, converts Rheb from its guanosine triphosphate (GTP) bound form (RhebGTP) into its guanosine diphosphate (GDP) bound form (RhebGDP). For mTORC1 to become activated through this axis, TSC1/2 must therefore be inhibited. The phosphorylation of different sites within the TSC1/2 complex in response to growth factors and mitogen-dependent pathways leads to activation of mTORC1; whereas in poor nutrient conditions, activation of 5′ AMP-activated protein kinase (AMPK) suppresses mTORC1. Independent of phosphorylation, TSC1/2 is also regulated by the availability of amino acids. In amino acid depleted conditions, the Ras-related GTP-binding proteins (Rags) can recruit TSC1/2 to the lysosome to bring it into close contact with Rheb, which in turn is converted into its inactive GDP-bound form. In amino acid replete conditions, the TSC complex is dissociated from the surface of lysosomes, therefore promoting mTORC1 activation via RhebGTP [6]. Rag complexes are also direct regulators of mTORC1; in the presence of amino acids they recruit mTORC1 to the lysosome surface where it binds to Rheb, and in the absence of amino acids Rags release mTORC1 from the lysosome [7].
FIGURE 1.
Simplified schematic diagram of the activators and substrates of mammalian target of rapamycin complexes (mTORC)1 and mTORC2 in the context of fibrosis. a) mTORC1 activity is highly dependent on RAS homologue enriched in brain (Rheb), which is under negative regulatory control by tuberous sclerosis complex (TSC)1/2. TSC1/2 converts RhebGTP into RhebGDP. For mTORC1 to become activated through this axis, TSC1/2 must therefore be inhibited. The phosphorylation of different sites within the TSC1/2 complex in response to extracellular signal-regulated kinaes (ERK) and phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT) pathways leads to activation of mTORC1; whereas activation of 5' AMP-activated protein kinase (AMPK) suppresses mTORC1. Transforming growth factor (TGF)-β1 can activate PI3K/AKT, ERK and AMPK (through TAK1), however, the inhibition of neither of those kinases is able to modulate TGFβ1-mediated mTORC1 activity. In the presence of amino acids, the Rags can release TSC1/2 from the lysosome and instead recruit mTORC1 to bring it into close contact with Rheb. Upon activation, mTORC1 phosphorylates its substrates Eukaryotic Translation Initiation Factor 4E Binding Protein 1 (4E-BP1), ribosomal protein S6 kinase beta-1 (p70S6K), sterol regulatory element-binding protein (SREBP) and Unc-51 Like Autophagy Activating Kinase 1 (ULK1) which in turn regulate extracellular matrix (ECM) deposition, senescence, lipid synthesis and autophagy. b) mTORC2 activity is highly dependent on PI3K activity. Upon activation, mTORC2 phosphorylates AKT, SGK1 and protein kinase C (PKC) to control cell growth, ion transport and cytoskeleton rearrangement.
The mTORC1 complex acts via a number of downstream substrates. It promotes de novo lipid synthesis through the sterol responsive element binding protein (SREBP), protein synthesis through the phosphorylation of Eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), autophagy through Unc-51 Like Autophagy Activating Kinase 1 (ULK1) and transcription factor EB (TFEB), and protein ubiquitination through a mechanism which remains unclear (figure 1a). The mTORC2 complex is less well understood but is felt to primarily function as an effector of insulin/PI3K signalling and a regulator of cell proliferation, survival and cytoskeletal remodelling (figure 1b) [8].
The mTOR signalling axis is commonly dysregulated in the setting of cancer and has been strongly implicated in coordinating metabolic reprogramming in order to optimise nutrient uptake and utilisation and meet the biosynthetic requirements of rapidly proliferating cancer cells [9]. This has led to an intense research effort aimed at developing ATP-competitive mTOR inhibitors that are either now approved or in clinical development (reviewed in [10]). In this review we will focus on emerging evidence supporting a role for mTOR as a potential druggable pathway in the context of IPF.
Role of mTOR in pulmonary fibrosis
The PI3K/mTOR pathway has been strongly implicated in promoting fibroblast proliferation, survival and differentiation in response to several mediators. The cellular responses elicited by the PI3K pathway are tightly regulated by the lipid phosphatase and tensin homolog (PTEN). Lung fibroblasts derived from IPF patients have been shown to display aberrant PI3K activity [11, 12] in that in normal human lung fibroblasts, the β1 integrin binds polymerised collagen and activates PTEN activity and thereby inhibits their proliferation. In contrast, IPF lung-derived fibroblasts circumvent the suppressive effects of polymerised collagen through reduced PTEN activity at the cell membrane. The functional role of unopposed PI3K/mTOR signalling during fibrogensesis is supported by evidence from the bleomycin model of lung injury and fibrosis. PTEN haploinsufficient mice develop exaggerated pulmonary fibrosis following bleomycin injury compared to wild-type controls, indicating that aberrant PI3K activity potentiates the fibrotic response to lung injury. Conversely, inhibition of PI3K is protective and PI3Kγ knockout mice, and rats treated with a specific PI3Kγ inhibitor, display evidence of reduced fibrosis secondary to bleomycin injury [13].
More recent studies from our laboratory added further evidence to support the rationale for targeting the PI3K/mTOR pathway in the context of IPF and provided key evidence for progressing the potent pan-PI3K-mTOR inhibitor, omipalisib, to a randomised, placebo-controlled, double-blind, repeat dose escalation, experimental medicine study in subjects with IPF (NCT01725139) [14], based on the potential of this compound to interfere with IPF-derived lung fibroblast proliferation and collagen deposition induced in response to TGFβ1 [15]. In this proof of mechanism study, exposure-dependent inhibition of phosphatidylinositol 3, 4, 5 trisphosphate and AKT in IPF patients treated with omipalisib confirmed the ability of this compound to engage the target both systemically and in the lung [14].
In extensive mechanistic follow-on studies, we subsequently went on to demonstrate that PI3K/AKT, ERK and AMPK-independent, rapamycin-insensitive mTORC1/4E-BP1 signalling, acting in cooperation with the Smad3 canonical pathway, is critical for mediating the potent stimulatory effects of TGFβ1 on fibroblast collagen I deposition. Our studies further revealed that this axis likely acts at the translational level by regulating the 4E-BP1-driven cap-dependent translation of a protein intermediate(s) which in turn influences maximal COL1A1 mRNA levels. Moreover, the inhibitory effect of mTOR inhibition was found to extend to other matrisome proteins that have been shown to play a role in the development of fibrosis, including the collagens III, V, VII, elastin, SPARC and the key procollagen processing enzyme, ADAMTS2 [14, 16]. Finally, further disease-relevant evidence for a role for mTOR signalling in IPF was provided by recent studies from our laboratory aimed at defining the major transcriptional programmes involved in fibrogenesis in IPF by profiling the transcriptome of un-manipulated (myo)fibroblasts within fibrotic foci in situ by laser capture microdissection [17]. We adapted the core principle of weighted gene correlation network analysis to create a bespoke collagen eigengene, corresponding to the expression pattern across patients from the first principal component of COL1A1 and COL3A1, and identified genes highly correlated with the collagen eigengene and transcriptional signatures which correlate with fibrillar collagen gene expression. Using this approach, we found that TGFβ1, RhoA kinase and the TSC2/Rheb axis formed major signalling clusters associated with collagen gene expression. Functional studies using CRISPR-Cas9 gene edited cells further confirmed a key role for the TSC2/Rheb axis in regulating TGFβ1-induced mTORC1 activation and collagen I deposition.
mTORC1 regulates fibrogenesis by reconfiguring fibroblast metabolism
Evidence for altered metabolism in the context of fibrosis has also recently emerged. 18F-fluorodeoxyglucose ([18F]-FDG) uptake by positron emission tomography, a commonly used imaging marker of enhanced glycolysis in cancer, has been reported to be increased in the lungs of IPF patients and is predictive of progression-free survival [18, 19]. A role for PI3K/mTOR in promoting [18F]-FDG uptake was further suggested by the omipalisib proof of mechanism study, which demonstrated an exposure-dependent reduction in [18F]-FDG uptake in fibrotic areas of the lung, as measured by target-to-background in IPF patients treated with the agent.
The cell type(s) responsible for increased uptake of [18F]-FDG in IPF is currently unknown; however, glycolysis has recently been shown to be increased during TGFβ1-induced myofibroblast differentiation in vitro and targeting glycolysis has been shown to be effective in attenuating experimentally induced lung fibrosis [20]. Recent RNA-Seq studies of TGFβ1-stimulated fibroblasts shed further mechanistic light on the potential role of mTORC1 in promoting glycolysis during fibrogenesis. Using an unbiased bioinformatics approach to identify the key transcriptional modules that are induced in fibroblasts in response to TGFβ1 and modulated by mTOR inhibition, we were able to uncover a critical mechanistic pathway linking mTORC1 activation to altered myofibroblast metabolism through the transcriptional master regulator of amino acid metabolism, activating transcription factor 4 (ATF4). Whilst Smad3 promotes ATF4 gene expression, mTORC1 was shown to play a critical role in promoting the translation of ATF4. ATF4 in turn promotes the transcription of the glucose transporter 1 (GLUT1), as well as the genes encoding enzymes of the de novo serine-glycine biosynthetic pathway in order to supply glucose-derived glycine to meet the amino acid requirements associated with enhanced collagen production. These observations therefore highlight a critical mechanism by which targeting the mTORC1 axis may offer therapeutic potential for interfering with myofibroblast function in IPF and potentially other fibrotic conditions [21].
In addition to glycolysis, glutaminolysis has also been implicated in TGFβ1-induced myofibroblast differentiation and activation [22, 23]. Glutamine is a known activator of mTORC1 through Rag-dependent and independent pathways [24] and a recent study revealed a critical role for glutaminase 1 (GLS1), the enzyme responsible for the hydrolysis of glutamine to glutamate in promoting collagen translation and stability of collagens in TGFβ1-induced myofibroblasts [25].
Finally, a role for dysregulated fatty acid metabolism via the induction/activation of fatty acid synthase through rapamycin sensitive TGFβ1/mTORC1 signalling in fibrogenesis has also recently emerged [26, 27]. Fatty acid metabolism consists of catabolic processes for energy production and anabolic processes for the synthesis of fatty acids that are integral components of the cell membrane and pulmonary surfactant. In our recent eigengene-based bioinformatics analysis of the transcriptome profile of IPF fibrotic foci, we found that the fatty acid metabolism pathway correlated with collagen I and III expression [17]. In addition, SREBP which is responsible for mTORC1-dependent lipid synthesis has also been linked to fibrosis [28, 29], so that mTORC1 likely regulates lipid synthesis at different levels in this disease context.
mTORC1 regulates autophagy and senescence
In response to nutrient limitation, cells use autophagy to degrade cell components and maintain essential activity and viability. Under glucose starvation, AMPK, a key energy sensor, promotes autophagy by directly activating ULK1. Under nutrient sufficiency, mTORC1 phosphorylates ULK1 preventing its activation and disrupting its interaction with AMPK [30], therefore preventing autophagy. As alluded to previously, during normal wound healing, fibroblasts sense collagen I polymerisation as a negative regulator of proliferation and a trigger for apoptosis, while in fibrosis, they continue to proliferate and are resistant to apoptosis [31]. There is mounting evidence that in IPF fibroblasts, decreased autophagy activity and increased resistance to apoptosis may be linked through aberrant mTORC1 activation [32].
mTORC1 has also been linked to cellular senescence, a process characterised by irreversible cell growth arrest in response to ageing or cellular damage, as a result of telomere attrition, oxidative stress, DNA damage and proteome instability. The senescence-associated secretory phenotype is characterised by the secretion of a range of pro-inflammatory cytokines, chemokines, matrix remodelling proteinases and growth factors (including TGFβ1, interleukin-6 and matrix metalloproteinase-12) by senescent cells and has been shown to be regulated by rapamycin sensitive mTORC1 signalling [33]. Cellular senescence is a feature of IPF, with both lung fibroblasts and epithelial cells displaying evidence of the acquisition of a senescent phenotype in the lung of patients with IPF [34, 35]. In recent years, mitochondrial dysfunction of the epithelium in IPF has also emerged as an important causative factor of cellular senescence. The mitochondrial biogenesis pathway downstream of the mTOR/PGC-1α/β axis is markedly upregulated in senescent lung epithelial cells, and inhibition of mTORC1 with rapamycin restores mitochondrial homeostasis and reduces cellular senescence to bleomycin in lung epithelial cells [36].
mTORC1 as a therapeutic target in IPF
The scientific rationale for targeting mTORC1 signalling in the context of IPF is continuing to gain support. However, the rapalog everolimus failed to show any benefit in an IPF clinical trial and was even found to be associated with various adverse effects [37], potentially at least in part related to downregulation of mTORC2 activity upon chronic treatment [38, 39]. Rapalogs are allosteric inhibitors that exclusively bind the FKBP12/rapamycin binding domain of mTOR to restrict substrates access to the catalytic site. Despite the presence of the mTOR protein in both complexes, rapalogs only inhibit mTORC1 activity due to different spatial conformations of the complexes. However, at least in some cell types, prolonged incubation with rapalogs can affect mTORC2, presumably by binding the newly synthesised mTOR and preventing complex formation [40]. Phosphoproteomic studies have shown that mTORC1 substrates, p70S6K and 4E-BP1, are differentially sensitive to rapamycin/rapalog treatment; while p70S6K is readily sensitive to rapamycin treatment, 4E-BP1 is only partially sensitive [41]. We found that TGFβ1-induced collagen deposition in IPF fibroblasts was 4E-BP1-mediated and therefore insensitive to rapamycin. Furthermore, this observation extended to other matrisome proteins induced in response to TGFβ1 treatment and implicated in fibrosis. We propose that this might, at least in part, explain the negative outcome of the everolimus IPF trial.
The next generation of mTOR inhibitors target the active site of the mTOR kinase and multiple orally available ATP competitive mTOR inhibitors are now in clinical development in the setting of treatment-unresponsive cancer (table 1) [42]. Proof-of-concept that such agents could hold promise in the setting of IPF was obtained by our recent data demonstrating that ATP-competitive mTOR inhibition was highly effective in blocking collagen synthesis in live precision-cut IPF lung tissue slices. However, the long-term tolerability of dual mTORC1 and mTORC2 inhibition remains unknown and the risk–benefit ratio for chronic fibrotic diseases remains to be established. Moreover, rapamycin/rapalogs at clinically approved doses have been reported to be associated with various adverse events, including wound-healing complications, diabetes/hyperglycaemia, dyslipidaemia, proteinuria, nephrotoxicity, pneumonitis, anaemia and hypertension [43]. Agents which inhibit mTORC1 without these associated adverse events are currently in development, including a small molecule inhibitor of Rheb, NR1, which inhibits mTORC1 mediated phosphorylation of ribosomal protein S6 kinase beta-1 (S6K1) without affecting mTORC2 signalling [44]. However, similarly to rapamycin, NR1 does not appear to inhibit the phosphorylation of the mTORC1 substrate 4E-BP1 and may therefore not impact on TGFβ1-induced ECM production.
TABLE 1.
Non-exhaustive list of mammalian target of rapamycin (mTOR) inhibitors in current and completed clinical trials
| Target | Compound | Clinical trials |
| PI3K/mTOR | GSK2126458 (omipalisib) | Dose escalation studies in solid cancer (NCT00972686) and proof of mechanism trial in IPF (NCT01725139) |
| mTORC1 | Everolimus | FDA-approved for pancreatic neuroendocrine tumours and advanced breast cancer |
| Sirolimus | Lymphangioleiomyomatosis (Phase I: NCT02432560) | |
| Organ rejection in kidney transplant (Phase I: NCT01236378; Phase II: NCT00076570) | ||
| mTORC1/2 | AZD8055 | Recurrent gliomas, liver cancer and advanced tumours (Phase I: NCT01316809) |
| AZD2014 (vistusertib) | Glioblastoma multiforme (Phase I: NCT02619864, NCT03071874) | |
| Meningiomas (Phase II: NCT02831257) | ||
| High-risk prostate cancer (Phase II: NCT02064608) | ||
| MLN0128 | Advanced solid tumours (Phase I: NCT02719691) | |
| Sarcoma (Phase I: NCT02987959) |
Conclusions
In this mini-review, we have described the role of mTORC1 in influencing ECM production, metabolism, autophagy and senescence, in the context of IPF. Current evidence supports the conclusion that carefully targeted inhibition of the TGFβ1-mTORC1 axis may hold considerable promise as an anti-fibrotic strategy with the potential to impact multiple IPF pathomechanisms.
Footnotes
Provenance: Commissioned article, peer reviewed.
Author contributions: Original draft, editing and review: R. Chambers and M. Platé; review and editing: D. Guillotin.
Conflict of interest: M. Platé has nothing to disclose.
Conflict of interest: D. Guillotin reports other funding from GlaxoSmithKline, during the conduct of the study.
Conflict of interest: R. Chambers reports grants from GlaxoSmithKline during the conduct of the study.
Support statement: R. Chambers gratefully acknowledges funding support received in this area of research from the Medical Research Council, UK (Ref #MR/K024078/1); NIHR University College London Hospitals Biomedical Research Centre /NIMR and Fonds de la Recherche en Santé, Québec, the Biotechnology and Biological Research Council (Ref #1353894 and #1488900); and funding from GlaxoSmithKline (GSK) under a collaborative framework agreement. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Lee AS, Mira-Avendano I, Ryu JH, et al. . The burden of idiopathic pulmonary fibrosis: an unmet public health need. Respir Med 2014; 108: 955–967. doi: 10.1016/j.rmed.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 2.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol 2013; 229: 298–309. doi: 10.1002/path.4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003; 425: 577–584. doi: 10.1038/nature02006 [DOI] [PubMed] [Google Scholar]
- 4.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res 2009; 19: 128–139. doi: 10.1038/cr.2008.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yip CK, Murata K, Walz T, et al. . Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell 2010; 38: 768–774. doi: 10.1016/j.molcel.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demetriades C, Doumpas N, Teleman AA. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 2014; 156: 786–799. doi: 10.1016/j.cell.2014.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sancak Y, Bar-Peled L, Zoncu R, et al. . Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010; 141: 290–303. doi: 10.1016/j.cell.2010.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 2017; 169: 361–371. doi: 10.1016/j.cell.2017.03.035 [DOI] [PubMed] [Google Scholar]
- 9.Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer 2018; 18: 744–757. doi: 10.1038/s41568-018-0074-8 [DOI] [PubMed] [Google Scholar]
- 10.Sun SY. mTOR kinase inhibitors as potential cancer therapeutic drugs. Cancer Lett 2013; 340: 1–8. doi: 10.1016/j.canlet.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White ES, Atrasz RG, Hu B, et al. . Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10). Am J Respir Crit Care Med 2006; 173: 112–121. doi: 10.1164/rccm.200507-1058OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia H, Diebold D, Nho R, et al. . Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med 2008; 205: 1659–1672. doi: 10.1084/jem.20080001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo RC, Garcia CC, Barcelos LS, et al. . Phosphoinositide 3-kinase gamma plays a critical role in bleomycin-induced pulmonary inflammation and fibrosis in mice. J Leukoc Biol 2011; 89: 269–282. doi: 10.1189/jlb.0610346 [DOI] [PubMed] [Google Scholar]
- 14.Lukey PT, Harrison SA, Yang S, et al. . A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur Respir J 2019; 53: 1801992. doi: 10.1183/13993003.01992-2018 [DOI] [PubMed] [Google Scholar]
- 15.Mercer PF, Woodcock HV, Eley JD, et al. . Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax 2016; 71: 701–711. doi: 10.1136/thoraxjnl-2015-207429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodcock HV, Eley JD, Guillotin D, et al. . The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nat Commun 2019; 10: 6. doi: 10.1038/s41467-018-07858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guillotin D, Taylor AR, Platé M, et al. . Transcriptome analysis of IPF fibroblastic foci identifies key pathways involved in fibrogenesis. bioRxiv 2020; preprint [ 10.1101/2020.03.10.984955]. [DOI] [PubMed] [Google Scholar]
- 18.Win T, Thomas BA, Lambrou T, et al. . Areas of normal pulmonary parenchyma on HRCT exhibit increased FDG PET signal in IPF patients. Eur J Nucl Med Mol Imaging 2014; 41: 337–342. doi: 10.1007/s00259-013-2514-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Justet A, Laurent-Bellue A, Thabut G, et al. . [(18)F]FDG PET/CT predicts progression-free survival in patients with idiopathic pulmonary fibrosis. Respir Res 2017; 18: 74. doi: 10.1186/s12931-017-0556-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie N, Tan Z, Banerjee S, et al. . Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med 2015; 192: 1462–1474. doi: 10.1164/rccm.201504-0780OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvarajah B, Azuelos I, Platé M, et al. . mTORC1 amplifies the ATF4-dependent de novo serine-glycine pathway to supply glycine during TGF-beta1-induced collagen biosynthesis. Sci Signal 2019; 12: eaav3048. 10.1126/scisignal.aav3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernard K, Logsdon NJ, Benavides GA, et al. . Glutaminolysis is required for transforming growth factor-beta1-induced myofibroblast differentiation and activation. J Biol Chem 2018; 293: 1218–1228. doi: 10.1074/jbc.RA117.000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai L, Bernard K, Tang X, et al. . Glutaminolysis epigenetically regulates antiapoptotic gene expression in idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol 2019; 60: 49–57. doi: 10.1165/rcmb.2018-0180OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jewell JL, Kim YC, Russell RC, et al. . Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 2015; 347: 194–198. doi: 10.1126/science.1259472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge J, Cui H, Xie N, et al. . Glutaminolysis promotes collagen translation and stability via alpha-ketoglutarate-mediated mTOR activation and proline hydroxylation. Am J Respir Cell Mol Biol 2018; 58: 378–390. doi: 10.1165/rcmb.2017-0238OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung MY, Kang JH, Hernandez DM, et al. . Fatty acid synthase is required for profibrotic TGF-beta signaling. FASEB J 2018; 32: 3803–3815. doi: 10.1096/fj.201701187R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao YD, Yin L, Archer S, et al. . Metabolic heterogeneity of idiopathic pulmonary fibrosis: a metabolomic study. BMJ Open Respir Res 2017; 4: e000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorotea D, Koya D, Ha H. Recent insights into SREBP as a direct mediator of kidney fibrosis via lipid-independent pathways. Front Pharmacol 2020; 11: 265. doi: 10.3389/fphar.2020.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertolio R, Napoletano F, Mano M, et al. . Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat Commun 2019; 10: 1326. doi: 10.1038/s41467-019-09152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Kundu M, Viollet B, et al. . AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011; 13: 132–141. doi: 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol 2020; 16: 11–31. doi: 10.1038/s41584-019-0324-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero Y, Bueno M, Ramirez R, et al. . mTORC1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell 2016; 15: 1103–1112. doi: 10.1111/acel.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laberge RM, Sun Y, Orjalo AV, et al. . MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 2015; 17: 1049–1061. doi: 10.1038/ncb3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schafer MJ, White TA, Iijima K, et al. . Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 2017; 8: 14532. doi: 10.1038/ncomms14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calhoun C, Shivshankar P, Saker M, et al. . Senescent cells contribute to the physiological remodeling of aged lungs. J Gerontol A Biol Sci Med Sci 2016; 71: 153–160. doi: 10.1093/gerona/glu241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summer R, Shaghaghi H, Schriner D, et al. . Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am J Physiol Lung Cell Mol Physiol 2019; 316: L1049–L1L60. doi: 10.1152/ajplung.00244.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malouf MA, Hopkins P, Snell G, et al. . An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. Respirology 2011; 16: 776–783. doi: 10.1111/j.1440-1843.2011.01955.x [DOI] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Sengupta S, et al. . Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006; 22: 159–168. doi: 10.1016/j.molcel.2006.03.029 [DOI] [PubMed] [Google Scholar]
- 39.Lamming DW, Ye L, Katajisto P, et al. . Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012; 335: 1638–1643. doi: 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans 2009; 37: 217–222. doi: 10.1042/BST0370217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang SA, Pacold ME, Cervantes CL, et al. . mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science 2013; 341: 1236566. doi: 10.1126/science.1236566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua H, Kong Q, Zhang H, et al. . Targeting mTOR for cancer therapy. J Hematol Oncol 2019; 12: 71. doi: 10.1186/s13045-019-0754-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando) 2014; 28: 126–133. doi: 10.1016/j.trre.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 44.Mahoney SJ, Narayan S, Molz L, et al. . A small molecule inhibitor of Rheb selectively targets mTORC1 signaling. Nat Commun 2018; 9: 548. doi: 10.1038/s41467-018-03035-z [DOI] [PMC free article] [PubMed] [Google Scholar]