Abstract
Most translesion DNA synthesis (TLS) in Escherichia coli is dependent upon the products of the umuDC genes, which encode a DNA polymerase, DNA polymerase V, with the unique ability to replicate over a variety of DNA lesions, including cyclobutane dimers and abasic sites. The UmuD protein is activated for its role in TLS by a RecA–single-stranded DNA (ssDNA)-facilitated self-cleavage event that serves to remove its amino-terminal 24 residues to yield UmuD′. We have used site-directed mutagenesis to construct derivatives of UmuD and UmuD′ with glycines in place of leucine-101 and arginine-102. These residues are extremely well conserved among the UmuD-like proteins involved in mutagenesis but are poorly conserved among the structurally related LexA-like transcriptional repressor proteins. Based on both the crystal and solution structures of the UmuD′ homodimer, these residues are part of a solvent-exposed loop. Our genetic and biochemical characterizations of these mutant UmuD and UmuD′ proteins indicate that while leucine-101 and arginine-102 are critical for the RecA-ssDNA-facilitated self-cleavage of UmuD, they serve only a minimal role in enabling TLS. These results, and others, suggest that the interaction of RecA-ssDNA with leucine-101 and arginine-102, together with numerous other contacts between UmuD2 and the RecA-ssDNA nucleoprotein filaments, serves to realign lysine-97 relative to serine-60, thereby activating UmuD2 for self-cleavage.
When an organism's DNA replication machinery encounters a lesion in the DNA that, for a variety of reasons, was not repaired by accurate repair pathways, it stalls, leading to one of two possible outcomes: (i) damage avoidance, a poorly understood set of processes, including daughter strand gap repair, that appear to utilize the information in the newly synthesized daughter strand of the DNA duplex to somehow bypass the lesion (17) or (ii) translesion synthesis (TLS), in which a specialized DNA polymerase is recruited for bypassing the damaged site (17, 21, 67, 69). The latter pathway is potentially mutagenic due to the miscoding or noncoding nature of the DNA lesion (17).
TLS in Escherichia coli is dependent on the umuDC and recA gene products (17, 23, 61). The umuDC operon encodes a DNA polymerase, DNA polymerase V, with the unique ability to replicate over particular types of DNA lesions, including abasic sites and thymine-thymine cyclobutane dimers (55, 64, 65). Recent work indicates that UmuC is the founding member of a diverse and ubiquitous family of DNA polymerases capable of copying imperfect templates (16, 18, 21, 69). In addition to their role in TLS, the umuDC gene products also participate in cell cycle checkpoint control (41, 46). In response to DNA damage, RecA protein nucleates on single-stranded DNA (ssDNA) that is generated by the cell's failed attempts to bypass lesions in its genome (17, 57). These RecA-ssDNA nucleoprotein filaments act to mediate the cleavage of the LexA repressor (33, 34). Cleavage of LexA inactivates it as a repressor, leading to the expression of the SOS regulon, a collection of ∼30 unlinked genes whose expression is coordinately regulated (11, 17, 25). The umuDC operon is among these ∼30 LexA-regulated genes (9, 17). Importantly, UmuD also undergoes RecA-ssDNA-mediated cleavage. This cleavage serves to remove its amino-terminal 24 residues to produce UmuD′ (5, 43, 58). It has been proposed that cleavage of UmuD serves to regulate the two physiological roles of the umuDC gene products so that they act in a temporally ordered fashion (41, 46), first by participating in a DNA damage checkpoint control system and second by participating in TLS.
UmuD is related to three distinct classes of proteins: (i) other UmuD-like proteins involved in mutagenesis, which also undergo RecA-ssDNA-facilitated cleavage (17, 29, 30, 52), (ii) transcriptional repressors belonging to the LexA-like family, which undergo RecA-ssDNA-facilitated cleavage (17, 52), and (iii) signal peptidases (49). Interestingly, despite the fact that UmuD′ and E. coli signal peptidase have little sequence identity apart from their lysine-serine dyad, they have a remarkable degree of structural identity. Comparison of their crystal structures revealed that 69 C-α atoms of UmuD′ are superimposable (with a root mean squared of 1.6 Å) on the E. coli signal peptidase crystal structure (49).
The E. coli UmuD and UmuD′ proteins interact with each other to form homo- and heterodimers (1, 5, 22) and also interact with UmuC (4, 22, 70), RecA-ssDNA nucleoprotein filaments (5, 32), and three components of the replicative DNA polymerase (62). Given the rather small sizes of UmuD and UmuD′, an important question is which part(s) of UmuD and UmuD′ is involved in interaction with each of these other proteins? A detailed understanding of these interactions will be required for a complete understanding of the molecular mechanisms underlying the roles of the umuDC gene products in checkpoint control and TLS. Determination of the crystal (10, 50) and solution (A. E. Ferentz, G. C. Walker, and G. Wagner, unpublished data) structures of the UmuD′2 homodimer and genetic studies (39, 45) have identified the residues involved in its dimerization interface. Furthermore, biochemical characterizations of single-cysteine derivatives of UmuD and UmuD′ by analyzing homo- and heterodimer cross-linking efficiency using thiol-specific cross-linking reagents and by studies of spin-labeled derivatives by electron spin resonance have identified important components of the dimerization interfaces of the UmuD-UmuD′ heterodimer and the UmuD2 homodimer (unpublished data). Finally, these single-cysteine derivatives of UmuD have also been used, in conjunction with the thiol-specific, photoactivatable, heterobifunctional cross-linking agent p-azidoiodoacetanilide (71), to identify residues of UmuD able to cross-link efficiently to RecA-ssDNA nucleoprotein filaments (32) (Fig. 1). Taken together, these studies have begun to provide a detailed molecular understanding of the roles of the umuD gene products in regulation of the cell cycle and TLS.
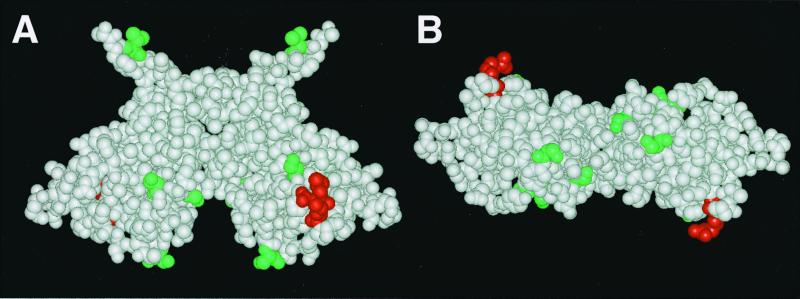
FIG. 1.
Side (A) and top (B) views of the UmuD′2 homodimer crystal structure reported by Peat et al. (50) with the homodimer interface reported by Ferentz et al. (10). Leu101 and Arg102 are shown in red. Val34, Ser57, Ser67, Ser81, and Ser112 are shown in green. These positions of UmuD2, when changed to cysteine and conjugated to the thiol-specific photoactivatable cross-linker p-azidoiodoacetanilide (71), were cross-linked efficiently to RecA-ssDNA in vitro (32).
As part of our ongoing effort to better understand how UmuD and UmuD′ interact with other proteins to enable checkpoint control and TLS, we have embarked on a site-directed mutational analysis of the umuD gene products (1, 45). Comparison of the deduced amino acid sequences of members of the UmuD-like mutagenesis proteins to those of the LexA-like transcriptional repressor family identified a small number of residues that were well conserved exclusively among the UmuD-like mutagenesis proteins (1) (see Fig. 2). This observation suggested that these might be residues that are important for the biological roles of the umuD gene products rather than for the RecA-ssDNA-facilitated cleavage of UmuD. Mutations affecting most of these highly conserved positions have already been characterized (19, 39, 45). Here we describe our construction and genetic and biochemical characterizations of a UmuD derivative in which two such highly conserved residues, leucine-101 (Leu101) and arginine-102 (Arg102), have been replaced with glycines. Our characterizations of this mutant UmuD protein, which we unexpectedly found to be deficient in RecA-ssDNA-facilitated cleavage, suggest a possible mechanism for how the interaction of UmuD2 with the RecA-ssDNA nucleoprotein filament results in the cleavage of one UmuD molecule by its intradimer partner to yield UmuD′ (10, 37).
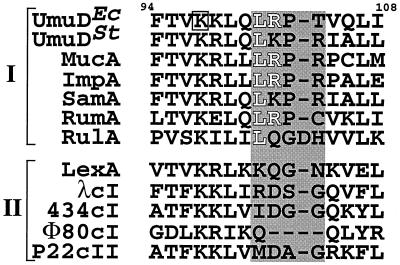
FIG. 2.
Partial amino acid alignment of proteins similar to E. coli UmuD. Shown is the region between amino acids 94 and 108 of UmuD. This figure is modified from references 1 and 51. UmuD-like mutagenesis proteins (I) and LexA-like transcriptional repressors (II) are grouped separately. The active-site lysine (Lys97) in E. coli UmuD is boxed. Residues 101 to 104 of E. coli UmuD, which are based on the crystal structure (10, 50) as well as the recently solved solution structure (Ferentz et al., unpublished data) form a solvent-exposed loop, as well as the corresponding residues in the related proteins, are indicated by the shaded box. Leu101 and Arg102 are represented as white letters. Ec, E. coli; St, Salmonella enterica serovar Typhimurium.
MATERIALS AND METHODS
Bacteriological techniques.
E. coli strains and plasmid DNAs used in this study are described in Table 1. Generalized transduction using P1vir was performed as described previously (40). E. coli was grown in either Luria-Bertani medium or M9 minimal medium (40) as indicated in Fig. 3 and 4. When required, the following antibiotics were used at the indicated concentrations: ampicillin, 150 μg/ml; kanamycin (KAN), 40 μg/ml; tetracycline, 20 μg/ml; and rifampin, 50 μg/ml. Bacterial transformation was by the calcium chloride technique (56). Plasmid DNAs were purified using the QIA-spin mini prep kit (Qiagen) per the manufacturer's recommendations. Missense mutations within the umuD and umuD′ coding regions were generated using the Quickchange kit (Stratagene) per the manufacturer's recommendations, and the nucleotide sequences of all constructs were verified by automated DNA sequence analysis.
TABLE 1.
Bacterial strains and plasmid DNAs used in this study
| Strain or plasmid | Relevant characteristics or comments | Source or reference |
|---|---|---|
| Strains | ||
| CAG12077 | Used as the donor for P1 vir-mediated transduction of the crcA280::Tn10 locusa | E. coli Genetic Stock Center |
| BL21(DE3) | Used for overexpression of UmuD+ and UmuD1012 | Novagen |
| GW8023b | recA+ lexA+ ΔumuDC595::cat | 47 |
| GW8024 | GW8023; lexA300(Def)::spec | 47 |
| GW8025 | GW8024; recA441 | 47 |
| GW8018 | GW8025; lexA(Def) | 47 |
| GW8040 | GW8024; recA430 srl::Tn10 | 41 |
| NR9350 | recA730 srl::Tn10c | R. Schaaper via V. Godoy and M. Fox (12) |
| GW8110 | GW8024; recA730 srl::Tn10 | This work |
| Plasmids | ||
| pBR322kan | Apr Kmr Tcs; pBR322 derivative expressing KAN resistance | 47 |
| pSE117 | Apr Kmr; pBR322 derivative carrying umuD+C+ | 9 |
| pSE117-1012 | Apr Kmr; pSE117 derivative carrying umuD1012C+ | This work |
| pGW3751 | Apr Kmr; pBR322 derivative carrying umuD′+C+ | 43 |
| pGW3751-1012 | Apr Kmr; pGW3751 derivative carrying umuD′1012C+ | This work |
| pAG98 | Apr; UmuD+ overproducer | 19 |
| pAG98-1012 | Apr; UmuD1012 overproducer | This work |
Complete genotype: crcA280::Tn10 LAM− rph-1.
All GW strains are isogenic with respect to each other except for loci indicated. The remaining genotype for all GW strains is xyl-5 mtl-1 galK2 rpsL31 kdgK51 Δ(argF-lacU)169 lacY1 tsx-33 supE44 thi-1 leuB6 hisG4(Oc) mgl-51 argE3(Oc) rfbD1 ara-14 thr-1 qsr′ qin-111 sulA11 ilvD(Ts) λ− Rac−.
Complete genotype: ara thi Δ(pro-lac) sulA366 recA730 srl::Tn10.
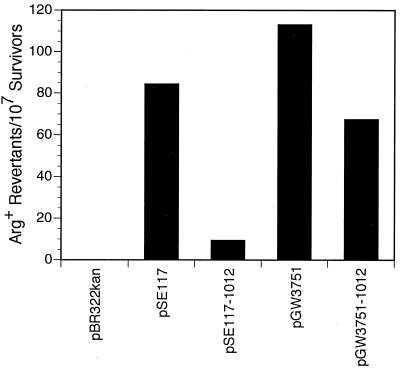
FIG. 3.
Effect of plasmids carrying either wild-type or mutant umuDC or umuD′C operons on UV-induced (20 J/m2) reversion of argE3(Oc)→Arg+ in a ΔumuDC E. coli strain (GW8023). UV mutability is expressed as the number of Arg+ revertants per 107 survivors (9). Plasmid genotypes are as follows: pBR322kan, ΔumuDC; pSE117, umuD+C+; pSE117–1012, umuD1012C+; pGW3751, umuD′+C+; pGW3751–1012, umuD′1012C+.
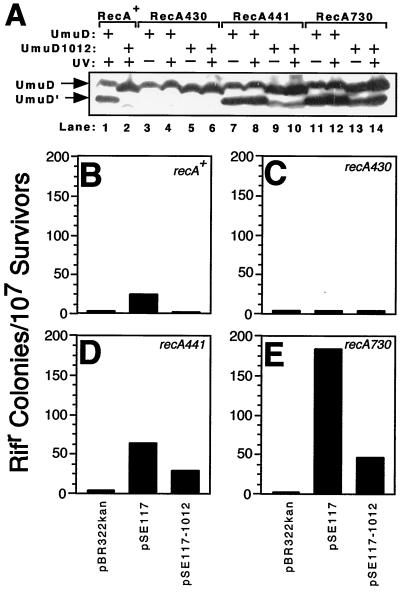
FIG. 4.
Relative in vivo cleavage efficiency and SOS mutator activity of umuD+C+ and umuD1012C+ as a function of the recA allele. (A) In vivo cleavage efficiency of UmuD and UmuD1012 was measured following UV irradiation (20 J/m2) or mock irradiation using four isogenic E. coli strains that differed in their recA allele as described in Materials and Methods [GW8023 differed additionally in that it was lexA+ and not lexA(Def); RecA430 protein cannot cleave LexA and therefore requires a lexA(Def) allele to express SOS-regulated functions (24)]. The RecA441 and RecA730 proteins are coprotease constitutive at 42°C and hence are phenotypically LexA(Def) regardless of the presence or absence of the lexA+ allele (reviewed in reference 28). All strains were grown at 42°C in M9 minimal medium supplemented with 0.4% Casamino Acids, 0.4% glucose, 100 μg of adenine per ml, and 40 μg of KAN per ml. These growth conditions have been previously reported as enhancing the coprotease activity of RecA protein (6). Note that UmuD1012 runs slightly ahead of UmuD+ in SDS-polyacrylamide gel electrophoresis. Aliquots of the same cultures that were UV irradiated and used for measuring cleavage efficiency were also used to measure SOS mutator activity (B to E). Thus, the results in panel A can be compared directly to those in panels B to E. Isogenic strains bearing either recA+ (GW8023 [B]), recA430 (GW8040 [C]), recA441 (GW8025 [D]), or recA730 (GW8110 [E]) were assayed for SOS mutator activity as described in Materials and Methods. SOS mutator activity is expressed as the number of rifampin-resistant colonies per 107 survivors.
Proteins and reagents.
The UmuD and UmuD1012 proteins were purified as described previously from 1-liter cultures of BL21(DE3) transformants except that Superose 12 chromatography was omitted (10). The chromatographic characteristics of UmuD1012 were essentially identical to those of the wild-type UmuD throughout its purification, suggesting that it had a native conformation and that the Leu101-to-glycine and Arg102-to-glycine substitutions had at most a minimal effect on the overall structure of the protein. RecA protein was purified as described elsewhere (15). M13mp18 ssDNA was from New England Biolabs, glutaraldehyde and adenosine 5′-O-(3-thiotriphosphate) were from Sigma, and the Western-Light chemiluminescence kit was from Tropix.
SOS mutagenesis assays and Western blot analysis.
SOS mutagenesis activity was measured by either the argE3(Oc)→Arg+ reversion assay as described elsewhere (9) or a rifampin resistance assay. For the rifampin resistance assay, 0.5 ml of cultures grown overnight at 42°C was used to inoculate 25 ml of M9 minimal medium supplemented with 0.4% Casamino Acids, 0.4% glucose, 100 μg of adenine per ml, and KAN. Cultures were grown at 42°C with shaking. We used these growth conditions because they have been previously demonstrated to enhance the coprotease activity of RecA protein (6). When cultures reached an optical density at 595 nm of ∼0.6, 6-ml aliquots of each were removed and either irradiated with UV (20 J/m2) or mock irradiated in sterile Pyrex dishes (100 mm by 15 mm). Irradiated and mock-irradiated cultures were then returned to 42°C and grown with aeration to allow the expression of SOS-regulated functions. Forty-five minutes after irradiation, 4-ml aliquots were removed from each culture for determination of their optical density at 595 nm and for preparation of whole-cell lysates to monitor the relative abundance of UmuD (or UmuD1012) and UmuD′ (or UmuD1012′) by Western blotting as described previously (45, 46). The remaining 2 ml of each culture was allowed to grow for an additional 1 h 15 min at 42°C to fix any induced mutations, and then serial dilutions of each were plated onto supplemented M9 minimal media containing adenine and KAN, with and without rifampin, to measure SOS mutagenesis activity. Plates were incubated at 42°C for 2 days prior to their counting.
In vitro cleavage of UmuD reconstituted with purified components.
In vitro cleavage of UmuD was reconstituted with purified components essentially as described elsewhere (5). Briefly, reaction mixtures (20 μl) containing the indicated amounts (indicated in Fig. 5) of purified UmuD, UmuD1012, and RecA; 1.7 μM ssDNA 20-mer oligonucleotide; 2.3 mM adenosine 5′-O-(3-thiotriphosphate); 50 mM Tris-HCl (pH 7.5); 100 mM NaCl; 20 mM MgCl2; 0.1 mM EDTA; 0.1 mM dithiothreitol, and 10% glycerol were assembled on ice. Reactions were initiated by incubation at 37°C and were quenched at the indicated times (indicated in Fig. 5) by addition of 0.25 volume of 4× sodium dodecyl sulfate (SDS) sample buffer (200 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol, 8% SDS, 0.8% bromophenol blue, and 40% glycerol) followed by heating to 95°C for 5 min. Aliquots of each sample were then electrophoresed in SDS–14% polyacrylamide gels followed by staining with Coomassie blue R-250. Cleavage efficiency was quantitated by densitometric analysis of the stained gels relative to appropriate standard curves (data not shown) using the Molecular Analyst software package (Bio-Rad). For time course experiments, reaction mixtures (140 μl) containing 24.5 μg of RecA (4.6 μM as a monomer) and 14 μg of either UmuD or UmuD1012 (3.3 μM as a dimer) were assembled on ice and then transferred to 37°C. Twenty-microliter aliquots were removed after 0, 15, 30, 60, 90, and 120 min of incubation at 37°C and quenched as described above. For RecA-ssDNA titrations, the ssDNA concentration was maintained at 1.7 μM. Reaction mixtures (20 μl) contained 0, 0.44, 0.9, 1.75, 3.5, or 7 μg of RecA (0.58 to 9.2 μM as a monomer) and 2 μg of UmuD or UmuD1012 (3.3 μM as a dimer). Incubation was at 37°C for 30 min.
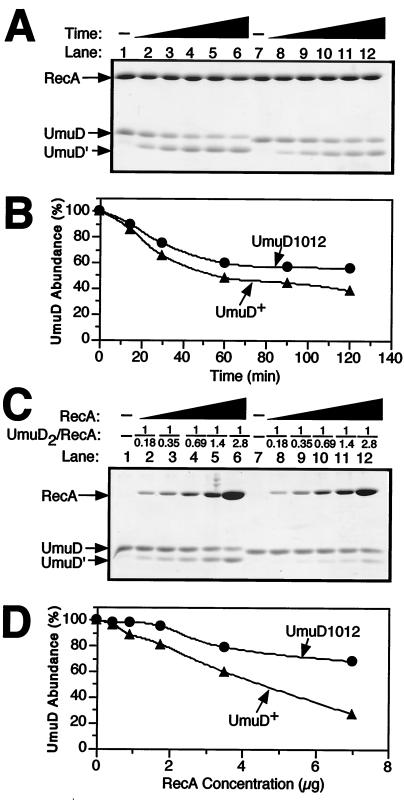
FIG. 5.
Relative in vitro cleavage efficiencies of UmuD and UmuD1012. RecA-ssDNA-facilitated cleavage of UmuD (lanes 1 to 6) or UmuD1012 (lanes 7 to 12) as a function of either time (A and B) or the RecA protein concentration (C and D) were measured in vitro as described in Materials and Methods. In both panels A and C, lanes 1 and 7 correspond to the 0-min and 0-μg RecA protein controls, respectively. Note that UmuD1012 runs slightly ahead of UmuD+ in SDS-polyacrylamide gel electrophoresis. The molar ratio of UmuD2 (as a dimer) to RecA (as a monomer) in panel A was 1:1.4. The molar ratios of UmuD2 (as a dimer) to RecA (as a monomer) in panel C are indicated (UmuD2/RecA). Densitometric analysis (B and D) of the results shown in panels A and C was performed using the Molecular Analyst software package (Bio-Rad).
RESULTS
umuD′1012, but not umuD1012, is active in SOS mutagenesis.
We employed site-directed mutagenesis to construct a plasmid-carried umuDC operon that expresses a UmuD derivative having glycines in place of Leu101 and Arg102. These residues are highly conserved among the UmuD-like mutagenesis proteins but not among members of the structurally related LexA-like transcriptional repressor family (Fig. 2), a fact that initially suggested to us that these residues might be important for the specific biological roles of the umuD gene products rather than for the RecA-ssDNA-facilitated self-cleavage of UmuD. We chose to change Leu101 and Arg102 to glycines instead of alanines because, based on the crystal structure of the UmuD′2 homodimer (10, 50) and the solution structure determined by nuclear magnetic resonance (Ferentz et al., unpublished data), they are located in a solvent-exposed loop of UmuD2 (Fig. 1 and 2). We chose to analyze the double mutant in this study because the close proximity of Leu101 and Arg102 together with their high degree of conservation among the UmuD-like mutagenesis proteins suggests their mutual participation in a single function(s). Thus, we reasoned that the double mutant would exhibit a more pronounced phenotype than either of the single mutants. Finally, for the sake of simplicity, we will ascribe all phenotypes unique to UmuD1012 and/or UmuD′1012 to both substitutions throughout this report.
In the context of this report, the resulting umuD allele will be referred to as umuD1012, and its gene product will be referred to as UmuD1012. In addition, a synthetically engineered plasmid-carried operon that directly expresses the UmuD′ protein together with UmuC (pGW3751) (43) was similarly mutagenized. The resulting umuD′ allele will be referred to as umuD′1012, and its gene product will be referred to as UmuD′1012. It is important to note that the expression of all of these operons was under the control of the native, LexA-regulated umuD+C+ promoter.
We first investigated whether UmuD1012 was competent in SOS mutagenesis. The ability of a pBR322 derivative lacking a umuDC operon (pBR322kan) or bearing the wild-type umuD+C+ (pSE117) or the mutant umuD1012C+ (pSE117–1012) operon to functionally complement a ΔumuDC host for SOS mutagenesis was assessed using an in vivo assay that quantitated the reversion frequency of the argE3(Oc) allele from an Arg− to an Arg+ phenotype following a UV dose (9). Compared to the wild-type umuDC operon, umuD1012C+ was essentially inactive in SOS mutagenesis (Fig. 3). The observed lack of SOS mutagenesis was not due to an instability of the UmuD1012 protein, as immunoblot analysis of whole-cell extracts indicated that it was as abundant as wild-type UmuD following UV irradiation (see below).
UmuD must undergo a posttranslational, RecA-ssDNA-facilitated self-cleavage reaction to activate it for its role in SOS mutagenesis (5, 43, 58). Interestingly, this cleavage reaction can occur in an intermolecular fashion in which the catalytic dyad of one UmuD molecule cleaves in between Cys24 and Gly25 of its intradimer partner (10, 37). Since it was unclear whether the UmuD1012 protein was deficient in cleavage or in its ability to participate in SOS mutagenesis, we tested the ability of the umuD′1012C+ operon to complement the ΔumuDC strain in SOS mutagenesis. Our observation that the strain expressing the precleaved UmuD′1012 protein together with UmuC was ∼60% as active in SOS mutagenesis as the control strain expressing wild-type UmuD′ together with UmuC (Fig. 3) suggests that the proximal cause of the nonmutability of the strain expressing umuD1012C+ was a deficiency of the UmuD1012 protein in undergoing RecA-ssDNA-facilitated cleavage. Nevertheless, the somewhat reduced level of SOS mutagenesis observed in the strain expressing the UmuD′1012 protein indicates that it is less efficient than the wild-type UmuD′ protein in promoting the TLS process that underlies SOS mutagenesis.
umuD1012C+ confers a cold sensitivity for growth slightly greater than that conferred by wild-type umuDC.
Another indication that the UmuD1012 protein was deficient in RecA-ssDNA-mediated cleavage came from our examination of the ability of the umuD1012C+ operon to cause umuD+C+-mediated cold sensitivity. This phenomenon refers to the inability of E. coli lexA(Def) strains that constitutively express a plasmid-carried umuDC operon to grow at 30°C; interestingly, these same strains exhibit no growth defects at 42°C (36, 47). A careful analysis of the genetic requirements for umuDC-mediated cold sensitivity has revealed that they are different from those for SOS mutagenesis in the sense that uncleaved UmuD, together with UmuC, confers a greater degree of cold sensitivity than does UmuD′, together with UmuC (47). One of the clearest illustrations of this point was the finding that UmuD derivatives containing missense mutations rendering them noncleavable by RecA-ssDNA conferred a more severe cold sensitive phenotype than did the wild-type operon (between ∼2- and ∼60-fold more severe) (47). The ability of uncleaved UmuD together with UmuC to cause cold sensitivity for growth when overproduced, by virtue of an increased gene dosage, appears to be related to the proteins' ability to participate in a DNA damage checkpoint (41, 46). We observed that the plasmid-carried umuD1012C+ operon confers an approximately 20-fold more severe cold sensitive phenotype than did the wild-type umuDC operon (Table 2). This finding strongly parallels our earlier results with the noncleavable umuD alleles (47) and provides an additional suggestion that the UmuD1012 protein is refractory to RecA-ssDNA-mediated cleavage. Incidentally, whatever properties of UmuD are necessary for it to participate in umuDC-mediated cold sensitivity must remain largely intact in the UmuD1012 protein.
TABLE 2.
umuD1012C+ confers a more severe cold sensitive phenotype than does umuD+C+
| GW8018 transformant | umuD allelea | CFU (30°C/42°C)b |
|---|---|---|
| pBR322kan | None | 0.9 |
| pSE117 | umuD+ | 4.6 × 10−4 |
| pSE117-1012 | umuD1012 | 2.3 × 10−5 |
pSE117 and pSE117-1012 both carry the wild-type umuC allele.
Representative transformants obtained at 42°C were picked and resuspended in 0.8% saline. After serial dilution, aliquots were plated in duplicate onto Luria-Bertani agar supplemented with KAN and then incubated at 30 or 42°C overnight prior to colony counting.
UmuD1012 is defective in RecA-ssDNA-facilitated self-cleavage in vivo.
As a direct test of the possibility that the UmuD1012 protein was defective in RecA-ssDNA-facilitated cleavage, we measured its cleavage in vivo to yield UmuD′1012 following a UV dose of 20 J/m2. Cleavage was monitored directly by Western blotting of whole-cell extracts using affinity-purified anti-UmuD-UmuD′ antibodies as previously described (45, 46). Preliminary experiments indicated that whereas wild-type UmuD was efficiently cleaved to yield UmuD′ after UV irradiation of a recA+ strain, UmuD1012 was not. We therefore investigated whether cleavage of UmuD1012 could be facilitated in vivo by RecA derivatives whose coprotease activity is altered. For these experiments we used culturing conditions previously demonstrated to enhance the coprotease activity of RecA protein (see Materials and Methods). Plasmids carrying umuD+C+ or umuD1012C+ were introduced into ΔumuDC derivatives of recA+, recA430, recA441, or recA730 strains. The recA430 allele encodes a mutant RecA protein containing a glycine-204-to-serine (G204S) substitution that renders it deficient in facilitating self-cleavage of UmuD, LexA, and λcI repressor while leaving it proficient in homologous recombination (24). The recA441 and recA730 alleles encode a common Q38K substitution, with the recA441 allele encoding a V298I substitution as well (26, 68). The recA441 mutant is coprotease constitutive at 42 but not 30°C, while the recA730 mutant is coprotease constitutive at both temperatures (68; reviewed in reference 28). Since the RecA441 mutant is coprotease constitutive at 42°C and since we wanted to maximize the coprotease activity of all RecA derivatives, we grew all cultures, regardless of their recA genotype, at the permissive temperature of 42°C (see Materials and Methods).
Essentially no UV-induced cleavage of UmuD1012 was observed in the recA+ strain (Fig. 4A, lane 2), and, as expected, neither UmuD nor UmuD1012 was cleaved in the recA430 strain (Fig. 4A, lanes 3 to 6). In contrast, however, the recA441 and recA730 genetic backgrounds were capable of facilitating cleavage of both UmuD and UmuD1012, with or without UV irradiation (Fig. 4A, lanes 7 to 14), with recA730 being slightly better than recA441 at mediating cleavage of UmuD1012. Nevertheless, this cleavage of UmuD1012 was still far less efficient than that observed for wild-type UmuD (Fig. 4A, compare lanes 9 and 10 and lanes 13 and 14 with lane 1). The ability of the recA441 and recA730 gene products to facilitate the cleavage of UmuD1012 in vivo suggests that the RecA441 and the RecA730 proteins interact with UmuD2 in such a way that, for them, Leu101 and Arg102 are less important for stimulating the cleavage reaction.
UmuD1012 is active in SOS mutagenesis in vivo in a recA allele-specific fashion.
In a parallel experiment, we compared the abilities of the UmuD and UmuD1012 proteins to participate in SOS mutagenesis as a function of the recA alleles discussed above. This was done by plating aliquots of the same UV-irradiated cultures used for the analysis of the levels of the umuD (or umuD1012) gene products onto solid medium containing rifampin. Quantitation of the rifampin-resistant CFU per survivor for each strain allowed a direct comparison of the umuD and umuD1012 alleles with respect to their activity in SOS mutagenesis. The presence of the wild-type umuDC operon efficiently complemented the ΔumuDC allele of the host strains, as indicated by the increased frequency of rifampin resistance with all recA alleles tested except for recA430, which is defective in promoting cleavage of UmuD (Fig. 4B to E). Furthermore, the frequency of umuDC-dependent rifampin resistance was itself enhanced relative to the recA+ strain by recA alleles exhibiting enhanced coprotease activity (i.e., recA441 and recA730 [Fig. 4, compare panels B, D, and E]), as expected (reviewed in references 28 and 66).
Consistent with our earlier observation indicating that UmuD1012 was essentially inactive in SOS mutagenesis in a recA+ genetic background using the argE3(Oc)→Arg+ reversion assay (Fig. 3), umuD1012C+ was similarly unable to enhance the frequency of rifampin resistance beyond that observed for the ΔumuDC strain lacking a plasmid-carried umuDC operon (Fig. 4B). This inactivity of umuD1012C+ in SOS mutagenesis correlates with the inability of recA+ to facilitate self-cleavage of UmuD1012 (Fig. 4A, lane 2) and was similar to the level of SOS mutagenesis observed for both umuD+C+ and umuD1012C+ in a recA430 (coprotease-deficient mutant) genetic background (Fig. 4C). In contrast to the recA+ genetic background, umuD1012C+ was proficient in SOS mutagenesis in both the recA441 and recA730 genetic backgrounds (Fig. 4D and E). This activity correlates with the abilities of these recA gene products to facilitate the self-cleavage of UmuD1012 (Fig. 4A, lanes 9 and 10 and lanes 13 and 14), a prerequisite for activation of UmuC as a lesion bypass DNA polymerase (43, 55), and is consistent with the comparatively robust activity of umuD′1012C+ in SOS mutagenesis as measured by the arginine reversion assay (Fig. 3). Taken together, these results confirm that umuD′1012C+ is active in SOS mutagenesis but umuD1012C+ is not and further support the notion that Leu101 and Arg102 of UmuD are critical for efficient RecA-facilitated self-cleavage of UmuD to yield UmuD′ but, subsequent to cleavage, are of only moderate importance for the role of UmuD′ in TLS.
UmuD1012 can undergo RecA-ssDNA-facilitated cleavage in vitro.
To follow up on our observation that wild-type RecA protein could not facilitate efficient cleavage of UmuD1012 in vivo, we investigated whether highly purified UmuD1012 would be cleaved when incubated with purified RecA protein and ssDNA in vitro. In our first experiment, we investigated the efficiency of self-cleavage of UmuD1012 relative to that of wild-type UmuD as a function of time using a fixed concentration of RecA protein (4.6 μM as a monomer) and ssDNA (1.7 μM). Under these conditions, the ssDNA was slightly more than twofold in excess of that necessary for complete nucleation of RecA (∼6 RecA molecules can bind to a single 20-mer ssDNA) (28). In striking contrast to our expectations based on in vivo observations, we did observe rather efficient cleavage of UmuD1012 to yield UmuD′1012 in vitro (Fig. 5A). Densitometric analysis of the Coomassie blue-stained gel relative to appropriate standard curves indicated that although UmuD1012 was activated for cleavage by RecA-ssDNA in vitro, it nevertheless underwent self-cleavage less efficiently than did wild-type UmuD under the identical conditions (Fig. 5B).
To further characterize the ability of UmuD1012 to be cleaved by RecA-ssDNA in vitro, we investigated the efficiency of UmuD1012 cleavage relative to that of wild-type UmuD as a function of the RecA concentration over a 16-fold range (from 0.58 to 9.2 μM) using a fixed level of ssDNA (1.7 μM) and a fixed time of incubation. Whereas the efficiency of wild-type UmuD cleavage mediated by RecA and ssDNA appeared to be directly proportional to the amount of RecA protein added starting at molar ratios of UmuD2 (as a dimer) to RecA (as a monomer) as low as 1:0.18 (Fig. 5C), appreciable cleavage of UmuD1012 was observed only at ratios of UmuD10122 to RecA between 1:0.69 and 1:1.4 (Fig. 5C). Furthermore, regardless of the concentration of RecA protein investigated, cleavage of UmuD1012 was significantly less efficient than that observed for wild-type UmuD (Fig. 5D), consistent with the results of the time course experiment (Fig. 5B).
Our inability to detect cleavage of UmuD1012 in vivo in a recA+ genetic background (Fig. 4A) contrasts with the rather efficient cleavage observed in vitro with a reconstituted self-cleavage assay comprising purified proteins (Fig. 5A and B). We have previously observed rather striking differences between cleavage of certain mutant UmuD proteins in vivo versus their cleavage in vitro under conditions similar to those used here (19, 31). Our inability to observe cleavage of UmuD1012 by wild-type RecA in vivo is presumably attributable to the numerous protein-protein interactions that occur in vivo between UmuD2, as well as RecA, and other proteins that are absent from our in vitro system, including RecA-ssDNA-facilitated cleavage of LexA (35) and RecA-ssDNA-dependent homologous recombination (54), both of which represent processes that compete directly with UmuD cleavage.
Finally, a rough estimation of the steady-state levels of UmuD, UmuD1012, and RecA under the conditions of our in vivo cleavage assay provides further support for this conclusion (data not shown). When UmuD and UmuD1012 were expressed from multicopy plasmids in vivo to measure cleavage efficiency, the approximate molar ratio was 1 molecule of UmuD2 (as a dimer) to 1.8 to 3.8 molecules of RecA (as a monomer). The molar ratio of UmuD2 to RecA (and UmuD10122 to RecA) analyzed in our in vitro system varied from a low of 1:2.8 to a high of 1:0.18 (Fig. 5C). Given that only a portion of the total RecA protein in vivo is likely to be available to facilitate UmuD cleavage (see above) and that cleavage of UmuD1012 in vitro requires higher levels of RecA than those required by wild-type UmuD (i.e., UmuD10122/RecA ratios on the order of 1:0.69 to 1.4 [or more] [Fig. 5C and D]), we suggest that UmuD1012 is not cleaved to an easily detectable level in vivo because of limiting levels of RecA-ssDNA nucleoprotein filaments and not because of widespread differences between our in vitro and in vivo systems with respect to their underlying mechanisms.
UmuD1012 can interact directly with the RecA-ssDNA nucleoprotein filament in vitro.
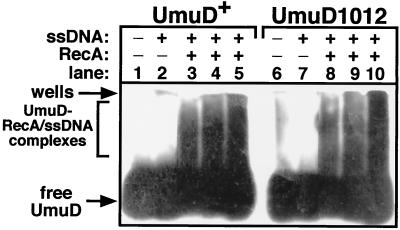
Cleavage of purified UmuD1012 in vitro was very sensitive to the concentration of RecA protein (Fig. 5C and D), suggesting that UmuD1012 might have a reduced affinity for the RecA-ssDNA nucleoprotein filament. To test whether UmuD1012 was affected in interaction with RecA, we employed a cross-linking assay developed by Frank et al. (14). Under the conditions used in this analysis, both UmuD and UmuD1012 were essentially comparable with respect to their abilities to be cross-linked to RecA-ssDNA nucleoprotein filaments in vitro (Fig. 6). Our finding that UmuD1012 was able to interact with the RecA-ssDNA nucleoprotein filament in a manner grossly similar to that of wild-type UmuD is consistent with our finding that purified UmuD1012 can be cleaved by RecA and ssDNA in vitro.
FIG. 6.
The ability of UmuD or UmuD1012 to interact with RecA-ssDNA was measured in vitro essentially as described previously (14). Reaction mixtures (10 μl) contained 100 ng of M13mp18 ssDNA and 8 μg of RecA protein (enough to coat ∼50% of the ssDNA) as indicated, as well as 0.3 (lanes 3 and 8), 0.6 (lanes 4 and 9), or 1.2 (lanes 5 and 10) μg of UmuD or UmuD1012. After glutaraldehyde cross-linking (0.01% glutaraldehyde for 10 min at room temperature), aliquots of each reaction mixture were electrophoresed in 0.9% TGE (TGE is 25 mM Tris-HCl, 190 mM glycine, and 1 mM EDTA)–agarose gels (20), transferred to polyvinylidene difluoride, and probed with affinity-purified anti-UmuD-UmuD′ antibodies as described elsewhere (45, 46). The positions of free and cross-linked UmuD and UmuD1012 are indicated.
UmuD′1012 is defective in inhibition of RecA-ssDNA-facilitated homologous recombination.
Although UmuD′1012 was active in SOS mutagenesis (Fig. 3), it was only ∼60% as active as wild-type UmuD′, suggesting that Leu101 and Arg102 might contribute in some relatively modest way towards TLS. Another biological property of UmuD′ and UmuC is their ability to inhibit RecA-mediated homologous recombination (53, 59, 63). In vivo, this inhibition requires that their expression be elevated to higher than physiological levels (3). Sommer and coworkers have proposed that this antirecombination activity of UmuD′ together with UmuC is physiologically relevant and important for regulating the activity of RecA protein such that homologous recombination can be attenuated to permit TLS once UmuD′ and UmuC have accumulated to sufficient levels (59).
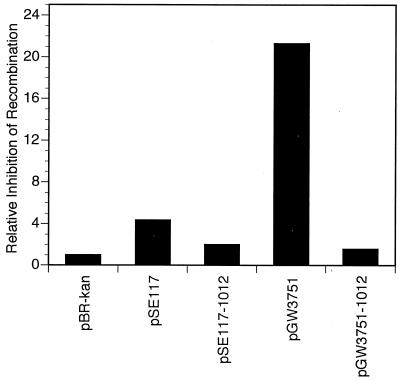
To investigate whether Leu101 and Arg102 are important for the antirecombination activity of UmuD′2C, we measured the effects of umuD1012C+ and umuD′1012C+ on homologous recombination relative to those of the wild-type operons. This was done by quantitating the respective efficiencies of P1vir-mediated transduction of the crcA280::Tn10 locus as a function of the various plasmid-carried umuDC operons. crcA is an unessential gene that confers camphor resistance (2). The host strain contained a deletion of the chromosomal umuDC locus and expressed elevated levels of the various plasmid-encoded Umu proteins by virtue of a lexA(Def) mutation. With this approach, we observed ∼4.3- and ∼21-fold inhibition of homologous recombination by wild-type umuDC and umuD′C, respectively, relative to that observed for the pBR322kan control (Fig. 7). Interestingly, whereas umuD1012C+ was only ∼2-fold less efficient at inhibiting homologous recombination than was umuD+C+, umuD′1012C+ was ∼13.5-fold less efficient than was umuD′C+ (Fig. 7), thus indicating that Leu101 and Arg102 of UmuD′ are important for its antirecombination activity. Consequently, although the inability of umuD′1012C+ to effectively antagonize homologous recombination might account for its modest defect in TLS (Fig. 3), the fact that it was ∼60% as active as wild-type umuD′C+ in SOS mutagenesis suggests that this antagonistic activity is not a strict requirement for TLS in vivo under our experimental conditions.
FIG. 7.
The effect of plasmids carrying either wild-type or mutant umuDC or umuD′C operons on RecA-dependent homologous recombination in vivo. The efficiency of P1vir-mediated transduction of the crcA280::Tn10 locus was measured as a function of the indicated plasmid-carried umuDC operon (40). The extent of inhibition of homologous recombination is expressed relative to that observed for the ΔumuDC control strain GW8024 bearing pBR322kan. The efficiency of transducing GW8024(pBR322kan) to tetracycline resistance by P1vir grown on the crcA280::Tn10 strain CAG12077 was 4.67 × 10−6 CFU per P1vir PFU. This value was normalized to 1.0, and the values obtained for GW8024 bearing the indicated plasmids were expressed relative to it as fold effects. For example, homologous recombination was inhibited ∼4.3-fold by pSE117 (umuD+C+) relative to inhibition by the pBR322kan control. Plasmid genotypes are as follows: pBR322kan, ΔumuDC; pSE117, umuD+C+; pSE117–1012, umuD1012C+; pGW3751, umuD′+C+; and pGW3751–1012, umuD′1012C+.
The fact that we saw less inhibition by umuD′C than that reported by Sommer and coworkers likely relates to differences in experimental design. Whereas Sommer and colleagues used either a lexA+ or a lexA1(Ind−) strain together with plasmids that expressed the Umu proteins by virtue of an operator constitutive mutation (59), we used a lexA(Def) strain. Consequently, since transcription of the recA gene is negatively regulated by LexA protein, our experimental conditions resulted in vastly higher levels of RecA protein. As maximal inhibition of RecA-mediated homologous recombination in vivo requires an optimal ratio of the umuDC gene products to RecA (3), the lesser degree of inhibition observed under our experimental conditions may be due to the vastly elevated levels of RecA protein, which is induced nearly 10-fold following derepression of the SOS regulon (60). However, despite these differences in experimental design, the two sets of results are similar in that the umuD′C gene products were significantly more effective than the umuDC gene products at inhibiting RecA-mediated homologous recombination.
DISCUSSION
Model for RecA-ssDNA-facilitated cleavage of UmuD.
In this report we describe our genetic and biochemical characterizations of the umuD1012 and umuD′1012 alleles, each encoding glycines in place of Leu101 and Arg102. Our findings that UmuD1012 undergoes RecA-ssDNA-facilitated cleavage less efficiently than wild-type UmuD, both in vivo and in vitro, and that umuD′1012C+ is severely impaired in inhibition of RecA-ssDNA-mediated homologous recombination in vivo indicate that Leu101 and Arg102 are important for interaction of the umuD gene products with RecA-ssDNA nucleoprotein filaments. In contrast, Leu101 and Arg102 are of only modest importance for TLS in vivo; umuD′1012C+ was ∼60% as proficient as wild-type umuD′C in SOS mutagenesis.
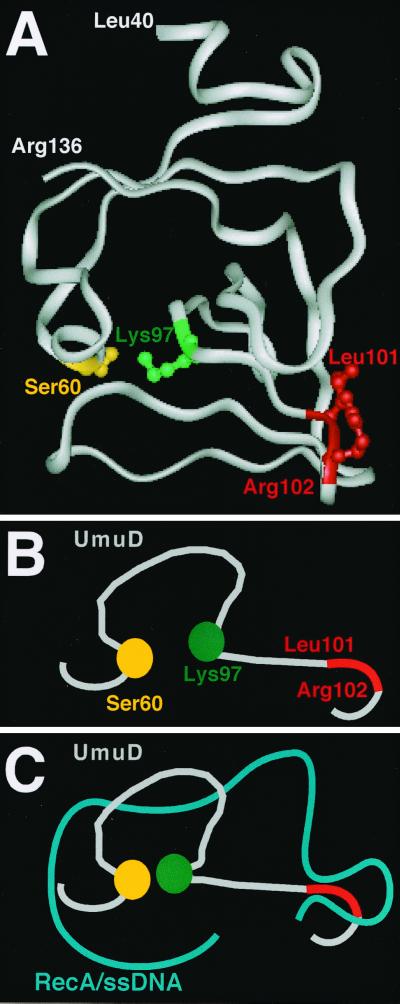
Our finding that UmuD1012 was not grossly affected in interaction with RecA-ssDNA nucleoprotein filaments in vitro, as measured by solution cross-linking, suggests that the deficiency of UmuD1012 in cleavage by RecA is due to a reduced ability of UmuD1012 to undergo the presumably subtle RecA-induced conformational change that leads to UmuD cleavage. In this respect, it is interesting that Leu101 and Arg102 are necessarily only a few residues away from Lys97, which is critical for UmuD cleavage (Fig. 8A). In the Lys-Ser dyad found both in the signal peptidases (48, 49) and in UmuD and related molecules (43), the exact position of the Lys clearly must be crucial for activating the Ser (Ser60 in UmuD) to act as a nucleophile in the cleavage reaction (33, 34). Thus, a plausible model to explain our results is that Leu101 and Arg102 interact with the RecA-ssDNA nucleoprotein filament in such a way that the loop containing Lys97 is pushed, thereby bringing Lys97 closer to Ser60 (Fig. 8B and C). This, together with numerous other contacts between the RecA-ssDNA nucleoprotein filament and both the amino-terminal arm (19, 31) and the carboxy-terminal globular domain of UmuD (32), is presumably sufficient for the proper alignment of the Ser60-Lys97 catalytic dyad with the cleavage site (located between residues 24 and 25) for activation of the intrinsic protease activity of UmuD. Consistent with this model, the recently solved solution structure of the UmuD′2 homodimer (Ferentz et al., unpublished data) indicates that in UmuD′2, the terminal group of Lys97 is not as ideally positioned to deprotonate Ser60 as suggested by the crystal (50) but rather is farther away from Ser60 and would thus require an external force to push them together.
FIG. 8.
A model to describe a possible role for Leu101 and Arg102 in RecA-ssDNA-facilitated cleavage of UmuD2. (A) Ribbon diagram of UmuD′ (residues 40 to 136) indicating the relative positions of the Ser60-Lys97 catalytic dyad and Leu101-Arg102 based on the crystal structure reported by Peat et al. (10, 50). (B and C) Cartoon representation of the Ser60-Lys97 catalytic dyad and Leu101-Arg102 region of UmuD (gray) alone (B) and in complex with RecA-ssDNA (blue) (C). In the absence of RecA-ssDNA (B), Lys97 is relatively far away from Ser60 (Ferentz et al., unpublished data). In the model (C), RecA-ssDNA pushes on Leu101-Arg102, leading to the repositioning of Lys97 relative to Ser60. This repositioning of Lys97, together with numerous other contacts between UmuD and RecA-ssDNA (19, 31, 32), leads to the activation of Ser60 and subsequent cleavage of UmuD to yield UmuD′. For simplicity, the cartoons depict the catalytic dyad and Leu101-Arg102 of a single UmuD protomer; its intradimer partner and the amino-terminal arms containing the cleavage sites are not shown. See the text for further details.
The UmuD-like mutagenesis proteins and the LexA-like transcriptional repressors utilize nonidentical sets of contacts with RecA-ssDNA to facilitate their cleavage.
Our finding that Leu101 and Arg102 are critical for cleavage of UmuD yet are not conserved among the structurally related transcriptional repressors that also undergo RecA-ssDNA-facilitated cleavage (1, 17) raises the question of why the UmuD-like and LexA-like subfamilies employ nonidentical sets of contacts to enable self-cleavage. Three possible explanations for this difference are as follows: (i) the UmuD-like proteins have a different and smaller amino-terminal domain than do the transcriptional repressors (52) and therefore require a different set of contacts with the RecA-ssDNA nucleoprotein filament to facilitate their cleavage; (ii) UmuD-like proteins undergo physical interactions with partner proteins (i.e., UmuC and its homologs) (4, 22, 70), as well as with other proteins involved in recombination (5, 32) and replication (62), that collectively demand that the UmuD-like proteins undergo interaction with RecA-ssDNA nucleoprotein filaments via a different set of contacts; and (iii) the use of a nonidentical set of contacts to differentially activate cleavage constitutes a device whereby the cell is able to achieve radically distinct cleavage kinetics for LexA relative to UmuD.
Given that LexA protein represses the SOS regulon while UmuD, although inactive in TLS (43), appears to act as part of a primitive cell cycle checkpoint control (41, 46), it seems reasonable that the cleavage kinetics of LexA and UmuD would differ. Whereas rapid cleavage of LexA in response to DNA damage is desirable for the timely derepression of the SOS-regulated gene products, the comparatively slow cleavage of UmuD presumably allows additional time for the repair of damaged DNA by accurate repair mechanisms such as nucleotide excision repair (46). Consequently, the timed delay in cleavage of UmuD to yield UmuD′ may have been optimized through evolution, in part through the use of a different set of contacts between UmuD and RecA-ssDNA nucleoprotein filaments, to allow maximum cell survival, first via a UmuD2C-dependent checkpoint control (41, 46) and second via UmuD′2C-dependent TLS (17). The finding by McDonald et al. that this comparatively low rate of UmuD self-cleavage is due primarily to its being a poor substrate and not to its being a poor enzyme (37, 38) is consistent with this model.
Our results, indicating that the UmuD-like mutagenesis proteins and the LexA-like transcriptional repressors utilize nonidentical sets of contacts with the RecA-ssDNA nucleoprotein filaments to promote self-cleavage, are consistent with earlier reports that RecA is similarly thought to use unique sets of contacts with the various repressors and UmuD to facilitate their cleavage. Four recA alleles, recA91 (G229S), recA430 (G204S), recA1730 (S117F), and recA1734 (R243L), exhibit differential coprotease activities towards the λcI repressor, the φ80 repressor, LexA, and UmuD (reviewed in reference 28). Although the RecA91 protein can promote the cleavage of the λcI repressor but not the φ80 repressor (8, 44), the RecA430 protein can promote the cleavage of the φ80 repressor but not the λcI repressor, LexA, or UmuD (5, 7). By contrast, the RecA1730 protein can promote cleavage of the λcI repressor, the φ80 repressor, and UmuD but not LexA (8). Finally, the RecA1734 protein can promote the cleavage of the λcI repressor and LexA but not the φ80 repressor or UmuD (8). Results similar to these have also been found with respect to differences in cleavage of UmuD and LexA in vivo by plasmid-carried recA alleles with P67D, P67R, E154D, or E154Q substitutions (27, 42).
Relationship between the roles of the umuD′C gene products in SOS mutagenesis and in inhibition of RecA-mediated homologous recombination.
In addition to its role in TLS, elevated levels of UmuD′ together with UmuC act to antagonize RecA-mediated homologous recombination in vivo (59). It has been suggested that this inhibition of homologous recombination might result from an effect on the formation of RecA-ssDNA filaments and may constitute a general mechanism by which RecA-mediated homologous recombination is attenuated to allow TLS (59, 63). Our finding that umuD′1012 is able to efficiently promote SOS mutagenesis, despite its inability to efficiently inhibit RecA-mediated homologous recombination in vivo, indicates that this inhibition of recombination is not a prerequisite for SOS mutagenesis under our experimental conditions. This interpretation is consistent with the finding that a recA mutation with an N113K change was refractory to the inhibitory effect of elevated levels of UmuD′2C but was nonetheless proficient in SOS mutagenesis (60). Although other recA alleles shown to be similarly refractory to the inhibitory effect of elevated levels of UmuD′2C were less active in SOS mutagenesis (60), it is possible that their defect in SOS mutagenesis was due to a deficiency of the mutant RecA proteins in TLS rather than to their reduced sensitivity to UmuD′2C.
Recent electron microscopy studies have suggested that UmuD′2C binds preferentially to the tip of the RecA-ssDNA filament but at higher levels can also bind within the helical groove of the filament (13). Based on this and other findings (53, 59, 60, 63), it has been suggested that the binding of UmuD′2C to the helical groove might competitively inhibit RecA-mediated homologous recombination, while its interaction with the tip of the RecA-ssDNA filament might deliver UmuD′2C to the site of the lesion. Further work will be required to see whether the UmuD′1012 protein is affected in its interaction with the groove and/or the tip of RecA-ssDNA nucleoprotein filaments. These and related studies may add additional insights into the molecular mechanism of RecA-ssDNA-mediated self-cleavage of UmuD2, thus allowing us to test further our model for the role(s) of Leu101 and Arg102 in self-cleavage (Fig. 8).
ACKNOWLEDGMENTS
We thank Mary Berlyn and Roel Schaaper for strains, Ann Ferentz for help in making Fig. 1 and 8, and members of our lab, in particular Brad Smith and Rachel Woodruff, for their comments on the manuscript.
This work was supported by Public Health Service grant CA21615 to G.C.W. from the National Cancer Institute. M.D.S. was supported by a fellowship (5 F32 CA79161-02) from the National Cancer Institute. M.K. carried out her research as part of the Undergraduate Research Opportunities Program at the Massachusetts Institute of Technology.
REFERENCES
- 1.Battista J R, Ohta T, Nohmi T, Sun W, Walker G C. Dominant negative umuD mutations decreasing RecA-mediated cleavage suggest roles for intact UmuD in modulation of SOS mutagenesis. Proc Natl Acad Sci USA. 1990;87:7190–7194. doi: 10.1073/pnas.87.18.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlyn M K B. Linkage map of Escherichia coli K-12, edition 10: the traditional map. Microbiol Mol Biol Rev. 1998;62:814–984. doi: 10.1128/mmbr.62.3.814-984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudsocq F, Campbell M, Devoret R, Bailone A. Quantitation of the inhibition of Hfr × F− recombination by the mutagenesis complex UmuD′C. J Mol Biol. 1997;270:201–211. doi: 10.1006/jmbi.1997.1098. [DOI] [PubMed] [Google Scholar]
- 4.Bruck I, Woodgate R, McEntee K, Goodman M F. Purification of a soluble UmuD′C complex from Escherichia coli. Cooperative binding of UmuD′C to single-stranded DNA. J Biol Chem. 1996;271:10767–10774. doi: 10.1074/jbc.271.18.10767. [DOI] [PubMed] [Google Scholar]
- 5.Burckhardt S E, Woodgate R, Scheuermann R H, Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc Natl Acad Sci USA. 1988;85:1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Ari R, Huisman O. Novel mechanism of cell division inhibition associated with the SOS response in Escherichia coli. J Bacteriol. 1983;156:243–250. doi: 10.1128/jb.156.1.243-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devoret R, Pierre M, Moreau P L. Prophage phi 80 is induced in Escherichia coli K12 recA430. Mol Gen Genet. 1983;189:199–206. doi: 10.1007/BF00337804. [DOI] [PubMed] [Google Scholar]
- 8.Dutreix M, Moreau P L, Bailone A, Galibert F, Battista J R, Walker G C, Devoret R. New recA mutations that dissociate the various RecA protein activities in Escherichia coli provide evidence for an additional role for RecA protein in UV mutagenesis. J Bacteriol. 1989;171:2415–2423. doi: 10.1128/jb.171.5.2415-2423.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elledge S J, Walker G C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983;164:175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- 10.Ferentz A E, Opperman T, Walker G C, Wagner G. Dimerization of the UmuD′ protein in solution and its implications for regulation of SOS mutagenesis [letter] Nat Struct Biol. 1997;4:979–983. doi: 10.1038/nsb1297-979. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez, De Henestrosa A R, Ogi T, Aoyagi S, Chafin D, Hayes J J, Ohmori H, Woodgate R. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol Microbiol. 2000;35:1560–1572. doi: 10.1046/j.1365-2958.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- 12.Fijalkowska I J, Dunn R L, Schaaper R M. Genetic requirements and mutational specificity of the Escherichia coli SOS mutator activity. J Bacteriol. 1997;179:7435–7445. doi: 10.1128/jb.179.23.7435-7445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank E G, Cheng N, Do C C, Cerritelli M E, Bruck I, Goodman M F, Egelman E H, Woodgate R, Steven A C. Visualization of two binding sites for the Escherichia coli UmuD′(2)C complex (DNA pol V) on RecA-ssDNA filaments. J Mol Biol. 2000;297:585–597. doi: 10.1006/jmbi.2000.3591. [DOI] [PubMed] [Google Scholar]
- 14.Frank E G, Hauser J, Levine A S, Woodgate R. Targeting of the UmuD, UmuD′, and MucA′ mutagenesis proteins to DNA by RecA protein. Proc Natl Acad Sci USA. 1993;90:8169–8173. doi: 10.1073/pnas.90.17.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freitag N, McEntee K. Affinity chromatography of RecA protein and RecA nucleoprotein complexes on RecA protein-agarose columns. J Biol Chem. 1988;263:19525–19534. [PubMed] [Google Scholar]
- 16.Friedberg E C, Gerlach V L. Novel DNA polymerases offer clues to the molecular basis of mutagenesis. Cell. 1999;98:413–416. doi: 10.1016/s0092-8674(00)81970-4. [DOI] [PubMed] [Google Scholar]
- 17.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 18.Gerlach V L, Aravind L, Gotway G, Schultz R A, Koonin E V, Friedberg E C. Human and mouse homologs of Escherichia coli DinB (DNA polymerase IV), members of the UmuC/DinB superfamily. Proc Natl Acad Sci USA. 1999;96:11922–11927. doi: 10.1073/pnas.96.21.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzzo A, Lee M H, Oda K, Walker G C. Analysis of the region between amino acids 30 and 42 of intact UmuD by a monocysteine approach. J Bacteriol. 1996;178:7295–7303. doi: 10.1128/jb.178.24.7295-7303.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann H J, Lyman S K, Lu C, Petit M A, Echols H. Activity of the Hsp70 chaperone complex—DnaK, DnaJ, and GrpE—in initiating phage lambda DNA replication by sequestering and releasing lambda P protein. Proc Natl Acad Sci USA. 1992;89:12108–12111. doi: 10.1073/pnas.89.24.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson R E, Washington M T, Prakash S, Prakash L. Bridging the gap: a family of novel DNA polymerases that replicate faulty DNA. Proc Natl Acad Sci USA. 1999;96:12224–12226. doi: 10.1073/pnas.96.22.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonczyk P, Nowicka A. Specific in vivo protein-protein interactions between Escherichia coli SOS mutagenesis proteins. J Bacteriol. 1996;178:2580–2585. doi: 10.1128/jb.178.9.2580-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato T, Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977;156:121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- 24.Kawashima H, Horii T, Ogawa T, Ogawa H. Functional domains of Escherichia coli recA protein deduced from the mutational sites in the gene. Mol Gen Genet. 1984;193:288–292. doi: 10.1007/BF00330682. [DOI] [PubMed] [Google Scholar]
- 25.Kenyon C J, Walker G C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci USA. 1980;77:2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight K L, Aoki K H, Ujita E L, McEntee K. Identification of the amino acid substitutions in two mutant forms of the recA protein from Escherichia coli: recA441 and recA629. J Biol Chem. 1984;259:11279–11283. [PubMed] [Google Scholar]
- 27.Konola J T, Guzzo A, Gow J B, Walker G C, Knight K L. Differential cleavage of LexA and UmuD mediated by recA Pro67 mutants: implications for common LexA and UmuD binding sites on RecA. J Mol Biol. 1998;276:405–415. doi: 10.1006/jmbi.1997.1531. [DOI] [PubMed] [Google Scholar]
- 28.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulaeva O I, Koonin E V, McDonald J P, Randall S K, Rabinovich N, Connaughton J F, Levine A S, Woodgate R. Identification of a DinB/UmuC homolog in the archeon Sulfolobus solfataricus. Mutat Res. 1996;357:245–253. doi: 10.1016/0027-5107(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 30.Kulaeva O I, Wootton J C, Levine A S, Woodgate R. Characterization of the umu-complementing operon from R391. J Bacteriol. 1995;177:2737–2743. doi: 10.1128/jb.177.10.2737-2743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee M H, Ohta T, Walker G C. A monocysteine approach for probing the structure and interactions of the UmuD protein. J Bacteriol. 1994;176:4825–4837. doi: 10.1128/jb.176.16.4825-4837.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee M H, Walker G C. Interactions of Escherichia coli UmuD with activated RecA analyzed by cross-linking UmuD monocysteine derivatives. J Bacteriol. 1996;178:7285–7294. doi: 10.1128/jb.178.24.7285-7294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little J W. LexA cleavage and other self-processing reactions. J Bacteriol. 1993;175:4943–4950. doi: 10.1128/jb.175.16.4943-4950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little J W. Mechanism of specific LexA cleavage: autodigestion and the role of RecA coprotease. Biochimie. 1991;73:411–421. doi: 10.1016/0300-9084(91)90108-d. [DOI] [PubMed] [Google Scholar]
- 35.Little J W, Edmiston S H, Pacelli L Z, Mount D W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci USA. 1980;77:3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh L, Walker G C. Cold sensitivity induced by overproduction of UmuDC in Escherichia coli. J Bacteriol. 1985;162:155–161. doi: 10.1128/jb.162.1.155-161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald J P, Frank E G, Levine A S, Woodgate R. Intermolecular cleavage by UmuD-like mutagenesis proteins. Proc Natl Acad Sci USA. 1998;95:1478–1483. doi: 10.1073/pnas.95.4.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald J P, Maury E E, Levine A S, Woodgate R. Regulation of UmuD cleavage: role of the amino-terminal tail. J Mol Biol. 1998;282:721–730. doi: 10.1006/jmbi.1998.2044. [DOI] [PubMed] [Google Scholar]
- 39.McLenigan M, Peat T S, Frank E G, McDonald J P, Gonzalez M, Levine A S, Hendrickson W A, Woodgate R. Novel Escherichia coli umuD′ mutants: structure-function insights into SOS mutagenesis. J Bacteriol. 1998;180:4658–4666. doi: 10.1128/jb.180.17.4658-4666.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 41.Murli S, Opperman T, Smith B T, Walker G C. A role for the umuDC gene products of Escherichia coli in increasing resistance to DNA damage in stationary phase by inhibiting the transition to exponential growth. J Bacteriol. 2000;182:1127–1135. doi: 10.1128/jb.182.4.1127-1135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nastri H G, Guzzo A, Lange C S, Walker G C, Knight K L. Mutational analysis of the RecA protein L1 region identifies this area as a probable part of the co-protease substrate binding site. Mol Microbiol. 1997;25:967–978. doi: 10.1111/j.1365-2958.1997.mmi533.x. [DOI] [PubMed] [Google Scholar]
- 43.Nohmi T, Battista J R, Dodson L A, Walker G C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci USA. 1988;85:1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogawa H, Ogawa T. General recombination: functions and structure of RecA protein. Adv Biophys. 1986;21:135–148. doi: 10.1016/0065-227x(86)90019-5. [DOI] [PubMed] [Google Scholar]
- 45.Ohta T, Sutton M D, Guzzo A, Cole S, Ferentz A E, Walker G C. Mutations affecting the ability of the Escherichia coli UmuD′ protein to participate in SOS mutagenesis. J Bacteriol. 1999;181:177–185. doi: 10.1128/jb.181.1.177-185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Opperman T, Murli S, Smith B T, Walker G C. A model for a umuDC-dependent prokaryotic DNA damage checkpoint. Proc Natl Acad Sci USA. 1999;96:9218–9223. doi: 10.1073/pnas.96.16.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Opperman T, Murli S, Walker G C. The genetic requirements for UmuDC-mediated cold sensitivity are distinct from those for SOS mutagenesis. J Bacteriol. 1996;178:4400–4411. doi: 10.1128/jb.178.15.4400-4411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paetzel M, Dalbey R E, Strynadka N C. Crystal structure of a bacterial signal peptidase in complex with a beta-lactam inhibitor. Nature. 1998;396:186–190. doi: 10.1038/24196. . (Erratum, 396:707.) [DOI] [PubMed] [Google Scholar]
- 49.Paetzel M, Strynadka N C. Common protein architecture and binding sites in proteases utilizing a Ser/Lys dyad mechanism. Protein Sci. 1999;8:2533–2556. doi: 10.1110/ps.8.11.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peat T S, Frank E G, McDonald J P, Levine A S, Woodgate R, Hendrickson W A. Structure of the UmuD′ protein and its regulation in response to DNA damage. Nature. 1996;380:727–730. doi: 10.1038/380727a0. [DOI] [PubMed] [Google Scholar]
- 51.Peat T S, Frank E G, McDonald J P, Levine A S, Woodgate R, Hendrickson W A. The UmuD′ protein filament and its potential role in damage induced mutagenesis. Structure. 1996;4:1401–1412. doi: 10.1016/s0969-2126(96)00148-7. [DOI] [PubMed] [Google Scholar]
- 52.Perry K L, Elledge S J, Mitchell B B, Marsh L, Walker G C. umuDC and mucAB operons whose products are required for UV light- and chemical-induced mutagenesis: UmuD, MucA, and LexA proteins share homology. Proc Natl Acad Sci USA. 1985;82:4331–4335. doi: 10.1073/pnas.82.13.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rehrauer W M, Bruck I, Woodgate R, Goodman M F, Kowalczykowski S C. Modulation of RecA nucleoprotein function by the mutagenic UmuD′C protein complex. J Biol Chem. 1998;273:32384–32387. doi: 10.1074/jbc.273.49.32384. [DOI] [PubMed] [Google Scholar]
- 54.Rehrauer W M, Lavery P E, Palmer E L, Singh R N, Kowalczykowski S C. Interaction of Escherichia coli RecA protein with LexA repressor. I. LexA repressor cleavage is competitive with binding of a secondary DNA molecule. J Biol Chem. 1996;271:23865–23873. [PubMed] [Google Scholar]
- 55.Reuven N B, Arad G, Maor-Shoshani A, Livneh Z. The mutagenesis protein UmuC is a DNA polymerase activated by UmuD′, RecA, and SSB and is specialized for translesion replication. J Biol Chem. 1999;274:31763–31766. doi: 10.1074/jbc.274.45.31763. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 57.Sassanfar M, Roberts J W. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J Mol Biol. 1990;212:79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- 58.Shinagawa H, Iwasaki H, Kato T, Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci USA. 1988;85:1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sommer S, Bailone A, Devoret R. The appearance of the UmuD′C protein complex in Escherichia coli switches repair from homologous recombination to SOS mutagenesis. Mol Microbiol. 1993;10:963–971. doi: 10.1111/j.1365-2958.1993.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 60.Sommer S, Boudsocq F, Devoret R, Bailone A. Specific RecA amino acid changes affect RecA-UmuD′C interaction. Mol Microbiol. 1998;28:281–291. doi: 10.1046/j.1365-2958.1998.00803.x. [DOI] [PubMed] [Google Scholar]
- 61.Steinborn G. Uvm mutants of Escherichia coli K12 deficient in UV mutagenesis. I. Isolation of uvm mutants and their phenotypical characterization in DNA repair and mutagenesis. Mol Gen Genet. 1978;165:87–93. doi: 10.1007/BF00270380. [DOI] [PubMed] [Google Scholar]
- 62.Sutton M D, Opperman T, Walker G C. The Escherichia coli SOS mutagenesis proteins UmuD and UmuD′ interact physically with the replicative DNA polymerase. Proc Natl Acad Sci USA. 1999;96:12373–12378. doi: 10.1073/pnas.96.22.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szpilewska H, Bertrand P, Bailone A, Dutreix M. In vitro inhibition of RecA-mediated homologous pairing by UmuD′C proteins. Biochimie. 1995;77:848–853. doi: 10.1016/0300-9084(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 64.Tang M, Pham P, Shen X, Taylor J-S, O'Donnell M, Woodgate M, Goodman M F. Roles of E. coli DNA polymerase IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature. 2000;404:1014–1018. doi: 10.1038/35010020. [DOI] [PubMed] [Google Scholar]
- 65.Tang M, Shen X, Frank E G, O'Donnell M, Woodgate R, Goodman M F. UmuD′(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci USA. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walker G C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- 67.Walker G C. Skiing the black diamond slope: progress on the biochemistry of translesion DNA synthesis. Proc Natl Acad Sci USA. 1998;95:10348–10350. doi: 10.1073/pnas.95.18.10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W B, Tessman E S. Evidence that the recA441 (tif-1) mutant of Escherichia coli K-12 contains a thermosensitive intragenic suppressor of RecA constitutive protease activity. J Bacteriol. 1985;163:407–409. doi: 10.1128/jb.163.1.407-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woodgate R. A plethora of lesion-replicating DNA polymerases. Genes Dev. 1999;13:2191–2195. doi: 10.1101/gad.13.17.2191. [DOI] [PubMed] [Google Scholar]
- 70.Woodgate R, Rajagopalan M, Lu C, Echols H. UmuC mutagenesis protein of Escherichia coli: purification and interaction with UmuD and UmuD′. Proc Natl Acad Sci USA. 1989;86:7301–7305. doi: 10.1073/pnas.86.19.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Lee M H, Walker G C. p-Azidoiodoacetanilide, a new short photocrosslinker that has greater cysteine specificity than p-azidophenacyl bromide and p-azidobromoacetanilide. Biochem Biophys Res Commun. 1995;217:1177–1184. doi: 10.1006/bbrc.1995.2893. [DOI] [PubMed] [Google Scholar]