Abstract
Morning symptoms are common in chronic obstructive pulmonary disease (COPD). Many COPD patients consider the morning as the most troublesome part of the day, in which they experience more symptoms and physical activity limitations.
To systematically report evidence of the association between morning symptoms and physical activity in COPD patients, a literature search was conducted using relevant MESH terms and text words in PubMed, Embase, Web of Science, COCHRANE, CINAHL and PsycINFO. Quality of the articles was assessed with validated checklists.
Eight studies were included. Morning symptoms were present in 39.8–94.4%. In 37.0–90.6% of all COPD patients, there was an association between physical activity and morning symptoms. However, causality could not be proved. Morning symptoms were associated with a sedentary lifestyle (p<0.05). Treatment in line with the guidelines improved the degree of activity limitations due to morning symptoms (p<0.0001).
Across all disease stages, COPD patients experience morning symptoms which are negatively associated with physical activity. Physicians should consider morning symptoms as a treatment goal. Pharmacotherapy may improve the degree of activity limitations due to morning symptoms. More objective research should focus on symptoms, activity limitations and physical inactivity of COPD patients, especially in the morning.
Short abstract
Association between morning symptoms and physical activity in COPD http://ow.ly/SJQi301fvdD
Introduction
Chronic obstructive pulmonary disease (COPD) has a huge socio-economic impact. COPD is the fourth cause of years of life lost according to the latest findings of the Global Burden of Disease Study [1] whereas the World Health Organization stated COPD as the third leading cause of death in the period 2000–2012 [2]. COPD is not a curable disease; therefore, the main focus of pharmacotherapy is to limit or reduce symptoms as much as possible and to prevent acute exacerbations. In addition, reduction of mortality is an important treatment goal. However, so far, no pharmacological intervention has been able to reduce mortality in COPD patients [3]. The actual consensus report of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend non-pharmacological interventions such as smoking cessation, avoiding exposure to air pollution and increased physical activity as well as pharmacological treatment and adequate use of medication [4]. In current guidelines, severity of disease is categorised by lung function as well as symptoms and the occurrence of acute exacerbations. Symptoms have especially been found to have an important impact in overall health status [5] and are therefore of importance from the patient's perspective. In addition, there is a strong association between increased shortness of breath and difficulty with physical activities [6, 7]. Frequently, symptoms occur in the morning, resulting in limitations of morning activities and often in work absenteeism as well [8]. Despite the large impact of morning symptoms on activities and quality of life, morning symptoms are not a focus of current treatment guidelines and have not been mentioned in the official European Respiratory Society statement on physical activity in COPD [3]. Still, there is growing awareness of the impact of COPD symptoms in the morning and an increasing number of tools to evaluate morning symptoms have been developed [8]. To further investigate the impact of morning symptoms on patients with COPD and especially on physical activity, we performed a structural literature review of the current knowledge of the association between morning symptoms and physical activity in patients with COPD.
Materials and methods
Data sources and searches
An electronic search of the literature was performed on October 27, 2015, using relevant MESH terms and text words. The search was performed using PubMed, Embase, Web of Science, COCHRANE, CINAHL and PsycINFO. There was no limitation for date of publication. All types of studies were included, except meeting abstracts, reviews and articles from non-peer-reviewed journals. Original complete texts in all languages were used and the actual studies may have been conducted in any country. The full search is available in the supplementary material.
Study selection
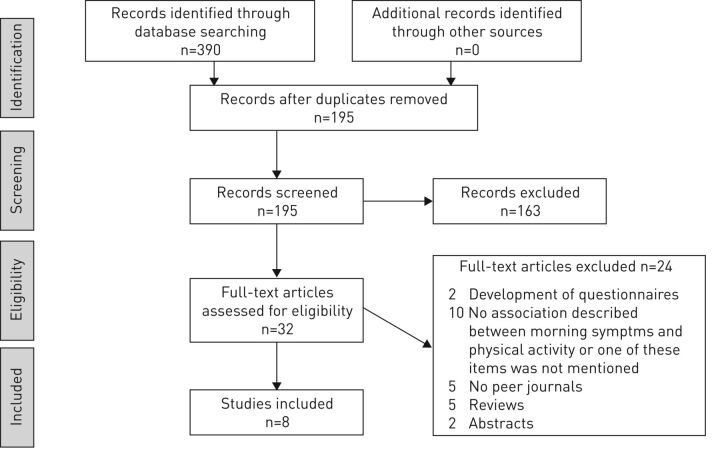
Two authors (A.R. van Buul and M.J. Kasteleyn) screened the titles to include the relevant articles. Studies were included if the study population consisted of patients with the diagnosis COPD. Studies were excluded when the predefined outcomes did not comprise either morning symptoms or physical activity. After this first screening, two authors (A.R. van Buul and M.J. Kasteleyn) screened the abstracts to only include relevant articles (figure 1). Abstracts that did not include both morning symptoms and physical activity were excluded. If an abstract suggested that a substantial part of the article concerned morning symptoms and physical activity, it was included. A third author (N.H. Chavannes) reviewed abstracts when there was a disagreement between A.R. van Buul and M.J. Kasteleyn. Of the remaining articles, A.R. van Buul and M.J. Kasteleyn read the full-texts. The same inclusion and exclusion criteria as for the abstracts were used for the full-texts.
Figure 1.
Study flow diagram with the use of the official PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart.
Synthesis and report strategy
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram [9] was used to give insight into the amount of excluded articles. To review the current knowledge of morning symptoms and physical activity in COPD patients, the occurrence of morning symptoms, the association of morning symptoms with physical activity limitations and the impact of medication on activity limitations associated with morning symptoms were evaluated. The occurrence of morning symptoms was studied in three ways. First, the incidence of morning symptoms in COPD was examined to determine the frequency of these symptoms. The occurrence of morning symptoms in all COPD patients and the type of morning symptoms was assessed in studies in which all COPD patients were included regardless whether they experienced symptoms or not. Second, in studies with patients with morning symptoms, the type of morning symptoms were examined to determine the most common. Third, in studies with patients with symptoms during any part of the day, the percentage of patients that reported morning as the worst time of the day was determined. Furthermore, the association between morning symptoms and physical activity was assessed. Thereafter, the impact of medication on physical activity limitations that were associated with morning symptoms was evaluated. No further statistical analysis or meta-analysis has been conducted, as all studies had different endpoints and the endpoints were measured with different tools.
Quality assessment
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [10] was used to assess the quality of observational, case control and cohort studies. The Consolidated Standards of Reporting Trials (CONSORT) checklist [11] was used for the quality assessment of randomised controlled trials. There is no consensus when an article may be indicated as high or low; however, previous research has shown that observational studies published in high-quality journals contain an average of 69% of the STROBE items [12]. Consistent with this study and other studies who support this interpretation [13, 14], a minimum of 15 (69%) reported items out of 22 indicated “high quality”, and lower than 15 out of 22 indicated “moderate-to-low quality”.
Results
Search strategy
The search identified 390 articles. After removing duplicates, 195 individual articles remained. After screening titles, 117 articles were considered relevant. After reading abstracts, 32 articles remained. Full texts of these articles have been read and 24 articles have been excluded. Eight remaining studies were included in this systematic review (figure 1). Of the eight included studies, seven studies were observational studies and one was a randomised controlled trial. The quality of the studies was assessed using the STROBE checklist. The scores ranged from 14 to 18 out of 22 points. This means that six observational studies had high quality and one observational study had a moderate quality. One article was a randomised controlled trial and was assessed by the CONSORT checklist. This article scored 17 out of 25 points. This is a study that pooled analysis from two studies. The full methods of the two studies are not described in the present study. When using the methods of the original studies, the study would score a higher amount of points. None of the included articles had a low quality (supplementary table S2).
General findings
The oldest study was published in 2009 and the most recent in 2015. The number of patients included in the studies ranged from 133 to 3394. Patients participating were at least 30 years old, 5.3–60.5% were female. In all studies, at least 24% of patients were current smokers. All stages of COPD were represented. The study population and the general conclusion of each study are reported in table 1.
Table 1.
Overall conclusion and quality of included studies
| First author [ref.] | Study design | Participants' characteristics at baseline | Overall conclusion | Quality |
| Bateman [ 15 ] | Pooled analysis from two phase III double-blind, randomised, parallel-group active- and placebo-controlled studies | n=3394; age ≥40 years; stable moderate-to-severe COPD | Aclidinium/formoterol 400/12 μg significantly improves 24-h symptom control compared with placebo or aclidinium or formoterol alone. The frequency of exacerbations was also reduced compared with placebo | 17/25#,¶ |
| Stephenson [ 20 ] | Cross-sectional survey study | n=752; 60.5% female; age ≥40 years; COPD plus at least one pharmacy claim for maintenance COPD medication | The majority of the patients with night time or morning symptoms experience at least three distinct types of symptoms a week. Approximately half of them consider their symptoms to be moderate to severe. They felt that their symptoms had impact on their sleep and morning activities, and they were anxious | 18/22+ |
| Miravitlles [ 16 ] | Observational study | n=727; 34.2% female; age ≥40 years; current of former smokers; stable mild-to-very-severe COPD | More than half of COPD patients experience symptoms throughout the whole day. There was a significant association between night time, early morning and daytime symptoms. In each period, symptoms were associated with worse patient-reported outcomes | 18/22+ |
| O’Hagan [ 19 ] | Observational study | n=811; 44% female; age 30–70 years; COPD diagnosed by a physician; at least one morning symptom | Morning symptoms can severely interfere with COPD patients’ ability to perform tasks throughout the day. Half of the patients had made changes in their morning routines | 15/22+ |
| Roche [ 17 ] | Cross-sectional observational study | n=1489; 34.3% female; age ≥40 years; with a history of smoking, airflow obstruction and the diagnosis of COPD | 39.8% of the COPD patients experience morning symptoms. Morning symptoms are associated with poorer health status, impaired daily activities and increased risk of exacerbations | 16/22+ |
| Kim [ 18 ] | Prospective non-interventional and observational study | n=133; 5.3% female; age >45 years; with a history of smoking; stable severe-to-very severe COPD | 57% of COPD patients experience limitation in their activities due to morning symptoms. These patients also have more prevalent and severe COPD symptoms | 14/22+ |
| Kessler [ 22 ] | Cross-sectional observational study | n=2441; 21.5% female; age >45 years; with a history of smoking; stable severe-to-very severe COPD | Patient-perceived COPD symptoms vary over the day and the week, and have impact on activities. The morning was considered the worst time of the day | 16/22+ |
| Partridge [ 21 ] | Quantitative internet interviews | n=803; 44% female; age ≥40 years; with a history of smoking; all stages of COPD | COPD are worst during the morning. Many patients consider the impact of COPD on morning activities to be extensive | 17/22+ |
COPD: chronic obstructive pulmonary disease; #: CONSORT (Consolidated Standards of Reporting Trials) was used as a tool to assess quality; ¶: pooled analysis from two studies; +: STROBE (Strengthening the Reporting of Observational studies in Epidemiology) was used as a tool used to assess quality.
Morning symptoms
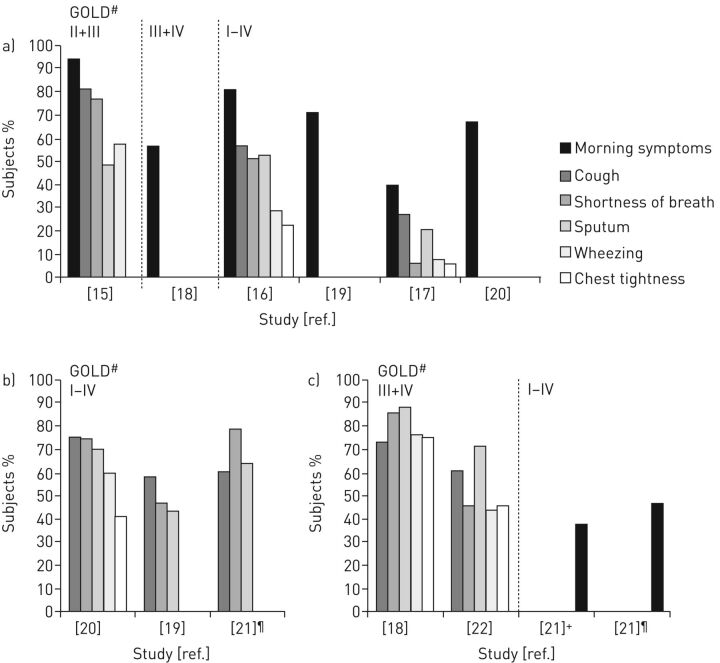
When analysing all patients with COPD, the most common morning symptoms were cough, sputum production and shortness of breath [15–17]. In this patient group the percentage of morning symptoms varied between 39.8 and 94.4% (figure 2a) [15–20]. When only assessing the group of patients with morning symptoms, again cough, sputum production and shortness of breath were frequently detected (figure 2b) [19–21]. In symptomatic severe and very severe COPD patients, it was found that the morning was the worst time of the day for the symptom sputum production (in 70.9–87.2%), for cough (in 60.1–72.6%) and for shortness of breath (in 45.4–85.1%) (figure 2c) [18, 21, 22]. More than half of patients considered the severity of their symptoms to be mild to moderate [15, 16, 20]. The association between COPD severity and morning symptoms was analysed. Interestingly, morning symptoms could be detected across all stages of disease. One study that included COPD patients with all stages of the disease, concluded that morning symptoms were associated with the severity of COPD [16]. However, when only patients with severe and very severe COPD were included, no difference in pulmonary function between patients with or without morning symptoms could be detected [18]. Nevertheless, when patients over all disease stages were included, a significantly decreased pulmonary function could be detected in patients with morning symptoms compared with those without morning symptoms [17]. Mostly, patients do not experience solely morning symptoms, but might experience daytime and night-time problems as well [16, 17, 20]. Most common night-time problems were night-time symptoms, sleep disturbances and early awaking [15–18, 20, 22].
Figure 2.
Occurrence of morning symptoms. a) In all studied chronic obstructive pulmonary disease (COPD) patients. b) Occurrence of different morning symptoms in COPD patients experiencing symptoms. c) Symptomatic COPD patients who report the morning as worst time of the day for that symptom (results for at waking and the rest of the morning are combined). #: Classification of airflow limitation according to Global initiative for Chronic Obstructive Lung Disease (GOLD); ¶: “severe” was defined in this study as regular use of COPD medication plus a third level of breathlessness or above using the Medical Research Council dyspnoea scale and one or more exacerbations in the preceding 12 months +: in all included COPD patients.
The association between morning symptoms and physical activity
All studies showed that COPD patients who experience morning symptoms also report a negative impact on physical activity. When analysing all patients with COPD, 37–90.6% of patients experienced physical activity limitations that were associated with morning symptoms (table 2) [15, 18, 21]. When just patients with morning symptoms were assessed, 34–79% of these patients reported limitations in morning activities due to these symptoms [19, 20]. Most mentioned limited activities were getting up, taking a shower and dressing [18, 19, 22]. Reported routine activities took at least 10 min longer compared with the situation before morning symptoms had increased [19]. Half of patients reported to have made changes to their morning routines because of morning symptoms [19]. Patients' core coping strategies were doing things slowly and taking more breaks [21]. Shortness of breath was the symptom most strongly correlated with the reduced ability to perform tasks [21]. One of the studies, which included only patients with severe to very severe COPD, reported that 9.4% was completely unable to exercise outside, 24.7% managing up to 30 min·day−1, 32.9% between 30 and 60 min·day−1 and 30.9% reported walking outdoors longer than 1 h·day−1 [22]. In another article, 30% of patients described themselves as sedentary, 38% as moderately active and 34% as active. There was a significant association between morning symptoms and patients' self-reported physical activity. Sedentary patients experience more morning symptoms and more symptoms the rest of the day when compared with moderately active or active patients (p<0.05) [16], although it was not reported whether inactivity was actually a result of morning symptoms. Besides morning activities, normal daily activities are also influenced by morning symptoms [17, 19, 22]. More detailed information about the sort of activities is given in supplementary table 3.
Table 2.
Influence of morning symptoms on physical activity
| First author [ref.] | Stage of COPD | Definition of the morning | Method to evaluate morning symptoms and activity limitations | Physical activity limitation associated with morning symptoms | Self-reported limitations | Conclusion |
| Bateman [ 15 ] | Moderate to severe | As described in the EMSCI and NiSCI | Questionnaires (EMSCI and NiSCI) for the patients | 90.6% of all patients with COPD | NA | Most COPD patients with morning symptoms considered that their symptoms affect their morning activities |
| Stephenson [ 20 ] | All stages | Time of getting out of bed and approximately 11:00 h | A 30-min questionnaire for patients about morning symptoms and the impact on morning activities | 60.4%# | Work§ | More than half of patients considered that their symptoms affect their morning activities |
| Miravitlles [ 16 ] | All stages | Time of getting out of bed and approximately 11:00 h | Patients filled out a Night-time, Morning and Daytime Symptoms of COPD questionnaire, developed by the sponsor | Patients who are sedentary experience more symptoms in any part of the day (also in the morning); p<0.05 | NA | In each part of the day (morning, daytime, night time) there was an significant association between symptoms and a low physical activity level |
| O’Hagan [ 19 ] | All stages | Not defined | Online questionnaire consisting predefined questions for patients | 34–79%# have problems with common morning activities; 56–70%# with more physically demanding activities | Self-care, domestic activities and work§ | Morning symptoms can severely compromise patients’ ability to perform, even simple tasks. Half of the patients had made changes to their morning routines |
| Roche [ 17 ] | All stages | Symptoms that are present when getting up in the morning, thus those symptoms present on waking, rather than those persisting through the morning | Questionnaires with predefined questions. Physicians gave information about severity grade of the symptoms; patients about the impact on daily life | Impact on normal activities was higher in those with morning symptoms (3.96 versus 3.29+; p=0.007) | Self-care and work§ | Impact on daily activities was significantly higher in patients with morning symptoms than without |
| Kim [ 18 ] | Severe to very severe | Not defined | Patients filled out the CSQ. Those who reported morning symptoms, subsequently completed the MAQ | 57% of all patients with COPD | Self-care and domestic activities§ | 57% of COPD patients had considerable impact on their morning activities |
| Kessler [ 22 ] | Severe to very severe | In the morning, after waking up and later in the morning | Interview over the telephone. Predefined questions developed by the sponsor | 35.4–41.0% of patients that experience any symptom felt that morning symptoms affect morning activities | Self-care§ | There was an association between morning symptoms and the impact on activities |
| Partridge [ 21 ] | All stages | From the time they woke up until they were dressed, had breakfast and were ready to start the day | Predefined questions were answered by the patient by an internet interview | 37% of all COPD patients and 73% of the severe¶ COPD patients regarded problems associated with morning routines as bothersome. 74% of all COPD patients and 96% of the severe¶ patients reported that they took longer to complete their morning routines | Self-care and domestic activities§ | Many patients considered the impact of COPD on morning activities to be extensive |
COPD: chronic obstructive pulmonary disease; CSQ: clinical symptom questionnaire; EMSCI: early-morning symptoms of COPD instrument; MAQ: morning activity questionnaire; NiSCI: night-time symptoms of COPD instrument. #: in patients with morning symptoms; ¶: “severe” was defined in this study as regular use of COPD medication plus a third level of breathlessness or above using the Medical Research Council dyspnoea scale and one or more exacerbations in the preceding 12 months; +: measured on a 7-point Likert scale of 0=no impact to 7=constant impact; §: more detailed information in supplementary table S3.
Table 3.
Impact of medication on morning symptoms and physical activity
| First author [ref.] | Medication | Morning symptoms | Physical activity limitation associated with morning symptoms | Effect medication on morning symptoms | Effect intervention/medication on physical activity limitation due to morning symptoms |
| Bateman [ 15 ] | Aclidinium bromide/formoterol | 94.4% of all patients | 90.6% of all patients | FDC 400/12 µg on severity scores: −0.23 units (−17.0%); aclidinium 400 µg: −0.14 units (−10.7%); formoterol 12 µg: −0.17 units (−13.6%) p<0.0001 versus aclidinium and p<0.01 versus formoterol#. Individual morning symptoms: p<0.05 versus aclidinium for cough and difficulty bringing up phlegm, and versus both monotherapies for wheezing and shortness of breath | Improvements in limitation of early morning activities: p<0.05 versus aclidinium and p<0.05 versus formoterol |
| O’Hagan [ 19 ] | Patients were allowed to select any of their applied medication | Morning symptoms was an inclusion criterion in this study | Impact on normal activities was higher in those with morning symptoms compared to those without (3.96 versus 3.29; p<0.007) | 79% of COPD patients who feel medications provides relief from symptoms in the morning enough | 33% of patients considered “improvement of ability to carry out morning activities” a key treatment goal. 21% of patients feel medication provides improvement in the ability to carry out morning activities |
| Kim [ 18 ] | No standard treatment for COPD was defined by the study protocol | 57% of all patients | 57% of all patients | LAMA and ICS plus LABA were used significantly less frequent in patients with morning symptoms. LAMA was a preventive factor for the presence of morning symptoms | Severity of all morning activities were significantly reduced after 2 months follow-up |
COPD: chronic obstructive pulmonary disease; FDC: fixed-dose combination; LAMA: long-acting muscarinic antagonists; ICS: inhaled corticosteroids; LABA: long-acting β2-agonist. #: symptom severity measured on a score from 0 (no symptoms) to 4 (very severe symptoms).
Treatment to improve activity limitations that are associated with morning symptoms
An internet survey stated that about 79% of COPD patients report that medication provides relief of symptoms in the morning [19]. A prospective non-interventional observational study in which patients were treated following present guidelines with pharmacological or non-pharmacological therapies, showed that the impairment of all activities that were associated with morning symptoms was significantly reduced (table 3) [18]. Recently, data from two phase III, double-blind, randomised, parallel-group active- and placebo-controlled studies were pooled to compare the effect of a fixed dose combination of long acting β2-agonist (LABA) and long-acting muscarinic antagonist (LAMA) versus monotherapy or placebo on morning symptoms and associated physical activity limitations [15]. In this analysis, there was a significant improvement of morning symptoms and the individual symptoms cough, wheezing, dyspnoea and sputum production in the morning after treatment with the fixed LABA/LAMA combination. In the same study, the fixed LABA/LAMA combination significantly improved severity scores for limitations of morning activities that are associated with morning symptoms when compared with LABA and LAMA alone (both p<0.05) (table 3). Furthermore, in an internet survey, it was found that improvement in the ability to perform morning activities is one of the patients' expectations of treatment [19]. Nevertheless, patients reported that physicians were unlikely to ask about morning symptoms and the ability to perform morning activities [19, 21]. Hence, physicians were unlikely to discuss how to cope with these symptoms and to describe what to expect from therapy for activity limitations.
Discussion
The aim of the present study was to systematically review the current evidence of the association between morning symptoms and physical activity in COPD patients. The most dated study included in this review was from 2009, suggesting that interest for the combination of these topics is relatively recent. None of the studies had a low quality when scored with the STROBE and the CONSORT tools. Therefore, all eligible articles were included in the present review. It was found that morning symptoms are common in patients with COPD across all stages. Importantly, these symptoms are associated with impaired physical activity.
Morning symptoms in COPD were detected frequently in different studies. However, the percentage of patients with symptoms varied widely. One explanation might be that different definitions for morning were used. In the studies a lower percentage of morning symptoms could reflect just the symptoms immediately on awaking and a higher percentage of morning symptoms reflects the symptoms on awaking plus the symptoms that develop throughout the rest of the morning. There are some tools developed to evaluate morning symptoms [23–25], but none of them have been adequately validated. In the included articles, mostly self-developed questionnaires were used, and only one article used one of the previously mentioned tools. In some studies, only patients with severe and very severe COPD were included; while, in other studies, mild to moderate COPD patients were also included. It is of note that morning symptoms were apparent in all stages of COPD and that activity limitations associated with morning symptoms were also detected in less severe COPD. This is in line with studies showing that physical activity of patients with COPD is already impaired in early disease stages [26]. Therefore, the difference in percentage of morning symptoms cannot be explained by the different study populations of the included studies. Currently, there is lack of evidence that morning symptoms increase as the degree of severity of COPD increases. In three included studies [19–21], lung function was unknown. In one study, the authors made their own definition for “severe COPD” that was not linked to pulmonary function [21]. In other studies the authors did not analyse the patients by pulmonary function [15, 19, 22]. One further explanation for the diverse results might be that questionnaires are not always filled out by the patients. In some studies the physician scored the severity of symptoms, which could further explain the wide range of incidences of morning symptoms. Previous research has shown that there is a low level of concordance in assessing disease impact between COPD patients and physicians [27]. Furthermore, it can also be stipulated that differences in methods of data collection (electronically or by paper) could affect the results. However, we believe that the impact of different data collection methods is minimal, since data administered on paper are quantitatively comparable with measures administered on an electronic device [28, 29]. There are some disorders and conditions that may affect symptoms in the morning as well. Mostly, patients do not experience only morning symptoms, but experience daytime and night-time symptoms as well. Symptoms during daytime or night-time negatively influence health status, anxiety and depression levels, sleep quality, physical activity levels and adherence to medication. Patients with morning symptoms were more likely to use oxygen in the previous week [20], to have poorer health status [16, 17, 20], to experience exacerbations [16, 17, 20], have a worse sleep quality [16] and have higher anxiety and depression levels [16]. It is not possible yet to distinguish whether morning symptoms are a particular phenotype in COPD or whether it is a feature of the disease.
A large group of COPD patients have physical activity limitations that are associated with morning symptoms. This raises the question whether people with morning symptoms are more inactive or inactive patients have more morning symptoms. Until now, prospective comparative studies using objective measures of physical activity levels in relation to morning symptoms are lacking. Existing evidence about physical activity and morning symptoms mostly comes from observational studies. Thus, causality between morning symptoms and limitations of activities cannot be proven. It is has been shown that people with COPD are more physically inactive compared with their healthy peers [30, 31]. However, it is not completely clear if the relationship between the level of inactivity is solely a result of COPD or if inactivity is a risk factor and contributes to the development of COPD itself. Indeed, patients report frequently that dyspnoea impairs everyday tasks [32]. Especially in the morning while performing tasks, patients' main coping strategies are doing things slowly and taking frequent breaks [21]. This is in line with previous research demonstrating a low walking speed is typical for COPD patients compared with healthy controls [3]. Therefore, it has been suggested that patients with COPD may be in a downward spiral of symptom-induced inactivity, as well as in the early stages of disease [30, 31]. However, impaired physical inactivity is not an exclusive feature of patients with COPD but has been reported in many different chronic diseases such as stroke, kidney disease, diabetes, coronary heart disease, hypertension and obesity [33]. This suggests that also other factors such as behavioural, genetic, social, environmental, cultural and policy factors could contribute to impaired physical activity in chronic disease [3].
Previous research has shown that the impairment in activity is progressive as activity levels in COPD patients further decreases over time [26, 34]. In only two of the studies included in this review, patients with COPD reported their activity levels [16, 22]. Interestingly, their activity levels were higher compared with previous findings reported in literature [34]. A potential explanation may be that physical activity was self-reported in the studies included in our review, while the lower levels in the literature were objectively measured by a validated accelerometer. Of self-reported physical activity, it is known that it is often misjudged by participants; patients tend to underestimate standing time and overestimate walking time [35]. Therefore, the use of objective measures, such as accelerometers, to adequately assess physical activity is recommended [36].
The present review showed that limitations in physical activity due to morning symptoms can be significantly reduced with medical treatment. This conclusion is based on two observational studies and one study that pooled two large phase III, double-blind, randomised, parallel-group active- and placebo-controlled study. Despite two out of three studies being observational studies without a prespecified intervention and a change of recall bias, all conclusions pointed in the same direction: medical treatment results in a reduction of physical activity limitations and a reduction of morning symptoms. This is in line with other studies that found positive effects of pharmacotherapy on symptoms and morning routines [37–39]. These studies were not included in this review, since the authors did not examine the association between morning symptoms and physical activity. Therefore, it is unclear whether the improvements in physical activities were the result of improvements in symptoms or the other way around, or if these effects are independent from each other.
One of the potential limitations of this review is that the search strategy could have missed some articles about morning symptoms if morning symptoms were not adequately highlighted in the abstract. However, this is unlikely because cross-references of the included articles were carefully checked as well. Another limitation of this review is the use of the STROBE and CONSORT checklists as a quality checklist for the included studies. These checklists were not developed to score the quality of reviews, but were developed as checklists to strongly report original studies. Therefore, there is no consensus when an article may be indicated has high or low and arbitrary rules are used to assess the quality. Another limitation of the included studies could be that some patients included in the studies had asthma. In most included articles, asthma was an exclusion criterion and only one study did not exclude patients with other lung diseases. In that study, 37% of the included patients had self-reported asthma as a co-diagnosis [19]. Also, it is possible that patients in the included studies have been misdiagnosed or wrongly coded as COPD patients. In one included study, 12.9% of patients had never smoked [20], which makes the diagnosis of COPD much more unlikely. It is presumable that the more asthma patients were wrongly included, the more morning symptoms occurred, since circadian variation of lung function and symptoms is well described in asthma [40]. Nevertheless, in COPD patients, symptoms vary as well, even with daily and weekly variation [21, 41]. More than half of patients experience COPD symptoms throughout the whole 24-h day [16] and only a few patients experienced solely morning symptoms. The morning is the most troublesome part of the day with limitations in activities, probably due to circadian variation in lung function or because the morning is the most active period of the day. The night is the second most troublesome part of the day for patients with COPD [41, 42]. Another limitation is that most included articles were observational studies, with potential recall bias; patients might inadequately report some events or symptoms. Two of the observational studies were internet surveys [19, 21]. Important limitations of internet surveys are that data is self-reported and there might be selection bias, since not all patients have access to internet, and internet access is influenced by age, social status and employment status. This may result in a younger population and a higher socio-economic status. Two of the included studies [19, 21] recruited patients from consumer panels. This might cause “self-selection bias” and the included patients are probably not representative for the whole COPD group. In one study [18], 94.7% of included patients were male and results of this study cannot be generalised to females.
In conclusion, across all disease stages, COPD patients experience morning symptoms that are negatively associated with physical activity. However, it is not possible to prove causality yet, because of the observational designs of these studies. The important finding that morning symptoms are negatively associated with morning symptoms suggests that physicians should include the evaluation of morning symptoms in their clinical assessment and they should include the control of morning symptoms as a goal of treatment, since there is evidence that treatment has positive impact on morning symptoms. There is also some evidence that pharmacotherapy improves morning symptoms and possibly reduces the degree of activity limitations by reducing morning symptoms. Up until now, studies using objective evaluations of physical activity levels and the association with morning symptoms are lacking. Future studies, preferably prospective randomised trials, should focus on objectively measured physical activity in COPD patients especially in the morning. We also recommend validation of a tool to evaluate morning symptoms, because a validated tool is lacking. If more evidence supports the finding that morning symptoms and physical activity are related, these factors will be more emphasised and will find a place in guidelines and statements.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0033-2016_Supplement (7KB, pdf)
Supplementary table S2 ERR-0033-2016_Supplementary_table_S2 (165.6KB, pdf)
Supplementary table S3 ERR-0033-2016_Supplementary_table_S3 (76KB, pdf)
Disclosures
N.H. Chavannes ERR-0033-2016_Chavannes (1.2MB, pdf)
C. Taube ERR-0033-2016_Taube (1.2MB, pdf)
Acknowledgements
We thank Jan W. Schoones (Leiden University Medical Center, Leiden, the Netherlands) for support with the search strategy. We also thank Steven J.H.A. McDowell (Leiden University Medical Center) for critically reading the manuscript.
Footnotes
This article has supplementary material available from err.ersjournals.com
Support statement: This work was supported with an unrestricted research grant from Novartis.
Conflict of interest: Disclosures can be found alongside this article at err.ersjournals.com
Provenance: Submitted article, peer reviewed.
References
- 1.Murray CJ, Richards MA, Newton JN, et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013; 381: 997–1020. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Top ten causes of death. www.who.int/mediacentre/factsheets/fs310/en/ Date last updated: May 2014. Date last accessed: March 14, 2016.
- 3.Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J 2014; 44: 1521–1537. [DOI] [PubMed] [Google Scholar]
- 4.Global Strategy for the Diagnosis MaPoC. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. www.goldcopd.org/. Date last updated: Dec 2015. Date last accessed: March 14, 2016.
- 5.Tsiligianni I, Kocks J, Tzanakis N, et al. Factors that influence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of Pearson correlations. Prim Care Respir J 2011; 20: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katajisto M, Kupiainen H, Rantanen P, et al. Physical inactivity in COPD and increased patient perception of dyspnea. Int J Chron Obstruct Pulmon Dis 2012; 7: 743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulich K, Keininger DL, Tiplady B, et al. Symptoms and impact of COPD assessed by an electronic diary in patients with moderate-to-severe COPD: psychometric results from the SHINE study. Int J Chron Obstruct Pulmon Dis 2015; 10: 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roche N, Chavannes NH, Miravitlles M. COPD symptoms in the morning: impact, evaluation and management. Respir Res 2013; 14: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med 2001; 134: 657–662. [DOI] [PubMed] [Google Scholar]
- 12.Poorolajal J, Cheraghi Z, Irani AD, et al. Quality of cohort studies reporting post the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Epidemiol Health 2011; 33: e2011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altmann VC, Hart AL, Vanlandewijck YC, et al. The impact of trunk impairment on performance of wheelchair activities with a focus on wheelchair court sports: a systematic review. Sports Med Open 2015; 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramesh A, Blanchet K, Ensink JH, et al. Evidence on the effectiveness of water, sanitation, and hygiene (WASH) interventions on health outcomes in humanitarian crises: a systematic review. PLoS One 2015; 10: e0124688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateman ED, Chapman KR, Singh D, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res 2015; 16: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miravitlles M, Worth H, Soler Cataluna JJ, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res 2014; 15: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roche N, Small M, Broomfield S, et al. Real world COPD: association of morning symptoms with clinical and patient reported outcomes. COPD 2013; 10: 679–686. [DOI] [PubMed] [Google Scholar]
- 18.Kim YJ, Lee BK, Jung CY, et al. Patient's perception of symptoms related to morning activity in chronic obstructive pulmonary disease: the SYMBOL Study. Korean J Intern Med 2012; 27: 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Hagan P, Chavannes NH. The impact of morning symptoms on daily activities in chronic obstructive pulmonary disease. Curr Med Res Opin 2014; 30: 301–314. [DOI] [PubMed] [Google Scholar]
- 20.Stephenson JJ, Cai Q, Mocarski M, et al. Impact and factors associated with nighttime and early morning symptoms among patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015; 10: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin 2009; 25: 2043–2048. [DOI] [PubMed] [Google Scholar]
- 22.Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J 2011; 37: 264–272. [DOI] [PubMed] [Google Scholar]
- 23.Partridge MR, Miravitlles M, Stahl E, et al. Development and validation of the Capacity of Daily Living during the Morning questionnaire and the Global Chest Symptoms Questionnaire in COPD. Eur Respir J 2010; 36: 96–104. [DOI] [PubMed] [Google Scholar]
- 24.Palsgrove A, Houghton K, Hareendran A, et al. The development of the early morning symptoms of COPD instrument (EMSCI). Value Health 2011; 14: A496–A497. [Google Scholar]
- 25.Garrow AP, Khan N, Tyson S, et al. The development and first validation of the Manchester Early Morning Symptoms Index (MEMSI) for patients with COPD. Thorax 2015; 70: 757–763. [DOI] [PubMed] [Google Scholar]
- 26.Waschki B, Kirsten AM, Holz O, et al. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 295–306. [DOI] [PubMed] [Google Scholar]
- 27.Miravitlles M, Ferrer J, Baro E, et al. Differences between physician and patient in the perception of symptoms and their severity in COPD. Respir Med 2013; 107: 1977–1985. [DOI] [PubMed] [Google Scholar]
- 28.Bjorner JB, Rose M, Gandek B Jr., et al. Difference in method of administration did not significantly impact item response: an IRT-based analysis from the Patient-Reported Outcomes Measurement Information System (PROMIS) initiative. Qual Life Res 2014; 23: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muehlhausen W, Doll H, Quadri N, et al. Equivalence of electronic and paper administration of patient-reported outcome measures: a systematic review and meta-analysis of studies conducted between 2007 and 2013. Health Qual Life Outcomes 2015; 13: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 972–977. [DOI] [PubMed] [Google Scholar]
- 31.Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med 2010; 104: 1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brawner CA, Churilla JR, Keteyian SJ. Prevalence of physical activity is lower among individuals with chronic disease. Med Sci Sports Exerc 2016. [DOI] [PubMed] [Google Scholar]
- 34.Watz H, Waschki B, Meyer T, et al. Physical activity in patients with COPD. Eur Respir J 2009; 33: 262–272. [DOI] [PubMed] [Google Scholar]
- 35.Wick K, Faude O, Schwager S, et al. Deviation between self-reported and measured occupational physical activity levels in office employees: effects of age and body composition. Int Arch Occup Environ Health 2016; 89: 575–82. [DOI] [PubMed] [Google Scholar]
- 36.Rabinovich RA, Louvaris Z, Raste Y, et al. Validity of physical activity monitors during daily life in patients with COPD. Eur Respir J 2013; 42: 1205–1215. [DOI] [PubMed] [Google Scholar]
- 37.Marth K, Schuller E, Pohl W. Improvements in patient-reported outcomes: a prospective, non-interventional study with aclidinium bromide for treatment of COPD. Respir Med 2015; 109: 616–624. [DOI] [PubMed] [Google Scholar]
- 38.Partridge MR, Schuermann W, Beckman O, et al. Effect on lung function and morning activities of budesonide/formoterol versus salmeterol/fluticasone in patients with COPD. Ther Adv Respir Dis 2009; 3: 1–11. [DOI] [PubMed] [Google Scholar]
- 39.Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180: 741–750. [DOI] [PubMed] [Google Scholar]
- 40.Clark TJ. Diurnal rhythm of asthma. Chest 1987; 91: 137S–141S. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Campos JL, Calero C, Quintana-Gallego E. Symptom variability in COPD: a narrative review. Int J Chron Obstruct Pulmon Dis 2013; 8: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agusti A, Hedner J, Marin JM, et al. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev 2011; 20: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0033-2016_Supplement (7KB, pdf)
Supplementary table S2 ERR-0033-2016_Supplementary_table_S2 (165.6KB, pdf)
Supplementary table S3 ERR-0033-2016_Supplementary_table_S3 (76KB, pdf)
N.H. Chavannes ERR-0033-2016_Chavannes (1.2MB, pdf)
C. Taube ERR-0033-2016_Taube (1.2MB, pdf)