Abstract
Oral appliances are increasingly recommended for selected patients with obstructive sleep apnoea (OSA) and those who do not tolerate nor prefer continuous positive airway pressure. The most commonly used oral appliance advances the lower jaw during sleep, the so-called mandibular advancement device (MAD). Patients seek treatment because of disturbing snoring, daytime symptoms, apnoeas that disturb sleep and the longer term consequences with regard to cardiovascular risks. MADs reduce the apnoea–hypopnoea index, although to various degrees among patients. Effects on daytime sleepiness have been observed mainly among the more severe OSA patients. Blood pressure may be reduced in MAD-treated OSA patients. There is, however, uncertainty about which patients will respond to this therapy in terms of apnoea reductions, decreased sleepiness and other symptoms, and reduced risk for future impaired health. The occurrence of side-effects also remains difficult to predict at present. The majority of sleep apnoea patients suffer from various comorbidities in terms of cardiovascular diseases, type 2 diabetes and depression. The most recent findings indicate that phenotyping of patients, considering various aspects of this multifaceted disease, will shed more light on the indications for MADs in patients with nightly sleep breathing disturbances. This review summarises the most recent knowledge about MAD treatment.
Short abstract
Oral appliances are increasingly used in the treatment of patients with obstructive sleep apnoea. This update highlights the most recent knowledge about this therapy. http://bit.ly/2Za1IGl
Introduction
Mandibular advancement devices (MADs) represent a well-tolerated treatment for selected patients with obstructive sleep apnoea (OSA) or those who do not tolerate nor want to use continuous positive airway pressure (CPAP) [1–6]. MADs reduce upper airway collapsibility, often in a dose-dependent manner, by increasing the pharyngeal dimensions upon protrusion of the lower jaw [7]. There are no effects on other OSA-related pathophysiological traits such as sensitivity of the respiratory control system [8]. Many randomised studies have confirmed that the apnoea–hypopnoea index (AHI) is reduced, although to different degrees among patients [1–6]. The effects on symptoms and longer term cardiovascular consequences are more variable and less studied [3, 6, 9–12]. More knowledge about phenotyping of patients will therefore become an important tool in improving the selection process for this non-CPAP therapy. In addition, there is a need for more standardisation of MAD therapy in terms of appliance selection and follow-up of the odontological consequences of treatment [13].
Patient selection
Patients with a moderate pharyngeal collapsibility and low loop gain are likely to respond to MAD therapy, i.e. characteristics that have been identified as beneficial with respect to the mechanism of action of MAD treatment [8, 14]. Milder OSA patients have also been recognised as responders. The common use of the AHI to identify this complex disease and make recommendations for treatment has, however, been questioned [15]. The AHI has been found to be a weaker predictor to identify MAD treatment responders than previously believed and severe patients might also have a good outcome [16–18]. Instead, more advanced ways to interpret sleep apnoea recordings are under development [19, 20]. This will allow the identification of various phenotypes of sleep apnoea patients and provide a more promising way forward to find patients who respond to OSA treatment in different ways.
Non-responders to MAD therapy are more likely among severe OSA patients with non-positional OSA [21–23]. Evaluation of the upper airways during drug-induced sleep endoscopy (DISE), showing a complete circular collapse or lack of widening of the upper airway during a mandibular advancement test, has been successful in order to identify poor responders [24, 25]. Also, when using a remotely controlled mandibular positioner, one is able to determine the most effective target protrusion during a single-night titration of the lower jaw [26]. Although major efforts are now being directed towards more accurate prediction of the required mandibular protrusion, at present there is unfortunately still no clinical routine protocol allowing this [27]. Obesity, older age and male sex relate to a poorer outcome, although with some variability in results [21, 28, 29]. However, early treatment might be beneficial in order to reduce the risk for systemic hypertension as a consequence of untreated OSA [30].
It will be important to define clusters of characteristics that represent phenotypes that respond to MAD therapy. Table 1 summarises the current knowledge about possible phenotypes of interest for identifying good and poor responders to MAD therapy and the efficacy of the device on comorbidities. New studies can, for instance, use the clusters that have been identified in the Icelandic Sleep Apnoea Cohort and include patients who are minimally symptomatic, sleepy or suffer from disturbed sleep, or those with cardiovascular or other identified OSA comorbidities [31, 32].
TABLE 1.
Phenotypes of obstructive sleep apnoea (OSA) patients of interest for mandibular advancement device therapy
| Phenotypes that have been related to treatment success | Phenotypes of interest to study | |
| Anthropometric | Females; younger age; less overweight/obesity | |
| Polysomnographic | Positional OSA | More sophisticated ways to interpret polysomnographic sleep recordings; simpler methods to evaluate treatment effects |
| Functional | DISE defining type of collapse and increase in airway size during advancement | |
| Symptomatic | Sleepiness and fatigue; headaches; restless legs; nasal symptoms; insomnia; disturbed sleep; minimally symptomatic | |
| Cardiovascular | Systemic hypertension; other cardiovascular diseases | |
| Metabolic/endocrine | Type 2 diabetes | |
| Other comorbidities | More overall knowledge on relationships between OSA and comorbidities |
DISE: drug-induced sleep endoscopy.
Symptoms
Symptomatic patients diagnosed with OSA complain about a combination of daytime sleepiness and fatigue.
The effect of OSA on daytime sleepiness, evaluated by the Epworth Sleepiness Scale, is uncertain and has mainly been found among severe OSA patients [3, 6, 12]. Milder OSA patients may become less sleepy during treatment, but the effect is probably more diluted by sleepiness from other causes [12, 33, 34]. A good approach in patients with mild symptomatic OSA is to test whether treatment with CPAP reduces daytime symptoms before treatment with an oral appliance is initiated [35].
The effect of OSA on fatigue is less well studied; furthermore, hypersomnolence and fatigue are often mixed up, although they are two distinct symptoms. Fatigue is defined as the subjective feeling of tiredness or exhaustion and is a commonly reported symptom among patients with chronic conditions such as OSA [36, 37]. Recent results indicated that the Checklist Individual Strength (a 20-item self-report questionnaire) was a reliable tool to demonstrate MAD treatment efficacy as well as improvement in health outcome characteristics such as fatigue upon MAD treatment; after 3 months of MAD treatment, fatigue was significantly reduced under the level of increased risk for prolonged absence at work [38, 39].
OSA patients may suffer from symptoms other than sleepiness, such as headaches, insomnia, restless legs, disturbed sleep and sleep bruxism. Few studies have evaluated the effects of MADs on such symptoms. Positive effects on symptoms of restless legs have been found in two studies [12, 40]. Some findings indicated that headaches, nasal congestion and insomnia were reduced with MAD treatment [12]. Short-term positive effects of MADs on sleep bruxism have been detected in small samples, although the longer term benefits are unclear [41]. Identification of various symptomatic phenotypes of OSA patients would be of interest in order to better understand the effects of MADs on various symptoms as well as comorbidities.
Blood pressure and cardiovascular health
Blood pressure is reduced from MAD treatment compared with placebo and mostly to a similar degree as with CPAP in the relatively small samples studied [5, 9, 11]. The blood pressure effects are particularly evident at night-time, in hypertensive patients and probably among females [18, 42–47]. Sex differences in effects from OSA treatments are mainly unknown, since mixed samples have included a majority of males (80% on average). In light of two recent studies, where untreated females with OSA were found to be particularly at risk to develop cardiovascular disease [48] or cancer [49], there is a risk of females being undertreated.
The effect of treatment of sleep apnoea in order to reduce future long-term consequences, in terms of cardiovascular risks, also needs to be studied in relation to OSA phenotype. For instance, other concomitant conditions to sleep apnoea such as periodic leg movements or disturbed sleep have been found to be more related to longer term risks for cardiovascular disease than the presence of apnoeas and hypopnoeas [50]. Another example would be to test whether sleepy OSA patients have a larger chance than non-sleepy OSA patients to reduce the risk for cardiovascular disease in terms of systemic hypertension during MAD treatment. This difference has been found for CPAP, possibly related to some degree to adherence to treatment [51], although it has now become clear that this must be studied in more detail given the heterogeneity in the types of MADs. Since adherence has been found to be better with MADs than with CPAP [18], such findings might be of help to find the best treatment for patients with comorbidities who need treatment for several diseases. The development of compliance monitors for MADs, in accordance with what is available for CPAP, will facilitate such comparisons [52].
No effect on inflammatory and metabolic markers has, however, been found during MAD treatment of severe OSA patients in a recent study, which suggests that earlier interventions are needed [53].
Device design and advancement
It is generally unknown if various brands of custom-made MADs produce significantly different effects on sleep apnoeas, although some design details have been found to be of importance. Custom-made devices have been found to be more effective and better tolerated than prefabricated devices [54–56]. A larger advancement of the mandible will generally produce a better effect of the MAD [7, 57, 58], but there is no precise linear relationship between mandibular advancement and treatment success [59, 60]. It is, however, important to secure the degree of mandibular advancement by the MAD in order to produce the intended outcome on sleep apnoeas. This can be achieved either by an adjustment mechanism that stabilises the lower jaw firmly to the upper jaw or attaches the lower part to the upper part by, for example, elastic bands [61, 62]. A stable position of the lower jaw will also be important when it comes to the prediction of responders, since otherwise efficacy in the supine position might be suboptimal [61, 62]. Indeed, during DISE it was found that increased mouth opening will counteract the stability of the upper airway in the large majority of patients [63].
Bite changes
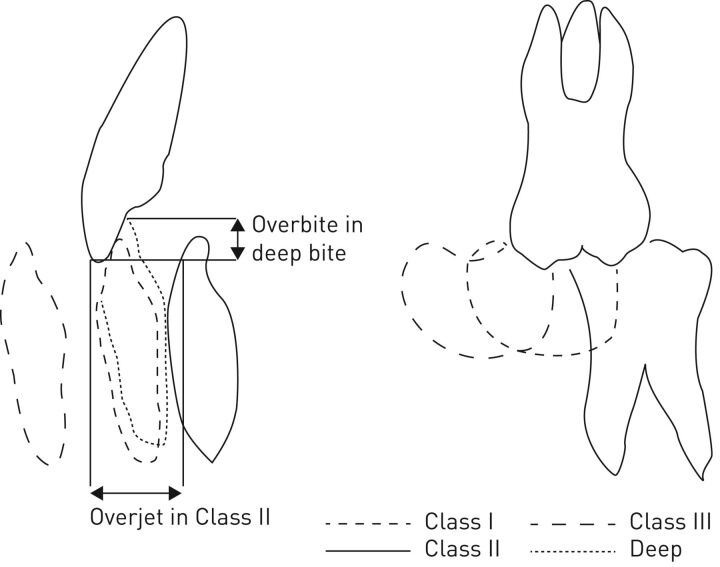
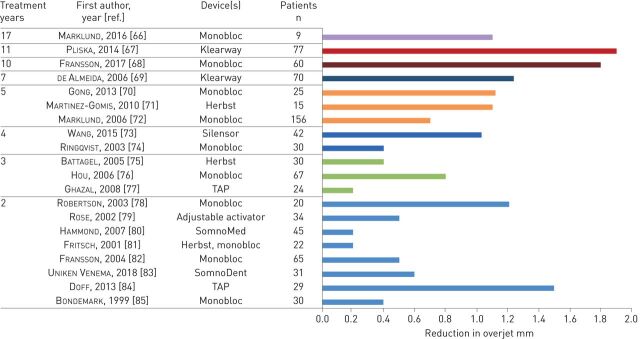
Oral appliances are attached to the teeth and therefore this therapy is highly dependent on a healthy dentition. Forces will arise on the teeth during appliance use and there is a risk for bite changes [64, 65]. The overjet, i.e. the horizontal distance between the upper and lower front teeth (figure 1), will decrease and patients may lose antagonistic contacts between the molar teeth, although to various degrees between patients. The bite changes are noticed early during the first years of treatment and will thereafter gradually continue (figure 2) [65–85]. The initial type of bite is associated with the degree of bite changes from MAD treatment: patients with a deep bite, i.e. a large vertical overlap between the front teeth, are to some degree protected from overjet changes (figure 1) [69, 72]. Those with normal bites or Angle Class III, i.e. lower front teeth located anterior to the upper front teeth, seem to be more at risk for negative bite changes. Patients with Angle Class II, i.e. lower front teeth much posteriorly located from the upper front, might receive positive orthodontic effects of oral appliance treatment. Consequently, bite changes do not necessarily need to be considered as disadvantageous to a particular dentition and, indeed, some patients could benefit.
FIGURE 1.
Various types of dental occlusion of interest for mandibular advancement device therapy.
FIGURE 2.
Reduction in overjet in various studies. TAP: Thornton Anterior Positioner.
Device design will also influence the risk of bite changes. One study describes that a device that is attached mainly to the front teeth will produce a faster and larger change in dental occlusion compared with a device that is attached to the whole dentition [83].
Although most patients are unaware of bite changes [86], the changed dental occlusion might influence the efficacy of the device. The advancement of the mandible by the device might diminish if the device is left unadjusted in patients with larger bite changes.
Conclusions
MADs represent an appealing treatment for selected patients with OSA. The variability in efficacy of MADs means, however, that identification of OSA phenotypes that respond to this treatment is urgently needed. Females and younger individuals may be currently undertreated. Standardisation of the methodology is required and the longer term health outcomes have to be explored further.
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: M. Marklund reports personal fees from ResMed, outside the submitted work.
Conflict of interest: M.J.A. Braem is President of the European Academy of Dental Sleep Medicine. He reports a research grant from the Flemish Government, and is an advisory board member of SomnoMed and ResMed Narval. He has also received lecture fees and travel reimbursement from dental professional organisations and national and international meeting organising committees.
Conflict of interest: J. Verbraecken reports grants and personal fees from ResMed, Bioprojet and Jazz Pharmaceutics, personal fees from Philips, Sanofi, Agfa-Gevaert and Springer, and grants from AirLiquide, Westfalen Medical, SomnoMed, Vivisol, Total Care, Medidis, Fisher & Paykel, Wave Medical, OSG, Mediq Tefa, NightBalance, Heinen & Löwenstein, AstraZeneca, Accuramed, Bekaert Deslee Academy and UCB Pharma, outside the submitted work.
References
- 1.Marklund M, Verbraecken J, Randerath W. Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy. Eur Respir J 2012; 39: 1241–1247. [DOI] [PubMed] [Google Scholar]
- 2.Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 2015; 11: 773–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharples LD, Clutterbuck-James AL, Glover MJ, et al. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med Rev 2016; 27: 108–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 2014; 10: 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu T, Li W, Zhou H, et al. Verifying the relative efficacy between continuous positive airway pressure therapy and its alternatives for obstructive sleep apnea: a network meta-analysis. Front Neurol 2017; 8: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koretsi V, Eliades T, Papageorgiou SN. Oral interventions for obstructive sleep apnea. Dtsch Arztebl Int 2018; 115: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bamagoos AA, Cistulli PA, Sutherland K, et al. Dose-dependent effects of mandibular advancement on upper airway collapsibility and muscle function in obstructive sleep apnea. Sleep 2019; 42: zsz049. [DOI] [PubMed] [Google Scholar]
- 8.Edwards BA, Andara C, Landry S, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2016; 194: 1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA 2015; 314: 2280–2293. [DOI] [PubMed] [Google Scholar]
- 10.Gao YN, Wu YC, Lin SY, et al. Short-term efficacy of minimally invasive treatments for adult obstructive sleep apnea: a systematic review and network meta-analysis of randomized controlled trials. J Formos Med Assoc 2019; 118: 750–765. [DOI] [PubMed] [Google Scholar]
- 11.Iftikhar IH, Hays ER, Iverson MA, et al. Effect of oral appliances on blood pressure in obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med 2013; 9: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marklund M, Carlberg B, Forsgren L, et al. Oral appliance therapy in patients with daytime sleepiness and snoring or mild to moderate sleep apnea: a randomized clinical trial. JAMA Intern Med 2015; 175: 1278–1285. [DOI] [PubMed] [Google Scholar]
- 13.Randerath W, Bassetti CL, Bonsignore MR, et al. Challenges and perspectives in obstructive sleep apnoea. Eur Respir J 2018; 52: 1702616.29853491 [Google Scholar]
- 14.Bamagoos AA, Cistulli PA, Sutherland K, et al. Polysomnographic endotyping to select obstructive sleep apnea patients for oral appliances. Ann Am Thorac Soc 2019; in press [ 10.1513/AnnalsATS.201903-190OC]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards BA, Redline S, Sands SA, et al. More than the sum of the respiratory events: personalized medicine approaches for obstructive sleep apnea. Am J Respir Crit Care Med 2019; 200: 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoekema A, Stegenga B, Wijkstra PJ, et al. Obstructive sleep apnea therapy. J Dent Res 2008; 87: 882–887. [DOI] [PubMed] [Google Scholar]
- 17.Gagnadoux F, Pepin JL, Vielle B, et al. Impact of mandibular advancement therapy on endothelial function in severe obstructive sleep apnea. Am J Respir Crit Care Med 2017; 195: 1244–1252. [DOI] [PubMed] [Google Scholar]
- 18.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2013; 187: 879–887. [DOI] [PubMed] [Google Scholar]
- 19.Genta PR, Sands SA, Butler JP, et al. Airflow shape is associated with the pharyngeal structure causing OSA. Chest 2017; 152: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Chazal P, Sutherland K, Cistulli PA. Advanced polysomnographic analysis for OSA: a pathway to personalized management? Respirology 2019; in press [ 10.1111/resp.13564]. [DOI] [PubMed] [Google Scholar]
- 21.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest 2004; 125: 1270–1278. [DOI] [PubMed] [Google Scholar]
- 22.Petri N, Christensen IJ, Svanholt P, et al. Mandibular advancement device therapy for obstructive sleep apnea: a prospective study on predictors of treatment success. Sleep Med 2019; 54: 187–194. [DOI] [PubMed] [Google Scholar]
- 23.Takaesu Y, Tsuiki S, Kobayashi M, et al. Mandibular advancement device as a comparable treatment to nasal continuous positive airway pressure for positional obstructive sleep apnea. J Clin Sleep Med 2016; 12: 1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastoer C, Op de Beeck S, Dom M, et al. Drug-induced sleep endoscopy upper airway collapse patterns and maxillomandibular advancement. Laryngoscope 2019; in press [ 10.1002/lary.28022]. [DOI] [PubMed] [Google Scholar]
- 25.Remmers JE, Topor Z, Grosse J, et al. A feedback-controlled mandibular positioner identifies individuals with sleep apnea who will respond to oral appliance therapy. J Clin Sleep Med 2017; 13: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kastoer C, Dieltjens M, Oorts E, et al. The use of remotely controlled mandibular positioner as a predictive screening tool for mandibular advancement device therapy in patients with obstructive sleep apnea through single-night progressive titration of the mandible: a systematic review. J Clin Sleep Med 2016; 12: 1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieltjens M, Vanderveken OM, Heyning PH, et al. Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing. Sleep Med Rev 2012; 16: 177–185. [DOI] [PubMed] [Google Scholar]
- 28.Lettieri CJ, Paolino N, Eliasson AH, et al. Comparison of adjustable and fixed oral appliances for the treatment of obstructive sleep apnea. J Clin Sleep Med 2011; 7: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vecchierini MF, Attali V, Collet JM, et al. Sex differences in mandibular repositioning device therapy effectiveness in patients with obstructive sleep apnea syndrome. Sleep Breath 2018; 23: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vgontzas AN, Li Y, He F, et al. Mild-to-moderate sleep apnea is associated with incident hypertension: age effect. Sleep 2019; 42: zsy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J 2014; 44: 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonsignore MR, Baiamonte P, Mazzuca E, et al. Obstructive sleep apnea and comorbidities: a dangerous liaison. Multidiscip Respir Med 2019; 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durán-Cantolla J, Crovetto-Martinez R, Alkhraisat MH, et al. Efficacy of mandibular advancement device in the treatment of obstructive sleep apnea syndrome: a randomized controlled crossover clinical trial. Med Oral Patol Oral Cir Bucal 2015; 20: e605–e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbruggen AE, Dieltjens M, Wouters K, et al. Prevalence of residual excessive sleepiness during effective oral appliance therapy for sleep-disordered breathing. Sleep Med 2014; 15: 269–272. [DOI] [PubMed] [Google Scholar]
- 35.McNicholas WT. Obstructive sleep apnoea of mild severity: should it be treated? Curr Opin Pulm Med 2017; 23: 506–511. [DOI] [PubMed] [Google Scholar]
- 36.Nordin A, Taft C, Lundgren-Nilsson A, et al. Minimal important differences for fatigue patient reported outcome measures – a systematic review. BMC Med Res Methodol 2016; 16: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichstein KL, Means MK, Noe SL, et al. Fatigue and sleep disorders. Behav Res Ther 1997; 35: 733–740. [DOI] [PubMed] [Google Scholar]
- 38.Kazemeini E, Braem MJ, Moorkens G, et al. Scoring of hypersomnolence and fatigue in patients with obstructive sleep apnea treated with a titratable custom-made mandibular advancement device. J Clin Sleep Med 2019; 15: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bultmann U, de Vries M, Beurskens AJ, et al. Measurement of prolonged fatigue in the working population: determination of a cutoff point for the checklist individual strength. J Occup Health Psychol 2000; 5: 411–416. [DOI] [PubMed] [Google Scholar]
- 40.Saletu A, Anderer P, Parapatics S, et al. Effects of a mandibular repositioning appliance on sleep structure, morning behavior and clinical symptomatology in patients with snoring and sleep-disordered breathing. Neuropsychobiology 2007; 55: 184–193. [DOI] [PubMed] [Google Scholar]
- 41.Jokubauskas L, Baltrusaityte A, Pileicikiene G. Oral appliances for managing sleep bruxism in adults: a systematic review from 2007 to 2017. J Oral Rehabil 2018; 45: 81–95. [DOI] [PubMed] [Google Scholar]
- 42.Andren A, Hedberg P, Walker-Engstrom ML, et al. Effects of treatment with oral appliance on 24-h blood pressure in patients with obstructive sleep apnea and hypertension: a randomized clinical trial. Sleep Breath 2013; 17: 705–712. [DOI] [PubMed] [Google Scholar]
- 43.Sekizuka H, Osada N, Akashi YJ. Effect of oral appliance therapy on blood pressure in Japanese patients with obstructive sleep apnea. Clin Exp Hypertens 2016; 38: 404–408. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int J Prosthodont 2006; 19: 61–66. [PubMed] [Google Scholar]
- 45.Zhang LQ, Zheng X, Wang JL, et al. [Effects of oral appliance treatment upon blood pressure in mild to moderate obstructive sleep apnea–hypopnea syndrome]. Zhonghua Yi Xue Za Zhi 2009; 89: 1807–1810. [PubMed] [Google Scholar]
- 46.Rietz H, Franklin KA, Carlberg B, et al. Nocturnal blood pressure is reduced by a mandibular advancement device for sleep apnea in women: findings from secondary analyses of a randomized trial. J Am Heart Assoc 2018; 7: e008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med 2004; 170: 656–664. [DOI] [PubMed] [Google Scholar]
- 48.Strausz S, Havulinna AS, Tuomi T, et al. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: a longitudinal population-based study in Finland. BMJ Open 2018; 8: e022752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pataka A, Bonsignore MR, Ryan S, et al. Cancer prevalence is increased in females with sleep apnoea: data from the ESADA study. Eur Respir J 2019; 53: 1900091. [DOI] [PubMed] [Google Scholar]
- 50.Zinchuk AV, Jeon S, Koo BB, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax 2018; 73: 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bratton DJ, Stradling JR, Barbe F, et al. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax 2014; 69: 1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanderveken OM, Braem MJ, Dieltjens M, et al. Objective measurement of the therapeutic effectiveness of continuous positive airway pressure versus oral appliance therapy for the treatment of obstructive sleep apnea. Am J Respir Crit Care Med 2013; 188: 1162. [DOI] [PubMed] [Google Scholar]
- 53.Recoquillon S, Pepin JL, Vielle B, et al. Effect of mandibular advancement therapy on inflammatory and metabolic biomarkers in patients with severe obstructive sleep apnoea: a randomised controlled trial. Thorax 2019; 74: 496–499. [DOI] [PubMed] [Google Scholar]
- 54.Johal A, Haria P, Manek S, et al. Ready-made versus custom-made mandibular repositioning devices in sleep apnea: a randomized clinical trial. J Clin Sleep Med 2017; 13: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178: 197–202. [DOI] [PubMed] [Google Scholar]
- 56.Gagnadoux F, Nguyen XL, Le Vaillant M, et al. Comparison of titrable thermoplastic versus custom-made mandibular advancement device for the treatment of obstructive sleep apnoea. Respir Med 2017; 131: 35–42. [DOI] [PubMed] [Google Scholar]
- 57.Aarab G, Lobbezoo F, Hamburger HL, et al. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin Oral Investig 2010; 14: 339–345. [DOI] [PubMed] [Google Scholar]
- 58.Kato J, Isono S, Tanaka A, et al. Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest 2000; 117: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 59.Anitua E, Durán-Cantolla J, Almeida GZ, et al. Minimizing the mandibular advancement in an oral appliance for the treatment of obstructive sleep apnea. Sleep Med 2017; 34: 226–231. [DOI] [PubMed] [Google Scholar]
- 60.Bartolucci ML, Bortolotti F, Raffaelli E, et al. The effectiveness of different mandibular advancement amounts in OSA patients: a systematic review and meta-regression analysis. Sleep Breath 2016; 20: 911–919. [DOI] [PubMed] [Google Scholar]
- 61.Milano F, Mutinelli S, Sutherland K, et al. Influence of vertical mouth opening on oral appliance therapy outcome in positional obstructive sleep apnea. J Dent Sleep Med 2018; 5: 17–23. [Google Scholar]
- 62.Norrhem N, Marklund M. An oral appliance with or without elastic bands to control mouth opening during sleep – a randomized pilot study. Sleep Breath 2016; 20: 929–938. [DOI] [PubMed] [Google Scholar]
- 63.Vroegop AV, Vanderveken OM, Van de Heyning PH, et al. Effects of vertical opening on pharyngeal dimensions in patients with obstructive sleep apnoea. Sleep Med 2012; 13: 314–316. [DOI] [PubMed] [Google Scholar]
- 64.Araie T, Okuno K, Ono Minagi H, et al. Dental and skeletal changes associated with long-term oral appliance use for obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2018; 41: 161–172. [DOI] [PubMed] [Google Scholar]
- 65.Bartolucci ML, Bortolotti F, Martina S, et al. Dental and skeletal long-term side effects of mandibular advancement devices in obstructive sleep apnea patients: a systematic review with meta-regression analysis. Eur J Orthod 2019; 41: 89–100. [DOI] [PubMed] [Google Scholar]
- 66.Marklund M. Long-term efficacy of an oral appliance in early treated patients with obstructive sleep apnea. Sleep Breath 2016; 20: 689–694. [DOI] [PubMed] [Google Scholar]
- 67.Pliska BT, Nam H, Chen H, et al. Obstructive sleep apnea and mandibular advancement splints: occlusal effects and progression of changes associated with a decade of treatment. J Clin Sleep Med 2014; 10: 1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fransson AMC, Kowalczyk A, Isacsson G, et al. A prospective 10-year follow-up dental cast study of patients with obstructive sleep apnoea/snoring who use a mandibular protruding device. Eur J Orthod 2017; 39: 502–508. [DOI] [PubMed] [Google Scholar]
- 69.de Almeida FR, Lowe AA, Otsuka R, et al. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: part 2. Study-model analysis. Am J Orthod Dentofacial Orthop 2006; 129: 205–213. [DOI] [PubMed] [Google Scholar]
- 70.Gong X, Zhang J, Zhao Y, et al. Long-term therapeutic efficacy of oral appliances in treatment of obstructive sleep apnea-hypopnea syndrome. Angle Orthod 2013; 83: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinez-Gomis J, Willaert E, Nogues L, et al. Five years of sleep apnea treatment with a mandibular advancement device. Side effects and technical complications. Angle Orthod 2010; 80: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2006; 129: 214–221. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Gong X, Yu Z, et al. Follow-up study of dental and skeletal changes in patients with obstructive sleep apnea and hypopnea syndrome with long-term treatment with the Silensor appliance. Am J Orthod Dentofacial Orthop 2015; 147: 559–565. [DOI] [PubMed] [Google Scholar]
- 74.Ringqvist M, Walker-Engstrom ML, Tegelberg A, et al. Dental and skeletal changes after 4 years of obstructive sleep apnea treatment with a mandibular advancement device: a prospective, randomized study. Am J Orthod Dentofacial Orthop 2003; 124: 53–60. [DOI] [PubMed] [Google Scholar]
- 75.Battagel JM, Kotecha B. Dental side-effects of mandibular advancement splint wear in patients who snore. Clin Otolaryngol 2005; 30: 149–156. [DOI] [PubMed] [Google Scholar]
- 76.Hou HM, Sam K, Hagg U, et al. Long-term dentofacial changes in Chinese obstructive sleep apnea patients after treatment with a mandibular advancement device. Angle Orthod 2006; 76: 432–440. [DOI] [PubMed] [Google Scholar]
- 77.Ghazal A, Jonas IE, Rose EC. Dental side effects of mandibular advancement appliances – a 2-year follow-up. J Orofac Orthop 2008; 69: 437–447. [DOI] [PubMed] [Google Scholar]
- 78.Robertson C, Herbison P, Harkness M. Dental and occlusal changes during mandibular advancement splint therapy in sleep disordered patients. Eur J Orthod 2003; 25: 371–376. [DOI] [PubMed] [Google Scholar]
- 79.Rose EC, Staats R, Virchow C Jr, et al. Occlusal and skeletal effects of an oral appliance in the treatment of obstructive sleep apnea. Chest 2002; 122: 871–877. [DOI] [PubMed] [Google Scholar]
- 80.Hammond RJ, Gotsopoulos H, Shen G, et al. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2007; 132: 806–814. [DOI] [PubMed] [Google Scholar]
- 81.Fritsch KM, Iseli A, Russi EW, et al. Side effects of mandibular advancement devices for sleep apnea treatment. Am J Respir Crit Care Med 2001; 164: 813–818. [DOI] [PubMed] [Google Scholar]
- 82.Fransson AM, Tegelberg A, Johansson A, et al. Influence on the masticatory system in treatment of obstructive sleep apnea and snoring with a mandibular protruding device: a 2-year follow-up. Am J Orthod Dentofacial Orthop 2004; 126: 687–693. [DOI] [PubMed] [Google Scholar]
- 83.Uniken Venema JAM, Stellingsma M, Doff MHJ, et al. Dental side-effects of long-term obstructive sleep apnea therapy; a comparison of three therapeutic modalities. J Dent Sleep Med 2018; 5: 39–46. [Google Scholar]
- 84.Doff MH, Finnema KJ, Hoekema A, et al. Long-term oral appliance therapy in obstructive sleep apnea syndrome: a controlled study on dental side effects. Clin Oral Investig 2013; 17: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bondemark L. Does 2 years' nocturnal treatment with a mandibular advancement splint in adult patients with snoring and OSAS cause a change in the posture of the mandible? Am J Orthod Dentofacial Orthop 1999; 116: 621–628. [DOI] [PubMed] [Google Scholar]
- 86.Marklund M. Subjective versus objective dental side effects from oral sleep apnea appliances. Sleep Breath 2019; in press [ 10.1007/s11325-019-01852-0]. [DOI] [PMC free article] [PubMed] [Google Scholar]