Abstract
Locally advanced nonsmall cell lung cancer, due to its varying prognosis, is grouped according to TNM stage IIIA, IIIB and IIIC. Developments over the last 3 years have been focused on the integration of immunotherapy into the combination treatment of a locally definitive therapy (surgery or radiotherapy) and chemotherapy. For concurrent chemoradiotherapy, consolidation therapy with durvalumab was established. Adjuvant targeted therapy has again gained increasing interest. In order to adapt treatment to the specific stage subgroup and its prognosis, fluorodeoxyglucose positron emission tomography/computed tomography and pathological evaluation of the mediastinum are important. Tumours should be investigated for immunological features and driver mutations. Regarding toxicity, evaluation of pulmonary and cardiac function, as well as symptoms and quality of life, is of increasing importance. To improve the management and prognosis of this heterogeneous entity, clinical trials and registries should take these factors into account.
Short abstract
Locally advanced NSCLC comprises TNM stage IIIA–C. Immune-checkpoint inhibition after chemoradiotherapy is standard and part of multimodal trials with surgery. Exact staging, genetics, immunology and functional testing are important. https://bit.ly/366lNQ2
Introduction
Locally advanced nonsmall cell lung cancer (NSCLC) comprises a variety of different entities, which are grouped according to the TNM system (version 8) in stage IIIA, IIIB and IIIC. This classification was chosen due to the varying prognosis in these substages (table 1) [1]. For years, a locally definitive therapy was combined with systemic platinum-based doublet chemotherapy. The locally definitive therapy depends on the functional situation, comorbidities, performance status, the amount of involvement of mediastinal lymph nodes and technical issues, as well as the patient's preference and will be discussed in the interdisciplinary tumour board, as outlined recently in the European Respiratory Review in accordance with international guidelines [2–4]. Some aspects of these discussions are summarised in table 2. There are still many questions in the optimal management regarding the various stages and histologies, the tumour biology and the condition of the individual patients [5–9]. Also, efforts to improve the functional situation pre-therapeutically may be relevant and need further evaluation [10]. Accordingly, there is also limited standardisation of the management of these patients in the clinical practice [11, 12]. Presently, following developments in stage IV, attempts have been made to integrate targeted therapy and immunotherapy into the management of this heterogenous group of NSCLC of usually poor prognosis. Consolidation immune-checkpoint inhibition after chemoradiotherapy is already a standard of care in many parts of the world [13]. But also new techniques for imaging, functional and biological assessment have to be taken into account. In this article we provide an overview of the developments in the field over the past 3 years.
TABLE 1.
5-year survival rates in stage III depending on sub-stage and specific staging methods
| TNM 8 | Clinical stage % | Pathological stage % |
| IIIA | 36 | 41 |
| IIIB | 26 | 24 |
| IIIC | 13 | 12 |
Data from [1].
TABLE 2.
Considerations in the multidisciplinary decision about locally definitive treatment
| Surgery | Radiotherapy | Combined |
| Adequate functional status | Multilevel or bulky N2 | Local tumour control is very important |
| Bulky, necrotic tumours with possible complications | (Small) tumours with multiple mediastinal lymph node involvement | Locally invasive tumours with slim possible resection margins (e.g. superior sulcus tumours) |
| Multiple nodules in the same lobe | Reasonable dose affections of lung and heart | |
| No pneumonectomy necessary | PD-L1 inhibition possible |
PD-L1: programmed death ligand 1.
Evaluation by imaging, function and metabolism in locally advanced NSCLC
Endobronchial ultrasound with transbronchial tissue probes is currently well established and standardised. Apart from mediastinal lymph node staging it can also be used for centrally located lung tumours [14]. These techniques have largely replaced more invasive techniques. Even in restaging after neoadjuvant treatment, endobronchial ultrasound has its place, where mediastinoscopy and even remediastinoscopy may be challenging [15].
For surgery, the preoperative evaluation of pulmonary and cardiac function is well established [16]. Following the discussions of the Radioation Therapy Oncology Group (RTOG) 0617 [6] and other radiation dose escalation trials, cardiac toxicity and pulmonary toxicity have also received more attention in the chemoradiotherapy setting. Right ventricular function seems to be correlated with prognosis after chemoradiotherapy [17, 18]. A pre-therapeutic reduced left ventricular ejection fraction may increase the risk of radiation pneumonitis [19]. Pulmonary and cardiac toxicities can be increased by chemo- and immunotherapy [20–24]. The frequencies in clinical scenarios may be higher than in the original licensing trials [25]. Pre-existing lung diseases and other clinical risk factors increase the probability of pulmonary toxicity [26, 27].
Toxicities may be reduced by taking into account these risk factors in chemoradiotherapy and by adapting the radiation prescription accordingly [28]. Image-guided adaptive replanning [29] and image-based lung functional radiotherapy planning [30] may be of help.
In imaging positron emission tomography (PET)-computed tomography (CT) and magnetic resonance imaging of the brain are usually regarded as standard modalities for staging in these tumour entities. In addition, metabolic tumour evaluation by PET-CT for chemoradiotherapy can be of help for radiation treatment planning and dose prescription and may be of prognostic and predictive relevance. The concept of reducing the target volume to avoid toxicity and the use of PET-CT has developed over recent years [31]. In a randomised trial, this approach demonstrated that imaging based target volume reduction is feasible, and may change the standard of care [32].
There are reports that associate the pre-therapeutic metabolic activity in chemoradiotherapy with response and prognosis [33]. This seems to primarily be the case for the adenocarcinoma type of NSCLC [34]. The relevance of metabolic response to induction chemotherapy before surgery [35] and (chemo)radiotherapy [36] or the response (chemo)radiotherapy [37–40] is further evaluated. In addition, radiomic and artificial intelligence approaches are being pursued in this setting [41].
Targeted therapy in locally advanced NSCLC
The application of genetic testing in stage IV NSCLC led to a substantial progress in a subgroup of these patients. There was hope early on that this would also lead to an improvement in the locally advanced disease stage. Until now, genetic testing in locally advanced NSCLC has not been the standard of care.
In resected patients, the presence of driver mutations was evaluated regarding prognosis. In 242 Japanese resected patients (stage I–III) whole-exome sequencing of the resected tumours was retrospectively performed and demonstrated prognostic relevance for recurrence-free survival [42]. In 213 resected patients in stage I–III from the Princess Margaret Cancer Center in Toronto (Canada), next-generation sequencing retrospectively demonstrated that the presence of known somatic mutations is associated with worse disease-free survival [43]; however, larger prospective trials are needed.
In the setting of chemoradiotherapy the presence of epidermal growth factor receptor (EGFR) mutations may be associated with lower locoregional recurrence and higher distant progression, especially brain metastasis [44]. Progression-free survival seems to be short, with the brain being the most common site of distant metastasis.
In early trials, targeted therapy after curative resection or chemoradiotherapy did not improve overall survival [45, 46]. In a Chinese population with stage II and III EGFR-mutated NSCLC, adjuvant gefitinib in comparison to vinorelbine and cisplatin improved progression-free survival [47]. This was also the case in a phase II trial with 100 patients in stage I–III [48]. A recent update of the Chinese trial did not demonstrate an advantage in overall survival [49]. Whether the advantage with osimertinib in progression-free survival in a similar trial setting as the Chinese trial leads to a better overall survival remains to be shown [50]. The results were presented at the congress of the American Society of Clinical Oncology 2020 and were rather good regarding progression-free survival, but the follow-up for other measures and especially overall survival was too short and not mature.
There are few reports about using targeted therapy as induction therapy [51, 52] and in combination with induction chemotherapy and resection [53], which presently allows no clear conclusion.
For the combination of EGFR inhibition with radiotherapy in EGFR-mutated patients, several small trials have been performed previously and a few are ongoing [54, 55]. There are some issues with toxicity [45] and the results to date do not allow a recommendation outside of clinical trials.
Addition of immunotherapy in the setting of surgery
Despite promising signals for vaccination in the setting of locally advanced NSCLC in phase II trials, randomised phase III trials could not demonstrate a relevant benefit. With the progress in stage IV being made by using immune-checkpoint inhibitors, there is renewed hope for immunotherapy in the operative setting [56, 57].
Adjuvant chemotherapy after curative resection improves overall survival, but only modestly. Therefore, prognostic or predictive biomarkers for tailoring systemic therapy would be helpful. The effect of driver mutations has already been discussed. Regarding immune markers, several biomarkers are being pursued. There is ongoing work for immune cells in the tumour and tumour–stroma interactions [58–62]. For tumour mutation burden (TMB) an analysis was performed in the Lung Adjuvant Cisplatin Evaluation (LACE)-Bio-II study [63]. In 908 samples, a high nonsynonymous TMB was associated with a better prognosis in patients with resected NSCLC. In addition, the benefit of adjuvant chemotherapy on lung cancer-specific survival was more pronounced in patients with low nonsynonymous TMBs. In a clinical trial with induction therapy using nivolumab, TMB was correlated with pathological response to programmed death ligand 1 (PD-L1) blockade [64].
Regarding PD-L1 expression, a meta-analysis concluded that high PD-L1 expression by immunohistochemistry was significantly associated with poor overall survival for patients with lung cancer, especially for Asian patients with surgically resected, early stage I–III tumours and using 5% as the cut-off value [65]. This negative association may primarily be present in never-smokers [66]. For adjuvant chemotherapy, PD-L1 expression is probably not predictive [67].
The correlation of PD-L1 expression with TMB is mostly absent [68]. Positive CD8 and negative PD-L1 expression together may be favourable prognostic markers in resectable NSCLC [69]. A firm conclusion regarding biomarkers cannot be drawn at the moment.
For proof of concept, immune-checkpoint inhibitors were used in clinical trials in the neoadjuvant setting with and without chemotherapy and produced promising preliminary results. In a trial with two preoperative doses of nivolumab in 21 resected patients, nine out of 20 patients had a major pathological response [64]. There was no correlation with PD-L1 expression, but pathological response correlated with the TMB. A complete response was reported by neoadjuvant treatment with chemotherapy and pembrolizumab [70]. Patients with resectable NSCLC received two cycles of atezolizumab pre-operatively in a multicentre trial and 15 out of 82 evaluable patients without driver mutations achieved a major pathological response [71]. Four of them had a complete pathological response and another four patients had progressive disease. Atezolizumab was also tested pre-operatively in combination with carboplatin and nab–paclitaxel on 30 patients with stage IB–IIIA NSCLC, who had a smoking history and had an Eastern Cooperative Oncology Group performance status of 0 or 1 [72]. Overall, 57% had a major pathological response. Pathological response does not correlate well with morphological imaging results. Therefore, for evaluation of response, a pathological analysis seems to be necessary. The relevance of a metabolic response has not yet been evaluated adequately in this setting.
Immune-checkpoint inhibition with or without chemotherapy is now being tested in the adjuvant and neoadjuvant setting and in a combination of neoadjuvant and adjuvant systemic therapies in various phase I–III trials, which are summarised in table 3. Usually they are not limited to stage III and not subclassified to the subtypes of stage III. The primary end-points also vary. These imprecise definitions will hinder the final interpretation of these trials.
TABLE 3.
Current clinical trials on immunotherapy in the setting of surgery in early and locally advanced nonsmall cell lung cancer (NSCLC)
| ClinicalTrials.gov ID | Phase | NSCLC stage | IO-drug | Protocol |
Primary
end-point |
| Induction therapy | |||||
| NCT03694236 | I/II | II/IIIA | Durvalumab | Chemoradiotherapy+durvalumab induction→surgery | pCR |
| NCT03237377 | II | III | Durvalumab/ tremelimumab |
Radiotherapy+durvalumab ± tremelimumab→surgery | pCR |
| NCT03197467 | II | II/IIIA | Pembrolizumab | Pembrolizumab induction→surgery | Safety |
| NCT02994576 | II | IB–IIIA | Atezolizumab | Atezolizumab induction→surgery | Safety |
| NCT03732664 | I | IA–IIIA | Nivolumab | Nivolumab induction→surgery | Safety |
| NCT04205552 | II | IB–IIIA | Nivolumab/ relatlimab |
Nivolumab ± relatlimab induction→surgery | Feasibility |
| NCT02259621 | II | IB–IIIA | Nivolumab/ ipilimumab |
Nivolumab ± ipilimumab induction→surgery | Safety |
| NCT04348292 | I | I–IIIA | Durvalumab/ sirolimus |
Durvalumab+sirolimus induction→surgery | Safety |
| Combined induction and adjuvant therapy | |||||
| NCT03871153 | II | Resectable III | Durvalumab | Chemoradiotherapy+durvalumab induction→surgery→durvalumab consolidation | pCR |
| NCT04062708 | II | III | Durvalumab | Chemotherapy+durvalumab induction→surgery→radiotherapy→durvalumab consolidation | pCR |
| NCT03871153 | II | Resectable III | Durvalumab | Chemotherapy+durvalumab induction→radiotherapy+durvalumab→surgery→ durvalumab consolidation |
pCR |
| NCT04202809 | II | IIIA–B | Durvalumab | Chemotherapy induction→chemoradiotherapy ± durvalumab→surgery if resectable | PFS |
| NCT02818920 | II | IB–IIIA | Pembrolizumab | Pembrolizumab induction→surgery→pembrolizumab consolidation | Surgical feasibility rate |
| NCT03838159 | II | IIIA | Nivolumab | Arm 1: chemotherapy+nivolumab induction→surgery→nivolumab consolidation Arm 2: chemotherapy induction→surgery |
pCR |
| NCT02572843 | II | IIIA | Durvalumab | Chemotherapy+durvalumab induction→surgery→durvalumab consolidation | DFS |
| NCT04025879# | III | II–IIIB | Nivolumab | Chemotherapy ± nivolumab→surgery→nivolumab consolidation or observation | DFS |
| Adjuvant therapy | |||||
| NCT02595944# | III | IB–IIIA | Nivolumab | Surgery→chemotherapy→nivolumab consolidation or observation | DFS, OS |
Data obtained from www.clinicaltrials.gov on 7 August 2020. IO: immune-oncology; pCR: pathological complete response; PFS: progression-free survival; DFS: disease-free survival; OS: overall survival. #: phase III trials.
Adjuvant radiotherapy
In stage III there is still discussion about adjuvant radiotherapy after surgery. In this context, it is of importance to understand which patients are at increased risk of relapse and the impact of local recurrence on the overall course of the disease. A further point of discussion is prophylactic cranial irradiation in locally advanced NSCLC. The technical progress in radiotherapy is rather relevant and is discussed in another article by Finazzi et al. (unpublished data).
Prophylactic cranial irradiation (PCI) is not routinely applied in curatively treated NSCLC, but there are still results of published clinical trials that add to the evidence base of this topic. The RTOG 0214 trial randomised PCI versus observation in 340 patients with stage III NSCLC [73]. PCI decreased the 5- and 10-year rate of brain metastasis and improved 5- and 10-year disease-free survival but did not improve overall survival. A multivariable analysis within the nonsurgical arm suggests that PCI also effectively prolongs overall survival. Younger patients (aged <60 years) and patients with nonsquamous disease developed more brain metastases. In the Dutch randomised phase III NVALT-11 trial, 175 patients with stage III disease were also randomised to either observation or PCI [74]. PCI significantly decreased the proportion of patients who developed symptomatic brain metastases with an increase of low-grade toxicity and without a statistically significant effect on overall survival.
Adjuvant post-operative radiotherapy (PORT), usually given in addition to adjuvant chemotherapy, is mostly given in N2 disease with increased risk of local recurrence. Recent data add to the current body of evidence. A recent study retrospectively analysed 183 Chinese patients with stage III–pN2 NSCLC regarding the status of applied post-operative radiotherapy [75]. A short local recurrence-free survival was correlated with multiple-station pN2, older age (>55 years), patients with a high positive lymph node ratio >1:3 and poor histological differentiation of the tumour. Post-operative radiotherapy reduced the risk of local recurrence and improved overall survival. In a retrospective analysis from New York (USA) of a prospectively maintained database for patients with cIII–N2 NSCLC who underwent induction chemotherapy followed by resection, 99 biopsy-proven cIII–N2 patients could be identified [76]. In this series, 95% of patients had an initial distant recurrence and on multivariable analysis PORT was not associated with locoregional recurrence or disease-free survival. An analysis of the SEER database investigated 5168 patients with stage IIIA, of whom 1711 received PORT [77]. PORT negatively influenced 3- and 5-year lung cancer-related mortality rates in N1 disease, but decreased the 3- and 5-year mortality rate by 4.67% and 10.08%, respectively, and improved overall survival in patients with N2 disease and at least six positive lymph nodes.
It seems clear that prognostic factors have to be taken into account, which influences prognosis and the relationship between local and systemic recurrence. There are some factors such as smoking, COPD or low forced expiratory volume in 1 s (FEV1) that have already been known for some time. A recent trial analysed the quantitative emphysema severity of the whole lung using CT in 45 patients [78]. After adjusting for age, sex, smoking status and FEV1 a more severe score was an independent predictor for recurrence and survival. For this trial, no details regarding local and systemic recurrence have been published. A Japanese study analysed the data of 1012 consecutive stage I–III NSCLC patients who underwent complete resection [79]. Local recurrence was identified in 9.4% of these patients. The most significant risk factor for local recurrence was lymph node metastasis (N1: HR=2.27, p=0.009; N2: HR=6.85, p<0.0001). For the subgroup of patients with lymph node metastasis (n=289), independent risk factors for local recurrence were N2 disease with N1 metastasis (N2 with N1: HR=3.46, p<0.0001) and no adjuvant platinum-based chemotherapy (HR=1.91, p=0.018).
Integration of immunotherapy in radiochemotherapy in locally advanced NSCLC
For years modifications of chemoradiotherapy using mostly cisplatin/etoposide or vinorelbine/cisplatin did not improve the outcome of locally advanced unresectable NSCLC [2, 80, 81]. This has now changed by integrating immunotherapy in definitive chemoradiotherapy [82, 83]. This has undoubtedly improved the prognosis of many patients with locally advanced NSCLC.
For chemoradiotherapy, consolidation with durvalumab improved progression-free and overall survival in stage III NSCLC [84]. In the PACIFIC trial, randomisation was performed after chemoradiotherapy, there were no specific staging requirements, detailed data before randomisation were missing and PETCT was not mandated.
In a retrospective subgroup analysis, patients with PD-L1 expression <1% did not have a benefit [85]. This led to some discussion and the European Medicines Agency did not approve durvalumab in this indication for tumours with a PD-L1 expression <1% [86]. However, in an analysis of long-term survival of 102 patients with inoperable locally advanced NSCLC and chemoradiotherapy without immunomodulation, overall survival was significantly shorter for patients with PD-L1 expression [87]. Of course, there is also some uncertainty about the possible heterogeneity of PD-L1 expression in small tumour samples [88] and further data are awaited.
In a Single Technology Appraisal for the UK National Institute for Health and Care Excellence (NICE) for patients with PD-L1-expressing tumours (≥1%) more serious adverse events were reported for durvalumab (64 (30%) out of 213 versus 18 (20%) out of 90) [89]. The Evidence Review Group raised some concerns regarding the economic analysis and NICE recommended durvalumab as an option in the subgroup of patients with concurrent platinum-based chemoradiation therapy, with a commercially managed access agreement in place. An analysis in the context of the US healthcare system concluded cost-effectiveness with an estimated incremental cost-effectiveness ratio of US$67 421 per quality-adjusted life-year and an additional US$768 million of national cancer spending in year 1 [90].
In a retrospective analysis of Japanese patients, 30% of patients were ineligible to receive durvalumab by using the criteria of the PACIFIC study [91]. In the group of 81 patients who received definitive chemoradiotherapy, radiation pneumonitis of any grade occurred in 73.9%. Of these, 12 (16.4%) developed radiation pneumonitis of grade 2 or more within 42 days after chemoradiotherapy, which is still in line with historical series. Another analysis in 82 Japanese patients found an ineligibility rate of 23% [92]. Old age (p=0.042), male sex (p=0.031) and radiation therapy with V20 (volume of the lung receiving ≥20 Gy) ≥35% (p=0.032) were associated with ineligibility after chemo-radio therapy. Moreover, ineligible patients showed shorter progression-free survival (6.6 versus 15.7 months, HR 2.61 (95% CI 1.16–5.89), p=0.016) and shorter overall survival (18.6 versus 44.3 months, HR 3.03 (95% CI 1.29–7.10), p=0.007) than eligible patients.
There are now several active clinical trials from phase I–III that use different immune-checkpoint inhibitors or different schedules, such as a concurrent application for integrating immune-checkpoint inhibitors in chemoradiotherapy schedules [72, 93–95], which are summarised in table 4. As with the trials that include surgery, various stages and conditions are included, which will impede clear interpretation of the results.
TABLE 4.
Current clinical trials on immunotherapy and chemoradiotherapy in mostly stage II and III nonsmall stage lung cancer (NSCLC)
| ClinicalTrials.gov ID | Phase | NSCLC stage | IO-drug | Protocol | Primary end-point |
| Concurrent (+consolidation) regimen | |||||
| NCT04202809 | II | IIIA–B | Durvalumab | Chemotherapy induction→chemoradiotherapy ± durvalumab→surgery if resectable | PFS |
| NCT03694236 | I/II | II/IIIA | Durvalumab | Chemoradiotherapy+durvalumab→surgery | pCR |
| NCT02343952 | II | IIIA–B | Pembrolizumab | Chemoradiotherapy→pembrolizumab consolidation | PFS |
| NCT02987998 | I | III | Pembrolizumab | Chemoradiotherapy+pembrolizumab | Safety |
| NCT03519971# | III | Unresectable | Durvalumab | Chemoradiotherapy ± durvalumab | PFS |
| NCT04013542 | I | II–III | Nivolumab/ ipilimumab |
Radiotherapy+nivolumab/ipilimumab | Safety |
| NCT03523702 | II | II–III | Pembrolizumab | Arm 1 (PD-L1 >50%): radiotherapy+pembrolizumab Arm 2 (PD-L1 1–49%): chemoradiotherapy |
PFS |
| NCT03631784 | II | III unresectable | Pembrolizumab | Chemoradiotherapy+pembrolizumab→ pembrolizumab consolidation |
Safety, ORR |
| NCT03644823 | II | III–IV | Atezolizumab | Radiotherapy+atezolizumab | Safety |
| Combination regimen | |||||
| NCT04085250 | II | III | Nivolumab | Chemotherapy ± nivolumab induction→ chemoradiotherapy→nivolumab consolidation or observation | PFS |
| NCT03589547 | II | III | Durvalumab | Durvalumab→chemoradiotherapy→durvalumab | Safety, PFS |
| NCT04380636# | III | III | Pembrolizumab | Arm 1: chemoradiotherapy+pembrolizumab→ pembrolizumab+placebo Arm 2: chemoradiotherapy+pembrolizumab→ pembrolizumab+olaparib Arm 3: chemoradiotherapy→durvalumab |
PFS, OS |
| NCT03871153 | II | Resectable III | Durvalumab | Chemoradiotherapy+durvalumab induction→surgery→durvalumab consolidation | pCR |
| NCT03693300 | II | III | Durvalumab | Chemoradiotherapy→durvalumab (fixed dose) | Safety |
| NCT03285321 | II | IIIA–B | Nivolumab/ ipilimumab |
Chemoradiotherapy→nivolumab ± ipilimumab consolidation | PFS |
| NCT04026412# | III | IIIA–C | Nivolumab/ ipilimumab |
Arm 1: chemoradiotherapy+nivolumab→ nivolumab/ipilimumab consolidation Arm 2: chemoradiotherapy+nivolumab→ nivolumab consolidation Arm 3: chemoradiotherapy→durvalumab consolidation |
PFS, OS |
| NCT04062708 | II | III resectable | Durvalumab | Chemotherapy+durvalumab induction→ surgery→radiotherapy→durvalumab consolidation | pCR |
| NCT03237377 | II | III resectable | Durvalumab/ tremelimumab |
Radiotherapy+durvalumab ± tremelimumab→surgery | pCR |
| NCT03871153 | II | III resectable | Durvalumab | Chemotherapy+durvalumab induction→radiotherapy+durvalumab→surgery→durvalumab consolidation | pCR |
| NCT03663166 | I/II | III | Nivolumab/ ipilimumab |
Chemoradiotherapy+ipilimumab→nivolumab consolidation | Safety, PFS |
| Induction regimen | |||||
| NCT04287894 | Ib | III | Durvalumab/ tremelimumab |
Durvalumab+tremelimumab induction→chemoradiotherapy | Safety |
| NCT03102242 | II | IIIA–B | Atezolizumab | Atezolizumab induction→chemoradiotherapy | DCR |
Data obtained from www.clinicaltrials.gov on 7 August 2020. IO: immune-oncology; pCR: pathological complete response; PFS: progression-free survival; OS: overall survival: PD-L1: programmed death ligand 1; ORR: overall response rate; DCR: disease control rate. #: phase III trials.
Many factors in the immunochemoradiotherapy setting have to be analysed in order to optimise the application to select the right patients for safety and efficacy. Chemoradiotherapy can have side-effects that can interfere with the effects of immune-checkpoint inhibition. There can be direct cardiac toxicity, [17–19] but radiotherapy of immune-relevant body systems may also induce immunosuppression, which can negatively influence patient outcomes [96, 97]. Concurrent chemoradiotherapy may dynamically alter PD-L1 expression and numbers of CD8+ tumour infiltrating lymphocytes [98, 99]. Pre-therapeutic blood-based parameters, or their change during chemoradiotherapy, may predict prognosis [100–102].
Chemotherapy and radiotherapy, as well as immune-checkpoint inhibition, can cause damage to the lung. Risk depends on pre-conditions of the patient and its reserve, the specific chemotherapy substances [103] and the mode of radiotherapy. With adequate techniques in radiotherapy these side-effects can be reduced [104]. But also, patient-related factors such as smoking history are of relevance [105]. Patient symptoms, performance status and quality of life before and during chemoradiotherapy influence the outcome in stage III NSCLC [106–108]. This all has to be taken into account and supportive measures are recommended for patients undergoing intensified treatment [109]. Relapses, radiation and immune-related side-effects occur primarily within the first year after immunochemoradiotherapy [110, 111], which suggests that more intensive follow-up during this period may be needed.
Conclusions
Recent years have seen a major wave of developments in treating locally advanced NSCLC. We have more knowledge about driver mutations and immune aspects in patients with stage III disease. With the broader availability of fluorodeoxyglucose PET-CT we have learned about the relevance of metabolic activity for radiotherapy planning and prognosis. In addition, PET-CT may determine the necessity of additional therapy after locally applied therapies or therapies in combination. New techniques allow definitive local treatments for more patients and the addition of immune-checkpoint inhibition to chemoradiotherapy improves progression-free and overall survival. However, there are also additional side-effects, which in combination with more aggressive local approaches, demand among others, consequent evaluation and monitoring of pulmonary and cardiac function. In all clinical trials and registries, quality of life of patients should be evaluated. Driver mutations seem to have an adverse effect on the overall outcome in locally advanced NSCLC. But more data and adequately specified data are needed. Whether adjuvant-targeted EGFR inhibition after surgery has to be given for tumours with classical activating EGFR mutations can be decided on the basis of more mature data. The advantage in progression-free survival with osimertinib after surgery is promising [50], but there was also a clear advantage with gefitinib in the Chinese trial, but this did not translate into an advantage for overall survival [49]. Therefore, at the moment the question remains, whether we only postpone metastasis for the time that an EGFR-tyrosine kinase inhibitor is given.
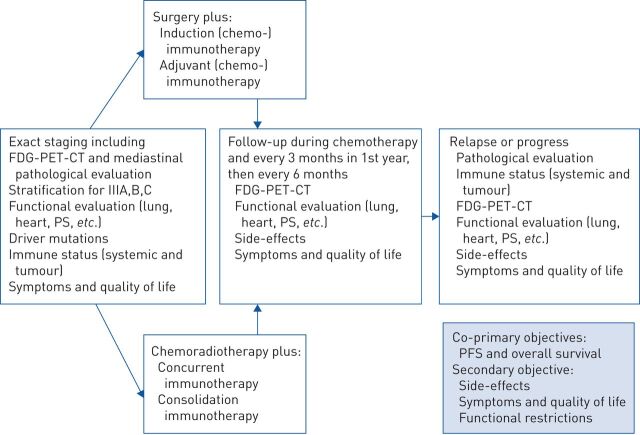
The biological and clinical picture in locally advanced NSCLC is very heterogenous. In stage IV NSCLC it is now common sense that we have to be precise regarding staging (such as for oligometastatic disease) and histological, molecular and immunological aspects. By this we can improve the management and outcome of our patients with metastasised NSCLC. Until now these aspects have mostly not been taken into consideration for clinical trials and registries of locally advanced NSCLC. We believe that this would also be necessary in these locally advanced situations and propose a stringent functional evaluation, staging and molecular and immunological evaluation before, during and after therapeutic interventions (figure 1).
FIGURE 1.
Proposal for further strategies in clinical trials and registries about the management of locally advanced nonsmall cell lung cancer (NSCLC). FDG: fluorodeoxyglucose; PET: positron emission tomography; CT: computed tomography; PS: performance status; PFS: progression-free survival.
Footnotes
Number 4 in the Series “Thoracic oncology” Edited by Rudolf Huber and Peter Dorfmüller
Previous articles in this series: No. 1: Eichhorn F, Winter H. How to handle oligometastatic disease in nonsmall cell lung cancer. Eur Respir Rev 30: 2021; 200234. No. 2: Asciak R, George V, Rahmna NM. Update on biology and management of mesothelioma. Eur Respir Rev 30: 2021; 200226. No. 3: Finazzi T, Schneiders FL, Senan S. Developments in radiation techniques for thoracic malignancies. Eur Respir Rev 30: 2021; 200224.
Provenance: Commissioned article, peer reviewed.
Conflict of interest: R.M. Huber reports grants from German Ministry of Education and Research and AstraZeneca, and personal fees from AstraZeneca, BMS, Bayer, Boehringer Ingelheim, Lilly, MSD, Takeda, Pfizer, Novartis, Roche, Celgene, Abbvie and Tesaro, outside the submitted work.
Conflict of interest: D. Kauffmann-Guerrero reports personal fees from AstraZeneca, BMS, Roche, MSD, Pfizer and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: H. Hoffmann has nothing to disclose.
Conflict of interest: M. Flentje has nothing to disclose.
References
- 1.Goldstraw P, Chansky K, Crowley J, et al. . The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016; 11: 39–51. doi: 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 2.Huber RM, De Ruysscher D, Hoffmann H, et al. . Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev 2019; 28: 190024. doi: 10.1183/16000617.0024-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postmus PE, Kerr KM, Oudkerk M, et al. . Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28: Suppl. 4: iv1–iv21. doi: 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 4.Kris MG, Gaspar LE, Chaft JE, et al. . Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non–small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline update. J Clin Oncol 2017; 35: 2960–2974. doi: 10.1200/JCO.2017.72.4401 [DOI] [PubMed] [Google Scholar]
- 5.Putora PM, Leskow P, McDonald F, et al. . International guidelines on stage III N2 nonsmall cell lung cancer: surgery or radiotherapy? ERJ Open Res 2020; 6: 00159-2019. doi: 10.1183/23120541.00159-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley JD, Hu C, Komaki RR, et al. . Long-term results of NRG oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol 2020; 38: 706–714. doi: 10.1200/JCO.19.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sculier JP, Lafitte JJ, Berghmans T, et al. . A phase III randomised study comparing concomitant radiochemotherapy with cisplatin and docetaxel as induction versus consolidation treatment in patients with locally advanced unresectable non-small cell lung cancer. Lung Cancer 2018; 117: 32–37. doi: 10.1016/j.lungcan.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 8.Kenmotsu H, Yamamoto N, Yamanaka T, et al. . Randomized phase III study of pemetrexed plus cisplatin versus vinorelbine plus cisplatin for completely resected stage II to IIIA nonsquamous non-small-cell lung cancer. J Clin Oncol 2020; 38: 2187–2196. doi: 10.1200/JCO.19.02674 [DOI] [PubMed] [Google Scholar]
- 9.Tan WL, Chua KLM, Lin C-C, et al. . Asian Thoracic Oncology Research Group expert consensus statement on optimal management of stage III NSCLC. J Thorac Oncol 2020; 15: 324–343. doi: 10.1016/j.jtho.2019.10.022 [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Lorente D, Navarro-Ripoll R, Guzman R, et al. . Prehabilitation in thoracic surgery. J Thorac Dis 2018; 10: Suppl. 22, S2593–S2600. doi: 10.21037/jtd.2018.08.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotarla I, Boron ML, Cullen SL, et al. . Treatment decision drivers in stage III non-small-cell lung cancer: outcomes of a web-based survey of oncologists in the United States. JCO Oncol Pract 2020; 16: e1232–e1242. [DOI] [PubMed] [Google Scholar]
- 12.Zemanova M, Pirker R, Petruzelka L, et al. . Care of patients with non-small-cell lung cancer stage III – the Central European real-world experience. Radiol Oncol 2020; 54: 209–220. doi: 10.2478/raon-2020-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsao AS, Jolly S, Lee JM. Updates in local-regionally advanced non–small cell lung cancer. Am Soc Clin Oncol Educ Book 2019; 39: 553–562. doi: 10.1200/EDBK_237839 [DOI] [PubMed] [Google Scholar]
- 14.Kuijvenhoven JC, Leoncini F, Crombag LC, et al. . Endobronchial ultrasound for the diagnosis of centrally located lung tumors: a systematic review and meta-analysis. Respiration 2020; 99: 441–450. doi: 10.1159/000500363 [DOI] [PubMed] [Google Scholar]
- 15.Muthu V, Sehgal IS, Dhooria S, et al. . Efficacy of endosonographic procedures in mediastinal re-staging of lung cancer after neoadjuvant therapy: a systematic review and diagnostic accuracy meta-analysis. Chest 2018; 154: 99–109. doi: 10.1016/j.chest.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 16.Brunelli A, Charloux A, Bolliger CT, et al. . ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009; 34: 17–41. doi: 10.1183/09031936.00184308 [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Huang J, Wu W, et al. . The impact of right ventricular function on prognosis in patients with stage III non-small cell lung cancer after concurrent chemoradiotherapy. Int J Cardiovasc Imaging 2019; 35: 1009–1017. doi: 10.1007/s10554-019-01590-0 [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Ta S, Wu W, et al. . Prognostic and added value of echocardiographic strain for prediction of adverse outcomes in patients with locally advanced non-small cell lung cancer after radiotherapy. Ultrasound Med Biol 2019; 45: 98–107. doi: 10.1016/j.ultrasmedbio.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 19.Cai G, Liang S, Li C, et al. . Left ventricular systolic dysfunction is a possible independent risk factor of radiation pneumonitis in locally advanced lung cancer patients. Front Oncol 2019; 9: 1511. doi: 10.3389/fonc.2019.01511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashdan S, Minna JD, Gerber DE. Diagnosis and management of pulmonary toxicity associated with cancer immunotherapy. Lancet Respir Med 2018; 6: 472–478. doi: 10.1016/S2213-2600(18)30172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Sanctis A, Taillade L, Vignot S, et al. . Pulmonary toxicity related to systemic treatment of nonsmall cell lung cancer. Cancer 2011; 117: 3069–3080. doi: 10.1002/cncr.25894 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Huang Z, Xing L, et al. . Radiation recall pneumonitis induced by anti-PD-1 blockade: a case report and review of the literature. Front Oncol 2020; 10: 561. doi: 10.3389/fonc.2020.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho JY, Kim J, Lee JS, et al. . Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 2018; 125: 150–156. doi: 10.1016/j.lungcan.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 24.Voong KR, Hazell SZ, Fu W, et al. . Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-small-cell lung cancer. Clin Lung Cancer 2019; 20: e470–e479. doi: 10.1016/j.cllc.2019.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cathcart-Rake EJ, Sangaralingham LR, Henk HJ, et al. . A population-based study of immunotherapy-related toxicities in lung cancer. Clin Lung Cancer 2020; 21: 421–427. doi: 10.1016/j.cllc.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Shimizu J, Hasegawa T, et al. . Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung Cancer 2018; 125: 212–217. doi: 10.1016/j.lungcan.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 27.Rades D, Glatzel E, Werner EM, et al. . Prevalence and characteristics of symptomatic pneumonitis after radiotherapy of patients with locally advanced lung cancer. Anticancer Res 2019; 39: 6909–6913. doi: 10.21873/anticanres.13911 [DOI] [PubMed] [Google Scholar]
- 28.O'Reilly S, Jain V, Huang Q, et al. . Dose to highly functional ventilation zones improves prediction of radiation pneumonitis for proton and photon lung cancer radiotherapy. Int J Radiat Oncol Biol Phys 2020; 107: 79–87. doi: 10.1016/j.ijrobp.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 29.Appel S, Bar J, Alezra D, et al. . Image-guidance triggered adaptive replanning of radiation therapy for locally advanced lung cancer: an evaluation of cases requiring plan adaptation. Br J Radiol 2020; 93: 20190743. doi: 10.1259/bjr.20190743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mounessi FS, Eckardt J, Holstein A, et al. . Image-based lung functional radiotherapy planning for non-small cell lung cancer. Strahlenther Onkol 2020; 196: 151–158. doi: 10.1007/s00066-019-01518-6 [DOI] [PubMed] [Google Scholar]
- 31.Flentje M, Huber RM, Engel-Riedel W, et al. . GILT – a randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non-small cell lung cancer. Strahlenther Onkol 2016; 192: 216–222. doi: 10.1007/s00066-016-0941-8 [DOI] [PubMed] [Google Scholar]
- 32.Nestle U, Schimek-Jasch T, Kremp S, et al. . Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): a multicentre, open-label, randomised, controlled trial. Lancet Oncol 2020; 21: 581–592. doi: 10.1016/S1470-2045(20)30013-9 [DOI] [PubMed] [Google Scholar]
- 33.Ercelep O, Alan O, Sahin D, et al. . Effect of PET/CT standardized uptake values on complete response to treatment before definitive chemoradiotherapy in stage III non-small cell lung cancer. Clin Transl Oncol 2019; 21: 499–504. doi: 10.1007/s12094-018-1949-6 [DOI] [PubMed] [Google Scholar]
- 34.Kim E, Wu HG, Keam B, et al. . Significance of (18)F-FDG PET parameters according to histologic subtype in the treatment outcome of stage III non-small-cell lung cancer undergoing definitive concurrent chemoradiotherapy. Clin Lung Cancer 2019; 20: e9–e23. doi: 10.1016/j.cllc.2018.08.018 [DOI] [PubMed] [Google Scholar]
- 35.Castello A, Toschi L, Rossi S, et al. . Predictive and prognostic role of metabolic response in patients with stage III NSCLC treated with neoadjuvant chemotherapy. Clin Lung Cancer 2020; 21: 28–36. doi: 10.1016/j.cllc.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 36.Ganem J, Thureau S, Gouel P, et al. . Prognostic value of post-induction chemotherapy 18F-FDG PET-CT in stage II/III non-small cell lung cancer before (chemo-) radiation. PLoS One 2019; 14: e0222885. doi: 10.1371/journal.pone.0222885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okazaki E, Seura H, Hasegawa Y, et al. . Prognostic value of the volumetric parameters of dual-time-point (18)F-FDG PET/CT in non-small cell lung cancer treated with definitive radiation therapy. AJR Am J Roentgenol 2019; 213: 1366–1373. doi: 10.2214/AJR.19.21376 [DOI] [PubMed] [Google Scholar]
- 38.Chen HHW, Su W-C, Guo H-R, et al. . Prognostic value of volumetric metabolic parameter changes determined by during and after radiotherapy-based 18F-FDG PET/CT in stage III non-small cell lung cancer. Kaohsiung J Med Sci 2019; 35: 151–159. doi: 10.1002/kjm2.12027 [DOI] [PubMed] [Google Scholar]
- 39.Roengvoraphoj O, Wijaya C, Eze C, et al. . Analysis of primary tumor metabolic volume during chemoradiotherapy in locally advanced non-small cell lung cancer. Strahlenther Onkol 2018; 194: 107–115. doi: 10.1007/s00066-017-1229-3 [DOI] [PubMed] [Google Scholar]
- 40.Roengvoraphoj O, Eze C, Wijaya C, et al. . How much primary tumor metabolic volume reduction is required to improve outcome in stage III NSCLC after chemoradiotherapy? A single-centre experience. Eur J Nucl Med Mol Imaging 2018; 45: 2103–2109. doi: 10.1007/s00259-018-4063-7 [DOI] [PubMed] [Google Scholar]
- 41.Arshad MA, Thornton A, Lu H, et al. . Discovery of pre-therapy 2-deoxy-2-18F-fluoro-D-glucose positron emission tomography-based radiomics classifiers of survival outcome in non-small-cell lung cancer patients. Eur J Nucl Med Mol Imaging 2019; 46: 455–466. doi: 10.1007/s00259-018-4139-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono A, Isaka M, Serizawa M, et al. . Genetic alterations of driver genes as independent prognostic factors for disease-free survival in patients with resected non-small cell lung cancer. Lung Cancer 2019; 128: 152–157. doi: 10.1016/j.lungcan.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 43.Jao K, Tomasini P, Kamel-Reid S, et al. . The prognostic effect of single and multiple cancer-related somatic mutations in resected non-small-cell lung cancer. Lung Cancer 2018; 123: 22–29. doi: 10.1016/j.lungcan.2018.06.023 [DOI] [PubMed] [Google Scholar]
- 44.Qin Q, Peng B, Li B. The impact of epidermal growth factor receptor mutations on the efficacy of definitive chemoradiotherapy in patients with locally advanced unresectable stage III non-small cell lung cancer: a systematic review and meta-analysis. Expert Rev Anticancer Ther 2019; 19: 533–539. doi: 10.1080/14737140.2019.1621754 [DOI] [PubMed] [Google Scholar]
- 45.Kelly K, Chansky K, Gaspar LE, et al. . Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non–small-cell lung cancer: SWOG S0023. J Clin Oncol 2008; 26: 2450–2456. doi: 10.1200/JCO.2007.14.4824 [DOI] [PubMed] [Google Scholar]
- 46.Goss GD, O'Callaghan C, Lorimer I, et al. . Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013; 31: 3320–3326. doi: 10.1200/JCO.2013.51.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong W-Z, Wang Q, Mao W-M, et al. . Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018; 19: 139–148. doi: 10.1016/S1470-2045(17)30729-5 [DOI] [PubMed] [Google Scholar]
- 48.Pennell NA, Neal JW, Chaft JE, et al. . SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol 2019; 37: 97–104. doi: 10.1200/JCO.18.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y-L, Zhong W, Wang Q, et al. . CTONG1104: adjuvant gefitinib versus chemotherapy for resected N1-N2 NSCLC with EGFR mutation – Final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial. J Clin Oncol 2020; 38: 15_suppl, 9005-9005.
- 50.Herbst RS, Tsuboi M, John T, et al. . Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol 2020; 38: 18_suppl, LBA5-LBA5. doi: 10.1200/JCO.2020.38.18_suppl.LBA5 [DOI] [Google Scholar]
- 51.Parikh AB, Hammons L, Gomez JE. Neoadjuvant tyrosine kinase inhibition in locally-advanced non-small cell lung cancer: two cases and a brief literature review. Anticancer Res 2019; 39: 897–902. doi: 10.21873/anticanres.13191 [DOI] [PubMed] [Google Scholar]
- 52.Zhong WZ, Chen KN, Chen C, et al. . Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol 2019; 37: 2235–2245. doi: 10.1200/JCO.19.00075 [DOI] [PubMed] [Google Scholar]
- 53.Cascone T, Gold KA, Swisher SG, et al. . Induction cisplatin docetaxel followed by surgery and erlotinib in non-small cell lung cancer. Ann Thorac Surg 2018; 105: 418–424. doi: 10.1016/j.athoracsur.2017.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akamatsu H, Harada H, Tokunaga S, et al. . A phase II study of gefitinib with concurrent thoracic radiotherapy in patients with unresectable, stage III non-small-cell lung cancer harboring EGFR mutations (WJOG6911L). Clin Lung Cancer 2019; 20: e25–e27. doi: 10.1016/j.cllc.2018.08.017 [DOI] [PubMed] [Google Scholar]
- 55.Desai S, Kim C, Veytsman I. Role of anti-EGFR targeted therapies in stage III locally advanced non-small cell lung cancer: give or not to give? Curr Oncol Rep 2019; 21: 84. doi: 10.1007/s11912-019-0835-x [DOI] [PubMed] [Google Scholar]
- 56.Ghysen K, Vansteenkiste J. Immunotherapy in patients with early stage resectable nonsmall cell lung cancer. Curr Opin Oncol 2019; 31: 13–17. doi: 10.1097/CCO.0000000000000497 [DOI] [PubMed] [Google Scholar]
- 57.Vansteenkiste J, Wauters E, Reymen B, et al. . Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol 2019; 30: 1244–1253. doi: 10.1093/annonc/mdz175 [DOI] [PubMed] [Google Scholar]
- 58.O'Brien SM, Klampatsa A, Thompson JC, et al. . Function of human tumor-infiltrating lymphocytes in early-stage non–small cell lung cancer. Cancer Immunol Res 2019; 7: 896–909. doi: 10.1158/2326-6066.CIR-18-0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S-S, Liu W, Ly D, et al. . Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol 2019; 16: 6–18. doi: 10.1038/s41423-018-0027-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brambilla E, Le Teuff G, Marguet S, et al. . Prognostic effect of tumor lymphocytic infiltration in resectable non-small-cell lung cancer. J Clin Oncol 2016; 34: 1223–1230. doi: 10.1200/JCO.2015.63.0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishihira M, Nakazato Y, Maeda S, et al. . Impact of tumor infiltrating lymphocytes and lymphoid follicle formation on patient survival following surgery for lung squamous cell carcinoma. Thorac Cancer 2019; 10: 219–225. doi: 10.1111/1759-7714.12935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui S, Dong L, Qian J, et al. . Classifying non-small cell lung cancer by status of programmed cell death ligand 1 and tumor-infiltrating lymphocytes on tumor cells. J Cancer 2018; 9: 129–134. doi: 10.7150/jca.21842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devarakonda S, Rotolo F, Tsao MS, et al. . Tumor mutation burden as a biomarker in resected non-small-cell lung cancer. J Clin Oncol 2018; 36: 2995–3006. doi: 10.1200/JCO.2018.78.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forde PM, Chaft JE, Smith KN, et al. . Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018; 378: 1976–1986. doi: 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H, Xu Y, Wan B, et al. . The clinicopathological and prognostic significance of PD-L1 expression assessed by immunohistochemistry in lung cancer: a meta-analysis of 50 studies with 11,383 patients. Transl Lung Cancer Res 2019; 8: 429–449. doi: 10.21037/tlcr.2019.08.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edlund K, Madjar K, Mattsson JSM, et al. . Prognostic impact of tumor cell programmed death ligand 1 expression and immune cell infiltration in NSCLC. J Thorac Oncol 2019; 14: 628–640. doi: 10.1016/j.jtho.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 67.Tsao MS, Le Teuff G, Shepherd FA, et al. . PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol 2017; 28: 882–889. doi: 10.1093/annonc/mdx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu H, Chen Z, Ballman KV, et al. . Correlation of PD-L1 expression with tumor mutation burden and gene signatures for prognosis in early-stage squamous cell lung carcinoma. J Thorac Oncol 2019; 14: 25–36. doi: 10.1016/j.jtho.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim SH, Go SI, Song DH, et al. . Prognostic impact of CD8 and programmed death-ligand 1 expression in patients with resectable non-small cell lung cancer. Br J Cancer 2019; 120: 547–554. doi: 10.1038/s41416-019-0398-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill A, Gong J, Wilczynski S, et al. . Complete pathologic response when adding pembrolizumab to neoadjuvant chemotherapy in stage IIIA non–small-cell lung cancer. J Oncol Pract 2018; 14: 569–571. doi: 10.1200/JOP.18.00127 [DOI] [PubMed] [Google Scholar]
- 71.Kwiatkowski DJ, Rusch V, Chaft J, et al. . Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol 2019; 37: abstr 8503. doi: 10.1200/JCO.2019.37.15_suppl.8503 [DOI] [Google Scholar]
- 72.Shu CA, Gainor JF, Awad MM, et al. . Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020; 21: 786–795. doi: 10.1016/S1470-2045(20)30140-6 [DOI] [PubMed] [Google Scholar]
- 73.Sun A, Hu C, Wong SJ, et al. . Prophylactic cranial irradiation vs observation in patients with locally advanced non-small cell lung cancer: a long-term update of the NRG Oncology/RTOG 0214 phase 3 randomized clinical trial. JAMA Oncol 2019; 5: 847–855. doi: 10.1001/jamaoncol.2018.7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Ruysscher D, Dingemans A-MC, Praag J, et al. . Prophylactic cranial irradiation versus observation in radically treated stage III non–small-cell lung cancer: a randomized phase III NVALT-11/DLCRG-02 study. J Clin Oncol 2018; 36: 2366–2377. doi: 10.1200/JCO.2017.77.5817 [DOI] [PubMed] [Google Scholar]
- 75.Wei W, Zhou J, Zhang Q, et al. . Postoperative intensity-modulated radiation therapy reduces local recurrence and improves overall survival in III-N2 non-small-cell lung cancer: a single-center, retrospective study. Cancer Med 2020; 9: 2820–2832. doi: 10.1002/cam4.2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brandt WS, Yan W, Leeman JE, et al. . Postoperative radiotherapy for surgically resected ypN2 non-small cell lung cancer. Ann Thorac Surg 2018; 106: 848–855. doi: 10.1016/j.athoracsur.2018.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao F, Li N, Xu Y, et al. . Effects of postoperative radiotherapy on survival of patients with stage IIIA resected non–small cell lung cancer: analysis of the SEER database. J Natl Compr Canc Netw 2020; 18: 718–727. doi: 10.6004/jnccn.2020.7537 [DOI] [PubMed] [Google Scholar]
- 78.Lee SJ, Yoo JW, Ju S, et al. . Quantitative severity of pulmonary emphysema as a prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. Thorac Cancer 2019; 10: 421–427. doi: 10.1111/1759-7714.12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Isaka M, Kojima H, Takahashi S, et al. . Risk factors for local recurrence after lobectomy and lymph node dissection in patients with non-small cell lung cancer: Implications for adjuvant therapy. Lung Cancer 2018; 115: 28–33. doi: 10.1016/j.lungcan.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 80.Govindan R, Senan S, Dickgreber N, et al. . Healthcare resource utilization and associated cost analysis of the PROCLAIM study in patients with stage III non-small-cell lung cancer. Curr Med Res Opin 2019; 35: 1761–1767. doi: 10.1080/03007995.2019.1623185 [DOI] [PubMed] [Google Scholar]
- 81.Ryan KJ, Skinner KE, Fernandes AW, et al. . Real-world outcomes in patients with unresected stage III non-small cell lung cancer. Med Oncol 2019; 36: 24. doi: 10.1007/s12032-019-1249-1 [DOI] [PubMed] [Google Scholar]
- 82.Rajappa S, Sharma S, Prasad K. Unmet clinical need in the management of locally advanced unresectable lung cancer: treatment strategies to improve patient outcomes. Adv Ther 2019; 36: 563–578. doi: 10.1007/s12325-019-0876-4 [DOI] [PubMed] [Google Scholar]
- 83.Levy A, Doyen J, Botticella A, et al. . [Role of immunotherapy in locally advanced non-small cell lung cancer]. Cancer Radiother 2020; 24: 67–72. doi: 10.1016/j.canrad.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 84.Antonia SJ, Villegas A, Daniel D, et al. . Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379: 2342–2350. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 85.Paz-Ares L, Spira A, Raben D, et al. . Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol 2020; 31: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peters S, Dafni U, Boyer M, et al. . Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann Oncol 2019; 30: 161–165. doi: 10.1093/annonc/mdy553 [DOI] [PubMed] [Google Scholar]
- 87.Vrankar M, Stanic K. Long-term survival of locally advanced stage III non-small cell lung cancer patients treated with chemoradiotherapy and perspectives for the treatment with immunotherapy. Radiol Oncol 2018; 52: 281–288. doi: 10.2478/raon-2018-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munari E, Zamboni G, Lunardi G, et al. . PD-L1 expression heterogeneity in non-small cell lung cancer: defining criteria for harmonization between biopsy specimens and whole sections. J Thorac Oncol 2018; 13: 1113–1120. doi: 10.1016/j.jtho.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 89.Witlox WJA, van Asselt ADI, Wolff R, et al. . Durvalumab for the treatment of locally advanced, unresectable, stage III non-small cell lung cancer: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics 2020; 38: 317–324. doi: 10.1007/s40273-019-00870-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Criss SD, Mooradian MJ, Sheehan DF, et al. . Cost-effectiveness and budgetary consequence analysis of durvalumab consolidation therapy vs no consolidation therapy after chemoradiotherapy in stage III non-small cell lung cancer in the context of the US health care system. JAMA Oncol 2019; 5: 358–365. doi: 10.1001/jamaoncol.2018.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sakaguchi T, Ito K, Furuhashi K, et al. . Patients with unresectable stage III non-small cell lung cancer eligible to receive consolidation therapy with durvalumab in clinical practice based on PACIFIC study criteria. Respir Investig 2019; 57: 466–471. doi: 10.1016/j.resinv.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 92.Hosoya K, Fujimoto D, Kawachi H, et al. . Ineligibility for the PACIFIC trial in unresectable stage III non-small cell lung cancer patients. Cancer Chemother Pharmacol 2019; 84: 275–280. doi: 10.1007/s00280-019-03885-4 [DOI] [PubMed] [Google Scholar]
- 93.Yeh J, Marrone KA, Forde PM. Neoadjuvant and consolidation immuno-oncology therapy in stage III non-small cell lung cancer. J Thorac Dis 2018; 10: Suppl. 3, S451–S459. doi: 10.21037/jtd.2018.01.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anouti B, Althouse S, Durm G, et al. . Prognostic variables associated with improved outcomes in patients with stage III NSCLC treated with chemoradiation followed by consolidation pembrolizumab: a subset analysis of a phase II study from the Hoosier Cancer Research Network LUN 14–179. Clin Lung Cancer 2020; 21: 288–293. doi: 10.1016/j.cllc.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 95.Peters S, Felip E, Dafni U, et al. . Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer 2019; 133: 83–87. doi: 10.1016/j.lungcan.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 96.Contreras JA, Lin AJ, Weiner A, et al. . Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother Oncol 2018; 128: 498–504. doi: 10.1016/j.radonc.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 97.Ladbury CJ, Rusthoven CG, Camidge DR, et al. . Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys 2019; 105: 346–355. doi: 10.1016/j.ijrobp.2019.05.064 [DOI] [PubMed] [Google Scholar]
- 98.Choe EA, Cha YJ, Kim JH, et al. . Dynamic changes in PD-L1 expression and CD8+ T cell infiltration in non-small cell lung cancer following chemoradiation therapy. Lung Cancer 2019; 136: 30–36. doi: 10.1016/j.lungcan.2019.07.027 [DOI] [PubMed] [Google Scholar]
- 99.Yoneda K, Kuwata T, Kanayama M, et al. . Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer 2019; 121: 490–496. doi: 10.1038/s41416-019-0541-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schernberg A, Mezquita L, Boros A, et al. . Neutrophilia as prognostic biomarker in locally advanced stage III lung cancer. PLoS One 2018; 13: e0204490. doi: 10.1371/journal.pone.0204490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Topkan E, Selek U, Ozdemir Y, et al. . Chemoradiotherapy-induced hemoglobin nadir values and survival in patients with stage III non-small cell lung cancer. Lung Cancer 2018; 121: 30–36. doi: 10.1016/j.lungcan.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 102.Park EY, Kim YS, Choi KH, et al. . Prognostic value of neutrophil-to-lymphocyte ratio in locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. Radiat Oncol J 2019; 37: 166–175. doi: 10.3857/roj.2019.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sheng L, Cui X, Cheng L, et al. . Risk factors of grade ≥2 radiation pneumonitis after gemcitabine induction chemotherapy for patients with non-small cell lung cancer. Radiat Oncol 2019; 14: 229. doi: 10.1186/s13014-019-1440-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grambozov B, Wolf F, Kaiser J, et al. . Pulmonary function decreases moderately after accelerated high-dose irradiation for stage III non-small cell lung cancer. Thorac Cancer 2020; 11: 369–378. doi: 10.1111/1759-7714.13276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luna JM, Chao HH, Diffenderfer ES, et al. . Predicting radiation pneumonitis in locally advanced stage II-III non-small cell lung cancer using machine learning. Radiother Oncol 2019; 133: 106–112. doi: 10.1016/j.radonc.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 106.Voong KR, Feliciano JL. Patient-reported outcome measures in definitive chemoradiation for non-small cell lung cancer. Transl Lung Cancer Res 2020; 9: 4–7. doi: 10.21037/tlcr.2019.10.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vogel J, Wang X, Troxel AB, et al. . Prospective assessment of demographic characteristics associated with worse health related quality of life measures following definitive chemoradiation in patients with locally advanced non-small cell lung cancer. Transl Lung Cancer Res 2019; 8: 332–339. doi: 10.21037/tlcr.2019.08.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kasmann L, Taugner J, Eze C, et al. . Performance status and its changes predict outcome for patients with inoperable stage III NSCLC undergoing multimodal treatment. Anticancer Res 2019; 39: 5077–5081. doi: 10.21873/anticanres.13701 [DOI] [PubMed] [Google Scholar]
- 109.De Ruysscher D, Faivre-Finn C, Nackaerts K, et al. . Recommendation for supportive care in patients receiving concurrent chemotherapy and radiotherapy for lung cancer. Ann Oncol 2020; 31: 41–49. doi: 10.1016/j.annonc.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 110.Grass GD, Naghavi AO, Abuodeh YA, et al. . Analysis of relapse events after definitive chemoradiotherapy in locally advanced non-small-cell lung cancer patients. Clin Lung Cancer 2019; 20: e1–e7. doi: 10.1016/j.cllc.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 111.Hong J, Liao Z, Zhuang Y, et al. . Prognostic factors as a function of disease-free interval after definitive (chemo)radiation for non-small cell lung cancer using conditional survival analysis. Am J Clin Oncol 2018; 41: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]