Abstract
Objectives
To determine the effects of electronic cigarettes (e-cigarettes) as a therapeutic intervention compared to nicotine replacement therapy (NRT) on nicotine abstinence.
Methods
Two authors independently searched the PubMed, Embase, PsycInfo and Cochrane Central Register of Controlled Trials databases for articles published up to and including 10 July 2021. We included randomised controlled trials (RCTs) in which nicotine e-cigarettes were compared to NRT among current cigarette users. The primary outcome was abstaining from all nicotine-delivery devices. Secondary outcomes were 1) allocated product use (e-cigarettes or NRT) among successful cigarette quitters and 2) quitting cigarettes at the end of the trial using fixed-effect Mantel–Haenszel models.
Results
We included four RCTs representing 1598 adult participants (51.0% females). The mean age of participants in these studies ranged from 41 to 54 years, while average baseline smoking ranged from 14 to 21 cigarettes per day. Compared to NRT, e-cigarette use was associated with lower nicotine abstinence rates at the longest follow-up (risk ratio 0.50 (95% CI 0.32–0.77)). Among successful cigarette quitters, the risk of allocated product use by the end of the observational time was higher for e-cigarette users compared to NRT (risk ratio 8.94 (95% CI 3.98–20.07)). E-cigarette users had higher cigarette smoking cessation rates compared to NRT users (risk ratio 1.58 (95% CI 1.20–2.08)).
Conclusions
The use of e-cigarettes as a therapeutic intervention for smoking cessation may lead to permanent nicotine dependence.
Short abstract
Clinicians should not recommend e-cigarettes for smoking cessation due to the risk of permanent nicotine dependence https://bit.ly/358oToy
Introduction
Electronic cigarettes (e-cigarettes) deliver an aerosol by heating a solution typically consisting of propylene glycol, glycerine, flavourings and nicotine [1]. In the United States, Europe, and many countries around the world, e-cigarettes are mass-marketed consumer products [2]. As consumer products, in observational studies, e-cigarettes were not associated with increased smoking cessation in the adult population [3]. Nevertheless, e-cigarettes have been promoted for smoking cessation even though, up to now, no e-cigarette has been approved as a smoking cessation medication by the Federal Drug Administration or the European Medicines Agency [3].
In 2014, a Cochrane review concluded that there was evidence from two randomised controlled trials (RCTs) that e-cigarettes help smokers to stop smoking [4]. The confidence in the result was rated “low”. In an updated 2016 Cochrane review, the same conclusion was reached [5]. Further, in the recently published 2021 update, based on the results of four RCTs with 1924 participants, the authors concluded that there is moderate-certainty evidence that e-cigarettes with nicotine increase quit rates compared to nicotine replacement therapy (NRT) [6]. However, the authors have noted an overall lack of available RCT studies, while existing studies generally had small sample sizes. Finally, another recently published meta-analysis found no difference in smoking cessation and smoking reduction between e-cigarette and NRT users [7]. Nevertheless, none of these review studies reported information on overall nicotine abstinence at the end of the observational period of these RCTs.
The Cochrane review suggests that an additional three people for every 100 would quit smoking with nicotine e-cigarettes compared to NRT [6]. Nonetheless, it has been questioned if the benefit of the three extra people who quit with e-cigarettes will outweigh the harms of long-term (maybe lifelong) use of e-cigarettes in the many people who continue using them after smoking cessation and the possible uptake of e-cigarettes among young people [8]. Unfortunately, neither the Cochrane review nor other systematic reviews provide any information on the long-term use of e-cigarettes or NRT of participants included in the RCTs.
The primary goal of this systematic review is to systematically analyse the evidence found in RCTs to evaluate the effectiveness of e-cigarettes compared to standard NRT for nicotine abstinence among current cigarette smokers. The secondary aims of this study are to examine the allocated product use (i.e. e-cigarettes and NRT) among successful cigarette quitters and cigarette cessation rates at the end of the observational period of these RCTs.
Methods
The systematic review and meta-analysis were conducted following the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [9] statement. The protocol for this study was registered in the International Prospective Register of Systematic Review with the registration number CRD42021268682.
Search methods for identification of studies
Two authors (KN and AG) independently searched PubMed, Embase, PsycINFO and Cochrane Central Register of Controlled Trials databases for articles published up to and including 10 July 2021. Search terms included: “electronic nicotine delivery system”, “ENDS”, “e-cig*”, “electr* cigar*”, “electronic nicotine”, “vape”, “vaping” AND “tobacco use cessation devices”, “NRT”, “nicotine replacement therapy”, “nicotine patch”, “nicotine gum”, “nicotine inhalator”, “nicotine lozenge*”, “nicotine nasal spray”, “nicotine mouth spray”, “nicotine mouth strips”, “nicotine microtab*”, “nicotine tablet*” AND “randomised controlled trial”, “clinical trial”, and “controlled trial”. Search results were not limited by publication year, language or for being an abstract only, but were limited to studies conducted in humans.
Records identified through the search were downloaded and imported into a reference manager database to remove duplicates. Two review authors (KN and AG) independently screened titles and abstracts for inclusion eligibility. Further, full texts of all potentially eligible manuscripts were screened independently by the authors to determine eligibility. Any inconsistencies were resolved by discussion with the third author (RH).
Eligibility criteria
Studies were included if they met the following criteria: 1) all included studies had to be RCTs; 2) the exposure was nicotine e-cigarette use, and NRT as therapeutic intervention (smoking cessation); 3) the primary outcome was quitting cigarette use; and 4) the target population was adults aged 18 years or older. We excluded studies that compared nicotine e-cigarettes to non-nicotine e-cigarettes and studies that compared different e-liquid nicotine concentrations only.
Study population
All participants were current cigarette smokers at enrolment into the trials and the amount of combustible cigarette use differed. Participants could be motivated or unmotivated to quit smoking. All healthcare and community settings were included.
Intervention of interest
The intervention of interest included the use of all types, models and brands of e-cigarettes for smoking cessation.
Comparators
All included studies compared e-cigarettes with all forms of NRT (e.g. nicotine patches, gums, inhalators, lozenges, nasal sprays, mouth sprays, mouth strips, microtabs, and combination of products). Studies that compared nicotine e-cigarettes to non-nicotine e-cigarettes and studies that only compared different e-liquid nicotine concentrations were excluded.
Outcome measures
The primary outcome measure was nicotine abstinence at the longest follow-up point, defined as abstinence from any nicotine-containing products, measured on an intention-to-treat basis (preferring biochemically validated results).
Secondary outcomes were: a) the use of allocated products (defined as the use of the intervention nicotine product (e-cigarettes or NRT) allocated to the participants at the beginning of the trial) at the end of the observational period among successful cigarette quitters, and b) smoking cessation at the longest follow-up point, defined as abstinence from combustible cigarette smoking, measured on an intention-to-treat basis (preferring biochemically validated results).
Study selection and data extraction
All studies that met the criteria for inclusion in this meta-analysis were full peer-reviewed journal publications. Two review authors (KN and AG) independently extracted data from included studies to a data collection form; any inconsistencies were resolved by discussion with the third author (RH). The following information was extracted: author, date and place of publication, study dates, design and setting, participants’ demographic characteristics (age and gender distribution), sample size (and number in each arm), a summary of intervention and control conditions, outcomes, and type biochemical validation (if any). All studies reported data on smoking cessation, but none of the eligible studies reported information on nicotine abstinence and the use of allocated products among successful cigarette quitters at the end of the trial in the original paper. For that reason, we contacted the corresponding author for further information via e-mail. Two reminder e-mails were sent if the corresponding author did not respond to the first e-mail. If no response was given, the studies were excluded from the nicotine abstinence and use of allocated products analyses.
Risk of bias assessment for included studies
The overall quality of evidence for each outcome was evaluated (independently by two review authors) using the five Grading of Recommendations Assessment, Development and Evaluation framework [10], which provides a systematic approach to presenting evidence summaries by rating the overall quality and risk of bias of all included studies jointly. Additionally, two authors independently assessed the risks of bias for each included study using the revised Cochrane Risk of Bias Tool for RCTs [11]. Any inconsistencies were resolved by discussion with the third author. Reporting bias can be assessed using funnel plots; however, there are insufficient studies to use this approach (at least 10 studies should be included in the meta-analysis) [12].
Data synthesis
We provide a narrative summary of the included studies. Where appropriate, data have been pooled for meta-analyses. Fixed-effect Mantel–Haenszel models were used to calculate the risk ratio with a 95% confidence interval [6]. Three meta-analyses were conducted: 1) a first meta-analysis synthesised the evidence from three RCTs to evaluate the effectiveness of e-cigarettes compared to standard NRT for achieving nicotine abstinence; 2) a second meta-analysis examined the allocated product use (i.e. e-cigarettes and NRT) among successful cigarette quitters at the end of the observational period; and 3) finally, a third meta-analysis assessed the effectiveness of e-cigarettes compared to standard NRT for achieving smoking cessation (four RCTs). Statistical heterogeneity between studies was assessed with I2 statistic [13] and chi-square test (Cochrane Q). An I2 value of >50% and a p-value of 0.10 for the Cochrane Q test were used as indicators of substantial heterogeneity. Additional sensitivity analyses were conducted to evaluate whether pooled results were sensitive to the removal of studies judged to be at high risk of bias. All statistical analyses were conducted using Stata software (version 16.0; Stata Corp, College Station, Texas, USA).
Results
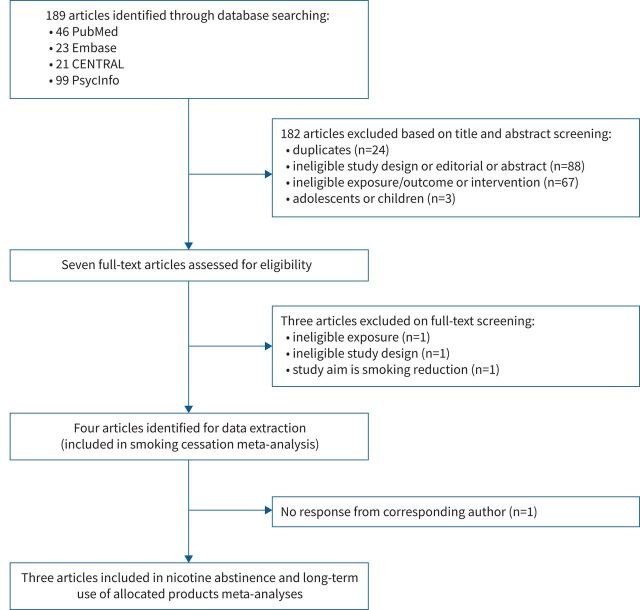
Our initial bibliographical search yielded 189 records. After excluding 24 duplicate records, 158 manuscripts were included in the title and abstract screening, and seven of those were selected for full-text screening. After screening and checking the full text of seven articles [14–20], we retained four RCT studies that were eligible for data extraction and were included in smoking cessation meta-analysis: Bonevski et al. [14], Bullen et al. [17], Hajek et al. [18] and Lee et al. [20] Reasons for exclusion are summarised in a flow diagram (see figure 1). Further, we e-mailed the corresponding author of each included study to obtain unavailable data; three authors [14, 17, 18] delivered data on nicotine abstinence and the use of allocated products for the present meta-analyses.
FIGURE 1.
Flow diagram of the systematic review process. CENTRAL: Cochrane Central Register of Controlled Trials.
The four included RCTs represented 1598 participants (51.0% female). These trials were conducted in Australia, New Zealand, the UK and the United States. All studies excluded potential participants if they reported pregnancy or breastfeeding, those with severe medical conditions, and those already enrolled in an existing cessation programme. Three of these studies were sampled among patient populations, including participants who visited cessation and withdrawal services, while one study recruited participants from the community via newspaper advertisements. The length of these trials varied between 6 and 12 weeks, while the longest follow-up point ranged between 12 weeks, 6 months and 52 weeks (see table 1). All these trials were conducted among adults (over 18 years old); the mean age of participants in these studies ranged from 41 to 54 years, while average baseline smoking ranged from 14 to 21 cigarettes per day. All studies evaluated tobacco smoking abstinence via self-reports, while three validated their results through exhaled breath carbon monoxide measurements [14, 17, 18]. More detailed information on included studies is presented in table 1; a summary of findings for each study outcome is presented in table 2.
TABLE 1.
Characteristics of included studies
| Authors (year) [ref.] | Design | Country | Number of participants | Eligibility criteria | Participants’ characteristics | Interventions | Main outcome |
| Bonevski et al. (2021) [ 14 ] | RCT: two parallel groups | New Zealand | Total n=100: 50 EC, 50 NRT | ≥18 years old; tobacco smokers and with capacity to provide informed consent | 67% women, mean age 41 years, smoking 21 (mean) cpd | Randomised to nicotine EC and NRT for 12 weeks | Self-reported 6-week continuous abstinence at 12 weeks (not validated) |
| Bullen et al. (2013) [ 17 ] | RCT: three parallel groups | Australia | Total n=100: 289 nicotine EC, 295 patch, 73 non-nicotine EC (this group was not included in this review) | ≥18 years old; smoked ≥10 cpd over past year, and wanted to stop smoking | 62% women, mean age 42 years, smoking 18 (mean) cpd | Randomised to nicotine EC, NRT, and non-nicotine EC for 12 weeks | Continuous abstinence 6 months after quit day (biochemically validated) |
| Hajek et al. (2019) [ 18 ] | RCT: two parallel groups | UK | Total n=100: 439 EC, 447 NRT | ≥18 years old; with no strong preference to EC and NRT, and were currently not using either type of product | 48% women; median age 41 years, smoking 15 (median) cpd | Randomised to nicotine EC and NRT (for at least 4 weeks and up to 3 months) | Continuous abstinence 52 weeks after quit day (biochemically validated) |
| Lee et al. (2018) [ 20 ] | RCT: two parallel groups | USA | Total n=100: 20 EC, 30 NRT | ≥18 years old; currently smoked ≥2 cpd and at least once in the last 7 days | 10% women; mean age 54 years, smoking 14 (mean) cpd | Randomised to nicotine EC (6-week supply) and NRT (5 weeks nicotine patch, 1 week placebo match) | 7-day point prevalence abstinence at 6 months (biochemically validated) |
cpd: cigarettes per day; EC: e-cigarette; NRT: nicotine replacement therapy; RCT: randomised controlled trial.
TABLE 2.
Summary of findings: e-cigarettes versus nicotine replacement therapy (NRT) for nicotine abstinence, allocated product use and smoking cessation
| Outcomes | Relative effect (risk ratio (95% CI)) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Nicotine abstinence | 0.50 (95% CI 0.32–0.77) | 1568 (three RCTs) | Moderate | Downgraded one level due to imprecision (i.e. overall, a small number of events) |
| Allocated product use among successful tobacco quitters at the end of the trial | 8.94 (95% CI 3.98–20.07) | 180 (three RCTs) | Low | Downgraded two levels due to imprecision (i.e. overall, a small number of events and too wide confidence intervals) |
| Smoking cessation | 1.58 (95% CI 1.20–2.08) | 1598 (four RCTs) | Moderate | Downgraded one level due to imprecision (i.e. overall, a small number of events) |
Patient or population: adult current smokers; setting: Australia, New Zealand, UK and USA; intervention: e-cigarettes; comparison: NRT. GRADE: Grading of Recommendations Assessment, Development and Evaluation; RCT: randomised controlled trial. GRADE Working Group grades of evidence [10]: high – the authors have a lot of confidence that the true effect is similar to the estimated effect; moderate – the true effect is probably close to the estimated effect; low – the true effect might be markedly different from the estimated effect; very low – the true effect is probably markedly different from the estimated effect.
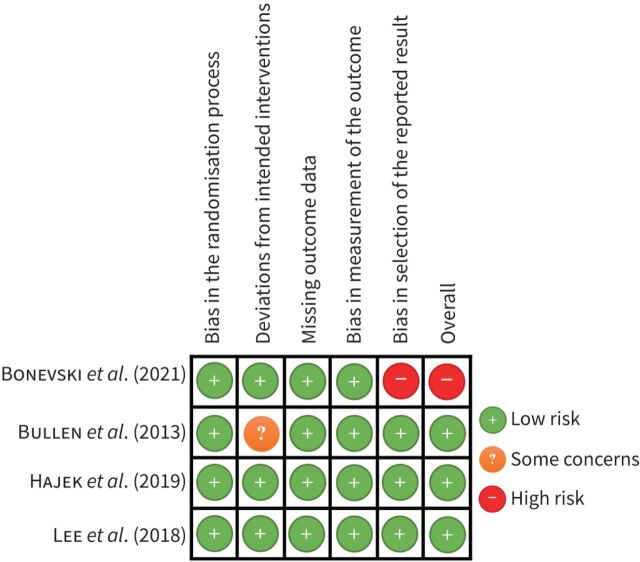
Risk of bias in included studies
Overall, the Bullen et al. [17], Hajek et al. [18] and Lee et al. [20] studies were judged be at low risk of bias, while the Bonevski et al. [14] study was judged to be at high risk of bias. Detailed information on the risk of bias assessment of each included study is reported in table 3. Figure 2 summarises the risk of bias in included studies.
TABLE 3.
Detailed information on the risk of bias in included studies
| Authors (year) [ref.] | Bias in the randomisation process | Deviations from intended interventions | Missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported result |
| Bonevski et al. (2021) [ 14 ] | Low risk | Low risk | Low risk | High risk: self-reported abstinence data without biochemical validation | Low risk |
| Bullen et al. (2013) [ 17 ] | Low risk | Some concerns: adherence to study treatments was significantly higher in the EC group compared to the NRT group due to the trial context: i.e. participants in the EC group were provided with ECs and replacement cartridges, while those in the NRT group were provided with exchange cards redeemable for patches from community pharmacies | Low risk | Low risk | Low risk |
| Hajek et al. (2019) [ 18 ] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lee et al. (2018) [ 20 ] | Low risk | Low risk | Low risk | Low risk | Low risk |
EC: e-cigarette; NRT: nicotine replacement therapy.
FIGURE 2.
Risk of bias for each study. Studies: Bonevski et al. (2021) [14], Bullen et al. (2013) [17], Hajek et al. (2019) [18], Lee et al. (2018) [20].
Effects of interventions
Nicotine abstinence
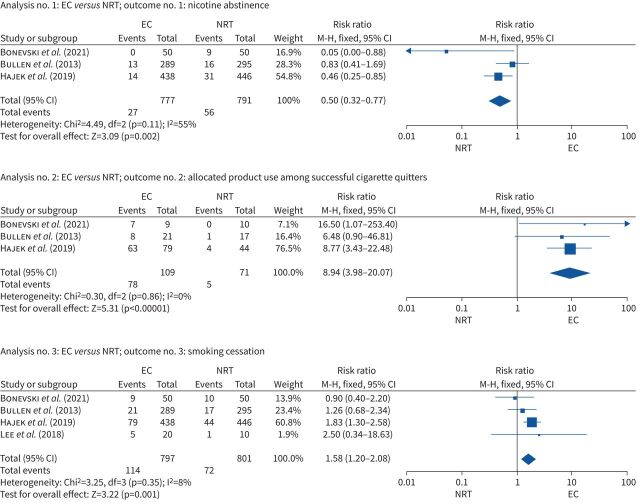
The corresponding authors for the three studies provided data on nicotine abstinence and the allocated product use at the end of the trial. Data from the Bonevski et al. [14] (risk ratio 0.05 (95% CI 0.01–0.88)) and Hajek et al. [18] (risk ratio 0.46 (95% CI 0.25–0.85)) studies demonstrated that participants randomised to e-cigarettes had significantly lower nicotine abstinence rates compared to those randomised to NTRs; in the Bullen et al. [17] study, this difference between two groups was not significant (risk ratio 0.83 (95% CI 0.41–1.69)). Pooled data from these three studies showed lower nicotine abstinence rates in participants randomised to e-cigarettes than in those randomised to NTRs (risk ratio 0.50 (95% CI 0.32–0.77); I2=55%, Cochrane Q p=0.11; 1568 participants; see figure 3).
FIGURE 3.
Pooled results for nicotine abstinence, allocated product use and smoking cessation. Studies: Bonevski et al. (2021) [14], Bullen et al. (2013) [17], Hajek et al. (2019) [18], Lee et al. (2018) [20]. EC: e-cigarette; df: degree of freedom; M–H: Mantel–Haenszel; NRT: nicotine replacement therapy.
Allocated product use among successful tobacco quitters
Data from the Hajek et al. [18] and Bonevski et al. [14] studies demonstrated that allocated product use among successful tobacco quitters at the end of the trial was statistically significantly higher among participants randomised to e-cigarettes (risk ratio 8.77 (95% CI 3.42–22.48) and risk ratio 16.50 (95% CI 1.07–253.40), respectively) than among those randomised to NTRs. This difference between the two groups was not found to be significant in the Bullen et al. [17] study (risk ratio 6.48 (95% CI 0.90–46.81)). Pooled data from these three studies showed higher allocated product use at the end of the trial in successful tobacco quitters randomised to e-cigarettes than in those randomised to NTRs (risk ratio 8.94 (95% CI 3.98–20.07); I2=0%; 180 participants; see figure 3).
Smoking cessation
All studies included in this review reported on tobacco smoking cessation outcomes. In the Bonevski et al. [14] study, there was no significant difference between the e-cigarette and NRT groups in self-reported 6-week continuous tobacco abstinence at 12 weeks (risk ratio 0.90 (95% CI, 0.40–2.02)). Similarly, there was no significant difference between groups in 7-day self-reported point prevalence smoking abstinence at 6 months (risk ratio 2.50 (95% CI 0.34–18.63)) in the Lee et al. [20] study, and in carbon monoxide validated self-reported continuous smoking abstinence at 6 months (risk ratio 1.26 [95% CI 0.68–2.34)) in the Bullen et al. [17] study. Contrary to the other three studies, in the Hajek et al. [18] study, participants randomised to the e-cigarette group had a higher self-reported carbon monoxide validated smoking cessation rate at 52 weeks (risk ratio 1.83 (95% CI 1.30–2.58)) than those randomised to the NRT group. Further, pooled data from these four studies demonstrated higher smoking cessation rates in participants randomised to e-cigarettes than to NTRs (risk ratio 1.58 (95% CI 1.20–2.08); I2=8%; 1598 participants; see figure 3).
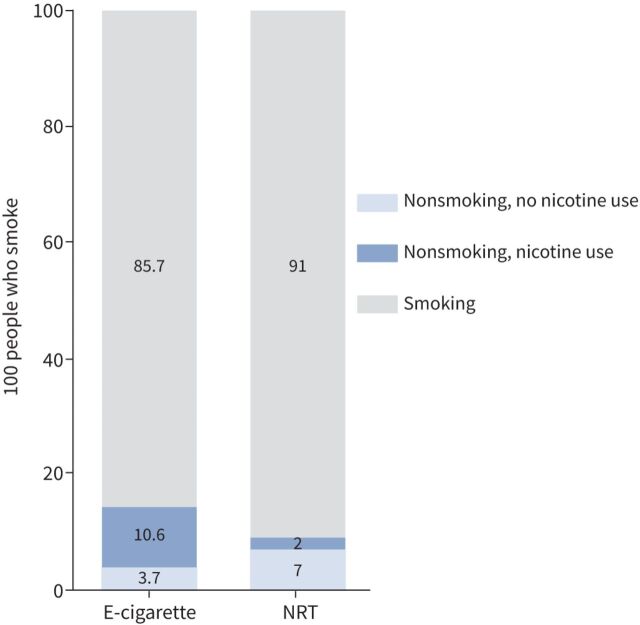
The data from these studies suggest that for every 100 people using e-cigarettes to stop smoking, 14 or 15 might successfully quit, but 10 or 11 of them will continue using e-cigarettes; whereas only nine out of 100 people might quit using NTRs, but only two of them will continue using nicotine (see figure 4).
FIGURE 4.
Anticipated absolute effects. NRT: nicotine replacement therapy.
Sensitivity analyses
Additional sensitivity analyses for all study outcomes were performed by removing the Bonevski et al. study [14], which was judged to be at high risk of bias and had the shortest follow-up period. After removing this study, the effect size for smoking cessation (risk ratio 1.69 (95% CI 1.26–2.27); I2=0%), nicotine abstinence (risk ratio 0.59 (95% CI 0.37–0.93); I2=33.5%), and allocated product use outcomes (risk ratio 8.37 (95% CI 3.57–19.59); I2=0%) remained in the same direction and significant.
Discussion
We summarised the literature comparing the effectiveness of e-cigarettes versus NRT for nicotine abstinence. The results of our meta-analyses based on RCTs indicate that using e-cigarettes as a therapeutic intervention decreased nicotine abstinence rates compared to standard NRT.
Results of a recent Cochrane review [6] and other systematic reviews and meta-analyses [3, 7, 21] indicating a therapeutic effect of e-cigarettes have been replicated by the present study. However, our study is the first to show that the use of e-cigarettes as a therapeutic intervention may have a negative effect on nicotine abstinence in RCTs compared to NRTs. In other words, most of the smokers who quit smoking with the help of e-cigarettes continue to use e-cigarettes until the end of the observational period of the RCTs. This can be seen as an indicator of nicotine dependence [22]. While there is a wealth of literature on the harmful effects of smoking, much less is known about nicotine itself isolated from tobacco. Nicotine is a stimulant in low doses and a depressant of nervous activity in very high doses [23].
Although e-cigarettes were introduced to help abstain from smoking and as a less harmful alternative for those not willing to quit cigarettes, there is growing evidence suggesting that thousands of chemicals can be found in the e-cigarette liquid and aerosol [24]. These include metal nanoparticles, propylene glycol, acrolein, diacetyl, and other additives which can cause toxic, carcinogenic and epigenetic modifications and adversely impact health [25–27]. Furthermore, it has been demonstrated that e-cigarette aerosol exposure could lead to increased epithelial cell and macrophage death in the lung and impair important macrophage functions that are essential for the maintenance of lung function [28]. Another review study reported that e-cigarette aerosol exposure might contribute to DNA damage, pulmonary inflammation, oxidative stress, and oral diseases [29]. In addition, there is consistent evidence that e-cigarette use is associated with asthma and COPD, even after controlling for cigarette use and other covariates, which is of concern for respiratory and public health [30].
It has consistently been shown that children and adolescents are highly susceptible to nicotine addiction, which affects their brain development, even in those who smoke infrequently. Furthermore, young people who become addicted to nicotine are at greater risk of becoming lifelong tobacco consumers [31]. Given this background, concerns have been raised that the use of nicotine-containing liquids in e-cigarettes could be a gateway to the use of conventional cigarettes [32].
The current evidence for this concern is strong. The latest meta-analysis reported the pooled results for 23 studies from the United States (n=13), Germany (3), UK (2), Canada (1), Mexico (1), Netherlands (1) and Scotland (1), Finland (1), Taiwan (1) and Romania (1) of young people up to age 20. Among young people who had never smoked a cigarette at baseline, the risk of smoking among e-cigarette users at follow-up was about tripled [33]. In line with the results of an earlier meta-analysis [34], it was shown that in all 23 individual studies, there were elevations in risk of ever-e-cigarette use at baseline and subsequent ever-cigarette use at follow-up.
A Cochrane Collaboration review lists nine main adverse events related to NRT: headache, dizziness/light-headedness, nausea/vomiting, gastrointestinal symptoms, sleep/dream problems, non-ischaemic palpitations and chest pain, skin reactions, oral/nasal reactions, and hiccups, but many of these side effects were also common in the placebo group without nicotine [35]. In a large-scale study of people with chronic obstructive pulmonary disease, users of nicotine gum (who used nicotine gum over a 5-year study period) showed no indication of harm and also had lower hospitalisation rates for cardiovascular conditions compared to those who did not use the gum [36]. NRTs are a proven, safe and effective method for quitting smoking; nonetheless, their persistent use remains low. For instance, two studies estimated that persistent use of over-the-counter nicotine gum for recommended 6 months (or more) was as low as 6% [37]. Thus, whenever possible, clinicians should encourage their patients to use NRT for an adequate duration, to not drop NRT when they lapse and to combine patch treatment with other NRT forms [37].
Our findings suggest that for every 100 people using e-cigarettes to stop smoking, only four might successfully quit and abstain from nicotine, compared with seven per 100 people in the NTR group. In such a way, a few systematic reviews demonstrated that there is an increased risk of subsequent combustible smoking initiation and smoking relapse among users of e-cigarettes [38, 39]. A substantial number of individuals using e-cigarettes as a cessation device may initiate dual use of e-cigarettes and combustible tobacco [40], which is the most common use pattern, and probably riskier for health than using tobacco or e-cigarettes alone [41].
Limitations
Publication bias is always a potential concern. We should be very cautious in drawing conclusions from the present meta-analyses’ results because they are only based on four RCTs with a small number of participants. Whether the results from these clinical trials can be extrapolated to other countries beyond Australia, New Zealand, the United States and the UK, or to the thousands of products available on the global market is unknown. Differences in e-cigarette products, the nicotine concentration of e-liquids, nicotine formulation (salt versus free-base), flavouring agents, distribution strategy (free e-liquid refills versus limited e-liquid refills; e-liquids with a consistent nicotine concentration versus e-liquids with a declining nicotine concentration) and cointerventions may reduce the external validity of our findings when extrapolated to different e-cigarette products or when extrapolated outside of the clinical trial setting. Biases within existing studies and their heterogeneity may have impacted our study results.
Policy implications
Before recommending e-cigarettes to smokers, we must remember the Hippocratic Oath: “first do no harm” [8]. A strong association between industry-related conflict of interests and tobacco- and e-cigarette industry-favourable results, indicating that e-cigarettes are harmless, have been found [42]. Industry-independent research indicates that e-cigarettes are not harmless, even though the long-term health consequences are unknown [43, 44]. The Cochrane review suggests that an additional three people for every 100 would quit smoking with nicotine e-cigarettes compared to NRTs [6]. Our data indicate that only a tiny minority of smokers will stay abstinent from nicotine. We must question ourselves if the benefit of the three extra people who quit smoking with e-cigarettes will outweigh the harms of long-term e-cigarette use. We estimate from our data that 10–11 people quit tobacco use but continue e-cigarette use after the end of the intervention. Data from the largest RCT [18] indicate that 80% of the e-cigarette intervention group participants who successfully quit cigarette smoking are still using e-cigarettes. Further, recent research findings demonstrate that compared to e-cigarette only or cigarette-only use, dual use substantially increases the odds of developing respiratory symptoms [45] and cardiovascular risk factors [46].
The Cochrane review did not detect any serious side effects of e-cigarette use [6], but long-term use has not been documented, nor have the (long-term) health consequences been discussed. We recommend that updated versions of the review should also report long-term use of e-cigarettes as an outcome of the trials in order to be able to further evaluate the benefits and harms of e-cigarettes as a therapeutic intervention.
Conclusions
NRT is a proven, safe and effective method for quitting smoking. As long as e-cigarette manufacturers, now to a large extent tobacco companies, are not willing to develop their “product” as a proven smoking cessation aid, it is an ethical question why public health advocates should promote an unsafe device [47] that may lead to permanent nicotine addiction.
Acknowledgements
We are grateful to Dunja Przulj (Health and Lifestyle Research Unit, Wolfson Institute of Population Health, Queen Mary University of London, London, UK), Billie Bonevski (School of Medicine and Public Health, University of Newcastle, Callaghan, NSW, Australia) and Chris Bullen (National Institute for Health Innovation, University of Auckland, Auckland, New Zealand) for providing data for this study.
Provenance: Submitted article, peer reviewed.
Conflicts of interest: R. Hanewinkel reports receiving grants from the German Ministries of Health and Research, German Cancer Aid and German Health Insurances, payments made to the institution, outside the submitted work. J.B. Unger reports receiving NIH grants, outside the submitted work. A. Galimov reports receiving NCI/FDA Grant #U54CA180905, paid to the University of Southern California, PIs: Mary Ann Pentz and Adam Leventhal, outside the submitted work. The remaining authors have nothing to disclose.
Support statement: A. Galimov and J.B. Unger are partially supported by the National Cancer Institute and the FDA Center for Tobacco Products (CTP) Award (NCI/FDA Grant #U54CA180905). NCI, or the FDA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.National Academies of Sciences, Engineering, and Medicine . Public Health Consequences of E-Cigarettes. Washington, DC, The National Academies Press, 2018. [PubMed] [Google Scholar]
- 2.Hansen J, Hanewinkel R, Morgenstern M. Electronic cigarette advertising and teen smoking initiation. Addict Behav 2020; 103: 106243. doi: 10.1016/j.addbeh.2019.106243 [DOI] [PubMed] [Google Scholar]
- 3.Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health 2021; 111: 230–246. doi: 10.2105/AJPH.2020.305999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev 2014; 12: CD010216. doi: 10.1002/14651858.CD010216.pub2 [DOI] [PubMed] [Google Scholar]
- 5.Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2016; 9: CD010216. doi: 10.1002/14651858.CD010216.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann-Boyce J, McRobbie H, Butler AR, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2021; 9: CD010216. doi: 10.1002/14651858.CD010216.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pound CM, Zhang JZ, Kodua AT, et al. Smoking cessation in individuals who use vaping as compared with traditional nicotine replacement therapies: a systematic review and meta-analysis. BMJ Open 2021; 11: e044222. doi: 10.1136/bmjopen-2020-044222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisinger C, Vestbo J. A new Cochrane review on electronic cigarettes for smoking cessation: should we change our practice? Eur Respir J 2020; 56: 2004083. doi: 10.1183/13993003.04083-2020 [DOI] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siemieniuk R, Guyatt G. What is GRADE? 2019. https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/
- 11.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Hoboken, John Wiley & Sons, 2019. [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonevski B, Manning V, Wynne O, et al. QuitNic: a pilot randomized controlled trial comparing nicotine vaping products with nicotine replacement therapy for smoking cessation following residential detoxification. Nicotine Tob Res 2021; 23: 462–470. doi: 10.1093/ntr/ntaa143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatsukami DK, Meier E, Lindgren BR, et al. A randomized clinical trial examining the effects of instructions for electronic cigarette use on smoking-related behaviors and biomarkers of exposure. Nicotine Tob Res 2020; 22: 1524–1532. doi: 10.1093/ntr/ntz233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers Smith K, Phillips-Waller A, Pesola F, et al. E-cigarettes versus nicotine replacement treatment as harm reduction interventions for smokers who find quitting difficult: randomized controlled trial. Addiction 2022; 117: 224–233. doi: 10.1111/add.15628 [DOI] [PubMed] [Google Scholar]
- 17.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382: 1629–1637. doi: 10.1016/S0140-6736(13)61842-5 [DOI] [PubMed] [Google Scholar]
- 18.Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019; 380: 629–637. doi: 10.1056/NEJMoa1808779 [DOI] [PubMed] [Google Scholar]
- 19.Lee S-H, Ahn S-H, Cheong Y-S. Effect of electronic cigarettes on smoking reduction and cessation in Korean male smokers: a randomized controlled study. J Am Board Fam Med 2019; 32: 567–574. doi: 10.3122/jabfm.2019.04.180384 [DOI] [PubMed] [Google Scholar]
- 20.Lee SM, Tenney R, Wallace AW, et al. E-cigarettes versus nicotine patches for perioperative smoking cessation: a pilot randomized trial. PeerJ 2018; 6: e5609. doi: 10.7717/peerj.5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan GCK, Stjepanović D, Lim C, et al. A systematic review of randomized controlled trials and network meta-analysis of e-cigarettes for smoking cessation. Addict Behav 2021; 119: 106912. doi: 10.1016/j.addbeh.2021.106912 [DOI] [PubMed] [Google Scholar]
- 22.Buu A, Cai Z, Li R, et al. Validating e-cigarette dependence scales based on dynamic patterns of vaping behaviors. Nicotine Tob Res 2021; 23: 1484–1489. doi: 10.1093/ntr/ntab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagerström K. Nicotine: pharmacology, toxicity and therapeutic use. J Smok Cessat 2014; 9: 53–59. doi: 10.1017/jsc.2014.27 [DOI] [Google Scholar]
- 24.Tehrani MW, Newmeyer MN, Rule AM, et al. Characterizing the chemical landscape in commercial e-cigarette liquids and aerosols by liquid chromatography–high-resolution mass spectrometry. Chem Res Toxicol 2021; 34: 2216–2226. doi: 10.1021/acs.chemrestox.1c00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caliri AW, Caceres A, Tommasi S, et al. Hypomethylation of LINE-1 repeat elements and global loss of DNA hydroxymethylation in vapers and smokers. Epigenetics 2020; 15: 816–829. doi: 10.1080/15592294.2020.1724401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao DJ, Aldy K, Hsu S, et al. Review of health consequences of electronic cigarettes and the outbreak of electronic cigarette, or vaping, product use-associated lung injury. J Med Toxicol 2020; 16: 295–310. doi: 10.1007/s13181-020-00772-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Connell G, Graff DW, D'Ruiz CD. Reductions in biomarkers of exposure (BoE) to harmful or potentially harmful constituents (HPHCs) following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Toxicol Mech Methods 2016; 26: 453–464. doi: 10.1080/15376516.2016.1196282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serpa GL, Renton ND, Lee N, et al. Electronic nicotine delivery system aerosol-induced cell death and dysfunction in macrophages and lung epithelial cells. Am J Respir Cell Mol Biol 2020; 63: 306–316. doi: 10.1165/rcmb.2019-0200OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javed F, Kellesarian SV, Sundar IK, et al. Recent updates on electronic cigarette aerosol and inhaled nicotine effects on periodontal and pulmonary tissues. Oral Dis 2017; 23: 1052–1057. doi: 10.1111/odi.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wills TA, Soneji SS, Choi K, et al. E-cigarette use and respiratory disorders: an integrative review of converging evidence from epidemiological and laboratory studies. Eur Respir J 2021; 57: 1901815. doi: 10.1183/13993003.01815-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferkol TW, Farber HJ, La Grutta S, et al. Electronic cigarette use in youths: a position statement of the Forum of International Respiratory Societies. Eur Respir J 2018; 51: 1800278. doi: 10.1183/13993003.00278-2018 [DOI] [PubMed] [Google Scholar]
- 32.Bals R, Boyd J, Esposito S, et al. Electronic cigarettes: a task force report from the European Respiratory Society. Eur Respir J 2019; 53: 1801151. doi: 10.1183/13993003.01151-2018 [DOI] [PubMed] [Google Scholar]
- 33.Yoong SL, Hall A, Turon H, et al. Association between electronic nicotine delivery systems and electronic non-nicotine delivery systems with initiation of tobacco use in individuals aged <20 years. A systematic review and meta-analysis. PLoS One 2021; 16: e0256044. doi: 10.1371/journal.pone.0256044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khouja JN, Suddell SF, Peters SE, et al. Is e-cigarette use in non-smoking young adults associated with later smoking? A systematic review and meta-analysis. Tob Control 2021; 30: 8–15. doi: 10.1136/tobaccocontrol-2019-055433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartmann-Boyce J, Chepkin SC, Ye W, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev 2018; 5: CD000146. doi: 10.1002/14651858.CD000146.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray RP, Bailey WC, Daniels K, et al. Safety of nicotine polacrilex gum used by 3,094 participants in the Lung Health Study. Chest 1996; 109: 438–445. doi: 10.1378/chest.109.2.438 [DOI] [PubMed] [Google Scholar]
- 37.Zapawa LM, Hughes JR, Benowitz NL, et al. Cautions and warnings on the US OTC label for nicotine replacement: what's a doctor to do? Addict Behav 2011; 36: 327–332. doi: 10.1016/j.addbeh.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 38.Baenziger ON, Ford L, Yazidjoglou A, et al. E-cigarette use and combustible tobacco cigarette smoking uptake among non-smokers, including relapse in former smokers: umbrella review, systematic review and meta-analysis. BMJ Open 2021; 11: e045603. doi: 10.1136/bmjopen-2020-045603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barufaldi LA, Guerra RL, Rita de Cássia R, et al. Risk of smoking relapse with the use of electronic cigarettes: a systematic review with meta-analysis of longitudinal studies. Tob Prev Cessat 2021; 7: 29. doi: 10.18332/tpc/132964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owusu D, Huang J, Weaver SR, et al. Patterns and trends of dual use of e-cigarettes and cigarettes among US adults, 2015–2018. Prev Med Rep 2019; 16: 101009. doi: 10.1016/j.pmedr.2019.101009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatta DN, Glantz SA. Association of e-cigarette use with respiratory disease among adults: a longitudinal analysis. Am J Prev Med 2020; 58: 182–190. doi: 10.1016/j.amepre.2019.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pisinger C, Godtfredsen N, Bender AM. A conflict of interest is strongly associated with tobacco industry-favourable results, indicating no harm of e-cigarettes. Prev Med 2019; 119: 124–131. doi: 10.1016/j.ypmed.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 43.Kavousi M, Pisinger C, Barthelemy JC, et al. Electronic cigarettes and health with special focus on cardiovascular effects: position paper of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol 2020; 28: 1552–1566. doi: 10.1177/2047487320941993 [DOI] [PubMed] [Google Scholar]
- 44.Pisinger C, Dagli E, Filippidis FT, et al. ERS and tobacco harm reduction. Eur Respir J 2019; 54: 1902009. doi: 10.1183/13993003.02009-2019 [DOI] [PubMed] [Google Scholar]
- 45.Reddy KP, Schwamm E, Kalkhoran S, et al. Respiratory symptom incidence among people using electronic cigarettes, combustible tobacco, or both. Am J Respir Crit Care Med 2021; 204: 231–234. doi: 10.1164/rccm.202012-4441LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim C-Y, Paek Y-J, Seo HG, et al. Dual use of electronic and conventional cigarettes is associated with higher cardiovascular risk factors in Korean men. Sci Rep 2020; 10: 5612. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teriba A, Mbama U, Sharma S, et al. Evidence against e-cigarettes for smoking cessation. J Am Pharm Assoc 2021; 61: e55–e58. doi: 10.1016/j.japh.2021.05.001 [DOI] [PubMed] [Google Scholar]