Abstract
The prenatal and perinatal environments can have profound effects on the development of chronic inflammatory diseases. However, mechanistic insight into how the early-life microenvironment can impact upon development of the lung and immune system and consequent initiation and progression of respiratory diseases is still emerging. Recent studies investigating the developmental origins of lung diseases have started to delineate the effects of early-life changes in the lung, environmental exposures and immune maturation on the development of childhood and adult lung diseases. While the influencing factors have been described and studied in mostly animal models, it remains challenging to pinpoint exactly which factors and at which time point are detrimental in lung development leading to respiratory disease later in life. To advance our understanding of early origins of chronic lung disease and to allow for proper dissemination and application of this knowledge, we propose four major focus areas: 1) policy and education; 2) clinical assessment; 3) basic and translational research; and 4) infrastructure and tools, and discuss future directions for advancement. This review is a follow-up of the discussions at the European Respiratory Society Research Seminar “Early origins of lung disease: towards an interdisciplinary approach” (Lisbon, Portugal, November 2019).
Short abstract
Future research into early origins of lung disease should be centred around four major focus areas: 1) policy and education, 2) clinical assessment, 3) basic and translational research and 4) infrastructure and tools https://bit.ly/2FHqWmP
Introduction
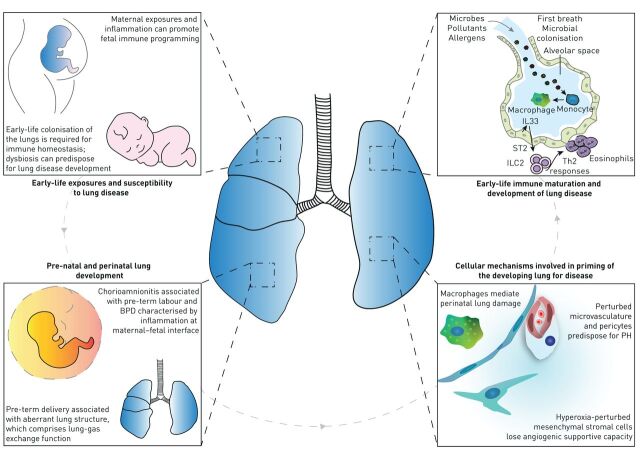
Chronic respiratory diseases, such as asthma and COPD, place an enormous burden on society. A better understanding of how early-life exposures may predispose people to develop chronic respiratory disease is key to developing prevention and treatment strategies. The prenatal and perinatal environments can have profound effects on the development and progression of chronic inflammatory diseases. However, mechanistic insight into how the early-life microenvironment can impact the development of the lung and immune system, and the consequent initiation and progression of respiratory diseases, is still emerging. Recent studies investigating the developmental origins of lung diseases have started to delineate the effects of early-life changes in 1) lung development, 2) environmental exposures and 3) immune maturation on the development of childhood and adult respiratory diseases. Alterations in what is considered “healthy” lung development may prime the neonate for increased susceptibility to develop respiratory complications in later life. Although this viewpoint is widely accepted, the exact mechanisms underlying and timeframe in which these alterations take place remain to be unravelled (figure 1).
FIGURE 1.
Early-life exposures, immune maturation and priming of the developing lung for disease. The prenatal and perinatal environments can have profound effects on the development and progression of respiratory diseases. Different maternal exposures (diet, smoking, medication usage) and maternal inflammation can promote fetal immune programming. Moreover, early-life colonisation of the lungs is imperative for shaping of the immune response. Early-life changes in lung development, specific environmental exposures and alterations in lung immune maturation following such changes and exposures can lead to the development of childhood and adult respiratory diseases. Alterations in what is considered “healthy” lung development, caused by, for example, chorioamnionitis-associated bronchopulmonary dysplasia (BPD), or aberrant lung structure associated with preterm birth may prime the neonate, via changes in immune maturation or cellular mechanisms in the lung, for increased susceptibility to develop respiratory complications in later life. IL: interleukin; ST: suppression of tumorigenicity; ILC: innate lymphoid cell; Th: T-helper; PH: pulmonary hypertension.
Prenatal and perinatal lung development
Premature delivery interrupts normal lung development
The development of the lung architecture results from the coordinated activity of several cell-driven mechanisms of lung development, together with physical forces such as those that result from breathing movements [1]. These cellular mechanisms include, for example, extracellular matrix (ECM) deposition and remodelling, primarily by myofibroblasts in the developing septa; coordination of epithelial–mesenchymal interactions by growth factors; cell–ECM interactions; and the proliferation, migration, apoptosis and transdifferentiation of the constituent cells of the developing lung at the correct time and place during lung maturation [2]. Any disturbances to these pathways result in an aberrant lung structure, which in turn compromises lung gas-exchange function, and contributes to the development of lung disease. One major disturbance is preterm delivery, which accounts for 6–12% of the 5.9 million live births annually in Europe [3]. Prematurity is a leading cause of perinatal and childhood mortality [4]. With intensive care in countries with a more advanced healthcare system, many preterm infants survive, but a major complication is bronchopulmonary dysplasia (BPD). Stunted alveolar lung development has emerged as the histopathological hallmark of BPD, which arises from accelerated lung maturation (alveolar epithelial type II cell maturation, surfactant production and alveolar wall thinning) following exposure to corticosteroids and/or (intrauterine) inflammation during prematurity. This is further complicated by the deleterious effects of prenatal and postnatal exposures to inflammatory, oxidant, stretch and other injurious agents [5, 6]. The consequences of aberrant lung development are evident in adolescent and adult survivors of BPD, who exhibit disturbances in breathing mechanics [7], as well as predisposition to lung disease [8–11]. Recent pre-clinical studies in experimental animal models of BPD support these conclusions, where neonatal exposure to hyperoxia resulted in persistent disturbances to pulmonary vascular function and right-heart remodelling [12, 13], long-term effects on airway hyperreactivity [14–16] and increased sensitivity to bleomycin-induced lung fibrosis in adult mice [17]. Thus, a solid body of evidence suggests that injury to the lung in the immediate postnatal period predisposes affected lungs to disease in later life.
A relatively common pregnancy complication associated with pre-term labour and BPD is acute chorioamnionitis, which is characterised by neutrophilic infiltration and inflammation at the maternal–fetal interface [18]. A surprising consequence of chorioamnionitis is increased production of surfactant in the fetal lung [19]. However, despite the associated increased lung compliance, chorioamnionitis inhibits alveolar and pulmonary vascular development, causing changes similar to BPD [20]. These include decreased and aberrant expression of elastin which identifies sites of secondary septation demonstrated in animal models of chorioamnionitis [21, 22]. Although the mechanisms are not entirely clear, NF-κB activation in innate immune cells and subsequent interleukin (IL)-1 signalling [23–25] inhibit FGF10 expression in mesenchymal cells. Inflammatory mediators induce transforming growth factor (TGF)-β1 and connective tissue growth factor signalling and decrease Shh mRNA levels and Gli1 protein expression in the distal lung [21, 26, 27].
Early-life exposures and susceptibility to lung disease
Maternal exposures and transplacental immune modulation
The maternal immune system plays a fundamental role in protecting the in utero environment [28, 29], and can provide signals that influence developmental trajectories within the fetal immune system, shaping immune functions post-birth [30–33]. Although mechanisms remain poorly defined, maternal inflammation can promote fetal programming that manifests as enhanced susceptibility to development of asthma (and related diseases) [31, 33, 34]. A broad spectrum of environmental maternal exposures have been implicated, including diet, stress, environmental toxins and pathogens and pre-existing clinical syndromes including obesity and asthma [34–37]. Notably, maternal inflammatory responses can be exaggerated during pregnancy (such as asthma [36, 37] and influenza [38]). The mechanisms involved in transplacental transmission of maternal-derived inflammatory immune signals are not well understood, but probably involve direct transfer and cell receptor mediated signalling [32]. Of note, fetal stem cells responsive to these programming signals contribute to development of a range of cellular subtypes that interact to play crucial roles in lung homeostatic processes and inflammation, including local lung tissue resident immune cells (e.g. alveolar macrophages and type 2 innate lymphoid cells (ILC2)) and haematopoietic stem cells in bone marrow that seed myeloid populations within respiratory tissues, providing multiple pathways for dysregulated immune function.
The capacity of the fetal immune system to be trained via maternal environmental signals provides exciting possibilities for prevention of lung diseases after birth. A seminal example is the profound reduction in allergic asthma (and related diseases) in children born to mothers exposed during pregnancy to benign microbial products from traditional farming environments [39–41]. Mechanistic studies using cord blood mononuclear cells from farmers’ children indicate that protection is associated with accelerated development of postnatal immunocompetence [42, 43]. These findings have been replicated in experimental animal models via exposure of pregnant mice to a variety of microbial extracts, and these processes were found to be Toll-like receptor-dependent [44–46]. The precise mechanisms that induce transplacental protection against disease are not well characterised, but recent evidence points to innate immune training via metabolic programming within the fetal bone marrow dendritic precursor populations [46–48]. Furthermore, exposure to microbial extracts during pregnancy may also provide potential benefits to support maternal immunity, as recently shown in pre-clinical animal models [49]. Harnessing the potential of immune training presents exciting opportunities to enhance control/regulation of maternal inflammatory responses for protection of pregnancy, fetal growth and development, in addition to training the fetal immune system for enhanced immunocompetence in the post-natal environment.
Neonatal and infant airway microbiota composition and development
Little is known about the crosstalk between immune cells and microbes (bacteria, viruses and fungi) in newborns, but evidence from mouse models suggests that immune priming in the lungs is essential for protection against infections and appropriate responses upon allergen exposure [33]. A study using lower-airways samples (tracheal aspirates) from children in the first year of life identified signs of bacterial colonisation as early as 1 day after birth [50]. The authors showed that the lower-airways microbiome developed within the first two postnatal months. During this period, three microbiome profiles were evident: two were dominated by either Staphylococcus or Ureaplasma, whereas the third was enriched with diverse bacterial genera such as Streptococcus, Prevotella, Porphyromonas and Veillonella, all known to be constituents of a healthy adult lung microbiome. Gestational age and delivery mode were important drivers of microbiome composition. The Ureaplasma profile was associated with pre-term vaginal delivery, whereas the Staphylococcus profile correlated with pre-term C-sections. The diverse microbiome profile was associated with term birth. Concomitant evaluation of the host gene expression revealed increased expression of IL33 and genes linked with IgA production pathway with increasing gestational age. This correlated with an increased predicted IgA1 protease function of the microbes. This concordance is suggestive of tightly regulated host-immune crosstalk, which may influence the development of the immune system and inform early-life interventions to prevent respiratory diseases.
In line with the lower respiratory tract microbiota, a birth cohort of 112 healthy infants indicated rapid development of the nasopharyngeal microbiota, characterised by early Staphylococcus-dominance, followed by enrichment of Corynebacterium/Dolosigranulum and a late introduction and predominance of Moraxella by the age of 3 months [51]. This microbial developmental trajectory is governed by birth mode, feeding type, antibiotics and crowding conditions, among others, and appears to be related to both bacterial and viral respiratory tract infection (RTI) susceptibility. Infants who suffered from more RTIs over the first year of life demonstrated reduced Corynebacterium/Dolosigranulum colonisation accompanied by an early Moraxella introduction at 1 month of age. These findings suggest a “window of opportunity”, within which timely microbial cues may modulate host immunity and determine RTI susceptibility [52]. Given the theory that lower RTIs may develop following micro-aspiration of upper respiratory tract pathogens, it was postulated that oral microbiota would show a similar pattern of maturation and association with RTI susceptibility as the nasopharyngeal microbiota. However, the oral microbiota assembles even more rapidly into highly stable and niche-specific microbial communities, showing no differences in maturation patterns related to RTI susceptibility. Instead, it was found that niche differentiation within the upper respiratory tract (nasopharynx versus oral cavity) is less explicit in children developing more RTIs over the first year of life. This phenomenon was even more pronounced shortly prior to RTI episodes, and characterised by an influx of oral bacteria such as Fusobacterium, Prevotella, Neisseria and streptococci into the nasopharynx [53]. These findings indicate that a “collapse” of bacterial community structure may precede RTI symptoms. Moreover, outgrowth of the number one cause of bacterial RTIs, Streptococcus pneumoniae, is controlled by the local respiratory microbiota, contributing to mucosal homeostasis [54].
However, the respiratory microbiota appears to not only be involved in protection against infections, but also play a role in controlling the severity of acute infections. In a recent study, particularly respiratory syncytial virus (RSV), Haemophilus influenzae and S. pneumoniae were indicative of infection, regardless of phenotype (pneumonia, bronchiolitis or mixed), suggesting a joint contribution of viruses and bacteria as the driver of these infections, whereas the absence of protective commensals was associated with severity [55]. Interestingly, children born pre-term are affected by respiratory viruses, including RSV, at a higher rate [56, 57]. The risk of readmission due to respiratory infections during the first year of life was shown to be 3.6 times higher in infants born in moderate or late pre-term stages [57]. One may speculate that the burden of RSV disease in these preterm infants may also be related to distorted microbial and/or immune maturation patterns.
Cellular mechanisms involved in priming of the developing lung for disease
Influence of vascular changes on lung development
The vasculature is important for branching morphogenesis of the future airways [58–61], and the pulmonary vessels are an integral part of the systemic circulation, and mainly expand through angiogenesis [62]. Angiogenesis is the process of the formation of new vessels through the expansion and sprouting of existing vessels. These new extended endothelial tubes are highly unstable and require perivascular cells, pericytes, to become fully functional vessels. The interaction between endothelial cells and pericytes is mediated through platelet-derived growth factor (PDGF)-β signalling, which induces stabilisation of endothelial cells and subsequent differentiation of pericytes into smooth muscle-like cells [63, 64].
Smooth muscle precursor cells (perivascular cells) are primed cells that express Kruppel-like factor-4. These cells respond to induced damage in adult mice, such as hyperoxia, by proliferation and migration, which eventually leads to pulmonary hypertension-like vascular abnormalities [65]. By studying paediatric congenital lung abnormalities, such as congenital diaphragmatic hernia (CDH), which is characterised by concomitant vascular problems (persistent pulmonary hypertension), it has become clear that vascular abnormalities associated with CDH develop in utero. Histological studies in premature and term CDH patients uncovered a thickening of the vascular smooth muscle layer in the mid-sized vessels as well as neo-muscularisation of capillaries. Additionally, smooth muscle cells expressed markers associated with a contractile phenotype [66]. Given the role of perivascular cells during angiogenesis, subsequent studies focused on these cells as a potential source of the vascular abnormalities observed in CDH. Clear vascular abnormalities already become apparent at the pseudoglandular and canalicular stage of development in a mouse model for CDH [67], including significant reductions in vessel length and number of branches from the main pulmonary vessels. Moreover, perivascular cells associated with vessels extended more distally compared to controls, indicative of a higher coverage of vessels by these cells, which was also shown for pulmonary hypertension in adults [68]. The perivascular cells in CDH subsequently expressed markers of advanced differentiation, as compared to controls.
Upon differentiation, perivascular cells lose the ability to support endothelial cells that form new branches during angiogenesis. As a result, lung development in CDH is characterised by a simplified microvascular development and subsequent hypoplasia, similar to BPD. In both diseases, retinoic acid signalling has been identified as a key pathway. Retinoic acid is required during the formation of the prospective lung field at the onset of lung development, and reduced retinoic acid signalling leads to increased TGF-β [69], which induces differentiation of pericytes [70]. Moreover, inhibiting retinoic acid signalling impaired vessel formation in vitro and caused a reduction in the production of collagen IV, which perivascular cells start to secrete upon interaction with endothelial cells, and has been observed in lungs of human CDH patients [67]. Considering the aberrant function of perivascular and endothelial cells in CDH and BPD patients, it will be important to create a better understanding of whether these patients are predisposed to chronic lung and vascular disease, and perhaps even infectious and inflammatory disease, in later life.
Mesenchymal cells in lung alveolar development and injury
The cellular mechanisms underlying the long-term effects of lung disease in infancy have not been clarified. However, recent studies have identified disease-relevant changes in an emerging spectrum of mesenchymal cells, namely fibroblast subsets, as well as in resident immune cells, particularly macrophages, as new cellular mechanisms on priming the developing lung for disease. Mesenchymal cells are important in orchestrating early lung development, by interacting with epithelial and endothelial cells, communicating back and forth to shape the branching structure that becomes the lung [2]. In saccular and alveolar lung development studying mesenchymal cells and their interplay with their niche has long been based on histological and in vitro studies. The mesenchymal compartment of the distal lung was long thought to consist of two types of fibroblasts based on structural properties: lipofibroblasts and myofibroblasts [71]. By studying lung regeneration after pneumonectomy in adult mice, it became clear that some lipofibroblasts function not only as progenitor cells for myofibroblasts, but also as orchestrators of new alveolar growth [72]. The notion that stromal stem cells can function as regulators of repair and regeneration, communicating with a variety of epithelial, endothelial, immune and even neuronal cell types, has been extensively studied in amphibians [73, 74]. The discovery of the potent regenerative and anti-inflammatory properties of bone marrow- and umbilical cord-derived mesenchymal stromal cells (MSCs) has led to a surge in pre-clinical and clinical studies showing the promise that these cells hold as a therapeutic agent [75]. In BPD, exogenous MSCs (derived from bone marrow or umbilical cord) have the potential to prevent and repair lung injury and perturbed alveolar development [76–78] and are currently the subject of a number of ongoing clinical trials worldwide (Clinicaltrials.gov identifiers NCT04062136, NCT03873506, NCT03558334, NCT03645525, NCT03378063, NCT03631420, NCT03392467, NCT03601416, NCT04255147, NCT02443961). However, this also leaves us to question why resident mesenchymal cell types are unable to fulfil this same role and highlights how little we really understand about the dynamics of mesenchymal cells with the developing alveolar niche. Using tracheal aspirates of pre-term infants, it was found that the shedding of mesenchymal cells into the airway compartment predicted the development of BPD [79]. Moreover, mesenchymal cells derived from the lungs of BPD patients have a strong pro-fibrotic phenotype [79] and lower expression of PDGF receptor-α and -β [80], similar to histological findings in the lungs of patients dying with BPD, suggesting that mesenchymal cells potentially contribute to BPD pathogenesis. This hypothesis is further supported by studies reporting that CD146+ lung MSCs exposed to hyperoxia in vivo [81] or in vitro [82] exhibited a diminished microvascular supportive capacity and an impaired secretion of factors that are important in orchestrating lung development. It has been suggested that exogenous MSC therapy restores the function of perturbed lung MSCs, but future studies will need to show whether this is indeed the case [83].
Mesenchymal cells are heterogeneous, making it hard to characterise and delineate mesenchymal cell interaction with the alveolar microenvironment. Lineage tracing studies and more recently single-cell RNA sequencing (scRNAseq) have allowed the identification of a variety of different mesenchymal subsets [84–88]. These mesenchymal subsets appear to be not only very heterogeneous, but also plastic in phenotype, as different subsets are found at different ages and after various exposures [88–90]. Defining how these expression-based subsets differ functionally, and what role they play in the cellular crosstalk during development, repair and susceptibility for chronic lung disease is a major challenge that remains to be addressed.
Macrophages in lung alveolarisation
Resident macrophages migrate into the lung in multiple waves during development, and have been hypothesised to contribute to lung development [26, 91]. Upon exposure to inflammation, fetal pulmonary macrophages play an active role in disrupting lung development by actively inhibiting expression of critical genes that are required for developmental processes [23]. While inflammation per se has long been considered a key pathological feature of BPD [92], a variety of inflammatory cell types have recently been implicated in perturbations to lung function, and the development of the lung structure associated with BPD. CD11b+ mononuclear cells have been demonstrated (using diphtheria toxin mediated depletion) to mitigate hyperoxia-induced lung injury in newborn mice [93]. These studies were expanded to reveal that an imbalance in Ly6Chi/Ly6Clo monocyte populations mediated hyperoxia-induced lung injury in the developing lung, which could be rescued by interferon-γ treatment [94].
Beyond these monocyte populations, alveolar macrophages were documented to mediate the deleterious impact of hyperoxia on lung alveolarisation in newborn mice (using Csf1r-lineage depletion) [95], in a study where neutrophils were documented to be without any effect (through antibody-mediated neutrophil depletion studies with anti-Ly6G IgG). These reports highlight the possibility that oxygen toxicity may reprogramme immune cells in hyperoxia-exposed developing lungs. These studies are important, since early-life exposure of mouse pups to hyperoxia profoundly impacted the adult response to influenza virus infection [96]. This suggests a persistent reprogramming of the inflammatory response during early life, which was attributed to intrinsic changes in hematopoietic cells, and changes in the reparative versus cytotoxic nature of natural killer cells [97]. The mechanisms underlying these persistent changes in immune cell behaviour that are maintained from infancy to adulthood have not been clarified. However, eosinophil-associated RNAse 1 (Ear1) in type 2 alveolar epithelial cells (AEC2) was suggested to play a role in this phenomenon [98]. There is clearly tremendous scope for the further exploration of terminal inflammatory cell programming in the lung in the immediate postnatal period, with consequences for both lung development and innate (and perhaps adaptive) immunity that last into adulthood. This is currently considered a priority area for investigational studies [99], and is explored further in the following section.
Early-life immune maturation and development of lung disease
Early-life development of the pulmonary immune system
The perinatal/postnatal period represents a critical window in the pulmonary immune development with enduring effects on pulmonary physiology, susceptibility to lung disease and resistance to infections. Postnatal lung developmental changes, genetic influences and novel (micro-) environmental cues shape the migration, expansion and/or maturation of pulmonary immune cells. Macrophage precursors, mainly originating from the fetal liver, seed the lungs during embryogenesis [100, 101]. These macrophage precursors at first reside in the interstitial space, to migrate to the alveolar space after birth, where they undergo final differentiation and remain with the potential of local self-renewal [102]. In line with the substantial adaptations of lung tissue at birth, several groups discovered the sudden release of the alarmin IL33 by AEC2 [103, 104]. By studying the impact of IL33 on post-natal immune development, it became clear that this phase is accompanied by an IL33-driven wave of type 2 immune cells, most prominently shown by the expansion and activation of ILC2s and eosinophils [103, 104]. The expansion of ILC2s shapes the functional performance of newly differentiating alveolar macrophages, by dampening their pro-inflammatory phenotype, at the cost of an increased susceptibility to bacterial pneumonia [103], and at the same time, skews scarcely present neonatal dendritic cells to drive T-helper type 2 (Th2) responses, thereby promoting asthma development at young age [104]. A scRNAseq study on mouse lungs before and after birth confirmed macrophage developmental trajectories and the IL33-driven ILC expansion [105]. Furthermore, examining the receptor–ligand interaction in lungs, a resident basophil population was revealed, which functions as an amplifier of the IL13 and colony-stimulating factor-2 driven maturation and expansion programme of alveolar macrophages after birth [105]. Another cell type shown to exert protective effects against bacterial pneumonia in neonates is pulmonary ILC3s, which develop postnatally upon the support of lung fibroblast derived insulin-like growth factor (IGF)1 [106]. Importantly, newborns with BPD exhibit reduced IGF1 levels and ILC3 numbers in their bronchoalveolar lavage fluid, as do neonatal mice with experimental BPD.
Allergic immunity
The relatively limited exposure to antigens in utero dictates that newborns are more reliant on innate immune pathways for protection against infections. However, the fetal immune system contains mature T- and B-cells that are actively suppressed by regulatory T-cells [107]. Moreover, nasal-associated lymphoid tissue is established before birth, while bronchus-associated lymphoid tissue expands rapidly postnatally [107]. This immature immune system is shaped following postnatal exposure to pathogens (bacteria, viruses and fungi), and inhaled particles such as dust, pollen and animal dander. Moreover, genetic makeup is also an important factor in shaping of the immune responses in early life and can consequently influence lung disease development, as evidenced by the high heritability of an allergic phenotype (and asthma). The timing and nature of the exposures has a significant impact on the developing immune system and may result in skewing towards health or disease. The majority of studies to date have used peripheral blood, and have shown that Th2-cell preference is required for a healthy pregnancy [108], but also that this preference is maintained during the neonatal period, reducing gradually during the first 2 years [109]. However, a deviation from this physiological Th2 skewing, with exaggerated Th2 responses in either pregnancy or the first 3 months of life has been associated with an increased risk of subsequent childhood asthma or wheeze [110]. Mouse models have shown that these Th2-cells are critical in the development of allergic inflammation [111]. Factors that may result in accentuated or prolonged Th2 skewing include maternal allergy [112], but environmental exposures are also critical, as has become apparent from farming exposure studies [113]. Similarly, diet has a profound influence on the systemic microbiome, which impacts upon the developing immune system and thus the trajectory towards disease or health [114]. Although it is apparent that the composition of inhaled exposures has a direct impact on the airway immune profile, mechanistic studies in the context of a developing immune system are scarce, while studies in children predominantly used peripheral blood rather than cells directly isolated from mucosal surfaces.
Therefore, it is apparent that early life represents a window of both vulnerability and opportunity that impact immune and tissue homeostasis, but it is not known whether pathology progresses because of an imbalance in regulatory cells versus effector cells. Peripheral blood isolated during the first postnatal year shows that the proportion of both resting naïve T-regulatory cells (Tregs; CD4+CD45RA+FoxP3+) and activated Tregs (CD4+CD45RA−FoxP3high) increased markedly from birth to 6 months of age [115]. In contrast, little is known regarding the phenotypes of cells within airways of neonates, although children who develop allergic disease in the first year of life have deficient Treg responses to microbial stimuli, but not allergens, from birth [116], highlighting the importance of skewed immunity in early life.
Inter-organ crosstalk
In recent years, the importance of inter-organ crosstalk and interactions in the initiation, development and progression of respiratory disease has become apparent, and the microbiome plays a central role in these processes [117, 118]. Alterations in the gut microbiome composition can influence early-life and adult immune development, and consequently affect the development of respiratory disease (gut–lung axis) [119–121]. Moreover, antibiotic use is strongly associated with the development of asthma, particularly when antibiotics were taken during the first 6 months of life [122]. In line with these observations, antibiotic treatment in the perinatal period enhances susceptibility to and severity of murine allergic airway inflammation [119, 123]. This effect was suggested to be linked to the loss of Tregs in the colon, which could enhance the development of allergic airway inflammation, probably mediated by the gut–lung axis. Dietary intake, in particular high fibre consumption, alters gut microbiome composition and consequently increases local and circulating short-chain fatty acid (SCFA) levels [34, 124]. Maternal high-fibre diet consumption and consequent reduction in allergic airway inflammation in offspring was found to be mediated by maternal–fetal transfer of SCFAs leading to altered gene expression in the fetal lung and changes in immune regulation [34].
Recent evidence has indicated that microbe- and age-mediated immune maturation in the skin can determine the nature and severity of allergic skin inflammation using a neonatal skin sensitisation model [125]. Interestingly, the nature of the observed skin inflammation (Th2 mediated in neonates and Th2/Th17 mediated in adults) determined the inflammatory profile (eosinophilic versus neutrophilic) in the lung following allergen challenge, suggestive of skin–lung interactions. Moreover, there is now first evidence of inflammatory immune cell seeding in remote non-allergen exposed mucosal tissues [126]. These data indicate that allergen challenge on the skin, in the gut or in the airway induces not only a local allergic inflammatory response, characterised by local eosinophil influx, but can also initiate increased eosinophil frequencies in distant non-allergen exposed mucosal tissues. There is strong evidence of interactions between the skin, gut and the lung. However, future mechanistic studies focusing on this inter-organ crosstalk are needed to dissect the mechanisms underlying these responses and their implications for disease development in early life [127].
Future perspectives
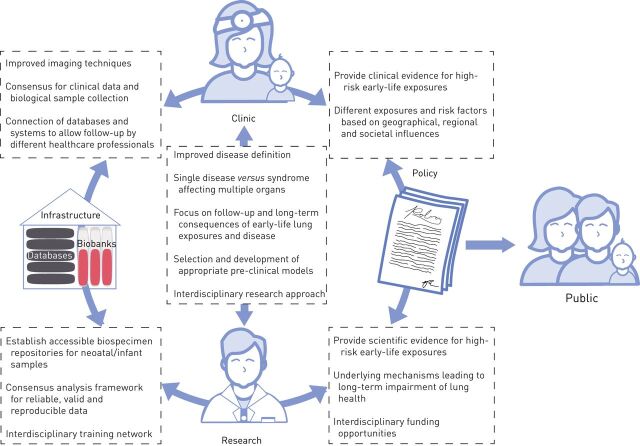
Lifelong respiratory health starts before birth. If we can provide each child with a healthy start, the chances of attaining maximal lung capacity in adult life are markedly increased. To advance our understanding of the early origins of lung disease and to allow for proper dissemination and implication of this knowledge, we propose four major focus areas and future directions for advancement within these areas (figure 2).
FIGURE 2.
Focus areas for future interdisciplinary research into early origins of chronic lung disease. To advance our understanding of the early origins of chronic lung disease four main focus areas and future directions for advancement within these areas have been identified: 1) policy; 2) clinical assessment; 3) research; and 4) infrastructure and tools. Central to these focus areas is education of researchers and clinicians, but also education of and communication to the general public. Clinical observations and research findings are required to inform policy making which consequently serves as a basis to inform, guide and educate the public. In order to facilitate proper clinical assessment and both basic and translational research, adequate infrastructure is needed in the form of databases and biobanks. Moreover, interdisciplinary training of professionals (clinicians, midwives, basic and translational researchers, and bioinformaticians and biostatisticians) working across the focus areas is required.
Policy
The largest impact of and earliest time for prevention of lung diseases is prior to birth. Education of mothers, especially by midwives, with respect to exposures to avoid prior to and during pregnancy can be complemented with advice on healthy behaviour. Such advice will require targeted evidence-based information. Policy makers need to be provided with scientific evidence of specific risk factors associated with explicit diseases. This research and policy advice should be an ongoing and active effort, as exposure to risk factors might fluctuate and change over time and display regional differences. For interventions and/or protective measures that will prevent disease development, scientific evidence is delivered by randomised controlled trials.
In underdeveloped and low-income countries, prenatal disease prevention is yet to become part of public health policies and more effort should be put into raising awareness. In these settings, cascading teaching methods and getting individuals with a respected position in society involved is imperative to spread good practices (education). It can be expected that if mothers adopt healthier lifestyles during pregnancy, they will raise their children under healthier circumstances, and this next generation will therefore take this as normal behaviour. This will not only affect early life, but also impact upon the development of adult-onset diseases. Moreover, given the lack of identified biomarkers so far, the focus should be on higher risk groups, with a prominent role for midwives.
Clinical assessment
The worst-case scenario of a difficult start to life with respect to lung function is the development of BPD, which we will use here as a prototypic early-life lung disease. As survival of pre-term infants at lower gestational ages has increased due to improvements in care and treatment, BPD has taken on new dimensions. There is a widespread consensus that the current definition is clinically useful, but too broad [128]. The fact that BPD is not a single disease, but rather a syndrome, is not captured by this definition. Moreover, the pathological features underlying the disease are difficult to assess in the clinic. In addition, the long-term consequences of BPD, such as airflow limitation, need to be clearly defined and the question remains whether this should be termed as an early COPD phenotype. Better imaging techniques, including magnetic resonance imaging, are needed to obtain greater insight in for instance alveolar simplification [129], Moreover, this will allow us to discern consequences of BPD from early COPD development and treat patients accordingly.
Awareness of the need for respiratory follow-up after suffering from early-life lung disease is only just emerging and local efforts are putting specific clinics into place. As the prognosis and long-term consequences of early-life lung disease are highly variable and cannot be predicted, these initiatives are essential in order to obtain better insight. To achieve this, the transfer of clinical data is an important issue. Patients and their data undergo transitions between multiple healthcare providers: from the obstetricians, the neonatologists and paediatricians to adult pulmonologists; and probably also between hospitals using separate databases and systems. Ethics and privacy regulations hinder the connection of these databases. For prospective monitoring, it will be essential to determine which clinical data and possibly biological samples should be collected and stored (infrastructure). Prior to establishing standardised prospective follow-up, adult pulmonologists should at least become aware of early-life events and exposures of their patients that might have significantly contributed to their current health problems (education). This insight into early-life events can offer extremely valuable information to help our understanding of the pathogenesis of adult lung diseases and the heterogeneous representation of each individual condition.
Research
A major challenge in a multidisciplinary research approach to early origins of lung disease is connecting basic and translational researchers to understand the clinical conditions and manifestations. Based on the clinical definition of the respective lung disease, various animal models are utilised for these studies. However, not a single model fully represents the complexity of the human situation. Each individual model simulates specific parts of the condition. These models are chosen based on their appropriateness to answer a specific research question and contribute to moving the field forward. However, we need to increase awareness of their limitations (such as phenotypic variability observed in patients) and search for alternatives to tackle them. Furthermore, better representative models for neonates should be established and coupled with ex vivo and in vitro models, including gut-on-a-chip or lung-on-a-chip.
Interdisciplinary approaches and collaborations will increase creativity and often the likelihood of major break-troughs. Here, “interdisciplinary” does not only relate to technologies, but also refers to the multisystemic dimensions of lung diseases and the use of multiple exposure models to reflect the complexity of the clinical situation, but also encompasses modelling and examination of the extrapulmonary effects and inter-organ crosstalk and their consequences for the disease. Furthermore, integration and connection of existing datasets from cohorts (infrastructure) should help to decipher sex-, ethnic-, age- and environment-specific effects as well as iatrogenic (for example antibiotics) causes linked to the onset of lung diseases.
To investigate how early-life exposures and alterations in the microbiome could interfere with developmental, cellular and immunological processes and ultimately contribute to development of lung disease, it is imperative that the composition of a physiologically “healthy” lung at different times in early life is defined on these different levels. This would allow for the recognition of altered processes that may predispose to the development of lung disease. Epidemiological and intervention studies (translational/clinical) are essential to define such physiological “healthy” conditions that lead to, for example, a health-promoting lung microbiota and absence of lung disease. These studies should highlight the functional role of these processes and the importance of investigating the underlying molecular mechanisms.
Finally, translational research should always aim for understanding, prevention and treatment of lung diseases. Endogenous and exogenous risk factors that negatively affect the development of the lung, pulmonary immune system and the lung microbiome and predispose to lung disease should be defined. Subsequently, new targets should be discovered to develop new treatment strategies, including maternal or post-natal nutritional interventions and antimicrobial treatments beyond antibiotics. Definition of nutritional aspects that promotes a healthy gut microbiota could enhance respiratory host defence mechanisms as part of immune homeostasis and decrease viral/bacterial infections/colonisations that may affect lung development, lung immune maturation and lung microbiome composition. Addressing these aims will not only provide new insights in the host–microbe interaction in the context of lung health and disease, but also offer new avenues for early intervention and prevention of respiratory disorders.
Infrastructure and tools
Without the proper infrastructure and tools, it is difficult to enhance and join our research efforts. Calls for interdisciplinary funding opportunities are scarce and it is imperative to join forces through collaborative networks. An important opportunity to move the field forward relies on the creation of a cohort of patients with a biospecimen repository, similar to the Global Alliance to Prevent Prematurity and Stillbirth repository (www.gapps.org/), where the focus has been placed on the sharing of samples. This will allow not only access to the samples, but will also forge a collaborative and potentially pluridisciplinary effort toward achieving common goals to understand the mechanisms underlying the early origins of lung diseases. Moreover, enhanced infrastructure is needed to facilitate the acquisition of cross-country ethical approvals to aid in these efforts.
To ensure the acquisition of more reliable, valid and reproducible data in this field between different research groups and countries, it is imperative to develop a consensus analysis framework with standard operating procedures for sample preparation and acquisition, methods, instruments and bioinformatics pipelines. This requires an interdisciplinary network including clinicians, microbiologists, immunologists, molecular and cell biologists, computational scientists and bioinformaticians.
Acknowledgements
On November 11–12, 2019, scientists from multiple disciplines gathered at a European Research Society (ERS) sponsored Research Seminar in Lisbon, Portugal, to discuss current investigations into the role of pre- and post-natal exposures, immune maturation and the influence of microbial composition on lung development and predisposition to disease from a multidisciplinary perspective. This meeting and the discussions that followed resulted in this review, including concerns and recommendations for the future of this multidisciplinary field of research. The authors acknowledge the participants of the ERS Research Seminar “Early origins of chronic lung disease: an interdisciplinary approach” and the associated scientific symposium at the annual ERS 2019 International Congress in Madrid, Spain, for their participation in the stimulating discussions leading to this review. The authors would like to thank Matthew Randall (Lausanne, Switzerland) for his contribution to figure 2.
Footnotes
Provenance: Commissioned article, peer reviewed
Conflict of interest: N.D.J. Ubags has nothing to disclose.
Conflict of interest: M.A. Alejandre Alcazar reports grants from Deutsche Forschungsgemeinschaft, Marga and Walter Boll Stiftung and Stiftung Oskar-Helene-Heim, during the conduct of the study.
Conflict of interest: S.G. Kallapur has nothing to disclose.
Conflict of interest: S. Knapp reports grants from Austrian Science Fund, during the conduct of the study.
Conflict of interest: S. Lanone has nothing to disclose.
Conflict of interest: C.M. Lloyd has nothing to disclose.
Conflict of interest: R.E. Morty has nothing to disclose.
Conflict of interest: C. Pattaroni has nothing to disclose.
Conflict of interest: N.L. Reynaert reports grants from Lung Foundation Netherlands, during the conduct of the study.
Conflict of interest: R.J. Rottier has nothing to disclose.
Conflict of interest: H.H. Smits has nothing to disclose.
Conflict of interest: W.A.A. de Steenhuijsen Piters has nothing to disclose.
Conflict of interest: D.H. Strickland has nothing to disclose.
Conflict of interest: J.J.P. Collins reports grants from Lung Foundation Netherlands, during the conduct of the study.
Support statement: This review is based on a Research Seminar funded by the European Respiratory Society. M.A. Alejandre Alcazar is supported by Deutsche Forschungsgemeinschaft (AL1632/2-1), Marga and Walter Boll Stiftung and Oskar Helene Heim Stiftung. S. Knapp is supported by the Austrian Science Fund (FWF) Special Research Program Chromatin Landscapes (L-Mac: F 6104-B21). R.E. Morty was supported by the Max Planck Society, the German Center for Lung Research and German Research Foundation (grants 390649896, 268555672, 284237345, 160966624 and 420759458). N.L. Reynaert is supported by the Lung Foundation Netherlands (6.1.16.088). J.J.P. Collins was supported by a Dirkje Postma Talent Award from the Lung Foundation Netherlands (11.1.16.152). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Whitsett JA, Kalin TV, Xu Y, et al. . Building and regenerating the lung cell by cell. Physiol Rev 2019; 99: 513–554. doi: 10.1152/physrev.00001.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 2010; 18: 8–23. doi: 10.1016/j.devcel.2009.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Euro-Peristat Project. 2018. European Perinatal Health Report: Core Indicators of the Health and Care of Pregnant Women and Babies in Europe in 2015. www.europeristat.com
- 4.Liu L, Oza S, Hogan D, et al. . Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385: 430–440. doi: 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- 5.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999; 46: 641–643. doi: 10.1203/00006450-199912000-00007 [DOI] [PubMed] [Google Scholar]
- 6.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007; 357: 1946–1955. doi: 10.1056/NEJMra067279 [DOI] [PubMed] [Google Scholar]
- 7.MacLean JE, DeHaan K, Fuhr D, et al. . Altered breathing mechanics and ventilatory response during exercise in children born extremely preterm. Thorax 2016; 71: 1012–1019. doi: 10.1136/thoraxjnl-2015-207736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tracy MK, Berkelhamer SK. Bronchopulmonary dysplasia and pulmonary outcomes of prematurity. Pediatr Ann 2019; 48: e148–e153. doi: 10.3928/19382359-20190325-03 [DOI] [PubMed] [Google Scholar]
- 9.Hadchouel A, Rousseau J, Rozé JC, et al. . Association between asthma and lung function in adolescents born very preterm: results of the EPIPAGE cohort study. Thorax 2018; 73: 1174–1176. doi: 10.1136/thoraxjnl-2017-211115 [DOI] [PubMed] [Google Scholar]
- 10.Urs R, Kotecha S, Hall GL, et al. . Persistent and progressive long-term lung disease in survivors of preterm birth. Paediatr Respir Rev 2018; 28: 87–94. [DOI] [PubMed] [Google Scholar]
- 11.Priante E, Moschino L, Mardegan V, et al. . Respiratory outcome after preterm birth: a long and difficult journey. Am J Perinatol 2016; 33: 1040–1042. doi: 10.1055/s-0036-1586172 [DOI] [PubMed] [Google Scholar]
- 12.Kumar VHS, Wang H, Kishkurno S, et al. . Long-term effects of neonatal hyperoxia in adult mice. Anat Rec 2018; 301: 717–726. doi: 10.1002/ar.23766 [DOI] [PubMed] [Google Scholar]
- 13.Menon RT, Shrestha AK, Reynolds CL, et al. . Long-term pulmonary and cardiovascular morbidities of neonatal hyperoxia exposure in mice. Int J Biochem Cell Biol 2018; 94: 119–124. doi: 10.1016/j.biocel.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Namba F, Ogawa R, Ito M, et al. . Sex-related differences in long-term pulmonary outcomes of neonatal hyperoxia in mice. Exp Lung Res 2016; 42: 57–65. doi: 10.3109/01902148.2016.1141264 [DOI] [PubMed] [Google Scholar]
- 15.Dylag AM, Haak J, Yee M, et al. . Pulmonary mechanics and structural lung development after neonatal hyperoxia in mice. Pediatr Res 2020; 87: 1201–1210. doi: 10.1038/s41390-019-0723-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regal JF, Lawrence BP, Johnson AC, et al. . Neonatal oxygen exposure alters airway hyper-responsiveness but not the response to allergen challenge in adult mice. Pediatr Allergy Immunol 2014; 25: 180–186. doi: 10.1111/pai.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yee M, Buczynski BW, Lawrence BP, et al. . Neonatal hyperoxia increases sensitivity of adult mice to bleomycin-induced lung fibrosis. Am J Respir Cell Mol Biol 2013; 48: 258–266. doi: 10.1165/rcmb.2012-0238OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallapur SG, Presicce P, Rueda CM, et al. . Fetal immune response to chorioamnionitis. Semin Reprod Med 2014; 32: 56–67. doi: 10.1055/s-0033-1361823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kallapur SG, Willet KE, Jobe AH, et al. . Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 2001; 280: L527–L536. doi: 10.1152/ajplung.2001.280.3.L527 [DOI] [PubMed] [Google Scholar]
- 20.Willet KE, Jobe AH, Ikegami M, et al. . Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res 2000; 48: 782–788. doi: 10.1203/00006450-200012000-00014 [DOI] [PubMed] [Google Scholar]
- 21.Collins JJ, Kuypers E, Nitsos I, et al. . LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 2012; 303: L778–L787. doi: 10.1152/ajplung.00280.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins JJ, Kallapur SG, Knox CL, et al. . Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol 2010; 299: L852–L860. doi: 10.1152/ajplung.00183.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackwell TS, Hipps AN, Yamamoto Y, et al. . NF-κB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol 2011; 187: 2740–2747. doi: 10.4049/jimmunol.1101495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallapur SG, Moss TJ, Ikegami M, et al. . Recruited inflammatory cells mediate endotoxin-induced lung maturation in preterm fetal lambs. Am J Respir Crit Care Med 2005; 172: 1315–1321. doi: 10.1164/rccm.200506-1007OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallapur SG, Nitsos I, Moss TJ, et al. . IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med 2009; 179: 955–961. doi: 10.1164/rccm.200811-1728OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins JJP, Tibboel D, de Kleer IM, et al. . The future of bronchopulmonary dysplasia: emerging pathophysiological concepts and potential new avenues of treatment. Front Med 2017; 4: 61. doi: 10.3389/fmed.2017.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins JJ, Kunzmann S, Kuypers E, et al. . Antenatal glucocorticoids counteract LPS changes in TGF-β pathway and caveolin-1 in ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 2013; 304: L438–L444. doi: 10.1152/ajplung.00251.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol 2013; 31: 387–411. doi: 10.1146/annurev-immunol-032712-100003 [DOI] [PubMed] [Google Scholar]
- 29.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med 2013; 19: 548–556. doi: 10.1038/nm.3160 [DOI] [PubMed] [Google Scholar]
- 30.Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clin Obstet Gynecol 2013; 56: 511–519. doi: 10.1097/GRF.0b013e31829cb9ca [DOI] [PubMed] [Google Scholar]
- 31.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol 2011; 12: 9–23. doi: 10.1038/nri3112 [DOI] [PubMed] [Google Scholar]
- 32.Apostol AC, Jensen KDC, Beaudin AE. Training the fetal immune system through maternal inflammation – a layered hygiene hypothesis. Front Immunol 2020; 11: 123. doi: 10.3389/fimmu.2020.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gollwitzer ES, Marsland BJ. Impact of early-life exposures on immune maturation and susceptibility to disease. Trends Immunol 2015; 36: 684–696. doi: 10.1016/j.it.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 34.Thorburn AN, McKenzie CI, Shen S, et al. . Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 2015; 6: 7320. doi: 10.1038/ncomms8320 [DOI] [PubMed] [Google Scholar]
- 35.Segovia SA, Vickers MH, Reynolds CM. The impact of maternal obesity on inflammatory processes and consequences for later offspring health outcomes. J Dev Orig Health Dis 2017; 8: 529–540. doi: 10.1017/S2040174417000204 [DOI] [PubMed] [Google Scholar]
- 36.Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax 2006; 61: 169–176. doi: 10.1136/thx.2005.049718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Agerbo E, Schlünssen V, et al. . Maternal asthma severity and control during pregnancy and risk of offspring asthma. J Allergy Clin Immunol 2018; 141: 886–892. doi: 10.1016/j.jaci.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 38.Siston AM, Rasmussen SA, Honein MA, et al. . Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 303: 1517–1525. doi: 10.1001/jama.2010.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ober C, Sperling AI, von Mutius E, et al. . Immune development and environment: lessons from Amish and Hutterite children. Curr Opin Immunol 2017; 48: 51–60. doi: 10.1016/j.coi.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 2010; 10: 861–868. doi: 10.1038/nri2871 [DOI] [PubMed] [Google Scholar]
- 41.Ege MJ, Mayer M, Normand AC, et al. . Exposure to environmental microorganisms and childhood asthma. N Engl J Med 2011; 364: 701–709. doi: 10.1056/NEJMoa1007302 [DOI] [PubMed] [Google Scholar]
- 42.Loss G, Bitter S, Wohlgensinger J, et al. . Prenatal and early-life exposures alter expression of innate immunity genes: the PASTURE cohort study. J Allergy Clin Immunol 2012; 130: 523–530. doi: 10.1016/j.jaci.2012.05.049 [DOI] [PubMed] [Google Scholar]
- 43.Schaub B, Liu J, Höppler S, et al. . Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol 2009; 123: 774–782. doi: 10.1016/j.jaci.2009.01.056 [DOI] [PubMed] [Google Scholar]
- 44.Conrad ML, Ferstl R, Teich R, et al. . Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med 2009; 206: 2869–2877. doi: 10.1084/jem.20090845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuijs MJ, Willart MA, Vergote K, et al. . Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015; 349: 1106–1110. doi: 10.1126/science.aac6623 [DOI] [PubMed] [Google Scholar]
- 46.Mincham KT, Scott NM, Lauzon-Joset JF, et al. . Transplacental immune modulation with a bacterial-derived agent protects against allergic airway inflammation. J Clin Invest 2018; 128: 4856–4869. doi: 10.1172/JCI122631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Netea MG, Joosten LA, Latz E, et al. . Trained immunity: a program of innate immune memory in health and disease. Science 2016; 352: aaf1098. doi: 10.1126/science.aaf1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitroulis I, Ruppova K, Wang B, et al. . Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018; 172: 147–161. doi: 10.1016/j.cell.2017.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott NM, Lauzon-Joset JF, Jones AC, et al. . Protection against maternal infection-associated fetal growth restriction: proof-of-concept with a microbial-derived immunomodulator. Mucosal Immunol 2017; 10: 789–801. doi: 10.1038/mi.2016.85 [DOI] [PubMed] [Google Scholar]
- 50.Pattaroni C, Watzenboeck ML, Schneidegger S, et al. . Early-life formation of the microbial and immunological environment of the human airways. Cell Host Microbe 2018; 24: 857–865. doi: 10.1016/j.chom.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 51.Man WH, de Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 2017; 15: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosch A, de Steenhuijsen Piters WAA, van Houten MA, et al. . Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. A prospective cohort study. Am J Respir Crit Care Med 2017; 196: 1582–1590. doi: 10.1164/rccm.201703-0554OC [DOI] [PubMed] [Google Scholar]
- 53.Man WH, Clerc M, de Steenhuijsen Piters WAA, et al. . Loss of microbial topography between oral and nasopharyngeal microbiota and development of respiratory infections early in life. Am J Respir Crit Care Med 2019; 200: 760–770. doi: 10.1164/rccm.201810-1993OC [DOI] [PubMed] [Google Scholar]
- 54.de Steenhuijsen Piters WAA, Jochems SP, Mitsi E, et al. . Interaction between the nasal microbiota and S. pneumoniae in the context of live-attenuated influenza vaccine. Nat Commun 2019; 10: 2981. doi: 10.1038/s41467-019-10814-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Man WH, van Houten MA, Mérelle ME, et al. . Bacterial and viral respiratory tract microbiota and host characteristics in children with lower respiratory tract infections: a matched case–control study. Lancet Respir Med 2019; 7: 417–426. doi: 10.1016/S2213-2600(18)30449-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broughton S, Roberts A, Fox G, et al. . Prospective study of healthcare utilisation and respiratory morbidity due to RSV infection in prematurely born infants. Thorax 2005; 60: 1039–1044. doi: 10.1136/thx.2004.037853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olabarrieta I, Gonzalez-Carrasco E, Calvo C, et al. . Hospital admission due to respiratory viral infections in moderate preterm, late preterm and term infants during their first year of life. Allergol Immunopathol 2015; 43: 469–473. doi: 10.1016/j.aller.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Tuyl M, Liu J, Wang J, et al. . Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol 2005; 288: L167–L178. doi: 10.1152/ajplung.00185.2004 [DOI] [PubMed] [Google Scholar]
- 59.Schwarz MA, Zhang F, Gebb S, et al. . Endothelial monocyte activating polypeptide II inhibits lung neovascularization and airway epithelial morphogenesis. Mech Dev 2000; 95: 123–132. doi: 10.1016/S0925-4773(00)00361-0 [DOI] [PubMed] [Google Scholar]
- 60.Schwarz MA, Caldwell L, Cafasso D, et al. . Emerging pulmonary vasculature lacks fate specification. Am J Physiol Lung Cell Mol Physiol 2009; 296: L71–L81. doi: 10.1152/ajplung.90452.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazarus A, Del-Moral PM, Ilovich O, et al. . A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development 2011; 138: 2359–2368. doi: 10.1242/dev.060723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parera MC, van Dooren M, van Kempen M, et al. . Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol 2005; 288: L141–L149. doi: 10.1152/ajplung.00148.2004 [DOI] [PubMed] [Google Scholar]
- 63.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. doi: 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 64.Sweeney M, Foldes G. It takes two: endothelial-perivascular cell cross-talk in vascular development and disease. Front Cardiovasc Med 2018; 5: 154. doi: 10.3389/fcvm.2018.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheikh AQ, Misra A, Rosas IO, et al. . Smooth muscle cell progenitors are primed to muscularize in pulmonary hypertension. Sci Transl Med 2015; 7: 308ra159. doi: 10.1126/scitranslmed.aaa9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sluiter I, van der Horst I, van der Voorn P, et al. . Premature differentiation of vascular smooth muscle cells in human congenital diaphragmatic hernia. Exp Mol Pathol 2013; 94: 195–202. doi: 10.1016/j.yexmp.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 67.Kool HM, Bürgisser PE, Edel GG, et al. . Inhibition of retinoic acid signaling induces aberrant pericyte coverage and differentiation resulting in vascular defects in congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol 2019; 317: L317–L331. doi: 10.1152/ajplung.00104.2018 [DOI] [PubMed] [Google Scholar]
- 68.Ricard N, Tu L, Le Hiress M, et al. . Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 2014; 129: 1586–1597. doi: 10.1161/CIRCULATIONAHA.113.007469 [DOI] [PubMed] [Google Scholar]
- 69.Chen F, Desai TJ, Qian J, et al. . Inhibition of Tgfβ signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 2007; 134: 2969–2979. doi: 10.1242/dev.006221 [DOI] [PubMed] [Google Scholar]
- 70.Bordenave J, Tu L, Berrebeh N, et al. . Lineage tracing reveals the dynamic contribution of pericytes to the blood vessel remodeling in pulmonary hypertension. Arterioscler Thromb Vasc Biol 2020; 40: 766–782. doi: 10.1161/ATVBAHA.119.313715 [DOI] [PubMed] [Google Scholar]
- 71.Collins JJ, Thébaud B. Lung mesenchymal stromal cells in development and disease: to serve and protect? Antioxid Redox Signal 2014; 21: 1849–1862. doi: 10.1089/ars.2013.5781 [DOI] [PubMed] [Google Scholar]
- 72.Perl AK, Gale E. FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am J Physiol Lung Cell Mol Physiol 2009; 297: L299–L308. doi: 10.1152/ajplung.00008.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science 2009; 324: 1666–1669. doi: 10.1126/science.1172687 [DOI] [PubMed] [Google Scholar]
- 74.Han M, Yang X, Taylor G, et al. . Limb regeneration in higher vertebrates: developing a roadmap. Anat Rec B New Anat 2005; 287: 14–24. doi: 10.1002/ar.b.20082 [DOI] [PubMed] [Google Scholar]
- 75.Caplan AI. Mesenchymal stem cells. J Orthop Res 1991; 9: 641–650. doi: 10.1002/jor.1100090504 [DOI] [PubMed] [Google Scholar]
- 76.van Haaften T, Byrne R, Bonnet S, et al. . Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 2009; 180: 1131–1142. doi: 10.1164/rccm.200902-0179OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pierro M, Ionescu L, Montemurro T, et al. . Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 2013; 68: 475–484. doi: 10.1136/thoraxjnl-2012-202323 [DOI] [PubMed] [Google Scholar]
- 78.Augustine S, Avey MT, Harrison B, et al. . Mesenchymal stromal cell therapy in bronchopulmonary dysplasia: systematic review and meta-analysis of preclinical studies. Stem Cells Transl Med 2017; 6: 2079–2093. doi: 10.1002/sctm.17-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Popova AP, Bozyk PD, Bentley JK, et al. . Isolation of tracheal aspirate mesenchymal stromal cells predicts bronchopulmonary dysplasia. Pediatrics 2010; 126: e1127–e1133. doi: 10.1542/peds.2009-3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Popova AP, Bentley JK, Cui TX, et al. . Reduced platelet-derived growth factor receptor expression is a primary feature of human bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2014; 307: L231–L239. doi: 10.1152/ajplung.00342.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collins JJP, Lithopoulos MA, Dos Santos CC, et al. . Impaired angiogenic supportive capacity and altered gene expression profile of resident CD146+ mesenchymal stromal cells isolated from hyperoxia-injured neonatal rat lungs. Stem Cells Dev 2018; 27: 1109–1124. doi: 10.1089/scd.2017.0145 [DOI] [PubMed] [Google Scholar]
- 82.Möbius MA, Freund D, Vadivel A, et al. . Oxygen disrupts human fetal lung mesenchymal cells. Implications for bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 2019; 60: 592–600. doi: 10.1165/rcmb.2018-0358OC [DOI] [PubMed] [Google Scholar]
- 83.Möbius MA, Rüdiger M. Mesenchymal stromal cells in the development and therapy of bronchopulmonary dysplasia. Mol Cell Pediatr 2016; 3: 18. doi: 10.1186/s40348-016-0046-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zepp JA, Zacharias WJ, Frank DB, et al. . Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 2017; 170: 1134–1148. doi: 10.1016/j.cell.2017.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peng T, Tian Y, Boogerd CJ, et al. . Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 2013; 500: 589–592. doi: 10.1038/nature12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Travaglini KJ, Nabhan AN, Penland L, et al. . A molecular cell atlas of the human lung from single cell RNA sequencing. bioRxiv 2020: doi:10.1101.742320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo M, Du Y, Gokey JJ, et al. . Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat Commun 2019; 10: 37. doi: 10.1038/s41467-018-07770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie T, Wang Y, Deng N, et al. . Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep 2018; 22: 3625–3640. doi: 10.1016/j.celrep.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ntokou A, Klein F, Dontireddy D, et al. . Characterization of the platelet-derived growth factor receptor-α-positive cell lineage during murine late lung development. Am J Physiol Lung Cell Mol Physiol 2015; 309: L942–L958. doi: 10.1152/ajplung.00272.2014 [DOI] [PubMed] [Google Scholar]
- 90.Al Agha E, Moiseenko A, Kheirollahi V, et al. . Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 2017; 20: 261–273. doi: 10.1016/j.stem.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan SY, Krasnow MA. Developmental origin of lung macrophage diversity. Development 2016; 143: 1318–1327. doi: 10.1242/dev.129122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol 2003; 8: 29–38. doi: 10.1016/S1084-2756(02)00190-2 [DOI] [PubMed] [Google Scholar]
- 93.Eldredge LC, Treuting PM, Manicone AM, et al. . CD11b+ mononuclear cells mitigate hyperoxia-induced lung injury in neonatal mice. Am J Respir Cell Mol Biol 2016; 54: 273–283. doi: 10.1165/rcmb.2014-0395OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eldredge LC, Creasy RS, Tanaka S, et al. . Imbalance of Ly-6Chi and Ly-6Clo monocytes/macrophages worsens hyperoxia-induced lung injury and is rescued by IFN-γ. J Immunol 2019; 202: 2772–2781. doi: 10.4049/jimmunol.1801374 [DOI] [PubMed] [Google Scholar]
- 95.Kalymbetova TV, Selvakumar B, Rodríguez-Castillo JA, et al. . Resident alveolar macrophages are master regulators of arrested alveolarization in experimental bronchopulmonary dysplasia. J Pathol 2018; 245: 153–159. doi: 10.1002/path.5076 [DOI] [PubMed] [Google Scholar]
- 96.Buczynski BW, Yee M, Martin KC, et al. . Neonatal hyperoxia alters the host response to influenza A virus infection in adult mice through multiple pathways. Am J Physiol Lung Cell Mol Physiol 2013; 305: L282–L290. doi: 10.1152/ajplung.00112.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reilly EC, Martin KC, Jin GB, et al. . Neonatal hyperoxia leads to persistent alterations in NK responses to influenza A virus infection. Am J Physiol Lung Cell Mol Physiol 2015; 308: L76–L85. doi: 10.1152/ajplung.00233.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O'Reilly MA, Yee M, Buczynski BW, et al. . Neonatal oxygen increases sensitivity to influenza A virus infection in adult mice by suppressing epithelial expression of Ear1. Am J Pathol 2012; 181: 441–451. doi: 10.1016/j.ajpath.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lignelli E, Palumbo F, Myti D, et al. . Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2019; 317: L832–L887. doi: 10.1152/ajplung.00369.2019 [DOI] [PubMed] [Google Scholar]
- 100.Ginhoux F. Fate PPAR-titioning: PPAR-γ ‘instructs’ alveolar macrophage development. Nat Immunol 2014; 15: 1005–1007. doi: 10.1038/ni.3011 [DOI] [PubMed] [Google Scholar]
- 101.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 2015; 16: 36–44. doi: 10.1038/ni.3052 [DOI] [PubMed] [Google Scholar]
- 102.Guilliams M, De Kleer I, Henri S, et al. . Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 2013; 210: 1977–1992. doi: 10.1084/jem.20131199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saluzzo S, Gorki AD, Rana BMJ, et al. . First-breath-induced type 2 pathways shape the lung immune environment. Cell Rep 2017; 18: 1893–1905. doi: 10.1016/j.celrep.2017.01.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Kleer IM, Kool M, de Bruijn MJ, et al. . Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity 2016; 45: 1285–1298. doi: 10.1016/j.immuni.2016.10.031 [DOI] [PubMed] [Google Scholar]
- 105.Cohen M, Giladi A, Gorki AD, et al. . Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 2018; 175: 1031–1044. doi: 10.1016/j.cell.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 106.Oherle K, Acker E, Bonfield M, et al. . Insulin-like growth factor 1 supports a pulmonary niche that promotes type 3 innate lymphoid cell development in newborn lungs. Immunity 2020; 52: 716–718. doi: 10.1016/j.immuni.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brugman S, Perdijk O, van Neerven RJ, et al. . Mucosal immune development in early life: setting the stage. Arch Immunol Ther Exp 2015; 63: 251–268. doi: 10.1007/s00005-015-0329-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marzi M, Vigano A, Trabattoni D, et al. . Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol 1996; 106: 127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prescott SL, Macaubes C, Yabuhara A, et al. . Developing patterns of T cell memory to environmental allergens in the first two years of life. Int Arch Allergy Immunol 1997; 113: 75–79. doi: 10.1159/000237512 [DOI] [PubMed] [Google Scholar]
- 110.Rothers J, Halonen M, Stern DA, et al. . Adaptive cytokine production in early life differentially predicts total IgE levels and asthma through age 5 years. J Allergy Clin Immunol 2011; 128: 397–402. doi: 10.1016/j.jaci.2011.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saglani S, Gregory LG, Manghera AK, et al. . Inception of early-life allergen-induced airway hyperresponsiveness is reliant on IL-13+CD4+ T cells. Sci Immunol 2018; 3: eaan4128. doi: 10.1126/sciimmunol.aan4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fu Y, Lou H, Wang C, et al. . T cell subsets in cord blood are influenced by maternal allergy and associated with atopic dermatitis. Pediatr Allergy Immunol 2013; 24: 178–186. doi: 10.1111/pai.12050 [DOI] [PubMed] [Google Scholar]
- 113.Stein MM, Hrusch CL, Gozdz J, et al. . Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med 2016; 375: 411–421. doi: 10.1056/NEJMoa1508749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lloyd CM, Marsland BJ. Lung homeostasis: influence of age, microbes, and the immune system. Immunity 2017; 46: 549–561. doi: 10.1016/j.immuni.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 115.Collier FM, Tang ML, Martino D, et al. . The ontogeny of naive and regulatory CD4+ T-cell subsets during the first postnatal year: a cohort study. Clin Transl Immunology 2015; 4: e34. doi: 10.1038/cti.2015.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ismail IH, Boyle RJ, Mah LJ, et al. . Reduced neonatal regulatory T cell response to microbial stimuli associates with subsequent eczema in high-risk infants. Pediatr Allergy Immunol 2014; 25: 674–684. doi: 10.1111/pai.12303 [DOI] [PubMed] [Google Scholar]
- 117.Nibbering B, Ubags NDJ. Microbial interactions in the atopic march. Clin Exp Immunol 2020; 199: 12–23. doi: 10.1111/cei.13398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol 2018; 48: 39–49. doi: 10.1002/eji.201646721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Russell SL, Gold MJ, Hartmann M, et al. . Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 2012; 13: 440–447. doi: 10.1038/embor.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol 2019; 12: 843–850. doi: 10.1038/s41385-019-0160-6 [DOI] [PubMed] [Google Scholar]
- 121.Marsland BJ, Trompette A, Gollwitzer ES. The gut–lung axis in respiratory disease. Ann Am Thorac Soc 2015; 12: Suppl. 2, S150–S156. [DOI] [PubMed] [Google Scholar]
- 122.Örtqvist AK, Lundholm C, Kieler H, et al. . Antibiotics in fetal and early life and subsequent childhood asthma: nationwide population based study with sibling analysis. BMJ 2014; 349: g6979. doi: 10.1136/bmj.g6979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deshmukh HS, Liu Y, Menkiti OR, et al. . The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med 2014; 20: 524–530. doi: 10.1038/nm.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trompette A, Gollwitzer ES, Yadava K, et al. . Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20: 159–166. doi: 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- 125.Ubags ND, Trompette A, Pernot J, et al. . Microbiome-induced antigen-presenting cell recruitment coordinates skin and lung allergic inflammation. J Allergy Clin Immunol 2020; in press [ 10.1016/j.jaci.2020.06.030]. doi: 10.1016/j.jaci.2020.06.030 [DOI] [PubMed] [Google Scholar]
- 126.Olbrich CL, Bivas-Benita M, Xenakis JJ, et al. . Remote allergen exposure elicits eosinophil infiltration into allergen nonexposed mucosal organs and primes for allergic inflammation. Mucosal Immunol 2020; 13: 777–787. doi: 10.1038/s41385-020-0310-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ubags ND. Remote tissue immune priming in allergic disease. Mucosal Immunol 2020; 13: 719–720. doi: 10.1038/s41385-020-0328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Higgins RD, Jobe AH, Koso-Thomas M, et al. . Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr 2018; 197: 300–308. doi: 10.1016/j.jpeds.2018.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Polverino F, Hysinger EB, Gupta N, et al. . Lung MRI as a potential complementary diagnostic tool for early COPD. Am J Med 2020; 133: 757–760. doi: 10.1016/j.amjmed.2019.12.009 [DOI] [PubMed] [Google Scholar]