Abstract
Liquid biopsy refers to the analysis of any tumour-derived material circulating in the blood or any other body fluid. This concept is particularly relevant in lung cancer as the tumour is often difficult to reach and may need an invasive and potentially harmful procedure. Moreover, the multitude of anticancer drugs and their sequential use underline the importance of conducting an iterative assessment of tumour biology. Liquid biopsies can noninvasively detect any targetable genomic alteration and guide corresponding targeted therapy, in addition to monitoring response to treatment and exploring the genetic changes at resistance, overcoming spatial and temporal heterogeneity.
In this article, we review the available data in the field, which suggest the potential of liquid biopsy in the area of lung cancer, with a particular focus on cell-free DNA and circulating tumour cells. We discuss their respective applications in patient selection and monitoring through targeted therapy, as well as immune checkpoint inhibitors. The current data and future applications of liquid biopsy in the early stage setting are also investigated.
Liquid biopsy has the potential to help manage nonsmall cell lung cancer throughout all stages of lung cancer: screening, minimal residual disease detection to guide adjuvant treatment, early detection of relapse, systemic treatment initiation and monitoring of response (targeted or immune therapy), and resistance genotyping.
Short abstract
Liquid biopsy has great potential for NSCLC screening, for treatment selection (adjuvant, targeted therapy and immunotherapy), to monitor response to treatment and to analyse and overcome acquired resistance http://bit.ly/2m26HGm
Introduction
Targeted therapies and, more recently, immune checkpoint inhibitors (ICIs), have transformed the treatment landscape of advanced nonsmall cell lung cancer (NSCLC). Response to these agents can be predicted by the use of companion biomarkers. There is currently a paradox between the need to obtain significant samples for multiple analyses for a growing number of molecular biomarkers and the development of minimally invasive or noninvasive techniques, resulting in small tissue samples with very small amounts of DNA. Cytological samples, such as endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), are often insufficient for a comprehensive molecular examination (10–20% of cases) [1, 2]. Moreover, a better understanding of the resistance to corresponding targeted therapies has led to a need for rapid, noninvasive, repeatable assays to assess and follow tumour biology through treatment.
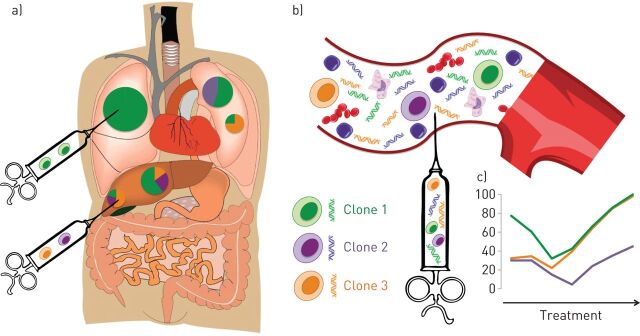
It is within this context that there has been renewed interest in liquid biopsy, a concept which refers to any tumour-derived material circulating through the blood or any other bodily fluid [3]. Circulating tumour cells (CTCs) and circulating tumour DNA (ctDNA), the most widely studied substrates in the field of NSCLC, have different advantages and disadvantages, and may be complementary. While tissue only offers a snapshot of the tumour at a given time and location (figure 1a), liquid biopsy has the potential to overcome both spatial and temporal tumour heterogeneity (figure 1b) and can noninvasively interrogate the molecular landscape of a tumour (taking into account different clones present within all metastatic sites) and can follow subclonal evolution through iterative blood draws (figure 1c).
FIGURE 1.
Liquid biopsy compared to tissue biopsy can capture both spatial (a versus b) and temporal (c) tumour heterogeneity and noninvasively follow the subclonal evolution of the disease through treatment.
This review focuses on the potential clinical applications of these two methods. Circulating exosomes, miRNA, RNA and tumour-educated platelets, which are not discussed in this article, are other appealing but immature approaches for tumour genotyping [4, 5].
Cell-free DNA
Terminology, physiopathology and biology
Cell-free DNA (cfDNA) corresponds to the cfDNA floating in the blood, but also other bodily fluids such as urine, pleural fluid, cerebrospinal fluid or cytology specimen-derived supernatant [6–9]. The first demonstration of circulating DNA was conducted in 1948 by Mandel and Metais [10]. These small DNA fragments (150–200 base pairs) can be passively released from apoptotic or necrotic cells or indirectly by tumour-associated macrophages [11, 12]. ctDNA tends to be more fragmented, with sizes ranging from 90 to 150 base pairs [13]. The amount of plasma DNA is much higher in patients with cancer (5–1500 ng·mL−1) than in healthy patients (1–5 ng·mL−1), with allelic frequency (AF; the fraction of mutated/wild type alleles) in blood being very variable in patients with cancer [14]. Nucleic acids can be found in blood bound to proteins, as oligo- or mono-nucleosomes, as a result of apoptosis, but also in membrane-bearing vesicles (exosomes, apoptotic bodies, microparticles) that are actively released by cells [15, 16]. An active secretion of tumour DNA into the bloodstream by the tumour has been demonstrated, albeit its pathophysiology has not yet been clearly deciphered. This release of nucleic acids might be used by the tumour to modify the profile of distant target cells and thus participate in the metastatic spread (genometastasis) [15, 17, 18].
Genotyping platforms studied in lung cancer
Earlier studies have shown that cfDNA concentration is increased in patients with lung cancer [19], with a high level being correlated with a poor prognosis [20, 21]. Nevertheless, multiple inflammatory or infectious diseases can increase this level [22]. This problem can be avoided by targeting mutated tumour-specific DNA, which requires highly sensitive genotyping assays given that ctDNA only accounts for a very small proportion of the whole cfDNA, which is dominated by the germline, wild-type DNA compartment (the low mutant allelic fraction). Pre-selection based on the size of DNA fragments could increase AF and thus sensitivity and specificity of plasma genotyping [13]. Moreover, because it aims to be used as a screening test, and most targetable genotypes are of low prevalence in NSCLC, the specificity must be prioritised over sensitivity. Table 1 summarises the characteristics of the main plasma genotyping platforms available for lung cancer genotyping.
TABLE 1.
Characteristics of the major plasma genotyping assays available for advanced nonsmall cell lung cancer
| Principle | Coverage | Diagnostic accuracy (tissue reference) | Advantages | Pitfalls | Preferred indications | |
| Targeted assays | Genotyping of pre-defined hotspots, exons or complete genes of interest | 1–7 hotspots of a gene (ddPCR) 7 hotspots (Therascreen) 42 mutations in four exons (Cobas) |
Sensitivity 60–80% (EGFR) Specificity (EGFR) 96% Cobas, 97% BEAMing 100% ddPCR |
Highly sensitive Highly specific Quantitative (except Cobas) Low turnaround time |
Only targets pre-defined regions of interest: Doesn't cover all targetable alterations Doesn't capture a potentially subclonal resistance |
Screening for pre-defined targetable mutations (i.e. EGFR activating mutation, EGFR resistance mutations (T790M, C797S)) Monitoring of response |
| ||||||
| NGS | ||||||
| Whole genome sequencing | Sequencing of the full genome | NA | Discovery of new targets (fusion genes involving intronic areas) | Risk of false positives (poor specificity) Risk of identifying germline mutations Heavy bioinformatics Low sensitivity |
None in routine, exploration of new targets in research (fusion genes involving intronic areas) | |
| Whole exome sequencing | Sequencing of the full exome (coding regions) | Discovery of new targets or mechanisms of resistance | Tumour mutation burden (but usually replaced by large gene panels) | |||
| Panels (hybrid capture) | Capture and hybridisation to probes of pre-determined regions of interest, then sequencing | Depend on the gene panels (usually hotspots, exons or full genes in 30–400 genes) | Sensitivity 70–90% for SNVs Sensitivity 50–80% for fusions Specificity 65% for hybrid capture Specificity >99% for amplicon |
Interrogates simultaneously pre-determined genes of interest Comprehensive detection of known and unknown mutations Detection of SNVs, CNVs, fusions Lower cost and less bioinformatics data compared to whole genome sequencing or whole exome sequencing |
Imperfect specificity and concordance with tissue, in particular for low AF variants | Initial and resistance genotyping (focusing on genes of therapeutic interest) Tumour mutation burden (large, >300 genes panels) |
| Panels (amplicon sequencing) | PCR amplifications of hotspots/exons/genes of interest, then sequencing | PCR amplification can bias CNVs and AFs Unable to detect fusions without prior knowledge of partners |
||||
ddPCR: digital-droplet PCR; NGS: next-generation sequencing; NA: not available; SNV: single-nucleotide variation; CNV: copy-number variation; AF: allelic fraction.
Targeted assays: EGFR sensitising mutations and T790M screening
Currently approved (US Food and Drug Administration (FDA) and European Medicines Agency) clinical use of cfDNA is limited to the cobas EGFR Mutation test v.2 CE-IVD (Roche, Basel, Switzerland) and Therascreen mutation kits (Qiagen, Hilden, Germany) for patients with NSCLC who are unable to undergo a tissue biopsy or with acquired resistance to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs). BEAMing (beads, emulsion, amplification and magnetics) [23] and digital-droplet PCR are other highly sensitive (0.01% AF) and quantitative approaches based on digital PCR [24]. Targeted assays can detect a known driver mutation (EGFR, KRAS, BRAF) [24–26], as well as the emergence of a pre-defined resistant clone (T790M) in blood weeks before clinical progression, and follow their variations through treatment [24, 27, 28].
Next-generation sequencing
The PCR-based assays described thus far only target one to three sites in a pre-defined gene, but fail to multiplex across several genes and to detect more complex genomic alterations, such as fusion genes. Next-generation sequencing (NGS) is a high-throughput sequencing method that can simultaneously interrogate variable areas of the genome and detect somatic mutations, including single-nucleotide variations (SNVs), copy number variations (CNVs), insertions/deletions or gene fusions.
Libraries preparation
The larger the genomic area an assay will target (coverage), the lower the depth (average number of reads obtained in a specific region of the genome) will be, which is a considerable criterion to take into account, since the lower the depth is, the more difficult it is to call a variant with confidence [29].
Whole-exome sequencing can simultaneously detect expected oncogenic drivers or mechanisms of resistance, and has the ability to discover/explore new molecular mechanisms of resistance. However, most clinically relevant fusions occur in noncoding regions and are missed by the whole-exome approach. Considering the trade-off between the depth and the amount of the genome covered, the use of panels of primers/probes targeting hotspots or exons of predetermined genes seems to be the most reasonable approach in the context of liquid biopsy, due to the very low fraction and amount of tumour DNA.
Hybrid capture NGS is a well-established approach in the liquid biopsy field [30, 31]. Predetermined DNA sequences are “captured” by hybridisation to biotinylated probes. The biotin is bound to streptavidin beads, allowing for the clearance of the remaining DNA. Because these technologies do not resort to prior amplification, they can reliably quantify copy number changes. For plasma genotyping, one disadvantage of hybrid capture is the inherent low input DNA, requiring platforms with a high depth of sequencing. This means there is a risk of sequencing error and false positives. For example, in a recent study, specificity, taking tissue as a reference, was 63.5%. The concordance rates between cfDNA and tissue were only 64.7% and 48.9% for pre-treatment and post-treatment specimens, respectively. Tumour heterogeneity and the time interval between tissue and blood sampling might partly explain these conflicting results, but since 100% of variants found at AF >1% in cfDNA were also found in tissue, sequencing errors might explain some of them [31]. Paweletz et al. [32] designed a bias-corrected NGS based on hybrid capture, but using single-primer amplification and tags for sample identification to reduce false positives.
Amplicon-based NGS enriches predefined sequences of interest by PCR amplification of exons/hotspots of specific genes [33]. This methodology appears to be particularly promising for cfDNA genotyping where the input DNA is low [34–36]. The use of molecular barcodes is needed to avoid false positives after PCR amplification: a PCR bias, present only on a very low fraction of reads carrying the same barcodes, will be distinguished from a true variant, whose number of reads will rise proportionally during amplification. However, PCR amplification can bias the observed allele frequency, but also the CNVs.
Data analysis
Sequencing will generate a massive amount of raw genomic data. Each read generated by the sequencing is assigned to a genetic location and aligned to the reference genome to detect any alteration [37]. In the field of liquid biopsy, variant calling is very challenging, and low AFs (<0.5%) of true mutations are particularly difficult to discern from sequencing artefacts [32]. Fusion genes will generate split reads, where both ends are mappable, but not the in-between (and potentially wide) sequence making them difficult to identify. Moreover, while hybrid capture has the ability to detect fusion without prior knowledge of the gene partner, amplicon-based NGS only covers previously known rearrangements.
How to interpret plasma NGS data
False negatives are the first limitation of cfDNA genotyping. If technical limits of the genotyping platforms can be a potential barrier, the main limitation remains the lack of DNA shed, which is strongly linked to the number and sites of metastases [24, 38].
The interpretation of “false positives”, assuming tumour tissue as a reference, can be more challenging, since they are not necessarily due to sequencing errors. As previously reported, a “false positive” can be the consequence of tissue heterogeneity, the molecular profile of a tissue biopsy potentially missing a clone present in other tumour sites (figure 1). Tumour temporal and spatial heterogeneity will become increasingly complex through the different lines of treatments, by two mechanisms potentially coexisting: selection of a minor clone (i.e. T790M mutation) and acquisition of de novo resistance mutations [36, 39, 40]. Concordance between tissue and plasma will thus become lower, and the physician will interpret the result of the assay depending on the relevance of the mutation within the context of the cancer (the example of T790M is detailed in the next section).
But a false positive can also be due to the detection of a variant linked to another tumour, or to pre-cancerous conditions. DNA shed from clonal haematopoiesis is the most common situation generating cancer-associated genes mutations (TP53, KRAS, JAK2) [41, 42]. Paired genotyping of peripheral blood cells can discriminate clonal haematopoiesis from cancer-derived variants.
Current and future applications of cfDNA for NSCLC genotyping
cfDNA to guide and monitor genotype-directed therapies
For the initial genotyping of the tumour, tissue is still the gold standard for NSCLC genotyping. However, technically challenging anatomy, biopsy complications, insufficient tumour tissue, low turnaround time, and sampling errors due to tumour heterogeneity limit this approach. Several plasma assays have been approved for EGFR when the genotyping cannot be assessed on these samples. Their specificity is strong enough to allow the prescription of EGFR-TKIs based on this result. However, the sensitivity is imperfect (∼70%) [43] and a negative result must be confirmed by a tissue biopsy, which will enable wider genotyping, including gene rearrangements (e.g. ALK, ROS1, etc.). To date, only EGFR mutational status is routinely assessed by a blood-based test.
Physicians are increasingly facing situations where there is a lack of tissue, given the paradox between an increasing number of targetable genotypes to track and the parallel development of minimally invasive bronchoscopic tools (EBUS-TBNA, radial EBUS-guided biopsy, electromagnetic navigation) that give access to small specimens [44–46]. Plasma NGS can noninvasively rapidly interrogate the genomic landscape of the tumour and, as previously proposed, targeted panels including genes of interest are preferred.
At progression, liquid biopsy constitutes a very appealing option to track mechanisms of resistance [27, 34]. This setting is different to initial diagnosis because tumour biology is more heterogeneous and subclonal [47]. The analysis of specificity is very tricky in this setting. In cases of EGFR mutated tumours, Oxnard et al. [27] showed that 31% of patients who test negative for the presence of T790M in tissue are positive in plasma (−/+). However, albeit lower than tissue/plasma (+/+) or tissue/plasma (+/−), these patients benefit from osimertinib treatment, establishing that these apparent false positives are not sequencing errors but the result of tumour heterogeneity [27]. The modest outcomes of patients designated as −/+ suggest more heterogeneous resistance, with the presence of other competing, coexistent clones. The relative AF of T790M (T790M AF/sensitising mutation AF) can be an additional tool in interpreting a plasma test, that informs the clinician whether T790M is the dominant mechanism of resistance or a subclonal phenomenon within a heterogeneous biology (T790M relative AF <10%). Also, the detection of the driver at high AF confirms that ctDNA is shed by the tumour and makes a negative T790M result more likely than a true negative, even if its presence as a minor subclone is still possible. Conversely, a test negative for both driver and T790M is not informative because it could potentially be linked to the absence of DNA shed, and indicates the need for tissue biopsy. A negative result must also be validated by tissue sampling, which will enable the detection of other mechanisms of resistance, not covered by currently approved cfDNA assays (small cell carcinoma transformation, MET or HER2 amplification, PIK3CA or BRAF mutations).
Overall, ∼30% of T790M-positive tissues are missed by plasma due to a lack of sensitivity/DNA shed, and ∼30% of cfDNA T790M-positive results would test negative on tissues due to tumour heterogeneity, making these two approaches complementary [27].
Oxnard et al. [27] proposed a new paradigm for detecting T790M mutations at progression: plasma genotyping should be proposed as a first-line screening test, with a tumour biopsy needed in the case of a negative result. Targeted plasma NGS covering a wider range of potential mechanisms of resistance to EGFR-TKIs (EGFR, MET, BRAF, PIK3CA, HER2) could, however, prevent the need for this repeat biopsy in future, with the only resistance mechanism that cannot be found in blood-based assays so far being small-cell transformation.
Monitoring plasma response throughout osimertinib treatment using amplicon-based NGS highlights the complexity of resistance biology in some cases where concomitant, competitive resistant clone (KRAS, BRAF, PIK3CA) can be seen in addition to T790M [36, 48]. This highlights the limitations of assays that only target T790M and can probably be extrapolated to any TKI resistance analysis, including front-line osimertinib, EGFR-TKIs targeting C797S, and other genotype-directed therapy [36]. The resistance to ALK inhibitors such as crizotinib is also too heterogeneous for targeted PCR-based assays, involving multiple on-target mechanisms (with variable sensitivities to second or third-generation anaplastic lymphoma kinase (ALK)-TKIs) also in addition to the upregulation of alternative pathways [49].
Analytical validation studies have now accumulated and clinical validation is strongly needed. Aggarwal et al. [50] demonstrated that plasma and tissue genotyping were complementary, with 20% of targetable variants detected in blood but not tissue, with sensitivity to the corresponding agents. Similarly, two recent prospective studies confirmed the complementary role of tissue and ctDNA for NSCLC genotyping [51, 52], with plasma having a lower turnaround time [52].
Potential future applications of cfDNA to guide and monitor immune therapy
While cfDNA has been widely studied in the field of targeted therapy, its potential to guide and follow the response to immune therapy is just beginning to be evaluated [53].
Tumour mutation burden (TMB) can be estimated from cfDNA NGS, with good concordance with tissue NGS provided that ctDNA is detected [54, 55]. In the only study showing discordant results between blood TMB and tissue TMB, two different platforms/panels were used for cfDNA and tissue [56]. A high blood TMB has proven to be correlated with response to inhibitors of programmed cell death (PD)1 and its ligand (PD-L1) [54, 57], in particular with NSCLC and atezolizumab in the POPLAR and OAK trials (high blood TMB being defined as >16 SNVs detected among 394 genes) [54, 58]. Of note, blood TMB is better correlated with metastatic tissue TMB (0.9 Pearson correlation versus 0.8 overall) than primary tumour, and concordance is better in cases with high ctDNA concentrations [59]. Also, in the study by Fabrizio et al. [54], the concordance was much better (64% positive agreement) when using a large gene panel (394 genes) than when using a smaller one (62 genes, 17% positive agreement).
However, the clinical adoption of TMB is challenging due to the high costs associated with whole-exome sequencing or broad panels. We performed targeted, amplicon-based, NGS of plasma cell-free DNA in 86 patients treated with PD1 inhibitors, using limited gene panels (n=36). Focusing on specific molecular determinants of response (KRAS and/or TP53) [60], or resistance (oncogenic drivers, STK11, PTEN) [60–63] to ICIs, we built a simple algorithm that could predict durable outcomes of patients with advanced NSCLC treated with immunotherapy [64].
Monitoring of response is another application of cfDNA during ICI treatment. Several reports have demonstrated a good correlation between ctDNA kinetics and clinical response. A complete or partial clearance at 4–8 weeks was strongly predictive of a durable response, whereas persistence of ctDNA had a detrimental impact [64–66]. We also showed that early changes in circulating tumour DNA burden has the ability to discriminate between pseudo-progression and true progression [67].
Other applications of cfDNA are likely to emerge in the near future, such as the detection of minimal residual disease (MRD) for adjuvant immunotherapy, or detection of mechanisms of resistance, like acquired JAK1/2 or B2M mutations [53].
Potential applications in early stage NSCLC: screening and residual disease
Screening is probably the most challenging area in lung cancer. The large National Lung Screening Trial [68] and more recently, the NELSON trial demonstrated that the use of computed tomography (CT) scans among asymptomatic men at a high risk for lung cancer led to a 26% reduction in lung cancer deaths at 10 years of study follow-up (41% for women) [69]. Nevertheless, because lung nodules have many benign differentials, this examination generates a considerable number of false positives and leads to useless and dangerous (28% complications in the NSLT trial) invasive diagnostic biopsies or even surgeries. Combining this high sensitivity of CT scans with the specificity of liquid biopsy is very promising, but it is limited by the fact that the ctDNA shed is either absent or very low in the earlier stages.
A screening cfDNA assay should cover a sufficiently wide array of recurrent mutations to capture all NSCLC tumours, but should stay focused on targeted areas that are of great concern and need to be banished in the screening setting.
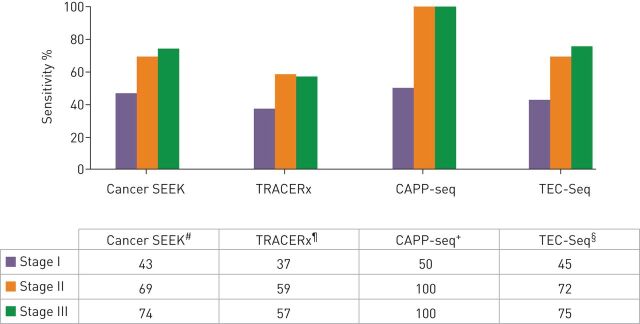
The principles and sensitivity of the four main platforms investigated for this purpose so far are reported in figure 2. Even the most sensitive assay, such as CAPP-seq, only detects 50% of stage I diseases (100% sensitivity for stage II and III) [70]. The CancerSEEK assay, combining genomic analysis of 16 genes in ctDNA and eight protein biomarkers, demonstrated compelling results in detecting eight types of nonmetastatic cancers (lung, colorectal, ovary, breast, liver, pancreas, oesophagus and stomach) [71]. However, while the test could reliably detect 70% of stage I–III cancers, its sensitivity for stage I cancers was low (43%), with lung and oesophagus showing the lowest rates of detection. Also, because lung cancer does not have a specific circulating protein marker, determining the site was only possible in 39% of cases versus 84% for colorectal, 79% for ovary and 81% for pancreatic cancers. Finally, the remarkable specificity (false positive rate <1%) may be higher in a population of healthy people (i.e. in a real screening setting).
FIGURE 2.
Summary of the technical properties and performances of the four main plasma genotyping platforms studied for early stage nonsmall cell lung cancer detection. #: early stages: hybrid capture, plasma next-generation sequencing (NGS) (16 genes) and 8 protein markers. ¶: minimal residual disease (MRD): plasma NGS, patient-specific multiplex PCR (10 to 22 single-nucleotide variations), subclonal evolution. +: early stage MRD: hybrid capture, plasma NGS (139 genes). §: early stage MRD: hybrid capture, plasma NGS (58 genes).
One other limitation of early detection of cancer is the possibility to detect mutations in cfDNA that are not derived from tumour but clonal haematopoiesis, a false positive result that can be avoided by combining peripheral blood cells and cfDNA genotyping [41].
MRD is a term extrapolated from haematological disorders, which refers to the detection of any tumour-derived material in the blood after curative treatment. CAPP-seq has been shown to be highly effective for MRD detection after treatment of localised lung cancer (94% of patients with recurrence had ctDNA detected in blood). However, most of them had stage II–III disease (33 out of 40) [72]. Again, it is more challenging here for stage I diseases because of low/no DNA shed. However, unlike in the screening setting, it is possible to access the molecular profile from the initial tumour, and thus follow the patient with simplified and personalised, multiplex PCR assays. One of the best examples is in breast cancer. Using the mutations detected in the tissue after surgery, a patient-specific digital PCR assay is designed to track MRD through time. The detection of ctDNA with this methodology can predict metastatic relapse with high accuracy (HR 25.1) [73]. Using a similar approach, the detection of MRD after stage II colon cancer curative surgery was also found to be a strong predictor of relapse [74].
Given the low benefit of adjuvant chemotherapy in NSCLC (5% absolute benefit [75]), new approaches to detect clinically indiscernible MRD and guide adjuvant therapy are strongly needed. The TRACERx team analysed cfDNA data from the first 100 patients enrolled in their study from diagnosis through treatment (including curative surgery) and beyond [76–78]. Personalised sequencing assays were built and used for each patient from multi-region exome sequencing of the tumour. Although potentially costly and time consuming, these assays had remarkable sensitivity and specificity (as the team set a threshold of two or more variants for positivity). While some predictors of DNA shed were already established, which included tumour size, number of metastatic sites, stage and organ of origin [24, 28, 79], this study discovered that necrosis, lymphovascular invasion, Ki67 labelling index, tumour size and nonadenocarcinoma status all predict ctDNA shed. However, they only identified tumour cfDNA in 48% of patients with early stage NSCLC, and discussed a theoretical limit of DNA shed for tumours of a given size, estimating that tumours <10 cm3 will not have detectable ctDNA [77]. This work confirms that plasma genotyping can detect MRD/recurrence weeks to months before imaging (median 70 days). Finally, in many patients this assay identified the phylogenetic subclone that was responsible for recurrence.
Methylation is an early and frequent epigenetic alteration that can be detected in cfDNA, including in plasma. A methylation panel of six genes showed a sensitivity of 72% (31 out of 41) for the detection of stage Ia NSCLC, but with a poor specificity (12 out of 42 control patients tested positive) [80]. A prospective trial is ongoing to investigate the ability of ctDNA mutations and methylation monitoring to detect MRD after surgery for stage Ia–III NSCLC [81].
In addition to the inherent low DNA shed, another limitation to the integration of plasma genotyping in early stage disease is the lack of CT targets to monitor treatment efficacy. If MRD detection was to guide systemic therapy, exclusive plasma monitoring should be chosen, as in chronic myeloid leukaemia.
Circulating tumour cells
Pathophysiology
The release of CTCs into the bloodstream benefits from a change in the tumour cell phenotype, characterised by a loss of the epithelial markers (cytokeratin filaments and E-cadherin) and the gain of some mesenchymal markers such as vimentin. This epithelial–mesenchymal transition (EMT) increases the plasticity of CTCs, facilitating migration and invasion [82, 83]. CTCs may migrate as clusters of cells, called circulating tumour microemboli, which are likely to survive better in the bloodstream than isolated CTCs [84]. They can also bring their own “soil” (stroma cells from the primary tumour) to increase their metastatic potential [85, 86].
Isolation/enrichment and analysis
Two main approaches have been developed for CTC isolation: 1) methods based on antigen expression (antigens not expressed on blood cells, but not specific to tumour cells); and 2) methods based on biophysical characteristics (density and size, higher in CTCs than in blood cells, along with cytopathological criteria of malignancy) [77, 82–86].
Cellsearch is a widely used and US FDA-approved method that isolates cells from blood using epithelial specific antibodies (epithelial cell adhesion molecue-positive selection, CD45-negative selection) [87]. However, malignant CTCs frequently lose their epithelial antigens and acquire some mesenchymal markers during EMT [88], and not all circulating epithelial cells are malignant, explaining the lack of sensitivity and specificity of these approaches [89, 90].
Isolation by size of epithelial tumour cells (ISET; Rarecells, Paris, France) is based on the isolation of CTCs according to their size (superior to 20 µm microns compared to 8–10 µm for white blood cells) [91, 92] and enables a reliable cytomorphological analysis of the isolated cells [91] and immunocytochemical, immunofluorescence or in situ hybridisation to detect rearrangements or amplifications [93, 94].
The microfluidic technologies probably represent the most appealing approach for CTC isolation and have been reported since 2007 with the “CTC-ship” [95]: CTCs are captured in antibody (epithelial cell adhesion molecule)-coated microposts under precisely controlled laminar flow conditions. This allowed for the detection of CTCs in 99% (115 out of 116) of patients with various solid tumours [95]. Pasortix uses a similar approach but isolates tumour cells based on compressibility as well as their size (microfluidic technology). This technology has been used in patients with prostate and breast cancer for single-cell genotyping, revealing molecular heterogeneity across CTCs [96].
The Vortex technology also exploits microfluidics and uses laminar microscale vortices to isolate and concentrate CTCs from blood, based on their physical properties (size, shape and compressibility) [97, 98]. This approach has the advantages of being automated, being independent of antibody expression (thus avoiding false negatives due to EMT), and results in high purity CTC samples with low white blood cell contamination and preserved cells, facilitating further molecular analyses or culture [97, 98].
Endeavours to improve the sensitivity of these technologies and increase the sample purity of CTCs, but also to miniaturise the microfluidics platforms are ongoing [99].
Current and potential applications of CTCs for NSCLC
Screening
In early stage cancer, the CTC detection rate is usually low, <30% [100, 101]. In the case of Cellsearch enrichment, this low sensitivity, combined with a poor specificity (any inflammatory disease can release circulating epithelial cells), probably makes this approach unsuitable for cancer screening. ISET has a better specificity and is currently being evaluated by Ilie et al. [102] as a screening tool in high-risk populations. In a cohort of 168 patients with COPD, CTCs were detected using ISET (RareCells, Paris, France) in five patients who were subsequently followed up with a yearly CT scan. All of these patients developed a lung nodule 1–4 years after, corresponding to stage Ia NSCLC that could be treated surgically, while none of the other patients with COPD or control patients developed lung cancer. Based on these positive results, the large AIR trial was launched. This prospective, multicentric study (21 centres) has enrolled 600 patients with COPD. Yearly screening with both low-dose CT scans and ISET blood treatment has been performed [103]. Results are not yet published. CTC detection after radical treatment (MRD) to guide adjuvant therapy or early detection of relapse is another potential application [84].
Prognosis
While the most robust data have been reported in patients with breast [104] and colorectal cancer using Cellsearch [105, 106], several studies have also established the burden of CTCs as an independent prognostic factor in NSCLC [107]. In particular, a high CTC count before, during or after surgery correlates with a worse prognosis, and could influence the adjuvant therapy decision [108–111].
Monitoring of response
A large study was performed on 326 patients with metastases enrolled in phase I studies in order to evaluate the potential of early CTC changes (Cellsearch) to predict response to treatment. However, CTC kinetics did not correlate with response according to RECIST criteria. Sensitivity and specificity of CTC count variations for response prediction was only 41% and 80%, respectively [112]. Accurate quantitative follow-up may be difficult due to the very low number of CTCs that are usually detected, and given the great potential of cfDNA to monitor tumour burden, CTCs should not be used for response follow-up.
Genomics
The detection of EGFR mutations in DNA extracted from CTCs [113] and the complementary role of cfDNA and CTCs for resistance genotyping, as demonstrated in several studies (in acquired resistance to EGFR-TKI in particular), created a strong interest in CTCs for genomics [79, 114] which diminished with the accumulation of highly sensitive, specific and rapid cfDNA genotyping platforms. CTC genotyping is less sensitive and more laborious [25, 26] and thus probably less suited for translation into routine. Although it is now widely accepted that cfDNA should be favoured over CTCs for detecting point mutations, CTCs may still play a role in detecting chromosomal rearrangements or CNVs because they can be subjected to immunocytochemical and fluorescence in situ hybridisation analyses, as demonstrated for ALK rearrangements and MET amplifications [115, 116]. Nevertheless, the ability of plasma NGS to detect a wide range of genomic alterations, including SNVs, CNVs and chromosomal rearrangements with high sensitivity has now been demonstrated [30–32, 36, 50, 117]. The yield, in addition to the cost and turnaround time of these two approaches, should be compared. Single-cell analysis of CTCs to better decipher resistance [118] is sometimes reported as an advantage of CTCs over ctDNA [119]. In particular, this approach has demonstrated that both on-target and off-target resistance mechanisms can be identified in the same CTC after acquired resistance to ALK–TKI [120]. It has, however, limited clinical impact and is technically highly challenging.
PD-L1 expression
Another potential application of CTCs is PD-L1 analysis, which cannot be explored in cfDNA. The hypothesis that PD-L1 expression on CTCs could be used as predictive marker of response to ICIs has unfortunately not been confirmed to date. Studies on PD-L1 expression on CTCs in advanced NSCLC have accumulated and while the feasibility is now well established, the rate of PD-L1+ CTCs has always been found to be very high and associated with poor prognosis, without a clear scientific explanation [121–128]. Only Ilie et al. [94] were able to show good concordance between tissue and CTCs, but the rate of positive samples was surprisingly low and correlation with response to PD1/PD-L1 inhibitors could not be investigated. The relationship between CTCs and circulating immune cells (liquid microenvironment) is extremely complex [129], making CTCs an interesting tool to study metastasis and immune escape mechanisms, but they seem far from being adopted into routine practice as a biomarker for cancer immunotherapy.
Drug screening
CTCs can be used ex vivo (CTC culture) or in vivo (CTC-derived xenograft) as an alternative to tissue patient-derived xenograft for drug screening. CTC-derived xenograft models have been studied by Hodgkinson et al. [130], mainly in small-cell lung cancer and will not be extensively reviewed here, particularly as they are much more challenging to generate from NSCLC-derived CTCs [131]. CTC-derived xenografts mirror the corresponding patient's response to chemotherapy [130], but not to immunotherapy (because the mice are deeply immunosuppressed).
Discussion
Is tissue still the issue?
In our opinion, liquid biopsy will never fully replace tissue biopsy, which will remain the gold standard for the pathological diagnosis of lung cancer, by clarifying the subtype of cancer using cytomorphological criteria and immunohistochemistry assays (adenocarcinoma versus squamous versus other). In addition, tissue sampling is sometimes the only way to assess the exact stage of cancer (e.g. EBUS-TBNA, mediastinoscopy, etc.) or to confirm disease recurrence. When some material is left after all the diagnostic steps, genotyping of DNA extracted from tissue is still the standard. A negative plasma test will always need tissue confirmation, due to its lack of sensitivity (inherent of the assay and due to the inconsistent DNA shed). Furthermore, in this context of immune therapy revolution, tumour biopsy will have more and more value, in both assessing PD-L1 expression on tumour cells and exploring the tumour microenvironment. Genetic mechanisms of primary or acquired resistance to ICIs have been reported, such as JAK and B2M acquired mutations, and may be detected in blood [132, 133]. However, acquired resistance to ICIs still needs to be explored and should involve dynamic changes in the microenvironment, such as loss of surface expression of major histocompatibility complex class I, changes in polarisation of immune cells, or involvement of other immune checkpoints, which are impossible to detect from a simple blood draw.
Can liquid biopsy replace imaging for response monitoring?
Based on the observations that cfDNA kinetics accurately monitor tumour burden through treatment, it has been suggested that repeated liquid biopsy could outclass a CT scan follow-up. Different situations should be discussed. For example, if a patient does not show any clinical changes and their plasma does not show any detectable tumour DNA, perhaps clinical and blood monitoring alone can indeed replace imaging. However, if a patient reports new symptoms, regardless of whether their plasma shows an increase in ctDNA or not, a CT scan should be performed, because plasma could potentially miss a recurrence due to poor sensitivity.
Nevertheless, the most challenging and still unanswered situation is the case of a clinically stable patient showing re-emergence of ctDNA (recurrence of the driver and/or emergence of a resistance clone and/or detection of nondriver variants). In this case, a CT scan should be performed. If the scan confirms disease progression, treatment should obviously be adapted. However, it is still not known whether isolated plasma progression should lead to treatment modification. Some dedicated trials such as the APPLE-EORTC trial are investigating the particular case of T790M-driven acquired resistance, and may be extrapolated to other similar contexts [134]. Our feeling is that plasma progression predicts clinical progression associated with deterioration in both quality of life and performance status, which can compromise the initiation of further treatment. This suggests that treatment modification could be considered from the time when ctDNA emerges. The management of this situation will obviously depend on the “targetability” of the alterations detected in blood.
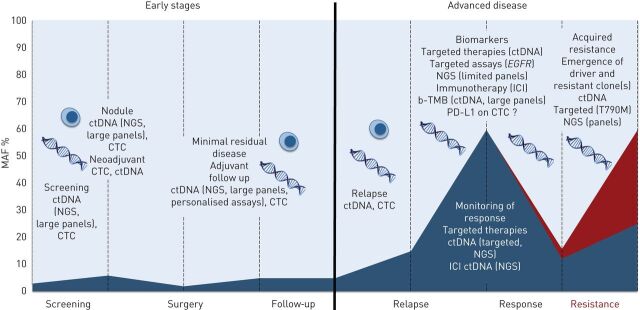
In conclusion, liquid biopsy has the potential to help manage NSCLC throughout all stages of lung cancer [135]: screening, MRD detection to guide adjuvant treatment, early detection of relapse, systemic treatment initiation and monitoring of response (targeted or immune therapy), and resistance genotyping (figure 3).
FIGURE 3.
Potential applications of liquid biopsy in nonsmall cell lung cancer throughout treatment. In early stage disease, screening requires plasma next-generation sequencing (NGS) using large panels, with both high sensitivity (limited by low tumour shed) and perfect specificity, or highly specific circulating tumour cell (CTC) detection platforms. To discriminate benign from malignant nodules, plasma NGS could be useful and avoid invasive biopsies. Circulating tumour (ct)DNA or CTCs burden before surgery have the potential to help guide neoadjuvant therapy, while minimal residual disease detection after surgery (large or patient-specific NGS panels) may guide adjuvant therapy. In advanced stage disease, plasma genotyping is well established in the epidermal growth factor receptor (EGFR) setting, and NGS platforms allow for a wider genotyping at both diagnosis (including other oncogenic drivers detection and blood tumour mutation burden (b-TMB) estimation) and resistance. MAF: mutant allele frequency; ICI: immune checkpoint inhibitor; PD-L1: programmed-death ligand 1.
Footnotes
Published in volume 29, issue 155 of the European Respiratory Review on 12 February 2020; republished 29 April 2021 with amendments to manufacturer details for ISET on page 8.
Provenance: Submitted article, peer reviewed.
Conflict of interest: N. Guibert reports non-financial support from BMS, MSD and Pfizer, and personal fees from AstraZeneca, BMS, and MSD, outside the submitted work.
Conflict of interest: A. Pradines has nothing to disclose.
Conflict of interest: G. Favre has nothing to disclose.
Conflict of interest: J. Mazieres has nothing to disclose.
References
- 1.Malapelle U, Bellevicine C, De Luca C, et al. . EGFR mutations detected on cytology samples by a centralized laboratory reliably predict response to gefitinib in non-small cell lung carcinoma patients. Cancer Cytopathol 2013; 121: 552–560. doi: 10.1002/cncy.21322 [DOI] [PubMed] [Google Scholar]
- 2.Folch E, Yamaguchi N, VanderLaan PA, et al. . Adequacy of lymph node transbronchial needle aspirates using convex probe endobronchial ultrasound for multiple tumor genotyping techniques in non-small-cell lung cancer. J Thorac Oncol 2013; 8: 1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siravegna G, Marsoni S, Siena S, et al. . Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017; 14: 531–548. doi: 10.1038/nrclinonc.2017.14 [DOI] [PubMed] [Google Scholar]
- 4.Inamura K. Diagnostic and therapeutic potential of microRNAs in lung cancer. Cancers 2017; 9: E49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best MG, Wesseling P, Wurdinger T. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res 2018; 78: 3407–3412. [DOI] [PubMed] [Google Scholar]
- 6.Reckamp KL, Melnikova VO, Karlovich C, et al. . A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol 2016; 11: 1690–1700. [DOI] [PubMed] [Google Scholar]
- 7.Guibert N, Tsukada H, Hwang DH, et al. . Liquid biopsy of fine-needle aspiration supernatant for lung cancer genotyping. Lung Cancer 2018; 122: 72–75. [DOI] [PubMed] [Google Scholar]
- 8.De Mattos-Arruda L, Mayor R, Ng CKY, et al. . Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015; 6: 8839. doi: 10.1038/ncomms9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura H, Fujiwara Y, Sone T, et al. . EGFR mutation status in tumour-derived DNA from pleural effusion fluid is a practical basis for predicting the response to gefitinib. Br J Cancer 2006; 95: 1390–1395. doi: 10.1038/sj.bjc.6603428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandel P, Metais P. Les acides nucléiques du plasma sanguin chez l'homme. C R Seances Soc Biol Fil 1948; 142: 241–243. [PubMed] [Google Scholar]
- 11.Crowley E, Di Nicolantonio F, Loupakis F, et al. . Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 2013; 10: 472–484. doi: 10.1038/nrclinonc.2013.110 [DOI] [PubMed] [Google Scholar]
- 12.Jahr S, Hentze H, Englisch S, et al. . DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001; 61: 1659–1665. [PubMed] [Google Scholar]
- 13.Mouliere F, Chandrananda D, Piskorz AM, et al. . Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med 2018; 10: eaat4921. doi: 10.1126/scitranslmed.aat4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouliere F, El Messaoudi S, Gongora C, et al. . Circulating cell-free DNA from colorectal cancer patients may reveal high KRAS or BRAF mutation load. Transl Oncol 2013; 6: 319–328. doi: 10.1593/tlo.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thierry AR, El Messaoudi S, Gahan PB, et al. . Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev 2016; 35: 347–376. doi: 10.1007/s10555-016-9629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rykova EY, Morozkin ES, Ponomaryova AA, et al. . Cell-free and cell-bound circulating nucleic acid complexes: mechanisms of generation, concentration and content. Expert Opin Biol Ther 2012; 12: Suppl. 1, S141–S153. doi: 10.1517/14712598.2012.673577 [DOI] [PubMed] [Google Scholar]
- 17.Gahan PB, Stroun M. The virtosome – novel cytosolic informative entity and intercellular messenger. Cell Biochem Funct 2010; 28: 529–538. doi: 10.1002/cbf.1690 [DOI] [PubMed] [Google Scholar]
- 18.Anker P, Lyautey J, Lefort F, et al. . Transformation de cellules NIH/3T3 et cellules SW 480 porteuses d'une mutation K-ras [Transformation of NIH/3T3 cells and SW 480 cells displaying K-ras mutation]. C R Acad Sci III 1994; 317: 869–874. [PubMed] [Google Scholar]
- 19.Leon SA, Shapiro B, Sklaroff DM, et al. . Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res 1977; 37: 646–650. [PubMed] [Google Scholar]
- 20.Gautschi O, Bigosch C, Huegli B, et al. . Circulating deoxyribonucleic acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J Clin Oncol 2004; 22: 4157–4164. doi: 10.1200/JCO.2004.11.123 [DOI] [PubMed] [Google Scholar]
- 21.Tissot C, Toffart A-C, Villar S, et al. . Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur Respir J 2015; 46: 1773–1780. doi: 10.1183/13993003.00676-2015 [DOI] [PubMed] [Google Scholar]
- 22.Rainer TH, Lam NYL. Circulating nucleic acids and critical illness. Ann NY Acad Sci 2006; 1075: 271–277. doi: 10.1196/annals.1368.035 [DOI] [PubMed] [Google Scholar]
- 23.Diehl F, Li M, He Y, et al. . BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods 2006; 3: 551–559. doi: 10.1038/nmeth898 [DOI] [PubMed] [Google Scholar]
- 24.Oxnard GR, Paweletz CP, Kuang Y, et al. . Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res 2014; 20: 1698–1705. doi: 10.1158/1078-0432.CCR-13-2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guibert N, Pradines A, Casanova A, et al. . Detection and monitoring of the BRAF mutation in circulating tumor cells and circulating tumor DNA in BRAF-mutated lung adenocarcinoma. J Thorac Oncol 2016; 11: e109–e112. [DOI] [PubMed] [Google Scholar]
- 26.Guibert N, Pradines A, Farella M, et al. . Monitoring KRAS mutations in circulating DNA and tumor cells using digital droplet PCR during treatment of KRAS-mutated lung adenocarcinoma. Lung Cancer 2016; 100: 1–4. doi: 10.1016/j.lungcan.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 27.Oxnard GR, Thress KS, Alden RS, et al. . Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 3375–3382. doi: 10.1200/JCO.2016.66.7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacher AG, Alden RS, Oxnard GR. Early intervention in lung cancers with rapid plasma genotyping for EGFR and KRAS mutations-reply. JAMA Oncol 2016; 2: 1096–1097. doi: 10.1001/jamaoncol.2016.1963 [DOI] [PubMed] [Google Scholar]
- 29.Gagan J, Van Allen EM. Next-generation sequencing to guide cancer therapy. Genome Med 2015; 7: 80. doi: 10.1186/s13073-015-0203-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson JC, Yee SS, Troxel AB, et al. . Detection of therapeutically targetable driver and resistance mutations in lung cancer patients by next-generation sequencing of cell-free circulating tumor DNA. Clin Cancer Res 2016; 22: 5772–5782. doi: 10.1158/1078-0432.CCR-16-1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwaederlé MC, Patel SP, Husain H, et al. . Utility of genomic assessment of blood-derived circulating tumor DNA (ctDNA) in patients with advanced lung adenocarcinoma. Clin Cancer Res 2017; 23: 5101–5111. doi: 10.1158/1078-0432.CCR-16-2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paweletz CP, Sacher AG, Raymond CK, et al. . Bias-corrected targeted next-generation sequencing for rapid, multiplexed detection of actionable alterations in cell-free DNA from advanced lung cancer patients. Clin Cancer Res 2016; 22: 915–922. doi: 10.1158/1078-0432.CCR-15-1627-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang F, Li MM. Clinical application of amplicon-based next-generation sequencing in cancer. Cancer Genet 2013; 206: 413–419. doi: 10.1016/j.cancergen.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 34.Remon J, Caramella C, Jovelet C, et al. . Osimertinib benefit in EGFR-mutant NSCLC patients with T790M-mutation detected by circulating tumour DNA. Ann Oncol 2017; 28: 784–790. [DOI] [PubMed] [Google Scholar]
- 35.Couraud S, Vaca-Paniagua F, Villar S, et al. . Noninvasive diagnosis of actionable mutations by deep sequencing of circulating free DNA in lung cancer from never-smokers: a proof-of-concept study from BioCAST/IFCT-1002. Clin Cancer Res 2014; 20: 4613–4624. doi: 10.1158/1078-0432.CCR-13-3063 [DOI] [PubMed] [Google Scholar]
- 36.Guibert N, Hu Y, Feeney N, et al. . Amplicon-based next-generation sequencing of plasma cell-free DNA for detection of driver and resistance mutations in advanced non-small cell lung cancer. Ann Oncol 2018; 29: 1049–1055. doi: 10.1093/annonc/mdy005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Allen EM, Wagle N, Levy MA. Clinical analysis and interpretation of cancer genome data. J Clin Oncol 2013; 31: 1825–1833. doi: 10.1200/JCO.2013.48.7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbosh C, Birkbak NJ, Swanton C. Early stage NSCLC – challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol 2018; 15: 577–586. [DOI] [PubMed] [Google Scholar]
- 39.Hata AN, Niederst MJ, Archibald HL, et al. . Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med 2016; 22: 262–269. doi: 10.1038/nm.4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez M, Rajaram S, Steininger RJ, et al. . Diverse drug-resistance mechanisms can emerge from drug-tolerant cancer persistent cells. Nat Commun 2016; 7: 10690. doi: 10.1038/ncomms10690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Y, Ulrich BC, Supplee J, et al. . False-positive plasma genotyping due to clonal hematopoiesis. Clin Cancer Res 2018; 24: 4437–4443. [DOI] [PubMed] [Google Scholar]
- 42.Paweletz CP, Lau CJ, Oxnard GR. Does testing error underlie liquid biopsy discordance? JCO Precis Oncol 2019: 1–3. [DOI] [PubMed] [Google Scholar]
- 43.Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci Rep 2014; 4: 6269. doi: 10.1038/srep06269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeow K-M, Su I-H, Pan K-T, et al. . Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004; 126: 748–754. doi: 10.1378/chest.126.3.748 [DOI] [PubMed] [Google Scholar]
- 45.Steinfort DP, Khor YH, Manser RL, et al. . Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011; 37: 902–910. doi: 10.1183/09031936.00075310 [DOI] [PubMed] [Google Scholar]
- 46.Asano F, Eberhardt R, Herth FJF. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respir Int Rev Thorac Dis 2014; 88: 430–440. [DOI] [PubMed] [Google Scholar]
- 47.Oxnard GR. The cellular origins of drug resistance in cancer. Nat Med 2016; 22: 232–234. doi: 10.1038/nm.4058 [DOI] [PubMed] [Google Scholar]
- 48.Blakely CM, Watkins TBK, Wu W, et al. . Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet 2017; 49: 1693–1704. doi: 10.1038/ng.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gainor JF, Dardaei L, Yoda S, et al. . Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov 2016; 6: 1118–1133. doi: 10.1158/2159-8290.CD-16-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aggarwal C, Thompson JC, Black TA, et al. . Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non-small cell lung cancer. JAMA Oncol 2019; 5: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li BT, Janku F, Jung B, et al. . Ultra-deep next-generation sequencing of plasma cell-free DNA in patients with advanced lung cancers: results from the Actionable Genome Consortium. Ann Oncol 2019; 30: 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leighl NB, Page RD, Raymond VM, et al. . Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res 2019; 25: 4691–4700. [DOI] [PubMed] [Google Scholar]
- 53.Cabel L, Proudhon C, Romano E, et al. . Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat Rev Clin Oncol 2018; 15: 639–650. doi: 10.1038/s41571-018-0074-3 [DOI] [PubMed] [Google Scholar]
- 54.Fabrizio D, Lieber D, Malboeuf C, et al. . Abstract 5706: a blood-based next-generation sequencing assay to determine tumor mutational burden (bTMB) is associated with benefit to an anti-PD-L1 inhibitor, atezolizumab. Cancer Res 2018; 78: 13 Suppl., 5706––5717..30115693 [Google Scholar]
- 55.Koeppel F, Blanchard S, Jovelet C, et al. . Whole exome sequencing for determination of tumor mutation load in liquid biopsy from advanced cancer patients. PLoS One 2017; 12: e0188174. doi: 10.1371/journal.pone.0188174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis AA, Chae YK, Agte S, et al. . Comparison of tumor mutational burden (TMB) across tumor tissue and circulating tumor DNA (ctDNA). J Clin Oncol 2017; 35: Suppl. 15, e23028. doi: 10.1200/JCO.2017.35.15_suppl.e23028 [DOI] [Google Scholar]
- 57.Khagi Y, Goodman AM, Daniels GA, et al. . Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor-based immunotherapy. Clin Cancer Res 2017; 23: 5729–5736. doi: 10.1158/1078-0432.CCR-17-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gandara DR, Kowanetz M, Mok TSK, et al. . Blood-based biomarkers for cancer immunotherapy: tumor mutational burden in blood (bTMB) is associated with improved atezolizumab (atezo) efficacy in 2L+ NSCLC (POPLAR and OAK). Ann Oncol 2017; 28: Suppl. 5, v460–v496. doi: 10.1093/annonc/mdx380 [DOI] [Google Scholar]
- 59.Yang N, Li Y, Liu Z, et al. . The characteristics of ctDNA reveal the high complexity in matching the corresponding tumor tissues. BMC Cancer 2018; 18: 319. doi: 10.1186/s12885-018-4199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skoulidis F, Goldberg ME, Greenawalt DM, et al. . STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 2018; 8: 822–835. doi: 10.1158/2159-8290.CD-18-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koyama S, Akbay EA, Li YY, et al. . STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res 2016; 76: 999–1008. doi: 10.1158/0008-5472.CAN-15-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng W, Chen JQ, Liu C, et al. . Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov 2016; 6: 202–216. doi: 10.1158/2159-8290.CD-15-0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazieres J, Drilon AE, Mhanna L, et al. . Efficacy of immune-checkpoint inhibitors (ICI) in non-small cell lung cancer (NSCLC) patients harboring activating molecular alterations (ImmunoTarget). J Clin Oncol 2018; 36: Suppl. 15, 9010. doi: 10.1200/JCO.2018.36.15_suppl.9010 [DOI] [Google Scholar]
- 64.Guibert N, Jones G, Beeler JF, et al. . Early prediction of outcomes to PD1 inhibitors in non-small cell lung cancer (NSCLC) using next generation sequencing (NGS) of plasma circulating tumor DNA (ctDNA). J Clin Oncol 2018; 36: Suppl. 15, 9078. doi: 10.1200/JCO.2018.36.15_suppl.9078 [DOI] [Google Scholar]
- 65.Anagnostou V, Forde PM, White JR, et al. . Dynamics of tumor and immune responses during immune checkpoint blockade in non-small cell lung cancer. Cancer Res 2019; 79: 1214–1225. doi: 10.1158/0008-5472.CAN-18-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cabel L, Riva F, Servois V, et al. . Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol 2017; 28: 1996–2001. doi: 10.1093/annonc/mdx212 [DOI] [PubMed] [Google Scholar]
- 67.Guibert N, Mazieres J, Delaunay M, et al. . Monitoring of KRAS-mutated ctDNA to discriminate pseudo-progression from true progression during anti-PD-1 treatment of lung adenocarcinoma. Oncotarget 2017; 8: 38056–38060. doi: 10.18632/oncotarget.16935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Koning H, Van Der Aalst C, Ten Haaf K, et al. . PL02.05 Effects of volume CT lung cancer screening: mortality results of the NELSON randomised-controlled population-based trial. J Thorac Oncol 2018; 13: Suppl., S185. doi: 10.1016/j.jtho.2018.08.012 [DOI] [Google Scholar]
- 70.Newman AM, Bratman SV, To J, et al. . An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med 2014; 20: 548–554. doi: 10.1038/nm.3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen JD, Li L, Wang Y, et al. . Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018; 359: 926–930. doi: 10.1126/science.aar3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. . Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 2017; 7: 1394–1403. doi: 10.1158/2159-8290.CD-17-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Murillas I, Schiavon G, Weigelt B, et al. . Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015; 7: 302ra133. doi: 10.1126/scitranslmed.aab0021 [DOI] [PubMed] [Google Scholar]
- 74.Tie J, Wang Y, Tomasetti C, et al. . Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 2016; 8: 346ra92. doi: 10.1126/scitranslmed.aaf6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pignon J-P, Tribodet H, Scagliotti GV, et al. . Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol 2008; 26: 3552–3559. doi: 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- 76.Jamal-Hanjani M, Wilson GA, McGranahan N, et al. . Tracking the evolution of non-small-cell lung cancer. N Engl J Med 2017; 376: 2109–2121. doi: 10.1056/NEJMoa1616288 [DOI] [PubMed] [Google Scholar]
- 77.Abbosh C, Birkbak NJ, Wilson GA, et al. . Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017; 545: 446–451. doi: 10.1038/nature22364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ulrich BC, Guibert N. Towards a comprehensive framework for cell-free DNA analysis: lessons from TRACERx. Ann Transl Med 2017; 5: 428. doi: 10.21037/atm.2017.08.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yanagita M, Redig AJ, Paweletz CP, et al. . A prospective evaluation of circulating tumor cells and cell-free DNA in EGFR-mutant non-small cell lung cancer patients treated with erlotinib on a phase II trial. Clin Cancer Res 2016; 22: 6010–6020. doi: 10.1158/1078-0432.CCR-16-0909 [DOI] [PubMed] [Google Scholar]
- 80.Ooki A, Maleki Z, Tsay J-CJ, et al. . A panel of novel detection and prognostic methylated DNA markers in primary non-small cell lung cancer and serum DNA. Clin Cancer Res 2017; 23: 7141–7152. doi: 10.1158/1078-0432.CCR-17-1222 [DOI] [PubMed] [Google Scholar]
- 81.Kang G, Chen K, Yang F, et al. . Monitoring of circulating tumor DNA and its aberrant methylation in the surveillance of surgical lung cancer patients: protocol for a prospective observational study. BMC Cancer 2019; 19: 579. doi: 10.1186/s12885-019-5751-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mani SA, Guo W, Liao M-J, et al. . The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715. doi: 10.1016/j.cell.2008.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer 2014; 14: 623–631. doi: 10.1038/nrc3820 [DOI] [PubMed] [Google Scholar]
- 84.Hofman V, Ilie M, Long E, et al. . Detection of circulating tumor cells from lung cancer patients in the era of targeted therapy: promises, drawbacks and pitfalls. Curr Mol Med 2014; 14: 440–456. doi: 10.2174/1566524014666140414205455 [DOI] [PubMed] [Google Scholar]
- 85.Duda DG, Duyverman AMMJ, Kohno M, et al. . Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA 2010; 107: 21677–21682. doi: 10.1073/pnas.1016234107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009; 10: 445–457. doi: 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- 87.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett 2007; 253: 180–204. doi: 10.1016/j.canlet.2006.12.014 [DOI] [PubMed] [Google Scholar]
- 88.Barrière G, Tartary M, Rigaud M. Epithelial mesenchymal transition: a new insight into the detection of circulating tumor cells. ISRN Oncol 2012; 2012: 382010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hofman V, Ilie MI, Long E, et al. . Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small cell lung carcinoma: comparison of the efficacy of the CellSearch AssayTM and the isolation by size of epithelial tumor cell method. Int J Cancer 2011; 129: 1651–1660. doi: 10.1002/ijc.25819 [DOI] [PubMed] [Google Scholar]
- 90.Krebs MG, Hou J-M, Sloane R, et al. . Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012; 7: 306–315. [DOI] [PubMed] [Google Scholar]
- 91.Hofman V, Long E, Ilie M, et al. . Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathol 2012; 23: 30–38. doi: 10.1111/j.1365-2303.2010.00835.x [DOI] [PubMed] [Google Scholar]
- 92.Vona G, Sabile A, Louha M, et al. . Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol 2000; 156: 57–63. doi: 10.1016/S0002-9440(10)64706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ilie M, Long E, Butori C, et al. . ALK-gene rearrangement: a comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann Oncol 2012; 23: 2907–2913. doi: 10.1093/annonc/mds137 [DOI] [PubMed] [Google Scholar]
- 94.Ilié M, Szafer-Glusman E, Hofman V, et al. . Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small cell lung cancer. Ann Oncol 2018; 29: 193–199. doi: 10.1093/annonc/mdx636 [DOI] [PubMed] [Google Scholar]
- 95.Nagrath S, Sequist LV, Maheswaran S, et al. . Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007; 450: 1235–1239. doi: 10.1038/nature06385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorges TM, Kuske A, Röck K, et al. . Accession of tumor heterogeneity by multiplex transcriptome profiling of single circulating tumor cells. Clin Chem 2016; 62: 1504–1515. doi: 10.1373/clinchem.2016.260299 [DOI] [PubMed] [Google Scholar]
- 97.Khamenehfar A, Li PCH. Microfluidic devices for circulating tumor cells isolation and subsequent analysis. Curr Pharm Biotechnol 2016; 17: 810–821. doi: 10.2174/1389201017666160301103509 [DOI] [PubMed] [Google Scholar]
- 98.Sollier-Christen E, Renier C, Kaplan T, et al. . VTX-1 liquid biopsy system for fully-automated and label-free isolation of circulating tumor cells with automated enumeration by BioView platform. Cytom Part J Int Soc Anal Cytol 2018; 93: 1240–1245. doi: 10.1002/cyto.a.23592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun Y, Haglund TA, Rogers AJ, et al. . Review: microfluidics technologies for blood-based cancer liquid biopsies. Anal Chim Acta 2018; 1012: 10–29. doi: 10.1016/j.aca.2017.12.050 [DOI] [PubMed] [Google Scholar]
- 100.Pierga J-Y, Bidard F-C, Mathiot C, et al. . Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res 2008; 14: 7004–7010. doi: 10.1158/1078-0432.CCR-08-0030 [DOI] [PubMed] [Google Scholar]
- 101.van Dalum G, Stam G-J, Scholten LFA, et al. . Importance of circulating tumor cells in newly diagnosed colorectal cancer. Int J Oncol 2015; 46: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 102.Ilie M, Hofman V, Long-Mira E, et al. . “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One 2014; 9: e111597. doi: 10.1371/journal.pone.0111597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leroy S, Benzaquen J, Mazzetta A, et al. . Circulating tumour cells as a potential screening tool for lung cancer (the AIR study): protocol of a prospective multicentre cohort study in France. BMJ Open 2017; 7: e018884. doi: 10.1136/bmjopen-2017-018884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cristofanilli M, Budd GT, Ellis MJ, et al. . Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351: 781–791. doi: 10.1056/NEJMoa040766 [DOI] [PubMed] [Google Scholar]
- 105.Bidard F-C, Peeters DJ, Fehm T, et al. . Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014; 15: 406–414. doi: 10.1016/S1470-2045(14)70069-5 [DOI] [PubMed] [Google Scholar]
- 106.Huang X, Gao P, Song Y, et al. . Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015; 15: 202. doi: 10.1186/s12885-015-1218-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lindsay CR, Faugeroux V, Michiels S, et al. . A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann Oncol 2017; 28: 1523–1531. doi: 10.1093/annonc/mdx156 [DOI] [PubMed] [Google Scholar]
- 108.Hofman V, Bonnetaud C, Ilie MI, et al. . Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011; 17: 827–835. doi: 10.1158/1078-0432.CCR-10-0445 [DOI] [PubMed] [Google Scholar]
- 109.Hou J-M, Krebs MG, Lancashire L, et al. . Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012; 30: 525–532. doi: 10.1200/JCO.2010.33.3716 [DOI] [PubMed] [Google Scholar]
- 110.Sher Y-P, Shih J-Y, Yang P-C, et al. . Prognosis of non-small cell lung cancer patients by detecting circulating cancer cells in the peripheral blood with multiple marker genes. Clin Cancer Res 2005; 11: 173–179. [PubMed] [Google Scholar]
- 111.Wu C, Hao H, Li L, et al. . Preliminary investigation of the clinical significance of detecting circulating tumor cells enriched from lung cancer patients. J Thorac Oncol 2009; 4: 30–36. [DOI] [PubMed] [Google Scholar]
- 112.Massard C, Borget I, Farace F, et al. . RECIST response and variation of circulating tumour cells in phase 1 trials: a prospective multicentric study. Eur J Cancer Oxf Engl 2017; 83: 185–193. [DOI] [PubMed] [Google Scholar]
- 113.Maheswaran S, Sequist LV, Nagrath S, et al. . Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008; 359: 366–377. doi: 10.1056/NEJMoa0800668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sundaresan TK, Sequist LV, Heymach JV, et al. . Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin Cancer Res 2016; 22: 1103–1110. doi: 10.1158/1078-0432.CCR-15-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pailler E, Adam J, Barthélémy A, et al. . Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J Clin Oncol 2013; 31: 2273–2281. doi: 10.1200/JCO.2012.44.5932 [DOI] [PubMed] [Google Scholar]
- 116.Ilie M, Szafer-Glusman E, Hofman V, et al. . Expression of MET in circulating tumor cells correlates with expression in tumor tissue from advanced-stage lung cancer patients. Oncotarget 2017; 8: 26112–26121. doi: 10.18632/oncotarget.15345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cui S, Zhang W, Xiong L, et al. . Use of capture-based next-generation sequencing to detect ALK fusion in plasma cell-free DNA of patients with non-small-cell lung cancer. Oncotarget 2017; 8: 2771–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alix-Panabières C, Pantel K. Characterization of single circulating tumor cells. FEBS Lett 2017; 591: 2241–2250. doi: 10.1002/1873-3468.12662 [DOI] [PubMed] [Google Scholar]
- 119.Pawlikowska P, Faugeroux V, Oulhen M, et al. . Circulating tumor cells (CTCs) for the noninvasive monitoring and personalization of non-small cell lung cancer (NSCLC) therapies. J Thorac Dis 2019; 11: Suppl. 1, S45–S56. doi: 10.21037/jtd.2018.12.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pailler E, Mezquita L, Faugeroux V, et al. . Analysis of single circulating tumor cells (CTCs) to identify resistance mutations to ALK-inhibitors in both ALK-gene and bypass oncogenic pathways. J Clin Oncol 2018; 36: 15 Suppl., 12038. doi: 10.1200/JCO.2018.36.15_suppl.12038 [DOI] [Google Scholar]
- 121.Dhar M, Wong J, Che J, et al. . Evaluation of PD-L1 expression on vortex-isolated circulating tumor cells in metastatic lung cancer. Sci Rep 2018; 8: 2592. doi: 10.1038/s41598-018-19245-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guibert N, Delaunay M, Lusque A, et al. . PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018; 120: 108–112. doi: 10.1016/j.lungcan.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 123.Kallergi G, Vetsika E-K, Aggouraki D, et al. . Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther Adv Med Oncol 2018; 10: 1758834017750121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koh Y, Yagi S, Akamatsu H, et al. . Comparison of PD-L1 expression between tumor tissues and circulating tumor cells in patients with lung cancer. Eur J Cancer 2016; 69: S14. doi: 10.1016/S0959-8049(16)32629-6 [DOI] [Google Scholar]
- 125.Mazel M, Jacot W, Pantel K, et al. . Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 2015; 9: 1773–1782. doi: 10.1016/j.molonc.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nicolazzo C, Raimondi C, Mancini M, et al. . Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor nivolumab. Sci Rep 2016; 6: 31726. doi: 10.1038/srep31726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Skibinski DA. Noninvasive detection of PD-L1 on circulating tumor cells in patient blood samples. Future Oncol 2018; 14: 1237–1240. doi: 10.2217/fon-2018-0150 [DOI] [PubMed] [Google Scholar]
- 128.Ulrich BC, Guibert N. Non-invasive assessment of tumor PD-L1 status with circulating tumor cells. Ann Transl Med 2018; 6: Suppl. 1, S48. doi: 10.21037/atm.2018.10.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leone K, Poggiana C, Zamarchi R, et al. . The interplay between circulating tumor cells and the immune system: from immune escape to cancer immunotherapy. Diagnostics 2018; 8: 59. doi: 10.3390/diagnostics8030059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hodgkinson CL, Morrow C, Li Y, et al. . Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 2014; 20: 897–903. [DOI] [PubMed] [Google Scholar]
- 131.Lallo A, Schenk MW, Frese KK, et al. . Circulating tumor cells and CDX models as a tool for preclinical drug development. Transl Lung Cancer Res 2017; 6: 397–408. doi: 10.21037/tlcr.2017.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. . Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov 2017; 7: 188–201. doi: 10.1158/2159-8290.CD-16-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. . Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016; 375: 819–829. doi: 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Remon J, Menis J, Hasan B, et al. . The APPLE trial: feasibility and activity of AZD9291 (osimertinib) treatment on positive plasma T790M in EGFR-mutant NSCLC patients. EORTC 1613. Clin Lung Cancer 2017; 18: 583–588. doi: 10.1016/j.cllc.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 135.Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov 2016; 6: 479–491. doi: 10.1158/2159-8290.CD-15-1483 [DOI] [PubMed] [Google Scholar]