Abstract
Sphingolipids are a distinct class of lipid molecules widely found in nature, principally as cell membrane constituents. After initial uncertainty about their function, sphingolipids have been increasingly recognised to be metabolically active entities involved in many biological processes, including the control of inflammation. Their role as mediators of inflammation may have significant implications for a range of lung diseases in which inflammation is a central element of pathogenesis. Chronic obstructive pulmonary disease (COPD), a highly prevalent and morbid condition predominantly affecting cigarette smokers, is a prime example of a respiratory illness with an inflammatory component. Understandably, sphingolipids have received growing attention for their increasingly demonstrated role in the pathophysiology of COPD. The present review aims to be among the first to focus exclusively on the connection between sphingolipids and lung inflammation in COPD, providing the reader with a clinically oriented synopsis of this intriguing association.
Short abstract
Sphingolipids have been hypothesised to play a role in the pathogenesis of COPD. Progress in the understanding of sphingolipid pathobiology in COPD has identified putative therapeutic targets. http://bit.ly/2KVwbj4
Introduction
Chronic obstructive pulmonary disease (COPD) is estimated to affect 30 million Americans [1]. In European adults, the reported prevalence of COPD ranges from 15% to 20% [2]. It is the third leading cause of death in the USA and the fourth worldwide [3]. COPD is a disease intimately linked to exposure to cigarette smoking and environmental pollutants, which can cause airway obstruction and inflammation [4]. The former component is typically treated with bronchodilation [5] and the latter with inhaled corticosteroids after consideration of symptoms and exacerbation burden [6]. Acute exacerbations of COPD call for the addition of systemic steroids, antibiotics and, in more severe cases, ventilator support [7]. In the emphysema phenotype, progressive destruction of the small airways and alveoli can lead to chronic respiratory failure due to irreversible loss of functional lung units [8]. Inflammation along with oxidative stress has been implicated in this destructive process through disruption of the balance between protease and antiprotease activity [9].
Sphingolipids are a unique category of lipids in which the backbone is a long chain (sphingoid) base with an attached fatty acid [10]. They are found in all types of eukaryotic cells, where they are uniquely suited as membrane components due to their amphipathic nature. Initially, their function was poorly understood, hence the root ‘sphinx’ (Greek for enigma) in their name [11]. Over time, sphingolipids have turned out to be active participants in a myriad of cellular functions, including growth, differentiation, apoptosis and protein trafficking [12]. They have also been found to play a role as mediators of cell signalling initiated by important regulators of inflammation such as tumour necrosis factor (TNF)-α and nuclear factor (NF)-κβ [13]. In medicine, sphingolipids have been implicated in the development of common disease states, among them cancer [14], diabetes [15] and neurodegenerative conditions [15]. They have been increasingly studied in the field of respiratory medicine [16, 17] for their link to lung inflammation and cell cycle control [18].
The present review briefly summarises the fundamentals of sphingolipid metabolism and then proceeds to discuss current perspectives on the relationship between sphingolipids and COPD with respect to pathogenesis and potential therapeutic interventions.
Sphingolipid biochemistry
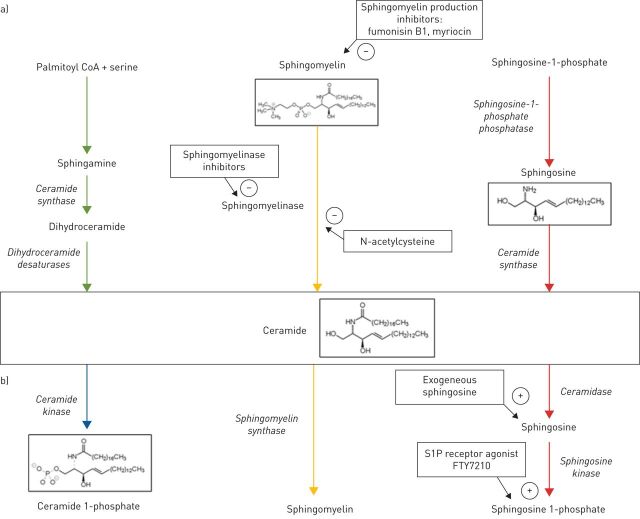
Ceramide is the most basic of the sphingolipids composed of sphingosine as the backbone attached to a fatty acid. It lies at the core of sphingolipid metabolism. There are three major pathways that can lead to the formation of ceramide (figure 1a): 1) de novo synthesis from serine and palmitoyl-CoA; 2) conversion from sphingomyelin by sphingomyelinase (SMase); and 3) conversion from sphingosine.
FIGURE 1.
a) Schematic depiction of the three major pathways capable of producing ceramide with relevant enzymes (italics) and therapeutic inhibition points (arrows) indicated. b) Schematic representation of the three major reactions involving ceramide as substrate. Relevant enzymes are listed in italics, and options for so-called “sphingosine 1-phosphate rescue” are indicated by arrows. −: inhibitory actions; +: promoter actions.
The important SMase family of enzymes is divided according to an optimal working pH into acidic, neutral and alkaline. Acid sphingomyelinase (ASMase) activity has been shown to increase in response to experimental lung injury with a resultant adverse effect on surfactant function [19]. Inflammatory mediators such as TNF-α can activate neutral sphingomyelinase (NSMase), which in turn is part of a pathway that increases cyclooxygenase (COX)-2 expression in macrophages [20, 21]. COX-2 is an inducible enzyme that generates pro-inflammatory prostaglandins from arachidonic acid [22].
Ceramide, in turn, serves as a substrate for three major reactions (figure 1b): 1) conversion to ceramide 1-phosphate (C1P) by ceramide kinase; 2) conversion to sphingomyelin by sphingomyelin synthase; and 3) conversion to sphingosine by ceramidase, followed by phosphorylation to become sphingosine 1-phosphate (S1P).
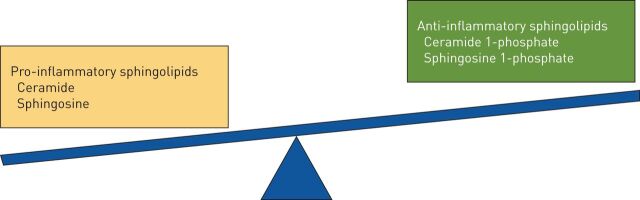
Of the many known sphingolipids, ceramide, C1P and S1P have been the most extensively studied. C1P plays an active role in inflammation and apoptosis [23], whereas S1P is a mediator of cell survival [12]. One theory gaining traction is that it is the balance among competing sphingolipids rather than the quantity of any specific sphingolipid that determines the net effect they will exert on a given system [24]. The term that has been applied to this concept is the “sphingolipid rheostat” (figure 2).
FIGURE 2.
Competing sphingolipids with predominantly pro-inflammatory and anti-inflammatory properties, the net balance of which constitutes the “sphingolipid rheostat”.
Sphingolipids and COPD pathogenesis
Chronic airway inflammation is a central element of the pathophysiology of COPD [8]. Cigarette smoking is the most common aetiologic agent for the inflammation, which persists even after smoking cessation [25]. However, COPD has also been detected in 25–45% of certain populations of never-smokers, such as those exposed to indoor consumption of biomass fuel or to high levels of outdoor air pollution [25].
The classic theory of COPD pathogenesis has held that respiratory irritants create an imbalance between protease and antiprotease activity, which results in the progressive destruction of the bronchoalveolar epithelium [4]. More recently, murine and human investigations have shown that cigarette smoke leads to NSMase activation, thereby increasing ceramide levels, which raises oxidative stress and promotes apoptosis [26]. The effect of ceramide on oxidative stress is multifactorial: it stimulates the synthesis of reactive oxygen species while at the same time suppressing antioxidants such as superoxide dismutase [27]. The mechanisms of ceramide-induced apoptosis are likewise diverse and include facilitation of the pro-apoptotic effects of TNF-α [28]. Conversely, ceramide inhibitors and antioxidants have been shown to protect against the deleterious effects of ceramide in experimental models [29]. Consistent with these properties of ceramide, lung ceramide levels have been found to be higher in emphysematous smokers than in those without emphysema [30]. Experimental data have also suggested that the effect of ceramide on lung epithelial and endothelial cells may be modulated by chronic cigarette smoke exposure, wherein the response of long-term smokers may actually be proliferative rather than apoptotic [31]. In the case of endothelial cells, this could have relevance to the pathogenesis of vascular remodelling in COPD-associated pulmonary hypertension [32].
It has been recognised that the alveolar macrophages of smokers are deficient in clearing apoptotic epithelial cells (a process known as efferocytosis) due to an impaired phagocytic function [33]. Accumulation of apoptotic cells in the lung has been hypothesised to contribute to the chronic inflammation seen in COPD. Acting through its close relative sphingosine, ceramide was found to inhibit efferocytosis, thus potentially contributing to lung destruction in COPD by both augmenting apoptosis and interfering with apoptotic cell clearance [32].
In contrast with ceramide, its derivative C1P is capable of suppressing inflammation. Specifically, it has been shown to dampen airway injury induced by cigarette smoke, possibly through inhibition of NF-κβ and NSMase [23]. Another product of ceramide metabolism, S1P, attenuated the pro-apoptotic activity of its parent compound in an animal study, an effect which was associated with reduced emphysema-like change on histological sections [34]. Recapitulating the concept of the so-called “sphingolipid rheostat” or balance as the determinant of net effect, augmentation of S1P activity in murine lung tissue overexpressing ceramide led to preservation of airspace morphology and blunting of apoptosis, as well as restoration of the proliferative ability of lung endothelial cells [34, 35].
Clinical evidence for the role of sphingolipids as a class in the pathogenesis of COPD builds on the finding that the expression of 168 different sphingolipid species is significantly higher in the sputum of smokers with COPD [36]. The sphingolipids whose expression was most dramatically elevated in people with COPD correlated strongly with spirometric impairment and inflammatory cell burden in the sputum [36]. Figure 3 schematically depicts the putative contributions of sphingolipids to the pathogenesis of COPD.
FIGURE 3.
Schematic summary of sphingolipid actions implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD) with ceramide as the central mediator. C1P: ceramide 1-phosphate; S1P: sphingosine 1-phosphate; nSMase: neutral sphingomyelinase; ROS: reactive oxygen species; SOD: superoxide dismutase; TNF-α: tumour necrosis factor-α. +: pro-inflammatory effects of ceramide derivatives; −: anti-inflammatory.
Therapeutic implications
The search for pharmaceutical agents capable of manipulating sphingolipid metabolism for therapeutic benefit has mainly focused on suppression of either de novo ceramide synthesis or its generation from sphingomyelin. Fungal metabolites fumonisin B1 and myriocin are effective synthesis inhibitors in vitro [37, 38], but their disadvantage is the accumulation of toxic precursors and the depletion of downstream anti-inflammatory ceramide derivatives, namely S1P [28]. One potential solution to the S1P depletion problem could be so-called “S1P rescue” in the form of exogenous S1P precursor sphingosine or the S1P receptor agonist FTY720, itself a fungal product derived from myriocin (figure 1a) [34].
Another possible therapeutic target is ASMase, which catalyses the conversion of sphingomyelin to ceramide [19]. Many widely used antidepressants, among them tricyclic compounds and selective serotonin reuptake inhibitors, can be considered functional inhibitors of acid sphingomyelinase (FIASMAs) [39]. They act selectively upon lysosomal ASMase, which is normally resistant to proteolysis but becomes vulnerable when detached from the lysosome by these agents. As opposed to FIASMAs, direct ASMase inhibitors such as the bisphosphonate drug family inhibit both lysosomal and plasma membrane ASMase [40]. Notably, NSMase appears to be more directly involved in cigarette smoke-related airway injury mediated by ceramide than ASMase [41]. The investigators who demonstrated this finding also showed that the antioxidant N-acetyl cysteine (NAC) is effective at downregulating NSMase [41]. The latter observation is intriguing in light of recent randomised controlled trial data suggesting a benefit of NAC in the prevention of acute exacerbations of COPD [42]. Continued progress in the understanding of sphingolipid biology and function has yielded numerous other candidate foci for pharmacological intervention, but no specific agent is currently nearing clinical application.
Conclusion
Sphingolipids have been the subject of active research pertinent to many lung diseases in which inflammation plays a pivotal role, among them COPD. There is mounting experimental evidence that ceramide, acting either by itself or through closely related sphingolipids, stimulates lung inflammation by a variety of mechanisms. However, other derivative sphingolipids such as S1P exert an anti-inflammatory effect, giving rise to the notion that the cumulative effect of sphingolipids on lung inflammation reflects a balance of competing influences. In the laboratory, it has been possible to effectively antagonise pro-inflammatory sphingolipids and promote anti-inflammatory ones, but the direct clinical application of these pathways for COPD therapy needs further investigation.
Footnotes
Submitted article, peer reviewed.
Conflict of interest: R.C. Chakinala has nothing to disclose.
Conflict of interest: A. Khatri has nothing to disclose.
Conflict of interest: K. Gupta has nothing to disclose.
Conflict of interest: K. Koike has nothing to disclose.
Conflict of interest: O. Epelbaum has nothing to disclose.
References
- 1.COPD Foundation. COPD Across America. www.copdfoundation.org/What-is-COPD/Understanding-COPD/Statistics.aspx Date last accessed: May 21, 2019.
- 2.Terzikhan N, Verhamme KM, Hofman A, et al. . Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam study. Eur J Epidemiol 2016; 8: 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2002. http://goldcopd.org [DOI] [PubMed]
- 4.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2009; 4: 435–459. [DOI] [PubMed] [Google Scholar]
- 5.Sestini P, Renzoni E, Robinson S, et al. . Short-acting beta 2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; 4: CD001495. [DOI] [PubMed] [Google Scholar]
- 6.Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev 2014; 3: CD010844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 2007; 29: 1224–1238. [DOI] [PubMed] [Google Scholar]
- 8.McDonough JE, Yuan R, Suzuki M, et al. . Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011; 365: 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003; 22: 672–688. [DOI] [PubMed] [Google Scholar]
- 10.Merrill AH Jr, Schmelz EM, Dillehay DL, et al. . Sphingolipids – the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol 1997; 142: 208–225. [DOI] [PubMed] [Google Scholar]
- 11.Grimal P. The Dictionary of Classical Mythology. Vol. 1. Oxford, Wiley-Blackwell, 1996. [Google Scholar]
- 12.Spiegel S, Merrill AH Jr. Sphingolipid metabolism and cell growth regulation. FASEB J 1996; 10: 1388–1397. [DOI] [PubMed] [Google Scholar]
- 13.Dbaibo GS, Obeid LM, Hannun YA. Tumor necrosis factor-α (TNF-α) signal transduction through ceramide. Dissociation of growth inhibitory effects of TNF-α from activation of nuclear factor-κB. J Biol Chem 1993; 268: 17762–17766. [PubMed] [Google Scholar]
- 14.Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 2018; 18: 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol 2008; 9: 162–176. [DOI] [PubMed] [Google Scholar]
- 16.Ammit AJ, Hastie AT, Edsall LC, et al. . Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J 2001; 15: 1212–1214. [DOI] [PubMed] [Google Scholar]
- 17.Jolly PS, Rosenfeldt HM, Milstien S, et al. . The roles of sphingosine-1-phosphate in asthma. Mol Immunol 2002; 38: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 18.Helke K, Angel P, Lu P, et al. . Ceramide synthase 6 deficiency enhances inflammation in the DSS model of colitis. Sci Rep 2018; 8: 1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Bismarck P, Wistädt CF, Klemm K, et al. . Improved pulmonary function by acid sphingomyelinase inhibition in a newborn piglet lavage model. Am J Respir Crit Care Med 2008; 177: 1233–1241. [DOI] [PubMed] [Google Scholar]
- 20.Clarke CJ, Guthrie JM, Hannun YA. Regulation of neutral sphingomyelinase-2 (NSMase2) by tumor necrosis factor-alpha involves protein kinase c-delta in lung epithelial cells. Mol Pharmacol 2008; 74: 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen BC, Chang HM, Hsu MJ, et al. . Peptidoglycan induces cyclooxygenase-2 expression in macrophages by activating the neutral sphingomyelinase-ceramide pathway. J Biol Chem 2009; 284: 20562–20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999; 18: 7908–7916. [DOI] [PubMed] [Google Scholar]
- 23.Baudiß K, Ayata CK, Lazar Z, et al. . Ceramide-1-phosphate inhibits cigarette smoke-induced airway inflammation. Eur Respir J 2015; 45: 1669–1680. [DOI] [PubMed] [Google Scholar]
- 24.Pyne S, Pyne NJ. Sphingosine 1 phosphate signalling in mammalian cells. Biochem J 2000; 349: 385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009; 374: 733–743. [DOI] [PubMed] [Google Scholar]
- 26.Levy M, Khan E, Careaga M, et al. . Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death. Am J Physiol Lung Cell Mol Physiol 2009; 297: L125–L133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrache I, Natarajan V, Zhen L, et al. . Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005; 11: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghidoni R, Caretti A, Signorelli P. Role of sphingolipids in the pathobiology of lung inflammation. Mediators Inflamm 2015; 2015: 487508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrache I, Medler TR, Richter AT, et al. . Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol 2008; 295: L44–L53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarpa MC, Baraldo S, Marian E, et al. . Ceramide expression and cell homeostasis in chronic obstructive pulmonary disease. Respiration 2013; 85: 342–349. [DOI] [PubMed] [Google Scholar]
- 31.Petrusca DN, Van Demark M, Gu Y, et al. . Smoking exposure induces human lung endothelial cell adaptation to apoptotic stress. Am J Respir Cell Mol Biol 2014; 50: 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrusca DN, Gu Y, Adamowicz JJ, et al. . Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J Biol Chem 2010; 285: 40322–40332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodge S, Hodge G, Ahern J, et al. . Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2007; 37: 748–755. [DOI] [PubMed] [Google Scholar]
- 34.Diab KJ, Adamowicz JJ, Kamocki K, et al. . Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med 2010; 181: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweitzer KS, Chen SX, Law S, et al. . Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol 2015; 309: L175–L187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Telenga ED, Hoffmann RF, Ruben T, et al. . Untargeted lipidomic analysis in chronic obstructive pulmonary disease. Uncovering sphingolipids. Am J Respir Crit Care Med 2014; 190: 155–164. [DOI] [PubMed] [Google Scholar]
- 37.Castillo SS, Levy M, Wang C, et al. . Nitric oxide-enhanced caspase-3 and acidic sphingomyelinase interaction: a novel mechanism by which airway epithelial cells escape ceramide-induced apoptosis. Exp Cell Res 2007; 313: 816–823. [DOI] [PubMed] [Google Scholar]
- 38.Schroeder JJ, Crane HM, Xia J, et al. . Disruption of sphingolipid metabolism and stimulation of DNA synthesis by fumonisin B1. A molecular mechanism for carcinogenesis associated with Fusarium moniliforme. J Biol Chem 1994; 269: 3475–3481. [PubMed] [Google Scholar]
- 39.Albouz S, Le Saux F, Wenger D, et al. . Modifications of sphingomyelin and phosphatidylcholine metabolism by tricyclic antidepressants and phenothiazines. Life Sci 1986; 38: 357–363. [DOI] [PubMed] [Google Scholar]
- 40.Kölzer M, Arenz C, Ferlinz K, et al. . Phosphatidylinositol-3,5-bisphosphate is a potent and selective inhibitor of acid sphingomyelinase. Biol Chem 2003; 384: 1293–1298. [DOI] [PubMed] [Google Scholar]
- 41.Filosto S, Castillo S, Danielson A, et al. . Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury. Am J Respir Cell Mol Biol 2011; 44: 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng JP, Wen FQ, Bai CX, et al. . Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014; 2: 187–194. [DOI] [PubMed] [Google Scholar]