Abstract
Recent evidence has demonstrated that mucin 1 (MUC1) is involved in many pathological processes that occur in the lung. MUC1 is a transmembrane protein mainly expressed by epithelial and hematopoietic cells. It has a receptor-like structure, which can sense the external environment and activate intracellular signal transduction pathways through its cytoplasmic domain. The extracellular domain of MUC1 can be released to the external environment, thus acting as a decoy barrier to mucosal pathogens, as well as serving as a serum biomarker for the diagnosis and prognosis of several respiratory diseases such as lung cancer and interstitial lung diseases. Furthermore, bioactivated MUC1-cytoplasmic tail (CT) has been shown to act as an anti-inflammatory molecule in several airway infections and mediates the expression of anti-inflammatory genes in lung diseases such as chronic rhinosinusitis, chronic obstructive pulmonary disease and severe asthma. Bioactivated MUC1-CT has also been reported to interact with several effectors linked to cellular transformation, contributing to the progression of respiratory diseases such as lung cancer and pulmonary fibrosis. In this review, we summarise the current knowledge of MUC1 as a promising biomarker and drug target for lung disease.
Short abstract
MUC1 is involved in most lung inflammatory, carcinogenic and fibrotic processes, thus representing a promising druggable target and useful biomarker for several lung diseases https://bit.ly/32gruZO
MUC1 overview
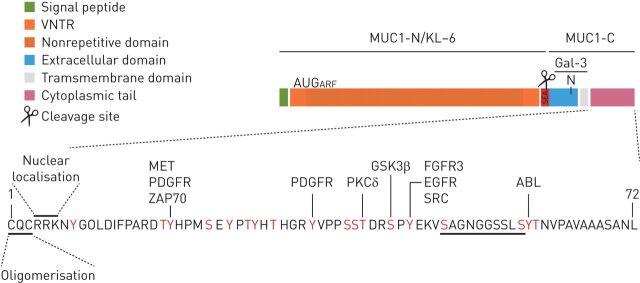
Mucin 1 (MUC1) is a large transmembrane O-glycoprotein (approximately 200 kDa) with a rigid structure that extends up to 200–500 nm from the cell surface of primarily epithelial and hematopoietic cells [1, 2]. As a tethered mucin, MUC1 is composed of dimers of two dissimilar subunits (α and β chains) held together by noncovalent, sodium dodecyl sulfate-labile bonds [1]. The larger subunit (α-chain or N-terminal subunit), which is extracellular and can be shed into the lumen, comprises a signal peptide; 25–125 tandem repeats of a 20-amino acid sequence of PTS domains (regions with high proportions of proline, threonine and serine residues) that are heavily O-glycosylated; and the sperm protein, enterokinase and agrin (SEA) domain. However, the smaller subunit (β-chain or C-terminal subunit) consists of a 58-amino acid extracellular domain, a single-pass 28-amino acid transmembrane domain and a 72-amino acid cytoplasmic tail (CT) [3], which contains 18 documented potential and putative tyrosine or serine/threonine phosphorylation sites (figure 1) [4].
FIGURE 1.
Structure of mucin 1 (MUC-1). MUC1 protein comprises an N-terminal subunit (also called KL-6) constituted by a signal peptide, a VNTR domain composed of 25–125 repeats of 20 amino acids and the SEA domain. MUC1-C-terminal subunit has a 58-amino acid extracellular domain, a single-pass 28-amino acid transmembrane domain and a 72-amino acid CT. The MUC1 extracellular domain can be shed into the lumen by auto-proteolytic cleavage in the SEA domain or by the action of metalloproteinase 14 in the region following the SEA domain. The MUC1-C extracellular domain is glycosylated on Asn-36 and then serves as a binding site for the profibrotic galectin 3 ligand. MUC1-CT serves as a substrate for phosphorylation (18 documented and putative tyrosine and serine/threonine potential phosphorylation sites) in response to activation of several growth factor receptors and/or kinases. The CQC motif is necessary for MUC1-C oligomerisation and the RRK motif is necessary for MUC1-C binding to importin β and targeting to the nucleus. β-catenin-binding site is also localised to MUC1-CT. An AUG codon downstream to the MUC1 initiation codon has been described to initiate an ARF thereby generating a novel protein, MUC1-ARF. ABL: tyrosine-protein kinase ABL1; ARF: alternate reading frame; CT: cytoplasmic tail; FGFR: fibroblast growth factor receptor; Gal-3: galectin-3; GSK3β: glycogen synthase kinase 3 beta; KL-6: Krebs von den Lungen-6; MET: tyrosine-protein kinase Met; MUC1-C: mucin 1 C-terminal; MUC1-N: mucin 1 N-terminal; PDGFR: platelet-derived growth factor receptor; PKCδ: delta isoform of protein kinase C; SEA: sperm protein, enterokinase and agrin; VNTR: variable number tandem repeat; ZAP70: zeta chain of T-cell receptor associated protein kinase 70.
At the plasma membrane, MUC1 acts as a membrane receptor, regulating several inflammatory processes through the phosphorylation and bioactivation of MUC1-CT. Nuclear translocation of bioactivated MUC1-CT has been demonstrated to mediate the expression of several anti-inflammatory genes, thus acting as an important mediator in respiratory diseases such as chronic rhinosinusitis [5], asthma [6] and COPD [7]. MUC1-CT has also been shown to play an anti-inflammatory role in several airway infections [8]. However, bioactivated MUC1-CT also interacts with various effectors linked to transformation [4], thus promoting carcinogenic and fibrotic processes in the lung.
The MUC1-N-terminal subunit has been shown to increase the invasive and metastatic abilities of tumour cells [1, 9, 10], as well as providing a releasable decoy barrier during airway infection [11]. Furthermore, the soluble MUC1-N-terminal subunits Krebs von den Lungen-6 (KL-6) and CA15-3 have been studied as serum biomarkers in several lung diseases [12, 13].
All these findings indicate the importance of MUC1 in the development of lung diseases and suggest that MUC1 may be useful in the diagnosis of many lung diseases, or as a therapeutic target.
MUC1 expression in lung tissue has been evaluated by immunohistochemistry analysis in several lung diseases [14–20]. However, depending on the anti-MUC1 antibody used, a large variability of MUC1 expression has been reported [21]. Therefore, immunohistochemistry analysis should be considered to be a weak point when evaluating MUC1 expression and its influence on mechanisms of disease.
MUC1 structure [22], interacting proteins [23], expression [24] and participation in several carcinogenic and inflammatory processes [4, 25], including inflammation in the respiratory tract [26], have been previously reviewed. However, the exact role of MUC1 in most of the currently known lung diseases has not been reviewed in detail yet. This review summarises the current state of MUC1 research in several respiratory diseases and provides an assessment of the potential of MUC1 as a diagnosis/prognosis tool and as a treatment target for lung disease.
MUC1 and airway inflammation and infection
MUC1 has been shown to be an important modulator of innate immunity, playing an important anti-inflammatory role during airway infection caused by bacterial and viral pathogens [27–29]. The suppressive effect of MUC1 has been attributed to Toll-like receptors (TLR) 2, 3, 4, 5, 7 and 9 signalling, which attenuates nuclear factor (NF)-κB activation and reduce tumour necrosis factor (TNF)-α and interleukin (IL)-8 secretion [8]. Furthermore, the anti-inflammatory effect of MUC1 has been reported to require only the MUC1 cytoplasmic domain, instead of the MUC1 entire molecule [8].
Immunoprecipitation and immunofluorescence studies revealed that MUC1-CT constitutively forms a protein complex with TLR5 [30]. Since all TLR adaptor proteins (myeloid differentiation primary response gene 88 (MyD88) for TLR2, 4, 5, 7, and 9 and TIR (Toll/interleukin-1 receptor)-domain-containing adapter-inducing interferon-β (TRIF) for TLR3) share the conserved TIR domain, it has been suggested that MUC1-CT might suppress TLR signalling by associating with the TIR domains of TLRs, acting as a steric hindrance/decoy receptor and preventing recruitment of adaptor proteins to their respective receptors (figure 2) [30]. Nevertheless, further research is needed to confirm that MUC1-CT forms protein complexes with all the TLRs, and, to confirm the direct interaction between MUC1-CT and TIR domains.
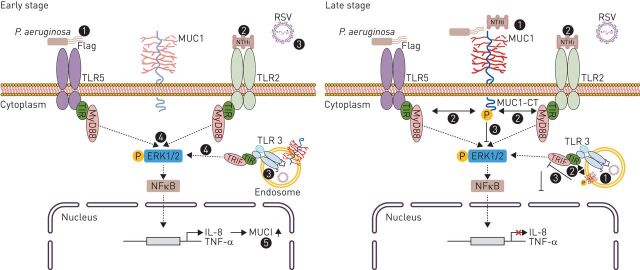
FIGURE 2.
The role of MUC1 in airway infection/inflammation. During early stage of infection, primary human airway epithelial cells sense Pseudomonas aeruginosa via TLR5 (1), Haemophilus influenzae (NTHi) via TLR2 (2), and RSV via TLR3 (3), which is expressed in endosomes. After recognition, MyD88/TRIF-dependent secretion of TNF-α and IL-8 is produced (4). Following it, IL-8 and TNF-α lead to an increased expression of MUC1 at the apical surface of lung epithelial cells (5). During the late-stage of infection, P. aeruginosa/NTHi/RSV-induced MUC1-CT tyrosine phosphorylation (1) leads to MUC1-CT interaction with TLR5/TLR2/TLR3 (2), leading to TLR signalling downregulation (3) and inhibition of inflammation (4). CT: cytoplasmic tail; ERK: extracellular signal-regulated kinase; NFκB: nuclear factor-κB; IL: interleukin; MUC1: mucin 1; MyD88: myeloid differentiation primary response gene 88; RSV: respiratory syncytial virus; TIR: Toll/interleukin-1 receptor; TLR: Toll-like receptor; TNF: tumour necrosis factor; TRIF: TIR (Toll/interleukin-1 receptor)-domain-containing adapter-inducing interferon-β.
As an example of bacterial airway infection, MUC1 has been reported to act as an extracellular adhesion site for Pseudomonas aeruginosa, an opportunistic pathogen that colonises the lungs in several airway diseases, including bronchiectasis, cystic fibrosis, and nosocomial pneumonia [31–34]. Initially, it was suggested that during early stage infection, primary human airway epithelial cells and macrophages sense P. aeruginosa infection via TLR5 recognition of flagellin and subsequent MyD88-dependent secretion of TNF-α and IL-8. Following this, IL-8 recruits neutrophils that release neutrophil elastase (NE) into the airway lumen. TNF-α and NE secretion results in increased expression of MUC1 at the apical surfaces of lung epithelial cells. Therefore, during late-stage infection, P. aeruginosa-induced MUC1-CT tyrosine phosphorylation leads to inhibition of TLR5 signalling, downregulation of inflammation and promotion of neutrophil apoptosis, which is important for the removal of inflammatory cells from the lung (figure 2) [35, 36]. By contrast, Kato et al. [37] recently suggested that stimulation of primary human bronchial epithelial cells with P. aeruginosa or flagellin increases the release of transforming growth factor (TGF)-α, which leads to autocrine activation of epidermal growth factor receptor (EGFR) at the cell surface. Furthermore, P. aeruginosa stimulation of human or mouse macrophages has also been reported to enhance TNF-α release. Therefore, TGF-α via autocrine EGFR activation as well as TNF-α via paracrine signalling have both been reported to increase MUC1 expression in bronchial epithelial cells, thus increasing MUC1-CT/TLR5 association and inhibiting TLR5 signalling [38]. P. aeruginosa has also been shown to upregulate the expression of MUC1 in primary human alveolar macrophages via activation of TLR4 and the p38 mitogen-activated protein kinase signalling pathway, preventing hyperactivation of macrophages and emphasising the importance of MUC1 as a negative regulator of macrophage-generated inflammatory responses [39].
Similarly, MUC1 has been shown to be part of a feedback loop that downregulates TLR2-induced IL-8 and TNF-α production during late-stage airway epithelial cell infection with nontypeable Haemophilus influenzae (figure 2) [27].
MUC1 has been shown to also exhibit anti-inflammatory effects during TLR3 activation by respiratory syncytial virus, which is considered the leading cause of viral bronchiolitis and pneumonia, both of which represent a major health and economic burden worldwide [40, 41]. Following viral infection, TLR3, which is expressed in endosomes by airway epithelial cells, senses viral-derived double-stranded RNA, triggering TRIF-dependent inflammatory and apoptosis signalling pathways that release TNF-α, which in turn upregulate MUC1. The interaction of MUC1-CT with TLR3 supresses TNF-α release, terminating the inflammatory response (figure 2) [28, 42].
The protective role of MUC1 during respiratory tract infections is further supported by evidence reported from research into MUC1 and certain gastrointestinal infections. Indeed, MUC1 has demonstrated to play a protective role during infection with gastrointestinal pathogens such as Helicobacter pylori [43, 44] and Campylobacter jejuni [45], which also induce inflammation via TLR signalling.
Altogether, these results suggest anti-inflammatory function of MUC1 in the host inflammatory response to microbial respiratory tract infection, specifically in the resolution phase of inflammation through its ability to inhibit TLR signalling. Therefore, genetic or epigenetic alterations of MUC1-CT might block its ability to inhibit inflammatory signalling, thus contributing to the progression of respiratory tract infections, as well as, to the pathophysiology of chronic inflammatory lung diseases.
MUC1 and chronic rhinosinusitis with nasal polyps
Chronic rhinosinusitis (CRS) encompasses a group of heterogeneous disorders associated with inflammation of the nasal and sinus mucosa with or without the presence of nasal polyps (NP). Different inflammatory phenotypes have been described in NP: NP without asthma or aspirin intolerance (NPwA), NP with aspirin-tolerant asthma (NP-ATA), and NP with aspirin-intolerant asthma (NP-AIA).
The proposed CRS aetiology includes both host and environmental factors that activate the host innate and adaptive immune responses involved in pathogenesis [46, 47]. However, depending on the ethology, CRS immune responses might be different.
Cigarette smoke (CS) has been identified as a significant risk factor for the development of CRS [48]. Cigarette smoke-associated pathological phenotype includes globet cell metaplasia (GCM) and overproduction of mucus among others [49]. Recently, it has been reported that in response to cigarette smoke exposure, total protein levels of MUC1 are not altered in the lung tissue [50]. However, an expansion of basal/intermediate cells enriched with MUC1 in the intracellular compartment is apparent following exposure to cigarette smoke, leading to GCM and MUC5AC overproduction [50].
Eosinophilic inflammation is another hallmark of chronic rhinosinusitis with nasal polyps (CRSwNP). Staphylococcus aureus exotoxins are among the best characterised elicitors of eosinophilic inflammation and are thought to be heavily involved in the pathogenesis of CRSwNP through their behaviour as superantigens [51]. S. aureus has been observed to promote the production of IL-22 by NP cells. In this context, IL-22 regulates chronic inflammation and has been shown to significantly enhance the levels of MUC1 expression in NP cells, which are significantly and positively correlated with IL-22 receptor mRNA levels [52].
Oral systemic corticosteroids are the first-line therapy for patients with CRSwNP [53]. Among responders, corticosteroids have been shown to upregulate membrane-tethered mucins (MUC1 and MUC4) while downregulating secreted mucins (MUC5AC and MUC5B) in NP [54]. No differences for MUC1 expression have been observed among responders in the NPwA, NP-ATA and NP-AIA groups [5]. However, a large number of patients show resistance to the effects of systemic corticosteroids (CR-CRSwNP).
Dexamethasone has been shown to induce the formation of a protein complex between MUC1-CT and glucocorticoid receptor α (GRα), which results in translocation of GRα into the nucleus to exert its anti-inflammatory effects (figure 3) [5]. However, since patients with CR-CRSwNP exhibit significantly lower expression of MUC1 in the NP epithelium, as well as upregulation of TLR2 and TLR5 [5], it results in overactivation of TLR signalling and subsequent inflammation, contributing to the loss of corticosteroid efficacy in these patients. The same pattern of MUC1 expression was also observed between patients with CR-CRSwNP showing NPwA, NP-ATA and NP-AIA [5].
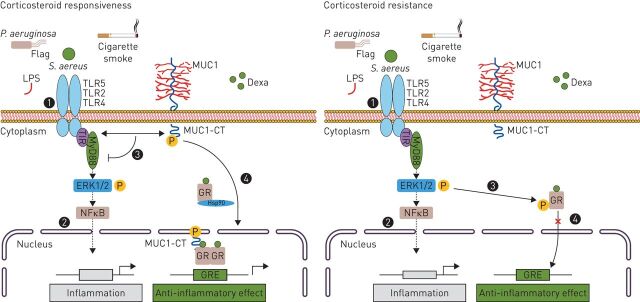
FIGURE 3.
The role of MUC1 in the corticosteroid mediated anti-inflammatory response in CRS, severe asthma and COPD. The TLR recognition of airway pathogens such as Pseudomonas aeruginosa or Staphylococcus aureus, as well as, cigarette smoke (1) triggers a MyD88-mediated inflammatory response (2). Among corticosteroid responder patients, corticosteroids increase MUC1 expression. The MUC1-CT interacts with TLR, inhibiting TLR inflammatory signalling (3). Dexamethasone (DEXA) treatment induces the formation of a protein complex between MUC1-CT and GRα, which helps GRα to translocate into the nucleus to exert its anti-inflammatory effects (4). Corticosteroid resistance patients show lower expression of MUC1. Therefore, it is produced an overactivation of TLR signalling (1) and subsequent inflammation (2), which leads to GR-Ser226 phosphorylation through ERK 1/2 hyper-phosphorylation (3). Therefore, MUC1-CT downregulation and GR hyperphosphorylation inhibit the corticosteroids-induced GRα nuclear translocation and GR anti-inflammatory mediated effects (4). CRS: chronic rhinosinusitis; CT: cytoplasmic tail; ERK: extracellular signal-regulated kinase; NFκB: nuclear factor-κB; GR: glucocorticoid receptor; GRE: glucocorticoid response element; Hsp: heat shock protein; IL: interleukin; LPS: lipopolysaccharide; MUC1: mucin 1; MUC1-CT: MUC1 cytoplasmic domain; MyD88: myeloid differentiation primary response gene 88; TIR: Toll/interleukin-1 receptor; TLR: Toll-like receptor; TRIF: TIR-domain-containing adapter-inducing interferon-β.
Patients with CR-CRSwNP show significantly higher extracellular signal-regulated kinase (ERK) 1/2 phosphorylation than responders [5]. Similarly, these patients also show higher glucocorticoid receptor (GR)-Ser226 phosphorylation [5], which has been reported to enhance GR nuclear export, thus contributing to termination of GR-mediated transcription [55]. It suggests that GR-Ser226 hyperphosphorylation is induced by ERK1/2 hyperphosphorylation, leading to an inhibition of corticosteroid-induced GRα nuclear translocation [56]. Therefore, MUC1 downregulation in patients with CR-CRSwNP has been suggested to contribute to the loss of corticosteroid efficacy, by GR-Ser226 hyperphosphorylation, which is consequence of the enhanced TLR-induced ERK1/2 phosphorylation, and by the reduced formation of the MUC1-CT-GRα transcription complex [5]. Furthermore, in the absence of ligand, GR resides predominantly in the cytoplasm, sequestered in a multimeric chaperone complex comprising heat shock protein (hsp) 90, hsp70, and other factors that prevent its degradation and assist in its maturation [57]. MUC1-CT has been shown to form a complex with hsp90 [58]. Therefore, MUC1-CT may bind the GRα-chaperone complex, leading to nuclear translocation of the mature complex (figure 3).
Overall, these findings suggest that increased or altered localisation of MUC1 expression might be a defence mechanism to compensate for excessive inflammation in patients with CRSwNP, modulating the anti-inflammatory effects of the corticosteroid therapy used and inhibiting overactivation of TLR-triggered inflammation.
MUC1 and asthma
Bronchial asthma is a chronic airway inflammatory disease whose strongest risk factors are a combination of genetic predisposition with environmental exposure to inhaled substances and particles that may provoke allergic reactions or irritate the airways.
The symptoms of patients with mild to moderate asthma are often well controlled with low doses of inhaled corticosteroids (ICS). However, the symptoms of most patients with severe asthma are not well controlled with ICS [59].
Four distinct asthma inflammatory phenotypes can be identified based on the presence (or absence) of increased levels of eosinophils and neutrophils. These phenotypes are termed eosinophilic (increased eosinophils); neutrophilic (increased neutrophils); granulocytic asthma (MGA) (increased both eosinophils and neutrophils); and paucigranulocytic (normal levels of eosinophils and neutrophils). Up to now, any association between MUC1 expression and the different asthma inflammatory phenotypes has not been reported yet. However, similar to patients with CR-CRSwNP, corticosteroid resistance in patients with uncontrolled severe asthma has been associated with MUC1 downregulation in human bronchial epithelial cells (HBECs) and blood neutrophils, as compared with patients with mild asthma and healthy controls [6]. Indeed, neutrophils and bronchial epithelial cells from patients with severe asthma are less sensitive to the anti-inflammatory effects of corticosteroids [6]. Similar results have been reported in an ovalbumin-induced inflammatory asthma model, in which wildtype (WT) mice, but not MUC1 knockout (KO) mice, responded to the anti-inflammatory effects of corticosteroids [6].
As it was described above, corticosteroids mediate some of their anti-inflammatory effects by forming MUC1-CT/GRα nuclear transcription complexes (figure 3) [5]. HBECs from patients with severe asthma exhibit increased GR-Ser226 phosphorylation compared with cells from patients with mild asthma and healthy controls. Furthermore, immunoprecipitation experiments revealed that the expression levels of GRα/MUC1-CT complexes are decreased in HBECs from severe asthmatics [6]. In this context, in an ovalbumin-induced inflammatory model of asthma, corticosteroids have been shown to promote bronchial epithelial nuclear translocation of the MUC1-CT/GRα complex in WT mice, inducing the expression of corticosteroid-inducible anti-inflammatory genes. By contrast, GRα nuclear translocation is not observed in MUC1-KO mice [6]. Corticosteroids also inhibit inflammatory cell extravasation to lung tissue. When pulmonary artery endothelial cells (HPAECs) are transfected with small interfering RNA targeting MUC1, they become resistant to the effects of corticosteroids inhibiting neutrophil adhesion and downregulation of intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM) and E-selectin. These results suggest that MUC1 not only mediates corticosteroid regulation of anti-inflammatory gene expression but also mediates the inhibitory effects of corticosteroids on neutrophil migration and adhesion to HPAECs [6].
Viral or bacterial infections are observed in 70% of inpatients with an asthma exacerbation [60]. Therefore, MUC1-CT downregulation in severe asthma patients may enhance viral and bacterial-induced TLR signalling (figure 3) [30], triggering sustained inflammation in the pathogenesis of asthma [61]. However, elevated KL-6 serum levels have been reported in acute exacerbations of paediatric patients with bronchial asthma [62], suggesting that KL-6 may become a useful biomarker for the diagnosis and prognosis of asthma.
The death of epithelial cells in asthmatic airways also plays a regulatory role in severe asthma [63]. Indeed, multiple mechanisms have been implicated in mediating epithelial apoptosis in asthma, including viral infection [64]. It has been demonstrated that asthmatic patients have an impaired antiviral response, and it has been hypothesised that this may allow certain viruses to proliferate in epithelial cells with consequential necrosis, the release of damage-associated molecular patterns (DAMPs), and persistent inflammation [65, 66]. In this context, MUC1 downregulation in HBECs has been shown to impair the antinecroptotic (necrosis regulated by orchestrated pathways) effects of glucocorticoids in these cells [67].
In summary, these findings suggest that MUC1 expression mediates the corticosteroids effects in patients patients with severe asthma.
MUC1 and COPD
COPD is a chronic inflammatory, progressive, and debilitating lung disease mainly caused by chronic exposure to cigarette smoke [68]. Anti-inflammatory options for COPD are based on corticosteroids. However, similar to severe asthma [6] and CRSwNP [5], the loss of corticosteroid anti-inflammatory efficacy is common in patients with COPD [69].
MUC1-CT has been shown to be downregulated in lung tissue, isolated bronchial epithelial cells and sputum neutrophils from smokers and corticosteroid resistant patients with COPD defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) as GOLD II (moderate disease) and GOLD III (severe disease), without recent exacerbations [7]. Additionally, the forced expiratory volume (FEV1%) of smokers and patients with COPD without recent exacerbations has been directly correlated with MUC1 lung tissue expression [7].
Similar to the mechanism of action of corticosteroids in asthma [6] and CRSwNP [5], it has been suggested that corticosteroids given to patients with COPD also partially mediate their anti-inflammatory effects by forming MUC1-CT-GRα complexes that translocate to the nucleus to increase the expression of anti-inflammatory genes [7]. Furthermore, TLR4, whose inflammatory downstream signalling is activated by cigarette smoke [70], is chronically elevated in bronchial epithelial cells and neutrophils from patients with COPD [7, 71]. Therefore, the increased expression of TLR4 and decreased expression of MUC1-CT could explain the greater inflammatory response in patients with COPD, as well as the lack of corticosteroid responsiveness (figure 3). Indeed, HBECs transiently transfected with siRNA-MUC1 have been reported to be resistant to the anti-inflammatory effects of corticosteroids in response to cigarette smoke extract [7]. Consistent with this, corticosteroids are unable to exert anti-inflammatory effects in lungs from a MUC1-KO model of acute cigarette smoke inflammation [7]. Nevertheless, the loss of MUC1 expression in the lungs of patients with COPD is still controversial because there is evidence of bronchial/alveolar epithelium of patients with COPD which display highly positive areas of KL-6 staining in contrast to the situation in nonsmokers and smokers [72]. Furthermore, as it was previously mentioned, it has been also reported that oxidative stress upregulates MUC1 expression [73] or modifies the localisation of MUC1 expression to the intracellular compartment of hyperplastic intermediate cells [50].
MUC1 downregulation is not the only cause for the lack of corticosteroid responsiveness in COPD. Indeed, the endogenous glucocorticoid hydrocortisone and the synthetic glucocorticoids administered to patients with COPD function via histone deacetylase 2 (HDAC2)-regulated epigenetic modifications to suppress pro-inflammatory gene transcription. Therefore, changes in the histone acetylation/deacetylation balance due to oxidative stress induced by cigarette smoke may be also an important factor in inducing glucocorticoid resistance in patients with COPD [74].
Similar to asthma, respiratory virus infections are a major cause of COPD exacerbations, sometimes resulting in secondary bacterial infections. In this context, MUC1-CT expression in sputum has been reported to be increased in acute phase COPD exacerbations (AECOPD) compared with sputum from the remission phase [75], thus suggesting a protective role of MUC1 during AECOPD. Furthermore, linear regression analysis has shown that, during COPD acute exacerbation, the increased levels of sputum MUC1-CT fragments are positively correlated with sputum neutrophil number [75].
The extracellular domain (EC) of MUC1 has also been shown to be elevated in sputum not only from patients with AECOPD, but also patients with GOLD I–III COPD [72, 75, 76]. The contribution of MUC1 shedding to mucous biology is not fully understood. Nevertheless, it might be hypothesised that the untethered MUC1-N-terminal subunit may contribute to mucus hypersecretion and increased amount of sputum in patients with COPD, likely serving as a protective barrier against invading pathogens. However, long-term mucous plugging, particularly of the small airways, might also contribute to airflow limitation. Therefore, elevated MUC1-EC levels in sputum can have beneficial and nonbeneficial effects in patients with COPD.
Elevated KL-6 serum levels have been also detected in patients with COPD, although elevated levels have also been observed in smokers regardless of COPD diagnosis [72].
In summary, these findings suggest that MUC1 expression levels in patients with COPD are dependent on the nature of patients with COPD, with differences reported between AECOPD and patients with stable (without recent exacerbations) COPD. It is possible that the different MUC1 subunits play different roles in COPD, as MUC1-CT has been shown to participate in modulation of the anti-inflammatory effects of corticosteroids in patients with stable COPD. However, the significance of the elevated levels of MUC1-EC/KL-6 in the development and progression of COPD is still unknown.
MUC1 and lung cancer
MUC1 basal expression is generally restricted to the apical/luminal side of cells [3]. However, MUC1 has long been viewed as a tumour-associated molecule due to its frequent overexpression and aberrant glycosylation in many carcinomas [3], in which there is loss of polarity and aberrant cell surface distribution [77]. In this context, several clinical studies based on immunohistochemical staining [14–19] and meta-analysis studies [78, 79] have demonstrated a negative prognostic association of tumour MUC1 overexpression and depolarised cellular distribution in patients with nonsmall cell lung cancer (NSCLC). Nevertheless, some contradictory findings have also been reported, although they represent minor contributions [80–82].
Elevated levels of MUC1 have been detected more frequently in adenocarcinoma (86.3%) and other NSCLCs (74.1%) compared with squamous cell carcinoma tissue (39.1%) [15]. However, some studies have shown that MUC1 expression is increased during the progression of pre-malignant lung lesions to invasive carcinoma; this increase is significant for squamous cell carcinoma but not for adenocarcinoma [83].
CA15-3 serum levels have also been reported to be significantly increased and correlated with metastasis in patients with lung cancer [84, 85]. Otherwise, preoperative serum KL-6 levels have been identified as a simple and novel predictor of recurrence following curative surgery in patients with NSCLC [86–88], while postoperative serum KL-6 levels have been identified as a prognostic factor for resected patients with NSCLC [89]. In this context, the degree of the decrease of natural autoantibody to KL-6/MUC1 has been correlated with impaired NSCLC prognosis [90]. By contrast, recent findings have reported no significant difference in serum MUC1 levels between patients with NSCLC and healthy individuals, although higher plasma exosomal MUC1 levels have been detected in patients with NSCLC [91].
In lung cancer cells, STAT3 [92] and 14-3-3ζ [93] oncogenes have been shown to regulate the expression of MUC1 in a promoter-dependent manner. Overexpressed and depolarised MUC1 has been shown to inhibit lung cancer cell apoptosis and to induce proliferation, the epithelial–mesenchymal transition (EMT), tumour growth and angiogenesis and metastasis [92–95]. The MUC1-N-terminal subunit has been shown to increase the invasive and metastatic capability of tumour cells by different mechanisms: 1) reducing cell–cell adhesion by disturbing E-cadherin interactions between adjacent cells [9]; 2) reducing cell–extracellular matrix adhesion through inhibition of integrin-mediated cell adhesion to extracellular matrix components [10]; and 3) interacting with adhesion molecules such as endothelial ICAM-1, which triggers invasion of MUC1-expressing tumour cells into the endothelium and reattachment at distant sites of metastasis (figure 4) [1].
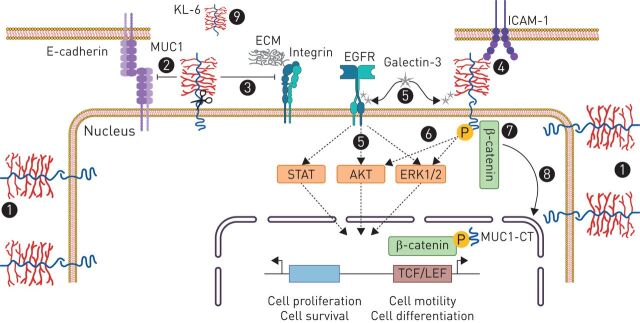
FIGURE 4.
The role of MUC1 in lung cancer. (1) MUC1 is overexpressed and depolarised in lung cancer. Therefore, MUC1 interactions with potential binding partners are increased under these circumstances, thus increasing the invasive and metastatic capability of tumour cells by different mechanisms: (2) reducing cell–cell adhesion through disturbing E-cadherin interactions between adjacent cells, (3) reducing cell–ECM adhesion through inhibition of integrin-mediated cell adhesion to ECM components, and (4) interacting with adhesion molecules such as the endothelial ICAM-1. (5) MUC1-C-terminal subunit interacts with galectin-3, increasing MUC1 association with the EGFR and leading to EGFR activation and signalling, which mediates epithelial cell proliferation and survival. (6) Galectin-3 binding to MUC1-C also phosphorylates MUC1-CT, activating mitogen-activated protein kinase and PI3K/AKT signalling pathways, which lead to cell proliferation and motility. (7) β-Catenin preferentially binds to the phosphorylated intracellular tail of MUC1, thus also contributing to the decrease of cell–cell adhesions through E-cadherin. (8) MUC1-CT/β-catenin complex translocates into the nucleus to regulate genes involved in cell proliferation and differentiation. (9) MUC1-N (KL6) is shed from the cell surface and has demonstrated to be a prognostic and treatment responsiveness serum biomarker in lung cancer. AKT: protein kinase B; ECM: extracellular matrix; EGFR: epidermal growth factor receptor; ERK: extracellular signal-regulated kinase; ICAM: intracellular adhesion molecule; KL-6: Krebs von den Lungen-6; LEF: lymphoid enhancer factor; MUC1: mucin 1; MUC1-CT: MUC1 cytoplasmic domain; TCF: T-cell factor protein.
MUC1-C-terminal subunit has been reported to interact with the β-galactosidase binding lectin galectin-3 (figure 1), increasing MUC1 association with EGFR and leading to EGFR activation and signalling, which mediates epithelial cell growth and survival [96]. Independently of EGFR, galectin 3 binding to MUC1-C has also been shown to promote MUC1-CT phosphorylation, activating mitogen-activated protein kinase (MAPK) and PI3K/AKT signalling pathways, resulting in cell proliferation, motility, and tumour angiogenesis [25, 97, 98]. Similarly, other transmembrane receptors such as platelet-derived growth factor receptor (PDGFR)-β [99], fibroblast growth factor receptor (FGFR) 3 [58] and Met [23] and ErbB1–4 receptors [77, 100] have also been shown to phosphorylate MUC1-CT (figure 1), promoting cell proliferation, migration, and transformation [4, 23]. Furthermore, β-catenin preferentially binds the phosphorylated intracellular tail of MUC1 (figure 1), also contributing to the decrease in cell–cell adhesions through E-cadherin [25]. MUC1-CT/β-catenin complexes translocate to the nucleus and regulate genes involved in cell proliferation and differentiation (figure 4) [101].
Recently, it has been shown that an AUG codon downstream to the MUC1 initiation codon initiates an alternate reading frame (ARF) thereby generating a novel protein, MUC1-ARF (figure 1). The amino acid sequence of the MUC1-ARF variable number tandem repeat (VNTR) as well as N- and C- sequences flanking it differ entirely from those of MUC1-transmembrane and it has been shown that MUC1-ARF protein mainly localises in the nucleus of malignant epithelial cells of pancreatic cancer and breast cancer. In this context, further research would be needed to address if MUC1-ARF is also expressed in lung cancer, as well as, to describe the link between MUC1-ARF and malignancy.
In the clinic, paclitaxel (PTX) is an important first-line chemotherapeutic agent against lung cancer [102, 103]. However, its treatment can generate resistance, which has been associated with enhanced MUC1-C expression in NSCLC [104]. Alternatively, among agents for the treatment of lung cancer, the EGFR tyrosine kinase inhibitors (EGFR-TKIs) gefitinib and erlotinib have been established as options for first-, second-, or third-line, or maintenance, treatment. Nevertheless, EGFR activating mutations in NSCLC cells frequently develop, resulting in resistance to EGFR-TKIs. In this context, targeting the oncogenic protein MUC1-C has been shown to inhibit mutant EGFR-mediated signalling and survival of NSCLC cells, suggesting a potential approach for the treatment of EGFR-TKI-resistant NSCLC [105]. Monitoring of circulating KL-6 levels in patients with NSCLC prior to and 2 weeks after the start of EGFR-TKI treatment revealed that serum KL-6 levels are increased in EGFR-TKI no-responder patients, which is accompanied by disease progression and reduced survival. By contrast, in partial-response patients, KL-6 serum levels significantly reduced after 2 weeks of EGFR-TKI treatment [106]. Furthermore, it has been suggested that patients with NSCLC with high serum KL-6 levels [106, 107] and MUC1 mRNA positivity in blood have unfavourable responses and poor clinical outcomes when treated with gefitinib [108]. Therefore, KL-6 serum levels and/or MUC1 mRNA blood levels may be promising noninvasive and repeatable markers for predicting the efficacy of EGFR-TKIs, which could help in the choice of treatment for different patients. By contrast, other findings have demonstrated that monitoring of KL-6 levels in the intrabronchial epithelial lining fluid near the tumour before and 2 weeks after the start of gefitinib treatment can predict tumour response at 4 weeks, whereas serum levels do not. However, the median KL-6 level near the tumour is significantly higher in treatment responders than in nonresponders prior to treatment [109].
Importantly, aberrant overexpression of MUC1-C in NSCLC has been linked to the induction of programmed death ligand 1 (PD-L1) [110], which promotes the evasion of NSCLC cells from immune recognition [111]. Therefore, several vaccines using MUC1 as an antigen have been developed and investigated as an immunomodulatory approach to the treatment and prevention of lung cancer. TG4010 (a modified vaccinia virus expressing MUC1 and IL-2) has been studied in a phase II trial in combination with first-line chemotherapy in patients with stage IV untreated NSCLC. This trial demonstrated a small but statistically significant improvement in progression-free survival [112]. Another vaccine, tecemotide (L-BLP25; a liposome-based vaccine consisting of a synthetic 25-amino acid lipopeptide derived from the tandem repeat region of MUC1, together with the nonspecific adjuvant monophosphoryl lipid A and three different lipids [113]), has been administered in a phase III study as maintenance therapy for unresectable stage III/IV NSCLC following treatment with platinum-based chemotherapy and radiation. Results from this clinical trial have already shown an improved overall survival [114, 115]. Additionally, two clinical trials are currently investigating the combination of MUC1 targeting vaccines with anti-PD1 therapy in advanced NSCLC: one using the above mentioned TG4010 vaccine (NCT02823990), and another using the CV301 vaccine (NCT02840994), which targets both MUC1 and carcinoembryonic antigen (CEA). Finally, preliminary studies have reported that the MUC1-targeted dendritic cell-based vaccine also significantly prolongs the survival of patients with MUC1-expressing lung cancer [116].
In summary, MUC1 is considered a lung cancer-associated molecule, since its overexpression and depolarisation have been shown to be linked to lung cancer progression. Therefore, part of the clinical efforts are currently focused on targeting MUC1 to treat lung cancer. Furthermore, numerous studies have demonstrated that CA-15-3 and KL-6 are reliable lung cancer prognostic and treatment responsiveness biomarkers, thus highlighting the potential use of MUC1 in the treatment of lung cancer.
MUC1 and interstitial lung disease
Elevated KL-6/MUC1 serum levels have been observed in >70% of patients with interstitial lung diseases (ILDs) [117, 118]; serum levels are negatively correlated with the diffusing capacity of lungs for carbon monoxide, as well as positively correlated with the high-resolution computerised tomography (HRCT) score [119]. Additionally, serum levels of KL-6 have been reported to be significantly correlated with KL-6 levels in bronchoalveolar lavage fluid (BALF), which are significantly correlated with the numbers of total cells, lymphocytes, and neutrophils in BALF from patients with ILD [120] and KL-6 expression in regenerating alveolar type II (ATII) cells from ILD tissue [117]. Therefore, KL-6 serum levels are useful for evaluating the extent of ILDs, predicting disease outcome, monitoring the clinical course, and identifying patients with ILDs who are at increased risk for subsequent mortality [117–119, 121]. Nevertheless, ILD is a term that comprises the pathologies described below.
Hypersensitivity pneumonitis
Measurement of serum KL-6 concentrations is useful to screen for hypersensitivity pneumonitis (HP) and to detect HP activity [122]. Indeed, bird-related and house-related HP show seasonal serum KL-6 variations, showing a significant increase in serum KL-6 concentrations in bird-related HP during the winter and in house-related HP during the summer. Furthermore, serum KL-6 variations have been reported to be significantly greater in acute HP than chronic HP [123].
Sarcoidosis
In sarcoidosis, serum levels of KL-6 have been reported to vary according to the genotypic distribution of rs4072037 [124], a single nucleotide polymorphism in exon 2 of the MUC1 gene that has been linked with MUC1 VNTR size class [125]. In this context, the large molecular size of KL-6/MUC1 has been associated with the high levels of serum KL-6 reported in sarcoidosis [126, 127]. However, these results are based on an exceptionally small case–control study subject to type 1 error. Therefore, further research would be needed to confirm rs4072037 MUC1 genetic linkage with sarcoidosis.
Radiation pneumonitis
Patients lung cancer who have received thoracic radiotherapy (TRT) with or without chemotherapy are at risk of developing radiation pneumonitis (RP). In this context, serum KL-6 levels have shown a consistent tendency to increase following clinical diagnosis of severe RP. By contrast, in patients with localised (within the irradiated field) RP, serum levels do not show a tendency to increase during or after TRT [128].
Drug-induced interstitial pneumonitis
Interstitial pneumonitis can be drug-induced and is characterised by different histological patterns. In the context of KL-6 serum levels, only diffuse alveolar damage (DAD) and chronic interstitial pneumonia (CIP) patterns exhibit KL-6 increased serum levels, which are closely correlated with their clinical course. By contrast, serum KL-6 levels in bronchiolitis obliterans organising pneumonia or eosinophilic pneumonia (BOOP/EP) patterns are within the normal range [129].
Collagen vascular disease-associated interstitial pneumonia
Collagen diseases are also frequently associated with interstitial pneumonia (IP); the mean serum KL-6 level of patients with both diseases is significantly higher than that of patients with collagen diseases and inactive IP. Furthermore, serum KL-6 levels have been shown to increase with the deterioration of IP, while successful treatment of IP results in a significant decrease in KL-6 serum levels [130].
Idiopathic interstitial pneumonias
Idiopathic pulmonary fibrosis (IPF) and patients with nonspecific interstitial pneumonia (NSIP) have also shown higher BALF KL-6 levels than healthy controls. These levels positively correlate with oxygen demand at rest and during a 6 min walk test [131], as well as with the extent of fibrotic abnormalities on HRCT [132].
In the clinic, initial evaluation of serum KL-6 levels has been suggested to predict survival in patients with IPF [133]. Furthermore, several studies have reported increased serum KL-6 levels during IPF acute exacerbations, correlating with a rapid decline in forced vital capacity (FVC) predicted and lower survival rates [134]. Interestingly, MT1-MMP or MMP14 proteins are an alternative mechanism to the MUC1 SEA self-cleaving domain and shedding to the plasma membrane [135, 136]. Thus, the elevated levels of MT1-MMP under lung fibrotic conditions [137] are hypothesised to mediate the release of the extracellular MUC1 domain (MUC1-N/KL-6) in many biological fluids in IPF. Recently, KL-6 serum levels have been considered a reliable prognostic biomarker indicative of the response to nintedanib treatment in patients with IPF [138].
There is evidence of weak MUC1/MUC1-CT expression mainly localised to the membrane of ATII cells and alveolar macrophages in control lung sections [139, 140]. However, under fibrotic conditions, MUC1/MUC1-CT is overexpressed and located mostly in hyperplastic ATII cells and fibroblasts of fibrotic areas, being mainly distributed in the cell cytoplasm and nucleus [117, 140] and contributing to IPF pathology. Furthermore, two phosphorylated/bioactivated forms of MUC1-CT (MUC1-P/T-1224 and MUC1-P/Y-1229) have been shown to be overexpressed in IPF lung tissue, with a similar pattern of distribution to that of nonphosphorylated MUC1-CT [140].
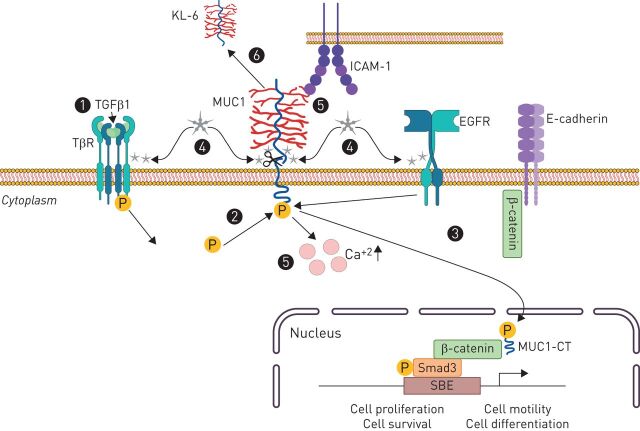
In IPF, MUC1-CT has been reported to collaborate with transforming growth factor (TGF)-β1, promoting disease progression [140]. Following MUC1-CT phosphorylation/activation via TGF-β1-induced Smad3 phosphorylation, MUC1-CT increases the active form of β-catenin, forming a MUC1-CT/phospho-Smad3/active-β-catenin nuclear protein complex that promotes fibrotic processes such as the EMT, the fibroblast–mesenchymal transition (FMT), fibroblast proliferation and ATII and fibroblast senescence [140]. Otherwise, MUC1-CT has also been shown to be activated by galectin 3 [97, 140], whose expression has been observed to be increased in BALF and serum of patients with stable IPF, as compared with those with nonspecific interstitial pneumonitis and controls [141]. Furthermore, galectin-3 has been shown to interact directly with MUC1-C (figure 1), functioning as a bridge between MUC1-C and EGFR, as well as other cell surface receptors [142]. Indeed, galectin-3 has been reported to promote IPF through stabilisation of the TGF-β1 receptor (TβR) [141]; it has been hypothesised that galectin-3 might also serve as a bridge between MUC1-C and TβR, suggesting MUC1-CT might be activated indirectly via p-Smad3 and directly via galectin 3 (figure 5). Importantly, a phase I/II clinical trial (NCT02257177) to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of TD139, a galectin 3 inhibitor, in patients with IPF has been successfully conducted.
FIGURE 5.
The role of MUC1 in IPF. Transforming growth factor β1 (TGF-β1) receptor (TβR) activation induces Smad3 phosphorylation (1), which phosphorylates MUC1-CT (2). (3) Bioactivated MUC1-CT has demonstrated to increase the active form of β-catenin, forming a MUC1-CT/phospho-Smad3/active-β-catenin nuclear protein complex that promotes fibrotic processes such as epithelial to mesenchymal transition, fibroblast to mesenchymal transition (FMT), fibroblast proliferation and alveolar type II and fibroblast senescence. (4) MUC1-CT is also activated by galectin 3, which has demonstrated to directly interact with MUC1-C, functioning as a bridge between MUC1-C and the epidermal growth factor receptor (EGFR), as well as, other cell surface receptors such as TβR. (5) MUC1-N-terminal binds to intracellular adhesion molecule-1 (ICAM-1) and the MUC1/ICAM-1 association results in a rapid increase of intracellular calcium in MUC1-expressing cells, inducing cytoskeletal changes and motility. (6) MUC1-N (KL6) is shed from the cell surface and has demonstrated to be a diagnosis and prognostic serum biomarker in idiopathic pulmonary fibrosis KL-6: Krebs von den Lungen-6; LEF: lymphoid enhancer factor; MUC1: mucin 1; SBE: Smad binding element; MUC1-CT: MUC1 cytoplasmic domain.
Additionally, the N-terminal binding of MUC1-N to ICAM-1 has also been related to IPF progression; ICAM-1 serum levels are elevated in patients with IPF [143] and the MUC1/ICAM-1 association results in a rapid increase of intracellular calcium in MUC1-expressing cells [144], inducing cytoskeletal changes and motility.
The effect of MUC1 targeting in the development of pulmonary fibrosis has been studied by using the MUC1-C peptide inhibitor GO-201 in vitro and in vivo, which has been shown to inhibit the targeting of MUC1-C to the nucleus and to attenuate the development of pulmonary fibrosis in a bleomycin-induced model [140]. Furthermore, antibodies against KL-6 have been shown to attenuate bleomycin-induced lung fibrosis [145], and MUC1-KO mice have been shown to exhibit resistance to bleomycin-induced pulmonary fibrosis, with improvements in lung function, survival, and fibrotic lung tissue remodelling [140]. By contrast, in a silica-induced in vivo model of lung fibrosis, it has been observed that MUC1-KO mice have increased lung fibrosis [146].
Previously to this paper, higher expression levels of MUC1 in MUC5B+ cells were already registered in the IPF Cell Atlas (www.ipfcellatlas.com/). MUC5B had been largely reported as a proven lung fibrosis mediator [147, 148]. Therefore, it further supports the participation of MUC1 in IPF.
In summary, these findings suggest that KL-6 may be a novel, noninvasive marker for the diagnosis and evaluation of severity in patients with ILD. Furthermore, the detection of activated MUC1-CT in lung tissue and cells from patients with IPF may be of scientific value in the development of new targets to treat this devastating disease.
Conclusions
Respiratory diseases include a group of complex diseases with diverse backgrounds. Therefore, diagnosis and/or treatment are often challenging. As MUC1 is broadly associated with airway defence, airway inflammation, cell growth, and tissue remodelling processes compatible with the processes observed in most respiratory diseases (table 1), analysing the expression, distribution, activation and function of MUC1 in lung pathological conditions may be a useful tool for lung disease diagnosis, follow-up and treatment. However, mucin biology is complex; in spite of numerous pre-clinical and clinical studies focused on MUC1, further in-depth studies are required before MUC1 can be robustly used as a biomarker for diagnosis, prognosis or treatment responsiveness or as a new therapeutic approach in lung diseases.
TABLE 1.
MUC1 expression and participation in respiratory diseases
| Disease | MUC1 expression | Role of MUC1 |
| Infectious diseases | ||
| Pseudomonas aeruginosa | Upregulated in lung epithelial cells [35, 36] and alveolar macrophages [39] | During late-stage infection:
|
| Haemophilus influenzae | Upregulated in airway epithelial cells [27] | During late-stage infection:
|
| Respiratory syncytial virus | Upregulated in airway epithelial cells [28, 42] | During last stage infection:
|
| CRSwNP | ||
| Corticosteroid responders | Upregulated in NP epithelium [54] | MUC1-CT/GRα nuclear translocation: anti-inflammatory effects [5] |
| Corticosteroid resistant | Downregulated in NP epithelium [5] |
|
| Asthma | ||
| Mild asthma (corticosteroid responders) | Normal expression levels in HBECs and neutrophils | MUC1-CT/GRα nuclear translocation: anti-inflammatory effects [5] |
| Severe asthma (corticosteroid resistant) | Downregulation in HBECs and neutrophils [6] |
|
| Paediatric acute exacerbation [62] | Elevated KL-6 serum levels | Biomarker |
| COPD | ||
| Corticosteroid responders | Normal expression levels in lung tissue, bronchial epithelial cells and sputum neutrophils | MUC1-CT/GRα nuclear translocation: anti-inflammatory effects [5] |
| Corticosteroid resistant without recent exacerbation | Downregulated in lung tissue, bronchial epithelial cells, and sputum neutrophils [7] |
|
| Acute exacerbation |
|
|
| Lung cancer | ||
| Paclitaxel no responders |
|
|
| EGFR-TKI no responders | Enhanced MUC1-C expression [104] | Biomarker for predicting the efficacy of lung cancer treatments |
| EGFR-TKI partial responders |
|
|
| Reduced serum KL-6 levels after EGFR-TKI treatment | ||
| ILDs | ||
| Hypersensitivity pneumonitis | Elevated KL-6 serum levels [123, 127–132] | Biomarker [122, 124, 128–132] |
| Sarcoidosis | ||
| Radiation pneumonitis | ||
| Drug-induced interstitial pneumonitis | ||
| Collagen vascular disease-associated IP | ||
| Nonspecific IP | ||
| IPF |
|
|
MUC1: mucin 1; TLR: toll-like receptor; CRSwNP: chronic rhinosinusitis with nasal polyps; NP: nasal polyps; MUC1-CT: MUC1 cytoplasmic tail; GR: glucocorticoid receptor; HBEC: human bronchial epithelial cell; ILD: interstitial lung disease; IP: interstitial pneumonia; KL-6: Krebs von den Lungen-6; MUC1-EC: MUC1 extracellular; EMT: epithelial to mesenchymal transition; PD-L1: programmed death ligand 1; EGFR-TKI: epidermal growth factor receptor–tyrosine kinase inhibitor; IPF: idiopathic pulmonary fibrosis; BALF: bronchoalveolar lavage fluid; TGF-β1: transforming growth factor-β1; FMT: fibroblast to mesenchymal transition; ATII: alveolar type II cells; ICAM-1: intracellular adhesion molecule-1.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: B. Ballester has nothing to disclose.
Conflict of interest: J. Milara Payá has nothing to disclose.
Conflict of interest: J. Cortijo Gimeno has nothing to disclose.
Support statement: This work was supported by Ministerio de Ciencia e Innovación (grants CB06/06/0027, FEDER, JR18/00050, SAF2017–82913-R and TRA2009–0311) Generalitat Valenciana (grants 2017/023/UV and ACIF/2016/341) and Instituto de Salud Carlos III (grant: FIS PI17/02158). Funding entities did not contribute to the study design or data collection, analysis and interpretation nor to the writing of the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol 2008; 70: 431–457. doi: 10.1146/annurev.physiol.70.113006.100659 [DOI] [PubMed] [Google Scholar]
- 2.Pemberton LF, Rughetti A, Taylor-Papadimitriou J, et al. . The epithelial mucin MUC1 contains at least two discrete signals specifying membrane localization in cells. J Biol Chem 1996; 271: 2332–2340. doi: 10.1074/jbc.271.4.2332 [DOI] [PubMed] [Google Scholar]
- 3.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010; 29: 2893–2904. doi: 10.1038/onc.2010.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kufe D. Oncogenic function of the MUC1 receptor subunit in gene regulation. Oncogene 2010; 29: 5663–5666. doi: 10.1038/onc.2010.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milara J, Peiro T, Armengot M, et al. . Mucin 1 downregulation associates with corticosteroid resistance in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2015; 135: 470–476. doi: 10.1016/j.jaci.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 6.Milara J, Morell A, de Diego A, et al. . Mucin 1 deficiency mediates corticosteroid insensitivity in asthma. Allergy 2019; 74: 111–121. doi: 10.1111/all.13546 [DOI] [PubMed] [Google Scholar]
- 7.Milara J, Diaz-Platas L, Contreras S, et al. . MUC1 deficiency mediates corticosteroid resistance in chronic obstructive pulmonary disease. Respir Res 2018; 19: 226. doi: 10.1186/s12931-018-0927-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueno K, Koga T, Kato K, et al. . MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol 2008; 38: 263–268. doi: 10.1165/rcmb.2007-0336RC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell–cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell 1996; 7: 565–577. doi: 10.1091/mbc.7.4.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wesseling J, van der Valk SW, Vos HL, et al. . Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 1995; 129: 255–265. doi: 10.1083/jcb.129.1.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhar P, McAuley J. The role of the cell surface mucin MUC1 as a barrier to infection and regulator of inflammation. Front Cell Infect Microbiol 2019; 9: 117. doi: 10.3389/fcimb.2019.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanzarone N, Gentili F, Alonzi V, et al. . Bronchoalveolar lavage and serum KL-6 concentrations in chronic hypersensitivity pneumonitis: correlations with radiological and immunological features. Intern Emerg Med 2020; 15(7): 1247–1254. [DOI] [PubMed] [Google Scholar]
- 13.Xue M, Guo Z, Cai C, et al. . Evaluation of the diagnostic efficacies of serological markers KL-6, SP-A, SP-D, CCL2, and CXCL13 in idiopathic interstitial pneumonia. Respiration 2019; 98: 534–545. doi: 10.1159/000503689 [DOI] [PubMed] [Google Scholar]
- 14.Guddo F, Giatromanolaki A, Koukourakis MI, et al. . MUC1 (episialin) expression in non-small cell lung cancer is independent of EGFR and c-erbB-2 expression and correlates with poor survival in node positive patients. J Clin Pathol 1998; 51: 667–671. doi: 10.1136/jcp.51.9.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Situ D, Wang J, Ma Y, et al. . Expression and prognostic relevance of MUC1 in stage IB non-small cell lung cancer. Med Oncol 2011; 28 Suppl 1: S596–S604. doi: 10.1007/s12032-010-9752-4 [DOI] [PubMed] [Google Scholar]
- 16.Sun ZG, Zhang M, Yang F, et al. . Clinical and prognostic significance of signal transducer and activator of transcription 3 and mucin 1 in patients with non-small cell lung cancer following surgery. Oncol Lett 2018; 15: 4278–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guddo F, Giatromanolaki A, Patriarca C, et al. . Depolarized expression of episialin (EMA, MUC1) in lung adenocarcinoma is associated with tumor progression. Anticancer Res 1998; 18: 1915–1920. [PubMed] [Google Scholar]
- 18.Woenckhaus M, Merk J, Stoehr R, et al. . Prognostic value of FHIT, CTNNB1, and MUC1 expression in non-small cell lung cancer. Hum Pathol 2008; 39: 126–136. doi: 10.1016/j.humpath.2007.05.027 [DOI] [PubMed] [Google Scholar]
- 19.Nagai S, Takenaka K, Sonobe M, et al. . A novel classification of MUC1 expression is correlated with tumor differentiation and postoperative prognosis in non-small cell lung cancer. J Thorac Oncol 2006; 1: 46–51. [PubMed] [Google Scholar]
- 20.Milara J, Ballester B, Montero P, et al. . MUC1 intracellular bioactivation mediates lung fibrosis. Thorax 2020; 75: 132–142. doi: 10.1136/thoraxjnl-2018-212735 [DOI] [PubMed] [Google Scholar]
- 21.Yonezawa S, Kitajima S, Higashi M, et al. . A novel anti-MUC1 antibody against the MUC1 cytoplasmic tail domain: use in sensitive identification of poorly differentiated cells in adenocarcinoma of the stomach. Gastric Cancer 2012; 15: 370–381. doi: 10.1007/s10120-011-0125-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albrecht H, Carraway KL. 3rd. MUC1 and MUC4: switching the emphasis from large to small. Cancer Biother Radiopharm 2011; 26: 261–271. doi: 10.1089/cbr.2011.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci 2010; 35: 236–245. doi: 10.1016/j.tibs.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodoropoulos G, Carraway KL. Molecular signaling in the regulation of mucins. J Cell Biochem 2007; 102: 1103–1116. doi: 10.1002/jcb.21539 [DOI] [PubMed] [Google Scholar]
- 25.van Putten JPM, Strijbis K. Transmembrane mucins: signaling receptors at the intersection of inflammation and cancer. J Innate Immun 2017; 9: 281–299. doi: 10.1159/000453594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato K, Lillehoj EP, Lu W, et al. . MUC1: the first respiratory mucin with an anti-inflammatory function. J Clin Med 2017; 6: 110. doi: 10.3390/jcm6120110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyo Y, Kato K, Park YS, et al. . Antiinflammatory role of MUC1 mucin during infection with nontypeable Haemophilus influenzae. Am J Respir Cell Mol Biol 2012; 46: 149–156. doi: 10.1165/rcmb.2011-0142OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Dinwiddie DL, Harrod KS, et al. . Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 2010; 298: L558–L563. doi: 10.1152/ajplung.00225.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu W, Hisatsune A, Koga T, et al. . Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 2006; 176: 3890–3894. doi: 10.4049/jimmunol.176.7.3890 [DOI] [PubMed] [Google Scholar]
- 30.Kato K, Lillehoj EP, Park YS, et al. . Membrane-tethered MUC1 mucin is phosphorylated by epidermal growth factor receptor in airway epithelial cells and associates with TLR5 to inhibit recruitment of MyD88. J Immunol 2012; 188: 2014–2022. doi: 10.4049/jimmunol.1102405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato K, Lillehoj EP, Kai H, et al. . MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front Biosi (Elite Ed.) 2010; 2: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lillehoj EP, Hyun SW, Kim BT, et al. . Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2001; 280: L181–L187. doi: 10.1152/ajplung.2001.280.1.L181 [DOI] [PubMed] [Google Scholar]
- 33.Lillehoj EP, Kim BT, Kim KC. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am J Physiol Lung Cell Mol Physiol 2002; 282: L751–L756. doi: 10.1152/ajplung.00383.2001 [DOI] [PubMed] [Google Scholar]
- 34.Bodey GP, Bolivar R, Fainstein V, et al. . Infections caused by Pseudomonas aeruginosa. Rev Infect Dis 1983; 5: 279–313. doi: 10.1093/clinids/5.2.279 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Louboutin JP, Weiner DJ, et al. . Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun 2005; 73: 7151–7160. doi: 10.1128/IAI.73.11.7151-7160.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi F, Smith KD, Ozinsky A, et al. . The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001; 410: 1099–1103. doi: 10.1038/35074106 [DOI] [PubMed] [Google Scholar]
- 37.Kato K, Lillehoj EP, Kim KC. Pseudomonas aeruginosa stimulates tyrosine phosphorylation of and TLR5 association with the MUC1 cytoplasmic tail through EGFR activation. Inflamm Res 2016; 65: 225–233. doi: 10.1007/s00011-015-0908-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koga T, Kuwahara I, Lillehoj EP, et al. . TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol 2007; 293: L693–L701. doi: 10.1152/ajplung.00491.2006 [DOI] [PubMed] [Google Scholar]
- 39.Kato K, Hanss AD, Zemskova MA, et al. . Pseudomonas aeruginosa increases MUC1 expression in macrophages through the TLR4-p38 pathway. Biochem Biophys Res Commun 2017; 492: 231–235. doi: 10.1016/j.bbrc.2017.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shay DK, Holman RC, Newman RD, et al. . Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 1999; 282: 1440–1446. doi: 10.1001/jama.282.15.1440 [DOI] [PubMed] [Google Scholar]
- 41.Hacking D, Hull J. Respiratory syncytial virus–viral biology and the host response. J Infect 2002; 45: 18–24. doi: 10.1053/jinf.2002.1015 [DOI] [PubMed] [Google Scholar]
- 42.Kato K, Lillehoj EP, Kim KC. MUC1 regulates epithelial inflammation and apoptosis by PolyI:C through inhibition of Toll/IL-1 receptor-domain-containing adapter-inducing IFN-beta (TRIF) recruitment to Toll-like receptor 3. Am J Respir Cell Mol Biol 2014; 51: 446–454. doi: 10.1165/rcmb.2014-0018OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGuckin MA, Every AL, Skene CD, et al. . Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 2007; 133: 1210–1218. doi: 10.1053/j.gastro.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 44.Linden SK, Sheng YH, Every AL, et al. . MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog 2009; 5: e1000617. doi: 10.1371/journal.ppat.1000617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAuley JL, Linden SK, Png CW, et al. . MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest 2007; 117: 2313–2324. doi: 10.1172/JCI26705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens WW, Lee RJ, Schleimer RP, et al. . Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol 2015; 136: 1442–1453. doi: 10.1016/j.jaci.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam K, Schleimer R, Kern RC. The etiology and pathogenesis of chronic rhinosinusitis: a review of current hypotheses. Curr Allergy Asthma Rep 2015; 15: 41. doi: 10.1007/s11882-015-0540-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christensen DN, Franks ZG, McCrary HC, et al. . A systematic review of the association between cigarette smoke exposure and chronic rhinosinusitis. Otolaryngol Head Neck Surg 2018; 158: 801–816. doi: 10.1177/0194599818757697 [DOI] [PubMed] [Google Scholar]
- 49.Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc 2014; 11 Suppl 5: S252–S258. doi: 10.1513/AnnalsATS.201402-049AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato K, Chang EH, Chen Y, et al. . MUC1 contributes to goblet cell metaplasia and MUC5AC expression in response to cigarette smoke in vivo. Am J Physiol Lung Cell Mol Physiol 2020; 319: L82–L90. doi: 10.1152/ajplung.00049.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okano M, Fujiwara T, Kariya S, et al. . Cellular responses to Staphylococcus aureus alpha-toxin in chronic rhinosinusitis with nasal polyps. Allergol Int 2014; 63: 563–573. doi: 10.2332/allergolint.14-OA-0703 [DOI] [PubMed] [Google Scholar]
- 52.Noyama Y, Okano M, Fujiwara T, et al. . IL-22/IL-22R1 signaling regulates the pathophysiology of chronic rhinosinusitis with nasal polyps via alteration of MUC1 expression. Allergol Int 2017; 66: 42–51. doi: 10.1016/j.alit.2016.04.017 [DOI] [PubMed] [Google Scholar]
- 53.Hissaria P, Smith W, Wormald PJ, et al. . Short course of systemic corticosteroids in sinonasal polyposis: a double-blind, randomized, placebo-controlled trial with evaluation of outcome measures. J Allergy Clin Immunol 2006; 118: 128–133. doi: 10.1016/j.jaci.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Anton A, de Bolos C, Alobid I, et al. . Corticosteroid therapy increases membrane-tethered while decreases secreted mucin expression in nasal polyps. Allergy 2008; 63: 1368–1376. doi: 10.1111/j.1398-9995.2008.01678.x [DOI] [PubMed] [Google Scholar]
- 55.Itoh M, Adachi M, Yasui H, et al. . Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol 2002; 16: 2382–2392. doi: 10.1210/me.2002-0144 [DOI] [PubMed] [Google Scholar]
- 56.Li LB, Goleva E, Hall CF, et al. . Superantigen-induced corticosteroid resistance of human T cells occurs through activation of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK-ERK) pathway. J Allergy Clin Immunol 2004; 114: 1059–1069. doi: 10.1016/j.jaci.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 57.Grad I, Picard D. The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol 2007; 275: 2–12. doi: 10.1016/j.mce.2007.05.018 [DOI] [PubMed] [Google Scholar]
- 58.Ren J, Raina D, Chen W, et al. . MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res 2006; 4: 873–883. doi: 10.1158/1541-7786.MCR-06-0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 2013; 131: 636–645. doi: 10.1016/j.jaci.2012.12.1564 [DOI] [PubMed] [Google Scholar]
- 60.Iikura M, Hojo M, Koketsu R, et al. . The importance of bacterial and viral infections associated with adult asthma exacerbations in clinical practice. PLoS One 2015; 10: e0123584. doi: 10.1371/journal.pone.0123584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crespo-Lessmann A, Mateus E, Vidal S, et al. . Expression of toll-like receptors 2 and 4 in subjects with asthma by total serum IgE level. Respir Res 2016; 17: 41. doi: 10.1186/s12931-016-0355-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imai T, Takase M, Takeda S, et al. . Serum KL-6 levels in pediatric patients: reference values for children and levels in pneumonia, asthma, and measles patients. Pediatr Pulmonol 2002; 33: 135–141. doi: 10.1002/ppul.10044 [DOI] [PubMed] [Google Scholar]
- 63.Juncadella IJ, Kadl A, Sharma AK, et al. . Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 2013; 493: 547–551. doi: 10.1038/nature11714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tesfaigzi Y. Roles of apoptosis in airway epithelia. Am J Respir Cell Mol Biol 2006; 34: 537–547. doi: 10.1165/rcmb.2006-0014OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Contoli M, Message SD, Laza-Stanca V, et al. . Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 2006; 12: 1023–1026. doi: 10.1038/nm1462 [DOI] [PubMed] [Google Scholar]
- 66.Edwards MR, Regamey N, Vareille M, et al. . Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol 2013; 6: 797–806. doi: 10.1038/mi.2012.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H, Liu Q, Kong L, et al. . Mucin 1 downregulation impairs the anti-necroptotic effects of glucocorticoids in human bronchial epithelial cells. Life Sci 2019; 221: 168–177. doi: 10.1016/j.lfs.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 68.Bagdonas E, Raudoniute J, Bruzauskaite I, et al. . Novel aspects of pathogenesis and regeneration mechanisms in COPD. Int J Chron Obstruct Pulmon Dis 2015; 10: 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tashkin DP, Strange C. Inhaled corticosteroids for chronic obstructive pulmonary disease: what is their role in therapy? Int J Chron Obstruct Pulmon Dis 2018; 13: 2587–2601. doi: 10.2147/COPD.S172240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doz E, Noulin N, Boichot E, et al. . Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol 2008; 180: 1169–1178. doi: 10.4049/jimmunol.180.2.1169 [DOI] [PubMed] [Google Scholar]
- 71.Di Stefano A, Ricciardolo FLM, Caramori G, et al. . Bronchial inflammation and bacterial load in stable COPD is associated with TLR4 overexpression. Eur Respir J 2017; 49: 1602006. doi: 10.1183/13993003.02006-2016 [DOI] [PubMed] [Google Scholar]
- 72.Ishikawa N, Mazur W, Toljamo T, et al. . Ageing and long-term smoking affects KL-6 levels in the lung, induced sputum and plasma. BMC Pulm Med 2011; 11: 22. doi: 10.1186/1471-2466-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hiraki M, Suzuki Y, Alam M, et al. . MUC1-C stabilizes MCL-1 in the oxidative stress response of triple-negative breast cancer cells to BCL-2 inhibitors. Sci Rep 2016; 6: 26643. doi: 10.1038/srep26643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adcock IM, Ito K, Barnes PJ. Glucocorticoids: effects on gene transcription. Proc Am Thorac Soc 2004; 1: 247–254. doi: 10.1513/pats.200402-001MS [DOI] [PubMed] [Google Scholar]
- 75.Zheng Z, Qi Y, Xu X, et al. . Sputum mucin 1 is increased during the acute phase of chronic obstructive pulmonary disease exacerbation. J Thorac Dis 2017; 9: 1873–1882. doi: 10.21037/jtd.2017.06.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ishikawa N, Hattori N, Tanaka S, et al. . Levels of surfactant proteins A and D and KL-6 are elevated in the induced sputum of chronic obstructive pulmonary disease patients: a sequential sputum analysis. Respiration 2011; 82: 10–18. doi: 10.1159/000324539 [DOI] [PubMed] [Google Scholar]
- 77.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009; 9: 874–885. doi: 10.1038/nrc2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu F, Liu F, Zhao H, et al. . Prognostic significance of mucin antigen MUC1 in various human epithelial cancers: a meta-analysis. Medicine 2015; 94: e2286. doi: 10.1097/MD.0000000000002286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang X, Sun Q, Chen C, et al. . MUC1 overexpression predicts worse survival in patients with non-small cell lung cancer: evidence from an updated meta-analysis. Oncotarget 2017; 8: 90315–90326. doi: 10.18632/oncotarget.19861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jarrard JA, Linnoila RI, Lee H, et al. . MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Can Res 1998; 58: 5582–5589. [PubMed] [Google Scholar]
- 81.Awaya H, Takeshima Y, Yamasaki M, et al. . Expression of MUC1, MUC2, MUC5AC, and MUC6 in atypical adenomatous hyperplasia, bronchioloalveolar carcinoma, adenocarcinoma with mixed subtypes, and mucinous bronchioloalveolar carcinoma of the lung. Am J Clin Pathol 2004; 121: 644–653. doi: 10.1309/U4WGE9EBFJN6CM8R [DOI] [PubMed] [Google Scholar]
- 82.Kuemmel A, Single K, Bittinger F, et al. . TA-MUC1 epitope in non-small cell lung cancer. Lung cancer 2009; 63: 98–105. doi: 10.1016/j.lungcan.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 83.Saltos A, Khalil F, Smith M, et al. . Clinical associations of mucin 1 in human lung cancer and precancerous lesions. Oncotarget 2018; 9: 35666–35675. doi: 10.18632/oncotarget.26278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X, Xu Y, Zhang L. Serum CA153 as biomarker for cancer and noncancer diseases. Prog Mol Biol Transl Sci 2019; 162: 265–276. doi: 10.1016/bs.pmbts.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 85.Chen ZQ, Huang LS, Zhu B. Assessment of seven clinical tumor markers in diagnosis of non-small-cell lung cancer. Dis Markers 2018; 2018: 9845123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shoji F, Yamazaki K, Kouso H, et al. . Predictive impact for postoperative recurrence of preoperative serum Krebs von den Lungen-6 concentration in pathologic stage IA non-small cell lung cancer. Ann Thorac Surg 2016; 101: 1903–1908. doi: 10.1016/j.athoracsur.2015.11.066 [DOI] [PubMed] [Google Scholar]
- 87.Tanaka S, Hattori N, Ishikawa N, et al. . Krebs von den Lungen-6 (KL-6) is a prognostic biomarker in patients with surgically resected nonsmall cell lung cancer. Int J Cancer 2012; 130: 377–387. doi: 10.1002/ijc.26007 [DOI] [PubMed] [Google Scholar]
- 88.Miyazaki K, Kurishima K, Kagohashi K, et al. . Serum KL-6 levels in lung cancer patients with or without interstitial lung disease. J Clin Lab Anal 2010; 24: 295–299. doi: 10.1002/jcla.20404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tomita M, Ayabe T, Chosa E, et al. . Prognostic significance of preoperative serum Krebs von den Lungen-6 level in non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2016; 64: 657–661. doi: 10.1007/s11748-016-0706-4 [DOI] [PubMed] [Google Scholar]
- 90.Hirasawa Y, Kohno N, Yokoyama A, et al. . Natural autoantibody to MUC1 is a prognostic indicator for non-small cell lung cancer. Am J Respir Crit Care Med 2000; 161: 589–594. doi: 10.1164/ajrccm.161.2.9905028 [DOI] [PubMed] [Google Scholar]
- 91.Pan D, Chen J, Feng C, et al. . Preferential localization of MUC1 glycoprotein in exosomes secreted by non-small cell lung carcinoma cells. Int J Mol Sci 2019; 20: 323. doi: 10.3390/ijms20020323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao J, McConnell MJ, Yu B, et al. . MUC1 is a downstream target of STAT3 and regulates lung cancer cell survival and invasion. Int J Oncol 2009; 35: 337–345. [PMC free article] [PubMed] [Google Scholar]
- 93.Xue M, Tao W. Upregulation of MUC1 by its novel activator 14-3-3ζ promotes tumor invasion and indicates poor prognosis in lung adenocarcinoma. Oncol Rep 2017; 38: 2637–2646. doi: 10.3892/or.2017.5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu T, Li D, Wang H, et al. . MUC1 downregulation inhibits non-small cell lung cancer progression in human cell lines. Exp Ther Med 2017; 14: 4443–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao M, Zhang W, Zhang Q, et al. . Overexpression of MUC1 enhances proangiogenic activity of non-small-cell lung cancer cells through activation of Akt and extracellular signal-regulated kinase pathways. Lung 2011; 189: 453–460. doi: 10.1007/s00408-011-9327-y [DOI] [PubMed] [Google Scholar]
- 96.Piyush T, Chacko AR, Sindrewicz P, et al. . Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ 2017; 24: 1937–1947. doi: 10.1038/cdd.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mori Y, Akita K, Yashiro M, et al. . Binding of galectin-3, a beta-galactoside-binding lectin, to MUC1 protein enhances phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt, promoting tumor cell malignancy. J Biol Chem 2015; 290: 26125–26140. doi: 10.1074/jbc.M115.651489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arsham AM, Plas DR, Thompson CB, et al. . Akt and hypoxia-inducible factor-1 independently enhance tumor growth and angiogenesis. Cancer Res 2004; 64: 3500–3507. doi: 10.1158/0008-5472.CAN-03-2239 [DOI] [PubMed] [Google Scholar]
- 99.Singh PK, Wen Y, Swanson BJ, et al. . Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res 2007; 67: 5201–5210. doi: 10.1158/0008-5472.CAN-06-4647 [DOI] [PubMed] [Google Scholar]
- 100.Schroeder JA, Masri AA, Adriance MC, et al. . MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene 2004; 23: 5739–5747. doi: 10.1038/sj.onc.1207713 [DOI] [PubMed] [Google Scholar]
- 101.Wen Y, Caffrey TC, Wheelock MJ, et al. . Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J Biol Chem 2003; 278: 38029–38039. doi: 10.1074/jbc.M304333200 [DOI] [PubMed] [Google Scholar]
- 102.Liu J, Meisner D, Kwong E, et al. . Translymphatic chemotherapy by intrapleural placement of gelatin sponge containing biodegradable paclitaxel colloids controls lymphatic metastasis in lung cancer. Cancer Res 2009; 69: 1174–1181. doi: 10.1158/0008-5472.CAN-08-1753 [DOI] [PubMed] [Google Scholar]
- 103.Hoang T, Dahlberg SE, Sandler AB, et al. . Prognostic models to predict survival in non-small-cell lung cancer patients treated with first-line paclitaxel and carboplatin with or without bevacizumab. J Thorac Oncol 2012; 7: 1361–1368. doi: 10.1097/JTO.0b013e318260e106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ham SY, Kwon T, Bak Y, et al. . Mucin 1-mediated chemo-resistance in lung cancer cells. Oncogenesis 2016; 5: e185. doi: 10.1038/oncsis.2015.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kharbanda A, Rajabi H, Jin C, et al. . Targeting the oncogenic MUC1-C protein inhibits mutant EGFR-mediated signaling and survival in non-small cell lung cancer cells. Clin Cancer Res 2014; 20: 5423–5434. doi: 10.1158/1078-0432.CCR-13-3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishikawa N, Hattori N, Yokoyama A, et al. . Usefulness of monitoring the circulating Krebs von den Lungen-6 levels to predict the clinical outcome of patients with advanced nonsmall cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. Int J Cancer 2008; 122: 2612–2620. doi: 10.1002/ijc.23411 [DOI] [PubMed] [Google Scholar]
- 107.Fujiwara Y, Kiura K, Toyooka S, et al. . Elevated serum level of sialylated glycoprotein KL-6 predicts a poor prognosis in patients with non-small cell lung cancer treated with gefitinib. Lung cancer 2008; 59: 81–87. doi: 10.1016/j.lungcan.2007.07.018 [DOI] [PubMed] [Google Scholar]
- 108.Li J, Hu YM, Du YJ, et al. . Expressions of MUC1 and vascular endothelial growth factor mRNA in blood are biomarkers for predicting efficacy of gefitinib treatment in non-small cell lung cancer. BMC cancer 2014; 14: 848. doi: 10.1186/1471-2407-14-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kamiya K, Watanabe M, Kohno M, et al. . KL-6 and CEA levels in epithelial lining fluid microsamples predict response to gefitinib in patients with advanced non-small cell lung cancer. Respirology 2011; 16: 976–982. doi: 10.1111/j.1440-1843.2011.02009.x [DOI] [PubMed] [Google Scholar]
- 110.Bouillez A, Adeegbe D, Jin C, et al. . MUC1-C promotes the suppressive immune microenvironment in non-small cell lung cancer. Oncoimmunology 2017; 6: e1338998. doi: 10.1080/2162402X.2017.1338998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bouillez A, Rajabi H, Jin C, et al. . MUC1-C integrates PD-L1 induction with repression of immune effectors in non-small-cell lung cancer. Oncogene 2017; 36: 4037–4046. doi: 10.1038/onc.2017.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quoix E, Lena H, Losonczy G, et al. . TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol 2016; 17: 212–223. doi: 10.1016/S1470-2045(15)00483-0 [DOI] [PubMed] [Google Scholar]
- 113.Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res 2007; 13(15 Pt 2): s4652–s4654. doi: 10.1158/1078-0432.CCR-07-0213 [DOI] [PubMed] [Google Scholar]
- 114.Butts C, Socinski MA, Mitchell PL, et al. . Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 59–68. doi: 10.1016/S1470-2045(13)70510-2 [DOI] [PubMed] [Google Scholar]
- 115.Mitchell P, Thatcher N, Socinski MA, et al. . Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol 2015; 26: 1134–1142. doi: 10.1093/annonc/mdv104 [DOI] [PubMed] [Google Scholar]
- 116.Kontani K, Taguchi O, Ozaki Y, et al. . Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin. Int J Mol Med 2003; 12: 493–502. [PubMed] [Google Scholar]
- 117.Ishikawa N, Hattori N, Yokoyama A, et al. . Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 2012; 50: 3–13. doi: 10.1016/j.resinv.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 118.Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J Med Invest 1999; 46: 151–158. [PubMed] [Google Scholar]
- 119.Qin H, Xu XP, Zou J, et al. . Krebs von den Lungen-6 associated with chest high-resolution CT score in evaluation severity of patients with interstitial lung disease. Pulmonology 2019; 25: 143–148. doi: 10.1016/j.pulmoe.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 120.Kohno N, Awaya Y, Oyama T, et al. . KL-6, a mucin-like glycoprotein, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am Rev Respir Dis 1993; 148: 637–642. doi: 10.1164/ajrccm/148.3.637 [DOI] [PubMed] [Google Scholar]
- 121.Satoh H, Kurishima K, Ishikawa H, et al. . Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med 2006; 260: 429–434. doi: 10.1111/j.1365-2796.2006.01704.x [DOI] [PubMed] [Google Scholar]
- 122.Miyazaki Y, Tsutsui T, Inase N. Treatment and monitoring of hypersensitivity pneumonitis. Expert Rev Clin Immunol 2016; 12: 953–962. doi: 10.1080/1744666X.2016.1182426 [DOI] [PubMed] [Google Scholar]
- 123.Ohnishi H, Miyamoto S, Kawase S, et al. . Seasonal variation of serum KL-6 concentrations is greater in patients with hypersensitivity pneumonitis. BMC Pulm Med 2014; 14: 129. doi: 10.1186/1471-2466-14-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Janssen R, Kruit A, Grutters JC, et al. . The mucin-1 568 adenosine to guanine polymorphism influences serum Krebs von den Lungen-6 levels. Am J Respir Cell Mol Biol 2006; 34: 496–499. doi: 10.1165/rcmb.2005-0151OC [DOI] [PubMed] [Google Scholar]
- 125.Imbert Y, Foulks GN, Brennan MD, et al. . MUC1 and estrogen receptor alpha gene polymorphisms in dry eye patients. Exp Eye Res 2009; 88: 334–338. doi: 10.1016/j.exer.2008.05.019 [DOI] [PubMed] [Google Scholar]
- 126.Shigemura M, Nasuhara Y, Konno S, et al. . Effects of molecular structural variants on serum Krebs von den Lungen-6 levels in sarcoidosis. J Transl Med 2012; 10: 111. doi: 10.1186/1479-5876-10-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kunitake R, Kuwano K, Yoshida K, et al. . KL-6, surfactant protein A and D in bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Respiration 2001; 68: 488–495. doi: 10.1159/000050556 [DOI] [PubMed] [Google Scholar]
- 128.Goto K, Kodama T, Sekine I, et al. . Serum levels of KL-6 are useful biomarkers for severe radiation pneumonitis. Lung cancer 2001; 34: 141–148. doi: 10.1016/S0169-5002(01)00215-X [DOI] [PubMed] [Google Scholar]
- 129.Ohnishi H, Yokoyama A, Yasuhara Y, et al. . Circulating KL-6 levels in patients with drug induced pneumonitis. Thorax 2003; 58: 872–875. doi: 10.1136/thorax.58.10.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakajima H, Harigai M, Hara M, et al. . KL-6 as a novel serum marker for interstitial pneumonia associated with collagen diseases. J Rheumatol 2000; 27: 1164–1170. [PubMed] [Google Scholar]
- 131.Bennett D, Salvini M, Fui A, et al. . Calgranulin B and KL-6 in bronchoalveolar lavage of patients with IPF and NSIP. Inflammation 2019; 42: 463–470. doi: 10.1007/s10753-018-00955-2 [DOI] [PubMed] [Google Scholar]
- 132.Sakamoto K, Taniguchi H, Kondoh Y, et al. . Serum KL-6 in fibrotic NSIP: correlations with physiologic and radiologic parameters. Respir Med 2010; 104: 127–133. doi: 10.1016/j.rmed.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 133.Yokoyama A, Kondo K, Nakajima M, et al. . Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 2006; 11: 164–168. doi: 10.1111/j.1440-1843.2006.00834.x [DOI] [PubMed] [Google Scholar]
- 134.Wakamatsu K, Nagata N, Kumazoe H, et al. . Prognostic value of serial serum KL-6 measurements in patients with idiopathic pulmonary fibrosis. Respir Invest 2017; 55: 16–23. doi: 10.1016/j.resinv.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 135.Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J Biol Chem 2003; 278: 3386–3394. doi: 10.1074/jbc.M208326200 [DOI] [PubMed] [Google Scholar]
- 136.Thathiah A, Carson DD. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem J 2004; 382(Pt 1): 363–373. doi: 10.1042/BJ20040513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pardo A, Cabrera S, Maldonado M, et al. . Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res 2016; 17: 23. doi: 10.1186/s12931-016-0343-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bergantini L, Bargagli E, Cameli P, et al. . Serial KL-6 analysis in patients with idiopathic pulmonary fibrosis treated with nintedanib. Respir Investig 2019; 57: 290–291. doi: 10.1016/j.resinv.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 139.Kato K, Uchino R, Lillehoj EP, et al. . Membrane-tethered MUC1 mucin counter-regulates the phagocytic activity of macrophages. Am J Respir Cell Mol Biol 2016; 54: 515–523. doi: 10.1165/rcmb.2015-0177OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Milara J, Ballester B, Montero P, et al. . MUC1 intracellular bioactivation mediates lung fibrosis. Thorax 2020; 75: 132–142. doi: 10.1136/thoraxjnl-2018-212735 [DOI] [PubMed] [Google Scholar]
- 141.Mackinnon AC, Gibbons MA, Farnworth SL, et al. . Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med 2012; 185: 537–546. doi: 10.1164/rccm.201106-0965OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ramasamy S, Duraisamy S, Barbashov S, et al. . The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell 2007; 27: 992–1004. doi: 10.1016/j.molcel.2007.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tsoutsou PG, Gourgoulianis KI, Petinaki E, et al. . ICAM-1, ICAM-2 and ICAM-3 in the sera of patients with idiopathic pulmonary fibrosis. Inflammation 2004; 28: 359–364. doi: 10.1007/s10753-004-6647-6 [DOI] [PubMed] [Google Scholar]
- 144.Rahn JJ, Shen Q, Mah BK, et al. . MUC1 initiates a calcium signal after ligation by intercellular adhesion molecule-1. J Biol Chem 2004; 279: 29386–29390. doi: 10.1074/jbc.C400010200 [DOI] [PubMed] [Google Scholar]
- 145.Xu L, Yang D, Zhu S, et al. . Bleomycin-induced pulmonary fibrosis is attenuated by an antibody against KL-6. Exp Lung Res 2013; 39: 241–248. doi: 10.3109/01902148.2013.798056 [DOI] [PubMed] [Google Scholar]
- 146.Kato K, Zemskova MA, Hanss AD, et al. . Muc1 deficiency exacerbates pulmonary fibrosis in a mouse model of silicosis. Biochem Biophys Res Commun 2017; 493: 1230–1235. doi: 10.1016/j.bbrc.2017.09.047 [DOI] [PubMed] [Google Scholar]
- 147.Seibold MA, Wise AL, Speer MC, et al. . A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 2011; 364: 1503–1512. doi: 10.1056/NEJMoa1013660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nakano Y, Yang IV, Walts AD, et al. . MUC5B promoter variant rs35705950 affects MUC5B expression in the distal airways in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2016; 193: 464–466. doi: 10.1164/rccm.201509-1872LE [DOI] [PMC free article] [PubMed] [Google Scholar]