Abstract
Interstitial lung disease (ILD) is the leading cause of morbidity and mortality in systemic sclerosis (SSc). We performed a systematic review to characterise the use and validation of pulmonary function tests (PFTs) as surrogate markers for systemic sclerosis-associated interstitial lung disease (SSc-ILD) progression.
Five electronic databases were searched to identify all relevant studies. Included studies either used at least one PFT measure as a longitudinal outcome for SSc-ILD progression (i.e. outcome studies) and/or reported at least one classical measure of validity for the PFTs in SSc-ILD (i.e. validation studies).
This systematic review included 169 outcome studies and 50 validation studies. Diffusing capacity of the lung for carbon monoxide (DLCO) was cumulatively the most commonly used outcome until 2010 when it was surpassed by forced vital capacity (FVC). FVC (% predicted) was the primary endpoint in 70.4% of studies, compared to 11.3% for % predicted DLCO. Only five studies specifically aimed to validate the PFTs: two concluded that DLCO was the best measure of SSc-ILD extent, while the others did not favour any PFT. These studies also showed respectable validity measures for total lung capacity (TLC).
Despite the current preference for FVC, available evidence suggests that DLCO and TLC should not yet be discounted as potential surrogate markers for SSc-ILD progression.
Short abstract
Despite the popularity of FVC, available evidence does not favour it as the best PFT surrogate marker for SSc-ILD http://ow.ly/l8vZ30j6kmj
Introduction
Systemic sclerosis (SSc) is a chronic and progressive autoimmune disease involving a complex interplay of microvasculopathy, disturbances in fibroblastic function and abnormalities of the immune system [1, 2]. In addition to disfiguring skin involvement, SSc patients can suffer from extensive internal organ damage, including interstitial lung disease (ILD) [3].
Systemic sclerosis-associated interstitial lung disease (SSc-ILD) is the leading cause of morbidity and mortality in SSc [4] and is estimated to occur in over 50% of patients [5]. The prognosis of patients with SSc-ILD can be poor and it is estimated that approximately 15% of patients will experience rapidly progressive ILD [4, 6]. For these reasons, the presence and progression of ILD are routinely monitored using pulmonary function tests (PFTs).
Since SSc-ILD was first described in 1949 [7], various PFT measures have been used to characterise its progression. However, forced vital capacity (FVC) has become the preferred surrogate marker for SSc-ILD despite the paucity of validation studies. To better understand the rationale behind FVC as the preferred outcome measure for SSc-ILD, we performed a systematic review of the literature aiming to outline the historical use and validation of PFT measures as surrogate markers for SSc-ILD progression.
The first objective was to determine the frequency at which the different PFT measures were used as outcomes in the study of SSc-ILD onset and progression. We then aimed to summarise the results of studies that validated PFT measures against either high-resolution computed tomography (HRCT) or lung biopsy results in SSc patients. The results of this systematic review would thus describe not only changing practices in the use of PFTs over time in SSc-ILD, but also assess the extent to which these trends were supported by available evidence.
Material and methods
The protocol for this review was registered with the Prospero international prospective register of systematic reviews (CRD42016039565). Institutional review board (IRB) approval was not required.
Eligibility criteria
Only studies published in English or in French were considered. To be as inclusive and comprehensive as possible all original research was eligible for inclusion, including published conference abstracts and clinical trial registrations. However, reviews, summary articles, case reports, commentaries, letters and editorials were excluded. No restrictions were placed on study design.
Eligible studies had to include patients with a diagnosis of SSc and, furthermore, SSc-ILD had to be the focus of the study. All included studies either 1) used at least one PFT measure as a longitudinal outcome for SSc-ILD; and/or 2) reported at least one classical measure of validity for the association between PFTs and either HRCT findings or lung biopsy results in SSc. The former studies are referred to hereafter as outcome studies and the latter as validation studies. Finally, a minimum of 20 SSc patients was required for inclusion in the review.
Information sources and search strategy
The MEDLINE (PubMed), Embase (Ovid), and Web of Science databases were searched on July 05, 2016 to identify all potentially relevant studies since January 1949. ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) were also searched for clinical trial registrations. Additionally, the reference lists of all studies identified via the electronic searches and adhering to the inclusion criteria were manually curated for further potentially eligible studies.
The search strategy, designed in consultation with a professional librarian, consisted of three main search terms: 1) systemic sclerosis; 2) interstitial lung disease; and 3) pulmonary function test. The detailed search strategy for the MEDLINE database is available in the supplementary material (e-Appendix 1).
Study selection
The title and abstract of each article were assessed independently for potential eligibility by two reviewers. Articles were only excluded if both reviewers came to this decision unanimously. Next, the reviewers performed a separate full-text assessment of the remaining articles to confirm their inclusion. Any disagreements were resolved by consensus. The kappa statistic was calculated at both stages of the screening process to assess inter-reviewer agreement.
Data collection
Data from the included studies was extracted using a standardised, pre-piloted form. Data extracted from the outcome studies included the PFT measures used as outcomes for SSc-ILD progression, as well as the reasoning provided for these choices. Data collected from the validation studies included the chosen clinical reference standard, the HRCT scoring system utilised (if applicable) and the reported measures of validity.
Synthesis of results
For the outcome studies, the cumulative use over time of the different PFT measures was depicted graphically. The reasoning provided for the choice in primary PFT outcome measure was also summarised. For all analyses, all variations of diffusing capacity of the lung for carbon monoxide (DLCO), including DLCO adjusted for alveolar volume (VA) and DLCO corrected for haemoglobin (Hb), were grouped together into one DLCO measure.
Among the validation studies, we identified those whose primary goal was specifically to validate the different PFT measures in SSc-ILD. We focused explicitly on these validation studies and summarised their reported measures of validity in Forest plots. Given the variability in study population and ILD scoring method, no attempts were made to subject the data to meta-analysis. The results of the remaining validation studies, whose aim was not specifically to validate PFT measures, are presented in the supplementary material (e-Appendix 6).
Risk of bias
The quality of the outcome studies was not assessed, as the purpose of this review was not to summarise measures of effect. The quality of the studies whose specific aim was to validate PFTs in SSc-ILD was evaluated using the quality assessment of diagnostic accuracy studies (QUADAS-2) tool [8]. Publication bias was not assessed as there are, to our knowledge, no available methods to do so in the context of screening/diagnostic test accuracy.
Results
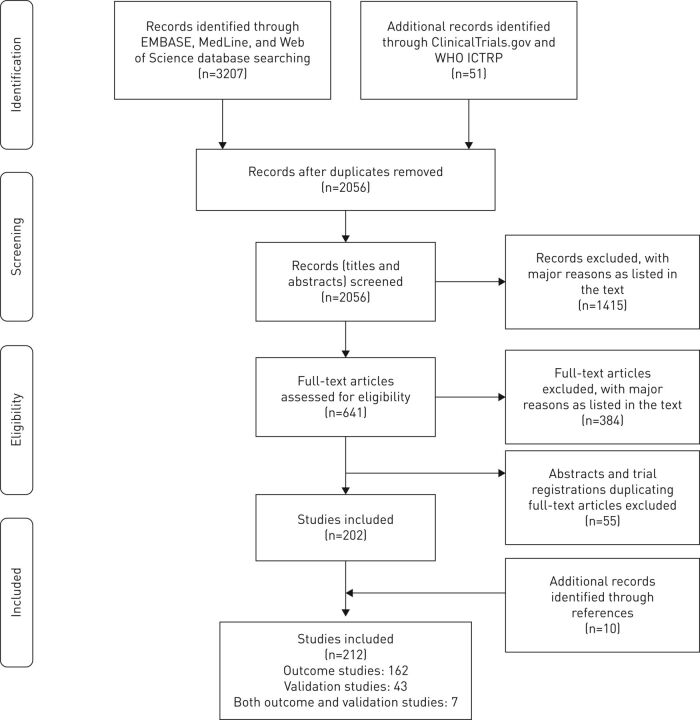
An adaptation of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram [9] outlining the study selection process is available in figure 1. The detailed search strategy retrieved 3258 records. Following removal of duplicates, the titles and abstracts of 2056 records were screened to assess eligibility. Both co-reviewers independently agreed on the exclusion of 1415 records, with an overall percent agreement of 0.91 and a kappa statistic of 0.78. To confirm inclusion, a full-text assessment was performed on the remaining 641 records of which 384 were excluded. The overall percent agreement and kappa statistic for the secondary screening were 0.85 and 0.69, respectively. The reasons for exclusion at both the primary and secondary screening stages are presented in the supplementary material (e-Appendix 2)
FIGURE 1.
A preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram outlining the study selection process. WHO: World Health Organization; ICTRP: International Clinical Trials Registry Platform. Adapted from [9].
Following the screening process, an additional 55 abstracts and clinical trial registrations were excluded since they matched subsequently published full-text articles. Finally, the reference lists of the 202 included studies were manually searched for further potentially relevant studies. This resulted in the addition of ten records, for a total of 212 studies included in this systematic review. Of the 212 included records, 169 qualified as outcome studies [10–178] while 50 were listed as validation studies [45, 73, 109, 146, 153, 159, 167, 179–221]. Seven records satisfied the inclusion criteria for both outcome and validation studies [45, 73, 109, 146, 153, 159, 167].
Outcome study results
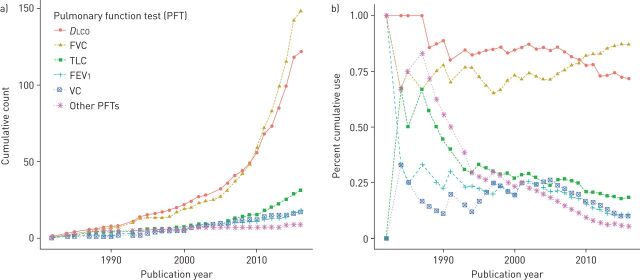
The relevant study characteristics of the 169 outcome studies are summarised in the supplementary material (e-Appendix 3). The earliest longitudinal study using PFTs as outcomes for SSc-ILD progression to be identified was published in 1982 [10]. For most of the study period, DLCO was cumulatively the most commonly used PFT outcome, followed closely by FVC. In 2010, FVC surpassed DLCO as the most often used PFT (figure 2a). Similarly, the percent cumulative use of DLCO (i.e. the yearly ratio of the cumulative count of a given PFT to the cumulative number of published articles) gradually decreased over time in favour of an increase in the percent cumulative use of FVC. These trends were especially prominent after 2005 (figure 2b). Interesting trends in the use of absolute and % predicted PFT values (the latter denoted by a % symbol after the PFT abbreviation) were also observed and are further described in the supplementary material (e-Appendix 4).
FIGURE 2.
a) Cumulative count and b) percent cumulative use of the different pulmonary function test (PFT) measures as longitudinal outcomes for systemic sclerosis-associated interstitial lung disease progression. For each year, percent cumulative use was calculated by dividing the cumulative use of each PFT measure by the cumulative number of published articles. No distinctions were made between absolute and % predicted PFT values. All variations of diffusing capacity of the lung for carbon monoxide (DLCO) were grouped together into one all-encompassing DLCO measure. Other PFTs included measures of forced expiratory flow over the mid-half of forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1)/FVC, FEV1/vital capacity (VC), functional residual capacity, residual volume and total lung capacity (TLC).
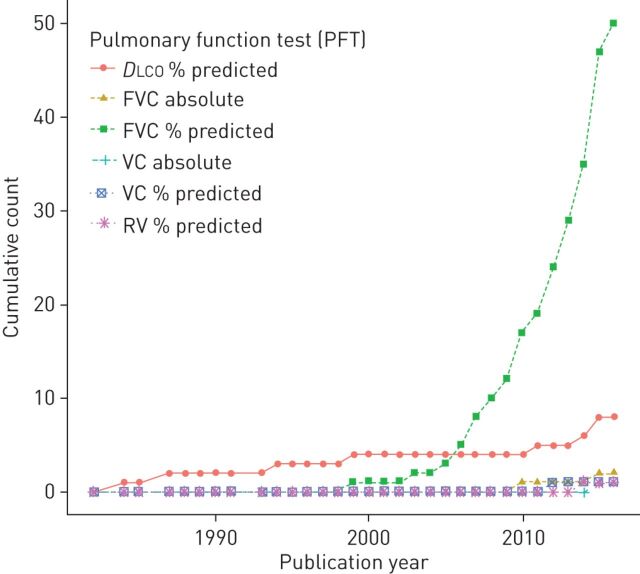
Among the 169 outcome studies, 71 clearly specified a single primary PFT endpoint for SSc-ILD progression. While % predicted DLCO comprised only a small proportion of primary PFT endpoints overall (11.3%), figure 3 reveals that it was a relatively important main outcome in the 1980s and 1990s, only to be surpassed by % predicted FVC in the mid-2000s (which accounted for 70.4% of studies overall). Of these 71 studies, 42 (59.2%) did not report a reason to support their choice in outcome, 20 (28.2%) alluded to the main PFT measure's use in previous SSc-ILD studies, five (7.0%) stated that the chosen PFT had been previously validated, while four (5.6%) reported using it either due to its high sensitivity or specificity.
FIGURE 3.
Cumulative count of the different primary pulmonary function test (PFT) outcomes in longitudinal studies of systemic sclerosis-associated interstitial lung disease progression. Four studies which used forced vital capacity (FVC) but which did not make a distinction between absolute and % predicted values were excluded from this figure. DLCO: diffusing capacity of the lung for carbon monoxide; VC: vital capacity; RV: residual volume.
Interestingly, of the five studies that cited prior validation to justify their choice of PFT (% predicted FVC in four cases [69, 111, 114, 149] and % predicted DLCO in one) [33], only the study using % predicted DLCO referenced an article with reported measures of validity. The four studies which chose their main PFT outcome measure based on its high sensitivity (in the case of DLCO) [11, 14] or its high specificity (in the case of FVC) [128, 169] either did not provide a citation to support this claim [169], referred to studies performed outside the field of SSc-ILD [11, 14, 128], or cited SSc studies with fewer than 20 patients [11, 14].
Validation study results
The relevant characteristics of the 50 validation studies and their measures of validity are summarised in the supplementary material (e-Appendix 5 and e-Appendix 6, respectively). Among these studies, only five had the clear objective of validating the different PFT measures in SSc-ILD [159, 180, 210, 213, 221]. Ideally, identifying the best surrogate marker for SSc-ILD progression should involve the comparison of longitudinal variations in PFT measures with concurrent variations in either HRCT scores or lung biopsy results. This would ensure that the surrogate marker changes along with the reference standard. However, all five of these validation studies performed cross-sectional assessments of PFT results against HRCT-assessed SSc-ILD severity.
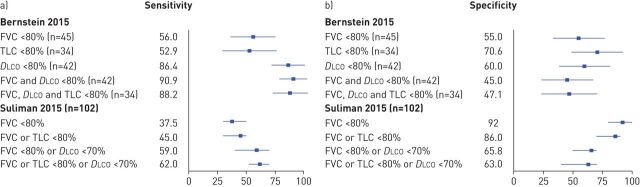
Three of the five studies correlated PFT values with HRCT scores [180, 210, 221] and Forest plots of the correlation coefficients are available in figure 4. The first of these studies, published in 1997, concluded that DLCO was the best measure of SSc-ILD extent (figure 4a) [180]. The authors of the second study, published in 2013, reported that PFTs alone may not be sufficient to identify cases of SSc-ILD (figure 4b) [210]. Finally, the third study, published in 2016, concluded in favour of % predicted DLCO being the best surrogate marker for SSc-ILD extent at any one point in time (figure 4c) [221]. The remaining two studies to have validated PFT measures reported measures of sensitivity and specificity (figure 5) [159, 213]. Neither study concluded in favour of any specific PFT. Rather, they both warned against using only PFTs to identify ILD in SSc patients and suggested evaluating more inclusive screening tools. The quality of these five validation studies is described in the supplementary material (e-Appendix 7). All studies exhibited some risk of bias, generally due to the potential for verification bias. Further issues included a lack of information about the timing of the PFTs and HRCT scans and about whether proper blinding was performed.
FIGURE 4.
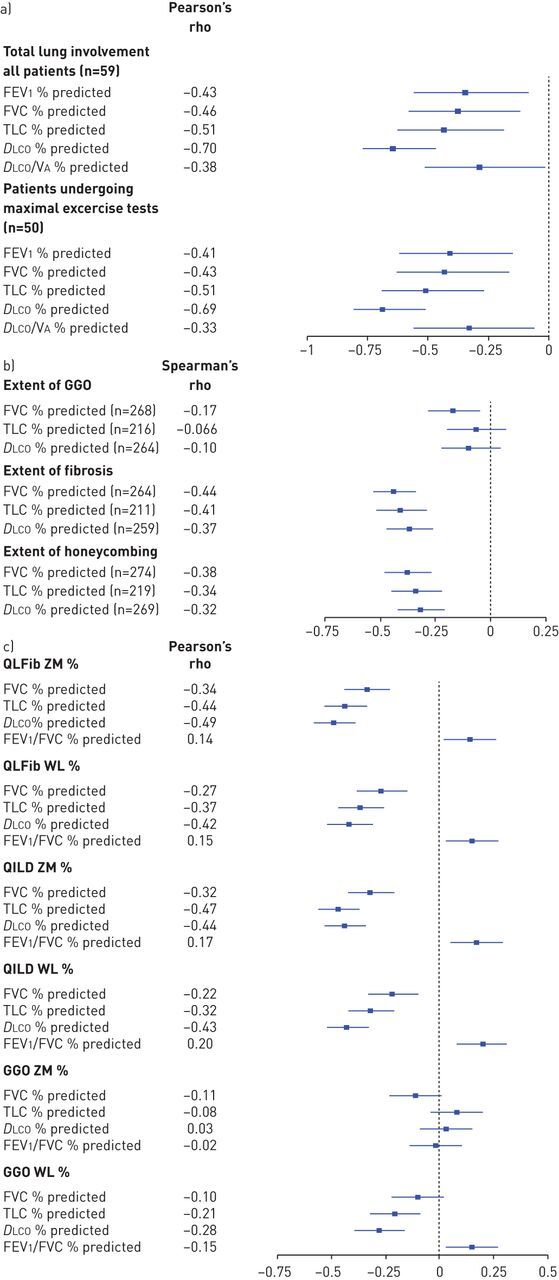
Forest plots of the correlation between high-resolution computed tomography (HRCT) scores and pulmonary function test values. The 95% CIs were calculated using the Fisher transformation. a) In the study by Wells [180] total abnormal lung involvement on HRCT was measured to the nearest 5%, while b) in the study by Zamora [210] the scoring method was not reported. c) Finally, in the study by Tashkin [221], HRCT scans were scored using quantitative imaging analyses and diffusing capacity of the lung for carbon monoxide (DLCO) was corrected for haemoglobin. While the number of subjects included in the analyses was not reported, it was assumed that all 261 systemic sclerosis subjects that underwent HRCT testing were included in the correlation analyses [221]. VA: alveolar volume; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GGO: ground-glass opacity; QILD: quantitative interstitial lung disease; QLFib: quantitative extent of lung fibrosis; TLC: total lung capacity; WL: whole lung; ZM: zone of maximal involvement.
FIGURE 5.
Forest plot of the a) sensitivity and b) specificity of different pulmonary function test screening algorithms for the presence of systemic sclerosis-associated interstitial lung disease [159, 213]. For the study by Bernstein [213], 95% CIs were calculated using reported measures of sensitivity, specificity, positive predictive value and negative predictive value. DLCO: diffusing capacity of the lung for carbon monoxide; FVC: forced vital capacity; TLC: total lung capacity.
Of the remaining 45 studies, more than half aimed to validate specific HRCT scoring systems using PFTs as reference standards. Thus, despite HRCT being the gold-standard for assessing SSc-ILD presence, no consensus exists on the best way in which to score SSc-ILD severity. This explains the large variety of different HRCT scoring systems used in SSc-ILD and renders it challenging to summarise results. It is also worth noting that only one study correlated PFTs with lung biopsy results [179] while six reported longitudinal correlations between PFTs and HRCT scores [73, 167, 183, 192, 197, 216]. While no formal synthesising analysis was performed on these studies, their results do not appear to overwhelmingly support the superiority of any particular PFT (supplementary material e-Appendix 6).
Discussion
We performed a comprehensive systematic review of the literature to explore the use of PFTs as surrogate markers for SSc-ILD onset and progression. This review confirmed the predominant selection of FVC as a primary endpoint in recent longitudinal SSc-ILD studies, despite a paucity of evidence from validation studies that FVC has better performance characteristics than other PFT measures.
Our results show that both FVC and DLCO were historically used to monitor SSc-ILD progression. However, given that many authors did not report why they chose a given PFT measure as an outcome, it is difficult to fully understand the recent shift in preference to FVC. A possible explanation is the widespread use of FVC as a therapeutic endpoint in a comparable disease, idiopathic pulmonary fibrosis (IPF) [222, 223], especially given the lack of connective tissue disease (CTD)-ILD trials. However, a lack of consensus still surrounds FVC as the best outcome measure for this disease [224, 225]. Furthermore, IPF is characterised by usual interstitial pneumonia, a pathologically different subtype of ILD than the non-specific interstitial pneumonia pattern commonly observed in SSc-ILD [226].
Here we suggest two additional reasons which may explain FVC's current popularity in SSc, as supported by the results of this systematic review. First, given that DLCO can be confounded by the presence of pulmonary arterial hypertension (PAH), FVC is believed to be more specific than DLCO to ILD [227, 228]. However, evidence suggests that DLCO may be more sensitive than FVC [11, 180, 221]. While the appropriate trade-off between sensitivity and specificity is debatable, it is worth noting that a lower specificity due to confounding by PAH can be corrected for in analyses by using simple epidemiological techniques such as restriction and adjustment. In addition to being less specific to ILD, DLCO is also regarded as being inherently more variable than FVC given its dependence on the measurement of both the transfer coefficient of the lung for carbon monoxide (KCO) and VA. Indeed, DLCO is often not chosen as a measure of SSc-ILD progression because it is considered to not be as reproducible as FVC. However, it is worth noting that variability in FVC can be equally concerning. In fact, a recent study calculated FVC's minimal clinically important difference in SSc-ILD to be well within the range of measurement error at the individual level [229].
Secondly, we suggest that the pivotal Scleroderma Lung Study I (SLS I) also played an important role in the sudden decrease in use of DLCO in favour of FVC. SLS I was the first double-blind, randomised, placebo-controlled trial to study the effect of oral cyclophosphamide on SSc-ILD. The baseline-adjusted mean absolute 12-month difference in FVC, the study's primary endpoint, was 2.53% (0.28–4.79%), favouring cyclophosphamide. This improvement persisted at 24 months, at which point the mean absolute difference in FVC was 1.95% (1.2–2.6%). Secondary outcomes included DLCO and total lung capacity (TLC) with adjusted mean 12-month differences of −1.04% (p=0.43) and 4.09% (0.49–7.65%), respectively [50]. Despite the slight variability in these results and the modest treatment effects observed, FVC was subsequently described as having been validated as an endpoint in randomised controlled trials [230]. In fact, the quality of FVC as a measure for SSc-ILD is often erroneously assessed by its observed treatment effect, rather than by proper validation techniques against a gold-standard. Interestingly, our analyses revealed a steep increase in the use of FVC after the 2006 publication of SLS I, as well as a steep decrease in the use of DLCO following years of relative stability (figure 2b).
It is likely that FVC's specificity for ILD and perceived validity led to its current acceptance as the best surrogate marker for ILD within the SSc community. However, we suggest that this preference is not yet fully warranted given the results of studies which validated PFT measures against HRCT-assessed SSc-ILD severity. In fact, these five studies revealed that FVC does not have conclusively better performance characteristics than other PFT measures. Moreover, two of these studies suggested that DLCO best captured SSc-ILD severity [180, 221].
Also noteworthy was TLC's performance, which overlapped considerably with those of FVC and DLCO (figures 4, 5). Despite these results and the fact that the American Thoracic Society (ATS) defines restrictive lung diseases such as ILD by a reduction in TLC in the presence of a preserved or elevated forced expiratory volume in 1 s (FEV1)/FVC ratio [231], TLC has rarely been used as an outcome in SSc-ILD (figure 2) and has never been used as a main outcome (figure 3).
Ultimately, the best surrogate marker for SSc-ILD onset and progression may be a composite outcome consisting of a combination of two or more PFT measures. In fact, composite outcomes were used in several studies included in this systematic review. The most common combinations included decreases in FVC or DLCO of ≥10% [45, 55, 67, 95, 143], a decline of ≥10% in FVC or 15% in DLCO [46, 54, 83–84, 94, 101, 109–110, 140–141] and decreases in FVC or DLCO of ≥15% [20–21, 76, 117, 125, 133]. Few studies used an FVC–TLC composite endpoint [12, 93, 105] and only one study jointly considered FVC, TLC and DLCO [58]. Nevertheless, the validity of such endpoints remains to be fully evaluated, as only cross-sectional validation studies have been performed so far. These studies found composite outcomes consisting of FVC and DLCO, as well as FVC, DLCO and TLC, to have high sensitivity but low specificity (figure 5).
This systematic review highlights the need to continue to focus efforts on identifying the best sole or composite PFT surrogate marker for SSc-ILD. Presently, the widespread use of FVC has translated into the identification of few predictors of SSc-ILD progression and into attenuated treatment effects in SSc-ILD randomised controlled trials [48, 50, 232], suggesting that FVC may only weakly reflect the extent of SSc-ILD. In fact, it is possible that FVC may also be affected by the cutaneous involvement of the chest wall in SSc [233]. Following a recent consensus exercise, the Outcome Measures in Rheumatology (OMERACT) CTD-ILD working group agreed to and proposed a clinically meaningful outcome in clinical trials of CTD-ILD (a ≥10% decline in FVC or a ≥5% to <10% decline in FVC in the presence of a ≥15% decline in DLCO). However, they also recognised that these measures remain to be validated [234]. Indeed, thorough PFT validation studies are needed to better assess the validity of different PFT measures. In particular, longitudinal studies should be carried out to explore the association between concurrent changes in PFTs and clinical reference standards. Future studies should also build upon recent research aiming to develop composite measures of SSc-ILD severity (e.g. measures of lung physiology, disease manifestations and patient-reported outcomes [162]). This will ultimately strengthen the quality of both clinical trials and epidemiological studies, as well as their results and assist in clinical decision-making.
Our systematic review is not without certain limitations. First and foremost, we did not include studies which validated PFT measures against mortality given the already large scope of this review. Such studies are also integral in identifying the best PFT surrogate marker for SSc-ILD progression. Despite this exclusion, our results are complemented by those of a 2010 systematic review which found that DLCO was more consistently associated with mortality in SSc-ILD than FVC [235]. Since the publication of this review, further evidence has been amassed favouring the utility of DLCO. Indeed, one recent study found a decline in DLCO and KCO to be the most predictive of adverse outcomes, including death [236]. Likewise, a second recent study suggested that 1-year trends in an FVC and DLCO composite endpoint and 2-year trends in measures of gas transfer were most predictive of mortality [237].
Additional limitations include the decision to restrict validation studies to those with classical measures of validity and to not account for studies which reported regression coefficients and p-values. While this translated into a loss of information, it permitted a better focus on higher quality validation data and allowed for standardisation across included studies. Finally, while there is potential for publication bias, we believe that the nature of our research question, in addition to the large number of included studies and the extensive searching of multiple databases, alleviates this concern.
In summary, available evidence does not overwhelmingly favour one PFT measure as the best surrogate marker for SSc-ILD, rendering it challenging to support the current preference for FVC. Indeed, the perceived superiority of FVC is not reflected in rigorous PFT validation studies. While FVC has the potential to be a viable surrogate marker for SSc-ILD, it would be ill-advised at this stage to discount other potentially interesting PFT measures, such as DLCO and TLC.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary Material ERR-0102-2017_Supplementary_Material (1MB, pdf)
Acknowledgements
The authors would like to thank the McGill University (Montreal, QC, Canada) epidemiology liaison librarian Genevieve Gore for her assistance in designing the detailed search strategy.
Footnotes
This article has supplementary material available from err.ersjournals.com
Provenance: Submitted article, peer reviewed.
Conflict of interest: M. Caron reports grants from the Fonds de Recherche du Québec (Santé PhD Studentship) and from the Canadian Institutes of Health Research (GSD–146268) during the conduct of the study. S. Hoa reports grants from the Université de Montréal Rheumatology Clinical Fellowship Program (Abbvie educational grant) and from the Arthritis Society's Postdoctoral Fellowship Award, during the conduct of the study.
References
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360: 1989–2003. [DOI] [PubMed] [Google Scholar]
- 2.Katsumoto TR, Whitfield ML, Connolly MK. The pathogenesis of systemic sclerosis. Annu Rev Pathol 2011; 6: 509–537. [DOI] [PubMed] [Google Scholar]
- 3.Seibold J. Scleroderma. In: Harris ED, Budd RC, Firestein GS, Genovese MC, Sergent JS, Ruddy S, Sledge CB, eds. Kelley's Textbook of Rheumatology. 7th Edn. Philadelphia, Elsevier, 2005; pp. 1279–1308. [Google Scholar]
- 4.Schoenfeld SR, Castelino FV. Interstitial lung disease in scleroderma. Rheum Dis Clin North Am 2015; 41: 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele R, Hudson M, Lo E, et al. Clinical decision rule to predict the presence of interstitial lung disease in systemic sclerosis. Arthritis Care Res (Hoboken) 2012; 64: 519–524. [DOI] [PubMed] [Google Scholar]
- 6.Khanna D, Denton CP. Evidence-based management of rapidly progressing systemic sclerosis. Best Pract Res Clin Rheumatol 2010; 24: 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin EF, Cournand A, Richards DW Jr. Pulmonary insufficiency; a study of 39 cases of pulmonary fibrosis. Medicine (Baltimore) 1949; 28: 1–25. [PubMed] [Google Scholar]
- 8.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider PD, Wise RA, Hochberg MC, et al. Serial pulmonary function in systemic sclerosis. Am J Med 1982; 73: 385–394. [DOI] [PubMed] [Google Scholar]
- 11.Konig G, Luderschmidt C, Hammer C. Lung involvement in scleroderma. Chest 1984; 85: 318–324. [DOI] [PubMed] [Google Scholar]
- 12.Peters-Golden M, Wise RA, Schneider P. Clinical and demographic predictors of loss of pulmonary function in systemic sclerosis. Medicine (Baltimore) 1984; 63: 221–231. [DOI] [PubMed] [Google Scholar]
- 13.Steen VD, Owens GR, Redmond C, et al. The effect of D-penicillamine on pulmonary findings in systemic sclerosis. Arthritis Rheum 1985; 28: 882–888. [DOI] [PubMed] [Google Scholar]
- 14.De Clerck LS, Dequeker J, Francx L, et al. D-Penicillamine therapy and interstitial lung disease in scleroderma. A long-term followup study. Arthritis Rheum 1987; 30: 643–650. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald GI, Tashkin DP, Gong H, et al. Longitudinal changes in lung function and respiratory symptoms in progressive systemic sclerosis. Prospective study. Am J Med 1987; 83: 83–92. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy DS, Baragar FD, Dhingra S, et al. The lungs in systemic sclerosis (scleroderma): a review and new information. Semin Arthritis Rheum 1988; 17: 271–283. [DOI] [PubMed] [Google Scholar]
- 17.Zarafonetis CJD, Dabich L, Devol EB, et al. Retrospective studies in scleroderma: pulmonary findings and effect of potassium p-aminobenzoate on vital capacity. Respiration 1989; 56: 22–33. [DOI] [PubMed] [Google Scholar]
- 18.Silver RM, Miller KS, Kinsella MB, et al. Evaluation and management of scleroderma lung disease using bronchoalveolar lavage. Am J Med 1990; 88: 470–476. [DOI] [PubMed] [Google Scholar]
- 19.Abramson MJ, Barnett AJ, Littlejohn GO, et al. Lung function abnormalities and decline of spirometry in scleroderma: an overrated danger? Postgrad Med J 1991; 67: 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells AU, Hansell DM, Rubens MB, et al. The predictive value of appearances on thin-section computed tomography in fibrosing alveolitis. Am Rev Respir Dis 1993; 148: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 21.Wells AU, Hansell DM, Harrison NK, et al. Clearance of inhaled 99mTc-DTPA predicts the clinical course of fibrosing alveolitis. Eur Respir J 1993; 6: 797–802. [PubMed] [Google Scholar]
- 22.Wells AU, Rubens MB, du Bois RM, et al. Serial CT in fibrosing alveolitis: prognostic significance of the initial pattern. AJR Am J Roentgenol 1993; 161: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 23.Dujic Z, Eterovic D, Tocilj J, et al. Increase of pulmonary diffusing capacity in systemic sclerosis. Br J Rheumatol 1994; 33: 437–441. [DOI] [PubMed] [Google Scholar]
- 24.Steen VD, Conte C, Owens GR, et al. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 1994; 37: 1283–1289. [DOI] [PubMed] [Google Scholar]
- 25.Steen VD, Lanz JK Jr, Conte C, et al. Therapy for severe interstitial lung disease in systemic sclerosis: a retrospective study. Arthritis Rheum 1994; 37: 1290–1296. [DOI] [PubMed] [Google Scholar]
- 26.Tashkin DP, Clements PJ, Wright RS, et al. Interrelationships between pulmonary and extrapulmonary involvement in systemic sclerosis. A longitudinal analysis. Chest 1994; 105: 489–495. [DOI] [PubMed] [Google Scholar]
- 27.Behr J, Adelmann-Grill BC, Hein R, et al. Pathogenetic and clinical significance of fibroblast activation in scleroderma lung disease. Respiration 1995; 62: 209–216. [DOI] [PubMed] [Google Scholar]
- 28.Behr J, Vogelmeier C, Beinert T, et al. Bronchoalveolar lavage for evaluation and management of scleroderma disease of the lung. Am J Respir Crit Care Med 1996; 154: 400–406. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen S, Halberg P, Ullman S, et al. A longitudinal study of pulmonary function in Danish patients with systemic sclerosis. Clin Rheumatol 1997; 16: 384–390. [DOI] [PubMed] [Google Scholar]
- 30.Greidinger EL, Flaherty KT, White B, et al. African-American race and antibodies to topoisomerase I are associated with increased severity of scleroderma lung disease. Chest 1998; 114: 801–807. [DOI] [PubMed] [Google Scholar]
- 31.Atamas SP, Yurovsky VV, Wise R, et al. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum 1999; 42: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 32.Kon OM, Daniil Z, Black CM, et al. Clearance of inhaled technetium-99m-DTPA as a clinical index of pulmonary vascular disease in systemic sclerosis. Eur Respir J 1999; 13: 133–136. [DOI] [PubMed] [Google Scholar]
- 33.Witt C, Borges AC, John M, et al. Pulmonary involvement in diffuse cutaneous systemic sclerosis: broncheoalveolar fluid granulocytosis predicts progression of fibrosing alveolitis. Ann Rheum Dis 1999; 58: 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White B, Moore WC, Wigley FM, et al. Cyclophosphamide is associated with pulmonary function and survival benefit in patients with scleroderma and alveolitis. Ann Intern Med 2000; 132: 947–954. [DOI] [PubMed] [Google Scholar]
- 35.Yuhara T, Takemura H, Akama T, et al. The relationship between serum immunoglobulin levels and pulmonary involvement in systemic sclerosis. J Rheumatol 2000; 27: 1207–1214. [PubMed] [Google Scholar]
- 36.Marie I, Dominique S, Levesque H, et al. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Care Res (Hoboken) 2001; 45: 346–354. [DOI] [PubMed] [Google Scholar]
- 37.Scorza R, Caronni M, Mascagni B, et al. Effects of long-term cyclic iloprost therapy in systemic sclerosis with Raynaud's phenomenon. A randomized, controlled study. Clin Exp Rheumatol 2001; 19: 503–508. [PubMed] [Google Scholar]
- 38.Bouros D, Wells AU, Nicholson AG, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 2002; 165: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 39.Giacomelli R, Valentini G, Salsano F, et al. Cyclophosphamide pulse regimen in the treatment of alveolitis in systemic sclerosis. J Rheumatol 2002; 29: 731–736. [PubMed] [Google Scholar]
- 40.Pakas I, Ioannidis JPA, Malagari K, et al. Cyclophosphamide with low or high dose prednisolone for systemic sclerosis lung disease. J Rheumatol 2002; 29: 298–304. [PubMed] [Google Scholar]
- 41.Kowal-Bielecka O, Distler O, Kowal K, et al. Elevated levels of leukotriene B4 and leukotriene E4 in bronchoalveolar lavage fluid from patients with scleroderma lung disease. Arthritis Rheum 2003; 48: 1639–1646. [DOI] [PubMed] [Google Scholar]
- 42.Yanaba K, Hasegawa M, Hamaguchi Y, et al. Longitudinal analysis of serum KL-6 levels in patients with systemic sclerosis: association with the activity of pulmonary fibrosis. Clin Exp Rheumatol 2003; 21: 429–436. [PubMed] [Google Scholar]
- 43.Airo P, Danieli E, Parrinello G, et al. Intravenous cyclophosphamide therapy for systemic sclerosis. A single-center experience and review of the literature with pooled analysis of lung function test results. Clin Exp Rheumatol 2004; 22: 573–578. [PubMed] [Google Scholar]
- 44.Yanaba K, Hasegawa M, Takehara K, et al. Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis. J Rheumatol 2004; 31: 1112–1120. [PubMed] [Google Scholar]
- 45.De Santis M, Bosello S, La Torre G, et al. Functional, radiological and biological markers of alveolitis and infections of the lower respiratory tract in patients with systemic sclerosis. Respir Res 2005; 6: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kodera M, Hasegawa M, Komura K, et al. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis: a sensitive indicator of active pulmonary fibrosis. Arthritis Rheum 2005; 52: 2889–2896. [DOI] [PubMed] [Google Scholar]
- 47.Kowal-Bielecka O, Kowal K, Rojewska J, et al. Cyclophosphamide reduces neutrophilic alveolitis in patients with scleroderma lung disease: a retrospective analysis of serial bronchoalveolar lavage investigations. Ann Rheum Dis 2005; 64: 1343–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 2006; 54: 3962–3970. [DOI] [PubMed] [Google Scholar]
- 49.Plastiras SC, Karadimitrakis SP, Ziakas PD, et al. Scleroderma lung: initial forced vital capacity as predictor of pulmonary function decline. Arthritis Care Res (Hoboken) 2006; 55: 598–602. [DOI] [PubMed] [Google Scholar]
- 50.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006; 354: 2655–2666. [DOI] [PubMed] [Google Scholar]
- 51.Beretta L, Bertolotti F, Cappiello F, et al. Interleukin-1 gene complex polymorphisms in systemic sclerosis patients with severe restrictive lung physiology. Hum Immunol 2007; 68: 603–609. [DOI] [PubMed] [Google Scholar]
- 52.Beretta L, Caronni M, Raimondi M, et al. Oral cyclophosphamide improves pulmonary function in scleroderma patients with fibrosing alveolitis: experience in one centre. Clin Rheumatol 2007; 26: 168–172. [DOI] [PubMed] [Google Scholar]
- 53.Clements PJ, Roth MD, Elashoff R, et al. Scleroderma lung study (SLS): differences in the presentation and course of patients with limited versus diffuse systemic sclerosis. Ann Rheum Dis 2007; 66: 1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goh NSL, Veeraraghavan S, Desai SR, et al. Bronchoalveolar lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum 2007; 56: 2005–2012. [DOI] [PubMed] [Google Scholar]
- 55.Mittoo S, Wigley FM, Wise R, et al. Persistence of abnormal bronchoalveolar lavage findings after cyclophosphamide treatment in scleroderma patients with interstitial lung disease. Arthritis Rheum 2007; 56: 4195–4202. [DOI] [PubMed] [Google Scholar]
- 56.Tashkin DP, Elashoff R, Clements PJ, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med 2007; 176: 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tzelepis GE, Plastiras SC, Karadimitrakis SP, et al. Determinants of pulmonary function improvement in patients with scleroderma and interstitial lung disease. Clin Exp Rheumatol 2007; 25: 734–739. [PubMed] [Google Scholar]
- 58.Berezne A, Ranque B, Valeyre D, et al. Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: a retrospective multicenter open-label study. J Rheumatol 2008; 35: 1064–1072. [PubMed] [Google Scholar]
- 59.Boin F, De Fanis U, Bartlett SJ, et al. T cell polarization identifies distinct clinical phenotypes in scleroderma lung disease. Arthritis Rheum 2008; 58: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goh NSL, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008; 177: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 61.Strange C, Bolster MB, Roth MD, et al. Bronchoalveolar lavage and response to cyclophosphamide in scleroderma interstitial lung disease. Am J Respir Crit Care Med 2008; 177: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Assassi S, Tan FK, McNearney TA, et al. The whole blood interferon score predicts progression of interstitial lung disease in systemic sclerosis. Arthritis Rheum 2009; 60: Suppl. 10, 443. [Google Scholar]
- 63.De Souza RB, Borges CTL, Capelozzi VL, et al. Centrilobular fibrosis: an underrecognized pattern in systemic sclerosis. Respiration 2009; 77: 389–397. [DOI] [PubMed] [Google Scholar]
- 64.Gordon J, Mersten J, Lyman S, et al. Imatinib mesylate (Gleevec) in the treatment of systemic sclerosis: interim results of a phase IIa, one year, open label clinical trial. Arthritis Rheum 2009; 60: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khanna D, Tseng CH, Furst DE, et al. Minimally important differences in the Mahler's Transition Dyspnoea Index in a large randomized controlled trial-results from the scleroderma lung study. Rheumatology (Oxford) 2009; 48: 1537–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ottewell L, Walker K, Griffiths B. Long-term outcome of a targeted treatment regimen with IV cyclophosphamide (CYC) in patients with systemic sclerosis (SSC) and interstitial lung disease (ILD). Rheumatology (Oxford) 2009; 48: Suppl. 1, i76. [Google Scholar]
- 67.Schmidt K, Martinez-Gamboa L, Meier S, et al. Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res Ther 2009; 11: R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wanchu A, Suryanaryana BS, Sharma S, et al. High-dose prednisolone and bolus cyclophosphamide in interstitial lung disease associated with systemic sclerosis: a prospective open study. Int J Rheum Dis 2009; 12: 239–242. [DOI] [PubMed] [Google Scholar]
- 69.Assassi S, Sharif R, Lasky RE, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther 2010; 12: R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boin F, Wigley F, Wise R, et al. Circulating T cell polarization is associated with respiratory decline in scleroderma patients with active interstitial lung disease. Arthritis Rheum 2010; 62: Suppl. 10, 1989. [Google Scholar]
- 71.Colaci M, Sebastiani M, Giuggioli D, et al. Bronchoalveolar lavage and response to cyclophosphamide in scleroderma alveolitis. Scand J Rheumatol 2010; 39: 155–159. [DOI] [PubMed] [Google Scholar]
- 72.Cuomo G, Abignano G, Iudici M, et al. Mycophenolate mophetil in the treatment of systemic sclerosis-interstitial lung disease (SSc-ILD) in patients unresponsive to cyclophosphamide (CYC). Clin Exp Rheumatol 2010; 28: S167. [Google Scholar]
- 73.Gilson M, Zerkak D, Wipff J, et al. Prognostic factors for lung function in systemic sclerosis: prospective study of 105 cases. Eur Respir J 2010; 35: 112–117. [DOI] [PubMed] [Google Scholar]
- 74.Mittoo S, Robinson D, Hudson M, et al. Predicting lung function decline in systemic sclerosis (SSC). Ann Rheum Dis 2010; 69: Suppl. 2, A27. [Google Scholar]
- 75.Schorr M, Wise RA, Wigley FM, et al. Long-term outcome of patients with an isolated low diffusing capacity. Clin Exp Rheumatol 2010; 28: S76.20868575 [Google Scholar]
- 76.Seibold JR, Denton CP, Furst DE, et al. Randomized, prospective, placebo-controlled trial of bosentan in interstitial lung disease secondary to systemic sclerosis. Arthritis Rheum 2010; 62: 2101–2108. [DOI] [PubMed] [Google Scholar]
- 77.Shahane A, Parambil J, Xu M, et al. Scleroderma lung disease: effect of co-existent pulmonary hypertension on progression of interstitial lung disease. Arthritis Rheum 2010; 62: Suppl. 10, 592. [Google Scholar]
- 78.Steen VD, Domsic RT, Medsger T. Severe pulmonary fibrosis is uncommon in scleroderma patients with RNA polymerase 3 antibody. Arthritis Rheum 2010; 62: Suppl. 10, 1231. [Google Scholar]
- 79.Theodore AC, Simms RW, Lafyatis RA, et al. A preliminary investigation of the predictive value and response to therapy with cyclophosphamide of interleukin-16 in bronchoalveolar lavage from patients with interstitial lung disease in systemic sclerosis. Clin Exp Rheumatol 2010; 28: S78–S79.21176425 [Google Scholar]
- 80.Abhishek A, Yazdani R, Pearce F, et al. Outcome of systemic sclerosis associated interstitial lung disease treated with intravenous cyclophosphamide. Clin Rheumatol 2011; 30: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 81.De Santis M, Inzitari R, Bosello SL, et al. Beta-thymosins and interstitial lung disease: study of a scleroderma cohort with a one-year follow-up. Respir Res 2011; 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Espinosa G, Simeon CP, Plasin MA, et al. Efficacy of cyclophospamide in the treatment of interstitial lung disease associated with systemic sclerosis. Arch Bronconeumol 2011; 47: 239–245. [DOI] [PubMed] [Google Scholar]
- 83.Goh NSL, Desai SR, Anagnostopoulos C, et al. Increased epithelial permeability in pulmonary fibrosis in relation to disease progression. Eur Respir J 2011; 38: 184–190. [DOI] [PubMed] [Google Scholar]
- 84.Hasegawa M, Fujimoto M, Hamaguchi Y, et al. Use of serum Clara cell 16-kDa (CC16) levels as a potential indicator of active pulmonary fibrosis in systemic sclerosis. J Rheumatol 2011; 38: 877–884. [DOI] [PubMed] [Google Scholar]
- 85.Hoshino K, Satoh T, Kawaguchi Y, et al. Association of hepatocyte growth factor promoter polymorphism with severity of interstitial lung disease in Japanese patients with systemic sclerosis. Arthritis Rheum 2011; 63: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 86.Jayaweera S, Sahhar J, Englert H, et al. Mycophenolate mofetil (MMF) in scleroderma (SSC) associated interstitial lung disease (ILD) and skin disease–the Australian experience. Intern Med J 2011; 41: 11. [Google Scholar]
- 87.Khanna D, Mayes MD, Abtin F, et al. A one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis Rheum 2011; 63: 3540–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khanna D, Tseng CH, Farmani N, et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: analysis of the Scleroderma Lung Study placebo group. Arthritis Rheum 2011; 63: 3078–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mittoo S, Wigley FM, Wise RA, et al. Long term effects of cyclophosphamide treatment on lung function and survival in scleroderma patients with interstitial lung disease. Open Rheumatol J 2011; 5: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poormoghim H, Lakeh MM, Mohammadipour M, et al. Pulmonary survival study in 91 patients with systemic sclerosis. Rheumatol Int 2011; 31: 1577–1582. [DOI] [PubMed] [Google Scholar]
- 91.Rosato E, Rossi C, Molinaro I, et al. Long-term N-acetylcysteine therapy in systemic sclerosis interstitial lung disease: a retrospective study. Int J Immunopathol Pharmacol 2011; 24: 727–733. [DOI] [PubMed] [Google Scholar]
- 92.Roth MD, Tseng CH, Clements PJ, et al. Predicting treatment outcomes and responder subsets in scleroderma-related interstitial lung disease. Arthritis Rheum 2011; 63: 2797–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tiev KP, Hua-Huy T, Kettaneh A, et al. Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur Respir J 2011; 38: 1355–1360. [DOI] [PubMed] [Google Scholar]
- 94.Volpinari S, Corte RL, Bighi S, et al. Bronchoalveolar lavage in systemic sclerosis with lung involvement: role and correlations with functional, radiological and scintigraphic parameters. Rheumatol Int 2011; 31: 1183–1188. [DOI] [PubMed] [Google Scholar]
- 95.Abignano G, Del Galdo F, Emery P, et al. Extended course cyclophosphamide and methylprednisolone pulse therapy can stabilize initially refractory interstitial lung disease in patients with SSc: a single-centre experience. Rheumatology (Oxford) 2012; 51: ii114. [DOI] [PubMed] [Google Scholar]
- 96.De Santis M, Bosello SL, Peluso G, et al. Bronchoalveolar lavage fluid and progression of scleroderma interstitial lung disease. Clin Respir J 2012; 6: 9–17. [DOI] [PubMed] [Google Scholar]
- 97.Hesselstrand R, Andreasson KA, Wuttge DM, et al. Increased serum COMP predicts mortality in ssc: results from a longitudinal study of interstitial lung disease. Rheumatology (Oxford) 2012; 51: 915–920. [DOI] [PubMed] [Google Scholar]
- 98.Kishore Babu KV, Srinivasa C, Sirisha K, et al. Outcome of immunosuppressive therapy in patients with scleroderma and interstitial lung disease, 2 years follow up data, a retrospective study. Indian J Rheumatol 2012; 7: S27. [Google Scholar]
- 99.Kuwana M, Kaburaki J. Natural history of pulmonary function in patients with SSc and interstitial lung disease. Rheumatology (Oxford) 2012; 51: ii83–ii84. [Google Scholar]
- 100.Kuwana M, Takeuchi T, Kaburaki J. Elevation of KL-6 at early disease course predicts subsequent deterioration of pulmonary function in patients with systemic sclerosis and interstitial lung disease. Arthritis Rheum 2012; 64: Suppl. 10, S371. [DOI] [PubMed] [Google Scholar]
- 101.Le Gouellec N, Faivre JB, Hachulla AL, et al. Prognosis factors for survival and progression-free survival in systemic sclerosis-associated interstitial lung disease. Ann Rheum Dis 2012; 71: Suppl. 3, 400.22233602 [Google Scholar]
- 102.Schupp J, Riemekasten G, Kollert C, et al. CCL18 as marker of disease progression in systemic sclerosis. Eur Respir J 2012; 40: Suppl. 56, P692. [Google Scholar]
- 103.Sfriso P, Cozzi F, Oliviero F, et al. CXCL11 in bronchoalveolar lavage fluid and pulmonary function decline in systemic sclerosis. Clin Exp Rheumatol 2012; 30: Suppl. 71, S71–S75. [PubMed] [Google Scholar]
- 104.Soriano A, Margiotta D, Marigliano B, et al. Pulmonary fibrosis and connective tissue diseases: follow-up of lung involvement in rheumatoid arthritis and systemic sclerosis. Ann Rheum Dis 2012; 71: Suppl. 3, 343. [Google Scholar]
- 105.Tiev KP, Hua-Huy T, Kettaneh A, et al. Alveolar concentration of nitric oxide predicts pulmonary function deterioration in scleroderma. Thorax 2012; 67: 157–163. [DOI] [PubMed] [Google Scholar]
- 106.Ananyeva L, Desinova O, Starovoytova M, et al. Rituximab for the treatment of systemic sclerosis associated interstitial lung disease: a case series. Ann Rheum Dis 2013; 72: Suppl. 3, 648. [Google Scholar]
- 107.Ando K, Motojima S, Doi T, et al. Effect of glucocorticoid monotherapy on pulmonary function and survival in Japanese patients with scleroderma-related interstitial lung disease. Respir Investig 2013; 51: 69–75. [DOI] [PubMed] [Google Scholar]
- 108.Burt RK, Oliveira MC, Shah SJ, et al. Cardiac involvement and treatment-related mortality after non-myeloablative haemopoietic stem-cell transplantation with unselected autologous peripheral blood for patients with systemic sclerosis: a retrospective analysis. Lancet 2013; 381: 1116–1124. [DOI] [PubMed] [Google Scholar]
- 109.Celeste S, Santaniello A, Caronni M, et al. Carbohydrate antigen 15.3 as a serum biomarker of interstitial lung disease in systemic sclerosis patients. Eur J Intern Med 2013; 24: 671–676. [DOI] [PubMed] [Google Scholar]
- 110.De Lauretis A, Sestini P, Pantelidis P, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol 2013; 40: 435–446. [DOI] [PubMed] [Google Scholar]
- 111.Elhaj M, Charles J, Pedroza C, et al. Can serum surfactant protein D or CC-chemokine ligand 18 predict outcome of interstitial lung disease in patients with early systemic sclerosis? J Rheumatol 2013; 40: 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Enghelmayer JI, Giovini V, Altube S, et al. Does the immunosuppressive therapy modify the pulmonary function parameters in interstitial lung disease (ILD) associated with scleroderma: our experience. Am J Respir Crit Care Med 2013; 187: A2919. [Google Scholar]
- 113.Koneva O, Ovsjannikova O, Ananjeva L, et al. Isolated reduction of diffusing lung capacity in patients with systemic sclerosis (SSC): evaluation covering a 5 year period. Ann Rheum Dis 2013; 72: Suppl. 3, A651–A652. [Google Scholar]
- 114.Liu X, Mayes MD, Pedroza C, et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis? Arthritis Care Res (Hoboken) 2013; 65: 1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Panopoulos ST, Bournia VK, Trakada G, et al. Mycophenolate versus cyclophosphamide for progressive interstitial lung disease associated with systemic sclerosis: a 2-year case control study. Lung 2013; 191: 483–489. [DOI] [PubMed] [Google Scholar]
- 116.Radic M, Becker MO, Adler S, et al. Cytokines in bronchoalveolar lavage fluid and serum predict deterioration of lung function and mortality in systemic sclerosis patients. Ann Rheum Dis 2013; 72: Suppl. 3, A501. [Google Scholar]
- 117.Stock CJ, Sato H, Fonseca C, et al. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax 2013; 68: 436–441. [DOI] [PubMed] [Google Scholar]
- 118.Vacca A, Garau P, Porru G, et al. Safety and efficacy of oral cyclophosphamide long-term therapy in systemic sclerosis: experience of a single-centre. Ann Rheum Dis 2013; 72: Suppl. 3, A503. [Google Scholar]
- 119.Wu M, Pedroza C, Salazar G, et al. Plasma MCP-1 and IL-10 levels predict long-term progression of interstitial lung disease in patients with early systemic sclerosis. Arthritis Rheum 2013; 65: Suppl. 10, S742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang XJ, Bonner A, Hudson M, et al. Association of gastroesophageal factors and worsening of forced vital capacity in systemic sclerosis. J Rheumatol 2013; 40: 850–858. [DOI] [PubMed] [Google Scholar]
- 121.Ananyeva L, Ovsyannikova O, Lesnyak V, et al. Slow progressive interstitial lung disease associated with systemic sclerosis: distinct disease phenotype? Ann Rheum Dis 2014; 73: Suppl. 2, 1013. [Google Scholar]
- 122.Chakr R, Gasparin A, Neves M, et al. Long-term effectiveness and safety of cyclophosphamide in systemic sclerosis lung disease. Ann Rheum Dis 2014; 73: Suppl. 2, 565–566. [Google Scholar]
- 123.Christmann RB, Sampaio-Barros P, Stifano G, et al. Association of interferon- and transforming growth factor β-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol 2014; 66: 714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cottrell TR, Wise RA, Wigley FM, et al. The degree of skin involvement identifies distinct lung disease outcomes and survival in systemic sclerosis. Ann Rheum Dis 2014; 73: 1060–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fraticelli P, Gabrielli B, Pomponio G, et al. Low-dose oral imatinib in the treatment of systemic sclerosis interstitial lung disease unresponsive to cyclophosphamide: a phase II pilot study. Arthritis Res Ther 2014; 16: R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guillen-Del Castillo A, Pilar Simeon-Aznar C, Fonollosa-Pla V, et al. Good outcome of interstitial lung disease in patients with scleroderma associated to anti-PM/Scl antibody. Semin Arthritis Rheum 2014; 44: 331–337. [DOI] [PubMed] [Google Scholar]
- 127.Hoffmann-Vold AM, Midtvedt O, Garen T, et al. Moderate decline in forced vital capacity is associated with a poor outcome in systemic sclerosis patients. Arthritis Rheumatol 2014; 66: Suppl. 10, S316–S317. [Google Scholar]
- 128.Kumanovics G, Gorbe E, Minier T, et al. Follow-up of serum KL-6 lung fibrosis biomarker levels in 173 patients with systemic sclerosis. Clin Exp Rheumatol 2014; 32: 6 Suppl. 86, S138–S144. [PubMed] [Google Scholar]
- 129.Kwon HM, Kang EH, Go DJ, et al. Efficacy and safety of long term cyclophosphamide treatment for interstitial lung disease in systemic sclerosis. Ann Rheum Dis 2014; 73: Suppl. 2, 1011. [Google Scholar]
- 130.Lambrecht S, Smith V, De Wilde K, et al. Growth differentiation factor 15, a marker of lung involvement in systemic sclerosis, is involved in fibrosis development but is not indispensable for fibrosis development. Arthritis Rheumatol 2014; 66: 418–427. [DOI] [PubMed] [Google Scholar]
- 131.Le Gouellec N, Duhamel A, Faivre JB, et al. Prognostic factors of functional outcome in systemic sclerosis-associated interstitial lung disease. Ann Rheum Dis 2014; 73: Suppl. 2, 1008–1009. [Google Scholar]
- 132.Narvaez J, Sancho JJA, Castellvi I, et al. Long-term efficacy of rituximab in systemic sclerosis. Arthritis Rheumatol 2014; 66: Suppl. 10, S737. [Google Scholar]
- 133.Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014; 66: 1625–1635. [DOI] [PubMed] [Google Scholar]
- 134.Parida J, Nath A, Neyaz Z, et al. A double blind randomized control trial of oral tadalafil in interstitial lung disease of scleroderma. Arthritis Rheumatol 2014; 66: Suppl. 10, S739. [Google Scholar]
- 135.Pham M, Griffing WL. Effects of mycophenolate mofetil on pulmonary lung function in interstitial lung disease of systemic sclerosis. Arthritis Rheumatol 2014; 66: Suppl. 10, S742. [Google Scholar]
- 136.Poormoghim H, Rezaei N, Sheidaie Z, et al. Systemic sclerosis: comparison of efficacy of oral cyclophosphamide and azathioprine on skin score and pulmonary involvement–a retrospective study. Rheumatol Int 2014; 34: 1691–1699. [DOI] [PubMed] [Google Scholar]
- 137.Rotondo C, Praino E, Lanciano E, et al. Residual volume: a candidate as early marker of interstitial lung disease in systemic sclerosis patients? Clin Exp Rheumatol 2014; 32: S83. [Google Scholar]
- 138.Ariani A, Silva M, Parisi S, et al. Can quantitative chest CT predict interstitial lung disease worsening in systemic sclerosis? Results from a multi-centre prospective cohort study. Ann Rheum Dis 2015; 74: Suppl. 2, 587–588.24326007 [Google Scholar]
- 139.Balbir-Gurman A, Yigla M, Guralnik L, et al. Long-term follow-up of patients with scleroderma interstitial lung disease treated with intravenous cyclophosphamide pulse therapy: a single-center experience. Isr Med Assoc J 2015; 17: 150–156. [PubMed] [Google Scholar]
- 140.Bosello SL, De Luca G, Rucco M, et al. Long-term efficacy of B cell depletion therapy on lung and skin involvement in diffuse systemic sclerosis. Semin Arthritis Rheum 2015; 44: 428–436. [DOI] [PubMed] [Google Scholar]
- 141.De Luca G, Bosello SL, Berardi G, et al. Tumour-associated antigens in systemic sclerosis patients with interstitial lung disease: association with lung involvement and cancer risk. Rheumatology (Oxford) 2015; 54: 1991–1999. [DOI] [PubMed] [Google Scholar]
- 142.Hoffmann-Vold AM, Aalokken TM, Lund MB, et al. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol 2015; 67: 2205–2212. [DOI] [PubMed] [Google Scholar]
- 143.Iudici M, Cuomo G, Vettori S, et al. Low-dose pulse cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSc-ILD): efficacy of maintenance immunosuppression in responders and non-responders. Semin Arthritis Rheum 2015; 44: 437–444. [DOI] [PubMed] [Google Scholar]
- 144.Jordan S, Distler JHW, Maurer B, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis 2015; 74: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 145.Khanna D, Albera C, Fischer A, et al. Safety and tolerability of pirfenidone in patients with systemic sclerosis interstitial lung disease. Arthritis Rheumatol 2015; 67: Suppl. 10, 816. [Google Scholar]
- 146.Khanna D, Nagaraja V, Tseng CH, et al. Predictors of lung function decline in scleroderma-related interstitial lung disease based on high-resolution computed tomography: implications for cohort enrichment in systemic sclerosis-associated interstitial lung disease trials. Arthritis Res Ther 2015; 17: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koneva O, Desinova O, Ovsyannikova O, et al. Impact of anti-B-cell therapy with rituximab on pulmonary function of the patients with systemic sclerosis and interstitial lung disease. Ann Rheum Dis 2015; 74: Suppl. 2, 603–604.24326009 [Google Scholar]
- 148.Lepri G, Avouac J, Airo P, et al. Mid-term effects of rituximab in connective tissue disorders related interstitial lung disease (ILD). Ann Rheum Dis 2015; 74: Suppl. 2, 1131. [PubMed] [Google Scholar]
- 149.Man A, Davidyock T, Ferguson LT, et al. Changes in forced vital capacity over time in systemic sclerosis: application of group-based trajectory modelling. Rheumatology (Oxford) 2015; 54: 1464–1471. [DOI] [PubMed] [Google Scholar]
- 150.Mani M, Sriram S, Saranya S, et al. Rituximab in systemic sclerosis with ILD-two year outcome from a tertiary care hospital in South India. Int J Rheum Dis 2015; 18: 114. [Google Scholar]
- 151.Mateos-Toledo H, Sarmiento-Padilla G, Mejia M, et al. Functional follow-up of diffuse interstitial lung disease (ILD) in phenotypes of systemic sclerosis (SSC). Am J Respir Crit Care Med 2015; 191: A1158. [Google Scholar]
- 152.Narvaez J, Heredia S, Panos HB, et al. Is the presence of esophageal dilation a poor prognostic factor in dilated interstitial lung disease associated with systemic sclerosis? Arthritis Rheumatol 2015; 67: Suppl. 10, 2267–2268. [Google Scholar]
- 153.Ninaber MK, Stoilk J, Smit J, et al. Lung structure and function relation in systemic sclerosis: application of lung densitometry. Eur J Radiol 2015; 84: 975–979. [DOI] [PubMed] [Google Scholar]
- 154.Radic M, Becker MO, Distler O, et al. Does angiotensin and endothelin receptor blockade have an impact on lung function? An analysis from the EUSTAR data base. Ann Rheum Dis 2015; 74: Suppl. 2, 817. [PubMed] [Google Scholar]
- 155.Sakamoto N, Kakugawa T, Hara A, et al. Association of elevated α-defensin levels with interstitial pneumonia in patients with systemic sclerosis. Respir Res 2015; 16: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saketkoo LA, Lammi MR, Fischer A, et al. Mycophenolate mofetil (MMF) use in scleroderma patients with pulmonary hypertension: FVC, outcomes and survival-observations from the Pulmonary Hypertension Recognition and Outcomes in Scleroderma (PHAROS) cohort. Ann Rheum Dis 2015; 74: Suppl. 2, 820. [Google Scholar]
- 157.Schulam P, Ligon C, Wise R, et al. A computational tool for individualized prognosis of percent of predicted forced vital capacity trajectories in systemic sclerosis. Arthritis Rheumatol 2015; 67: Suppl. 10, 1123–1124. [Google Scholar]
- 158.Shirai Y, Takeuchi T, Kuwana M. Clinical utility of serial KL-6 measurement in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2015; 67: Suppl. 10, 115. [Google Scholar]
- 159.Suliman YA, Dobrota R, Huscher D, et al. Pulmonary function tests: high rate of false-negative results in the early detection and screening of scleroderma-related interstitial lung disease. Arthritis Rheumatol 2015; 67: 3256–3261. [DOI] [PubMed] [Google Scholar]
- 160.Tanaseanu CM, Tiglea IA, Marta DS, et al. Lactate dehydrogenase a possible marker of progressive microvasculopathy and interstitial lung disease in systemic sclerosis. Acta Med Mediterr 2015; 31: 941–946. [Google Scholar]
- 161.Tashkin D, Roth M, Clements P, et al. Efficacy and safety of mycophenolate (MMF) vs oral cyclophosphamide (CYC) for treatment of scleroderma-interstitial lung disease (SscILD): results of scleroderma lung study II. Chest 2015; 148: 637A–637B. [Google Scholar]
- 162.Volkmann E, Tashkin D, Li N, et al. Development of a composite outcome measure for systemic sclerosis related interstitial lung disease. Rheumatology (Sunnyvale) 2015; 5: 1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Volkmann ER, Tashkin DP, Roth M, et al. CXCL4 does not predict extent or progression of interstitial lung disease in systemic sclerosis. Arthritis Rheumatol 2015; 67: Suppl. 10, 1406–1407. [Google Scholar]
- 164.Wallace B, Kafaja S, Furst DE, et al. Reliability, validity and responsiveness to change of the Saint George's Respiratory Questionnaire in early diffuse cutaneous systemic sclerosis. Rheumatology (Oxford) 2015; 54: 1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fava A, Cimbro R, Wigley FM, et al. Frequency of circulating topoisomerase-I-specific CD4 T cells predicts presence and progression of interstitial lung disease in scleroderma. Arthritis Res Ther 2016; 18: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hoffmann-Vold AM, Tennoe AH, Garen T, et al. High level of chemokine CCL18 is associated with pulmonary function deterioration, lung fibrosis progression and reduced survival in systemic sclerosis. Chest 2016; 150: 299–306. [DOI] [PubMed] [Google Scholar]
- 167.Kloth C, Maximilian Thaiss W, Preibsch H, et al. Quantitative chest computed tomography analysis in patients with systemic sclerosis before and after autologous stem cell transplantation: comparison of results with those of pulmonary function tests and clinical tests. Rheumatology (Oxford) 2016; 55: 1763–1770. [DOI] [PubMed] [Google Scholar]
- 168.Owen C, Ngian GS, Elford K, et al. Mycophenolate mofetil is an effective and safe option for the management of systemic sclerosis-associated interstitial lung disease: results from the Australian Scleroderma Cohort Study. Clin Exp Rheumatol 2016; 34: 170–176. [PubMed] [Google Scholar]
- 169.Shenoy PD, Bavaliya M, Sashidharan S, et al. Cyclophosphamide versus mycophenolate mofetil in scleroderma interstitial lung disease (SSc-ILD) as induction therapy: a single-centre, retrospective analysis. Arthritis Res Ther 2016; 18: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.U.S. National Library of Medicine (ClinicalTrials.gov) . Open-label study with bosentan in interstitial lung disease (BUILD 2 OL). http://clinicaltrials.gov/show/NCT00319033 Date last updated: April 28, 2015. Date last accessed: February 23, 2017.
- 171.EU Clinical Trials Register . Systemic sclerosis associated interstitial lung disease: a longitudinal observational study assessing optimum treatment regimens. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2008-000224-27 Date last accessed: February 23, 2017.
- 172.U.S. National Library of Medicine (ClinicalTrials.gov) . Intravenous cyclophosphamide for the treatment of systemic sclerosis associated interstitial lung disease. https://clinicaltrials.gov/show/NCT01570764 Date last updated: September 14, 2016. Date last accessed: February 23, 2017.
- 173.U.S. National Library of Medicine (ClinicalTrials.gov) . Study of pomalidomide (CC-4047) to evaluate safety, tolerability, pharmacokinetics, pharmacodynamics and effectiveness for subjects with systemic sclerosis with interstitial lung disease. https://clinicaltrials.gov/show/NCT01559129 Date last updated: April 05, 2017. Date last accessed: February 23, 2017.
- 174.U.S. National Library of Medicine (ClinicalTrials.gov) . Treatment and prevention of progression of interstitial lung disease in systemic sclerosis. http://clinicaltrials.gov/show/NCT01858259 Date last updated: August 12, 2014. Date last accessed: February 24, 2017.
- 175.U.S. National Library of Medicine (ClinicalTrials.gov) . A trial to compare nintedanib with placebo for patients with scleroderma related lung fibrosis. https://clinicaltrials.gov/show/NCT02597933 Date last updated: August 21, 2017. Date last accessed: February 23, 2017.
- 176.U.S. National Library of Medicine (ClinicalTrials.gov) . A double-blinded study to evaluate the safety, tolerability, and efficacy of BMS-986020 versus placebo in diffuse cutaneous systemic sclerosis (dcSSc). https://clinicaltrials.gov/show/NCT02588625 Date last updated: July 20, 2016. Date last accessed: February 23, 2017.
- 177.U.S. National Library of Medicine (ClinicalTrials.gov) . Comparing and combining bortezomib and mycophenolate in SSc pulmonary fibrosis. https://clinicaltrials.gov/show/NCT02370693 Date last updated: January 20, 2017. Date last accessed: February 24, 2017.
- 178.U.S. National Library of Medicine (ClinicalTrials.gov) . Abituzumab in SSc-ILD. https://clinicaltrials.gov/show/NCT02745145 Date last updated: July 27, 2017. Date last accessed: February 24, 2017.
- 179.Harrison NK, Myers AR, Corrin B, et al. Structural features of interstitial lung disease in systemic sclerosis. Am Rev Respir Dis 1991; 144: 706–713. [DOI] [PubMed] [Google Scholar]
- 180.Wells AU, Hansell DM, Rubens MB, et al. Fibrosing alveolitis in systemic sclerosis: indices of lung function in relation to extent of disease on computed tomography. Arthritis Rheum 1997; 40: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 181.Wells AU, Rubens MB, Du Bois RM, et al. Functional impairment in fibrosing alveolitis: relationship to reversible disease on thin section computed tomography. Eur Respir J 1997; 10: 280–285. [DOI] [PubMed] [Google Scholar]
- 182.Diot E, Boissinot E, Asquier E, et al. Relationship between abnormalities on high-resolution CT and pulmonary function in systemic sclerosis. Chest 1998; 114: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 183.Kim EA, Johkoh T, Lee KS, et al. Interstitial pneumonia in progressive systemic sclerosis: serial high-resolution CT findings with functional correlation. J Comput Assist Tomogr 2001; 25: 757–763. [DOI] [PubMed] [Google Scholar]
- 184.Shahin AA, Sabri YY, Mostafa HA, et al. Pulmonary function tests, high-resolution computerized tomography, α1-antitrypsin measurement, and early detection of pulmonary involvement in patients with systemic sclerosis. Rheumatol Int 2001; 20: 95–100. [DOI] [PubMed] [Google Scholar]
- 185.Han S, Kim S, Kang Y. DLCO as a predictor of pulmonary fibrosis on high resolution CT in systemic sclerosis. Ann Rheum Dis 2003; 62: 230. [Google Scholar]
- 186.Ooi GC, Mok MY, Tsang KW, et al. Interstitial lung disease in systemic sclerosis. Acta Radiol 2003; 44: 258–264. [DOI] [PubMed] [Google Scholar]
- 187.Orlandi I, Camiciottoli G, Diciotti S, et al. Thin-section and low-dose volumetric computed tomographic densitometry of the lung in systemic sclerosis. J Comput Assist Tomogr 2006; 30: 823–827. [DOI] [PubMed] [Google Scholar]
- 188.Beretta L, Santaniello A, Lemos A, et al. Validity of the Saint George's Respiratory Questionnaire in the evaluation of the health-related quality of life in patients with interstitial lung disease secondary to systemic sclerosis. Rheumatology (Oxford) 2007; 46: 296–301. [DOI] [PubMed] [Google Scholar]
- 189.Camiciottoli G, Orlandi I, Bartolucci M, et al. Lung CT densitometry in systemic sclerosis: correlation with lung function, exercise testing, and quality of life. Chest 2007; 131: 672–681. [DOI] [PubMed] [Google Scholar]
- 190.Goldin JG, Lynch DA, Strollo DC, et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 2008; 134: 358–367. [DOI] [PubMed] [Google Scholar]
- 191.Bellia M, Cannizzaro F, Scichilone N, et al. HRCT and scleroderma: semiquantitative evaluation of lung damage and functional abnormalities. Radiol Med 2009; 114: 190–203. [DOI] [PubMed] [Google Scholar]
- 192.Goldin J, Elashoff R, Kim HJ, et al. Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: findings from the scleroderma lung study. Chest 2009; 136: 1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vonk MC, Broers B, Heijdra YF, et al. Systemic sclerosis and its pulmonary complications in The Netherlands: an epidemiological study. Ann Rheum Dis 2009; 68: 961–965. [DOI] [PubMed] [Google Scholar]
- 194.Ananyeva L, Teplova LV, Lesnyak VN, et al. Relationship between computed tomography and lung function parameters in patients with different duration of systemic sclerosis. Clin Exp Rheumatol 2010; 28: S151.21044450 [Google Scholar]
- 195.Peng M, Xu W, Wang Q, et al. Pulmonary involvement in Chinese patients with SSc and correlations between the imaging and functional abnormalities. Clin Exp Rheumatol 2010; 28: S150. [Google Scholar]
- 196.Kim H, Brown M, Abtin F, et al. Association of texture-based quantitative fibrotic patterns and pulmonary function test in a new validation set. Eur Respir J 2011; 38: 1440. [Google Scholar]
- 197.Kim HJ, Brown MS, Elashoff R, et al. Quantitative texture-based assessment of one-year changes in fibrotic reticular patterns on HRCT in scleroderma lung disease treated with oral cyclophosphamide. Eur Radiol 2011; 21: 2455–2465. [DOI] [PubMed] [Google Scholar]
- 198.Moghadam KG, Gharibdoost F, Parastandechehr G, et al. Assessments of pulmonary involvement in patients with systemic sclerosis. Arch Iran Med 2011; 14: 22–26. [PubMed] [Google Scholar]
- 199.Parra ER, Oliveira e. Silva L, Rangel M, et al. Increase alpha-smooth muscle actin, telomerase, interleukin-4 and endothelin-1 expression in progressive pulmonary fibrosis of systemic sclerosis (SSc). Virchows Archiv 2011; 459: S144–S145. [Google Scholar]
- 200.Assayag D, Hudson M, Tatibouet S, et al. Clinical correlates of lung scan abnormalities in patients with systemic sclerosis-associated interstitial lung disease. Am J Respir Crit Care Med 2012; 185: A6616. [Google Scholar]
- 201.Mantero M, Aliberti S, Erba G, et al. Cytokines panel in exhaled breath condensate in SSc. Rheumatology (Oxford) 2012; 51: ii69. [Google Scholar]
- 202.Mittal S, Kumar U, Guleria R, et al. HRCT chest score and bronchoalveolar lavage fluid cytology in assessment of disease activity of systemic sclerosis associated interstitial lung disease. Indian J Rheumatol 2012; 7: S5. [Google Scholar]
- 203.Pernot J, Puzenat E, Magy-Bertrand N, et al. Detection of interstitial lung disease in systemic sclerosis through partitioning of lung transfer for carbon monoxide. Respiration 2012; 84: 461–468. [DOI] [PubMed] [Google Scholar]
- 204.Perrin F, Chambellan A, Hardouin JB, et al. Membrane diffusion and capillary blood volume measurements in patients with SSc. Rheumatology (Oxford) 2012; 51: ii71–ii72. [Google Scholar]
- 205.Wilsher M, Good N, Hopkins R, et al. The six-minute walk test using forehead oximetry is reliable in the assessment of scleroderma lung disease. Respirology 2012; 17: 647–652. [DOI] [PubMed] [Google Scholar]
- 206.Zimmermann A, Pizzichini E, Nobre LF, et al. Semiquantitative evaluation of high resolution CT in SSc: preliminary applicability of a simple score in clinical practice. Rheumatology (Oxford) 2012; 51: ii46–ii47. [Google Scholar]
- 207.Gatta G, Di Grezia G, Iacomino A, et al. HRCT in systemic sclerosis: correlation between respiratory functional indexes and extension of lung failure. J Biol Regul Homeost Agents 2013; 27: 579–587. [PubMed] [Google Scholar]
- 208.Nguyen-Kim TDL, Maurer Bm Suliman YA, Morsbach F, et al. Histogram-based quantification of fibrosis in systemic sclerosis on sequential low dose HRCT of 9 CT slices. J Thorac Imaging 2013; 28: W74–W75. [Google Scholar]
- 209.Piorunek T, Kuznar-Kaminska B, Cofta S, et al. Lung impairment in scleroderma. In: Pokorski M, ed. Respiratory regulation–clinical advances. New York, Springer, 2013; pp. 149–154. [DOI] [PubMed] [Google Scholar]
- 210.Zamora FD, Kim HJ, Wang Q. Prevalence of pulmonary function test abnormalities and their correlation to high resolution computer tomography in a large scleroderma population. Am J Respir Crit Care Med 2013; 187: A2920. [Google Scholar]
- 211.Colaci M, Sebastiani M, Manfredi A, et al. Lung involvement in systemic sclerosis: role of high resolution computed tomography and its relationship with other pulmonary and clinico-serological features. J Biol Regul Homeost Agents 2014; 28: 481–488. [PubMed] [Google Scholar]
- 212.Ariani A, Silva M, Bravi E, et al. Operator-independent quantitative chest computed tomography versus standard assessment of interstitial lung disease related to systemic sclerosis: a multi-centric study. Mod Rheumatol 2015; 25: 724–730. [DOI] [PubMed] [Google Scholar]
- 213.Bernstein EJ, Berrocal VJ, Steen VD, et al. The predictive value of pulmonary function tests to diagnose interstitial lung disease in adults with early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol 2015; 67: Suppl. 10, 2268–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ghandour AM, Gamal RM, Seifeldein GS, et al. MDCT staging of disease extent in patients with systemic sclerosis: a pilot study. Ann Rheum Dis 2015; 74: Suppl. 2, 601–602. [Google Scholar]
- 215.Guarnieri G, Zanatta E, Mason P, et al. Determinants of impairment in lung diffusing capacity in patients with systemic sclerosis. Clin Exp Rheumatol 2015; 33: 4 Suppl. 91, S80–S86. [PubMed] [Google Scholar]
- 216.Kim HJG, Tashkin DP, Brown MS, et al. Systemic sclerosis interstitial lung disease evaluation: comparison between two quantitative computed tomography for the change assessments. Am J Respir Crit Care Med 2015; 191: A1161. [Google Scholar]
- 217.Salaffi F, Carotti M, Bosello S, et al. Computer-aided quantification of interstitial lung disease from high resolution computed tomography images in systemic sclerosis: correlation with visual reader-based score and physiologic tests. Biomed Res Int 2015: 834262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Antoniou KM, Margaritopoulos GA, Goh NS, et al. Combined pulmonary fibrosis and emphysema in scleroderma-related lung disease has a major confounding effect on lung physiology and screening for pulmonary hypertension. Arthritis Rheumatol 2016; 68: 1004–1012. [DOI] [PubMed] [Google Scholar]
- 219.Cetincakmak MG, Goya C, Hamidi C, et al. Quantitative volumetric assessment of pulmonary involvement in patients with systemic sclerosis. Quant Imaging Med Surg 2016; 6: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Salaffi F, Carotti M, Di Donato E, et al. Computer-aided tomographic analysis of interstitial lung disease (ILD) in patients with systemic sclerosis (SSc). Correlation with pulmonary physiologic tests and patient-centred measures of perceived dyspnea and functional disability. PLoS One 2016; 11: e0149240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tashkin DP, Volkmann ER, Tseng CH, et al. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann Rheum Dis 2016; 75: 374–381. [DOI] [PubMed] [Google Scholar]
- 222.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 223.Richeldi L, Du Bois R, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 224.Wells AU. Forced vital capacity as a primary end point in idiopathic pulmonary fibrosis treatment trials: making a silk purse from a sow's ear. Thorax 2013; 68: 309–310. [DOI] [PubMed] [Google Scholar]
- 225.Raghu G, Collard HR, Anstrom KJ, et al. Idiopathic pulmonary fibrosis: clinically meaningful primary endpoints in phase 3 clinical trials. Am J Respir Crit Care Med 2012; 185: 1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Herzog EL, Mathur A, Tager AM, et al. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct? Arthritis Rheumatol 2014; 66: 1967–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wells AU, Behr J, Silver R. Outcome measures in the lung. Rheumatology (Oxford) 2008; 47: Suppl. 5, v48–v50. [DOI] [PubMed] [Google Scholar]
- 228.Khanna D, Seibold JR, Wells A, et al. Systemic sclerosis-associated interstitial lung disease: lessons from clinical trials, outcome measures, and future study design. Curr Rheumatol Rev 2010; 6: 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kafaja S, Clements PJ, Wilhalme H, et al. Reliability and minimal clinically important differences of forced vital capacity: results from the Scleroderma Lung Studies (SLS-I and SLS-II). Am J Respir Crit Care Med 2018; 197: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Furst D, Khanna D, Matucci-Cerinic M, et al. Systemic sclerosis-continuing progress in developing clinical measures of response. J Rheumatol 2007; 34: 1194–1200. [PubMed] [Google Scholar]
- 231.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. [DOI] [PubMed] [Google Scholar]
- 232.Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016; 4: 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Solomon JJ, Olson AL, Fischer A, et al. Scleroderma lung disease. Eur Respir Rev 2013; 22: 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Khanna, D, Mittoo S, Aggarwal R, et al. Connective tissue disease-associated interstitial lung diseases (CTD-ILD)-report from OMERACT CTD-ILD working group. J Rheumatol 2015; 42: 2168–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Winstone TA, Assayag D, Wilcox PG, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest 2014; 146: 422–436. [DOI] [PubMed] [Google Scholar]
- 236.Moore OA, Proudman SM, Goh N, et al. Quantifying change in pulmonary function as a prognostic marker in systemic sclerosis-related interstitial lung disease. Clin Exp Rheumatol 2015; 33: Suppl. 91, S111–S116. [PubMed] [Google Scholar]
- 237.Goh NS, Hoyles RK, Denton CP, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017; 69: 1670–1678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary Material ERR-0102-2017_Supplementary_Material (1MB, pdf)