Abstract
Tyrosine kinase 2 (TYK2), a key part of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway, plays an integral role in the differentiation and immune responses of intrinsic immune cells and regulates the mediation of cytokines. TYK2 leads to inflammatory cascade responses in the pathogenesis of immune-mediated inflammatory diseases (IMIDs), especially psoriasis. Small-molecule TYK2 inhibitors are considered to be an effective strategy for modulating psoriasis. Here, we attempt to review the pro-inflammatory mechanisms of the JAK-STAT signaling pathway, the regulatory roles of TYK2 in the pathogenesis of psoriasis, and provide updates on ongoing and recently completed trials of TYK2 inhibitors.
Keywords: TYK2, psoriasis, Janus kinase-signal transducer and activator of transcription, autoimmunity, inhibition
Introduction
Psoriasis is a chronic, multifactorial, immune-mediated inflammatory skin disease with a global prevalence of approximately 2%, affecting approximately 125 million people worldwide.1,2 Psoriasis is characterized by persistent inflammation leading to uncontrolled proliferation and dysfunctional differentiation of keratinocytes.3,4 The pathogenesis of psoriasis has not been fully elucidated. It is considered that the interaction between innate and adaptive immunity mediated by inflammatory mediators has been the focus of research in recent years. Among them, the Janus kinase-signal transducer and activator of the transcription (JAK-STAT) pathway is involved at multiple levels of the IL-23/Th17 signaling pathway, which is thought to be the core of the pathogenesis.5–8 A member of the JAK family, Tyrosine kinase 2 (TYK2) transmits a variety of extracellular cytokine signals, which contribute to disorders of immune-mediated inflammatory diseases (IMIDs), leading to inflammatory cascade responses, followed by induction of keratinocyte proliferation and other distinguishing features of psoriasis.9–11
Current treatments for psoriasis include topical drugs, phototherapy, traditional systemic drugs, biological agents, and novel small-molecule drugs. Biological agents such as TNF-α inhibitors, IL-17 inhibitors, and IL-23 inhibitors have been used for the clinical treatment of psoriasis. However, due to the adverse events (AEs) intolerable in some patients, the decrease in long-term application effect, and the limitations of biological agents in drug administration,12 people have begun to pay attention to small-molecular drugs that can be administered orally or topically. JAK inhibitors can block the Janus signaling pathway and thus improve the clinical symptoms of psoriasis. This review attempts to provide an overview of the pro-inflammatory mechanisms of the JAK-STAT signaling pathway, the role of the TYK2 signaling pathway in immune responses, and also presents recent advances in the treatment of immune-mediated inflammatory diseases (IMIDs) with JAK inhibitors, focusing on the efficacy and safety of three TYK2 inhibitors (BMS-986165, PF-06700841, PF-06826647) in clinical trials.
JAK-STAT Signaling Pathway
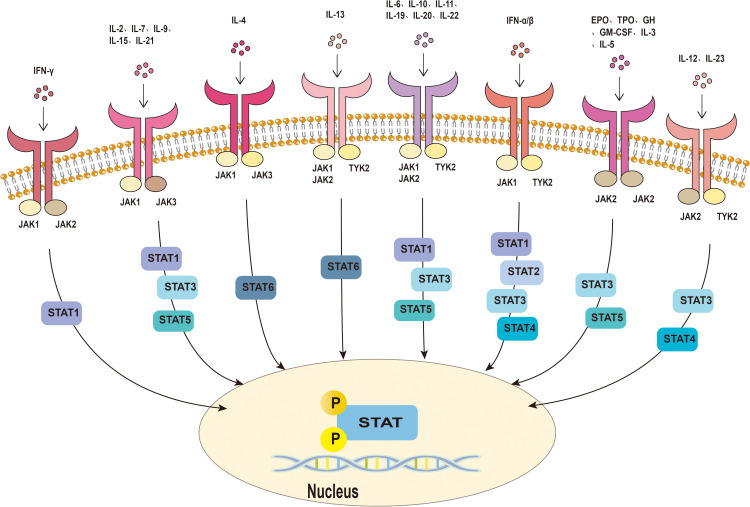
The members of the JAK-STAT family include four JAKs (JAK1, JAK2, JAK3, and TYK2), plus seven STAT proteins (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6).6,13 The JAK-STAT pathway mediates the signal transduction downstream of type I and type II cytokine receptors, including ILs, IFNs, and various growth factors.13,14 When the intracellular domain of the cytokine receptor binds to JAK, JAK is phosphorylated, which leads to the attachment of signal transmitter and transcription activator STAT protein. Subsequently, STAT becomes phosphorylated and dimerized. The dimerized STAT translocates into the nucleus of the cell to modify and regulate gene transcription and expression.15–17 Each member of the STAT family can be activated by multiple cytokines and their related JAKs8 (Figure 1).
Figure 1.
Janus kinase-signal transducer and activator of transcription pathway. Cytokines bind to receptors on the cell membrane, activating JAKs and phosphorylating them. This subsequently leads to phosphorylation and dimerization of STAT. Activated STAT dimers translocate to the nucleus and regulate gene transcription and expression, ultimately leading to changes in cellular function.
Abbreviations: JAK, Janus kinase; STAT, signal transducer and activator of transcription; TYK2, tyrosine kinase 2; IFN, interferon; IL, interleukin; EPO, erythropoietin; GH, growth hormone; TPO, thrombopoietin; GM-CSF, granulocyte macrophage colony-stimulating factor.
The pathological mechanisms of psoriasis include pathogenic T cell drive, the interaction between innate and adaptive immunity, and the “feed-forward” inflammatory loop of adaptive immunity.1,18 The activation of the Th17 cytokine pathway mediated by IL-23 is the key.12,19 IL-23 and many pathogenic factors of psoriasis mediate intracellular conduction through the JAK-STAT pathway, resulting in inflammatory cascade reactions, leading to the proliferation of epidermal cells and the recruitment of leukocyte subpopulation, and increased expression of vascular endothelial factors and endothelial adhesion molecules.5,20 Therefore, the JAK-STAT pathway is involved in many critical targets of regulating the pathogenesis of psoriasis, and the small-molecule JAK inhibitor is considered an effective treatment.
TYK2 Signaling Pathway in Psoriasis
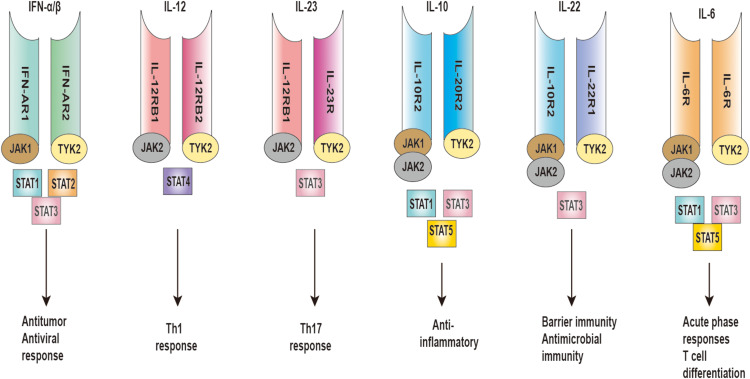
TYK2, a non-receptor tyrosine-protein kinase encoded by the TYK2 gene, induces intracellular signal transduction.21 TYK2 plays an integral role in the differentiation and immune responses of innate immunity cells, regulates the mediation of type I IFNs, IL-12, IL-23, IL-10, and IL-6, and participates in the regulation of multiple vital targets in the pathogenesis of psoriasis22–24 (Figure 2).
Figure 2.
TYK2 mediates IFN-α/β, IL-12, IL-23, IL-10, IL-22, and IL-6 signaling.
Abbreviations: TYK2, tyrosine kinase 2; IFN, interferon; IL, interleukin; JAK, Janus kinase; STAT, signal transducer and activator of transcription; Th, T-helper; R, receptor.
TYK2 and Innate Immunity
In psoriasis, the crosstalk between innate and adaptive immunity leads to the production of inflammatory mediators, causing the induction of inflammation with keratinocyte hyper-proliferation.25 DCs, NK cells, macrophages, and neutrophils produce TNF-α and IFN-γ.26 DC subsets present antigens to T cells and trigger up-regulation of inflammatory cytokines to be increased in psoriatic skin lesions.27
Adoptive transfer experiments showed that the generation of IFN-γ producing specific CD8+ T (Tc1) cells was impaired in the spleen of Tyk2−/− mice infected with Listeria monocytogenes. It was confirmed that Tyk2 signaling was important in inducing restricted CD8+ T (Tc1) cells.28 In DCs pre-infected with Dengue virus (DV), the antiviral response is antagonized by DV due to inhibition of TYK2 phosphorylation.29 It has been reported that in Tyk2−/− DCs, the ability to differentiate Th1 and Th17 cells are diminished, as well as the regulatory mechanisms of multiple cytokines and interferon production are affected.30,31 Therefore, we have reasons to believe that the immune responses of DCs against pathogens are partly dependent on the presence of TYK2, and TYK2 affects the tandem between DCs and T cells.
Current animal experiments confirmed that IFN-g production by NK cell activity induced by IL-12 and IL-18 were reduced in knockout mice for Tyk2. TYK2 promotes NK cell maturation and anti-cytotoxicity, which is also indispensable for immune defense against Listeria monocytogenes and Lactobacillus monocytogenes.32,33 Another study showed that NK cell immunity was not particularly affected in patients lacking TYK2. TYK2 deficiency does not affect IL-12-mediated activation of NK cells. This is contrary to the experimental results of animal trials. This may be due to the presence of two different antiviral defense mechanisms in NK cells that attenuate the viral susceptibility. However, the relevant mechanisms are still unclear.34 We still need to continue exploring the significance of TYK2 for NK cells, but at present, it seems that TYK2 may not be necessary for NK cells to perform functions such as immune defense and tumor surveillance in humans.
In macrophages, TYK2 mainly affects IFN signaling. TYK2-deficient macrophages exhibited loss of IFN-α, IFN-β or IFN-γ mediated responses and reduced gene expression. Furthermore, TYK2 is necessary for the activation of STAT1 and STAT2.35,36
TYK2 Mediates IFN-α/β Receptors
Different types of type I IFNs show different tissue expression and binding affinities for the heterodimeric receptor complex (IFNAR1/2) so that the different isoforms produce various outcomes in terms of antiviral, antiproliferative, and immunomodulatory activities.37 In psoriasis, type I IFNs promote significant inflammatory effects, including DCs maturation and activation, Th1 and Th17 polarization, reduced regulatory T cells (Treg) function, B cell activation, and subsequent increased antibody production.38
Different types of IFNs signals are emitted through different receptor complexes on the cell surface. IFN-α/β binds to receptor IFNAR1/2 complex and activates JAK1 and TYK2 to phosphorylate them, which in turn allowing reposition and phosphorylate STAT1, STAT2, and STAT3 proteins through Src homology 2 (SH2) domain interactions, and interacting with IFNs regulatory factor (IRF) to translocate to the nucleus.37 STAT1 homodimers bind to gamma-activated sequences (GASs) to induce pro-inflammatory genes. STAT3 homodimers indirectly inhibit the expression of pro-inflammatory genes, possibly by inducing unknown transcription repressors.39
TYK2 Mediates IL-12/IL-23 Receptors
IL-12 binds to IL-12Rb1 and IL-12Rb2 receptors to induce TYK2 and JAK2, resulting in the activation of STAT4.40 Then they bind to the STAT binding site in the IFN-g promoter to induce the transcription of the IFN-g gene. IL-12 promotes the differentiation of CD4+ T cells into Th1 cells, which produce IFN-g.41 The receptors of IL-23 consist of IL-12Rb1 and IL-23R, with STAT3 as their main STAT. IL-23 also activates STAT4, but to a lesser extent than IL-12.10,42 IL-23 is central to the value-added of innate immune Th17 cells, resulting in the secretion of IL-17, IL-22, and TNF-α by Th17 cells.40,41
Recent studies have demonstrated that TYK2 deficiency leads to specific impairment of immune responses pathways in mice. Reduced ability of IFN-α to induce gene expression, antiviral and immune responses, an inability of IL-12 to promote Th1 cell differentiation and IFN-g gene transcription, and an inability of IL-23 to stimulate the secretion of other inflammatory factors by Th17 cells, which subsequently leads to the absence of adaptive immune responses.43 The experiment also showed that the IL-6 and IL-10 responses of Tyk2−/− mice were not affected.43 The studies of TYK2 in human cytokine responses are still insufficient. A patient with TYK2 protein deficiency due to mutation of the TYK2 is related to the defects of IFN-α, IL-23, IL-12, IL-6, and IL-10 pathways.44,45 It is now considered that TYK2 protein plays a broader role in human cytokine pathways compared to mice. In another study, by establishing a mouse model of psoriasis-like skin inflammation induced by imiquimod (IMQ) and IL-23, the results showed that Tyk2−/− mice could eliminate the thickening of the ear margins due to epidermal hyperplasia caused by IMQ-induced inflammatory cell infiltration. Also, in the IL-23-induced mouse model, ear margin swelling was significantly reduced in Tyk2−/− mice compared to Tyk2+/+ mice. The absence of TYK2 could reduce the production of psoriasis-related cytokines and antibacterial peptides. IL-23 and IL-22 synergistically induce psoriasis skin inflammation through the TYK2 signaling pathway, leading to epidermal hyperplasia, infiltration of large numbers of leukocytes, and production of inflammatory factors.46
TYK2 Mediates Other Cytokines Receptors
IL-10 binds to IL-10R2 and IL-20R2 receptors to induce JAK1, JAK2, and TYK2, resulting in the activation of STAT1, STAT3, and also in STAT5.47 IL-22 belongs to the IL-10 cytokine family and mediates its action through a heterodimeric transmembrane receptor complex composed of IL-22R1 and IL-10R2, activating JAK1 and JAK2, and TYK2.48 Like other members of the IL-10 cytokine family, it mediates a common pathway of signaling through STAT3.47 In the mouse model of psoriasis-like disease, IL-22 deficient mice significantly reduced squamous skin injury, inflammatory cell infiltration, and the expression of chemokines.49–51
In addition, TYK2 is an important signaling sensor for IL-6.24 IL-6 binds to the receptor IL-6R on the cell surface to form a complex. Then the complex binds to the receptor subunit homodimer gp130 of IL-6, resulting in selective activation of JAK1, JAK2, and TYK2, which in turn promotes the recruitment and phosphorylation of STAT1, STAT3, and to a lesser extent STAT5.52–54 Ishizaki et al found various inflammatory cytokines, such as IL-6, IL-1b, and matrix metalloproteinases (MMPs), such as MMP3 and MMP9, were significantly reduced in Tyk2−/− mice.55
JAK Inhibitors Progress
In recent years, clinical trials of several oral JAK inhibitors for the treatment of psoriasis have been underway. First-generation JAK inhibitors, non-selective JAK inhibitors, include Tofacitinib, Baricitinib, and Ruxolitinib. Tofacitinib is a JAK1/3 inhibitor, which has been approved by the United States Food and Drug Administration (FDA) for ulcerative colitis, psoriatic arthritis, and rheumatoid arthritis.24,56,57,83,84 Baricitinib and Ruxolitinib are JAK1/2 inhibitors. A Phase 2 clinical trial of Baricitinib in patients with psoriasis is underway.58 Ruxolitinib has been approved by the FDA and the European Medicines Agency (EMA) to treat myelodysplasia. In psoriasis, its use has only been tested in topical cream, and clinical trials of related oral drugs are still in progress.59 Among the first-generation JAK inhibitors, although their efficacy has been confirmed to some extent, the safety problems related to AEs still exist. For example, changes in laboratory parameters including neutrophils, platelets, and creatinine, increased probability of herpes zoster, and gastric perforation.58,60–64 The mechanisms of many AEs are still unclear, but some of them are due to the inhibition of related cytokine transduction pathways by non-selective JAK inhibitors. Hyperlipidemia, neutropenia, and elevated liver enzymes are thought to be due to the inhibition of IL-6 signaling by JAK1/2 inhibitors. The reduction in platelet, hemoglobin, and reticulocyte counts is due to the inhibition of the EPO-JAK2 pathway by JAK2 inhibitors.15,65
The second-generation JAK inhibitors are highly selective for related JAKs. Theoretically, different cytokine receptor signal through different JAKs. Selective blockade of one JAK with a JAK inhibitor thus inhibits the function transduced by specific cytokines while allowing other irrelevant cytokines to continue to signal normally. Among them, TYK2 selective inhibitors have attracted much attention in recent years.
TYK2 Inhibitors Progress
A genome-wide association study (GWAS) in 2016 demonstrated that TYK2 transduced signals downstream of type I IFNs, gp130, IL-10R2, IL-13Ra, and IL-12Rb1 cytokine receptors, thus TYK2 gene is associated with several autoimmune diseases. As the pathogenesis of psoriasis is related to the cytokine signal transduction pathway, the deletion of the TYK2 gene locus is related to the susceptibility of psoriasis.66 Although IL-17 does not activate the JAK-STAT pathway, TYK2 inhibitors can indirectly suppress IL-23/IL-17 signaling by inhibiting other STAT-dependent cytokines, namely IL-23.67 Meanwhile, TYK2 inhibitors lead to decreased IL-12 and type I IFNs signaling, improving inflammatory cell infiltration, epidermal proliferation, and thus psoriasis symptoms.68 The latest clinical trial progress of TYK2 inhibitors will be described below (Table 1).
Table 1.
Clinical Trials of TYK2 Inhibitors
| Drug | Target | Clinical Trial | Research Phase | Description | Primary Endpoint | Key Results |
|---|---|---|---|---|---|---|
| PF-06700841 | JAK1/TYK2 | NCT02310750 | 1 | 30 patients with psoriasis received PF-06700841 30 or 100 mg or placebo QD for 28 days. | __ | Change from baseline in PASI for 30 mg and 100 mg QD were −67.92% and −96.31%, respectively in week 4. |
| NCT02969018 | 2a | 212 Patients were randomized to PF-06700841 30 mg QD, 60 mg QD, or placebo (4-week induction), then 10 mg QD, 30 mg QD, 100 mg QW, or placebo (8-week maintenance). | PASI score at week 12 | Achieving PASI 75 and PASI 90 was the highest in the 30-mg QD(86.2% and 51.7%) for 12 week. | ||
| NCT03850483 (topical) | 2b | 240 patients received PF-06700841 cream 0.1% QD,0.3% QD,1% QD,3% QD,0.3% BID,1% BID,3% BID or placebo. | PASI score at week 12 | __ | ||
| NCT03963401 | 2b | 219 patients with active PsA received PF-06700841 30mg QD, 60mg QD for 52 weeks or 10 mg QD for 16 weeks, followed by 30mg,60mg QD until 52 weeks or placebo. | ACR20 response at week 16 | __ | ||
| PF-06826647 | TYK2 | NCT03895372 | 2 | 178 patients with moderate-to-severe psoriasis received PF-06826647 50mg QD, 100mg QD, 200mg QD,400mg QD or placebo for 16 weeks. | PASI 90 at week 16 | A greater proportion of patients achieved PASI90 in the 200mg and 400mg groups(33.0% and 46.5%, respectively) versus placebo at week 16._ |
| BMS-986165 | TYK2 | NCT02931838 | 2 | 267 patients with psoriasis received BMS-986165 3mg every other day, 3mg QD,3mg BID,6mg BID,12mg QD or placebo for 12 weeks. | PASI 75 at week 12 | BMS-986165 at doses of 3 mg daily and higher resulted in greater PASI75 than placebo for 12 weeks. PASI score was 9% (3mg every other day),39%(3mg QD), 9% (3mg BID),67%(6mg BID),75%(12mg QD),7%(placebo). |
| NCT03881059 | 2 | 203 patients with PSA received BMS-986165 6mg QD,12mg QD, or placebo for 16 weeks. | ACR20 response at week 16 | BMS-986165 at doses of 6 mg and 12mg QD were more likely to achieve ACR20 response at week 16 compared with placebo. ACR20 response was 52.9%(6 mg QD) and 62.7%(12 mg QD). | ||
| NCT03624127 | 3 | 666 patients with psoriasis received BMS-986165 6mgQD or Apremilast 30mg BID or placebo for 52 weeks. | sPGA 0/1 at week 16 PASI75 at week 16 | BMS-986165 groups resulted in higher response rates versus Apremilast or placebo for PASI75 (58.4% vs 35.1% vs 12.7%); and sPGA 0/1 (53.6% vs 32.1% vs 7.2%). | ||
| NCT03611751 | 3 | 1020 patients with psoriasis received BMS-986165 6mgQD or Apremilast 30mg BID or placebo for 16 weeks. | sPGA 0/1 at week 16 PASI75 at week 16 | __ | ||
| NCT04167462 | 3 | 220 patients with psoriasis received BMS-986165. | sPGA 0/1 at week 16 PASI75 at week 16 | __ | ||
| NCT03924427 | 3 | 74 patients with psoriasis received BMS-986165 for 16 weeks. | sPGA 0/1 at week 16 PASI75 at week 16 | __ | ||
| NCT04036435 | 3 | 1470 patients with psoriasis received BMS-986165. | Incidence of AEs up to 244 weeks. | __ |
Abbreviations: PASI, Psoriasis Area and Severity Index; PASI75, 75% improvement from baseline PASI; ACR, American College of Rheumatology score; sPGA, static Physician’s Global Assessment; AEs, Adverse events; PsA, psoriatic arthritis.
Bms-986165
BMS-986165 is an emerging, highly selective, and potent oral TYK2 inhibitor that binds to the residual ATP-binding site of the TYK2 pseudokinase domain, also known as the JH2 domain, via an allosteric mechanism rather than to the highly conserved ATP-binding sites in the catalytic domain. This mode of binding, which differs from that of JAK1/2/3 inhibitors, is associated with the high selectivity of BMS-986165.9,69
In a phase 2 clinical trial, adults with moderate to severe plaque psoriasis were randomized into six groups. Patients received oral medication at 3 mg every other day, 3 mg once daily (QD), 3 mg twice daily (BID), 6 mg BID, 12 mg QD, or placebo. The results showed that, in the 12th week, the proportion of patients who reached the 75% improvement from baseline Psoriasis Area and Severity Index (PASI75) in the above treatment groups were: 9%, 39%, 69%, 67%, 75%, and 7%. AEs occurred in 55–80% of patients in the active drug groups, with the highest incidence in the 6 mg BID group, where nasopharyngitis, headache, nausea, diarrhoea, and upper respiratory tract infections were more common.70 Meanwhile, the clinical efficacy of BMS-986165 is associated with a decrease in IL-23/Th17 and IFNs pathway markers.71 In addition, in the phase 2 clinical trial for patients with psoriatic arthritis, patients were randomly divided into 3 groups to receive oral drugs. The results showed that patients treated with BMS-986165 were more likely to achieve ≥20% improvement in the American College of Rheumatology score (ACR20) than those treated with placebo at week 16.85
A large Phase 3 trial demonstrated that a significantly higher proportion with moderate-to-severe psoriasis achieved PASI75 and SPGA 0/1 at week 16 in the BMS-986165 6 mg QD (58.4% and 53.6%) dosage groups compared with Apremilast 30 mg QD (35.1% and 32.1%) or placebo (12.7% and 7.2%). In addition to the primary endpoint, BMS-986165 groups also showed greater improvements compared to Apremilast and placebo, including PASI90, quality of life, psoriasis symptoms, and improvements in scalp psoriasis. The incidence of AE was not significantly different among all 3 treatment groups, and the most common AEs in BMS-986165 groups were nasopharyngitis and upper respiratory tract infection. This experiment further confirmed that no meaningful changes in laboratory parameters associated with JAK1/2/3 inhibitors were detected during treatment with BMS-986165.72
The clinical trial data further confirmed the efficacy, safety and durability of BMS-986165 oral administration compared to placebo and Apremilast, which provides sufficient evidence for its use in the treatment of psoriasis. Three phases 3 trials for plaque psoriasis are still currently underway (NCT03611751, NCT04036435, and NCT03924427).24
Pf-06700841
PF-06700841 is a dual inhibitor of TYK2 and JAK1, inhibiting cytokines mediated through TYK2 and JAK1 signaling pathways.73
In a Phase 1 clinical trial, patients with moderate to severe psoriasis were randomized to 30 mg QD, 100 mg QD, and placebo.74 By comparing the changes of gene expression in lesional skin at weeks 2 and 4 with placebo, the changes were significant dose-dependent from baseline in both the 30 mg and 100 mg groups. By week 4, the overall improvement of the IL-17 gene in the 100 mg group reached 124%, while the reduction in IL-17 gene expression was strongly correlated with PASI and Body Surface Area (BSA). No serious AEs occurred in this study, with decreases in reticulocyte, platelet, and neutrophil counts, increases in lymphocyte counts, and increases in serum creatinine observed in healthy subjects and patients with psoriasis.73 In a phase 2a trial, patients with moderate-to-severe plaque psoriasis treated with the oral PF-06700841 were divided into seven treatment groups. Among the seven treatment groups, a higher percentage of patients achieved PASI 75 and PASI90 than in the placebo group, and at week 12, the proportion of patients achieving PASI75 and PASI90 were the highest in the 30 mg QD treatment group, respectively, 86.2% and 51.7%.75
A phase 2b clinical trial is currently underway in patients with psoriatic arthritis. Topical application of PF-06700841 cream is also being conducted in patients with mild-to-moderate psoriasis to evaluate its safety and efficacy.
Pf-06826647
PF-06826647 is a selective oral TYK2 inhibitor.76 Several phases 1 studies have been conducted to evaluate its safety and pharmacokinetics in healthy participants and patients with plaque psoriasis.77,86 The severity of AEs in the current study is mild, but the safety still needs to be further determined.77 In a phase 2 clinical trial, patients with moderate to severe plague psoriasis were randomized to 50 mg, 100 mg, 200 mg, 400 mg QD or placebo for 16 weeks. The trial demonstrated that a greater proportion of patients achieving PASI90 in the 200 mg (33.0%) and 400 mg (46.5%) groups versus placebo. Significant increases in secondary endpoints (PASI75, PASI50/100 and PGA responses) compared to placebo could be observed in all treatment groups. The most common AEs were upper respiratory tract infection, nasopharyngitis, and increased blood pressure. There are no serious AEs occurred in this trial.78
Discussion
Systemic treatment options for moderate to severe psoriasis include traditional oral immunosuppressive drugs, biologics, and oral small molecule drugs. The efficacy of traditional oral drugs is generally lower than that of biologics, but they are still widely used due to their low price.79 Among biologic agents, TNF antagonists, IL-17 inhibitors, and IL-23 are the predominant drug classes currently available for the treatment of moderate-to-severe psoriasis. Though biologics are currently among the most effective options for the treatment of psoriasis, they still have many limitations. A subset of patients with malignancies and chronic infections have restricted access, while others fade of efficacy over time. Additional limitations of biologics include intravenous or subcutaneous administration and expensive expenses, which have kept biologics from being preferred by some patients.80,81 Oral administration of small molecules, which can partially and reversibly inhibit multiple cytokine pathways with a single small molecule compared to inhibiting a single cytokine pathway with a biologic agent, could provide new treatment ideas for psoriasis. Apremilast, a phosphodiesterase inhibitor, may not be as effective as some biologics, but its lower cost of treatment and fewer AEs have kept its use in a stable range.82 JAK inhibitors offer the benefits of Apremilast while providing better efficacy than Apremilast. TYK2 inhibitors bind allosterically to the residual ATP-binding site of the JH2 domain, reducing the AEs such as hematological toxicity caused by the inhibition of the JAK1/2/3 signaling pathway compared with other pan-JAK inhibitors.87 Among these, TYK2 inhibitors are considered potential drugs for the treatment of psoriasis. In Table 2, we compare the efficacy, dosage and limitations of several representative systemic treatments.
Table 2.
Biologic and Oral Systemic Treatments for Psoriasis
| Systemic Treatments | Mechanism of Action | Dosage | PASI75 | Safety Considerations |
|---|---|---|---|---|
| Biologics | ||||
| Adalimumab | anti-TNF- antibody | 80 mg SQ first week, 40 mg SQthe second week, then 40 mg SQevery two weeks | 71% at week 16 | Serious infection, cancer, induction of autoimmunity,new onset demyelinating disorders;Contraindications: demyelinatingdiseases hepatitis B |
| Secukinumab | IL-17 monoclonal antibody | 300 mg SQ at weeks 0–4; then 300mg every 4 weeks | 82% at week 12 | Monilial infections;Contraindications: IBD |
| Ustekinumab | IL-12/23 monoclonal antibody | <100 kg: 45 mg SQ at weeks 0 and4, then every 12 weeks; >100kg: 90mg SQ at the same intervals | 67% and 76% at week 12, patients weighing <100kg and ≥100kg, respectively | Cancer, induction of autoimmunity,Candida infections, cardiovascular events;Contraindications: serious infection |
| Guselkumab | IL-23 monoclonal antibody | 100 mg SQ at weeks 0–4; then 100mg every 8 weeks | 73% achieve PASI 90 at week 16 | Monilial infections;Contraindications: serious infection |
| Oral systemic | ||||
| Methotrexate | Dihydrofolate reductase inhibitor | 15–20 mg once weekly with folic acid supplementation | 36% at week 16 | Bone marrow toxicity, hepatotoxic,lymphoma, drug-drug interactions;Contraindications: Pregnancy/nursing, alcoholism |
| Cyclosporine | Calcineurin inhibitor | 5mg/kg as a twice-daily divided dose | 65% achieve IGA 0/1 at week 8 | Nephrotoxicity, gastrointestinal disturbances,headache, myalgia, cancer, lethargy |
| Apremilast | Phosphodiesterase-4 inhibitor | Gradual increasing doseto 30 mg PO twice daily | 33% at week 16 | Gastrointestinal disturbances and weight loss |
| Tofacitinib | JAK1 and JAK3inhibitor | 5 or 10 mg, twice daily | 39.9–59.6% at week 12 | Increase in LDL, HDL, and Cr, sporadic neutropenia, anemia, possibly increase therisk for infections with varicella zostervirus and malignancies. |
| BMS-986165 | TYK2 inhibitor | 6 mg once daily | 58.4% at week 16 | Nasopharyngitis and upper respiratory tract infection |
Abbreviations: TNF, tumor necrosis factor; JAK, Janus kinase; TYK2, tyrosine kinase 2; SQ, subcutaneous; IBD, inflammatory bowel disease; PASI, Psoriasis Area and Severity Index; PASI75, 75% improvement from baseline PASI; IGA, Investigator’s Global Assessment scale.
Conclusion
TYK2, the key part of the JAK-STAT pathway, regulates the mediation of cytokines such as type I IFNs, IL-12, IL-23, IL-10, and IL-6 and plays an indispensable role in the maturation, differentiation, and immune responses of innate immune cells. There are few studies on the concrete functionary mechanisms related to the association of TYK2 and immune immunity, and the physiological significance of TYK2 expression in DCs, NK cells, and macrophages should be explored in depth, then search for new therapeutic targets related to TYK2 and innate immune cells. Small-molecule drugs that selectively inhibit TYK2 may become a new strategy for the long-term treatment of moderate-to-severe psoriasis in the future. We expect the performance of TYK2 inhibitors in clinical trials, and to obtain outstanding results in the future treatment of psoriasis and psoriatic arthritis.
Abbreviations
TYK2, Tyrosine kinase 2; JAK-STAT, Janus kinase-signal transducer and activator of transcription; IFNs, interferons; ILs, interleukins; DCs, Dendritic cells; NK cells, Natural killer cells; IMIDs, immune-mediated inflammatory diseases; TNF-α, tumor necrosis factor-α; AEs, adverse events; DV, Dengue virus; Treg, regulatory T cells; IMQ, imiquimod; MMPs, matrix metalloproteinases; FDA, Food and Drug Administration; EMA, European Medicines Agency; GWAS, genome-wide association study; QD, once daily; BID, twice daily; QW, once weekly; SQ, subcutaneous; PASI, Psoriasis Area and Severity Index; PASI75, 75% improvement from baseline PASI; PASI90, 90% improvement from baseline PASI; ACR, American College of Rheumatology score; ACR20, achieve ≥20% improvement in the ACR; BSA, Body Surface Area; sPGA, static Physician’s Global Assessment; PsA, psoriatic arthritis; IGA, Investigator’s Global Assessment scale; IBD, inflammatory bowel disease.
Ethical Approval
Submitted paper is a mini-review of relevant literature. This article does not contain any studies with human participants or animals performed by any of the authors.
Author Contributions
All authors contributed to conception, data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version for publication, and agree to be accountable for the contents of the article.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Armstrong AW, Read C. Read, pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 2.Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212. doi: 10.1111/jdv.13854 [DOI] [PubMed] [Google Scholar]
- 3.Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi: 10.1016/S0140-6736(14)61909-7 [DOI] [PubMed] [Google Scholar]
- 4.Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. doi: 10.1038/nrdp.2016.82 [DOI] [PubMed] [Google Scholar]
- 5.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–653. doi: 10.1016/j.jaci.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell MD, Kuo FI, Smith PA. Targeting the Janus Kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342. doi: 10.3389/fimmu.2019.02342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz DM, Bonelli M, Gadina M, et al. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12(1):25–36. doi: 10.1038/nrrheum.2015.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11:502. doi: 10.1126/scitranslmed.aaw1736 [DOI] [PubMed] [Google Scholar]
- 10.Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168(11):5699–5708. doi: 10.4049/jimmunol.168.11.5699 [DOI] [PubMed] [Google Scholar]
- 11.Boutet MA, Nerviani A, Gallo Afflitto G, et al. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int J Mol Sci. 2018;19:2. doi: 10.3390/ijms19020530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghoreschi K, Balato A, Enerbäck C, et al. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397(10275):754–766. doi: 10.1016/S0140-6736(21)00184-7 [DOI] [PubMed] [Google Scholar]
- 13.Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18(4):374–384. doi: 10.1038/ni.3691 [DOI] [PubMed] [Google Scholar]
- 14.Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(4):234–243. doi: 10.1038/nrrheum.2017.23 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843–862. doi: 10.1038/nrd.2017.201 [DOI] [PubMed] [Google Scholar]
- 16.McNally R, Eck MJ. JAK-cytokine receptor recognition, unboxed. Nat Struct Mol Biol. 2014;21(5):431–433. doi: 10.1038/nsmb.2824 [DOI] [PubMed] [Google Scholar]
- 17.Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1(10):979–993. doi: 10.1177/1947601910397187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:20. doi: 10.3390/ijms21207488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605–1613. doi: 10.4049/jimmunol.1800013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595 [DOI] [PubMed] [Google Scholar]
- 21.He X, Chen X, Zhang H, et al. Selective Tyk2 inhibitors as potential therapeutic agents: a patent review (2015–2018). Expert Opin Ther Pat. 2019;29(2):137–149. doi: 10.1080/13543776.2019.1567713 [DOI] [PubMed] [Google Scholar]
- 22.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–287. doi: 10.1111/j.1600-065X.2008.00754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Y, Zhu Y, Xia Y, et al. Therapeutic potential of tyrosine kinase 2 in autoimmunity. Expert Opin Ther Targets. 2014;18(5):571–580. doi: 10.1517/14728222.2014.892925 [DOI] [PubMed] [Google Scholar]
- 24.Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective TYK2 Inhibitors. Drugs. 2020;80(4):341–352. doi: 10.1007/s40265-020-01261-8 [DOI] [PubMed] [Google Scholar]
- 25.Schön MP, Erpenbeck L. The interleukin-23/interleukin-17 axis links adaptive and innate immunity in psoriasis. Front Immunol. 2018;9:1323. doi: 10.3389/fimmu.2018.01323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobin AM, Lynch L, Kirby B, et al. Natural killer cells in psoriasis. J Innate Immun. 2011;3(4):403–410. doi: 10.1159/000328011 [DOI] [PubMed] [Google Scholar]
- 27.Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54(3 Suppl):S67–80. doi: 10.1016/j.jaad.2005.10.057 [DOI] [PubMed] [Google Scholar]
- 28.Aizu K, Li W, Yajima T, et al. An important role of Tyk2 in APC function of dendritic cells for priming CD8+ T cells producing IFN-gamma. Eur J Immunol. 2006;36(11):3060–3070. doi: 10.1002/eji.200636173 [DOI] [PubMed] [Google Scholar]
- 29.Ho LJ, Hung L-F, Weng C-Y, et al. Dengue virus type 2 antagonizes IFN-alpha but not IFN-gamma antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cell. J Immunol. 2005;174(12):8163–8172. doi: 10.4049/jimmunol.174.12.8163 [DOI] [PubMed] [Google Scholar]
- 30.Conzelmann M, Wagner AH, Hildebrandt A, et al. IFN-γ activated JAK1 shifts CD40-induced cytokine profiles in human antigen-presenting cells toward high IL-12p70 and low IL-10 production. Biochem Pharmacol. 2010;80(12):2074–2086. doi: 10.1016/j.bcp.2010.07.040 [DOI] [PubMed] [Google Scholar]
- 31.Tokumasa N, Suto A, Kagami S-I, et al. Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-gamma production and the induction of Th1 cell differentiation. Blood. 2007;110(2):553–560. doi: 10.1182/blood-2006-11-059246 [DOI] [PubMed] [Google Scholar]
- 32.Simonović N, Witalisz-Siepracka A, Meissl K, et al. NK cells require cell-extrinsic and -intrinsic TYK2 for full functionality in tumor surveillance and antibacterial immunity. J Immunol. 2019;202(6):1724–1734. doi: 10.4049/jimmunol.1701649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimoda K, Tsutsui H, Aoki K, et al. Partial impairment of interleukin-12 (IL-12) and IL-18 signaling in Tyk2-deficient mice. Blood. 2002;99(6):2094–2099. doi: 10.1182/blood.V99.6.2094 [DOI] [PubMed] [Google Scholar]
- 34.Fuchs S, Kaiser-Labusch P, Bank J, et al. Tyrosine kinase 2 is not limiting human antiviral type III interferon responses. Eur J Immunol. 2016;46(11):2639–2649. doi: 10.1002/eji.201646519 [DOI] [PubMed] [Google Scholar]
- 35.Strobl B, Bubic I, Bruns U, et al. Novel functions of tyrosine kinase 2 in the antiviral defense against murine cytomegalovirus. J Immunol. 2005;175(6):4000–4008. doi: 10.4049/jimmunol.175.6.4000 [DOI] [PubMed] [Google Scholar]
- 36.Novak U, Harpur AG, Paradiso L, et al. Colony-stimulating factor 1-induced STAT1 and STAT3 activation is accompanied by phosphorylation of Tyk2 in macrophages and Tyk2 and JAK1 in fibroblasts. Blood. 1995;86(8):2948–2956. doi: 10.1182/blood.V86.8.2948.2948 [DOI] [PubMed] [Google Scholar]
- 37.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rönnblom L. The importance of the type I interferon system in autoimmunity. Clin Exp Rheumatol. 2016;34(4 Suppl):21–24. [PubMed] [Google Scholar]
- 39.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. doi: 10.1038/nri3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, Zhang R, Hu C, et al. Irradiation haematopoiesis recovery orchestrated by IL-12/IL-12Rβ1/TYK2/STAT3-initiated osteogenic differentiation of mouse bone marrow-derived mesenchymal stem cells. Front Cell Dev Biol. 2021;9:729293. doi: 10.3389/fcell.2021.729293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watford WT, Hissong BD, Bream JH, et al. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x [DOI] [PubMed] [Google Scholar]
- 42.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/S1074-7613(00)00070-4 [DOI] [PubMed] [Google Scholar]
- 43.Sohn SJ, Barrett K, Van Abbema A, et al. A restricted role for TYK2 catalytic activity in human cytokine responses revealed by novel TYK2-selective inhibitors. J Immunol. 2013;191(5):2205–2216. doi: 10.4049/jimmunol.1202859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13(4):549–560. doi: 10.1016/S1074-7613(00)00054-6 [DOI] [PubMed] [Google Scholar]
- 45.Shaw MH, Boyartchuk V, Wong S, et al. A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity. Proc Natl Acad Sci U S A. 2003;100(20):11594–11599. doi: 10.1073/pnas.1930781100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishizaki M, Muromoto R, Akimoto T, et al. Tyk2 is a therapeutic target for psoriasis-like skin inflammation. Int Immunol. 2014;26(5):257–267. doi: 10.1093/intimm/dxt062 [DOI] [PubMed] [Google Scholar]
- 47.Trivella DB, Ferreira-Júnior JR, Dumoutier L, et al. Structure and function of interleukin-22 and other members of the interleukin-10 family. Cell Mol Life Sci. 2010;67(17):2909–2935. doi: 10.1007/s00018-010-0380-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolk K, Witte E, Witte K, et al. Biology of interleukin-22. Semin Immunopathol. 2010;32(1):17–31. doi: 10.1007/s00281-009-0188-x [DOI] [PubMed] [Google Scholar]
- 49.Van Belle AB, de Heusch M, Lemaire MM, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188(1):462–469. doi: 10.4049/jimmunol.1102224 [DOI] [PubMed] [Google Scholar]
- 50.Wolk K, Haugen HS, Xu W, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl). 2009;87(5):523–536. doi: 10.1007/s00109-009-0457-0 [DOI] [PubMed] [Google Scholar]
- 51.Ma HL, Liang S, Li J, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118(2):597–607. doi: 10.1172/JCI33263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–248. doi: 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol. 2015;34:75–82. doi: 10.1016/j.coi.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 54.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–457. doi: 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 55.Ishizaki M, Muromoto R, Akimoto T, et al. Tyk2 deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice. Int Immunol. 2011;23(9):575–582. doi: 10.1093/intimm/dxr057 [DOI] [PubMed] [Google Scholar]
- 56.Berekmeri A, Mahmood F, Wittmann M, et al. Tofacitinib for the treatment of psoriasis and psoriatic arthritis. Expert Rev Clin Immunol. 2018;14(9):719–730. doi: 10.1080/1744666X.2018.1512404 [DOI] [PubMed] [Google Scholar]
- 57.Traynor K. FDA approves tofacitinib for rheumatoid arthritis. Am J Health Syst Pharm. 2012;69(24):2120. [DOI] [PubMed] [Google Scholar]
- 58.Papp KA, Menter MA, Raman M, et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol. 2016;174(6):1266–1276. doi: 10.1111/bjd.14403 [DOI] [PubMed] [Google Scholar]
- 59.Hosking AM, Juhasz M, Mesinkovska NA. Topical Janus kinase inhibitors: a review of applications in dermatology. J Am Acad Dermatol. 2018;79(3):535–544. doi: 10.1016/j.jaad.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 60.Xie F, Yun H, Bernatsky S, et al. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 2016;68(11):2612–2617. doi: 10.1002/art.39761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winthrop KL, Lebwohl M, Cohen AD, et al. Herpes zoster in psoriasis patients treated with tofacitinib. J Am Acad Dermatol. 2017;77(2):302–309. doi: 10.1016/j.jaad.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 62.Olivera PA, Lasa JS, Bonovas S, et al. Safety of Janus Kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1554–1573.e12. doi: 10.1053/j.gastro.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 63.Pérez-Jeldres T, Tyler CJ, Boyer JD, et al. Targeting cytokine signaling and lymphocyte traffic via small molecules in inflammatory bowel disease: JAK inhibitors and S1PR agonists. Front Pharmacol. 2019;10:212. doi: 10.3389/fphar.2019.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danese S, Argollo M, Le Berre C, et al. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut. 2019;68(10):1893–1899. doi: 10.1136/gutjnl-2019-318448 [DOI] [PubMed] [Google Scholar]
- 65.Gadina M, Chisolm DA, Philips RL, et al. Translating JAKs to Jakinibs. J Immunol. 2020;204(8):2011–2020. doi: 10.4049/jimmunol.1901477 [DOI] [PubMed] [Google Scholar]
- 66.Dendrou CA, Cortes A, Shipman L, et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci Transl Med. 2016;8(363):363ra149. doi: 10.1126/scitranslmed.aag1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol. 2011;186(7):4234–4243. doi: 10.4049/jimmunol.1003668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bellinato F, Gisondi P, Girolomoni G. Latest advances for the treatment of chronic plaque psoriasis with biologics and oral small molecules. Biologics. 2021;15:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus Kinase 1/2/3 Inhibitors. Dermatol Ther. 2021;11(5):1763–1776. doi: 10.1007/s13555-021-00596-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379(14):1313–1321. doi: 10.1056/NEJMoa1806382 [DOI] [PubMed] [Google Scholar]
- 71.Catlett IM, Hu Y, Gao L, et al. Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. J Allergy Clin Immunol. 2021;149:2010–2020. [DOI] [PubMed] [Google Scholar]
- 72.Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2022. doi: 10.1016/j.jaad.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 73.Page KM, Suarez-Farinas M, Suprun M, et al. Molecular and cellular responses to the TYK2/JAK1 Inhibitor PF-06700841 reveal reduction of skin inflammation in plaque psoriasis. J Invest Dermatol. 2020;140(8):1546–1555.e4. doi: 10.1016/j.jid.2019.11.027 [DOI] [PubMed] [Google Scholar]
- 74.Banfield C, Scaramozza M, Zhang W, et al. The safety, tolerability, pharmacokinetics, and pharmacodynamics of a TYK2/JAK1 Inhibitor (PF-06700841) in healthy subjects and patients with plaque psoriasis. J Clin Pharmacol. 2018;58(4):434–447. doi: 10.1002/jcph.1046 [DOI] [PubMed] [Google Scholar]
- 75.Forman SB, Pariser DM, Poulin Y, et al. TYK2/JAK1 Inhibitor PF-06700841 in patients with plaque psoriasis: phase IIa, randomized, double-blind, placebo-controlled trial. J Invest Dermatol. 2020;140(12):2359–2370.e5. doi: 10.1016/j.jid.2020.03.962 [DOI] [PubMed] [Google Scholar]
- 76.Gerstenberger BS, Ambler C, Arnold EP, et al. Discovery of tyrosine kinase 2 (TYK2) Inhibitor (PF-06826647) for the treatment of autoimmune diseases. J Med Chem. 2020;63(22):13561–13577. doi: 10.1021/acs.jmedchem.0c00948 [DOI] [PubMed] [Google Scholar]
- 77.Singh R, Pradhan V, Roberts ES, et al. Safety and pharmacokinetics of the oral TYK2 inhibitor PF-06826647: a Phase I, randomized, double-blind, placebo-controlled, Dose-Escalation Study. Clin Transl Sci. 2021;14(2):671–682. doi: 10.1111/cts.12929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87(2):333–342. doi: 10.1016/j.jaad.2022.03.059 [DOI] [PubMed] [Google Scholar]
- 79.Bissonnette R, Bourcier M, Gooderham M, et al. Management of moderate to severe plaque psoriasis: the emerging role of IL-17 inhibition. J Cutan Med Surg. 2017;21(2_suppl):2S–40S. doi: 10.1177/1203475417722552 [DOI] [PubMed] [Google Scholar]
- 80.Menter A, Thaçi D, Wu JJ, et al. Long-term safety and effectiveness of adalimumab for moderate to severe psoriasis: results from 7-year interim analysis of the ESPRIT registry. Dermatol Ther (Heidelb). 2017;7(3):365–381. doi: 10.1007/s13555-017-0198-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151(9):961–969. doi: 10.1001/jamadermatol.2015.0718 [DOI] [PubMed] [Google Scholar]
- 82.Van Voorhees AS, Stein Gold L, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83(1):96–103. doi: 10.1016/j.jaad.2020.01.072 [DOI] [PubMed] [Google Scholar]
- 83.European Medicines Agency. Xeljanz: product information; 2017. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/.xeljanz. Accessed August 3, 2022.
- 84.Food and Drug Administration. Xeljanz: FDA approves new treatment for moderately to severely active ulcerative colitis; 2018. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-moderately-severely-active-ulcerative-colitis. Accessed August 3, 2022.
- 85.FitzGerald O, Gladman D, Mease P, et al. Biomarker changes with selective tyrosine kinase 2 inhibitor, deucravacitinib, in PsA: effects on disease markers and tyrosine kinase 2‒ versus Janus Kinase 1/2/3‒mediated Pathways [abstract]. Arthritis Rheumatol. 2021;73(suppl 10):1305–1458 [Google Scholar]
- 86.Tehlirian C, Peeva E, Kieras E, et al. Safety, tolerability, efficacy, pharmacokinetics, and pharmacodynamics of the oral TYK2 inhibitor PF-06826647 in participants with plaque psoriasis: a phase 1, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Rheumatol. 2021;3(3):e204–e213. doi: 10.1016/S2665-9913(20)30397-0 [DOI] [PubMed] [Google Scholar]
- 87.Gordon K, Gooderham M, Papp K, et al. BMS- 986165, an oral, selective tyrosine kinase 2 (TYK2) inhibitor: evaluation of changes in laboratory parameters in response to treatment in a Phase 2 trial in psoriasis [poster]. Presented at: the American Academy of Dermatology Annual Meeting. Denver, CO; 2020. [Google Scholar]