Abstract
The aim of this study was to examine the use of pedometers as a tool to promote daily physical activity levels in patients with COPD.
A systematic review meta-analysis of pedometer physical activity promotion in patients with COPD was conducted. Medline/PubMed, Cochrane Library, Web of Science and CINAHL were searched from inception to January 2019. The search strategy included the following keywords: physical activity promotion, pulmonary rehabilitation and daily physical activity. The eligibility criteria for selecting studies were randomised controlled trials reporting pedometer physical activity promotion in patients with COPD.
Improvements in steps per day were found with pedometer physical activity promotion either standalone (n=12, mean 0.53 (95% CI 0.29–0.77); p=0.00001) or alongside pulmonary rehabilitation (n=7, 0.51 (0.13–0.88); p=0.006). A subgroup analysis reported significant differences in the promotion of physical activity based on baseline physical activity levels and the type of instrument used to assess levels of physical activity.
Future trials should consider the way in which pedometers are used to promote physical activity to inform clinical practice in the setting of pulmonary rehabilitation.
Short abstract
Pedometer based physical activity promotion as a standalone intervention or alongside pulmonary rehabilitation induces meaningful improvements in daily physical activity levels (steps per day) in patients with COPD. http://bit.ly/2LnxM2o
Introduction
Interventions to promote levels of daily physical activity are becoming important in the management of patients with COPD [1, 2]. Studies comparing the levels of physical activity in patients with COPD with healthy age-matched controls have reported significantly lower levels in those with COPD [3–5]. In addition, low levels of physical activity in patients with COPD are associated with an increased risk of hospitalisation and mortality [3, 6, 7]. Therefore, effective approaches to improve daily physical activity are needed in patients with COPD.
Pulmonary rehabilitation has shown substantial improvements in exercise tolerance; however, these findings have not consistently progressed into improvements in daily physical activity [8]. One reason for this may link to physical activity being a complex health behaviour, with the determinants of physical activity influenced by personal, interpersonal, environmental, regional and/or national and global factors [9].
Physical activity promotion through the use of pedometers encompasses the stimulation of patients towards higher levels of daily physical activity by modifying their behaviour, with many versions of this intervention also using elements of the self-regulatory theory [10]. This theory involves a process of guiding an individual's own thoughts, behaviours and feelings towards achieving specific goals [11]. Incorporating the use of pedometers as a real-time feedback tool for improving daily steps allows patients the ability to follow individualised physical activity goals, which can be assessed and improved alongside techniques of motivational interviewing [12].
Implementing behaviour strategies using pedometer feedback can be done a number of ways, including face-to-face contact between patients and clinicians, group contact during rehabilitation sessions and through electronic information and communication technologies (tele-coaching) [13].
Studies have, however, provided inconsistent findings towards the implementation of pedometer-based feedback and motivational interviewing as part of physical activity promotion [14, 15]. Moreover, when the same intervention was added to standard care pulmonary rehabilitation, results remained inconclusive [1]. The most updated systematic review and meta-analysis has found high levels of heterogeneity regarding physical activity promotion, both as a standalone intervention and alongside pulmonary rehabilitation [1]. The existence of such heterogeneity is predominantly due to both methodological variables (types of goal setting, provided feedback and length of intervention) and patient demographics (severity and baseline physical activity levels). Hence, the aim of this systematic review and meta-analysis was to elucidate on aspects of physical activity promotion related to the way that pedometers are used to optimise physical activity in patients with COPD. In this context, we investigated the optimal frequency of goal setting, the type of patient feedback, the optimal length of interventions, the type of instrument used for assessing physical activity, and associations between baseline activity levels and the magnitude of improvement in daily physical activity.
Review objective
The aim of this review was to systematically review and meta-analyse aspects of physical activity promotion, specifically regarding how pedometers are used to optimise physical activity in interventions which incorporate the use of pedometers as a key component for improving levels of daily physical activity in patients with COPD.
Methods
The Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16] guidelines for reporting systematic review and meta-analyses were followed when conducting and reporting this prospectively registered systematic review (identifier CRD42018103893; www.crd.york.ac.uk/prospero/).
Eligibility criteria
The review team conducted a computerised literature search beginning in March 2018 in the following databases: Medline/PubMed, Cochrane Review, Web of Science and CINAHL. The final search of the literature took place on 18 January, 2019. Pre-piloted literature searches prior to the final search strategy were conducted based on two previously published systematic reviews on a related topic [1, 17]. The full search strategy can be found in table 1. It included a wide range of modalities; using terms associated with “chronic obstructive pulmonary disease”, “physical exercise training”, “physical activity promotion, physical activity counselling” and “randomized controlled trial”. Bibliographic details of all articles from the different databases were stored in the reference software file EndNote.
TABLE 1.
Search criteria for computerised literature search conducted in PubMed
| Search | Query |
| 1 | (“Chronic obstructive pulmonary disease” [Text Word] OR “COPD” [Text Word] OR “Chronic Lung Disease” [Text Word] OR “Chronic Obstructive Lung Disease” [Text Word] OR “Emphysema” [Text Word] OR “Chronic Bronchitis” [Text Word]) |
| 2 | (“exercise” [Text Word] OR “rehabilitation” [Text Word] OR “exercise training” [Text Word] OR “pulmonary exercise training” [Text Word] OR “physical exercise training” [Text Word] OR “pulmonary rehabilitation” [Text Word] OR “exercise rehabilitation” [Text Word] OR “cardiopulmonary rehabilitation” [Text Word] OR “rehabilitation program#” [Text Word] OR “exercise program#” [Text Word] OR “physical activity advice” [Text Word] OR “physical activity counselling” [Text Word] OR “physical activity promotion” [Text Word] OR “accelerometer#” [Text Word] OR “Pedometer#” [Text Word] OR “activity monitor#” [Text Word] OR “step count#” [Text Word] OR [Text Word] OR “telerehabilitation” [Text Word] OR “e-Health intervention” [Text Word]) |
| 3 | (“Activity” [Text Word] OR “Motor activity” [Text Word] OR “physical inactivity” [Text Word] OR “risk factor” [Text Word] OR “outcome assessment” [Text Word] OR “activity” [Text Word] OR “step#” [Text Word] OR “walk#” [Text Word]) |
| 4 | (Randomised controlled trial OR clinical trial OR experimental study) |
| 5 | 1 AND 2 AND 3 AND 4 |
Text word includes all words and numbers in the title, abstract, other abstract, MeSH terms, MeSH subheadings, publication types, substance names, personal name as subject, corporate author, secondary source, comment/correction notes. #: all terms that begin with specific word.
On completion of the literature search, all stored references were exported from EndNote to the systematic review management software programme, Covidence. Eligible studies published in the English language were included if they fulfilled the predetermined PICOS criteria. 1) Population/participants: individuals with COPD defined by spirometry (i.e. forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7). 2) Interventions or exposures: patients with COPD who were enrolled onto a programme of physical activity promotion, which included the use of a tool that provides real-time feedback on steps per day (i.e. pedometer screen). This included standalone interventions or those incorporated into pulmonary rehabilitation. 3) Comparison or control groups: patients not receiving any physical activity promotion intervention. 4) Outcomes of interest: the effect of physical activity promotion on steps per day as a measure of daily physical activity. 5) Setting: certified research studies. 6) Study design: randomised controlled trials (RCTs), both arms (intervention plus control).
Data extraction
After removing the duplicates and based on the inclusion criteria, two authors (M. Armstrong and N. Chynkiamis) independently and blinded, reviewed the title and abstract of trials and assessed the full text of articles. Any possible disagreement between both authors during the study selection process was discussed with a third author (I. Vogiatzis) for resolution.
For each eligible study, a pre-designed standardised Microsoft Excel form was used to collect data by a single author (M. Armstrong) on the following subheadings: author information (including name of first author and date of publication), blindness, participant characteristics (including age, FEV1 % pred, FVC, 6-min walk distance (6MWD), baseline daily steps, total lung capacity and residual volume, intervention details, physical activity measurements, primary outcomes and results). Two blinded reviewers (M. Armstrong and N. Chynkiamis) screened all articles independently, any disagreements were sent to a third independent author (I. Vogiatzis) to make a majority agreement.
Quality assessment
Quality assessment was performed using the PEDro quality scale, which is an 11-item scale assessing internal and external validity of clinical trials [18]. Two authors (M. Armstrong and N. Chynkiamis) independently reviewed the following domains employed by this scale: eligibility criteria, random allocation, concealed allocation, baseline similarity, blinding (subject, therapist and assessor), and measures recorded from at least 85% of participants, full intention to treat, group comparison and point measure. The higher the given score, the better the quality. Cut-off points of the scale were excellent (9–10), good (6–8), fair (4–5) and poor (<3) [18].
Data synthesis
Meta-analyses were undertaken using Review Manager (RevMan v.5.3; Cochrane Collaboration, Oxford, UK). Change scores or end of intervention values with the corresponding standard deviation for the outcomes of interest were used to obtain the overall effect size represented by standard mean difference with 95% confidence interval, with a threshold p<0.05 considered as significant. Heterogeneity in this meta-analysis was assessed by I2 value as follows: 0–40%, might not be important; 30%–60%, moderate heterogeneity; 50%–90% substantial heterogeneity; and 75%–100% considerable heterogeneity [19]. A fixed-effects model was used for the meta-analysis; however, if statistical heterogeneity was noted (I2 >40%), meta-analyses were performed using the random effects model. Sensitivity analysis was used if substantial heterogeneity (I2 >75%) was reported in meta-analyses.
Results
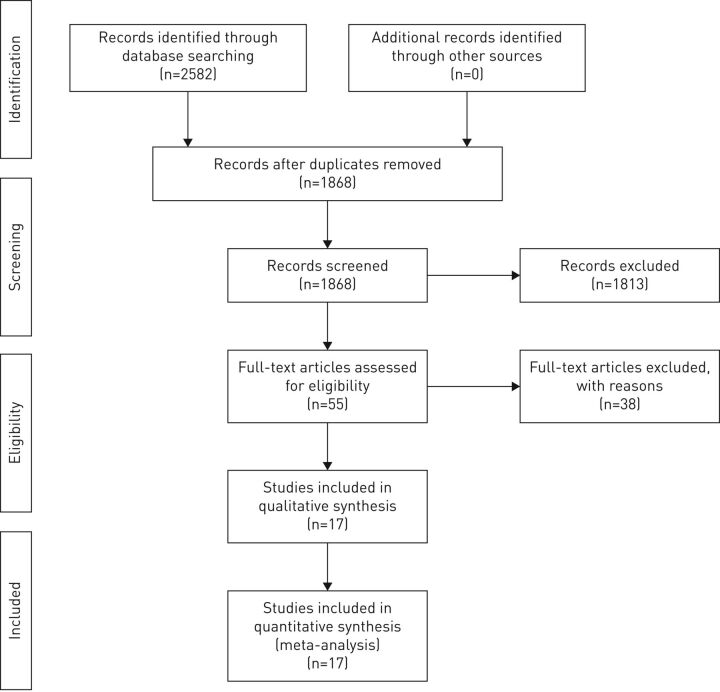
The search strategy yielded 2582 potentially relevant articles. After removing 714 duplicates and screening 1868 abstract/titles, 55 articles remained for the full-text screening. On completion of full-text screening, 38 studies were excluded. Therefore, 17 studies were considered eligible for inclusion in this systematic review and meta-analysis. One article provided three different comparisons, resulting in three RCTs. A full PRISMA flow diagram of the screening process is shown in figure 1. Participants were individually randomised in all included trials (i.e. there were no cluster RCTs). Characteristics of included RCTs are summarised in table 2 and all were published between 2006 and 2018.
FIGURE 1.
PRISMA flow diagram for database search and study selection process.
TABLE 2.
Characteristics of included studies
| First author [ref.] | I/C | Age years | FEV1 % pred L | Male/female | Patient recruitment | Intervention arm | Control arm | Type of feedback | Weekly goals | Time-points/outcomes |
| Altenburg [14] | 24/24 | 65 (58–72) | 78 (66–95) | 32/16 | General practices (primary care) | PA counselling 30 min ×5 sessions using MI, GS and pedometer: 12 weeks | Received care appropriate to their health status | Face-to-face | No | 3 months/daily steps |
| Altenburg [14] | 23/23 | 68 (61–72) | 58 (40–69) | 34/12 | Outpatient hospital clinics (secondary care) | PA counselling 30 min ×5 sessions using MI, GS and pedometer: 12 weeks Patients were recruited from outpatient hospital clinics (secondary care) |
Received care appropriate to their health status | Face-to-face | No | 3 months/daily steps |
| Altenburg [14] | 22/15 | 54±9.6 | 43±25.9 | 32/25 | Pulmonary rehabilitation centre | PA counselling (30 min ×5 sessions using MI, GS and pedometer 12 weeks) added to PR 2 h 3 times per week: 9 weeks | PR 2 h 3 times per week: 9 weeks | Face-to-face | No | 3 months/daily steps |
| Arbillaga-Etxarri [20] | 220/293 | 69±9 | 58±17 | 448/65 | Primary care and 5 hospital care centres | Six components: MI, urban walking training walking, pedometer and personalised calendar, phone updates, exercise leaflet, group walking sessions | General health counselling and info booklet | Remote | No | 12 months/daily steps |
| Bender [21] | 57/58 | 65±7 | 54.3±11 | 48/67 | Pulmonary outpatient clinics | Pedometer and personally selected goals involving enjoyed activities of daily living A target of increasing 15% daily steps per month for 3 months |
Pedometer with no goal setting or communication about physical activity. A small 1-1 telephone call to communicate daily steps |
Remote | No | 3 months/daily steps |
| Cruz [15] | 16/16 | 66.5±8.4 | 66.9±20.1 | 27/5 | 3 primary care centres and a district hospital | PA-focused behavioural counselling (average 25 min ×8 sessions using SCT, SE, MI and pedometer and diary feedback: 6 months) added to PR (1 h 3 times per week ET and 1.5 h once a week EDU session: 3 months) | PR (1 h 3 times per week ET and 1.5 h once a week EDU session: 3 months) | Face-to-face | Yes | 3 months/daily steps |
| De Blok [22] | 172/171 | 67±8 | 56±20 | 219/124 | N/A | Lifestyle PA counselling (3 min ×4 sessions using MI, GS and pedometer: 9 weeks) added to PR (9 weeks) | PR (9 weeks) | Face-to-face | No | 9 weeks/daily steps |
| Demeyer [13] | 172/171 | 67±8 | 56±20 | 219/124 | 6 rehabilitation centres across Europe | Received the usual care plus the tele-coaching platform This includes a one-to-one interview, a step counter and smartphone coaching application |
Received a standard leaflet explaining the importance of PA in COPD as well as information about PA recommendations | Remote | Yes | 3 months/daily steps |
| Holland [23] | 80/86 | 69±11 | 50±19 | 99/67 | Pulmonary rehabilitation waiting list | Home rehabilitation, which involved a pedometer and 7-weekly structured telephone calls based around motivational interviewing to improve walking fitness | Centre-based rehabilitation with encouragement to exercise at home, no pedometer issued | Remote | No | 12 months/daily steps |
| Horrnix [24] | 15/15 | 67±7 | 43±17 | 17/13 | Hospitalised exacerbation patients | Pedometer worn with telephone calls three times per week for 1 month to motivate and stimulate patients to increase their PA levels | No contact and didn't received any motivational messages, just advice about increasing PA before hospital discharge | Remote | Yes | 1-month, daily steps |
| Hospes [25] | 18/17 | 62±8 | 64±15 | 21/14 | Outpatient clinic | 12-week customised exercise counselling to enhance daily physical activity Based on principles of goal setting and implementation of goals |
No counselling programmes | Face-to-face | No | 3 months/daily steps |
| Kawagoshi [26] | 12/15 | 75±9 | 59.3±22 | 24/3 | N/A | Home-based rehabilitation in addition to monitored daily physical activity using pedometer and received monthly feedback on physical activity levels | Multidisciplinary home-based PR programme for 12 months | Face-to-face | No | 12 months, daily steps |
| Mendoza [27] | 52/50 | 68±8 | 66±19 | 62/40 | Outpatient clinics at private and public hospitals | Received pedometer and physical activity diary alongside counselling to improve physical activity | Received counselling at each visit to increase their physical activity levels and advised to walk for at least 30 min per day | Face-to-face | No | 3 months/daily steps |
| Moy [28] | 154/84 | 66±9 | 223/15 | National Database of Veterans | Pedometer and access to a website with components including; step count feedback, weekly goals, motivational content to enhance activity levels | Wore pedometer and recorded steps. Received no instruction about exercise and were not assigned step goals or website |
Remote | Yes | 4 months/daily steps | |
| Nolan [29] | 76/76 | 69.0±9.0 | 50.5±21.2 | 110/42 | Hospital-based PR unit | Lifestyle PA counselling (30 mins ×8 sessions using GS and pedometer: 8 weeks) added to PR (1 h ×2 times per week: 8 weeks) | PR (1 h ×2 time per week: 8 weeks) | Face-to-face | Yes | 9 weeks/daily steps |
| Tabak [30] | 14/16 | 66±7 | 52±13 | 19/11 | Hospital clinic | Tele-rehabilitation intervention for 4 weeks | Received no tele-rehabilitation Usual care was defined as usual medication/physiotherapy |
Remote | Yes | 1 month/daily steps |
| Varas [31] | 21/19 | 67±8 | 49±16 | 31/9 | Pulmonology consultants | 5 group physiotherapy sessions, 8 weeks counselling to increase daily activity levels, through telephone meetings | Informative sessions on the benefits of exercise, pedometer issued but no additional support | Remote | Yes | 8 weeks/daily steps |
| Vorrnik [32] | 84/73 | 62±9 | 55±17 | 78/79 | Outpatient physiotherapy practises | Consisted of two compartments: 1) smartphone application; 2) physiotherapist-based website for providing real-time goals and feedback for 6 months | No intervention | Remote | Yes | 3 months/daily steps |
| Wan [33] | 57/52 | 68±8 | 61±21 | 95/14 | General pulmonary clinics | Pedometer and received access to a website which provided four key components; individualised goal setting, iterative step-count feedback, motivational content and online community forum for 3 months | Received a pedometer and written material about exercise but weren't assigned step-count goals | Remote | Yes | 3 months, daily steps |
Data are presented as n, mean±sd or mean (range). FEV1: forced expiratory volume in 1 s; PA: physical activity; N/A: not applicable; PR: pulmonary rehabilitation; MI: motivational interviewing; GS: goal setting; SCT: social cognitive theory; SE: self-efficacy; ET: exercise training; EDU: education.
Characteristics of included subjects
All of the included trials comprised 1677 patients (45% male), with a median (range) sample size of 72 (16–343). Included patients had a mean (range) age of 66 (54–75) years and average FEV1 % pred ranged from 43 to 78, indicative of mild-to-moderate COPD [34]. Patients were reported as physically inactive at baseline with an average mean (range) value of 4365 (1557–7161) steps·day−1.
Characteristics of included/excluded trials
A total of 38 studies were excluded from this review on completion of full-text screening. The reasons for exclusion include: the wrong intervention (n=11), duplicates (n=9), wrong study design (n=6), wrong outcomes (n=6), wrong comparators (n=2), no full-text availability (n=2) and no reported data for daily steps (n=2).
Quality assessment
Table 3 provides a summary of the risk of bias decision made for each category for the included studies. In line with the PEDro scale, the quality of included studies ranged from good to excellent (mean (interquartile range) PEDro score 9.29 (1)); suggesting a low risk of bias towards the main outcome measure.
TABLE 3.
Qualitative synthesis of included studies using PEDro scale for the quality of randomised controlled trials
| First author [ref.] | Eligibility criteria | Random allocation | Concealed allocation | Baseline similarity | Blinding (subject) | Blinding (therapist) | Blinding (assessor) | Measure >85% | ITT | Group comparison | Point measure | Quality score |
| Altenburg [14] | * | * | * | * | * | * | * | * | 8 | |||
| Arbillaga-Etxarri [20] | * | * | * | * | * | * | * | * | * | 9 | ||
| Bender [21] | * | * | * | * | * | * | * | 7 | ||||
| De Blok [22] | * | * | * | * | * | * | * | * | 8 | |||
| Cruz [15] | * | * | * | * | * | * | * | * | * | * | 10 | |
| Demeyer [13] | * | * | * | * | * | * | * | * | * | 9 | ||
| Holland [23] | * | * | * | * | * | * | * | * | * | 9 | ||
| Horrnix [24] | * | * | * | * | * | * | * | 7 | ||||
| Hospes [25] | * | * | * | * | * | * | * | 7 | ||||
| Kawagoshi [26] | * | * | * | * | * | * | * | * | 8 | |||
| Mendoza [27] | * | * | * | * | * | * | * | * | 8 | |||
| Moy [28] | * | * | * | * | * | * | * | * | 8 | |||
| Nolan [29] | * | * | * | * | * | * | * | * | * | * | 10 | |
| Tabak [30] | * | * | * | * | * | * | * | * | 8 | |||
| Varas [31] | * | * | * | * | * | * | * | * | 8 | |||
| Vorrnik [32] | * | * | * | * | * | * | * | * | * | 9 | ||
| Wan [33] | * | * | * | * | * | * | * | * | * | 9 |
ITT: intention to treat. *: yes, score=1. The higher the given score, the better the quality. Cut-off points of the scale were: excellent (9–10), good (6–8), fair (4–5) and poor (3).
Meta-analyses of included studies
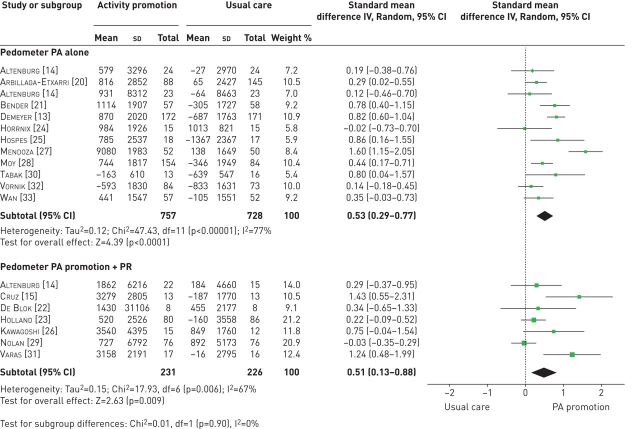
When observing the effects of physical activity promotion, there was a positive effect on steps per day compared with usual care (n=12 RCTs; 0.53 (0.29–0.77), p<0.00001) (figure 2) [13, 14, 20, 21, 24, 25, 27, 28, 30, 32, 33], which equated to an improvement of ∼1000 steps·day−1. A positive effect on steps per day was also found when pedometer physical activity promotion was added to pulmonary rehabilitation versus pulmonary rehabilitation alone (n=7 RCTs; 0.51 (0.13–0.88), p=0.006) (figure 2) [14, 15, 22, 23, 26, 30, 31]. However, the pooled analysis of pedometer physical activity promotion compared with usual care reported considerable heterogeneity (I2=77%).
FIGURE 2.
Effect sizes of pedometer-based physical activity (PA) promotion on steps per day in patients with COPD. PR: pulmonary rehabilitation.
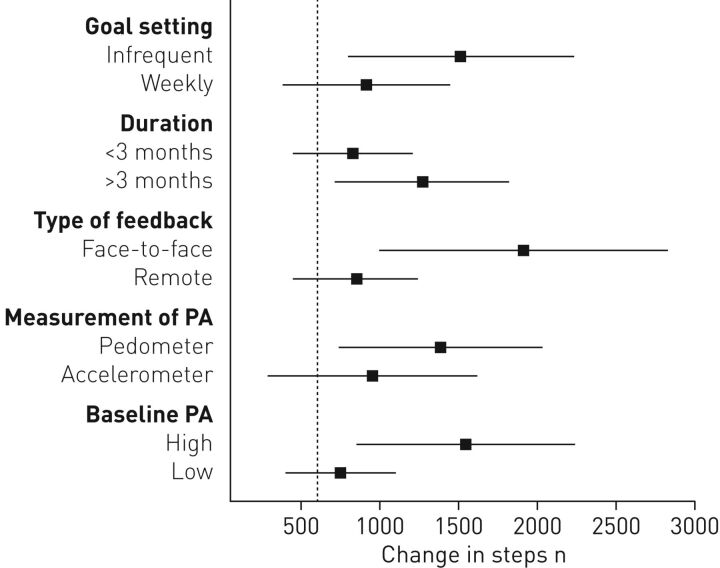
Moreover, the increases in daily physical activity induced by pedometer physical activity promotion (both alone and alongside pulmonary rehabilitation), were comparable among studies that provided: 1) weekly or infrequent goal setting; 2) an intervention length <3 months or >3 months; and 3) remote or face-to-face contact following overall or subgroup analysis (all p<0.05) (table 4). In contrast, studies employing accelerometers to measure physical activity were less effective compared with those employing pedometers. Furthermore, patients with greater baseline physical activity levels (>4000 steps·day−1) exhibited greater improvements in daily physical activity compared with those with lower baseline physical activity levels (<4000 steps·day−1) (table 4).
TABLE 4.
Subgroup analysis on physical activity (PA) outcomes of included studies
| Subgroups | Overall analysis | Pedometer PA alone | Pedometer PA promotion + PR | |||||||||
| n | Effect size | I2 % | n | Effect size | I2 % | n | Effect size | I2 % | ||||
| SMD | 95% CI | SMD | 95% CI | SMD | 95% CI | |||||||
| Goal setting | ||||||||||||
| Weekly | 9 | 0.50 | 0.21–0.78 | 77 | 6 | 0.44 | 0.16–0.71 | 70 | 3 | 0.82 | −0.23–1.88 | 88 |
| Infrequent | 10 | 0.55 | 0.24–0.85 | 75 | 6 | 0.64 | 0.19–1.09 | 84 | 4 | 0.29 | 0.04–0.54 | 0 |
| Duration | ||||||||||||
| <3 months | 15 | 0.57 | 0.31–0.84 | 78 | 10 | 0.57 | 0.27–0.88 | 79 | 5 | 0.60 | 0.00–1.20 | 76 |
| >3 months | 4 | 0.34 | 0.18–0.50 | 0 | 2 | 0.36 | 0.17–0.55 | 0 | 2 | 0.35 | −0.10–0.80 | 34 |
| Type of feedback | ||||||||||||
| Remote | 10 | 0.47 | 0.27–0.67 | 68 | 8 | 0.46 | 0.24–0.68 | 67 | 2 | 0.67 | −0.32–1.66 | 83 |
| Face-to-face | 9 | 0.60 | 0.14–1.06 | 81 | 3 | 0.70 | −0.06–1.46 | 86 | 5 | 0.48 | −0.03–0.99 | 65 |
| Measure of PA | ||||||||||||
| Accelerometer | 8 | 0.38 | 0.09–0.67 | 79 | 4 | 0.36 | −0.03–0.75 | 83 | 4 | 0.44 | −0.04–0.91 | 74 |
| Pedometer | 11 | 0.64 | 0.35–0.92 | 69 | 8 | 0.64 | 0.31–0.97 | 75 | 3 | 0.63 | 0.00–1.26 | 48 |
| Baseline PA levels | ||||||||||||
| Low baseline PA | 7 | 0.32 | 0.10–0.53 | 51 | 4 | 0.46 | 0.21–0.70 | 36 | 3 | 0.11 | −0.10–0.33 | 0 |
| High baseline PA | 11 | 0.67 | 0.36–0.98 | 80 | 8 | 0.59 | 0.24–0.94 | 83 | 3 | 0.95 | 0.22–1.67 | 63 |
PR: pulmonary rehabilitation; SMD: standard mean difference.
Sensitivity analysis removing a single study [27] from the pooled analysis of pedometer-based physical activity promotion reduced heterogeneity (I2=60%). The sensitivity analysis did not statistically affect the pooled analysis of the remaining 11 studies in pedometer-based physical activity promotion (0.44 (0.25–0.63); p<0.05).
Discussion
Summary of the main findings
This systematic review and meta-analysis of 19 RCTs provides evidence that pedometer physical activity promotion as a standalone intervention compared with usual care or alongside pulmonary rehabilitation compared with pulmonary rehabilitation alone improves steps per day by a magnitude that is within the minimal important difference (MID) of 600–1100 steps·day −1 reported by Demeyer et al. [35] (table 3). Moreover, this meta-analysis suggests that pedometer physical activity promotion was more effective in patients with greater baseline physical activity levels and when pedometers were used to measure improvements in physical activity compared with accelerometers (table 4).
The addition of a sensitivity analysis reducing levels of heterogeneity provided no additional effects to the pooled analysis of pedometer physical activity promotion.
Interpretation of the results
Previous literature surrounding the effects of physical activity promotion has reported inconclusive evidence of the effectiveness of this intervention on steps per day. In agreement with the findings of our review, Qui et al. [1] found that physical activity promotion improved steps per day compared with usual care in nine studies. However, significant heterogeneity (I2=81%) may have affected the overall analysis of those studies. The increase in steps per day reported as a result of pedometer physical activity promotion seems much larger than those from other methods including exercise training as part of pulmonary rehabilitation, health monitoring, long-term oxygen therapy or neuromuscular electrical stimulation [12, 17].
However, Lahham et al. [17] reported that physical activity promotion was not an effective standalone intervention towards improving steps per day. A number of disparities are apparent between review articles. First, Lahham et al. [17] based their analysis of physical activity promotion on a subgroup analysis of subjective and objective measures. Both our study and that of Qui et al. [36] only included studies reporting objective measures of daily physical activity due to limited validity and inaccuracy of subjective measures of activity levels in patients with COPD [37]. Secondly, the number of included studies varied across separate meta-analyses. In our review, a total of 12 studies with an average total sample size of 120 were included in the pooled analysis of pedometer physical activity promotion. Meanwhile, Lahham et al. [17] reported only two studies on objective measures of physical activity, with an average total sample size of 17. With the significant benefits of collecting and reporting objective measures of physical activity in both healthy individuals and patients with COPD, and a much greater sample size across pooled analyses, our review and that of Qui et al. [1] could be argued to have more valid findings for patients with COPD than Lahham et al. [17]. Benefits of pedometer physical activity promotion have also been reported in patients with type 2 diabetes [36]. A meta-analysis including 11 RCTs reported a significant increase in physical activity with an average magnitude of improvement of 1822 steps·day −1, which is greater than we found in patients with COPD (figure 3).
FIGURE 3.
Comparison between improvements in daily steps shown by various implementation modalities. Vertical dashed line indicates the lower levels of minimal important difference in patients with COPD. PA: physical activity.
When observing the effects of physical activity promotion alongside pulmonary rehabilitation, the present study and that by Lahham et al. [17] and Qui et al. [1] have shown statistically significant effects on steps per day. Lahham et al. [17] stated that providing persistent and individualised feedback on activity levels in conjunction with pulmonary rehabilitation, achieved significant effects that exceeded both physical activity promotion alone and pulmonary rehabilitation alone. However, Lahham et al. [17] were unable to include a recent RCT [29]. Within that study, the authors reported evidence questioning the effectiveness of physical activity promotion on daily physical activity in patients attending pulmonary rehabilitation [29]. It was determined that the routine use of this intervention should not be included in standard care pulmonary rehabilitation because levels of daily physical activity were greater after pulmonary rehabilitation alone when compared with baseline measures [29]. These results were based upon this study being the first to include a large sample size, suggesting other studies were underpowered. In addition, that study [29] scored highly on the PEDro scale, suggesting it had a low level of bias and the results reported were of high quality. The present study and that by Qui et al. [1] have been able to incorporate the study by Nolan et al. [29] into separate meta-analyses. A contrast in reporting physical activity between our study and that by Qui et al. [1] has provided two interpretations of the study by Nolan et al. [29]. Qui et al. [1] provided accelerometer step counts from baseline as a measure of steps per day from the study by Nolan et al. [29], reporting a small positive effect on physical activity levels. In contrast, we have chosen to report steps per day from pedometer step counts, resulting in a neutral effect on physical activity. We agree with Qui et al. [1] that accelerometers provide a more accurate measure of physical activity; however, the majority of studies in our meta-analysis have primarily used pedometers to report physical activity levels, so this may falsify results [29].
Our meta-analysis also suggests a number of important principles surrounding the way in which pedometers have been used for promoting physical activity. An overall analysis of patients with greater baseline physical activity levels (>4000 steps·day −1) showed greater improvements in steps per day compared with those with lower baseline physical activity (≤4000 steps·day−1) (figure 3). Of further interest is the influence that baseline physical activity had on the effects of pedometer physical activity promotion alongside pulmonary rehabilitation (table 4). In studies that implemented physical activity promotion alongside pulmonary rehabilitation, an insignificant effect on steps per day was reported when patients had a baseline physical activity ≤4000 steps·day −1 [14, 15, 31]. It must be outlined that there was only a small number of studies in this subgroup analysis with a small mean sample size; however, such differences in effect size warrants closer scrutiny.
Osadnik et al. [38] proposed that patients with COPD who exhibit greater exercise capacity prior to pulmonary rehabilitation are more likely to achieve greater improvements in daily physical activity. They reported clinically meaningful improvements in steps per day with patients reporting a 6MWD >350 m compared with <350 m (707±1780 versus 157±1694 steps·day −1). This higher likelihood of improvement in physical activity in patients preserving a greater exercise tolerance may also provide an explanation for those patients exhibiting a higher baseline physical activity. However, in contrast with this notion, a recent study from Gulart et al. [39] suggests that patients with lower values of FEV1 and steps per day were more likely to achieve MID in steps per day. This finding was attributed to the notion that patients with more severe disease have a greater potential for improvement as they are further from their “maximal” capacity, compared with patients with less severe disease.
In addition, our meta-analysis has found that the primary measure of physical activity (i.e. through accelerometers or pedometers) may have marked influences on the effects of physical activity promotion (table 4). Specifically, significant improvements in steps per day were shown in those studies reporting physical activity via a pedometer compared with an accelerometer (figure 3). The finding in physical activity outcomes may be due to accelerometers being a validated tool for measuring steps per day in patients with COPD and therefore pedometers may overestimate physical activity [40]. However, a number of previous studies, including Qui et al. [1], disagree with this finding. Both that study [1] and a meta-analysis in patients with type 2 diabetes [41] have shown no significant differences between accelerometers and pedometers. Consideration must be made in relation to these comparisons being indirect, with such confirmation potentially required through a future 1 versus 1 design.
In contrast, pedometer physical activity promotion in patients with type 2 diabetes presents different findings. For instance, it has been reported that patients with type 2 diabetes should initially set their own activity goals, before they set to increase their goals with the assistance of healthcare professionals [36]. We were unable to confirm this hypothesis among patients with COPD as many of the reported studies do not provide definitive step goal descriptions. In addition, studies have shown that the use of step diaries alongside pedometers as a source of motivation were imperative to increase physical activity levels [36, 42, 43].
Finally, it has become evident that regardless of the way pedometers are used (i.e. frequency of goal setting, type of patient feedback, length of intervention, the instrument used to assess physical activity) or the baseline activity levels, the improvement in steps per day is within the MID (figure 3) [35]. This finding has strong implications for the use of pedometers as part of the comprehensive management of patients with COPD.
Quality of the evidence
The overall quality of evidence from included studies was good, in line with the PEDro scale for quality assessment. The inability to blind subjects reduced the overall quality of evidence and increased the risk of bias towards the intervention procedure and may increase the chances of a placebo effect when using the pedometer. Future research reporting the effects of physical activity promotion may improve quality scoring by blinding all subjects from the intervention procedure. However, a concern remains that blinding patients from the intervention would require a pedometer being issued to a control group, which may present the control group with a level of physical activity promotion as they are able to monitor their daily steps. A number of studies were unable to blind any members of the study from patient allocation [14, 21, 24, 25, 28, 30]. In any clinical trial, blinding of at least the researcher is desirable and the blinding of subjects is warranted in order to decrease bias within the findings. When blinding is not used or the subject group status is easily detectable, subjects will generally try to fulfil the perceived expectation of the researcher [44].
Strength and limitations
This systematic review and meta-analysis is the first to include two recently published RCTs reporting pedometer-based physical activity promotion implemented either alone [20] or alongside a combined pulmonary rehabilitation programme [31]. Moreover, we are the first to report that, regardless of how pedometers are used in the implementation of physical activity promotion, they can provide improvements in daily physical activity (steps per day) which exceed the MID. Several limitations should be noted. First, some heterogeneity existed in the outcomes of pedometer physical activity promotion, which was partially explained by our findings on the modalities of pedometer use. Secondly, we cannot be certain of the specific improvement a pedometer intervention can have alongside pulmonary rehabilitation on daily physical activity without knowing the exact progression of exercise training for individual patients during pulmonary rehabilitation. Finally, despite a comprehensive search of the literature using the main scientific search databases, there is still a possibility that studies eligible for inclusion may have been missed. The search restriction on English written studies and the failure to search for unpublished studies and/or abstracts/conference papers may have resulted in selection and publication bias.
Conclusion
In conclusion, our systematic review and meta-analysis provides evidence that pedometer-based physical activity promotion promotes steps per day when it is used as an intervention alone or alongside pulmonary rehabilitation, including two recently published RCTs [20, 31]. Future trials should concentrate on high-quality study designs, with specific thought towards the optimal way of using pedometers during physical activity promotion (i.e. consider frequency of goal setting, type of patient feedback, length of intervention and instrument used for assessing physical activity). This review has found further evidence that patients benefit more from physical activity promotion when baseline levels of physical activity are >4000 steps·day−1. Therefore, consideration of baseline daily physical activity levels and/or exercise tolerance [38], should feature prominently in future studies. Furthermore, future studies should investigate the combined benefits of pulmonary rehabilitation, physical activity promotion and cognitive behavioural therapy for those patients with severe COPD who are anxious and depressed and therefore exhibit limitations in improving daily physical activity. Moreover, future studies could incorporate the addition of semi-automated tele-coaching as delivered by Demeyer et al. [7], as a low maintenance approach to providing continued support towards daily physical activity feedback [38].
Footnotes
This study is registered at www.crd.york.ac.uk/prospero/ with identifier CRD42018103893.
Provenance: Submitted article, peer reviewed.
Author contributions: M. Armstrong conducted the study, collected and analysed the data, and wrote the manuscript. N. Chynkiamis supported the collection of data. I. Vogiatzis, A. Winnard, S. Boyle and C. Burtin contributed to the review/editing of the manuscript. All authors read and approved the final manuscript.
Conflict of interest: M. Armstrong has nothing to disclose.
Conflict of interest: A. Winnard has nothing to disclose.
Conflict of interest: N. Chynkiamis has nothing to disclose.
Conflict of interest: S. Boyle has nothing to disclose.
Conflict of interest: C. Burtin has nothing to disclose.
Conflict of interest: I. Vogiatzis has nothing to disclose.
References
- 1.Qiu S, Cai X, Wang X, et al. . Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis 2018; 12: 10.1177/1753466618787386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watz H, Pitta F, Rochester CL, et al. . An official European Respiratory Society statement on physical activity in COPD. Eur Respir J 2014; 44: 1521–1537. [DOI] [PubMed] [Google Scholar]
- 3.Pitta F, Troosters T, Spruit MA, et al. . Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 972–977. [DOI] [PubMed] [Google Scholar]
- 4.Van Remoortel H, Hornikx M, Demeyer H, et al. . Daily physical activity in subjects with newly diagnosed COPD. Thorax 2013; 68: 962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vorrink SN, Kort HS, Troosters T, et al. . Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res 2011; 12: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Aymerich J, Lange P, Benet M, et al. . Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population-based cohort study. Thorax 2006; 61: 772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demeyer H, Donaire-Gonzalez D, Gimeno-Santos E, et al. . Physical activity is associated with attenuated disease progression in COPD. Med Sci Sports Exerc 2018; 51: 833–840. [DOI] [PubMed] [Google Scholar]
- 8.Spruit MA, Singh SJ, Garvey C, et al. . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. [DOI] [PubMed] [Google Scholar]
- 9.Bauman AE, Reis RS, Sallis JF, et al. . Correlates of physical activity: why are some people physically active and others not? Lancet 2012; 380: 258–271. [DOI] [PubMed] [Google Scholar]
- 10.Baumeister RF, Vohs KD, DeWall CN, et al. . How emotion shapes behavior: feedback, anticipation, and reflection, rather than direct causation. Pers Soc Psychol Rev 2007; 11: 167–203. [DOI] [PubMed] [Google Scholar]
- 11.Vohs KD, Baumeister RF, eds. Handbook of self-regulation: research, theory, and applications. 3rd Edn. New York, Guilford Publications, 2016. [Google Scholar]
- 12.Mantoani LC, Rubio N, McKinstry B, et al. . Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J 2016; 48: 69–81. [DOI] [PubMed] [Google Scholar]
- 13.Demeyer H, Louvaris Z, Frei A, et al. . Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax 2017; 72: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altenburg W, ten Hacken NH, Bossenbroek L, et al. . Short- and long-term effects of a physical activity counselling program in COPD. Eur Respir J 2014; 44: 3490. [DOI] [PubMed] [Google Scholar]
- 15.Cruz J, Brooks D, Marques A. Walk2Bactive: a randomized controlled trial of a physical activity-focused behavioural intervention beyond pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron Respir Dis 2016; 13: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 17.Lahham A, McDonald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis 2016; 11: 3121–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maher CG, Sherrington C, Herbert RD, et al. . Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003; 83: 713–721. [PubMed] [Google Scholar]
- 19.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions 5.1. 0. Cochrane Collaboration 2011; 33–49. [Google Scholar]
- 20.Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, et al. . Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (urban training) to increase physical activity in patients with COPD: a randomised controlled trial. Eur Respir J 2018; 52: 1800063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bender BG, Depew A, Emmett A, et al. . A patient-centered walking program for COPD. Chronic Obstr Pulm Dis 2016; 3: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Blok BM, de Greef MH, ten Hacken NH, et al. . The effects of a lifestyle physical activity counselling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD a pilot study. Patient Educ Couns 2006; 61: 48–55. [DOI] [PubMed] [Google Scholar]
- 23.Holland AE, Mahal A, Hill CJ, et al. . Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax 2017; 72: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornikx M, Demeyer H, Camillo CA, et al. . The effects of a physical activity counseling program after an exacerbation in patients with chronic obstructive pulmonary disease: a randomized controlled pilot study. BMC Pulm Med 2015; 15: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hospes G, Bossenbroek L, Ten Hacken NH, et al. . Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns 2009; 75: 274–278. [DOI] [PubMed] [Google Scholar]
- 26.Kawagoshi A, Kiyokawa N, Sugawara K, et al. . Effects of low-intensity exercise and home-based pulmonary rehabilitation with pedometer feedback on physical activity in elderly patients with chronic obstructive pulmonary disease. Respir Med 2015; 109: 364–371. [DOI] [PubMed] [Google Scholar]
- 27.Mendoza L, Horta P, Espinoza J, et al. . Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J 2015; 45: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moy ML, Collins RJ, Martinez CH, et al. . An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD. Chest 2015; 148: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nolan CM, Maddocks M, Canavan JL, et al. . Pedometer step count targets during pulmonary rehabilitation in chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med 2017; 195: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabak M, Vollenbroek-Hutten MM, van der Valk PD, et al. . A telerehabilitation intervention for patients with chronic obstructive pulmonary disease: a randomized controlled pilot trial. Clin Rehabil 2014; 28: 582–591. [DOI] [PubMed] [Google Scholar]
- 31.Varas AB, Córdoba S, Rodríguez-Andonaegui I, et al. . Effectiveness of a community-based exercise training programme to increase physical activity level in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Physiother Res Int 2018; 23: e1740. [DOI] [PubMed] [Google Scholar]
- 32.Vorrink SN, Kort HS, Troosters T, et al. . Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. Eur Respir J 2016; 48: 1019–1029. [DOI] [PubMed] [Google Scholar]
- 33.Wan ES, Kantorowski A, Homsy D, et al. . Promoting physical activity in COPD: insights from a randomized trial of a web-based intervention and pedometer use. Respir Med 2017; 130: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vestbo J, Hurd SS, Agustí AG, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 35.Demeyer H, Burtin C, Hornikx M, et al. . The minimal important difference in physical activity in patients with COPD. PLoS One 2016; 11: e0154587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu S, Cai X, Chen X, et al. . Step counter use in type 2 diabetes: a meta-analysis of randomized controlled trials. BMC Med 2014; 12: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitta F, Troosters T, Spruit MA, et al. . Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2005; 86: 1979–1985. [DOI] [PubMed] [Google Scholar]
- 38.Osadnik CR, Loeckx M, Louvaris Z, et al. . The likelihood of improving physical activity after pulmonary rehabilitation is increased in patients with COPD who have better exercise tolerance. Int J Chron Obstruct Pulmon Dis 2018; 13: 3515–3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulart AA, Munari AB, Santos Silva IJC, et al. . Baseline characteristics associated to improvement of patients with COPD in physical activity in daily life level after pulmonary rehabilitation. Respir Med 2019; 151: 142–147. [DOI] [PubMed] [Google Scholar]
- 40.Van Remoortel H, Raste Y, Louvaris Z, et al. . Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PLoS One 2012; 7: e39198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baskerville R, Ricci-Cabello I, Roberts N, et al. . Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2017; 34: 612–620. [DOI] [PubMed] [Google Scholar]
- 42.Bravata DM, Smith-Spangler C, Sundaram V, et al. . Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007; 298: 2296–2304. [DOI] [PubMed] [Google Scholar]
- 43.Coffman MJ, Ferguson BL, Steinman L, et al. . A health education pilot for Latina women with diabetes. Clin Nurs Res 2013; 22: 70–81. [DOI] [PubMed] [Google Scholar]
- 44.Clark GT, Mulligan R. Fifteen common mistakes encountered in clinical research. J Prosthodont Res 2011; 55: 1–6. [DOI] [PubMed] [Google Scholar]