Abstract
Asthma is a chronic, inflammatory lung disease affecting around 235 million people worldwide. Conventional medications in asthma are not curative and patients have significant concerns regarding their side-effects. Consequently, many asthma patients turn to complementary and alternative medicine (CAM) for a more holistic approach to care. We systematically reviewed the available evidence on the effectiveness of CAM in the management of asthma in adults.
We searched the MEDLINE, EMBASE, CINAHL, AMED and Cochrane databases for randomised controlled trials published in English between 1990 and 2016 investigating the effectiveness of oral or topical CAM in asthmatic adults. The quality of the studies was assessed using the Cochrane Risk of Bias Assessment Tool.
In all, 23 eligible trials were identified covering 19 different CAMs. Overall, there was limited evidence on the effectiveness of CAM in adult asthma as most CAMs were only assessed in a single trial. CAMs with multiple trials provided null or inconsistent results. Many of the trials were rated as having high risk of bias.
The existing evidence is insufficient to recommend any of the oral and topical CAMs in the management of asthma in adults.
Short abstract
Evidence is insufficient to recommend complementary and alternative medicines in the management of asthma http://ow.ly/xfcg306FSli
Introduction
Asthma is a chronic, inflammatory lung disease affecting around 235 million people worldwide [1]. Conventional medications in asthma are not curative and patients have significant concerns regarding their side-effects. Consequently, complementary and alternative medicine (CAM) is increasingly used in the management of asthma. Evidence suggests that up to 79% of patients use CAM [2]. The “natural”, noninvasive appeal of CAM combined with previous positive experience, consultation quality and personal beliefs are cited as the main reason for CAM use in asthma patients [3].
There have been several systematic reviews to assess the use of CAM in the management of asthma [4–9]. However, the existing reviews have been limited by poor methodology (study design and outcome measures) and conflicting results, thus preventing any definitive conclusions. Also, these reviews have made a broad assessment of CAM use amongst asthmatics and have not always isolated practitioner-based CAM from complementary medicines and, therefore, have not been able to attribute any benefit to one or the other. This review aims to address these problems by focusing on specific complementary medicines in the adult asthmatic population with clear outcome measures related to efficacy.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and recommendations of the Cochrane Collaboration were followed for the reporting of this review (www.prisma-statement.org).
Eligibility criteria
Studies were included if they: 1) were a randomised controlled trial (RCT) published in English between 1990 and 2015; 2) investigated a self-administered oral or topically applied CAM (i.e. not practitioner-based therapy); and 3) included only asthmatic participants aged ≥18 years with a diagnosis of asthma by a general practitioner, consultant or standard guidelines (e.g. British Thoracic Society). Trials with healthy controls, animals, pregnant women or children were excluded.
Information sources
During October 2016 the following databases were searched: AMED, EMBASE, MEDLINE and the Cochrane Library. The most recent search was conducted on October 7, 2016. References in each of the identified articles were further screened for relevant articles.
Search
The database search was constructed around three themes: 1) identification of relevant oral and topical interventions using 76 names of CAMs commonly taken in asthma; 2) identification of the disease of interest using “asthma” or other relevant terms; and 3) identification of relevant study design (RCTs). The search terms were adapted to suit each database and connected by Boolean terms “OR” and “AND”. The details of the search strategy can be found in the supplementary material.
Study selection
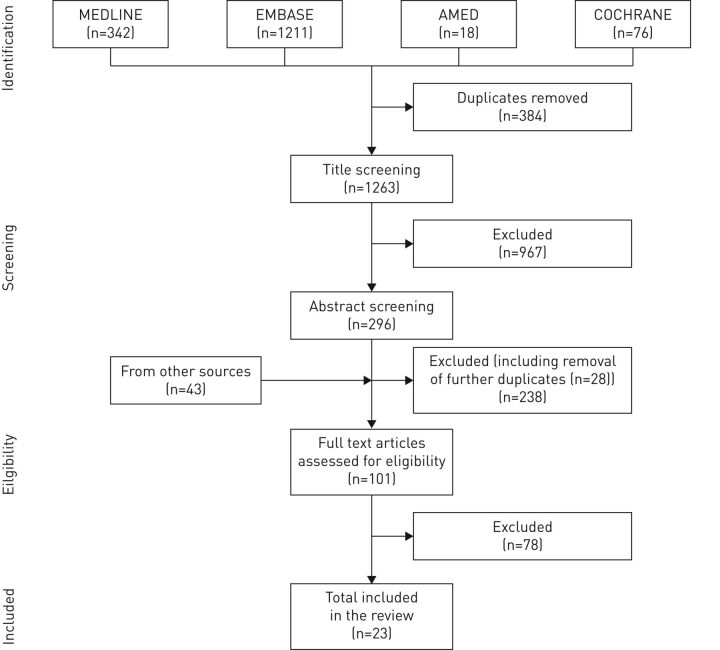
Study titles were double screened (by both authors) and irrelevant or duplicate articles removed. The abstracts of the remaining articles were reviewed using the selection criteria. During this stage, the criteria were adapted to exclude trials involving magnesium sulfate, biologic agents and monoclonal antibodies, as these form part of the national management guidelines and therefore do not conform to the World Health Organization definition of CAM. If the abstract did not provide sufficient information then the full text article was reviewed. Trials were excluded where the intervention was not self-administered or the patient group had comorbid lung disease. The identification of relevant studies is presented in figure 1.
FIGURE 1.
Flow chart of article selection process for this review.
Data extraction and items
Data from each eligible study were extracted and details of the author, date, country, setting, participant characteristics, intervention, control, duration of intervention, outcome measures and outcomes were placed in summary tables. The risk of bias was assessed using the Cochrane Risk of Bias Assessment Tool [10]. An “unclear” judgement was made if insufficient detail was provided. The raw data on change of outcome measures from each study was summarised in table form to enable easier comparison of outcome measures between studies. The results are summarised in tables 1 and 2.
TABLE 1.
Summary of results for complementary and alternative medicine (CAM) tested in single trials
| CAM | Intervention group | Control group | Treatment duration | Outcome | First author [ref.] | Country |
| Curcumin | “Standard” asthma treatment + 500 mg curcumin twice daily | “Standard” asthma treatment | 4 weeks | Difference (+): pre/post-bronchodilator FEV1 No difference (−): clinical symptom severity, adverse events |
Abidi [11] | India |
| New Zealand green-lipped mussel | Two 150 mg capsules of New Zealand green-lipped mussel twice daily | Two 150 mg placebo capsules twice daily | 8 weeks | Difference (+): mean daytime wheeze, mean morning PEFR No difference (−): nocturnal awakening, use of short-acting inhaled β2-agonists, mean FEV1, evening PEFR |
Emelyanov [12] | Russia |
| Solanum xanthocarpum | Solanum xanthocarpum 300 mg orally once daily | Salbutamol 4 mg once daily or deriphylline 220 mg | 1 day | Difference (+): FVC, FEV1, PEFR No difference (−): none |
Govindan [13] | India |
| Solanum trilobatum | Solanum trilobatum 300 mg orally once daily | Salbutamol 4 mg once daily or deriphylline 220 mg | 1 day | Difference (+): none No difference (−): FVC, FEV1, PEFR |
Govindan [13] | India |
| Coenzyme Q10 | 120 mg Coenzyme Q10 + 400 mg α-tocopherol + 250 mg vitamin C + “standard” asthma treatment | “Standard” asthma treatment | 32 weeks (16-week crossover) | Difference (+): total usage of corticosteroids No difference (−): FEV1, FEV1/FVC, PEFR |
Gvozdjakova [14] | Slovakia |
| Selenium | Daily oral 100 μg sodium selenite | Daily oral placebo | 14 weeks | Difference (+): none No difference (−): FEV1, PEFR |
Hasselmark [15] | Sweden |
| Auranofin | 3 mg oral auranofin twice daily | 3 mg oral placebo twice daily | 12 weeks | Difference (+): none No difference (−): FEV1, FVC |
Honma [16] | Japan |
| Pyridoxine (vitamin B6) | 300 mg oral pyridoxine (mixed with lactose) daily + oral prednisolone | Oral placebo daily + oral prednisolone | 9 weeks | Difference (+): none No difference (−): morning/evening PEFR, FEV1, morning/evening symptom scores, nocturnal asthma awakening |
Kaslow [17] | USA |
| Aqueous extract of propolis | 13% aqueous extract of propolis in water orally once daily + oral theophylline | Placebo sachet in water orally once daily + oral theophylline | 8 weeks | Difference (+): need for rescue medication, number of nocturnal attacks per week, FVC, FEV1, PEFR No difference (−): none |
Khayyal [18] | Egypt |
| Vitamin E | Two capsules (250 mg vitamin E + soya bean oil) | Two placebo capsules (gelatine base) | 6 weeks | Difference (+): none No difference (−): FEV1, FVC, mean morning peak flow, symptom score, bronchodilator use |
Pearson [19] | UK |
| AKL1# | Two AKL1 capsules twice daily | Two placebo capsules twice daily | 36 weeks | Difference (+): none No difference (−): PEFR, FEV1, AQLQ, ACQ, LCQ |
Thomas [20] | UK |
| Lactose powder | 6.25 mg, 12.5 mg, 25 mg, 50 mg or 100 mg of lactose powder in rotadisk | Inhaled placebo in rotadisk | 1 day | Difference (+): none No difference (−): FEV1 |
Thoren [21] | Sweden |
| TJ-96 | 2.5 g of oral Saiboku-to TJ-96 three times daily | Oral placebo three times daily | 8 weeks | Difference (+): symptom score No difference (−): FEV1 |
Urata [22] | Japan |
| Pingchuan Yiqi Granule | Two oral Pingchuan Yiqi Granule capsules three times daily | Four oral Ruyi Dingchuan pills three times daily | 1 week | Difference (+): none No difference (−): FEV1, PEFR |
Zhang [23] | China |
FEV1: forced expiratory volume in 1 s; PEFR: peak expiratory flow rate; FVC: forced vital capacity; AQLQ: Asthma Quality of Life Questionnaire; ACQ: Asthma Control Questionnaire; LCQ: Leicester Cough Questionnaire. #: Picrorrhiza kurroa, Zingiber officinale, Ginkgo biloba and apocynin.
TABLE 2.
Summary of results for complementary and alternative medicines tested in multiple trials
| Intervention group | Control group | Treatment duration | Outcome | First author [ref.] | Country |
| Magnesium | |||||
| Daily oral magnesium amino chelate 450 mg (27.6 mmol) + oral vitamin C placebo | Daily oral vitamin C placebo + oral magnesium placebo | 16 weeks | Difference (+): none No difference (−): FEV1, FVC, PEFR, symptom control, bronchodilator use |
Fogarty [24] | UK |
| 400 mg oral magnesium supplement daily | Oral placebo daily | 8 weeks | Difference (+): asthma symptom score No difference (−): FEV1, PEFR, bronchodilator use |
Hill [25] | UK |
| Vitamin C | |||||
| Daily oral vitamin C 1 g (5.6 mmol) + oral magnesium placebo | Daily oral vitamin C placebo + oral magnesium placebo | 16 weeks | Difference (+): none No difference (−): FEV1, FVC, PEFR, symptom control, bronchodilator use |
Fogarty [24] | UK |
| 1000 mg oral vitamin C daily + “standard” asthma treatment | Oral placebo daily + “standard” asthma treatment | 4 weeks | Difference (+): none No difference (−): FEV1, FVC, FEV1/FVC |
Nadi [26] | Iran |
| n-3 Polyunsaturated fatty acids | |||||
| Two fish oil capsules (455 mg EPA + 325 mg DHA + 10 mg vitamin E) once daily | Two capsules of amide once daily | 2 weeks | Difference (+): none No difference (−): FEV1, ACQ score |
Moreira [27] | Portugal |
| 10–20 g of perilla seed oil daily (n-3 fatty acids) | 10–20 g of corn oil daily (n-6 fatty acids) | 4 weeks | Difference (+): FVC, FEV1 No difference (−): PEFR |
Okamoto [28] | Japan |
| ASHMI | |||||
| Oral ASHMI 600 mg, 1200 mg or 1800 mg twice daily + “standard asthma treatment” | Oral placebo twice daily + “standard asthma treatment” | 1 week | Kelly-Pieper [29] | USA | |
| Four oral ASHMI capsules 3 times a day + prednisone placebo | Prednisone 20 mg once daily + ASHMI placebo | 4 weeks | Difference (+): FEV1, PEFR No difference (−): Symptom score, use of bronchodilators |
Wen [30] | China |
| Vitamin D | |||||
| Six 2-monthly oral doses 6 mL vigantol oil containing 3 mg (120 000 IU) vitamin D3 | Six 2-monthly oral doses 6 mL vigantol oil containing 6 mL organolpetically identical placebo | 52 weeks | Difference (+): SGRQ score No difference (−): ACT score, FEV1, PEFR, symptom control |
Martineau [31] | UK |
| 2 weeks 0.25 mcg oral calcitrol twice daily then 2 weeks combination with oral prednisolone | 2 weeks organoleptically identical lactose placebo twice daily then 2 weeks combination with oral prednisolone | 10 weeks | No difference (−): ACQ score, change in FEV1 | Nanzer [32] | UK |
| 28 weeks 4000 IU once daily with ciclesonide 320 mcg controller therapy | 28 weeks placebo with ciclesonide 320 mcg controller therapy | 32 weeks | No difference (−): lung function (FEV1, PEFR), asthma control/symptom score | Castro [33] | USA |
ASHMI: anti-asthma herbal medicine intervention; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PEFR: peak expiratory flow rate; ACQ: Asthma Control Questionnaire; SGRQ: St George's Respiratory Questionnaire; ACT: Asthma Control Test; EPA: eicosapentaenoic acid.
Outcome measures and analysis
The primary measure was lung function. Secondary outcomes included quality of life, asthma control, medication usage, healthcare utilisation and adverse effects.
For the meta-analysis, as all data were continuous, end-point scores were expressed as mean differences or standardised mean differences (SMDs) with associated 95% confidence intervals. Meta-analysis was performed using RevMan 5.3 software.
Results
The literature search revealed a total of 1263 non-duplicate records. A total of 967 articles were excluded after title screening and 296 abstracts were reviewed. At this stage, 238 articles were excluded, including 28 additional duplicates, leaving 101 full text articles to be assessed for eligibility. These 101 articles included 43 additional studies identified from the bibliographies of already finalised articles. After examination of the full text, 78 more articles were excluded. 23 full text articles were included in the review. Two trials appeared twice, as they both investigated multiple interventions [13, 24]. The methodological quality of the included trials varied. Details of the risk of bias assessment for each trial are summarised in table 3 and 4.
TABLE 3.
Summary of the trials of complementary and alternative medicines (CAM) with significant positive outcomes
| CAM | Intervention group | Control group | Treatment duration | Sample size n | Positive outcome# | Intervention group | Control group | p-value | Overall risk of bias score¶ | First author [ref.] | Country |
| Curcumin | “Standard” asthma treatment + 500 mg curcumin twice daily | “Standard” asthma treatment | 4 weeks | 60 | Change in FEV1 | −0.72±0.69 | −0.83±0.63 | <0.05 | Low | Abidi [11] | India |
| New Zealand green-lipped mussel | Two 150 mg capsules of New Zealand green-lipped mussel twice daily | Two 150 mg placebo capsules twice daily | 8 weeks | 46 | Mean morning PEFR | 47.0±11.7 | −33.4±6.2 | <0.001 | Low | Emelyanov [12] | Russia |
| Solanum xanthocarpum | Solanum xanthocarpum 300 mg orally once daily | Salbutamol 4 mg once daily or deriphylline 220 mg | N/A (1 day) | 60 | % increase predicted FVC, % increase predicted FEV1, % increase PEFR | 22.4±2.3 25.3±2.7 20.8±2.4 |
32.2±3.7 41.9±6.9 37.5±6.0 |
<0.05 <0.05 <0.01 |
Unclear | Govindan [13] | India |
| Aqueous extract of propolis | 13% aqueous extract of propolis in water orally once daily + oral theophylline | Placebo sachet in water orally once daily + oral theophylline | 8 weeks | 45 | % increase FVC, % predicted FEV1, % increase PEFR | 18.7% 29.5% 29.8% |
Not available Not available Not available |
0.0001 0.0001 0.0001 |
Unclear | Khayyal [18] | Egypt |
| n-3 Polyunsaturated fatty acids | 10–20 g of perilla seed oil daily (n-3 fatty acids) | 10–20 g of corn oil daily (n-6 fatty acids) | 4 weeks | 14 | FVC, % predicted FEV1 | Exact data not available 96.7 (85.4–108.0) |
Exact data not available 90.9 (75.9–105.8) |
<0.05 <0.05 |
Unclear | Okamoto [28] | Japan |
| ASHMI | Four oral ASHMI capsules 3 times a day + prednisone placebo | Prednisone 20 mg once daily + ASHMI placebo | 4 weeks | 91 | Mean change in FEV1 and PEFR |
19.4±5.5 20.1±5.6 |
23.2±8.9 23.0±7.5 |
0.02 0.04 |
Unclear | Wen [30] | China |
ASHMI: anti-asthma herbal medicine intervention; FEV1: forced expiratory volume in 1 s; PEFR: peak expiratory flow rate; FVC: forced vital capacity. #: lung function only; ¶: the risk of bias was assessed using the Cochrane Risk of Bias Assessment Tool [10]. An “unclear” judgement was made if insufficient detail was provided. The mean judgement from the seven domains was used to determine the “overall” score.
TABLE 4.
Risk of bias summary: author's judgement for each risk of bias domain and included study
| First author [ref.] | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Abidi [11] | + | − | − | ? | + | + | + |
| Castro [33] | + | ? | + | ? | + | + | + |
| Emelyanov [12] | + | ? | + | ? | + | + | + |
| Fogarty [24] | + | ? | + | ? | + | + | + |
| Govindan [13] | ? | ? | − | − | ? | + | + |
| Gvozdjakova [14] | ? | ? | − | ? | ? | − | + |
| Hasselmark [15] | + | ? | + | ? | + | − | + |
| Hill [25] | + | ? | + | ? | − | + | + |
| Honma [16] | ? | ? | + | ? | − | + | + |
| Kaslow [17] | ? | ? | + | ? | + | + | + |
| Kelly-Pieper [29] | ? | ? | + | ? | − | − | − |
| Khayyal [18] | ? | ? | ? | ? | + | + | + |
| Martineau [31] | + | ? | + | ? | + | + | + |
| Moreira [27] | + | + | + | ? | − | + | + |
| Nadi [26] | ? | ? | + | ? | ? | + | + |
| Nanzer [32] | + | + | + | + | + | + | + |
| Okamoto [28] | ? | ? | ? | ? | ? | − | + |
| Pearson [19] | + | ? | + | + | + | + | + |
| Thomas [20] | ? | ? | + | ? | − | + | + |
| Thoren [21] | ? | ? | + | ? | + | + | + |
| Urata [22] | ? | ? | + | ? | − | + | + |
| Wen [30] | ? | ? | + | ? | + | + | − |
| Zhang [23] | + | ? | ? | ? | − | + | + |
+: low risk; ?: unclear risk; −: high risk.
CAM tested in single trials
Curcumin
Curcumin is a natural product from the rhizome turmeric (Curcuma longa) [11]. A trial of 60 adults aged 18–55 years with bronchial asthma randomised participants to receive either 500 mg curcumin twice daily and standard asthma therapy or standard asthma therapy alone for 30 days [13]. Lung function was assessed using pre- and post-bronchodilator forced expiratory volume in 1 s (FEV1). The study reported significant improvement in FEV1 in the intervention group compared to the control group (p<0.05).
New Zealand green-lipped mussel
This CAM contains the lipid extract of Perna canaliculus (omega-3 fatty acids) [12]. A total of 46 adults aged 18–65 years with mild-to-moderate atopic asthma were randomised into either an intervention or control group for 8 weeks [12]. The intervention was two 150-mg capsules twice daily containing 50 mg omega-3 polyunsaturated fatty acids and 100 mg olive oil plus inhaled salbutamol as rescue medication. The control group took two placebo capsules twice daily containing 150 mg olive oil and rescue salbutamol when required. FEV1 and peak expiratory flow rate (PEFR) were used to measure lung function. Morning PEFR was significantly improved in the intervention group compared to placebo (p<0.001). There was no difference in FEV1 and evening PEFR.
Solanum xanthocarpum and Solanum trilobatum
In this trial, 60 adults aged 18–50 years with mild-to-moderate asthma took part in a single day trial [13]. Participants received either 300 mg oral Solanum xanthocarpum or 300 mg oral Solanum trilobatum (CAM commonly used in southern Indian Siddha medicine) as the intervention and 200 mg deriphylline or 4 mg salbutamol as the control. Lung function was assessed using forced vital capacity (FVC), FEV1 and PEFR. Compared to salbutamol, post-intervention FEV1 and FVC was significantly greater with S. xanthocarpum (p<0.05). PEFR improvement was significantly greater compared to deriphylline (p<0.01). There was no significant difference between the S. trilobatum intervention group and the two controls (salbutamol and deriphylline).
Coenzyme Q10
The antioxidants coenzyme Q10 and α-tocopherol were assessed in this crossover trial of 41 adults aged 25–50 years with mild-to-moderate bronchial asthma. A combination of 120 mg of coenzyme Q10 gel, 400 mg α-tocopherol, 250 mg vitamin C and standard asthma therapy were compared with standard asthma therapy (inhaled corticosteroids and short-acting β-agonists) alone. Participants received either the intervention or control for 16 weeks before crossing over to the other trial arm. Lung function was assessed with FEV1 and PEFR; neither of which showed significant changes compared to the placebo.
Selenium
In this small trial, 24 adults aged 18–75 years with intrinsic asthma were randomised to receive either 100 µg of oral sodium selenite (containing chemical element selenium) or oral placebo for 14 weeks [15]. FEV1 and PEFR were used to measure lung function. There was no statistically significant difference between the intervention and control groups for these parameters.
Auranofin
Auranofin is an oral gold compound that was used as the intervention in this 12-week trial of 19 adults with asymptomatic bronchial asthma [16]. Participants received either 3 mg oral auranofin twice daily or 3 mg placebo twice daily. FEV1 and FVC were outcome measures of interest. There was no statistically significant difference between the auranofin and placebo group.
Pyridoxine
31steroid-dependent adults aged 19–56 years with a diagnosis of asthma were included in this 9-week trial [17]. Participants were randomised to take 300 mg oral pyridoxine (vitamin B6 mixed with lactose) once daily alongside oral prednisolone or daily oral placebo and prednisolone. FEV1 and PEFR were measured. Although morning PEFR improved in the pyridoxine group in the last 2 weeks of the trial (p=0.02), compared to the placebo it was insignificant. There were no significant differences in any other outcome measure.
Aqueous extract of propolis
45 adults aged 19–52 years with a 2–5 year history of mild-to-moderate asthma were randomised to either the intervention or control arm [18]. Participants received a sachet of either 13% aqueous extract of propolis or placebo suspended in water as a milk drink once daily with oral theophylline for 2 months. Lung function was measured by FVC, FEV1 and PEFR. There was a significant improvement in FVC, FEV1 and PEFR during the trial period (p=0.0001). However, these results were not compared to the placebo and therefore the effectiveness of aqueous extract of propolis compared to the placebo remains unclear.
Vitamin E
This high-quality trial randomised participants to receive either two capsules containing 250 mg of natural vitamin E (D-α-tocopherol) in soya bean oil or two capsules of gelatine-based placebo daily for 6 weeks [19]. The 64 participants had physician-diagnosed asthma and were aged between 18 and 60 years. Each was using at least one dose of inhaled corticosteroid daily. FEV1, FVC and morning PEFR were used to assess lung function. There was no significant difference between the placebo and vitamin E groups in any outcome measure.
AKL1
AKL1 is a botanical product containing Picrorrhiza kurroa, Zingiber officinale, Ginkgo biloba and apocynin [20]. In this crossover trial, 32 participants were randomised to receive either two AKL1 capsules twice daily or two placebo capsules twice daily for 12 weeks before an 8 week wash-out period and a further 12 week crossover period. Lung function was measured by PEFR and FEV1. There was no statistically significant difference between the two groups.
Lactose powder
18 participants aged 18–57 years completed this trial. Four rotadisks were provided to each, containing either placebo (control), 6.25 mg, 12.5 mg, 25 mg, 50 mg or 100 mg lactose in total [21]. All were to be self-administered (via inhalation) in quick succession during one study visit. On a separate visit, all participants took another four rotadisks containing only placebo. FEV1 was used to assess lung function. No significant difference was observed in FEV1 between the control and intervention arms.
TJ-96
TJ-96 (or Saiboku-to) is a herbal compound used in kampo medicine [22]. 32 participants with mild-to-moderate atopic asthma were randomised to receive either 2.5 g of oral TJ-96 three times daily or oral placebo three times daily for 8 weeks. Outcome measures included FEV1 and FVC, which did not improve significantly when compared to placebo.
Pingchuan Yiqi Granule
This is a Chinese compound containing ephedra herb, red ginseng, Japanese Yam rhizome, apricot seed, magnolia bark, common perilla leaf, thorowax root, tangerine peel and liquorice root [23]. In this 7-day trial of 80 adults aged 33–60 years with mild-to-moderate bronchial asthma, participants were randomised to receive either two oral Pingchuan Yiqi Granule capsules three times daily or four control capsules three times daily. The control was Ruyi Dingchuan, another Chinese compound, containing gecko, toad venom, milkvetch root, earthworm, ephedra herb, asiabell root, apricot seed, gingko seed, immature bitter orange, asparagus root, schisandra fruit, lilyturf root, tatarian aster root, rhizome, stemona root, Barbary wolfberry, prepared rehmannia root, polygala root, pepperweed seed, hindu datura dried flower, gypsum fibrosum, and honey grilled liquorice root [23]. Lung function was assessed through FEV1 and PEFR. There was no significant difference between the control and intervention groups for these outcomes.
CAM tested in multiple trials
Magnesium
The first trial of 234 participants aged 18–60 years were randomised to receive either 450 mg daily oral magnesium amino chelate and oral vitamin C placebo or daily oral magnesium and vitamin C placebo for 16 weeks [24]. FEV1, FVC and PEFR were used to assess lung function. There was no significant difference between the control and intervention groups for these outcomes.
The second trial of 17 steroid-dependant asthmatic adults aged 25–60 years observed the effect of 400 mg daily oral magnesium versus daily placebo on FEV1 and PEFR [25]. This 8-week crossover trial had a 1-week run-in period followed by 3 weeks of intervention, 1 week of wash out and 3 more weeks of intervention. The outcome measures of interest were insignificant. Meta-analysis was conducted for FEV1.
Vitamin C
Neither of the two included trials found any difference in outcome measures between the control and vitamin C groups. The first trial of 234 participants aged 18–60 years were randomised to receive either 1 g daily oral vitamin C and oral magnesium placebo or daily oral vitamin C and magnesium placebo for 16 weeks [24]. Lung function was measured with FEV1, FVC and PEFR. The second trial of 60 adults aged 30–57 years with severe bronchial asthma measured FEV1, FVC and FEV1/FVC [26]. For 1 month, participants continued standard asthma therapy with either 1 g oral vitamin C daily or oral placebo. There was no significant difference in any of the outcomes between the intervention and control group. Meta-analysis was conducted for FEV1 and FVC.
n-3 Polyunsaturated fatty acids
20 females aged 24–46 years with stable, persistent asthma were randomised to receive two capsules of amide once daily (as the control) or two fish-oil capsules containing 455 mg eicosapentaenoic acid, 325 mg docosahexaenoic acid and 10 mg vitamin E daily [27]. This 2-week trial measured FEV1, which did not change significantly.
The second trial observed the effect of 10–20 g of perilla seed oil daily (n-3 polyunsaturated fatty acid) on PEFR, FEV1 and FVC compared to 10–20 g of corn oil daily (n-6 fatty acids) [28]. 14 adults aged 22–84 years with moderate asthma were recruited to this 4-week trial. Participants received oral theophylline, inhaled short-acting β-agonists and steroids regularly. A significant difference was noted between the control and intervention groups with regard to FEV1 (p<0.05) and FVC (p<0.05).
ASHMI
ASHMI (anti-asthma herbal medicine intervention) is a Chinese herbal formula containing Ku-Shen (Sophora flavescens), Gan-Cao (Glycyrrhiza uralensis) and Ling-Zhi (Ganoderma lucidum). The first of the two included studies did not provide details of their lung function results so could not be fully analysed [30]. The 20 participants in this trial, aged 18–55 years received 600 mg, 1200 mg or 1800 mg oral ASHMI twice daily with standard asthma therapy for 1 week. The control was a corn starch capsule taken twice daily alongside standard asthma treatment.
The second trial of 91 adults aged 18–65 years with moderate-to-severe asthma took either four ASHMI capsules three times a day with a prednisone placebo or 20 mg prednisone once daily with an ASHMI placebo [29]. This 4-week trial assessed lung function using FEV1 and PEFR, which improved significantly throughout the trial (p=0.02 and p=0.04, respectively).
Vitamin D
The three included trials found no difference in lung function between control and vitamin D groups. The first trial of 250 participants aged 16–80 years were randomised to receive 6 mL of vigantol oil containing either 3 mg vitamin D3 or 6 mL organoleptically identical placebo every 2 months for 12 months [31]. FEV1 and PEFR were used to assess lung function. No significant difference was observed in either outcome. The second trial of 23 asthmatic adults were randomised to receive either 4 weeks of 0.25 mcg oral calcitrol or lactose placebo twice-daily following 2 weeks of oral prednisolone and a 4-week wash-out period [32]. Adjunctive oral prednisolone was also taken in the last 2 weeks of the trial. FEV1 was used to assess lung function; no significant comparisons were found between groups. The final trial assessed lung function in 408 asthmatic adults taking 4000 IU of oral vitamin D for 28 weeks alongside regular ciclenoside controller therapy [33]. No significant results were found for FEV1 or PEFR when comparing groups.
Meta-analysis
Magnesium
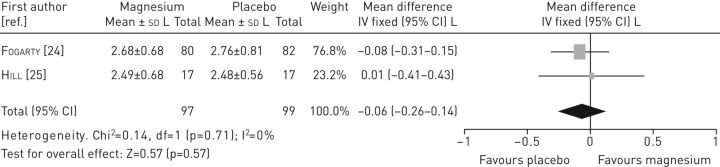
Data from two studies [24, 25] were pooled to assess the effectiveness of magnesium on FEV1 (figure 2). The result indicated no statistically significant differences in FEV1 between the intervention and the control group (mean difference −0.06, 95% CI −0.26–0.14). Data could not be pooled for any other outcomes.
FIGURE 2.
Forest plot of comparison: magnesium versus control (outcome: forced expiratory volume in 1 s).
Vitamin C
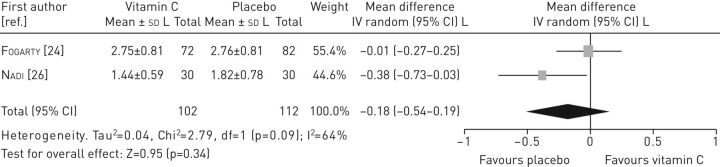
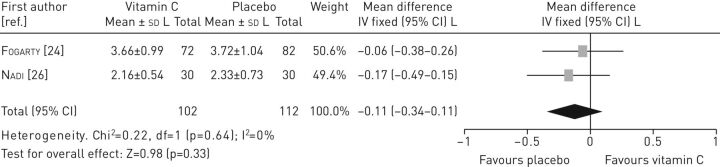
Data from two studies [11, 26] were pooled to assess the effectiveness of vitamin C on FEV1 (figure 3), which indicated no statistically significant differences in FEV1 between the intervention and the control group (mean difference −0.18, 95% CI −0.54–0.19). Also, no statistically significant differences in FVC were found between the intervention and the control group (mean difference −0.11, 95% CI −0.34–0.11) (figure 4).
FIGURE 3.
Forest plot of comparison: vitamin C versus control (outcome: forced expiratory volume in 1 s).
FIGURE 4.
Forest plot of comparison: vitamin C versus control (outcome: forced vital capacity).
Vitamin D
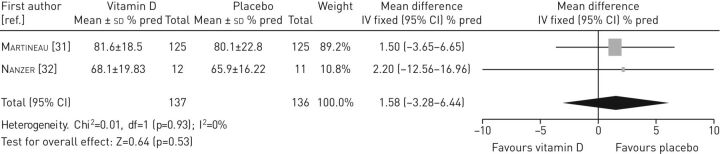
Data from two studies [31, 32] were pooled to assess the effectiveness of vitamin D on FEV1 (figure 5). The result indicated no statistically significant differences in FEV1 between the intervention and the control group (mean difference 1.58, 95% CI −3.28–6.44).
FIGURE 5.
Forest plot of comparison: vitamin D versus control (outcome: forced expiratory volume in 1 s).
Quality of evidence
A large number of included studies were of poor quality. Individual studies were small, ranging from 14 to 232 participants. 12 trials did not detail sequence generation [13, 14, 16–18, 20–22, 26, 28–30] and 20 trials did not adequately detail allocation concealment [11–26, 28, 29, 30, 33]. Complete blinding (of participants, personnel and outcome assessors) was only reported in two trials [19, 32]. 11 trials had incomplete outcome data [13, 14, 16, 20, 22, 23, 25, 26–29] and four selective reporting [14, 15, 28, 29]. Three of the trials [15–17] were published before the introduction of the CONSORT guidelines in 1996 which could explain their poor reporting of randomisation method and blinding [34]. This globally low level of methodological quality is a significant limitation of this review. Table 3 details the results of those trials with positive results and their overall risk of bias score.
Discussion
This review identified 23 trials measuring the effectiveness of 19 CAMs in the treatment of asthma in adults. Overall, this review found limited evidence that oral and topical CAMs have any effect on lung function, symptom control, quality of life, asthma medication usage, healthcare utilisation and adverse events. Meta-analysis was performed on magnesium, vitamin C and vitamin D, the results of which indicated that neither magnesium, vitamin C or D were beneficial in improving the lung function of asthma patients. Eight single trials showed improvement in lung function (table 3) and symptom control but there was no overall trend in one outcome measure across trials. In addition, many of the trials had a poor methodological quality, restricting the reliability and applicability of these results. These limitations have been mirrored in previous reviews.
Passalacqua et al. [4] found positive results but were limited by “methodological flaws”, such as poor randomisation, lack of blinding and no quantitative measurement. Many of the results derived in this review described themselves as double-blind, placebo-controlled trials but on further investigation were actually of very low quality. The majority of included studies had predominantly unclear or high-risk domains in their risk of bias assessment. This globally low level of methodological quality is a significant limitation of this review.
This review found 10 of the 23 included trials had some positive results, which were both primary and secondary outcome measures. In a review by Huntley et al. [6], approximately half of the trials had some positive outcomes, where the intervention group improved more than the placebo. Many of these findings were from a single trial with a high risk of bias assessment and, similar to this review, must be taken in this context. Huntley et al. [6] also highlighted the problem of using products with non-standardised quality and therefore even positive results for CAMs tested in multiple trials should be viewed critically.
Manufacturers of CAM compounds are not always required to prove the safety of their products [8], which are often poorly standardised and may contain harmful substances [4]. The low number of reports in included studies could be due to the search strategy of this review where the primary outcome of interest was lung function. Therefore, some trials focusing on adverse events may not have met entry criteria and been excluded. This deficit in information regarding adverse events and their frequency means CAM cannot be recommended for use in routine clinical practice.
14 of the included CAMs were only tested in one trial, which often also had a small sample size. This limited the data that could be pooled for meta-analysis and therefore the review. Furthermore, the type of outcome measure, outcome measurement tool and study duration varied across trials. The search was restricted to trials published in English after 1990, which assumes that no significant evidence was published before this date or in another language and therefore trials may have been missed. Procedural differences, participant compliance, smoking, concomitant medication usage and varying asthma severity further limited comparison between trials, the reliability of results and the validity of this review.
Overall, good quality RCTs assessing the efficacy of CAM in the management of asthma are rare. The included studies cover a wide range of outcomes and although this highlights the potential impact of CAM in future practice, it is unclear which asthma-related outcome CAM benefits the most. This review does not provide sufficient evidence to recommend any of the included CAM compounds for asthma. However, it also does not demonstrate that they are ineffective and suggests a need for further, higher quality RCTs with validated outcome measurement tools and formal safety assessments.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0092-2016_supplementary_material (112KB, pdf)
Footnotes
This article has supplementary material available from err.ersjournals.com
Conflict of interest: None declared.
Provenance: Submitted article, peer reviewed.
References
- 1.World Health Organization. Asthma. www.who.int/mediacentre/factsheets/fs307/en/ Date last accessed: January 4 2016. Date last updated: November 2013.
- 2.Chen W, FitzGerald J, Rousseau R, et al. . Complementary and alternative asthma treatments and their association with asthma control: a population-based study. BMJ Open 2013; 3: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst E. Complementary therapies for asthma: what patients use. J Asthma 1998; 35: 667–671. [DOI] [PubMed] [Google Scholar]
- 4.Passalacqua G, Bousquet P, Carlsen K, et al. . Aria update: I – systematic review of complementary and alternative medicine for rhinitis and asthma. J Allergy Clin Immunol 2006; 117: 1054–1062. [DOI] [PubMed] [Google Scholar]
- 5.Slader C, Reddel H, Jenkins C, et al. . Complementary and alternative medicine use in asthma: who is using what? Respirology 2006; 11: 373–387. [DOI] [PubMed] [Google Scholar]
- 6.Huntley A, Ernst E. Herbal medicines for asthma: a systematic review. Thorax 2000; 55: 925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarney RW, Linde K, Lasserson TJ. Homeopathy for chronic asthma. Cochrane Database Syst Rev 2004; 1: CD000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George M, Topaz M. A systematic review of complementary and alternative medicine for asthma self-management. Nurs Clin North Am 2013; 48: 1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CE, Arnold E, Lasserson TJ, et al. . Herbal interventions for chronic asthma in adults and children: a systematic review and meta-analysis. Prim Care Respir J 2010; 19: 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. 2011. [Google Scholar]
- 11.Abidi A, Gupta S, Agarwal M, et al. . Evaluation of efficacy of curcumin as an add-on therapy in patients of bronchial asthma. J Clin Diagn Res 2014; 8: HC19–HC24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emelyanov A, Fedoseev G, Krasnoschekova O, et al. . Treatment of asthma with lipid extract of New Zealand green-lipped mussel: a randomised clinical trial. Eur Respir J 2002; 20: 596–600. [DOI] [PubMed] [Google Scholar]
- 13.Govindan S, Viswanathan S, Vijayasekaran V, et al. . A pilot study on the clinical efficacy of Solanum xanthocarpum and Solanum trilobatum in bronchial asthma. J Ethnopharmacol 1999; 66: 205–210. [DOI] [PubMed] [Google Scholar]
- 14.Gvozdjakova A, Kucharska J, Bartkovjakova M, et al. . Coenzyme Q10 supplementation reduces corticosteroids dosage in patients with bronchial asthma. Biofactors 2005; 25: 235–240. [DOI] [PubMed] [Google Scholar]
- 15.Hasselmark L, Malmgren R, Zetterstrom O, et al. . Selenium supplementation in intrinsic asthma. Allergy: Eur J Allergy Clin Immunol 1993; 48: 30–36. [PubMed] [Google Scholar]
- 16.Honma M, Tamura G, Shirato K, et al. . Effect of an oral gold compound, auranofin, on non-specific bronchial hyperresponsiveness in mild asthma. Thorax 1994; 49: 649–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaslow JE. Double-blind trial of pyridoxine (vitamin B6) in the treatment of steroid-dependent asthma. Ann Allergy 1993; 71: 492. [PubMed] [Google Scholar]
- 18.Khayyal MT, el-Ghazaly MA, el-Khatib AS, et al. . A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam Clin Pharmacol 2003; 17: 93–102. [DOI] [PubMed] [Google Scholar]
- 19.Pearson PJK, Lewis SA, Britton J, et al. . Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax 2004; 59: 652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas M, Sheran J, Smith N, et al. . AKL1, a botanical mixture for the treatment of asthma: a randomised, double-blind, placebo-controlled, cross-over study. BMC Pulm Med 2007; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thoren P, Wallin A, Whitehead PJ, et al. . The effect of different concentrations of lactose powder on the airway function of adult asthmatics. Respir Med 2001; 95: 870–875. [DOI] [PubMed] [Google Scholar]
- 22.Urata Y, Yoshida S, Irie Y, et al. . Treatment of asthma patients with herbal medicine TJ-96: a randomized controlled trial. Respir Med 2002; 96: 469–474. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Chang J, Chi HH, et al. . Randomized controlled trial on treatment of bronchial asthma of qi-deficiency cold syndrome type by pingchuan yiqi granule. Chin J Integr Med 2007; 13: 27–32. [DOI] [PubMed] [Google Scholar]
- 24.Fogarty A, Lewis SA, Scrivener SL, et al. . Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy 2003; 33: 1355–1359. [DOI] [PubMed] [Google Scholar]
- 25.Hill J, Micklewright A, Lewis S, et al. . Investigation of the effect of short-term change in dietary magnesium intake in asthma. Eur Respir J 1997; 10: 2225–2229. [DOI] [PubMed] [Google Scholar]
- 26.Nadi E, Tavakoli F, Zeraati F, et al. . Effect of vitamin C administration on leukocyte vitamin C level and severity of bronchial asthma. Acta Med Iran 2012; 50: 233–238. [PubMed] [Google Scholar]
- 27.Moreira A, Moreira P, Delgado L, et al. . Pilot study of the effects of n-3 polyunsaturated fatty acids on exhaled nitric oxide in patients with stable asthma. J Investig Allergol Clin Immunol 2007; 17: 309–313. [PubMed] [Google Scholar]
- 28.Okamoto M, Mitsunobu F, Ashida K, et al. . Effects of dietary supplementation with N-3 fatty acids compared with N-6 fatty acids on bronchial asthma. Intern Med 2000; 39: 107–111. [DOI] [PubMed] [Google Scholar]
- 29.Kelly-Pieper K, Patil P, Busse P, et al. . Safety and tolerability of an antiasthma herbal formula (ASHMI) in adult subjects with asthma: a randomized, double-blinded, placebo-controlled, dose-escalation phase I study. J Altern Complement Med 2009; 15: 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen M-C, Wei C-H, Hu Z-Q, et al. . Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol 2005; 116: 517–524. [DOI] [PubMed] [Google Scholar]
- 31.Martineau AR, MacLaughlin BD, Hooper RL, et al. . Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015; 70: 451–457. [DOI] [PubMed] [Google Scholar]
- 32.Nanzer AM. The Calcitriol Study A Randomised Placebo Controlled Clinical Trial to Test the Effects of Calcitriol in Steroid Resistant Asthma. PhD thesis. Queen Mary University of London, London, UK, 2015. [Google Scholar]
- 33.Castro M, King TS, Kunselman SJ, et al. . Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D Levels: the VIDA randomized clinical trial. JAMA 2014; 311: 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CONSORT. History. www.consort-statement.org/about-consort/history Date last accessed: May 7 2016. Date last updated: 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0092-2016_supplementary_material (112KB, pdf)