Abstract
The results of the randomised controlled trials investigating the bronchoscopic lung volume reduction treatment using endobronchial valves (EBV) are promising, and have led to their inclusion in treatment guidelines, US Food and Drug Administration approval and inclusion in routine care in an increasing number of countries. The one-way valve treatment has advanced and is now a regular treatment option. However, this new phase will lead to new challenges in terms of implementation. We believe that key issues in future research concern advanced patient selection, improved methods for target lobe selection, increased knowledge on the predictive risk of a pneumothorax, positioning of pulmonary rehabilitation in conjunction with the EBV treatment, the positioning of lung volume reduction surgery versus EBV treatment, and the long-term efficacy, adverse events, impact on exacerbations and hospitalisations, costs and survival. Hopefully, the increasing number of patients treated, the setup of (inter)national registries and future research efforts will further optimise all aspects of this treatment.
Short abstract
EBV treatment for severe emphysema patients improves clinical and patient reported outcomes and has become a guideline treatment, but also has future challenges. http://ow.ly/Jskd30o3ufm
Introduction
There is increasing evidence that patients with advanced emphysema can experience meaningful clinical benefit after bronchoscopic lung volume reduction treatment with endobronchial valves (EBV) [1–5]. These results have recently resulted in both Global Initiative for Chronic Obstructive Lung Disease and National Institute for Health and Clinical Care Excellence recommendations, as well as US Food and Drug Administration approval [6–8]. This new phase of entering the routine treatment space for severe emphysema comes with challenges. To address these, in this paper we will review the development and current state of the treatment and discuss its challenges and future directions.
Four types of valves have been reported in the literature: the Zephyr one-way EBV (PulmonX Corp., Redwood City, CA, USA) [9]; the Spiration valve system (Spiration, Inc., Redmond, WA, USA) [10]; MedLung EBV (MedLung, Barnaul, Russia) [11]; and the endobronchial Miyazawa valve (Novatech, La Ciotat, France) [12]. The literature was systematically searched for randomised controlled trials (RCT) investigating these valves. RCT results are currently only published for the Zephyr and Spiration valves and are, therefore, included in this review. Little information can be found in literature about the MedLung EBV and Miyazawa valve and whether these are currently used or not and, therefore, these valves are not included in this review.
Bronchoscopic lung volume reduction treatment using EBV
As with lung volume reduction surgery (LVRS), bronchoscopic EBV treatment is aimed at “removing” less functional and hyperinflated areas of emphysematous lung; however, it is less invasive, has a lower mortality rate and is potentially cheaper than surgery [13–15]. The EBV allows expiration of air from a treated lobe but prevents re-inflation. In a completely treated lobe this will result in reducing the lobar volume and eventually in a full lobar collapse, causing reduced lung hyperinflation leading to improvements in breathlessness, exercise capacity, physical activity and quality of life [1–5, 16]. Over the years important steps in identifying the treatment responders have been made. Crucial for treatment success are: presence of an emphysematous treatment target lobe, a fully occluded lobe, and the absence of interlobar collateral ventilation measured with the Chartis system (PulmonX Corp., Redwood City, CA, USA) [3, 17, 18].
In our systematic literature search for the Zephyr EBV we focused on RCTs that used the Chartis system to include patients with functionally intact interlobar fissures [1–4]. Currently, four RCTs have been published with a total of 448 patients randomised, showing comparable significant and clinically relevant improvements in lung function, exercise capacity, physical activity, dyspnoea severity and quality of life (table 1) [1–4, 16]. In these trials the between group difference in favour of the EBV group was an, on average, improvement in forced expiratory volume in 1 s (FEV1) by 17–29%, residual volume (RV) by −522−831 mL, 6-min walk distance (6MWD) by 39–79 m and St George's Respiratory Questionnaire (SGRQ) by −6.5–14.7 points between 3- and 12-months follow-up. Importantly, these trials also revealed the 18–34% risk of treatment-related pneumothorax as the most prevalent and clinically relevant complication (table 1), which in expert centres is manageable and not related to worse outcome or worse survival [9, 19, 20]. Importantly, since EBV treatment is reversible and adjustable in these four RCTs, revision bronchoscopies were necessary in 19–35% of the patients up to 1 year after treatment, with adjustment or removal of the valves.
TABLE 1.
Overview of results of the four randomised controlled trials performed to date using the Chartis measurement
| STELVIO [3] | IMPACT [4] | TRANSFORM [1] | LIBERATE [2] | |
| Patients n | EBV: 34; SoC: 34 | EBV: 43; SoC: 50 | EBV: 65; SoC: 32 | EBV: 128; SoC: 62 |
| Emphysema distribution | Heterogeneous and homogeneous | Homogeneous | Heterogeneous | Heterogeneous |
| Procedure | ||||
| Procedure time min | 18 (6–51) | NR | NR | 29 (4–123) |
| Valves used n | 4 (2–7) | 4 | 4 (2–8) | 4 (2–8) |
| Hospital stay days | 1 (1–13) | 6 (3–40) | 4 (1–49) | NR |
| Efficacy | ||||
| Target lobe volume reduction# mL | −1366 | −1195 | −1090 | −1142 |
| Between group difference | 6 months follow-up | 3 months follow-up | 6 months follow-up | 12 months follow-up |
| Change in lung function | ||||
| FEV1 | +17.8%§ | +17%§ | +29%§ | +18%§ |
| RV mL | −831 | −480§ | −700§ | −522§ |
| Change in exercise capacity | ||||
| 6MWD m | +74§ | +40§ | +79§ | +39§ |
| Change in patient-centred outcomes | ||||
| SGRQ total score | −14.7 | −9.7§ | −6.5§ | −7.1§ |
| mMRC change | −0.61 | −0.57§ | −0.6§ | −0.8§ |
| Physical activity steps per day | +1340 (+57%) | NR | NR | NR |
| Responder rates¶ | ||||
| FEV1 | 72% | 40% | 66% | 56% |
| RV | 71% | 44% | 68% | 62% |
| 6MWD | 79% | 57% | 66% | 42% |
| SGRQ | 87% | 50% | 65% | 56% |
| Safety+ | ||||
| Pneumothorax | 18% | 26% | 29% | 34% |
| Valve retainment | 79% | 93% | 97% | 94% |
| Re-bronchoscopy | 35% | 19% | 28% | 27% |
| Deaths | 3% | 0% | 2% | 4% |
Data are presented as median (range) or mean change, unless otherwise stated. FEV1: forced expiratory volume in 1 s; RV: residual volume; 6MWD: 6-min walk distance; SGRQ: St George's Respiratory Questionnaire; mMRC: modified Medical Research Council scale; EBV: endobronchial valve; SoC: standard of care; NR: not reported. #: change in the EBV group only; ¶: percentage of patients who reached the earlier established minimal important difference: FEV1 ≥12% (STELVIO ≥10%), RV ≥430 mL (LIBERATE ≥310 mL), 6MWD ≥25 m, SGRQ ≥4 points; +: EBV group only; §: intention to treat analyses.
Currently, there are two RCTs that investigated the Spiration valve system. In the REACH study [21] (EBV: n=66; standard of care: n=33), the patients treated with valve significantly improved after 6 months in FEV1 (+91 mL), SGRQ (−8.4 points) and 6MWD (+21 m) and after 12 months the improvements were +40 mL, −3.8 points and +4.5 m, respectively [22]. The pneumothorax rate was 7.5% in the REACH trial up to 6 months follow-up. In the EMPROVE trial (clinicaltrials.gov NCT01812447) [23], which is not published but was presented in abstract form at the 2018 International European Respiratory Society Congress (EBV: n=113; standard of care: n=59), the EBV group improved after 12 months in FEV1 (+99 mL), SGRQ (−9.5 points) and 6MWD (+6.9 m).
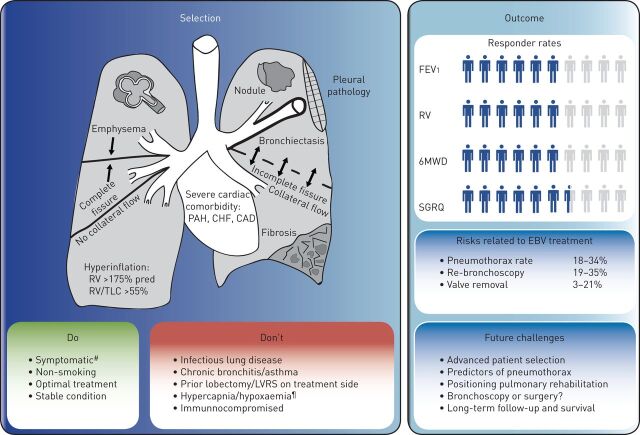
Recently, a group of experienced interventional pulmonologists in Europe have published “best practice recommendations” for EBV treatment [9]. These include key selection criteria, procedure recommendations and a pneumothorax management algorithm. The key selection criteria for success (do) and restraints for treatment (don't) are summarised in figure 1. Precise disease phenotyping is necessary to accurately target this treatment and achieve results, which makes this treatment a unique example of personalised medicine.
FIGURE 1.
Endobronchial valve (EBV) treatment for emphysema, summary of treatment selection and outcome. The key selection criteria for success (do) and restraints for treatment (don't) are shown. The criteria for success (do) are: severe emphysema; complete interlobar fissure (no collateral flow); severe hyperinflation (residual volume (RV) >175% pred, RV/total lung capacity (TLC) >55%); symptomatic; non-smoking; on optimal treatment; and stable condition. The criteria for restraints (don't) are: presence of a suspect nodule; pleural pathology; severe bronchiectasis; incomplete fissure; fibrosis; severe cardiac comorbidity (i.e. pulmonary arterial hypertension (PAH), congestive heart failure (CHF) and coronary artery disease (CAD)); infectious lung disease; chronic bronchitis or asthma; prior lobectomy or lung volume reduction surgery (LVRS) on treatment side; hypercapnia/hypoxaemia; and immunocompromised. The figure also shows the mean responder rates and percentage of risk related to EBV treatment from the four published randomised controlled trials. Responder rates are the percentage of patients who reached the earlier established minimal important difference: forced expiratory volume in 1s (FEV1) ≥12% (STELVIO ≥10%), RV ≥430 mL (LIBERATE ≥310 mL), 6-min walk distance (6MWD) ≥25 m, St George's Respiratory Questionnaire (SGRQ) ≥4 points. #: modified Medical Research Council scale ≥2 or 100 m<6MWD<500 m; ¶: partial pressure of carbon dioxide >60 mmHg/partial pressure of oxygen <45 mmHg.
With consistent efficacy, balanced adverse events and now market introduction, cost-effectiveness comes into play and the need to convince the payers that the procedure is of worthwhile utility. Two studies investigated the cost-effectiveness of the treatment and found a favourable cost-effectiveness profile in the long-term compared with other treatments for severe emphysema patients, such as bronchoscopic lung volume reduction with coils or LVRS. These studies included the costs of revision bronchoscopies. Both studies found comparable incremental cost-effectiveness ratios of approximately €40 000 per quality-adjusted life-year gained over 5 years and €25 000 over 10 years [13, 24]. One of the studies showed that reaching a minimal important difference (MID) in 6MWD of 26 m would cost €4160 extra in comparison with standard of care [13]. Furthermore, although not powered to reach significance, the LIBERATE trial found in the long-term follow-up (after 45 days) a strong signal that EBV treatment might lead to a lower frequency of severe COPD exacerbations (EBV: 23.0%, control: 30.6%; p=0.053), and thus lower healthcare costs [2].
Challenges and future directions of the treatment
The results of the EBV RCTs are promising, but also revealed future challenges. We believe that key issues in future research pertain to advanced patient selection, improved methods for target lobe selection, increased knowledge on the predictive risk of a pneumothorax, positioning of pulmonary rehabilitation in conjunction with the EBV treatment, positioning of LVRS versus EBV treatment and the long-term efficacy, adverse events, impact on exacerbations and hospitalisations, costs and survival (table 2).
TABLE 2.
Challenges and future directions of endobronchial valve (EBV) treatment
| Advanced patient selection | |
| Clinical patient characteristics | To define the cut-offs regarding clinical patient characteristics such as degree of obstruction, static hyperinflation, diffusion capacity, blood oxygen tension, exercise capacity, pulmonary hypertension, comorbidities, etc. for procedure-related risk estimation and efficacy outcome. |
| Emphysema severity | To define the exact role of quantitative lobar tissue destruction scores related to outcomes. |
| Prediction of collateral ventilation | To define the optimal cut-off (both lower and upper limit) for the degree of fissure integrity calculated on CT. |
| To establish the optimal method of measurement and interpretation of the Chartis system signal in the measurement of collateral ventilation. | |
| Prediction of response using quantification | To further develop quantitative HRCT software analysis with accurate assessment of fissure integrity, emphysema scores, the amount of air trapping and lung perfusion, all on a lobar level. |
| Multidisciplinary team | To establish a solid base for an emphysema multidisciplinary team like our lung cancer and ILD multidisciplinary team meetings. |
| Therapeutic challenges | |
| The positioning of LVRS versus EBV treatment | Not all patients that are good candidates for surgery are good candidates for valves and vice versa. |
| To create decision making guidance for candidate patients for both techniques. | |
| To create a step-up treatment guidance for initial good responders to EBV treatment. | |
| Closing the interlobar collateral channels | To develop treatments that successfully close the collateral channels which would significantly increase the patient population that could potentially benefit from EBV treatment. |
| Treatment decisions in patients with homogeneous disease | To identify the best treatment option for patients with real homogeneous disease. EBV treatment or coil treatment? |
| Granulation tissue | To identify predictors or risk factors for the development of granulation tissue after EBV treatment (and in fact after every implantable device in the human airways). |
| Burning questions | |
| Long-term effects | To establish the long-term efficacy, cost-efficiency, effect on exacerbations, hospitalisations, survival and adverse events. |
| The interaction with pulmonary rehabilitation. | The combination of EBV treatment and pulmonary rehabilitation could strengthen the effect of the EBV treatment. The best timing of the rehabilitation programme has not been investigated to date. |
| A look in the crystal globe | |
| Potential future developments | To develop new or customised valves. |
| To develop an advanced Chartis device that could be helpful in target lobe selection. | |
| To establish the role of advanced functional imaging with patient selection | |
| To combine different endoscopic and/or surgical techniques. |
LVRS: lung volume reduction surgery; CT: computed tomography; HRCT: high-resolution CT; ILD: interstitial lung disease.
It is well known that patients with advanced emphysema, severe hyperinflation and absence of collateral ventilation in the treatment target lobe have a high likelihood to respond to EBV treatment. However, much is still unknown about the exact amount of lobar tissue destruction scores that relates to positive outcomes and the optimal cut-off for the degree of fissure integrity. Also, most patients have collateral ventilation between ipsilateral lobes, and are not eligible for valve treatment, while often having a very suitable treatment target lobe. Developing successful treatments that close the collateral channels would significantly increase the number of patients that can benefit from this therapy (e.g. MIND THE GAP trial; www.trialregister.nl NTR5007). Furthermore, only one trial (IMPACT [4]) was specifically aimed at investigating the EBV treatment in homogenous patients (defined as <15% difference in emphysema destruction score between target lobe and ipsilateral lobe). This trial found significant efficacy results, but only results up to 3 months after treatment are published. Long-term effectiveness and responder profiles have to be further investigated to establish a solid patient profile in homogeneous emphysema.
Furthermore, it can be challenging to select the most diseased lobe to collapse, which is key in the success of EBV treatment. Further development of quantitative high-resolution computed tomography (HRCT) software analysis with accurate assessment of fissure integrity, emphysema score, amount of air trapping and vascular volume, all on a lobar level and combining imaging techniques such as lung perfusion scans with in- and expiratory HRCT scans, will increase the knowledge on the “best” lobe to treat [25–27]. These efforts could also result in more insight to predict patients who are at risk of a pneumothorax after treatment. Currently, the predictors of the risk of a pneumothorax are not clear and studies reported even contradictory results [28, 29].
In the Global Initiative for Chronic Obstructive Lung disease guidelines [8], pulmonary rehabilitation is an important part of integrated patient management. The combination of EBV treatment and a pulmonary rehabilitation programme has not been investigated to date. Combining both treatments could strengthen the effect of the EBV treatment, especially when the patients' most limiting factor, hyperinflation, has been significantly reduced. Hypothetically, the best timing of the pulmonary rehabilitation programme would be after EBV treatment instead of before as is more common in current practice. This is currently being investigated in the SOLVE trial (clinicaltrials.gov NCT03474471) [30]. However, as the sustainability of the EBV effect is still uncertain, in the case that pulmonary rehabilitation before EBV treatment leads to a significant higher functional status, patients could also choose to delay the EBV treatment.
The direct comparison of LVRS and EBV treatment is currently being investigated in the CELEB trial (www.isrctn.com 19684749). However, only a very small group of patients would be suitable for both treatments due to the very strict selection criteria for the two treatments, making this a hard comparison. The majority of our current EBV candidates are far from eligible for surgery because of a variety of factors such as homogeneous emphysema, lower lobe predominant disease, comorbidity, age, and especially patient preferences.
It has been shown that the treatment is safe and beneficial for patients with functionally intact fissures or a collapsed target lobe at least up to 1 year] [2, 3]. However, only single centre experiences have been published showing significant improvements beyond 1 year following treatment [31–33] and a few other small studies have shown favourable survival effects of the treatment for these patients [34–36]. Recently, Gompelmann et al. [19] showed that patients with valve-induced lobar atelectasis had a significant survival benefit compared to patients without atelectasis (5-year survival rate: 65% versus 44%, p=0.009). In the STELVIO trial it was shown that 78% of patients retained the valves after 1 year. However, not much is known about the sustainability of the valves in the longer term; how many re-bronchoscopies are necessary to achieve this, and how long-term data on efficacy, adverse events and survival will look. This all is important for further development and optimisation of EBV treatment.
After EBV treatment, there is a 40–80% responder rate for the individual end-points FEV1, RV, 6MWD and SGRQ depending on the trial and follow-up duration (table 1). Overall, the responder rate for reaching one of these four end-points at 1 year will be ∼80%. Even if the response is below the MID, the patient often still reports clinical benefit and does not want to have the valves removed. If a patient does not respond, a control HRCT should be performed to check for valve placement and follow-up bronchoscopy to restore valve function. In cases of definite non-response, the valves can be electively removed by bronchoscopy. After this, and only if requested by the patient, other treatment options can be discussed/explored (LVRS, lobectomy, other bronchoscopic lung volume reduction technique, lung transplant).
The inclusion of valve treatment in routine use will lead to an increase in the number of treatments performed per year. However, it will be important to guide the implementation of this new technique and this might significantly differ per country. The implementation of the treatment should be performed in selected expert centres with the availability of other bronchoscopic and preferably surgical treatments to be able to maintain an adequate patient flow, treatment volume and, thus, experienced physicians. The guidance of the implementation should include multidisciplinary meetings in and between centres to discuss patient selection, treatment challenges and complications. Also, it is recommended to set up nationwide or even international registries to monitor the treatment in routine clinical practice. For example, like the LIVE study in Germany [37], the BreathGroup registry in Italy [38] and the BREATH registry in The Netherlands (clinicaltrials.govNCT02815683). This will further optimise the treatment in terms of complications, patient selection and clinical outcomes in the short- and long-term.
In conclusion, the promising results of the efficacy of bronchoscopic lung volume reduction treatment using EBV has led to its inclusion in treatment guidelines, US Food and Drug Administration approval and inclusion in routine care in an increasing number of countries. The one-way valve treatment is advanced and now a regular treatment option. However, this new phase will lead to new challenges in terms of implementation. Hopefully, the increasing number of patients treated, the setup of (inter)national registries and future research efforts will further optimise all aspects of this treatment.
Acknowledgements
The authors would like to thank Judith Hartman for producing the figure.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: J.E. Hartman has nothing to disclose.
Conflict of interest: L.E.G.W. Vanfleteren reports personal fees from PulmonX, outside the submitted work.
Conflict of interest: E.M. van Rikxoort reports other funding from Thirona, during the conduct of the study.
Conflict of interest: K. Klooster has nothing to disclose.
Conflict of interest: D-J. Slebos reports grants, personal fees, non-financial support and other funding from PulmonX Inc., CA, USA, during the conduct of the study; and grants, personal fees, non-financial support and other funding from PneumRx/BTG, CA, USA, outside the submitted work.
Support statement: All authors received a grant from the Dutch Lung foundation for the SOLVE trial (grant number: 5.1.17.171).
References
- 1.Kemp SV, Slebos DJ, Kirk A, et al. . A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med 2017; 196: 1535–1543. [DOI] [PubMed] [Google Scholar]
- 2.Criner GJ, Sue R, Wright S, et al. . A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med 2018; 198: 1151–1164. [DOI] [PubMed] [Google Scholar]
- 3.Klooster K, ten Hacken NH, Hartman JE, et al. . Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015; 373: 2325–2335. [DOI] [PubMed] [Google Scholar]
- 4.Valipour A, Slebos DJ, Herth F, et al. . Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016; 194: 1073–1082. [DOI] [PubMed] [Google Scholar]
- 5.Davey C, Zoumot Z, Jordan S, et al. . Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015; 386: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration . FDA approval: Zephyr Endobronchial Valve System. www.accessdata.fda.gov/cdrh_docs/pdf18/P180002a.pdf Date last updated: June 29 2018. Date last accessed: October 14, 2018.
- 7.National Institute for Health and Care Excellence . Endobronchial valve insertion to reduce lung volume in emphysema. www.nice.org.uk/guidance/IPG600/chapter/1-Recommendations Date last updated: December 2017. Date last accessed: October 14, 2018.
- 8.Vogelmeier CF, Criner GJ, Martinez FJ, et al. . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195: 557–582. [DOI] [PubMed] [Google Scholar]
- 9.Slebos DJ, Shah PL, Herth FJ, et al. . Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from Expert Panel on Endoscopic Lung Volume Reduction. Respiration 2017; 93: 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterman DH, Mehta AC, Wood DE, et al. . A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration 2010; 79: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin A, Sklyuev S, Felker I, et al. . Endobronchial valve treatment of destructive multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2016; 20: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluccio G, Lucantoni G. Bronchoscopic lung volume reduction for pulmonary emphysema: preliminary experience with a new NOVATECH endobronchial silicone one-way valve. Interact Cardiovasc Thorac Surg 2010; 11: 213–215. [DOI] [PubMed] [Google Scholar]
- 13.Hartman JE, Klooster K, Groen H, et al. . Cost-effectiveness of endobronchial valve treatment in patients with severe emphysema compared to standard medical care. Respirology 2018; in press [ 10.1111/resp.13295]. [DOI] [PubMed] [Google Scholar]
- 14.Criner GJ, Cordova F, Sternberg AL, et al. . The National Emphysema Treatment Trial (NETT) part II: lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011; 184: 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishman A, Martinez F, Naunheim K, et al. . A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348: 2059–2073. [DOI] [PubMed] [Google Scholar]
- 16.Hartman JE, Klooster K, Slebos DJ, et al. . Improvement of physical activity after endobronchial valve treatment in emphysema patients. Respir Med 2016; 117: 116–121. [DOI] [PubMed] [Google Scholar]
- 17.Herth FJ, Eberhardt R, Gompelmann D, et al. . Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. Eur Respir J 2013; 41: 302–308. [DOI] [PubMed] [Google Scholar]
- 18.Sciurba FC, Ernst A, Herth FJ, et al. . A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010; 363: 1233–1244. [DOI] [PubMed] [Google Scholar]
- 19.Gompelmann D, Benjamin N, Bischoff E, et al. . Survival after endoscopic valve therapy in patients with severe emphysema. Respiration 2019: 145–152. [DOI] [PubMed] [Google Scholar]
- 20.Valipour A, Slebos DJ, de Oliveira HG, et al. . Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema – potential mechanisms, treatment algorithm, and case examples. Respiration 2014; 87: 513–521. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Wang G, Wang C, et al. . The REACH Trial: A Randomized Controlled Trial Assessing the Safety and Effectiveness of the Spiration Valve System in the Treatment of Severe Emphysema. Respiration 2018; in press [ 10.1159/000494327]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G, Li S, Wang C, et al. . The REACH study, a randomized controlled trial assessing the safety and effectiveness of the Spiration Valve System endobronchial therapy for severe emphysema: 12 month follow-up results. Eur Respir J 2017: 50; Suppl. 61, OA1465. [Google Scholar]
- 23.Criner G, Delage A, Voelker K. Endobronchial Valves for Severe Emphysema – 12-month results of the EMPROVE Trial. Eur Respir J 2018: 52: Suppl. 62, OA4928. [Google Scholar]
- 24.Pietzsch JB, Garner AM, Herth FJ. Cost-effectiveness of endobronchial valve therapy for severe emphysema: a model-based projection based on the Vent study. Value Health 2014; 17: A598. [DOI] [PubMed] [Google Scholar]
- 25.Charbonnier JP, Brink M, Ciompi F, et al. . Automatic pulmonary artery-vein separation and classification in computed tomography using tree partitioning and peripheral vessel matching. IEEE Trans Med Imaging 2016; 35: 882–892. [DOI] [PubMed] [Google Scholar]
- 26.Charbonnier JP, Rikxoort EMV, Setio AAA, et al. . Improving airway segmentation in computed tomography using leak detection with convolutional networks. Med Image Anal 2017; 36: 52–60. [DOI] [PubMed] [Google Scholar]
- 27.Koster TD, van Rikxoort EM, Huebner RH, et al. . Predicting lung volume reduction after endobronchial valve therapy is maximized using a combination of diagnostic tools. Respiration 2016; 92: 150–157. [DOI] [PubMed] [Google Scholar]
- 28.Gompelmann D, Lim HJ, Eberhardt R, et al. . Predictors of pneumothorax following endoscopic valve therapy in patients with severe emphysema. Int J Chron Obstruct Pulmon Dis 2016; 11: 1767–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Geffen WH, Klooster K, Hartman JE, et al. . Pleural adhesion assessment as a predictor for pneumothorax after endobronchial valve treatment. Respiration 2017; 94: 224–231. [DOI] [PubMed] [Google Scholar]
- 30.Hartman JE, Vanfleteren LEGW, van Rikxoort EM, et al. . Bronchoscopic lung volume reduction treatment using endobronchial valves for emphysema: emerging questions. Respiration 2018; 96: 588–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klooster K, Hartman JE, ten Hacken NHT, et al. . One-year follow-up after endobronchial valve treatment in patients with emphysema without collateral ventilation treated in the STELVIO trial. Respiration 2017; 93: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venuta F, Anile M, Diso D, et al. . Long-term follow-up after bronchoscopic lung volume reduction in patients with emphysema. Eur Respir J 2012; 39: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 33.Fiorelli A, Santoriello C, De Felice A, et al. . Bronchoscopic lung volume reduction with endobronchial valves for heterogeneous emphysema: long-term results. J Vis Surg 2017; 3: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garner J, Kemp SV, Toma TP, et al. . Survival after endobronchial valve placement for emphysema: a 10-year follow-up study. Am J Respir Crit Care Med 2016; 194: 519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klooster K, Hartman JE, Ten Hacken NHT, et al. . Improved predictors of survival after endobronchial valve treatment in patients with severe emphysema. Am J Respir Crit Care Med 2017; 195: 1272–1274. [DOI] [PubMed] [Google Scholar]
- 36.Hopkinson NS, Kemp SV, Toma TP, et al. . Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J 2011; 37: 1346–1351. [DOI] [PubMed] [Google Scholar]
- 37.Skowasch D, Fertl A, Schwick B, et al. . A long-term follow-up investigation of endobronchial valves in emphysema (the LIVE Study): study protocol and six-month interim analysis results of a prospective five-year observational study. Respiration 2016; 92: 118–126. [DOI] [PubMed] [Google Scholar]
- 38.BreathGroup . Registry. www.breathgroup.eu/registry.html Date last accessed: January 30, 2019.