Abstract
Bronchiectasis is an increasing clinical problem, but multiple recent clinical trials have failed to reach their primary end-point. Difficulties in achieving “positive” bronchiectasis trials is reflected in a lack of agreement from trialists and regulators on what are the optimal end-points.
To evaluate the use of end-points in bronchiectasis trials, we conducted a systematic review of published bronchiectasis trials from 2008 to 2018 and extracted end-points used, definitions, methods of analysis and responsiveness.
Our analysis shows that quality of life and exacerbation end-points are most frequently used. Trials using exacerbation end-points have been characterised by varying definitions, multiple methods of analysis and durations of follow-up. There are multiple quality of life tools for bronchiectasis (Quality of Life – Bronchiectasis questionnaire, St George's Respiratory Questionnaire, etc.). The majority of studies measure lung function (e.g. forced expiratory volume in 1 s), but this is shown to be nonresponsive to the majority of interventions. Microbiology end-points frequently show statistically significant differences in phase 2 antibiotic studies but their correlation with clinical end-points is unknown.
This systematic review demonstrates a need for guidance to standardise definitions and design features to improve reproducibility and increase the likelihood of demonstrating statistically significant benefits with new therapies.
Short abstract
There is an urgent need to standardise clinical trial end-points in bronchiectasis. This systematic review shows the diversity of end-points used in bronchiectasis and suggests approaches that may improve the success rate and reproducibility of trials. http://ow.ly/d4HR30nvvS3
Introduction
Bronchiectasis is a chronic respiratory disease that causes persistent cough, sputum production and recurrent chest infections. It can be caused by a variety of genetic, autoimmune, inflammatory, allergic and degenerative conditions. The prevalence of bronchiectasis is increasing worldwide [1, 2].
Bronchiectasis is an orphan disease with few randomised controlled trials (RCTs) having been conducted and a limited understanding of its pathophysiology. The 2017 European Respiratory Society (ERS) guidelines for bronchiectasis were unable to recommend any pharmacotherapy with strong evidence because of a lack of trials demonstrating statistically significant benefits [3]. In contrast, there have been many randomised trials in chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF) over the past 20 years, leading to a tendency for bronchiectasis researchers to use end-points and therapies previously developed in these two diseases. The failure of some therapies to show benefit in bronchiectasis, notably recombinant DNAse in the late 1990s, has led many to suggest this approach is flawed and that specific trial end-points and approaches are needed for bronchiectasis [4].
The US Food and Drug Administration (FDA) defines a valid end-point as something that measures how a patient “feels, functions or survives”. To license a medication, the FDA requires substantial evidence of efficacy and safety which usually means demonstrating statistically significant benefits versus placebo in terms of the primary end-point and no major issues with safety. European regulators have similar requirements [5]. In addition, substantial evidence of efficacy usually requires consistency of effect in two well-controlled RCTs [6].
Inhaled antibiotics, macrolides, mucoactive drugs, chest physiotherapy, pulmonary rehabilitation and anti-inflammatory drugs are all used in clinical practice and many are believed to benefit patients. Few have shown consistent benefit in large randomised trials. The reasons for this may be that the population most likely to benefit has not been identified or that the optimal end-points and end-point definitions have not been identified. The repeated challenges encountered in bronchiectasis trials requires a thorough re-evaluation of the selected end-points, their definitions and other aspects of trial design.
In this study, we conducted a systematic review of the end-points used in bronchiectasis clinical trials with the goal of identifying the most widely used end-points, their definitions and responsiveness, and of identifying research priorities for optimising pharmacotherapy trials.
Methods
Search strategy
A systematic review was conducted on the reporting of RCTs in bronchiectasis and is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched PubMed using the keyword “bronchiectasis” and filtering for clinical trials in humans, published in English, published between January 01, 2008 and July 31, 2018. This search was supplemented using the COCHRANE ultra-sensitivity search: (((((randomised controlled trial[pt] OR controlled clinical trial[pt] OR randomised[tiab] OR placebo[tiab] OR clinical trials as topic[mesh:noexp] OR randomly[tiab] OR trial[ti] NOT (animals[mh] NOT humans [mh])))) AND “bronchiectasis” AND (“2008/01”[Date – Publication] : “2018/07”[Date - Publication])).
An EMBASE sensitivity search was also conducted using the same criteria.
The review was registered with PROSPERO (www.crd.york.ac.uk/prospero) with identifier number CRD42018106167).
Inclusion criteria
The review included RCTs defined as per the International Committee of Medical Journal Editors definition [7]. This includes parallel group, crossover or any other controlled trial design. Studies enrolling a minimum of 25 participants were selected as adequately powered efficacy and safety studies. For this review, only studies including patients aged 18 years and over and diagnosed with bronchiectasis not related to CF were included. The interventions may include inhaled antibiotics, macrolides, mucoactive drugs or anti-inflammatory therapies. We excluded trials testing dietary, exercise or physiotherapy interventions as the objective of the study is to examine end-points specifically for pharmacotherapy trials. We included studies where the control was placebo, no intervention, standard care or an active comparator, but excluded studies without a control group. Studies which included more than one disease where the bronchiectasis cohort could not be clearly separated were excluded, as were secondary analyses of previously reported studies and those which only reported laboratory or experimental end-points as the review focused on end-points with the potential for clinical benefit.
For data extraction, articles were reviewed by two independent reviewers and confirmed by a third. Extracted data included end-points used, method of assessment and the definition of end-point assessment (where necessary). Trials were classified as “positive” or “negative” depending on whether there was a statistically significant difference between intervention and control in the primary outcome. Trials where the primary outcome was positive but there were significant findings in adverse events and/or patient safety were classified as “negative” overall. Any disagreements between reviewers were resolved by discussion.
Outcomes
The main aim of this review was to identify the most commonly used end-points in randomised controlled pharmacotherapeutic trials of bronchiectasis. Once established, these were analysed for responsiveness, defined as demonstration of statistically significant differences between groups, and consistency of definition across trials.
Statistical analysis
A priori, we determined that no meta-analysis would be undertaken. Data were collected and reported into tables for each individual trial and then summarised by end-point. For analysis purposes end-points were grouped into end-point categories: exacerbation, microbiological, sputum characteristics, lung function, health-related quality of life (HRQoL) and biomarkers. For example, forced expiratory volume in 1 s (FEV1) and peak flow rate would be grouped as lung function end-points, frequency and time to first exacerbation would be grouped as exacerbation end-points and patient-reported outcomes would be grouped under HRQoL. A further category “other” was added to capture less frequently used end-points such as changes to nitric oxide levels and radiological changes.
A further search of clinicaltrials.gov and ISRCTN (www.isrctn.com) was made to capture any trials that may have been conducted within our review period and that may not have been published. We identified 81 studies, of which 70 were excluded as they were published (n=14), did not relate specifically to bronchiectasis (n=15), were outside the study period (n=2), had a study population less than 25 (n=2) or were not a pharmacotherapeutic agent (n=37). The 11 remaining trials (table 1) were eligible for our review but were unpublished and could not be fully analysed, suggesting that at least a third of bronchiectasis research data is not being made available. Similar to the main analysis, the most frequently used primary end-point (n=12) was exacerbations (n=5) and the most commonly used end-point overall (n=34) was lung function (n=8), closely followed by health-related quality of life (HRQoL) questionnaires (n=7).
TABLE 1.
Summary of randomised controlled trials found not to be published (2008–2018)
| NCT number | Trial title | Year # | Phase | Primary end-point group(s) | Secondary end-point group(s) |
| NCT00775138 | A study to determine the safety and tolerability of arikace versus placebo in patients who have bronchiectasis | 2008–2009 | 2 | Lung function, other | Lung function, microbiology, HRQoL |
| NCT01677403 | A study to access safety and efficacy of nebulized tobramycin in patients with bronchiectasis | 2012–2014 | 4 | Microbiology | Sputum, lung function, HRQoL |
| NCT01684683 | The effect of theophylline in the treatment of bronchiectasis | 2012–2014 | 4 | HRQoL | Exacerbations, sputum, HRQoL, lung function, microbiology, biomarkers, other |
| NCT01580748 | Phase 2, single group, open clinical trial to evaluate the efficacy and safety of roflumilast in symptomatic bronchiectasis patients | 2012–2013 | 2 | HRQoL | Lung function, biomarkers |
| NCT01818544 | Safety and efficacy of oral BAY85–8501 in patients with non-CF (cystic fibrosis) bronchiectasis | 2013–2014 | 2 | Other | Lung function, HRQoL, sputum, biomarkers |
| NCT01769898 | The role of theophylline plus low-dose formoterol-budesonide in treatment of bronchiectasis | 2013–2014 | 4 | HRQoL | Exacerbations, sputum, lung function, biomarkers |
| NCT02088216 | Effect of long-term, high-dose N-acetylcysteine on exacerbations of bronchiectaisis (BENE) | 2014–2016 | 4 | Exacerbations | Sputum, exacerbations, lung function, HRQoL, other |
| NCT02104245 | Phase 3 study with ciprofloxacin dispersion for inhalation in non-CF bronchiectasis (ORBIT-4) | 2014–2016 | 3 | Exacerbations | No information |
| NCT01515007 | Phase 3 study with ciprofloxacin dispersion for inhalation in non-CF bronchiectasis (ORBIT-3) | 2014–2016 | 3 | Exacerbations | No information |
| NCT02546297 | Comparisons of inhaled LAMA or ICS+LABA for COPD with bronchiectasis | 2015–2017 | 4 | Exacerbations | Lung function, HRQoL, other |
| NCT02507843 | Vitamin D as an adjunctive treatment in patients with non-cystic fibrosis bronchiectasis (VIDB) | 2015–2017 | 4 | Exacerbations | Exacerbations, HRQoL, other |
All trials were registered at clinicaltrials.gov. Primary end-points (n=12): exacerbations (n=5), health-related quality of life (HRQoL) (n=3), microbiology (n=1), other (n=2) and lung function (n=1). Secondary end-points (n=34): lung function (n=8), HRQoL (n=7), sputum (n=5), exacerbations (n=4), biomarkers (n=4), other (n=4) and microbiology (n=2). “Other” end-points include FACED score, vital signs and adverse events. LAMA: long-acting muscarinic antagonists; ICS: inhaled corticosteroid; LABA: long-acting β-agonists; COPD: chronic obstructive pulmonary disease. #: it is recommended that trials are reported within 1 year of completion, and so trials are listed here if completed earlier than November 2017.
Results
Selection process
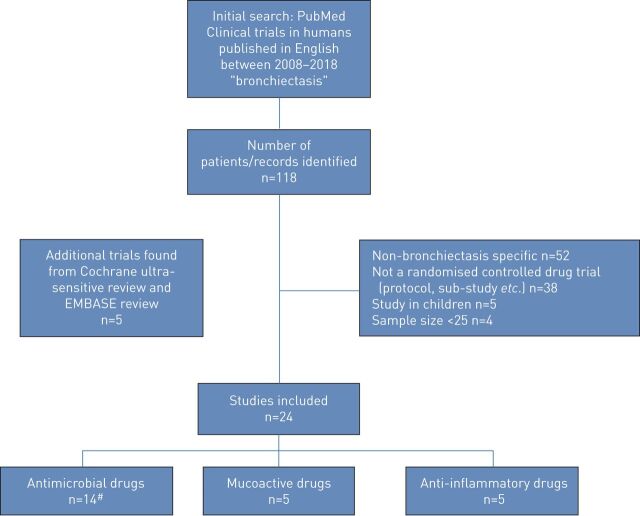
The primary search identified 118 papers. Following exclusion of 52 studies which were not specific to bronchiectasis, five paediatric studies, 38 non-pharmacotherapy trials and four studies which involved recruitment of less than 25 participants, the remaining 19 studies were eligible for end-point analysis.
The additional Cochrane ultra-sensitive search in PubMed identified 225 manuscripts of which only a further five were eligible for inclusion in this review. The EMBASE search did not identify any additional eligible trials.
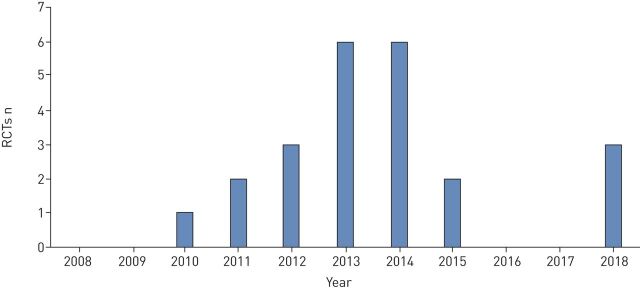
The final 24 trials are analysed as 23 trials, as two replicate trials were described in two manuscripts but as these trials ran in parallel using an identical protocol they are reported together [8, 9]. Figure 1 shows the flow of study selection using the above criteria, while figure 2 shows the chronological progress of publications on pharmacotherapeutic RCTs in bronchiectasis from 2008.
FIGURE 1.
Flow diagram of the study selection process. #: antimicrobial drugs trials n=14, were analysed as n=13 due to combined analysis of two manuscripts.
FIGURE 2.
Number of published pharmacotherapeutic randomised controlled trials (RCTs) conducted since 2008.
Studies
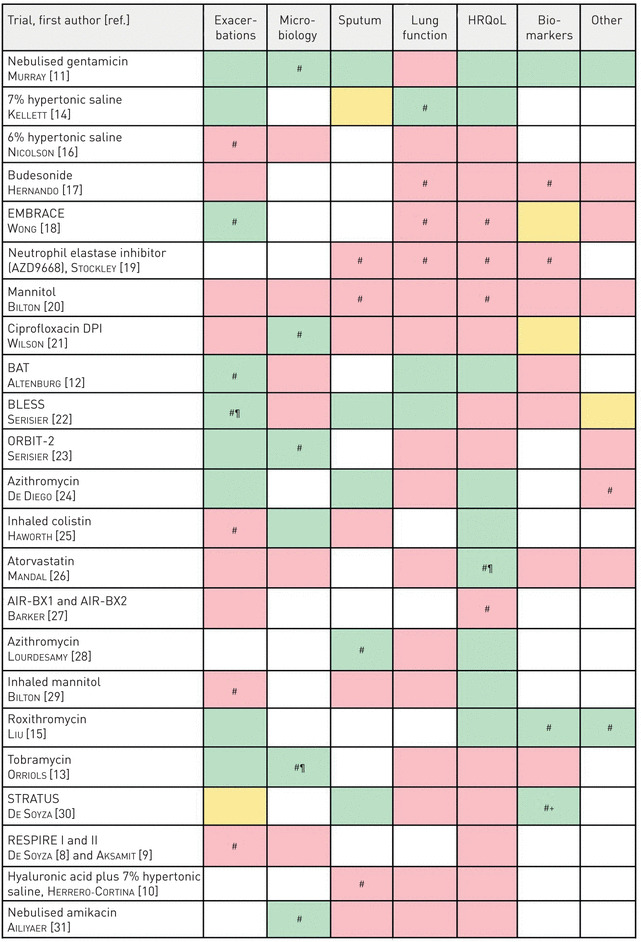
23 RCTs were identified meeting the review inclusion criteria. The shortest trial duration was 26 days for a crossover study looking at the effects of 7% hypertonic saline versus hyaluronic acid [10], while the longest trials were all antibiotic interventions for 15 months [11–13]. The inclusion criteria for this review required a minimum 25 participants in the trial, of the 23 RCTs reviewed the minimum study population was 28 [10, 14] while the largest study included 937 participants globally across two replicate trials [8, 9]. To categorise each trial by intervention: we reviewed 13 antibiotic trials, five mucolytic/mucoactive drug trials and five anti-inflammatory drug trials. Macrolides are typically referred to as both antibiotic and anti-inflammatory. We classified them as antibiotics for the purposes of analysis, except in one case where the investigators specifically referred to the trial as testing the anti-inflammatory effect [15]. Table 2 shows an overview of the selected trials used in this review and the end-points which each trial used grouped into seven main categories.
TABLE 2.
Summary of end-points for the articles eligible for review
|
Green: end-point(s) passed; pink: end-point(s) failed; orange: not clear or mixed results. HRQoL: health-related quality of life. #: primary end-point; ¶: safety issues; +: no clinical benefit.
Current end-points
Of the 23 RCTs identified by our search, we counted 31 primary end-points (five studies used co-primary end-points: Hernando et al. [17], Bilton et al. [20] and Liu et al. [15] each used two, Wong et al. [18] measured three, while Stockley et al. [19] used four). 14 of these primary end-points demonstrated a statistically significant difference versus placebo or control (48%). Accounting for the study by Wong et al. [18] using multiple primary end-points, 12 out of 23 studies successfully met their primary end-points. Of all 107 end-points reviewed, a total of 37 demonstrated statistically significant differences versus placebo or control (table 3).
TABLE 3.
Summary of frequency with which trials met their end-points comparing active treatment with placebo or control in each end-point category
| End-point group | Exacerbation-related | Microbiology | Sputum-related | Lung function | HRQoL | Biomarkers (blood and sputum) | Other |
| Primary end-points | 3/7# | 5/5 | 1/4 | 1/4 | 1/5 | 2/4 | 1/2 |
| Accumulative end-points | 9/19 | 6/12 | 5/13 | 3/19 | 9/23¶ | 3/12 | 2/9 |
Data are presented as n/N. HRQoL: health-related quality of life. #: most commonly used primary end-point; ¶: most commonly used end-point (primary and secondary).
Reviewing the trials by type of intervention, we see that antibiotic trials favour primary end-points in exacerbations and microbiology and are quite frequently successful in phase 2, whereas primary end-points in HRQoL rarely demonstrate benefits (table 4). Anti-inflammatory trials trend towards biomarker end-points and symptoms. The majority of trials were not successful in lung function end-points and had low success in all other categories. The mucoactive drug trials showed few positive results in all end-point categories with only 18% of their end-points showing statistically significant differences.
TABLE 4.
Summary of the end-points favoured by each type of intervention
| Exacerbation-related | Microbiology | Sputum-related | Lung function | HRQoL | Biomarkers (blood and sputum) | Other | ||
| Antibiotic trials (n=13) | Primary | 3/5 | 5/5 | 1/1 | 0/1 | 0/2 | N/A | 0/1 |
| Accumulative | 7/11 | 6/9 | 4/7 | 2/10 | 5/13 | 1/6 | 1/5 | |
| Anti-inflammatory trials (n=5) | Primary | N/A | N/A | 0/1 | 0/2 | 1/2 | 2/4 | 1/2 |
| Accumulative | 1/4 | 0/1 | 1/2 | 0/4 | 2/5 | 2/5 | 1/3 | |
| Mucolytic trials (n=5) | Primary | 0/2 | N/A | 0/2 | 1/1 | 0/1 | N/A | N/A |
| Accumulative | 1/4 | 0/2 | 0/4 | 1/5 | 2/5 | 0/1 | 0/1 |
Data are presented as n/N. HRQoL: health-related quality of life; N/A: not applicable.
In the following sections, we report the findings of each defined end-point used in the aforementioned eligible studies. The definition and method of assessment are detailed before discussing the experience of using them in existing bronchiectasis clinical trials.
Individual end-points
Time to next exacerbation and exacerbation frequency
While reviewing the exacerbation end-points, we noted four different measures: time to next exacerbation, number of subjects experiencing exacerbations, rate and duration, and with these came a variety of methods for statistical analysis. Using exacerbation as a primary end-point had a similar (∼50%) success rate as when used as a secondary end-point. In terms of primary end-points, attempting to reduce exacerbation frequency is the most frequently used [8, 9, 12, 16, 22]. Throughout the review process we identified many variations of a protocol-defined exacerbation and methods of corresponding analysis, these can be seen in table 5. Definitions of exacerbation were highly variable between studies and many criteria appeared to be subjective, for example, one study defined an exacerbation as an “increase in dyspnoea, sputum production and sputum purulence”. Some definitions relied not only on a minimum number of symptoms but also a minimum period of time experiencing them (varying up to 48 h). Other studies had multiple definitions within the same protocol, some of which were relatively complex, where investigators had to account for the previous week's entries of a daily diary card [18]. Diary cards were used in only two trials to assist symptom monitoring [12, 18]. Some studies did not use an exacerbation definition but used a surrogate of “antibiotic use” as an isolated end-point. However, in some cases, exacerbations could be diagnosed without a physician decision to treat while in others the symptom definition had to be accompanied by a decision to prescribe antibiotics and/or corticosteroids.
TABLE 5.
Summary of the studies which looked at exacerbations as end-points, demonstrating that each study used a different exacerbation definition with potential implications for the study results
| Trial, first author [ref.] | Exacerbation definition | Method of assessment/measurement |
|
Nebulised gentamicin,
Murray [ 11 ] |
A clinical deterioration with all of the following: increasing cough, increasing sputum volume and worsening sputum purulence All patients subsequently received antibiotics for 14 days |
TTF: median Number: count |
|
7% hypertonic saline,
Kellett [ 14 ] |
Unscheduled primary or secondary events leading to provision of rescue medications, either steroid or antibiotic, Accident and Emergency attendance or hospitalisation | Number: count |
|
6% hypertonic saline,
Nicolson [ 16 ] |
≥3 symptoms in 1 day for two or more consecutive days | Number: median (IQR) |
|
Budesonide,
Hernando [17] |
Worsening of at least 3 out of 4 symptoms for at least 48 h | Number: mean Duration: mean |
|
EMBRACE,
Wong [18] |
Two definitions Event-based exacerbation: an increase in or new onset of more than one pulmonary symptom (sputum volume, sputum purulence, or dyspnoea) requiring treatment with antibiotics Symptom-based exacerbation: an increase in or new onset of more than one pulmonary symptom reported on the daily diary card and the mean of the three symptom scores from the daily diary card on two consecutive days had to increase by at least one point (on a five-point scale) compared with the same calculation 1 week earlier |
Median TTF: Cox proportional hazard presented as Kaplan–Meier plots Number: rate ratio (Poisson regression model frequency) |
|
Mannitol,
Bilton [20] |
Treated with parenteral antibiotics for any four of the following 12 signs or symptoms: change in sputum; new or increased haemoptysis; increased cough; increased dyspnoea; malaise, fatigue or lethargy; temperature above 38°C; anorexia or weight loss; sinus pain or tenderness; change in sinus discharge; change in physical examination of the chest; decrease in pulmonary function by 10% or more from a previously recorded value; or radiographic changes indicative of pulmonary infection | TTF: presented as Kaplan–Meier plots |
|
Ciprofloxacin DPI,
Wilson [21] |
Not clear | TTF: method not reported |
|
BAT,
Altenburg [12] |
Two definitions Protocol defined exacerbation: at least four of the following nine symptoms, signs or findings were present: 1) change in sputum production (consistency, colour, volume or haemoptysis); 2) increased dyspnoea (chest congestion or shortness of breath); 3) increased cough; 4) fever (>38°C); 5) increased wheezing; 6) decreased exercise tolerance, malaise, fatigue or lethargy; 7) FEV1 or FVC decreased by at least 10% from previous recorded value; 8) radiographic changes indicative of a new pulmonary infectious process; or 9) changes in chest sounds Non-protocol defined exacerbation: fewer than four of the above abnormalities |
Number: median (IQR) TTF: Cox proportional hazard presented as Kaplan–Meier plots |
|
BLESS,
Serisier [22] |
Antibiotic treatment for a sustained (more than 24 h) increase in sputum volume or purulence accompanied by new deteriorations in at least two additional symptoms: sputum volume, sputum purulence, cough, dyspnoea, chest pain or haemoptysis | Number: rate ratio (Poisson regression model frequency) Frequency: rate ratio (Poisson regression model frequency) |
|
ORBIT-2,
Serisier [23] |
Deterioration in ≥4 of the following nine signs or symptoms: sputum production (volume, colour, consistency or haemoptysis), dyspnoea, cough, fever, wheezing, exercise tolerance (or fatigue/lethargy/malaise), FEV1 or FVC fall of at least 10%, new changes on chest radiograph and changes in chest sounds on auscultation | Median TTF: Kaplan–Meier plot |
|
Azithromycin,
De Diego [24] |
No definition stated | Number: mean |
|
Inhaled colistin,
Haworth [25] |
Presence of ≥3 of the following signs or symptoms for at least 24 h: increased cough, increased sputum volume, increased sputum purulence, haemoptysis, increased dyspnoea, increased wheezing, fever (>38°C) or malaise, and the treating physician agreed that antibiotic therapy was required | TTF: median (IQR) presented as Kaplan–Meier plots |
|
Atorvastatin,
Mandal [26] |
As per British Thoracic Society guidelines and treated them according to baseline sputum bacteriological findings and administered 14 days of oral antibiotic treatment | Frequency: median (IQR) |
|
AIR-BX1 and AIR-BX2,
Barker [27] |
Acute worsening of respiratory disease meeting ≥3 major (or two major and at least two minor) criteria Major criteria were increased sputum production, change in sputum colour, dyspnoea and cough Minor criteria were fever (>38°C) at clinic visit, increased malaise or fatigue, FEV1 (L) or FVC decreased by more than 10% from baseline, and new or increased haemoptysis |
TTF: median presented as Kaplan–Meier plots Rate: negative binomial model |
|
Inhaled mannitol,
Bilton [29] |
Worsening of signs and symptoms requiring changes in treatment | Number: count Rate: negative binomial model TTF: Cox proportional hazard |
|
Roxithromycin,
Liu [15] |
At least four of the following nine symptoms, signs or findings: 1) change in sputum production (consistency, colour, volume or haemoptysis); 2) increased dyspnoea (chest congestion or shortness of breath); 3) increased cough; 4) fever (>38°C); 5) increased wheezing; 6) decreased exercise tolerance, malaise, fatigue or lethargy; 7) FEV1 or FVC decreased by at least 10% from previous recorded value; 8) radiographic changes indicative of a new pulmonary infectious process; or 9) changes in chest sounds | TTF: Kaplan–Meier plot |
|
Tobramycin,
Orriols [13] |
More frequent coughing, greater dyspnoea and increased sputum volume and purulence | TTF: Kaplan–Meier plot Number: count |
|
STRATUS,
De Soyza [30] |
Increased dyspnoea, sputum production and sputum purulence | Not clear |
|
RESPIRE I and II,
De Soyza [8] and Aksamit [9] |
Two definitions For primary end-point: exacerbations had to meet three criteria: 1) worsening of at least three signs or symptoms (dyspnoea, wheezing, cough, 24-h sputum volume or sputum purulence) beyond normal day-to-day variations for at least 2 consecutive days; 2) fever (body temperature >38.0°C) or malaise/fatigue; and 3) systemic antibiotic treatment For secondary end-point: respiratory event with worsening of at least one of the aforementioned signs or symptoms and systemic antibiotic use |
TTF: (HR) Frequency: ratio (IRR) Number: mean |
TTF: time to first; DPI: dry powder inhaler; IQR: interquartile range; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; HR: hazard ratio; IRR: incidence rate ratio.
Inclusion in exacerbation studies tends to rely on exacerbation history, i.e. it is assumed that a frequent exacerbator in the previous year will exacerbate in the current year making this person the ideal candidate for an exacerbation study. From 19 trials using exacerbation end-point criteria, 11 defined exacerbation history as part of their protocol inclusion criteria and only two requested exacerbation history for more than 12 months [16, 29].
Recording of exacerbations requires a sufficiently long follow-up time to capture a meaningful number of events. We found, however, that exacerbation end-points were also used in short studies where the chance of a patient exacerbating within such a brief time frame is low [17, 20, 21, 23, 24]. From the trials reviewed, we noted eight with a maximum duration of 6 months which used various exacerbation end-points (one of which was the primary). Shorter studies which use exacerbations as end-points fall into the bias of recruitment periods, for example, a study running during a viral season or the winter months may have a higher volume of exacerbations than when running during summer months suggesting exacerbation frequency or time to next exacerbation may not be an ideal end-point for a short study. Of these eight studies under discussion, five were analysing time to next exacerbation, two were analysing frequency and the last was not clearly specified. There did not appear to be any preference as to which exacerbation definition was used by each of the intervention groups (antibiotics, anti-inflammatories or mucolytics). Methods of statistical analysis can have a major impact on the outcome of exacerbation studies and we also identified a high degree of heterogeneity in the analysis methods used in bronchiectasis trials (table 5).
Decrease in bacterial load or eradication of pathogens (predominantly Gram-negatives)
The most common microbes taken from positive cultures in bronchiectasis are Haemophilus influenzae and Pseudomonas aeruginosa [3]. A reduction in sputum bacterial load by greater than or equal to one log unit is deemed the minimum change necessary for clinical significance [32].
Microbiology as a primary end-point was exclusively used in antibiotic trials and demonstrated statistically significant differences versus placebo or control in 100% of trials reviewed. When including studies that used microbiology as a secondary end-point the success rate was 50% (table 3).
In general, antibiotic treatments do reduce bacterial load, as shown by positive results in six out of nine microbiology end-points within the antibiotic trials. However, the value of this end-point is questioned as it is not known to what extent reductions in bacterial load correlate with improvements in clinical outcomes. Of the six trials we identified in which CFU reductions were demonstrated, only two had positive HRQoL outcomes [11, 25] and three had positive exacerbation end-points [11, 13, 23].
Several studies have shown good reductions in bacterial load with no clear or consistent clinical efficacy raising questions about the value of this standard end-point for phase 2 antibiotic trials [11, 21, 23, 25, 31].
Sputum colour or weight
For treatments that aim to improve lung clearance by way of sputum expectoration, there is a degree of logic in trying to demonstrate that drugs increase the volume of sputum removed from the lungs. We identified 13 studies that used sputum characteristics as an end-point. The typical sputum characteristics were sputum volume, sputum weight and sputum colour.
There are known issues with the collection and characterisation of sputum including the high degree of patient compliance required when collecting sputum over 24 h. Sputum weight can be affected by multiple variables including weather, exercise, hydration status, diet and saliva contamination. The process of separating the weight of sputum from the lungs (desirable) from the weight of saliva from the mouth (undesirable) has not yet been standardised, and theoretically, some treatments that initially increase sputum weight because they are clearing the lungs in the short term, may actually reduce sputum volume over the long term as lung inflammation reduces. Examples of the challenges of using sputum weight were demonstrated in a study by Bilton et al. [20], where mannitol was used for 12 weeks with the hypothesis this would increase sputum weight compared with placebo. The sputum weight was significantly higher in the treatment group after 12 weeks, but primarily because sputum weight decreased in the placebo group, who had received more antibiotics [20]. This study demonstrates that other factors, such as antibiotic use, can influence sputum characteristics and make this a challenging end-point. We did not identify data on the variability of sputum volume as an end-point in bronchiectasis or a minimal clinically important difference (MCID).
Spirometry
Improvements in FEV1 have historically been the primary outcome for most COPD and CF clinical trials and a variety of drugs have consistently improved this end-point in those diseases. We found this was not the case in bronchiectasis as it has been shown to be poorly responsive to multiple interventions. The reasons for this are not clear and require further study. From the 23 studies reviewed, 19 had at least one end-point related to improvements in spirometry; however, only three obtained a statistically significant beneficial outcome. Four trials used lung function as the primary outcome, but only one had a positive outcome [28].
From the three trials showing a positive outcome in their lung function end-point, the variation in results is remarkable. The BAT trial conducted by Altenburg et al. [12] in the Netherlands was a 12-month study of 250 mg daily azithromycin. Visits were conducted every 3 months and lung function showed statistical difference between the treatment arm and placebo. For each 3-month interval, it was reported that FEV1 % predicted in the treatment arm increased by 1.03% while the placebo group decreased by 0.1%. The % predicted forced vital capacity (FVC) showed a similar trend, increasing 1.33% in each 3-month interval in the treatment arm and decreasing 0.3% in the placebo arm. BLESS was also a 12-month placebo-controlled trial conducted in Australia [22]. The study treatment was erythromycin 400 mg twice daily. By week 48 (end of treatment), erythromycin had reduced the rate of decline significantly in comparison to placebo. FEV1 % predicted had declined on average by 1.6% in the treatment arm; however, the placebo group showed a much faster decline with an average of 4.0%. The most remarkable results came from the study by Kellett et al. [14], who performed an 8-month crossover study between 7% hypertonic saline and 0.9% sodium chloride for 3 months each. The 0.9% saline arms showed increases of 1.8% and 0.72% in FEV1 % predicted and FVC % predicted, respectively, whereas the treatment arm saw large increases of 15.1% and 11.23% in FEV1 and FVC % predicted. Such a high increase sets this study out from the rest and raises questions about the characteristics of the included patients as other studies of hypertonic saline have not demonstrated similar improvements.
Most of the reviewed studies did not observe a change in FEV1 or other lung function parameters. Descriptions of the methods used for spirometry were lacking in most studies, including which guidelines were being followed. This has significant effects on reproducibility as there are substantial differences across % predicted calculations. The test itself is both technician and patient dependent, and as patients do not complain of reduced lung function, the clinical relevance of a change in this end-point is not known. With respect to this view, many treatments are not designed to improve lung function, i.e. they are not bronchodilators.
To date, lung function has not been found to be responsive in bronchiectasis in a manner similar to that observed in COPD and CF and does not appear to be a useful efficacy end-point. Further research on the prognosis of spirometry in relation to patient health status is required if lung function is to continue being used as a clinical trial end-point.
QoL and symptom questionnaires
The QoL of bronchiectasis patients can be evaluated using self-completed questionnaires. The questionnaires that we identified as being used in bronchiectasis trials were St George's Respiratory Questionnaire (SGRQ), Quality of Life – Bronchiectasis (QOL-B) and Leicester Cough Questionnaire (LCQ). A further bronchiectasis QoL questionnaire has been described in the literature but we did not find any trials to have used this tool as yet, the Bronchiectasis Health Questionnaire (BHQ) [33–36]. Most questionnaires have only been minimally validated in bronchiectasis and all except the QOL-B and BHQ were not specifically designed for bronchiectasis.
Clinical trials have failed to show improvements in QoL, despite it being the most commonly used end-point and SGRQ being used most often in bronchiectasis trials [37]. However, the validity of current questionnaires such as SGRQ and QOL-B are becoming frequently challenged as more trials show positive outcomes in areas such as exacerbations but lack the correlation in patient responses [13, 21–23, 26, 30, 31].
Five studies used HRQoL for their primary end-point with only one demonstrating a statistically significant difference when compared with placebo [26], although there was a statistically significant improvement in the AIR-BX2 study of inhaled aztreonam it did not reach the MCID [27].
More interestingly, following the failure of a trial in 6% hypertonic saline to show superiority over 0.9% placebo, Nicolson et al. [16] reported 29 (72.5%) of the study participants chose to remain on nebulised saline after study completion suggesting there was an overall acceptance and perception of benefit despite a significant decrease in self-reported compliance after 6 months and failure of the study to meet any end-points. This trial included SGRQ and LCQ as QoL measures [16].
Conversely, some studies have shown the opposite effect with improvement from a patient perspective despite failing due to quantitative findings in the primary end-points [24, 25, 29].
From the 23 studies we reviewed, only three had a positive primary end-point with a corresponding positive HRQoL result [12, 15, 28]. There was a disconnect between QoL tools and exacerbation reduction, but also a surprising disconnect in the RESPIRE trials of inhaled dry powder ciprofloxacin where two tools designed to measure symptoms (QOL-B and SGRQ) showed divergent results. RESPIRE1 saw a significant improvement in SGRQ scores for both the 14-day and 28-day on–off cycles (−7.59, p=0.0085 and −5.21, p=0.0636, respectively); however, this same improvement was not seen in the RESPIRE2 trial (−1.40, p=0.5446 and −1.44, p=0.5302, respectively) [8, 9]. In neither RESPIRE1 nor RESPIRE2, and for neither the 14-day nor the 28-day on–off cycle, were there any improvements measured by QOL-B. This suggests there is a need for further research into the representativeness and responsiveness of questionnaires in bronchiectasis.
Biomarkers (sputum and blood)
Biomarkers measured in RCTs include neutrophil markers and cytokines, such as interleukin (IL)-8, IL-1β, C-reactive protein and tumour necrosis factor-α, in sputum or serum. 12 out of the 23 reviewed studies contained biomarker end-points, with only three of these producing statistically beneficial outcomes. Of the four studies using biomarkers for primary end-points, half produced statistically significant results [15, 30]. Biomarkers are an area of growing interest and will probably be used more frequently in future studies especially as assays become more clearly validated and practical to perform.
Because the FDA and European regulators require end-points that reflect how a patient feels function and survives, biomarkers are only ever likely to be early surrogate end-points.
Other
Some trials had primary end-points which fell under this “other” category, e.g. cough, computed tomography changes and number of symptoms [15, 16]. These studies investigated changes in nitric oxide, nitrites and nitrates when treated with azithromycin and bronchus remodelling measured radiographically by the changes in wall thickening due to roxithromycin. The latter trial was regarded as positive in that it met the primary end-point with a statistically positive outcome and was the only trial in the review to show positive results in all end-points used. The remaining “other” end-points in use included exercise tolerance tests (6-min walk test and incremental field walking test) and the remaining trial monitored the number of bronchiectasis symptoms using a 0–3 scale [17].
Discussion
This systematic review has described the most frequently used end-points in bronchiectasis trials and identified those which have been most responsive to intervention. It is crucial to re-evaluate the conduct of bronchiectasis clinical trials in view of the failure of multiple recent large-scale trials to achieve consistent, statistically significant results. While it is possible that these trials represent appropriate failures because the drugs are ineffective, this seems unlikely since the majority of these drug therapies are either used in clinical practice with subjective patient benefit or have been shown to improve outcomes in CF or other diseases. Alternative hypotheses for the repeated challenges in bronchiectasis trials are therefore that the disease is too heterogeneous and therefore the responsive populations have not been identified, or that the clinical trial end-points have not been sufficiently characterised and defined. Our review focuses on this latter point which is crucial for future trial design.
As bronchiectasis has been a neglected disease, trial design has borrowed heavily from CF and COPD. There is limited longitudinal data for bronchiectasis patients and therefore little is known about disease prognosis. Welsh et al. [38] produced an overview of Cochrane systematic reviews and clearly concluded the need for a national or international organisation with patient values at its core and highlighted the need for developing improved outcome measures for future research. In September 2017, the ERS released the first bronchiectasis guidelines which were approved by an international patient advisory group [3]. The guidelines confirmed the nine most important Patient/problem, Intervention, Comparison, Outcome (PICO) topics agreed between patients and clinicians (aetiology, exacerbations, eradication, long-term anti-inflammatories, long-term antibiotics, long-term mucoactives, long-term bronchodilators, thoracic surgery and respiratory physiotherapy). These topics were unanimously decided as the most important factors and therefore it makes sense to develop future study outcomes with these in mind.
Chalmers et al. [37] reviewed 10 major bronchiectasis RCTs and ran a population of 1672 patients through the main inclusion and exclusion criteria of each trial. The outcome of this showed the high selectivity of RCTs do not represent the general bronchiectasis population as up to 93% of the reviewed population were not eligible for some of the studies. The group expressed their concern about licensing therapies based on this evidence; underestimating the efficacy of a drug and not licensing it despite the possibility of a positive result if a wider cohort had been used, or overestimating the efficacy leading to a licence and distribution to a population of bronchiectasis patients where there is no evidence of benefit [37].
Table 6 summarises the main end-point categories we have reviewed, and which should produce outcomes fitting both patient PICO expectations and the FDA requirement. The table also summarises initial possible approaches to optimising each end-point.
TABLE 6.
Potential solutions to problems found in the current end-points
| Current trial end-points | Problems identified | Possible solutions |
| Exacerbations | Lack of definition Seasonal effects in short-term studies Variable patient reporting including underreporting |
Validate EMBARC/BRR definition Study the impact of this on exacerbation reporting Patient diaries or symptom scales to capture nonreported exacerbations Develop an exacerbation prediction tool |
| Microbiology | Unknown clinical benefit | Study the correlation of microbiome with clinical burden |
| Sputum | Dependent on patient compliance Inter- and intra-day variation |
Develop a scale indicating burden of expectoration rather than specific volumes, weights, etc. Alternative methods of measuring airway clearance, e.g. imaging |
| Lung function | Multiple guidelines Patient and technician dependent Therapies are not designed to improve lung function Patients do not ask for improvement |
Improve reporting and standardisation of methods Study the impact of lung function on quality of life Qualitative data gathering on what quality of life means to patients themselves Identify patient subgroups who show lung function improvements in response to therapies as in cystic fibrosis |
| HRQoL (questionnaires) | Multiple variations Currently anchored to clinical markers with no validated significance in bronchiectasis patients Not shown to be responsive |
Further validation required Further work may involve optimising existing questionnaires or developing new responsive scales Establish MCID specific for bronchiectasis |
| Biomarkers | No direct clinical benefit | Remain as early phase end-point May be useful for selecting patients for entry into trials |
HRQoL: health-related quality of life; EMBARC: the European Bronchiectasis Registry; BRR: American Bronchiectasis Research Registry; MCID: minimal clinically important difference.
Currently there is no validated exacerbation prediction tool and therefore studies are hard to power accurately for this end-point. Clinically, a frequent exacerbator will have three or more exacerbations in a 12-month period [3]; however, eligibility to these studies usually only requires one documented exacerbation requiring antibiotics which is neither representative of the patient population nor likely to capture an event during the study period. Reviewing only the past 12 months of patients' exacerbation history could risk overestimating the severity of patients and prevent true reflection of any clinical benefit a therapy may have. Another critical issue that has not been addressed in these trials is the potential underreporting of exacerbations by patients. The exacerbation threshold (the point at which a patient seeks medical help) will vary from patient to patient.
In summary, exacerbations are a very important end-point, but insufficient research has been performed to understand how they should be defined, detected in trials, and to account for potential confounders in trials such as seasonality, underpowering and underreporting. We identified a high degree of heterogeneity in exacerbation analysis. There is an emerging consensus that exacerbation frequency is the most clinically relevant end-point, not least because it correlates with clinically important outcomes such as mortality and hospitalisation. However, even analysis of the frequency end-point is not straightforward as the negative binomial modelling approach which is most frequently used makes some assumptions, such as that exacerbations are independent events, which we know are not true. Alternative analysis approaches to exacerbation modelling such as the Andersen and Gill [39] “counting process” may be advantageous and better reflect the biology of the disease.
It is true that research should be centred on the patient and that patients will best answer whether there is a positive difference between an active intervention and a control. The most likely way of capturing this result is through HRQoL or symptom questionnaires.
Dudgeon et al. [40] conducted a qualitative study asking eight bronchiectasis patients to review the standard questionnaires (SGRQ, QOL-B, LCQ and COPD Assessment Test). The results showed a remarkable agreement in that none of the questionnaires captured a “baseline” result for later comparison. The patients themselves did not understand how the questions could assess changes in their health without this information. In addition to this, recall and content were most relevant to the reviewers. A 1-week recall time in the QOL-B was preferred over the 24 h in LCQ or 3 months in the SGRQ. The patients also preferred questionnaires with multiple choice or Likert scale answers as opposed to true and false options, again reflecting the heterogeneity of approaches to measuring symptoms and HRQoL in bronchiectasis.
In most cases the MCIDs for end-points in bronchiectasis have not been defined. It is important that a thorough evaluation of end-points for bronchiectasis is undertaken to better design future trials and understand their results. We also urge the companies and investigators that have undertaken trials in bronchiectasis to make their datasets available to academic researchers in order that we can learn from prior studies and to better inform future trial design.
Our review has limitations. We focused only on adequately powered efficacy and safety studies of pharmacotherapies and our results should not be generalised to airway clearance or other studies in bronchiectasis. We included an arbitrary cut-off of 25 subjects enrolled to the selected studies, but acknowledge that it is theoretically possible to have informative data from smaller studies.
Conclusion
This systematic review identified which end-points are used in bronchiectasis trials, which have been shown to be responsive to intervention and identified gaps and research priorities in our understanding of bronchiectasis end-points.
This systematic review demonstrates a need for guidance to standardise definitions and design features to improve reproducibility and increase the likelihood of demonstrating statistically significant benefits with new therapies.
Footnotes
This study is registered with PROSPERO (www.crd.york.ac.uk/prospero) with identifier number CRD42018106167.
Provenance: Submitted article, peer reviewed.
Conflict of interest: M.L. Crichton has nothing to disclose.
Conflict of interest: S. Aliberti has nothing to disclose.
Conflict of interest: J.D. Chalmers reports grants and personal fees from GlaxoSmithKline (research grants for COPD), grants and personal fees from Boehringer-Ingelheim (research grants for COPD studies), grants from AstraZeneca (research grants for COPD), grants and personal fees from Pfizer (research grants for COPD), grants and personal fees from Bayer Healthcare (research into bronchiectasis), grants and personal fees from Grifols (research into bronchiectasis), personal fees from Napp (consulting), personal fees from Aradigm corporation (consulting), and grants and personal fees from Insmed, outside the submitted work.
Support statement: EMBARC2 is supported by the European Respiratory Society through the Clinical Research Collaboration. The project is supported by project partners: Chiesi, Grifols, Insmed, Novartis and Zambon. EMBARC is supported by EU/EFPIA IMI iABC grant agreement no. 115721. J.D. Chalmers is supported by the GSK/British Lung Foundation Chair of Respiratory Research. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Ringshausen FC, de Roux A, Diel R, et al. Bronchiectasis in Germany: a population-based estimation of disease prevalence. Eur Respir J 2015; 46: 1805–1807. [DOI] [PubMed] [Google Scholar]
- 2.Quint JK, Millett ERC, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell AE, Barker AF, Ilowite JS, et al. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. Chest 1998; 113: 1329–1334. [DOI] [PubMed] [Google Scholar]
- 5.European Medicines Agency. Authorisation of medicines. www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines Date last accessed: November 5, 2018.
- 6.US Food and Drug Administration. New Drug Application (NDA). www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/NewDrugApplicationNDA/default.htm Date last updated: March 29, 2016.
- 7.ICMJE. Clinical Trials Registration. www.icmje.org/about-icmje/faqs/clinical-trials-registration/ Date last accessed: October 30, 2018.
- 8.De Soyza A, Aksamit T, Bandel T-J, et al. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51: 1702052. [DOI] [PubMed] [Google Scholar]
- 9.Aksamit T, De Soyza A, Bandel T-J, et al. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51: 1702053. [DOI] [PubMed] [Google Scholar]
- 10.Herrero-Cortina B, Alcaraz V, Vilaro J, et al. Impact of hypertonic saline solutions on sputum expectoration and their safety profile in patients with bronchiectasis: a randomized crossover trial. J Aerosol Med Pulm Drug Deliv 2018; 31: 281–289. [DOI] [PubMed] [Google Scholar]
- 11.Murray MP, Govan JRW, Doherty CJ, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2011; 183: 491–499. [DOI] [PubMed] [Google Scholar]
- 12.Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non–cystic fibrosis bronchiectasis. JAMA 2013; 309: 1251–1259. [DOI] [PubMed] [Google Scholar]
- 13.Orriols R, Hernando R, Ferrer A, et al. Eradication therapy against Pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis. Respiration 2015; 90: 299–305. [DOI] [PubMed] [Google Scholar]
- 14.Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir Med 2011; 105: 1831–1835. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Zhong X, He Z, et al. Effect of low-dose, long-term roxithromycin on airway inflammation and remodeling of stable noncystic fibrosis bronchiectasis. Mediators Inflamm 2014; 2014: 708608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolson CHH, Stirling RG, Borg BM, et al. The long term effect of inhaled hypertonic saline 6% in non-cystic fibrosis bronchiectasis. Respir Med 2012; 106: 661–667. [DOI] [PubMed] [Google Scholar]
- 17.Hernando R, Drobnic ME, Cruz MJ, et al. Budesonide efficacy and safety in patients with bronchiectasis not due to cystic fibrosis. Int J Clin Pharm 2012; 34: 644–650. [DOI] [PubMed] [Google Scholar]
- 18.Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012; 380: 660–667. [DOI] [PubMed] [Google Scholar]
- 19.Stockley R, De Soyza A, Gunawardena Ket al. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis Respiratory Medicine 2013; 107: 524–533. [DOI] [PubMed] [Google Scholar]
- 20.Bilton D, Daviskas E, Anderson SD, et al. Phase 3 randomized study of the efficacy and safety of inhaled dry powder mannitol for the symptomatic treatment of non-cystic fibrosis bronchiectasis Chest 2013; 144: 215–225. [DOI] [PubMed] [Google Scholar]
- 21.Wilson R, Welte T, Polverino E, et al. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: A phase II randomised study. Eur Respir J 2013; 41: 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013; 309: 1260–1267. [DOI] [PubMed] [Google Scholar]
- 23.Serisier DJ, Bilton D, De Soyza A, et al. Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial. Thorax 2013; 68: 812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Diego A, Milara J, Martinez-Moragon E, et al. Effects of long-term azithromycin therapy on airway oxidative stress markers in non-cystic fibrosis bronchiectasis. Respirology 2013; 18: 1056–1062. [DOI] [PubMed] [Google Scholar]
- 25.Haworth CS, Foweraker JE, Wilkinson P, et al. Inhaled colistin in patients with bronchiectasis and chronic pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2014; 189: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandal P, Chalmers JD, Graham C, et al. Atorvastatin as a stable treatment in bronchiectasis: a randomised controlled trial. Lancet Respir Med 2014; 2: 455–463. [DOI] [PubMed] [Google Scholar]
- 27.Barker AF, O'Donnell AE, Flume P, et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2014; 2: 738–749. [DOI] [PubMed] [Google Scholar]
- 28.Lourdesamy Anthony AI, Muthukumaru U. Efficacy of azithromycin in the treatment of bronchiectasis. Respirology 2014; 19: 1178–1182. [DOI] [PubMed] [Google Scholar]
- 29.Bilton D, Tino G, Barker AF, et al. Inhaled mannitol for non-cystic fibrosis bronchiectasis: a randomised, controlled trial. Thorax 2014; 69: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 30.De Soyza A, Pavord I, Elborn JS, et al. A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur Respir J 2015; 46: 1021–1032. [DOI] [PubMed] [Google Scholar]
- 31.Ailiyaer Y, Wang X, Zhang Y, et al. A prospective trial of nebulized amikacin in the treatment of bronchiectasis exacerbation. Respiration 2018; 95: 327–333. [DOI] [PubMed] [Google Scholar]
- 32.Hill AT, Campbell EJ, Hill SL, et al. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med 2000; 109: 288–295. [DOI] [PubMed] [Google Scholar]
- 33.Wilson CB, Jones PW, O'Leary CJ, et al. Validation of the St. George's respiratory questionnaire in bronchiectasis. Am J Respir Crit Care Med. 1997; 156: 536–541. [DOI] [PubMed] [Google Scholar]
- 34.Quittner AL, Marciel KK, Salathe MA, et al. A preliminary quality of life questionnaire-bronchiectasis: a patient-reported outcome measure for bronchiectasis. Chest 2014; 146: 437–448. [DOI] [PubMed] [Google Scholar]
- 35.Berkhof FF, Boom LN, ten Hertog NE, et al. The validity and precision of the Leicester cough questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes 2012; 10: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spinou A, Siegert RJ, Guan W-j, et al. The development and validation of the Bronchiectasis Health Questionnaire. Eur Respir J 2017; 49: 1601532. [DOI] [PubMed] [Google Scholar]
- 37.Chalmers JD, McDonnell MJ, Rutherford R, et al. The generalizability of bronchiectasis randomized controlled trials: a multicentre cohort study. Respir Med 2016; 112: 51–58. [DOI] [PubMed] [Google Scholar]
- 38.Welsh EJ, Evans DJ, Fowler SJ, et al. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane database Syst Rev 2015; 7: CD010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Stat 1982; 10: 1100–1120. [Google Scholar]
- 40.Dudgeon EK, Crichton M, Chalmers JD. “The missing ingredient”: the patient perspective of health related quality of life in bronchiectasis: a qualitative study. BMC Pulm Med 2018; 18: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]