Abstract
Pathogenic genetic variants (formerly called mutations) present in the germline of some individuals are associated with a clinically relevant increased risk of developing lung cancer. These germline pathogenic variants are hereditary and are transmitted in an autosomal dominant fashion. There are two major lung cancer susceptibility syndromes, and both seem to be specifically associated with the adenocarcinoma subtype. Li-Fraumeni syndrome is caused by variants in the TP53 tumour-suppressor gene. Carriers are mainly at risk of early-onset breast cancer, sarcoma, glioma, leukaemia, adrenal cortical carcinoma and lung cancer. EGFR variants, T790M in particular, cause the EGFR susceptibility syndrome. Risk seems limited to lung cancer. Emerging data suggest that variants in ATM, the breast and pancreatic cancer susceptibility gene, also increase lung adenocarcinoma risk. As for inherited lung disease, cancer risk is increased in SFTPA1 and SFTPA2 variant carriers independently of the underlying fibrosis. In this review, we provide criteria warranting the referral of a lung cancer patient to the cancer genetics clinic. Pathogenic variants are first identified in patients with cancer, and then in a subset of their relatives. Lung cancer screening should be offered to asymptomatic carriers, with thoracic magnetic resonance imaging at its core.
Short abstract
A proportion of lung cancers are hereditary. This includes patients with Li-Fraumeni syndrome and patients with EGFR-associated genetic susceptibility. They are mainly young patients with adenocarcinoma regardless of smoking history. https://bit.ly/2QAfjnB
Introduction
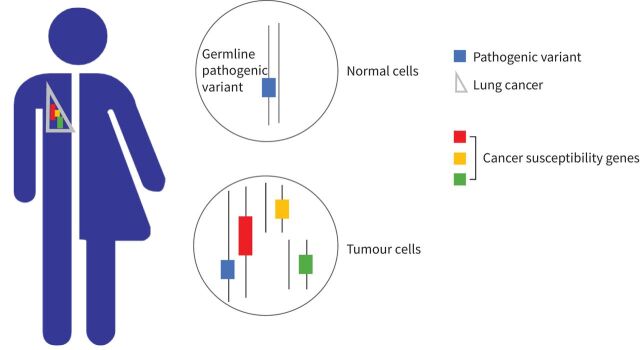
Approximately 10–15% of lung cancers in Western countries occur in never-smokers [1]. These cancers are accounted for by other occupational and environmental risk factors, such as passive smoking, radon and air pollution [2–5] and, importantly, by genetic factors. We mean here variations at a specific position within a gene that are rare in the population, and that are directly associated with a clinically relevant risk increase in carriers. We refer to these genetic variations as pathogenic variants, formerly called mutations. Pathogenic variants are present in the germline of an individual, i.e. in every cell throughout the body (figure 1). They are therefore hereditary, since they can be transmitted to the offspring and are in most cases inherited from the father or the mother in an autosomal dominant fashion. In a minority of cases, pathogenic variants appear de novo, i.e. in the first stages of embryogenesis, or in one of the parent's germ cells.
FIGURE 1.
Patient with a germline pathogenic variant increasing lung cancer risk. The pathogenic variant is present in every cell throughout the body. When lung cancer does develop, it accumulates somatic activating variants in a variety of genes, including the cancer susceptibility gene.
Interestingly, the first publication pointing towards a substantial contribution of genetic factors to lung cancer causality dates back almost 60 years [6]. In a comparison of relatives of 270 lung cancer cases with relatives of matched controls, Tokuhata et al. [6] observed a significant excess in lung cancer mortality among relatives of cases. It was not accounted for by smoking history. Genetics are indeed a major lung cancer risk factor, as illustrated by two seminal studies in the field. First, a pooled analysis from the International Lung Cancer Consortium concluded that individuals with a first-degree relative with lung cancer had a 50% increase in risk compared to individuals with no family history, after adjustment for smoking and other confounders [7]. Secondly, the prospective Nordic twin study estimated heritability for a variety of cancers by comparing cancer incidence in monozygotic versus dizygotic twins of cancer cases, heritability being the proportion of the variation in risk in a population accounted for by genetic factors [8]. It was 18% for lung cancer, higher than for colorectal cancer, in which screening for genetic susceptibility is now universal [8, 9].
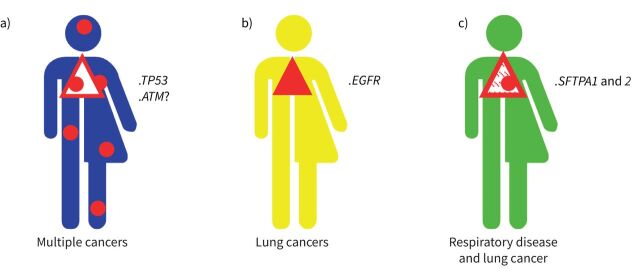
Genetic susceptibility to lung cancer occurs mainly through rare germline pathogenic variants specifically associated with cancer risk (figure 2a and b). They are considered clinically relevant if asymptomatic carriers can be offered personalised lung cancer screening, for example regular thoracic magnetic resonance imaging (MRI) or low-dose computed tomography (CT). That is the main focus of this review. In addition, we cover the topic of lung cancer risk in patients with other genetic conditions involving the lungs, such as pulmonary fibrosis (figure 2c). Finally, we address susceptibility through genetic variants that are common in the population. Individually, each variant only increases risk marginally, but taken together they might have clinical pertinence in the future.
FIGURE 2.
Different types of genetic susceptibility to lung cancer. a) The pathogenic variant is associated with a multicancer risk syndrome that includes an increased risk of lung cancer; b) the risk is limited to lung cancer; c) the pathogenic variant is associated with inherited lung disease such as fibrosis, and lung cancer risk is also increased.
Genetic susceptibility to lung cancer has recently become an issue of interest among thoracic oncologists and geneticists, through growing attention given to lung cancer in young nonsmokers, and via the generalisation of somatic next-generation sequencing using panels that include cancer susceptibility genes (TP53, BRCA1/2, ATM).
Genetic susceptibility through rare germline variants
Li-Fraumeni syndrome
Li-Fraumeni is a rare cancer susceptibility syndrome associated with germline pathogenic variants in the TP53 tumour-suppressor gene. Individuals are at increased risk of cancer in general and at all ages (including paediatric cancers), but more specifically of sarcoma, leukaemia, brain tumours, adrenal cortical carcinoma, pre-menopausal breast cancer, choroid plexus tumours and lung adenocarcinoma [10]. In a recent consensus paper, experts recommended that an alternative name, heritable TP53-related cancer syndrome, be used in attenuated forms of the syndrome [11]. For convenience, we refer to Li-Fraumeni throughout this section.
For reasons unknown, the majority of Li-Fraumeni-associated lung adenocarcinomas harbour EGFR (endothelial growth factor receptor) somatic activating variants, with the expected associated sensitivity to anti-EGFR tyrosine kinase inhibitors (TKIs). Such variants were indeed identified in eight out of nine and 18 out of 21 lung adenocarcinomas in Brazilian and a European Li-Fraumeni series, respectively [12, 13]. As for the prevalence of Li-Fraumeni among patients with EGFR-activated lung adenocarcinoma, it was high in a series of Brazilian patients: 5% (six out of 114) for the entire cohort, and 13% for cases aged ≤50 years [12]. This probably represents an overestimate, given the existence of a frequent founder TP53 variant in southern Brazil. Prospective systematic exploration in Caucasian (and other) populations would be highly informative.
Regarding lung adenocarcinoma risk in Li-Fraumeni patients, rough estimates can be derived from cancer screening studies using whole-body MRI. 2% (four out of 198) of adult cases had lung adenocarcinoma at baseline whole-body MRI in a meta-analysis, with ages at diagnosis ranging from 43 to 64 years [14]. The proportion was 6% (five out of 88) in a French series not included in the meta-analysis [15]. Most cancers reported were in nonsmokers.
The modified Chompret criteria normally determine who should be referred to the cancer genetics clinic for Li-Fraumeni exploration [16]. In our opinion, too little prominence is given to lung adenocarcinoma. Thus, we have adapted them to lung cancer patients in table 1, taking into account recent data from the literature, and have added a criterion based on the EGFR status of the tumour [14, 15].
TABLE 1.
TP53-associated genetic susceptibility to lung cancer: criteria for referral to the cancer genetics clinic for genetic counselling and germline testing
| 1. Lung adenocarcinoma age <46 years regardless of smoking history and |
| personal history of pre-menopausal breast cancer, glioma, sarcoma, adrenocortical carcinoma, acute leukaemia, choroid plexus tumour, OR |
| family history in first- or second-degree relatives of pre-menopausal breast cancer, glioma, sarcoma, adrenocortical carcinoma, acute leukaemia, choroid plexus tumour, lung adenocarcinoma age <56 years |
| 2. Lung adenocarcinoma age <50 years with a somatic EGFR-activating variant or deletion, regardless of smoking and family history |
Information from [16].
Annual whole-body MRI is now the cornerstone of cancer screening in Li-Fraumeni patients [11, 17]. It is probably the most appropriate tool for lung cancer screening, given the radiosensitivity observed in these patients and the reluctance in this context to recommend CT scans on a regular basis. Detailed cancer screening protocols are beyond the scope of this review; they can be found in the relevant publications [11, 17].
EGFR-associated genetic susceptibility
Germline EGFR pathogenic variants located in sequences coding for the tyrosine kinase domain of the protein are associated with lung cancer risk, the main variant by far being T790M. T790M is mainly known as an acquired somatic variant following administration of first- and second-generation anti-EGFR TKIs. Its identification on a tissue or liquid biopsy in a lung cancer patient not previously exposed to these drugs raises the possibility of a germline origin, even if associated with a common activating mutation. Between 73% and 100% of cancers associated with the T790M germline contain a second somatic EGFR-activating variant, most often L858R or exon 19 deletion [18–21]. Accordingly, these tumours are less sensitive to first- and second-generation anti-EGFR TKIs, but are expected to be sensitive to the third-generation osimertinib [21].
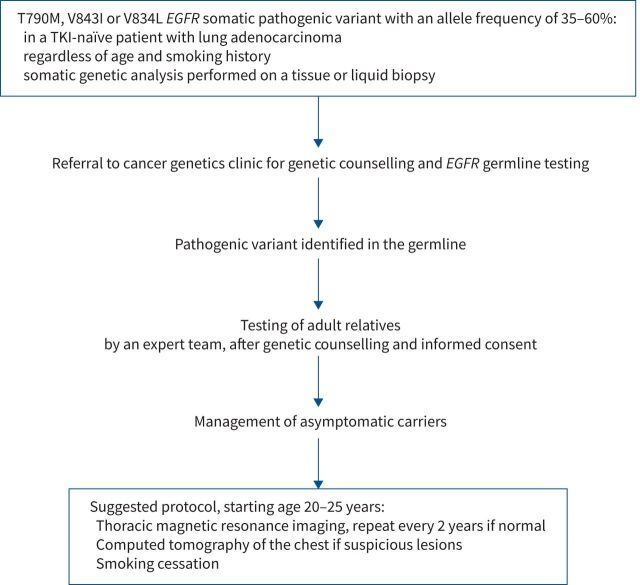
If the T790M variant allele frequency (VAF) is 35–60%, the patient should be referred to a clinical cancer genetics team (figure 3) [22]. Indeed, a VAF ∼50% suggests that it is present at the heterozygote state, at roughly equal proportions to the wild-type allele, as seen with germline variants. Interestingly, the VAF in this case is not influenced by therapy, and is therefore stable throughout the course of treatment.
FIGURE 3.
Epithelial growth factor receptor (EGFR)-associated genetic susceptibility to lung cancer: referral criteria, assessment and testing pathway, and management of asymptomatic carriers. TKI: tyrosine kinase inhibitor.
The EGFR T790M germline variant accounts for 0.3–0.9% of lung adenocarcinomas, as detailed later [19, 22, 23]. This represents up to 2000 new cases a year in Europe [24]; 140 in France [25]. The overall proportion and associated numbers might seem modest, but nevertheless it is essential to identify these cases given the possibility of downstream genetic testing of relatives, and then of personalised cancer screening in asymptomatic carriers. Transmission is autosomal dominant, and each first-degree relative has therefore a 50% risk of carrying the variant.
In a series of 12 774 nonsquamous nonsmall cell lung cancers (NSCLC) in which plasma cell-free DNA analysis was followed by bioinformatics exploration and germline testing of selected cases, 43 (0.34%) germline T790M variants were identified [22]. Assuming a liquid biopsy sensitivity of 70%, the overall proportion of cases would be 0.49% [23]. Lou et al. [19] evaluated 427 patients with lung adenocarcinoma. Four (0.9%) had a likely or confirmed germline T790M variant.
The T790M variant is associated with cancer risk regardless of smoking history. Indeed, the first reported case was a 50-year-old male smoker, and affected relatives in one of the families reported by Lou et al. had a history of smoking [19, 26].
We know that cancer risk starts at a young age, with reported cases as young as 29 and 34 years old [18, 19]. This leads us to the notion of penetrance; more precisely, the lung adenocarcinoma lifetime risk in a carrier. Based on a large family and on a literature review, Gazdar et al. [18] estimated it to be 23%.
The most striking aspects of EGFR T790M-associated lung adenocarcinoma are its sometimes indolent behaviour, and the multiplicity of pulmonary nodules in carriers undergoing radiological exploration. A female patient with multifocal disease opted for a strategy of expectant observation [27]. After 4 years, lesions had only grown minimally (11.4 mm diameter versus 8.54 mm for the target lesion, for example). Five asymptomatic carriers in a large EGFR T790M family were offered screening CT; all had pulmonary lesions of uncertain diagnosis [18]. The subcentimetre or ground-glass appearance observed in these cases probably corresponds to pre- or minimally invasive adenocarcinomas [18]. Biopsies of multiple nodules in this family's proband confirmed a spectrum of pathologies ranging from atypical adenomatous hyperplasia to adenocarcinoma in situ and minimally invasive adenocarcinoma.
Considering the high frequency of incidental nodules in asymptomatic carriers compared to the moderate estimated disease penetrance, it is likely that a proportion of lesions remain quiescent for years, if not decades; perhaps even a lifetime. A similar pattern is observed in other cancer predisposition syndromes, most notably the hereditary diffuse gastric cancer syndrome, where 95% of carriers of pathogenic variants in CDH1 have multiple cancer foci in their stomach, but only a proportion develop clinically manifest gastric cancer [28].
There are no data suggesting that the T790M variant is associated with other cancers, nor is there biological plausibility supporting an association. Its prevalence was only 0.04% among >65 000 cases tested for genetic susceptibility to cancer, mainly women with breast and ovarian cancer [29]. The majority of carriers had a history of lung cancer with the variant identified initially at the somatic level. In a distinct series, only one in 1000 cases tested for genetic susceptibility to cancer carried the variant, a woman with breast cancer in her seventies [30]. The incidental identification of the variant in cases with a history of cancer other than lung is expected, given its incomplete penetrance.
Other EGFR germline variants located in the tyrosine kinase domain have been reported in lung cancer patients, although their role as susceptibility variants could not always be firmly established in the absence of evidence of pathogenicity or co-segregation within the family. Among those, V834L and V843I can be confidently associated with susceptibility to lung adenocarcinoma [31–33]. Indeed, they co-segregated with the disease within families and/or were identified by multiple research teams in distinct families.
Figure 3 lists criteria warranting referral of lung adenocarcinoma patients to clinical cancer genetics and germline EGFR testing. Asymptomatic carriers of EGFR susceptibility variants are likely to benefit from personalised lung cancer screening. We would suggest biennial thoracic MRI starting at age 20 years with complementary CT only in cases of suspicious lesions, to spare carriers long-term exposure to ionising radiation (figure 3) [34]. If relevant, smoking cessation should be encouraged, and advice regarding occupational and environmental exposure given.
Lung cancer risk in other cancer susceptibility syndromes
Large studies of lung cancer cases are bound to identify germline variants in cancer susceptibility genes other than EGFR and TP53, given that variants are present in the general population regardless of medical history, albeit at a low frequency. An association between these genes and lung cancer risk requires a higher frequency of germline pathogenic variants in lung cancer cases compared to the general population (table 2). It must be ensured that the variants reported in these studies are pathogenic or likely to be pathogenic, and not of unknown significance. In addition, cases should not be enriched in patients with a personal or family history of cancers associated with the incriminated susceptibility genes (e.g. breast or ovarian and BRCA1/2), in which case the pathogenic variant might be associated with these other cancers and not the lung cancer itself. Finally, variants present in lung cancer cases at the heterozygote state, but that normally predispose to disease when homozygous (e.g. MUTYH and colorectal cancer) should be ignored.
TABLE 2.
Key points in assessing the validity of a study claiming an association between gene variants and lung cancer
| The frequency of the variants is higher than in the general population. |
| The variants reported are (probably) pathogenic, and not of unknown significance. |
| Lung cancer cases are not enriched in cases with a personal or family history of cancer associated with the incriminated genes. Should that be the case, the authors must take it into account in their statistical analyses. |
| Heterozygous variants are associated with susceptibility to disease in heterozygotes, and not only in homozygotes. |
ATM variants in the heterozygote state increase moderately the risk of breast and pancreatic cancer, while homozygotes have ataxia-telangectasia, a rare recessive neurodegenerative paediatric disease [35, 36]. Although replication studies are needed before it can be considered a definite lung adenocarcinoma susceptibility gene, ATM is clearly an excellent candidate at this stage. In a study of 555 lung adenocarcinomas undergoing germline gene panel testing, seven (1.3%) heterozygote pathogenic germline variants in the ATM gene were observed [37]. More recently, in a rigorous multistep association study an excess of ATM pathogenic variants in adenocarcinoma cases was observed compared to controls [38], with combined odd ratio of 4.6 (95% CI 2.2–9.5). Finally, in an analysis of rare variants in 39 146 individuals of European ancestry, Ji et al. [39] observed an odds ratio of 3.66 (95% CI 2.36–5.69) for lung adenocarcinoma among heterozygotes for the ATM L2307F variant. Frequent loss of heterozygosity in the tumour supported an association with disease risk. Interestingly, while rare in the general population, this variant is common in Ashkenazi Jews (4%). Population-wide genetic testing in Ashkenazis for ATM L2307F could be envisaged in the future, should the association with lung adenocarcinoma be confirmed, and if the increase in risk is sufficient to warrant cancer screening. Of note, population-based testing for the three BRCA1-BRCA2 Ashkenazi founder variants is already advocated by some experts [40].
Overall, there is no established association between lung cancer risk and major genes involved in other cancer susceptibility syndromes, for example hereditary breast and ovarian cancer (BRCA1, BRCA2, PALB2) or Lynch syndrome (MLH1, MSH2, MSH6, PMS2).
The association between BRCA2 and lung cancer suggested in a recent Chinese study remains exploratory [41], since other studies are negative. In the Chinese paper, BRCA2 germline pathogenic variants were found in 0.8% (49 out of 6220) of cases with advanced NSCLC, the majority of whom were smokers, higher than the estimated frequency in an East Asian population (0.2%) [41, 42]. The vast majority of included cases had adenocarcinoma. In contrast, among 4459 mostly Californian patients with advanced lung cancer (all types) undergoing plasma cell-free DNA analysis, only 17 (0.38%) carried BRCA2 pathogenic variants, close to that seen in 1997 healthy Australian controls (0.45%) in a breast cancer study [43, 44]. In the aforementioned retrospective study of 555 lung adenocarcinoma cases in the United States, there was only one BRCA2 germline pathogenic variant identified by sequencing of nontumoural lung tissue (0.2%), less than the general population frequency [37, 42, 44]. Finally, among 379 advanced NSCLC cases with a molecular profile included in the SAFIR02-lung trial, there were only two germline BRCA2 pathogenic variant carriers and both had a family history of BRCA-associated cancers [45]. In accordance with these observations, there was no increased incidence of lung cancer in a prospective cohort of nearly 4000 BRCA2 female carriers of breast/ovarian cancer susceptibility variants [46].
While the lack of association between BRCA2 and lung cancer is observed across the majority of studies, a pattern seems to emerge regarding histological subtype. Indeed, among eight Israeli BRCA2 pathogenic variant carriers with NSCLC [47], all had adenocarcinomas. Similarly, the two BRCA2 pathogenic variant carriers identified in the SAFIR02-lung study also had adenocarcinoma. These preliminary observations must be approached with caution, since they are based on small number of cases, and as adenocarcinomas represent the majority of lung cancers in the general population anyway. Further data from larger series would be highly informative.
If BRCA2 germline pathogenic variants involved in breast and ovarian cancer susceptibility do not seem to increase lung cancer risk, there is however one variant, K3326*, that is specifically associated with squamous cell lung cancer, as well as squamous cell carcinoma of the upper aerodigestive tract in general (e.g. head and neck, oesophagus) and of the skin. The K3326* stop variant (rs11571833) close to the 3′ end of BRCA2 is present in <1% of the general population and is considered nonpathogenic for breast and ovarian cancer. The odds ratio for squamous cell lung carcinoma in heterozygotes was 2.47 (95% CI 2.03–3.0) in a meta-analysis of four case–control studies, and 1.66 (95% CI 1.27–2.16) in a subsequent multinational collaboration that included >400 000 individuals [48, 49]. Risk of adenocarcinoma and of small cell lung cancer could be increased by a similar magnitude, although these observations have not been replicated [48, 49].
From a clinical point of view, we believe the utility of BRCA2 K3326* genotyping in patients with squamous cell lung cancer should be explored in prospective cohorts. At this stage, should the variant be identified, it would be premature to recommend testing or relatives and lung cancer screening to those who turn out to be asymptomatic carriers, given the only modest increase in risk. It is worth restating here that K3326* is neither a breast nor an ovarian cancer susceptibility variant, as there is no increase in disease risk, or depending on the study, an increase that is too small to have any clinical consequences [49, 50]. The highest reported odds ratio was <1.3, while bona fide pathogenic susceptibility variants increase breast and ovarian cancer risk between five- and 50-fold [46, 50].
As for other genes, Selvan et al. [51] compared whole-exome datasets from <1000 cases with squamous cell lung carcinoma and >4000 controls, and claimed to have found an increased risk associated with rare variants in the Fanconi anaemia genes when taken together. We consider these findings as hypothesis-generating, given the multiplicity of statistical hypotheses, the relatively high p-values when BRCA2K3326* was excluded, the low sample size and the fact that no gene came out as a susceptibility gene on its own apart from BRCA2 through the effect of K3326*.
Lung cancer and genetic susceptibility to malignant mesothelioma
There is no evidence of an increased lung cancer risk in patients with genetic susceptibility to pleural malignant mesothelioma, another thoracic malignancy. Indeed, a small proportion of mesotheliomas are also hereditary. The only validated susceptibility gene at this stage is BAP1, the gene encoding BRCA1-associated protein-1. Germline pathogenic variants were first reported in two families in the United States with multiples cases of mesothelioma [52]. Subsequently, Panou et al. [53] identified germline variants in six (3%) out of 198 nonfamilial malignant mesothelioma cases, sometimes in conjunction with previous asbestos exposure. Of note, patients with mesothelioma of the peritoneum and tunica vaginalis were also included in this series. Importantly, BAP1 loss by immunohistochemistry is predictive of a germline pathogenic variant, although it is not diagnostic, as it can also be due to two purely somatic events [52–54]. Interestingly, BAP1 variant carriers seem to have better survival after platinum-based chemotherapy, reflecting the underlying deficits in homologous recombination DNA repair [55]. Carriers are at risk of other tumours, more precisely uveal and cutaneous melanoma, skin basal cell carcinoma, renal cell carcinoma and meningioma [56, 57]. There are no data or recommendations regarding mesothelioma screening in asymptomatic carriers.
Lung cancer risk in inherited lung disease
Cystic fibrosis
Cystic fibrosis is the most common genetic disorder affecting the lungs. It is caused by pathogenic variants in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, and transmission is autosomal recessive. Lung cancer risk in cystic fibrosis patients is similar to the general population [58].
Alpha-1 antitrypsin deficiency
Alpha-1 antitrypsin deficiency (AATD) results in COPD, emphysema and cirrhosis. AATD is due to pathogenic variants in SERPINA1. Transmission is autosomal recessive [59]. Apart from small case–control studies prone to false positives, there is no evidence that SERPINA1 variants are associated with lung cancer risk [60–62].
Birt–Hogg–Dubé
Pathogenic variants in FLCN, the gene coding for the folliculin protein, are responsible for Birt–Hogg–Dubé, a renal cell carcinoma susceptibility syndrome in which lung bullae (often referred to as cysts, despite the absence of fluid) are frequently observed [63]. Bullae have an irregular shape with internal septation and are located below the carina [64]. They are sometimes subpleural, and as a result are associated with a risk of spontaneous pneumothorax. There is no increased risk of lung cancer.
Lymphangioleiomyomatosis
Lymphangioleiomyomatosis (LAM) is a diffuse cystic lung disease predominantly affecting females of reproductive age [65]. It is sometimes associated with the tuberous sclerosis complex (TSC), which is caused by germline pathogenic variants in the TSC1 and TSC2 genes [65]. LAM is characterised by small thin-walled cysts throughout the lungs resulting in progressive dyspnoea and renal angiomyolipomas [64, 65]. Pneumothorax occurs frequently. As in Birt–Hogg–Dubé, lung cancer risk is not increased.
Pulmonary fibrosis
Pulmonary fibrosis is characterised by the accumulation and the persistent activation of fibroblasts with a capacity to resist apoptosis, a process that bears similarities with carcinogenesis. Furthermore, events such as chronic repeated inflammation, as seen for example in smokers, can lead to multiple genetic alterations involving mutations in tumour suppressor genes or the activation of oncogenes affecting growth, differentiation and cell survival.
In the United States, the prevalence of interstitial lung diseases (ILDs) ranges from 67 to 80 cases per 100 000, and idiopathic pulmonary fibrosis (IPF) has an annual incidence of 7–16 cases per 100 000 [66, 67]. In a retrospective Italian cohort of 181 IPF patients, the prevalence of histologically proven lung cancer was 13% [68]. In a Japanese cohort, the incidence of lung cancer among IPF patients was 22.4 per 1000 person-years [69]. A meta-analysis adjusted for age, sex and smoking and based on 26 cohorts included 11 976 people, among them 1854 with IPF. The lung cancer incidence rate ratio associated with IPF was 6.42 (95% CI 3.21–9.62) [70].
Approximately 5–20% of IPF are familial [71]. As for lung cancer risk, the simultaneous occurrence of IPF and alveolar cell lung carcinoma in multiple members from the same family was described as far back as 1981 [72]. Pathogenic variants in telomerase complex (RTEL1, TERC, TERT) and surfactant genes (ABCA3, SFTPA1, SFTPA2, SFTPC) are associated with pulmonary fibrosis [73–80]. Among these genes, TERT pathogenic variants are most frequently involved and account for ∼15% of familial ILDs [71, 77]. Transmission is autosomal dominant. While pathogenic variants in the SFTPA1 and SFTPA2 surfactant genes have been identified in families with IPF and lung cancer, that does not seem to be the case for the telomerase complex genes [75, 76, 79, 81, 82].
When there is a germline pathogenic variant in a surfactant gene, the protein is not secreted by type II pneumocytes. The absence of surfactant induces endoplasmic reticulum stress via soluble mediators’ secretions such as transforming growth factor-β, which then promotes fibrosis and carcinogenesis [83, 84]. Using a linkage approach, Wang et al. [76] first identified a rare missense SFTPA2 variant in a family with multiple members affected by early-onset pulmonary fibrosis and lung cancer. The variant co-segregated with IPF in an autosomal-dominant pattern. Spectacularly, seven relatives had a diagnosis of lung cancer, mostly bronchoalveolar carcinoma, with or without underlying fibrosis. More recently, in a cohort of 39 unrelated patients with familial IPF, van Moorsel et al. [85] identified three index cases with SFTPA2 pathogenic variants, all located in exon 6. Two of them developed lung cancer. The first patient was a 40-year-old male who benefited from unilateral lung transplantation, but rapidly developed severe primary graft dysfunction and respiratory failure. Pathological examination of the explanted native right lung revealed a poorly differentiated adenocarcinoma situated in the right lower lobe with invasive growth into the pleural wall. The second case was a 43-year-old male who died of respiratory failure. On autopsy, an adenocarcinoma with a poorly differentiated invasive component and a component of lepidic growth (equivalent to bronchioloalveolar cell carcinoma) was observed in the left lower lobe. Regarding SFTPA1, it was identified later as a susceptibility gene in 2016 in a candidate-gene study involving 12 patients with familial IPF and a personal or familial history of lung cancer [79]. Nine members from a mutigenerational family carried a heterozygous missense variant. Co-segregation, impaired SP-A1 secretion and altered protein expression provided evidence for pathogenicity. To date, no association between SFPTC mutation and lung cancer has been reported.
The observation of IPF and lung cancer in an individual, especially if aged <50 years, should warrant referral to a clinical genetics team with expertise in the field for genetic counselling and SFPA1/SFPA2 germline testing. As for the surveillance of IPF patients, French guidelines recommend, regardless of genetic results, annual lung cancer screening of IPF patients using CT scan [86]. Of note, no such recommendations have been made by the American Thoracic Society [87].
Common low-risk susceptibility variants
Common low-risk susceptibility variants (CLRVs) are identified through genome-wide association studies (GWAS), where their frequencies are compared in large series (>10 000) of cancer cases and controls. Variants are considered common if their frequency in the general population is >0.5–1%. Taken individually, each CLRV only increases risk marginally (e.g. relative risk 1.1). However, the combined effect of a number of CLRVs, in the form of a polygenic risk score (PRS), could help stratify individuals from the general population into different categories of risk [88].
Regarding specifically lung cancer, one could imagine that those with a high PRS could be offered annual or biennial screening. However, that would imply that their risk is high enough to warrant screening. This is far from the case. In 2020, Jia et al. [89] compiled information for lung cancer CLRVs by reviewing the GWAS catalogue and previous PubMed publications. 19 CLRVs were genotyped and PRS scores calculated in 400 000 participants from the UK biobank cohort study, who were then followed prospectively. Lung cancer hazard ratios (HR) for individuals in the highest quintile were only 1.24 (95% CI 1.07–1.44) compared to the middle quintile. Improvement was only minor when participants from the top 5% PRS group were selected, with HR 1.54 (95% CI 1.24–1.91) compared to the average population risk. Admittedly, the number of CLRVs was low, much lower for example that numbers used to compile PRS in breast cancer, but increasing numbers would not necessarily mean increased accuracy [88, 90]. Zhang et al. [91] estimated the predictive performance of PRS as a function of increasing sample sizes for future GWAS, and of the associated increased number of susceptibility single nucleotide variants. Assuming a four-fold increase in GWAS sample sizes, individuals ⩾99th percentile of PRS would have a relative risk for lung cancer of ∼2.4 compared to the average population risk. While a relative risk of 2.4 might seem high enough to justify regular screening, only 1% of the population would have this relative risk. One can only wonder whether offering CLRV genotyping to the whole population when only 1% will benefit from it would be reasonable. The future lies in the combination of PRS with other risk factors, mainly smoking and family history.
Conclusion
The multidisciplinary management of lung cancer patients should progressively incorporate clinical cancer genetics. Lung cancer has indeed a genetic origin in a subset of cases, mainly in young patients with adenocarcinoma and regardless of smoking history. Pulmonologists, thoracic oncologists and cancer geneticists should be familiar with genetic susceptibility to the disease, while genetics laboratories need ensure that EGFR and TP53 testing is made routinely available. It is likely that additional genes such as ATM, perhaps BRCA2, will be soon be considered lung cancer susceptibility genes and that, in parallel, advances will be made in the surveillance of asymptomatic carriers. Large, multicentre and international collaborations are warranted in this rapidly expanding and fascinating field.
Footnotes
Provenance: Commissioned article, peer reviewed.
Number 10 in the Series “Thoracic oncology” Edited by Rudolf Huber and Peter Dorfmüller
Previous articles in this series: No. 1: Eichhorn F, Winter H. How to handle oligometastatic disease in nonsmall cell lung cancer. Eur Respir Rev 2021; 30: 200234. No. 2: Asciak R, George V, Rahman NM. Update on biology and management of mesothelioma. Eur Respir Rev 2021; 30: 200226. No. 3: Finazzi T, Schneiders FL, Senan S. Developments in radiation techniques for thoracic malignancies. Eur Respir Rev 2021; 30: 200224. No. 4: Huber RM, Kauffmann-Guerrero D, Hoffmann H, et al. New developments in locally advanced nonsmall cell lung cancer. Eur Respir Rev 2021; 30: 200227. No. 5: Rittmeyer A, Schiwitza A, Sahovic L, et al. Update on recent key publications in lung oncology: picking up speed. Eur Respir Rev 2021; 30: 200300. No. 6: Abdayem P, Planchard D. Update on molecular pathology and role of liquid biopsy in nonsmall cell lung cancer. Eur Respir Rev 2021; 30: 200294. No. 7: Lam S, Tammemagi M. Contemporary issues in the implementation of lung cancer screening. Eur Respir Rev 2021; 30: 200288. No. 8: Ghigna M-R, Thomas de Montpreville V. Mediastinal tumours and pseudo-tumours: a comprehensive review with emphasis on multidisciplinary approach. Eur Respir Rev 2021; 30: 200309. No. 9: Remon J, Facchinetti F, Besses B. The efficacy of immune checkpoint inhibitors in thoracic malignancies. Eur Respir Rev 2021; 30: 200387.
Conflict of interest: P.R. Benusiglio has received honoraria from AstraZeneca and Roche Diagnostics. He is also a member of the scientific committee of the Geneticancer patient association.
Conflict of interest: V. Fallet has nothing to declare.
Conflict of interest: M. Sanchis-Borja has nothing to declare.
Conflict of interest: F. Coulet has received funding from AstraZeneca.
Conflict of interest: J. Cadranel has nothing to declare.
References
- 1.Couraud S, Zalcman G, Milleron B, et al. Lung cancer in never-smokers – a review. Eur J Cancer 2012; 48: 1299–1311. doi: 10.1016/J.EJCA.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 2.Ou JY, Fowler B, Ding Q, et al. A statewide investigation of geographic lung cancer incidence patterns and radon exposure in a low-smoking population. BMC Cancer 2018; 18: 115. doi: 10.1186/s12885-018-4002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaskin J, Coyle D, Whyte J, et al. Global estimate of lung cancer mortality attributable to residential radon. Environ Health Perspect 2018; 126: 057009. doi: 10.1289/EHP2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamra GB, Guha N, Cohen A, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect 2014; 122: 906–911. doi: 10.1289/ehp.1408092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gharibvand L, Shavlik D, Ghamsary M, et al. The association between ambient fine particulate air pollution and lung cancer incidence: results from the AHSMOG-2 study. Environ Health Perspect 2017; 125: 378–384. doi: 10.1289/EHP124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokuhata GK, Lilienfeld AM. Familial aggregation of lung cancer in humans. J Natl Cancer Inst 1963; 30: 289–312. doi: 10.1093/jnci/30.2.289 [DOI] [PubMed] [Google Scholar]
- 7.Coté ML, Liu M, Bonassi S, et al. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. Eur J Cancer 2012; 48: 1957–1968. doi: 10.1016/J.EJCA.2012.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 2016; 315: 68–76. doi: 10.1001/jama.2015.17703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015; 110: 223–262. doi: 10.1038/ajg.2014.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bougeard G, Renaux-Petel M, Flaman JM, et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol 2015; 33: 2345–2352. doi: 10.1200/JCO.2014.59.5728 [DOI] [PubMed] [Google Scholar]
- 11.Frebourg T, Bajalica Lagercrantz S, Oliveira C, et al. Guidelines for the Li–Fraumeni and heritable TP53-related cancer syndromes. Eur J Hum Genet 2020; 28: 1376–1386. doi: 10.1038/s41431-020-0638-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbosa MVR, Cordeiro de Lima VC, Formiga MN, et al. High prevalence of EGFR mutations in lung adenocarcinomas from Brazilian patients harboring the TP53 p.R337H variant. Clin Lung Cancer 2020; 21: e37–e44. doi: 10.1016/j.cllc.2019.11.012 [DOI] [PubMed] [Google Scholar]
- 13.Mezquita L, Jové M, Nadal E, et al. High prevalence of somatic oncogenic driver alterations in patients with NSCLC and Li-Fraumeni syndrome. J Thorac Oncol 2020; 15: 1232–1239. doi: 10.1016/j.jtho.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 14.Ballinger ML, Best A, Mai PL, et al. Baseline surveillance in Li-Fraumeni syndrome using whole-body magnetic resonance imaging: a meta-analysis. JAMA Oncol 2017; 3: 1634–1639. doi: 10.1001/jamaoncol.2017.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caron O, Frebourg T, Benusiglio PR, et al. Lung adenocarcinoma as part of the Li-Fraumeni syndrome spectrum: preliminary data of the LIFESCREEN randomized clinical trial. JAMA Oncol 2017; 3: 1736–1737. doi: 10.1001/jamaoncol.2017.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bougeard G, Renaux-Petel M, Flaman JM, et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol 2015; 33: 2345–2352. doi: 10.1200/JCO.2014.59.5728 [DOI] [PubMed] [Google Scholar]
- 17.Kratz CP, Achatz MI, Brugières L, et al. Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin Cancer Res 2017; 23: e38–e45. doi: 10.1158/1078-0432.CCR-17-0408 [DOI] [PubMed] [Google Scholar]
- 18.Gazdar A, Robinson L, Oliver D, et al. Hereditary lung cancer syndrome targets never-smokers with germline EGFR gene T790M mutations. J Thorac Oncol 2014; 9: 456–463. doi: 10.1097/JTO.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lou Y, Pecot CV, Tran HT, et al. Germline mutation of T790M and dual/multiple EGFR mutations in patients with lung adenocarcinoma. Clin Lung Cancer 2016; 17: e5–e11. doi: 10.1016/J.CLLC.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu HA, Arcila ME, Hellmann MD, et al. Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann Oncol 2014; 25: 423–428. doi: 10.1093/annonc/mdt573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang JCH, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015; 16: 830–838. doi: 10.1016/S1470-2045(15)00026-1 [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Alden RS, Odegaard JI, et al. Discrimination of germline EGFR T790M mutations in plasma cell-free DNA allows study of prevalence across 31,414 cancer patients. Clin Cancer Res 2017; 23: 7351–7359. doi: 10.1158/1078-0432.CCR-17-1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 3375–3382. doi: 10.1200/JCO.2016.66.7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Agency for Research on Cancer . Lung Cancer Fact Sheet. 2020. https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf Date last accessed: 23 December 2020.
- 25.Defossey G, Le Guyadier-Peyrou S, Uhry Z, et al. Estimations Nationales de l'incidence et de La Mortalité Par Cancer En France Métropolitaine Entre 1990 et 2018. Santé Publique France, Saint-Maurice, 2019. [Google Scholar]
- 26.Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet 2005; 37: 1315–1316. doi: 10.1038/ng1671 [DOI] [PubMed] [Google Scholar]
- 27.Oxnard GR, Miller VA, Robson ME, et al. Screening for germline EGFR T790M mutations through lung cancer genotyping. J Thorac Oncol 2012; 7: 1049–1052. doi: 10.1097/JTO.0B013E318250ED9D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blair VR, McLeod M, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 2020; 21: e386–e397. doi: 10.1016/S1470-2045(20)30219-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry DK, Wang X, Michalski ST, et al. Clinical cohort analysis of germline EGFR T790M demonstrates penetrance across ethnicities and races, sexes, and ages. JCO Precis Oncol 2020; 4: 170–175. doi: 10.1200/po.19.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ileana Dumbrava E, Brusco L, Daniels MS, et al. Expanded analysis of secondary germline findings from matched tumor/normal sequencing identifies additional clinically significant mutations. JCO Precis Oncol 2019; 3: PO.18.00143. doi: 10.1200/po.18.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Leest C, Wagner A, Pedrosa RM, et al. Novel EGFR V834L germline mutation associated with familial lung adenocarcinoma. JCO Precis Oncol 2018; 2: PO.17.00266. doi: 10.1200/po.17.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtsuka K, Ohnishi H, Fujiwara M, et al. Predisposition to lung adenocarcinoma in a family harboring the germline EGFR V843I mutation. JCO Precis Oncol 2019; 3: PO.19.00104. doi: 10.1200/po.19.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demierre N, Zoete V, Michielin O, et al. A dramatic lung cancer course in a patient with a rare EGFR germline mutation exon 21V843I: is EGFR TKI resistance predictable? Lung Cancer 2013; 80: 81–84. doi: 10.1016/J.LUNGCAN.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 34.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020; 382: 503–513. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 35.Hu C, Hart SN, Polley EC, et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 2018; 319: 2401–2409. doi: 10.1001/jama.2018.6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renault AL, Mebirouk N, Fuhrmann L, et al. Morphology and genomic hallmarks of breast tumours developed by ATM deleterious variant carriers. Breast Cancer Res 2018; 20: 28. doi: 10.1186/s13058-018-0951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parry EM, Gable DL, Stanley SE, et al. Germline mutations in DNA repair genes in lung adenocarcinoma. J Thorac Oncol 2017; 12: 1673–1678. doi: 10.1016/J.JTHO.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esai Selvan M, Zauderer MG, Rudin CM, et al. Inherited rare, deleterious variants in ATM increase lung adenocarcinoma risk. J Thorac Oncol 2020; 15: 1871–1879. doi: 10.1016/j.jtho.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji X, Mukherjee S, Landi MT, et al. Protein-altering germline mutations implicate novel genes related to lung cancer development. Nat Commun 2020; 11: 2220. doi: 10.1038/s41467-020-15905-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manchanda R, Burnell M, Gaba F, et al. Randomised trial of population-based BRCA testing in Ashkenazi Jews: long-term outcomes. BJOG 2020; 127: 364–375. doi: 10.1111/1471-0528.15905 [DOI] [PubMed] [Google Scholar]
- 41.Hu X, Yang D, Li Y, et al. Prevalence and clinical significance of pathogenic germline BRCA1/2 mutations in Chinese non-small cell lung cancer patients. Cancer Biol Med 2019; 16: 556–564. doi: 10.20892/j.issn.2095-3941.2018.0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maxwell KN, Domchek SM, Nathanson KL, et al. Population frequency of germline BRCA1/2 mutations. J Clin Oncol 2016; 34: 4183–4185. doi: 10.1200/JCO.2016.67.0554 [DOI] [PubMed] [Google Scholar]
- 43.Slavin TP, Banks KC, Chudova D, et al. Identification of incidental germline mutations in patients with advanced solid tumors who underwent cell-free circulating tumor DNA sequencing. J Clin Oncol 2018; 36: 3459–3465. doi: 10.1200/JCO.18.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson ER, Rowley SM, Li N, et al. Panel testing for familial breast cancer: calibrating the tension between research and clinical care. J Clin Oncol 2016; 34: 1455–1459. doi: 10.1200/JCO.2015.63.7454 [DOI] [PubMed] [Google Scholar]
- 45.Remon J, Besse B, Leary A, et al. Somatic and germline BRCA 1 and 2 mutations in advanced NSCLC from the SAFIR02-lung trial. JTO Clin Res Rep 2020; 1: 100068. doi: 10.1016/j.jtocrr.2020.100068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017; 317: 2402–2416. doi: 10.1001/jama.2017.7112 [DOI] [PubMed] [Google Scholar]
- 47.Kadouri L, Rottenberg Y, Zick A, et al. Homologous recombination in lung cancer, germline and somatic mutations, clinical and phenotype characterisation. Lung Cancer 2019; 137: 48–51. doi: 10.1016/j.lungcan.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, McKay JD, Rafnar T, et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat Genet 2014; 46: 736–741. doi: 10.1038/ng.3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rafnar T, Sigurjonsdottir GR, Stacey SN, et al. Association of BRCA2 K3326* with small cell lung cancer and squamous cell cancer of the skin. J Natl Cancer Inst 2018; 110: 967–974. doi: 10.1093/jnci/djy002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meeks HD, Song H, Michailidou K, et al. BRCA2 polymorphic stop codon K3326X and the risk of breast, prostate, and ovarian cancers. J Natl Cancer Inst 2015; 108: djv315. doi: 10.1093/jnci/djv315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selvan ME, Klein RJ, Gümüş ZH. Rare, pathogenic germline variants in Fanconi anemia genes increase risk for squamous lung cancer. Clin Cancer Res 2019; 25: 1517–1525. doi: 10.1158/1078-0432.CCR-18-2660 [DOI] [PubMed] [Google Scholar]
- 52.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011; 43: 1022–1025. doi: 10.1038/ng.912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panou V, Gadiraju M, Wolin A, et al. Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J Clin Oncol 2018; 36: 2863–2871. doi: 10.1200/JCO.2018.78.5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HE, Molina JR, Sukov WR, et al. BAP1 loss is unusual in well-differentiated papillary mesothelioma and may predict development of malignant mesothelioma. Hum Pathol 2018; 79: 168–176. doi: 10.1016/j.humpath.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 55.Hassan R, Morrow B, Thomas A, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci USA 2019; 116: 9008–9013. doi: 10.1073/pnas.1821510116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walpole S, Pritchard AL, Cebulla CM, et al. Comprehensive study of the clinical phenotype of germline BAP1 variant-carrying families worldwide. J Natl Cancer Inst 2018; 110: 1328–1341. doi: 10.1093/jnci/djy171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wadt KAW, Aoude LG, Johansson P, et al. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet 2015; 88: 267–272. doi: 10.1111/cge.12501 [DOI] [PubMed] [Google Scholar]
- 58.Neglia JPP, FitzSimmons SCC, Maisonneuve P, et al. The risk of cancer among patients with cystic fibrosis. N Engl J Med 1995; 332: 494–499. doi: 10.1056/NEJM199502233320803 [DOI] [PubMed] [Google Scholar]
- 59.Strnad P, McElvaney NG, Lomas DA. Alpha1-antitrypsin deficiency. N Engl J Med 2020; 382: 1443–1455. doi: 10.1056/NEJMra1910234 [DOI] [PubMed] [Google Scholar]
- 60.Torres-Durán M, Ruano-Ravina A, Parente-Lamelas I, et al. Alpha-1 antitrypsin deficiency and lung cancer risk: a case–control study in never-smokers. J Thorac Oncol 2015; 10: 1279–1284. doi: 10.1097/JTO.0000000000000609 [DOI] [PubMed] [Google Scholar]
- 61.Broer L, Lill CM, Schuur M, et al. Distinguishing true from false positives in genomic studies: p values. Eur J Epidemiol 2013; 28: 131–138. doi: 10.1007/s10654-012-9755-x [DOI] [PubMed] [Google Scholar]
- 62.Yang P, Sun Z, Krowka MJ, et al. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch Intern Med 2008; 168: 1097–1103. doi: 10.1001/archinte.168.10.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt LS, Linehan WM. Molecular genetics and clinical features of Birt–Hogg–Dubé syndrome. Nat Rev Urol 2015; 12: 558–569. doi: 10.1038/nrurol.2015.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park HJ, Chae EJ, Do KH, et al. Differentiation between lymphangioleiomyomatosis and Birt–Hogg–Dubé syndrome: analysis of pulmonary cysts on CT images. AJR Am J Roentgenol 2019; 212: 766–772. doi: 10.2214/AJR.18.20232 [DOI] [PubMed] [Google Scholar]
- 65.O'Mahony AM, Lynn E, Murphy DJ, et al. Lymphangioleiomyomatosis: a clinical review. Breathe 2020; 16: 200007. doi: 10.1183/20734735.0007-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coultas DB, Zumwalt RE, Black WC, et al. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994; 150: 967–972. doi: 10.1164/ajrccm.150.4.7921471 [DOI] [PubMed] [Google Scholar]
- 67.Raghu G, Weycker D, Edelsberg J, et al. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006; 174: 810–816. doi: 10.1164/rccm.200602-163OC [DOI] [PubMed] [Google Scholar]
- 68.Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015; 147: 157–164. doi: 10.1378/CHEST.14-0359 [DOI] [PubMed] [Google Scholar]
- 69.Kato E, Takayanagi N, Takaku Y, et al. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. ERJ Open Res 2018; 4: 00111–2016. doi: 10.1183/23120541.00111-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown SAW, Dobelle M, Padilla M, et al. Idiopathic pulmonary fibrosis and lung cancer. A systematic review and meta-analysis. Ann Am Thorac Soc 2019; 16: 1041–1051. doi: 10.1513/AnnalsATS.201807-481OC [DOI] [PubMed] [Google Scholar]
- 71.Borie R, Kannengiesser C, Nathan N, et al. Familial pulmonary fibrosis. Rev Mal Respir 2015; 32: 413–434. doi: 10.1016/j.rmr.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 72.Beaumont F, Jansen HM, Elema JD, et al. Simultaneous occurrence of pulmonary interstitial fibrosis and alveolar cell carcinoma in one family. Thorax 1981; 36: 252–258. doi: 10.1136/THX.36.4.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diaz de Leon A, Cronkhite JT, Katzenstein ALA, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One 2010; 5: e10680. doi: 10.1371/journal.pone.0010680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nogee LM, Dunbar AE, Wert SE, et al. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 2001; 344: 573–579. doi: 10.1056/NEJM200102223440805 [DOI] [PubMed] [Google Scholar]
- 75.Kannengiesser C, Borie R, Ménard C, et al. Heterozygous RTEL1 mutations are associated with familial pulmonary fibrosis. Eur Respir J 2015; 46: 474–485. doi: 10.1183/09031936.00040115 [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 2009; 84: 52–59. doi: 10.1016/J.AJHG.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J 2016; 48: 1721–1731. doi: 10.1183/13993003.02115-2015 [DOI] [PubMed] [Google Scholar]
- 78.Armanios MY, Chen JJL, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007; 356: 1317–1326. doi: 10.1056/nejmoa066157 [DOI] [PubMed] [Google Scholar]
- 79.Nathan N, Giraud V, Picard C, et al. Germline SFTPA1 mutation in familial idiopathic interstitial pneumonia and lung cancer. Hum Mol Genet 2016; 25: 1457–1467. doi: 10.1093/hmg/ddw014 [DOI] [PubMed] [Google Scholar]
- 80.Bullard JE, Nogee LM. Heterozygosity for ABCA3 mutations modifies the severity of lung disease associated with a surfactant protein C gene (SFTPC) mutation. Pediatr Res 2007; 62: 176–179. doi: 10.1203/PDR.0b013e3180a72588 [DOI] [PubMed] [Google Scholar]
- 81.Morales CP, Holt SE, Ouellette M, et al. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet 1999; 21: 115–118. doi: 10.1038/5063 [DOI] [PubMed] [Google Scholar]
- 82.van Moorsel CHM, van Oosterhout MFM, Barlo NP, et al. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a Dutch cohort. Am J Respir Crit Care Med 2010; 182: 1419–1425. doi: 10.1164/rccm.200906-0953OC [DOI] [PubMed] [Google Scholar]
- 83.Naccache JM, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018; 10: 3829–3844. doi: 10.21037/jtd.2018.05.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Albert RK, Schwartz DA. Revealing the secrets of idiopathic pulmonary fibrosis. N Engl J Med 2019; 380: 94–96. doi: 10.1056/nejmcibr1811639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Moorsel CHM, Ten Klooster L, van Oosterhout MFM, et al. SFTPA2 mutations in familial and sporadic idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2015; 192: 1249–1252. doi: 10.1164/rccm.201504-0675LE [DOI] [PubMed] [Google Scholar]
- 86.Cottin V, Crestani B, Cadranel J, et al. Recommandations pratiques pour le diagnostic et la prise en charge de la fibrose pulmonaire idiopathique – actualisation 2017. Version longue. [French practical guidelines for the diagnosis and management of idiopathic pulmonary fibrosis. 2017 update. Full-length update]. Rev Mal Respir 2017; 30: 879–902. doi: 10.1016/j.rmr.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 87.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res 2020; 22: 21. doi: 10.1186/s13058-020-01260-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia G, Lu Y, Wen W, et al. Evaluating the utility of polygenic risk scores in identifying high-risk individuals for eight common cancers. JNCI Cancer Spectr 2020; 4: pkaa021. doi: 10.1093/JNCICS/PKAA021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mavaddat N, Michailidou K, Dennis J, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet 2019; 104: 21–34. doi: 10.1016/j.ajhg.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang YD, Hurson AN, Zhang H, et al. Assessment of polygenic architecture and risk prediction based on common variants across fourteen cancers. Nat Commun 2020; 11: 3353. doi: 10.1038/s41467-020-16483-3 [DOI] [PMC free article] [PubMed] [Google Scholar]