Abstract
Neurofibromatosis type 1 (NF1), also known as von Recklinghausen disease, is a frequent autosomal dominant genetic disorder with a prevalence of 1 in 3000. Pulmonary hypertension (PH) associated with NF1 (PH-NF1) is a rare but severe complication of NF1 and is classified as Group 5 PH, defined as “PH with unclear and/or multifactorial mechanisms”. A literature review in PubMed on the association between NF1 and PH identified 18 articles describing 31 cases. PH-NF1 was characterised by a female predominance, an advanced age at diagnosis, an association with parenchymal lung disease in two out of three cases and poor long-term prognosis. NF1 is generally associated with interstitial lung disease but some cases of severe PH without parenchymal lung disease suggest that there could be a specific pulmonary vascular disease. There is no data available on the efficacy of specific pulmonary arterial hypertension treatment in PH-NF1. Therefore, these patients should be evaluated in expert PH centres and referred for lung transplantation at an early stage. As these patients have an increased risk of malignancy, careful assessment of the post-transplant malignancy risk prior to listing for transplantation is necessary. Clinical trials are needed to evaluate promising treatments targeting the RAS-downstream signalling pathways.
Short abstract
Pulmonary hypertension is a rare but severe complication of neurofibromatosis type 1. There are no data about the efficacy of specific PAH treatment in this disease and lung transplantation should be discussed at an early stage. http://ow.ly/JMU030lezfY
Introduction
Neurofibromatosis type 1 (NF1), also known as von Recklinghausen disease, is a frequent autosomal dominant disorder which can require the respiratory physician's expertise. Indeed, NF1 is a multisystem disease and lung disease, especially pulmonary hypertension (PH), can be one of the most severe complications. PH associated with NF1 (PH-NF1) is classified as Group 5 PH, defined as “PH with unclear and/or multifactorial mechanisms” (table 1) [1]. This is a heterogeneous group including several disorders with multiple mechanisms, for which there is neither data nor recommendations regarding the use of pulmonary arterial hypertension (PAH) approved drugs. The only treatments available target the underlying disease. An improved understanding of PH-NF1 should help advance the pathophysiology of PH in general. This article is about NF1, however, it is interesting to note that neurofibromatosis type 2 is a different disease, caused by a different genetic mutation, and that to our knowledge, there is no described case of PH in neurofibromatosis type 2.
TABLE 1.
Classification of group 5 pulmonary hypertension according to European Society for Cardiology/European Respiratory Society guidelines
| 5. Pulmonary hypertension with unclear and/or multifactorial mechanisms |
| 5.1 Haematologic disorders: chronic haemolytic anaemia, myeloproliferative disorders, splenectomy |
| 5.2 Systemic disorders: sarcoidosis, pulmonary histiocytosis, lymphangioleiomyomatosis, neurofibromatosis |
| 5.3 Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders |
| 5.4 Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure, segmental pulmonary hypertension |
Reproduced from [1] with permission.
Clinical presentation of NF1
NF1 is characterised by multiple café au lait spots which occur in 95% of patients, axillary and inguinal freckling in 70% of patients, several discrete benign neurofibromas within the dermis in 95% of patients, and also iris Lisch nodules in 95% of patients [2]. Less common but potentially more severe manifestations can also occur, in particular with a predisposition for tumours. The most frequent tumours are nodular neurofibromas which occur in peripheral nerves and can grow to an enormous size, as well as plexiform neurofibromas which are usually congenital and occur in 30% of patients. These tumours are an important cause of morbidity as they affect long portions of nerves, can infiltrate the nerve and surrounding tissue and in ∼2–16% of patients transform to malignant peripheral nerve sheath tumours. Optic and other central nervous system gliomas occur in 15% of patients with potentially severe complications as they produce symptoms in 2–5% of cases. Phaeochromocytoma is rarely associated with the disorder, affecting between 0.1 to 5.7% of patients [3]. Intestinal tumours like carcinoids are also more frequent than the general population, and there is a higher incidence of malignancy in general in NF1.
NF1 is associated with osseous lesions like scoliosis, dysplasia of the sphenoid wing and thinning of long bones. Hypertension is present in 6% of patients and can be caused by renovascular disease, coarctation of the aorta and phaeochromocytoma. Learning disabilities are present in at least 50% of patients [4] and there is also an increased risk of epilepsy and headaches.
Cardiac abnormalities like secundum atrial septal defect, ventricular septal defect, mitral or aortic insufficiency, hypertrophic cardiomyopathy, and intracardiac tumours have also been described and are severe complications of NF1 [5, 6].
Finally vascular lesions are less frequently reported but are some of the most severe complications of NF1 [7, 8]. These include occlusive or aneurysmal arterial lesions, arterio-venous malformations, coarctation or segmental hypoplasia of the abdominal aorta with or without renal artery ostial stenosis (which can cause renovascular hypertension), occlusive coronary artery disease, visceral vasculopathy causing ischaemic bowel disease and retroperitoneal or abdominal bleeding, and peripheral vascular disease [7].
The diagnosis of NF1 is clinical, based on criteria proposed by the National Institute of Health (NIH) Consensus Development Conference [9] (table 2). If two or more of the seven criteria of the NIH conference are present in the same patient, the diagnosis is established. Diagnosis by genetic testing is possible but is usually not required because of the typical clinical features of the disease and of the great variety of mutations of the neurofibromin 1 (NF1) gene.
TABLE 2.
Diagnostic criteria for neurofibromatosis type 1 (NF1) [9]
| Two or more of the criteria below are required for diagnosis: |
| Six or more café au lait macules (>0.5 cm in children or >1.5 cm in adults) |
| Two or more cutaneous or subcutaneous neurofibromas or one plexiform neurofibroma |
| Axillary or groin freckling |
| Optic pathway glioma |
| Two or more Lisch nodules (iris hamartomas seen on slit lamp examination) |
| Bony dysplasia (sphenoid wing dysplasia, bowing of long bone±pseudoarthrosis) |
| First-degree relative with NF1 |
NF1 is fully penetrant in adults, but many manifestations of the disease increase in frequency or severity with age [10]. The disease features are extremely varied, even within the same family. Most studies have not found an evident relationship between particular NF1 mutations and the resulting clinical phenotype. The average life expectancy of patients with NF1 is reduced by 10–15 years and cancer is the most common cause of death [11, 12]. Disease treatment requires multidisciplinary life-long follow-up adapted to the patient's age [13]. It includes referral to specialists for treatment of complications. Surgery to remove both benign and malignant tumours or to correct skeletal manifestations is sometimes warranted, as well as arterial reconstruction, excision of arterio-venous malformations, clipping or embolisation of vascular lesions [8]. Annual physical examination by a physician familiar with the disorder is recommended. Other recommendations include ophthalmological examinations annually in children and less frequently in adults, regular developmental assessment in children, regular blood pressure monitoring, and magnetic resonance imaging for follow-up of clinically suspected intracranial and other internal tumours. Regular pulmonary examination, chest imaging and echocardiographic follow-up are also warranted because lung and cardiovascular complications are now well-documented complications of NF1.
Genetics, biology and pathophysiology in NF1
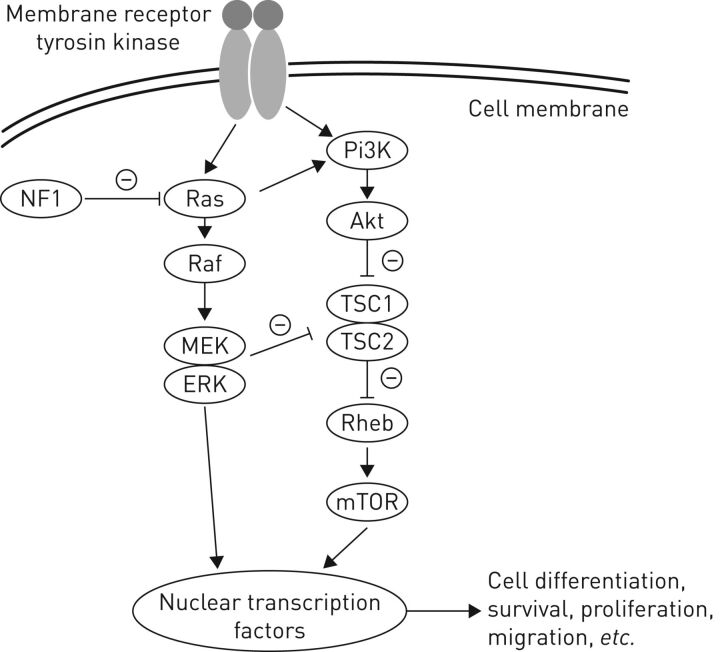
NF1 is a frequent autosomal dominant genetic disorder with a prevalence of 1 in 3000 [10, 14] and near-complete penetrance before the age of 5 years. The disease is caused by mutations of the NF1 gene, identified in 1990, which is located at chromosome 17q11.2 [11] and comprises 60 exons. It encodes a cytoplasmic protein named neurofibromin, which holds 2818 amino acids and has a role in tumour suppression [2]. Indeed, neurofibromin has a guanosine triphosphatase (GTPase)-activating protein domain that is responsible for decreasing the level of Ras bound to guanosine triphosphate (GTP) by hydrolysing GTP bound to small monomeric GTP-bound Ras [15]. This GTPase activity acts as a negative regulator of signal transmitted by Ras [16] and its loss is associated with the activation of several transcription pathways: the mitogen-activated protein kinase (MAPK) pathway ending by ERK activation [17] and also the mammalian target of rapamycin (mTOR) pathway, mediated by an activation of the PI3kinase-AKT pathway [18, 19] and by the tuberous sclerosis protein 1–tuberous sclerosis protein 2 complex (figure 1) [20].
FIGURE 1.
Schematic representation of the downstream signalling pathways of neurofibromin. Neurofibromin inhibits the activity of Ras by its guanosine triphosphatase activity. Loss of activity of neurofibromin in neurofibromatosis type 1 (NF1) leads to the activation of different pathways mediated by RAS, namely the mitogen-activated protein kinase cascade leading to activation of ERK and mammalian target of rapamycin (mTOR) pathway. These pathways activate nuclear transcription factors and are responsible for endothelial cell differentiation, proliferation, increased survival and migration. PI3K: phosphatidylinositol 3-kinase; Rheb: Ras homolog enriched in brain; TSC: tuberous sclerosis protein.
Inactivation of the gene through mutation leads to a loss of neurofibromin and to a constitutive activation of these pathways. It leads to the deregulation of cell proliferation and differentiation and to the development of benign neurofibroma-like tumours and malignant peripheral nerve sheath tumours, and probably also to the lung complications seen in NF1. About half of all cases result from neomutations [10]. A wide variety of mutations in the NF1 gene have been found in NF1 patients, but no recurring mutation has been identified.
Pulmonary hypertension
A PubMed literature review of the association between NF1 and PH found 18 articles describing 31 cases of pre-capillary PH (defined by a mean pulmonary artery pressure (mPpa) ≥25 mmHg and pulmonary artery wedge pressure <15 mmHg) measured by right heart catheterisation (table 3) [21–38]. None of these patients had identified risk factors for PAH, including anorexigen use, conditions associated with PAH (portal hypertension, HIV infection, connective tissue diseases, congenital heart disease) or chronic thromboembolic disease.
TABLE 3.
Published articles on pulmonary hypertension associated with neurofibromatosis type 1
| First author [ref.] | Sex | Age years | CT scan | NYHA | PO2 mmHg | FVC % | FEV1 % | DLCO % | mPpa mmHg | CI L·min·m−2 | PVR Wood units | Treatment | Course |
| Porterfield [21] | Female | 56 | Interstitial markings in lower lobes. bullae in apex (radio) | III | 33 | 7 | 35 | Calcium antagonist | |||||
| Samuels [22] | Male | 51 | Bilateral perfusion defects | III | 57 | 77 | 71 | 49 | 3.4 | 3.0 | Endarterectomy | Improvement after surgery | |
| Aoki [23] | Female | 16 | Normal | II | 67 | 116 | 84 | 79 | 49 | 2.1 | 15.1 | Calcium antagonist and anticoagulants | Aggravation and then treatment with i.v. prostanoids. Death 2 years after the diagnosis |
| Female | 70 | Normal | III | 48 | 38 | 2.3 | 12.0 | Anticoagulant and isosorbide dinitrate | Aggravation and then treatment with i.v. protanoids | ||||
| García Hernandez [24] | Male | 44 | Normal | III | 30 | 107 | 110 | 62 | i.v. prostanoids | Improvement and then death some years after diagnosis | |||
| Engel [25] | Female | 60 | Lung cysts and T-7 schwannoma | III | 85 | 90 | 48 | 50 | 2.3 | 10 | ERA, PDE5 inhibitor and i.v. prostanoids | Clinical improvement | |
| Female | 69 | Normal | III | 105 | 119 | 66 | 60 | 2.3 | 11 | PDE5 inhibitor | Clinical improvement | ||
| Stewart [26] | Female | 72 | Mosaic perfusion | IV | 66 | 73 | 35 | 2.7 | 5.4 | Calcium antagonists and i.v. prostanoids | Death from respiratory failure | ||
| Female | 56 | Mild ground-glass attenuation in the upper lobes and lung cysts | III | 83 | 76 | 41 | 68 | 1.6 | i.v. prostanoids | Death 2 years after diagnosis from respiratory failure | |||
| Male | 68 | Lung cysts | 49 | 2.4 | 5.4 | Calcium antagonists | Death 6 years after diagnosis from RH failure | ||||||
| Female | 33 | Mosaic perfusion | 34 | 38 | 53 | 1.3 | 24.4 | ERA and PDE5 inhibitor | Death 1 year after starting treatment | ||||
| Simeoni [27] | Female | 51 | Nodular lesions and schwannoma in the upper mediastinum | III | 72 | 65 | ERA | Stable after 2 years of treatment | |||||
| Montani [28] | Female | 59 | Normal | III | 73 | 104 | 59 | 48 | 2.0 | 12.0 | Anticoagulants, ERA and i.v. prostanoids | Death after 6 months | |
| Female | 63 | Moderate pulmonary fibrosis with large bullae | III | 58 | 52 | 45 | 27 | 52 | 1.9 | 14.4 | Anticoagulants, PDE5 inhibitor, ERA and i.v. prostanoids | Death after 42 months | |
| Female | 53 | Lung cysts and interstitial infiltrate | III | 109 | 25 | 47 | 2.7 | 8.0 | Anticoagulants, PDE5 inhibitor, ERA and i.v. prostanoids | Death after 46 months | |||
| Female | 69 | Normal | III | 67 | 103 | 39 | 54 | 2.3 | 14.4 | PDE5 inhibitor and ERA, prostanoids declined | Alive at 36 months but more severe | ||
| Male | 66 | Mosaic perfusion and mild emphysema | III | 69 (3 L) | 104 | 48 | 43 | 2.1 | 9.3 | Anticoagulants, PDE5 inhibitor and ERA | Alive at 8 months | ||
| Female | 63 | Lung cysts | III | 51 | 69 | 69 | 23 | 36 | 3.3 | Anticoagulants and ERA | Alive at 18 months. On a waiting list for lung transplant | ||
| Female | 53 | Lung cysts | III | 79 (4 L) | 83 | 29 | NA | 31 | 4.7 | No | Alive at 3 months | ||
| Female | 61 | Lung cysts and interstitial infiltrate | III | 40 | 95 | 24 | 37 | 2.3 | 7.1 | Anticoagulants, ERA, i.v. prostanoids then lung transplantation | Alive 8 months after diagnosis and 1 month after lung transplantation | ||
| Gumbiene [29] | Female | 30 | Mosaic perfusion | IV | 115 | 111 | 55 | 49 | 2.5 | 15 | Anticoagulants, PDE5 inhibitor and ERA | Death after 3 months | |
| Malviya [30] | Male | 34 | Mosaic perfusion | II | 58 | 2.7 | 19 | PDE5 inhibitor and balloon atrial septostomy | Alive 8 months after diagnosis | ||||
| Male | 44 | Mosaic perfusion and localised fibrotic lesion | IV | 74 | 66 | 44 | 48 | 1.7 | 26.5 | ERA and PDE5 inhibitor | Alive after 15 months and improved | ||
| Tamura [31] | Female | 30 | NA | II | 39 | i.v. prostanoid then PDE5 inhibitor and ERA then sorafenib | Alive 6 years after the diagnosis | ||||||
| Martignac [32] | Female | 64 | Lung cysts, ground-glass opacities and suspect mass | III | 64 | 72 | 26 | 62 | 1.9 | 21.4 | Anticoagulants, ERA and PDE5 inhibitor | Death after 4 months | |
| Kamdar [33] | Female | 69 | NA | 39 | 23 | PDE5 inhibitor and i.v. prostanoid | Improvement and alive after 12 months | ||||||
| Giannakoulas [34] | Female | 57 | Normal | 84 | 1.9 | 27.1 | Anticoagulants, ARE and PDE5 inhibitor then balloon atrial septostomy followed by i.v. prostanoids | Improvement and alive after 24 months | |||||
| Chaddha [35] | Female | 63 | Lung cysts, ground-glass opacities and interlobular septal thickening | IV | 60 | 2.1 | 14 | PDE5 inhibitor and i.v. prostanoids (stopped after pulmonary oedema) | Pulmonary oedema under vasodilator. Death after 3 months | ||||
| Küçük [36] | Female | 46 | Mosaic perfusion pattern | II | 91 | 100 | 93 | 85 | 2.9 | 16 | Calcium antagonists and ERA | Improvement | |
| Poble [37] | Female | 55 | Lung cysts | III | 68 | 93 | 104 | 49 | 41 | 2 | 19 | Anticoagulants, PDE5 inhibitor and ERA | Improvement and alive after 9 months |
| Palot [38] | Female | 55 | Intrathoracic meningocele and scoliosis | IV | 53 | 51 | 68 | 30 | 4.9 | Noninvasive ventilation | Improvement after 1 month |
CT: computed tomography; NYHA: New York Heart Association; PO2: oxygen tension; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide; mPpa: mean pulmonary artery pressure; CI: cardiac index; PVR: pulmonary vascular resistance; NA: not available; ERA: endothelin receptor antagonist; PDE5: phosphodiesterase-5.
Although NF1 affects male and female patients without sex predominance, there were 25 females but only six males (male:female sex ratio was 1:4.2), suggesting a female predominance in PH-NF1, as is generally seen in idiopathic and heritable PAH [39, 40].
It is interesting to note that PH occurred late in the course of NF1, with a median (range) age at diagnosis of 57 (50–65) years, in contrast with heritable PAH, which is characterised by a younger age at diagnosis (mean age 35.7 years in BMPR2 mutation carriers and 21.8 years in ACVRL1 mutation carriers), or with patients with idiopathic PAH (without an identified mutation, mean age 47.6 years) [41]. Dyspnoea and signs of right heart failure were the principal symptoms leading to evaluation for associated PH. Most patients had severe haemodynamic impairment at diagnosis, with a low cardiac index (median (range), 2.3 (2.0–2.7) L·min−1·m−2) and high levels of mPpa (median (range) 49 (39–60) mmHg) and pulmonary vascular resistance (median (range) 14.2 (8.9–19.0) Wood unit). None of the patients had a vasodilator response to nitric oxide. Acute vasodilator response with nitric oxide is reported in about 10% of idiopathic PAH, and is associated with long-term response to calcium channel blockers and an excellent prognosis [42]. However, it has been shown that the proportion of acute vasodilator responders was low in patients with heritable PAH [41], as well as in PAH associated with other conditions [43].
Moreover, most of the patients had severe exercise limitation with 75% of the patients in New York Heart Association functional class III or IV at diagnosis and a median (range) 6-min walk distance of 230 (153–300) m. As described later in this article, NF1 may be associated with parenchymal lung involvement. In the 31 reported cases of PH-NF1, lung involvement was reported in 22 patients and was in accordance with data reported by Zamora et al. [44]. Lung involvement manifested itself by mosaic perfusion with ground-glass opacities (n=10), lung cysts (n=10), interstitial septal infiltrates (n=5), large bullae (n=2), mediastinal schwannomas (n=2), pneumothorax (n=1), mild emphysema (n=1), intra-thoracic meningocele (n=1), lung nodules (n=1) and a suspicious lung mass (n=1). It could be suggested that precapillary PH observed in these patients may be due to vascular rarefaction and hypoxic vasoconstriction associated with parenchymal lung disease. However, nine (29%) out of 31 patients with confirmed severe PH had no significant lung involvement on high-resolution computed tomography (HRCT) of the chest and had normal pulmonary functional tests (PFTs). Furthermore, in most patients, the median (range) spirometry and lung volume measurements were in the normal range: forced expiratory volume in 1 s was 93 (71–104) %, forced vital capacity was 83 (72–99) % and total lung capacity was 94 (78–103) %. However, diffusing capacity of the lung for carbon monoxide was importantly decreased in most patients (median (range) 48 (27–57) %) suggesting significant pulmonary capillary involvement.
In this article, we found that most patients received conventional therapy for PH including oxygen and diuretics if needed and sometimes anticoagulation. In addition, despite the absence of recommendations, 22 patients received specific PAH therapies, including endothelin receptor antagonists (n=17), phosphodiesterase type-5 inhibitors (n=16), and i.v. prostanoids (n=13). Four patients also received calcium antagonists although they had no acute vasodilator response with nitric oxide. Two patients had balloon atrial septostomy and only one had lung transplantation. Another patient with PH predominantly due to restriction from an intrathoracic meningocele and scoliosis was successfully treated by noninvasive ventilation alone [38].
The outcomes of these patients reported in the literature were characterised by a limited response to specific PAH therapy and poor outcomes. Indeed, 13 out of 31 patients died with a median (range) delay of 24 (5–39) months. Of note, one patient was treated with the tyrosine kinase inhibitor (TKI) sorafenib and experienced mild clinical and haemodynamic improvement after 3 months; however, no data on long-term response was available [31]. Nevertheless, the benefit/risk ratio of sorafenib in PH is debatable due to possible impairment of cardiac output [45] and imatinib, another TKI tested in a PAH clinical trial, was associated with an increased incidence of subdural haemorrhage [46, 47]. Furthermore, it has been demonstrated that the second-generation TKI dasatinib may induce pulmonary endothelial dysfunction and severe PAH [48–50]. Another patient developed pulmonary oedema with specific PAH treatment and died 3 months later [35]. An autopsy confirmed the diagnosis of capillary haemangiomatosis, a condition similar to pulmonary veno-occlusive disease, known to have a bad response to specific PAH treatment and a poor prognosis [51]. Pulmonary veno-occlusive disease has also been suspected in another case with respiratory failure and death a short time after beginning a specific PH-treatment [32]. This limited response to specific PAH therapies and the poor prognosis of PH-NF1 emphasise the importance of referral for lung transplant assessment early in the course of the disease in eligible patients. While evaluating a patient with NF1 for lung transplantation, one must keep in mind that these patients have an increased risk of cancers and that the immunosuppression required for transplantation increases this risk. Among the 31 reported cases of PH-NF1, one had lung transplantation and was making satisfactory progress after 8 months [28]. Merlo et al. [52] described two other cases of lung transplantation in patients with NF1 because of advanced tobacco-induced chronic obstructive pulmonary disease but without PH. One patient was doing well after 5 years but the second patient developed post-transplant lymphoproliferative disorder and a massive intra-abdominal sarcoma consistent with a malignant nerve sheath tumour 9 months after lung transplantation and died 2 months later [52]. This emphasises the risk of immunosuppressive treatment in these patients and the attentive follow-up needed after lung transplantation.
Four patients had vascular histologic assessment: one pulmonary tissue biopsy [27], one after lung transplantation [28] and two after autopsy [23, 35]. The presence of vascular remodelling of the small pulmonary arteries with intimal and medial thickening and fibrosis was the common finding in these samples. Montani et al. [28] showed that the pulmonary vascular involvement included arterial remodelling with eccentric thickening of the intimal layer and uniform wall thickening through hyperplasia of pericytes/smooth muscle cells in most of the small pulmonary arterioles. Plexiform lesions were also found [23], along with areas of alveolar capillary engorgement and tortuosity with a diagnosis of pulmonary capillary haemangiomatosis [35] and lung interstitial fibrosis with partial loss of parenchymal architecture [28]. These observations reinforce the hypothesis of a disproportionate pulmonary vascular involvement in the case of interstitial lung disease in NF1.
The NF1 gene was screened in nine patients and heterozygous germline mutations of the NF1 gene were identified in all cases. NF1 mutations were of different types, including short deletions, nonsense and missense mutations, and a complete deletion of the gene. All these mutations were located in various exons of the gene and no relationship was found between the occurrence of PH and a specific position of the mutation. Similarly no relationship has ever been found between the position of the truncating mutation and the features of the NF1 phenotype [53].
There are, therefore, some differences between PH-NF1 and idiopathic and heritable PH. The distinguish features between PH-NF1 and idiopathic and heritable PAH are summarised in table 4.
TABLE 4.
Comparison of key distinguishing features between pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with neurofibromatosis type 1 (PH-NF1)
| Idiopathic or heritable PAH | PH-NF1 | |
| Genetics | ||
| Main gene mutation | BMPR2, ACVRL1, ENG, KCNK3, CAV-1, SMAD9 | NF1 |
| Genetic transmission | Autosomal dominant | Autosomal dominant |
| Epidemiology | ||
| Estimated prevalence | 15 cases per million | Prevalence of NF1 is 1 per 3000, prevalence of PH is unknown |
| Sex ratio | Female predominance (∼2:1) | Female predominance (∼4:1) |
| Clinical examination | ||
| Dermatological signs | Absent | Café au lait macules, axillary or groin freckling, subcutaneous neurofibroma |
| Neurological signs | Absent | Possible optic glioma |
| Right heart catheterisation | ||
| mPpa, PAWP, PVR | Increased mPpa, normal PAWP, increased PVR | More severely increased mPpa, normal PAWP, more severely increased PVR |
| Acute vasoreactivity testing | ∼10% in idiopathic PAH (predicts long-term CCB response) | No case reported |
| Pulmonary function | ||
| FEV1, FVC, TLC | Normal (possible mild reduction) | Normal (possible mild reduction) |
| DLCO | Normal (possible mild reduction) | Severe reduction |
| Imaging | ||
| Chest HRCT | Usually normal parenchyma | Ground-glass opacities, lung cysts, lung reticulations |
| V′/Q′ lung scan | Usually normal | Usually normal |
| Treatment | ||
| Targeted PAH therapy | Improved haemodynamics, functional capacity and clinical outcomes | Poor response (based on isolated case reports) |
BMPR2: bone morphogenetic protein receptor type 2; ACVRL1: activin A receptor type II-like kinase 1; ENG: endoglin; KCNK3: potassium channel subfamily K member 3; CAV-1: caveolin 1; mPpa: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; DLCO: diffusing capacity of the lung for carbon monoxide; HRCT: high-resolution computed tomography; V′/Q′: ventilation/perfusion; CCB: calcium channel blocker.
Pathophysiology of PH in NF1
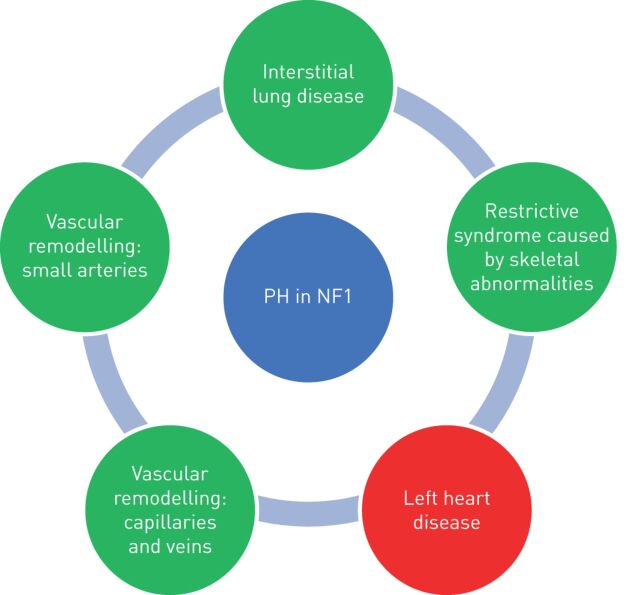
PH-NF1 is classified as group 5 PH, defined as “PH with unclear and/or multifactorial mechanisms”, because the mechanisms of PH remain poorly understood. Indeed, it may include different mechanisms such as lung parenchymal destruction, respiratory insufficiency secondary to restriction caused by skeletal abnormalities, left heart disease, but also pulmonary vascular remodelling of the pulmonary arteries and veins. The different mechanisms of development of PH-NF1 are shown in figure 2.
FIGURE 2.
Mechanisms leading to development of pulmonary hypertension (PH) in neurofibromatosis type 1 (NF1). Green indicates pre-capillary hypertension and red indicates post-capillary hypertension.
It is well known that the course of NF1 is often complicated by systemic vasculopathy affecting all the systemic arteries of the body and resulting, for instance, in renovascular hypertension, myocardial infarction, cerebral infarction and ischaemic bowel disease [7]. Lie et al. [7] described three basic types of vascular lesions: 1) zonal intimal vascular smooth muscle cell proliferation in large elastic arteries; 2) intimal vascular smooth muscle cell proliferation, with associated fibrosis and neoangiogenesis of medium-sized elastic and muscular arteries; and 3) plexiform or angiomatoid intimal proliferation in small arteries and arterioles. Knowledge of these systemic vascular complications, has led to the belief that a pulmonary vasculopathy might cause PH-NF1. The description of lung histology which shows the presence of arterial intimal and medial thickening and plexiform lesions are in agreement with this hypothesis, such as the mosaic pattern of lung attenuation seen on the chest HRCT scan of most patients. Veno-occlusive disease has also been suspected or documented [32, 35].
Neurofibromin is known to have a role in tumour suppression. Since neurofibromin is expressed in endothelial and smooth muscle cells of blood vessels [54], its loss is likely to be the key to the development of the vasculopathy associated with NF1. It is interesting that other multiple congenital anomaly syndromes that are due to Ras/MAPK activation by germline mutations (Noonan syndrome, Costello syndrome and Leopard syndrome), are also often associated with PH [55–57]. The mechanisms leading to PH in NF1 and in these diseases may be the same. Molecular mechanisms and cell abnormalities have been studied on systemic endothelial cells showing that loss of NF1 is responsible for an increased proliferation and migration of systemic endothelial cells depending on ERK activation [58]. It has also been shown that systemic vascular smooth muscle cells have an abnormal proliferative phenotype in case of loss of NF1, depending on ERK pathway [59]. In addition, another study showed that there is an increase in reactive oxygen species production in NF1 that could participate in the development of a systemic vasculopathy [60]. However, these mechanisms have not been studied on pulmonary vascular cells.
Moreover, as only a minority of NF1 patients will develop PH, one can hypothesise that a second genetic hit might be necessary to lead to the development of pulmonary vasculopathy. Stewart et al. [26] hypothesised that the pathogenesis of PH-NF1 stems from the loss of heterozygosis of NF1 in pulmonary endothelial cells, the subsequent dysregulation of the RAS pathway, monoclonal expansion of the endothelial cells, abnormal vascular cell proliferation and misguided angiogenesis. Additional unidentified factors may be also involved, such as inflammatory, infectious or autoimmune hits.
Treatment of PH associated with NF1
There is neither long-term data nor recommendations regarding the use of PAH-approved drugs in group 5 PH or in NF1 in particular [1, 61]. Indeed there is only minimal data on the use of these drugs in group 5 PH patients in general and there is the potential for pulmonary venous involvement in this group, which could be aggravated by pulmonary vasodilators [61].
When the diagnosis of PH-NF1 is suspected on transthoracic echocardiography, right heart catheterisation is required to confirm the diagnosis and assess the mechanism (pre- or post-capillary) and severity of the PH. In case of mild PH with severe lung disease, symptomatic treatment should be proposed including long-term oxygen therapy, diuretics and noninvasive ventilation in case of alveolar hypoventilation. In the presence of severe PH without extensive lung involvement, patients should be referred to a PH expert centre and specific PAH treatment should be discussed, based on an understanding of the potential underlying mechanisms and a proper assessment of haemodynamics, with attentive follow-up. Indeed, the benefit/risk ratio of these treatments in PH-NF1 is largely unknown, in particular because of the risk of pulmonary oedema associated with venous and capillary involvement. As PH-NF1 is generally severe, people should be referred early to a lung transplantation centre, with careful assessment of the increased post-transplant cancer risk.
From the known effects of neurofibromin on growth regulation signalling, several currently available agents have been suggested as potentially beneficial in PH-NF1. However, no treatments have been evaluated for this particular indication. Drugs that can inhibit the dysregulation induced by neurofibromin deficiency are potential targets, such as rapamycin, an mTOR inhibitor that has been shown to attenuate PH and neo-intimal formation in rats [62]. A recent study also showed that a stronger inhibition of mTOR by a dual mTORC1/2 inhibitor induced an anti-proliferative effect in NF1-associated plexiform neurofibroma and malignant peripheral nerve sheath tumour cells compared to rapamycin which is only an inhibitor of mTORC1 [63]. Attenuation of the vascular wall proliferation that characterises NF1 using statins (3-hydroxy-3-methylglutarylcoenzyme A reductase inhibitors) is another possible approach, since this class of agent is known to inhibit Ras activity through prevention of its lipid modification [64]. Targeting the MAPK pathway is also promising. Sorafenib is an oral inhibitor of multiple kinases, including Raf, the downstream target of Ras in the MAPK cascade and as described earlier, it improved a refractory PH-NF1 in one case study [31]. However, it has not yet been evaluated in a clinical trial. MEK inhibitors could also be a future strategy, as it has recently been shown that MEK inhibitors, like selumetinib, could be beneficial for plexiform neurofibromas [65] and malignant peripheral nerve sheath tumours in vitro [66]. Clinical trials are warranted to get new effective treatment for PH-NF1 by acting on its molecular mechanisms.
Interstitial lung disease and thoracic complications
Interstitial lung disease
Interstitial lung diseases were the first described lung complications of NF1 [67, 68]. A radiographic study published in 1977 reported that seven out of 70 patients with NF1 had radiographic evidence of “fibrosing alveolitis” with increased interstitial markings, bullous areas of lung destruction or both, and histologic specimens obtained in two patients confirmed lung fibrosis [69]. Bullous and cystic changes, mostly in the upper lobes, were then confirmed to be the most frequent lung manifestations characteristic of NF1, associated or not with interstitial abnormalities [70–72]. In a retrospective single-centre study of 156 NF1 patients, chest radiographs revealed abnormal findings in 70 (44.9%) patients but bilateral interstitial lung infiltrates or cystic airspaces were only demonstrated in eight (5.1%) patients [73]. However, HRCT scanning was not performed in all patients. Another retrospective study, reported three cases of interstitial lung disease associated with NF1 among 55 patients and reviewed 61 other cases described in the literature [44]. Overall, eight (37%) patients had HRCTs demonstrating ground-glass opacities, bibasilar reticular opacities (50%), bullae (50%), cysts (25%) and emphysema (25%); none had honeycombing. In these patients with diffuse lung disease, PFTs showed an obstructive pattern in 43%, restrictive pattern in 37%, and a mixed pattern in 17%, with a decreased diffusion capacity of the lung for carbon monoxide in the vast majority of cases. A group of 14 patients had surgical biopsy results that showed findings of interstitial fibrosis (100%) and interstitial inflammation (93%), consistent with nonspecific interstitial pneumonia. In conclusion, interstitial lung disease is a real complication of NF1 and is characterised by lung cysts or bullae in the upper lobes, diffuse ground-glass opacities sometimes with mosaic pattern and reticular opacities, sometimes with pathologic evidence of fibrosis but without honeycombing on HRCT scans. Another interesting point is that interstitial lung disease has been found to develop only in adulthood whereas NF1 exists from birth.
Other thoracic complications
Thoracic complications of NF1 are multiple and also include the development of airway plexiform neurofibromas, plexiform neurofibromas in the posterior mediastinum, spinal dumb-bell neurofibromas, intercostal neurofibromas, intrathoracic meningoceles, increased risk of pneumothorax or haemothorax [74, 75] and skeletal abnormalities [76]. An increased risk of cancer is known in NF1 and cases of lung cancer have also been described in NF1 although they seem to be rare [77, 78].
Conclusion
PH is a rare but potentially severe complication of NF1. PH-NF1 is classified in group 5 PH, defined as “PH with unclear and/or multifactorial mechanisms”. Severe PH-NF1 is generally associated with lung lesions, mostly cysts or bullae in the upper lobes, diffuse ground-glass opacities sometimes with mosaic pattern and reticular opacities. In one-third of PH-NF1 cases there is no lung parenchymal disease, suggesting specific vascular lesions. There are no guidelines regarding the treatment of PH in these patients and they need to be referred early to expert PH centres and considered for lung transplantation. There are several promising treatments targeting the RAS signalling downstream pathways which will require clinical trials.
Footnotes
Number 1 in the Series “Group 5 Pulmonary Hypertension” Edited by Yochai Adir and Laurent Savale
Provenance: Commissioned article, peer reviewed.
Conflict of interest: E-M. Jutant has nothing to disclose.
Conflict of interest: B. Girerd has nothing to disclose.
Conflict of interest: X. Jäis reports grants, personal fees and non-financial support from Actelion, GSK, Bayer and MSD, outside the submitted work.
Conflict of interest: L. Savale reports grants and personal fees from Actelion, grants, personal fees and non-financial support from MSD and Bayer, and personal fees and non-financial support from GSK, outside the submitted work.
Conflict of interest: C. O'Connell has nothing to disclose.
Conflict of interest: F. Perros has nothing to disclose.
Conflict of interest: O. Sitbon reports grants, personal fees and non-financial support from Actelion Pharmaceuticals, Merck and GSK, grants and personal fees from Bayer, and personal fees from Arena Pharmaceuticals and Acceleron Pharmaceuticals, outside the submitted work.
Conflict of interest: M. Humbert reports personal fees from Actelion, Merck and United Therapeutics, and grants and personal fees from Bayer and GSK, outside the submitted work.
Conflict of interest: D. Montani reports grants and personal fees from Actelion and personal fees from BMS, GSK, MSD and Pfizer, outside the submitted work.
References
- 1.Galiè N, Humbert M, Vachiery J-L, et al. . ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds RM, Browning GGP, Nawroz I, et al. . Von Recklinghausen's neurofibromatosis: neurofibromatosis type 1. Lancet Lond Engl 2003; 361: 1552–1554. [DOI] [PubMed] [Google Scholar]
- 3.Tate JM, Gyorffy JB, Colburn JA. The importance of pheochromocytoma case detection in patients with neurofibromatosis type 1: a case report and review of literature. SAGE Open Med Case Rep 2017; 5: 2050313X17741016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med 2010; 12: 1–11. [DOI] [PubMed] [Google Scholar]
- 5.Tedesco MA, Di Salvo G, Natale F, et al. . The heart in neurofibromatosis type 1: an echocardiographic study. Am Heart J 2002; 143: 883–888. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen R, Mir TS, Kluwe L, et al. . Cardiac characterization of 16 patients with large NF1 gene deletions. Clin Genet 2013; 84: 344–349. [DOI] [PubMed] [Google Scholar]
- 7.Lie JT. Vasculopathies of neurofibromatosis type 1 (von Recklinghausen Disease). Cardiovasc Pathol 1998; 7: 97–108. [DOI] [PubMed] [Google Scholar]
- 8.Oderich GS, Sullivan TM, Bower TC, et al. . Vascular abnormalities in patients with neurofibromatosis syndrome type I: clinical spectrum, management, and results. J Vasc Surg 2007; 46: 475–484. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, MD., USA, July 13–15, 1987. Neurofibromatosis 1988; 1: 172–178. [PubMed] [Google Scholar]
- 10.Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet 1999; 89: 1–6. [PubMed] [Google Scholar]
- 11.Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol 2000; 151: 33–40. [DOI] [PubMed] [Google Scholar]
- 12.Sørensen SA, Mulvihill JJ, Nielsen A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N Engl J Med 1986; 314: 1010–1015. [DOI] [PubMed] [Google Scholar]
- 13.Pinson S, Créange A, Barbarot S, et al. . [Recommendations for the treatment of neurofibromatosis type 1]. J Fr Ophtalmol 2002; 25: 423–433. [PubMed] [Google Scholar]
- 14.Huson SM, Compston DA, Clark P, et al. . A genetic study of von Recklinghausen neurofibromatosis in South East Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet 1989; 26: 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballester R, Marchuk D, Boguski M, et al. . The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell 1990; 63: 851–859. [DOI] [PubMed] [Google Scholar]
- 16.Basu TN, Gutmann DH, Fletcher JA, et al. . Aberrant regulation of Ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 1992; 356: 713–715. [DOI] [PubMed] [Google Scholar]
- 17.Donovan S, See W, Bonifas J, et al. . Hyperactivation of protein kinase B and ERK have discrete effects on survival, proliferation, and cytokine expression in NF1-deficient myeloid cells. Cancer Cell 2002; 2: 507–514. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Viciana P, Warne PH, Dhand R, et al. . Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 1994; 370: 527–532. [DOI] [PubMed] [Google Scholar]
- 19.Johannessen CM, Reczek EE, James MF, et al. . The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci USA 2005; 102: 8573–8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004; 18: 1926–1945. [DOI] [PubMed] [Google Scholar]
- 21.Porterfield JK, Pyeritz RE, Traill TA. Pulmonary hypertension and interstitial fibrosis in von Recklinghausen neurofibromatosis. Am J Med Genet 1986; 25: 531–535. [DOI] [PubMed] [Google Scholar]
- 22.Samuels N, Berkman N, Milgalter E, et al. . Pulmonary hypertension secondary to neurofibromatosis: intimal fibrosis versus thromboembolism. Thorax 1999; 54: 858–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aoki Y, Kodama M, Mezaki T, et al. . von Recklinghausen disease complicated by pulmonary hypertension. Chest 2001; 119: 1606–1608. [DOI] [PubMed] [Google Scholar]
- 24.García Hernández FJ, Sánchez Román J, Ocaña Medina C, et al. . [Pulmonary hypertension in a patient with neurofibromatosis]. Med Clin (Barc) 2002; 118: 78–79. [PubMed] [Google Scholar]
- 25.Engel PJ, Baughman RP, Menon SG, et al. . Pulmonary hypertension in neurofibromatosis. Am J Cardiol 2007; 99: 1177–1178. [DOI] [PubMed] [Google Scholar]
- 26.Stewart DR, Cogan JD, Kramer MR, et al. . Is pulmonary arterial hypertension in neurofibromatosis type 1 secondary to a plexogenic arteriopathy? Chest 2007; 132: 798–808. [DOI] [PubMed] [Google Scholar]
- 27.Simeoni S, Puccetti A, Chilosi M, et al. . Type 1 neurofibromatosis complicated by pulmonary artery hypertension: a case report. J Med Investig JMI 2007; 54: 354–358. [DOI] [PubMed] [Google Scholar]
- 28.Montani D, Coulet F, Girerd B, et al. . Pulmonary hypertension in patients with neurofibromatosis type I. Medicine (Baltimore) 2011; 90: 201–211. [DOI] [PubMed] [Google Scholar]
- 29.Gumbiene L, Petrulioniene Z, Rucinskas K, et al. . Pulmonary hypertension: a fatal complication of neurofibromatosis type 1. Respir Care 2011; 56: 1844–1848. [DOI] [PubMed] [Google Scholar]
- 30.Malviya A, Mishra S, Kothari SS. Type 1 neurofibromatosis and pulmonary hypertension: a report of two cases and a review. Heart Asia 2012; 4: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura Y, Ono T, Sano M, et al. . Favorable effect of sorafenib in a patient with neurofibromatosis-associated pulmonary hypertension. Am J Respir Crit Care Med 2012; 186: 291–292. [DOI] [PubMed] [Google Scholar]
- 32.Martignac B, Gagnadoux F, Trzepizur W, et al. . [Severe pulmonary involvement in the course of type 1 neurofibromatosis]. Rev Mal Respir 2014; 31: 621–623. [DOI] [PubMed] [Google Scholar]
- 33.Kamdar F, Thenappan T, Missov E. Pulmonary arterial hypertension in von Recklinghausen's disease. Am J Med 2015; 128: e39–e40. [DOI] [PubMed] [Google Scholar]
- 34.Giannakoulas G, Savvoulidis P, Grosomanidis V, et al. . Atrial septostomy and disease targeting therapy in pulmonary hypertension secondary to neurofibromatosis. BMC Pulm Med 2016; 16: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaddha U, Puscas I, Prosper A, et al. . A 63-year-old woman with neurofibromatosis type 1 and pulmonary hypertension with worsening hypoxemia. Chest 2017; 152: e89–e93. [DOI] [PubMed] [Google Scholar]
- 36.Küçük M, Öncel CR, Uçar M, et al. . Type 1 neurofibromatosis complicated by pulmonary arterial hypertension: a case report. Turk Kardiyol Dem Ars 2017; 45: 458–461. [DOI] [PubMed] [Google Scholar]
- 37.Poble PB, Dalphin JC, Degano B. Severe dyspnea in a patient with neurofibromatosis type 1. Respir Med Case Rep 2017; 22: 74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palot A, Ferrandez C, Alagha K, et al. . [Neurofibromatosis as a cause of breathlessness]. Rev Mal Respir 2018; 35: 338–341. [DOI] [PubMed] [Google Scholar]
- 39.Humbert M, Sitbon O, Chaouat A, et al. . Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 40.Austin ED, Cogan JD, West JD, et al. . Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J 2009; 34: 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girerd B, Montani D, Coulet F, et al. . Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am J Respir Crit Care Med 2010; 181: 851–861. [DOI] [PubMed] [Google Scholar]
- 42.Sitbon O, Humbert M, Jaïs X, et al. . Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111: 3105–3111. [DOI] [PubMed] [Google Scholar]
- 43.Montani D, Savale L, Natali D, et al. . Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur Heart J 2010; 31: 1898–1907. [DOI] [PubMed] [Google Scholar]
- 44.Zamora AC, Collard HR, Wolters PJ, et al. . Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J 2007; 29: 210–214. [DOI] [PubMed] [Google Scholar]
- 45.Weatherald J, Humbert M, Guignabert C, et al. . Response to the article “Sorafenib as a potential strategy for refractory pulmonary arterial hypertension”. Pulm Pharmacol Ther 2017; 45: 11–12. [DOI] [PubMed] [Google Scholar]
- 46.Hoeper MM, Barst RJ, Bourge RC, et al. . Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation 2013; 127: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 47.Humbert M. Impression, sunset. Circulation 2013; 127: 1098–1100. [DOI] [PubMed] [Google Scholar]
- 48.Montani D, Bergot E, Günther S, et al. . Pulmonary arterial hypertension in patients treated by dasatinib. Circulation 2012; 125: 2128–2137. [DOI] [PubMed] [Google Scholar]
- 49.Guignabert C, Phan C, Seferian A, et al. . Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest 2016; 126: 3207–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weatherald J, Chaumais M-C, Savale L, et al. . Long-term outcomes of dasatinib-induced pulmonary arterial hypertension: a population-based study. Eur Respir J 2017; 50: 1700217. [DOI] [PubMed] [Google Scholar]
- 51.Montani D, Price LC, Dorfmuller P, et al. . Pulmonary veno-occlusive disease. Eur Respir J 2009; 33: 189–200. [DOI] [PubMed] [Google Scholar]
- 52.Merlo CA, Studer SM, Conte JV, et al. . The course of neurofibromatosis type 1 on immunosuppression after lung transplantation: report of 2 cases. J Heart Lung Transplant 2004; 23: 774–776. [DOI] [PubMed] [Google Scholar]
- 53.Fahsold R, Hoffmeyer S, Mischung C, et al. . Minor lesion mutational spectrum of the entire NF1 gene does not explain its high mutability but points to a functional domain upstream of the GAP-related domain. Am J Hum Genet 2000; 66: 790–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamilton SJ, Friedman JM. Insights into the pathogenesis of neurofibromatosis 1 vasculopathy. Clin Genet 2000; 58: 341–344. [DOI] [PubMed] [Google Scholar]
- 55.Hopper RK, Feinstein JA, Manning MA, et al. . Neonatal pulmonary arterial hypertension and Noonan syndrome: two fatal cases with a specific RAF1 mutation. Am J Med Genet A 2015; 167A: 882–885. [DOI] [PubMed] [Google Scholar]
- 56.O'Shea J, Lynch SA, Macken S. A case of persistent pulmonary hypertension in a newborn with Costello syndrome. Clin Dysmorphol 2008; 17: 287–288. [DOI] [PubMed] [Google Scholar]
- 57.Blieden LC, Schneeweiss A, Neufeld HN. Primary pulmonary hypertension in leopard syndrome. Br Heart J 1981; 46: 458–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munchhof AM, Li F, White HA, et al. . Neurofibroma-associated growth factors activate a distinct signaling network to alter the function of neurofibromin-deficient endothelial cells. Hum Mol Genet 2006; 15: 1858–1869. [DOI] [PubMed] [Google Scholar]
- 59.Li F, Munchhof AM, White HA, et al. . Neurofibromin is a novel regulator of RAS-induced signals in primary vascular smooth muscle cells. Hum Mol Genet 2006; 15: 1921–1930. [DOI] [PubMed] [Google Scholar]
- 60.Bessler WK, Hudson FZ, Zhang H, et al. . Neurofibromin is a novel regulator of Ras-induced reactive oxygen species production in mice and humans. Free Radic Biol Med 2016; 97: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weatherald J, Savale L, Humbert M. Medical management of pulmonary hypertension with unclear and/or multifactorial mechanisms (group 5): is there a role for pulmonary arterial hypertension medications? Curr Hypertens Rep 2017; 19: 86. [DOI] [PubMed] [Google Scholar]
- 62.Nishimura T, Faul JL, Berry GJ, et al. . 40-O-(2-hydroxyethyl)-rapamycin attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med 2001; 163: 498–502. [DOI] [PubMed] [Google Scholar]
- 63.Varin J, Poulain L, Hivelin M, et al. . Dual mTORC1/2 inhibition induces anti-proliferative effect in NF1-associated plexiform neurofibroma and malignant peripheral nerve sheath tumor cells. Oncotarget 2016; 7: 35753–35767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finlay GA, Malhowski AJ, Liu Y, et al. . Selective inhibition of growth of tuberous sclerosis complex 2 null cells by atorvastatin is associated with impaired Rheb and Rho GTPase function and reduced mTOR/S6 kinase activity. Cancer Res 2007; 67: 9878–9886. [DOI] [PubMed] [Google Scholar]
- 65.Dombi E, Baldwin A, Marcus LJ, et al. . Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med 2016; 375: 2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer-Huchzermeyer S, Dombrowski A, Wilke G, et al. . MEK inhibitors enhance therapeutic response towards ATRA in NF1 associated malignant peripheral nerve sheath tumors (MPNST) in vitro. PloS One 2017; 12: e0187700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Massaro D, Katz S. Fibrosing alveolitis: its occurrence, roentgenographic, and pathologic features in von Recklinghausen's neurofibromatosis. Am Rev Respir Dis 1966; 93: 934–942. [DOI] [PubMed] [Google Scholar]
- 68.Patchefsky AS, Atkinson WG, Hoch WS, et al. . Interstitial pulmonary fibrosis and von Recklinghausen's disease. An ultrastructural and immunofluorescent study. Chest 1973; 64: 459–464. [DOI] [PubMed] [Google Scholar]
- 69.Webb WR, Goodman PC. Fibrosing alveolitis in patients with neurofibromatosis. Radiology 1977; 122: 289–293. [DOI] [PubMed] [Google Scholar]
- 70.Davis SA, Kaplan RL. Neurofibromatosis and interstitial lung disease. Arch Dermatol 1978; 114: 1368–1369. [PubMed] [Google Scholar]
- 71.Burkhalter JL, Morano JU, McCay MB. Diffuse interstitial lung disease in neurofibromatosis. South Med J 1986; 79: 944–946. [DOI] [PubMed] [Google Scholar]
- 72.Yokoyama A, Kohno N, Sakai K, et al. . Distal acinar emphysema and interstitial pneumonia in a patient with von Recklinghausen's disease: five-year observation following quitting smoking. Intern Med Tokyo Jpn 1997; 36: 413–416. [DOI] [PubMed] [Google Scholar]
- 73.Ryu JH, Parambil JG, McGrann PS, et al. . Lack of evidence for an association between neurofibromatosis and pulmonary fibrosis. Chest 2005; 128: 2381–2386. [DOI] [PubMed] [Google Scholar]
- 74.Fdil S, Bouchikhi S, Bourkadi J-E. [Spontaneous hemothorax: a rare complication of neurofibromatosis type 1]. Pan Afr Med J 2017; 28: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen KA, Elnaggar M, Gallant NM, et al. . Neurofibromatosis type 1: a case highlighting pulmonary and other rare clinical manifestations. BMJ Case Rep 2018; 2018: [ 10.1136/bcr-2017-222614]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsirikos AI, Saifuddin A, Noordeen MH. Spinal deformity in neurofibromatosis type-1: diagnosis and treatment. Eur Spine J 2005; 14: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brasfield RD, Das Gupta TK. Von Recklinghausen's disease: a clinicopathological study. Ann Surg 1972; 175: 86–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uusitalo E, Rantanen M, Kallionpää RA, et al. . Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol 2016; 34: 1978–1986. [DOI] [PubMed] [Google Scholar]