Abstract
Cigarette smoke (CS) exposure is a key risk factor for both active and latent tuberculosis (TB). It is associated with delayed diagnosis, more severe disease progression, unfavourable treatment outcomes and relapse after treatment. Critically, CS exposure is common in heavily populated areas with a high burden of TB, such as China, India and the Russian Federation. It is therefore prudent to evaluate interventions for TB while taking into account the immunological impacts of CS exposure. This review is a mechanistic examination of how CS exposure impairs innate barrier defences, as well as alveolar macrophage, neutrophil, dendritic cell and T-cell functions, in the context of TB infection and disease.
Short abstract
This review provides a mechanistic examination of how cigarette smoke exposure impairs innate and adaptive responses to tuberculosis infection and disease https://bit.ly/3tJba18
Introduction
Tuberculosis (TB) has been a disease of pandemic proportions for over 200 years, and was declared a global health emergency by the World Health Organization (WHO) in 1993. However, TB continues to devastate the most populous regions of the world today. With 10 million new cases and >1.4 million deaths each year [1], TB was the leading cause of death from a single pathogen until the COVID-19 pandemic and is estimated to cost the global economy over one trillion USD in lost productivity and healthcare expenses between 2015 and 2030 [2]. Treatment of active TB remains notoriously complex and lengthy, particularly in the case of multidrug-resistant TB (MDR-TB), greatly exacerbating the burden of human hardship, loss of education and poverty.
TB is spread by aerosol droplets expelled by actively infected individuals and is primarily a pulmonary infection. Mycobacterium tuberculosis causes disease in 5–10% of infected individuals, incubating for long periods or replicating soon after primary infection (figure 1). The intersection of cigarette smoke (CS) exposure and TB is a significant global health concern. Despite heavy tariffs and public health campaigns, there remain 1.3 billion smokers worldwide according to the WHO, and they are largely clustered in TB-endemic regions such as China, India, Bangladesh, Vietnam, the Philippines and the Russian Federation [3]. Of the 47 countries reporting the highest incidence of TB, 34 also report an adult male smoking prevalence of >20% (figure 2) [3]. For example, Indonesia reported a male adult smoking rate of 70.7% and TB incidence of 391 per 100 000 in 2018. Similarly, China reports one of the highest smoking rates in men as well as the highest ratio of male to female TB cases in the world [1].
FIGURE 1.
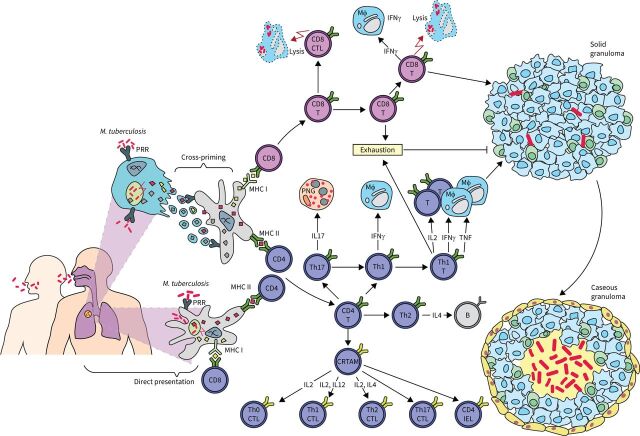
A representative flow of immunological mechanisms for countering infection with Mycobacterium tuberculosis. Upon inhalation, M. tuberculosis enters the lungs, reaches the alveolar space and encounters macrophages (MΦ). Cross-priming of dendritic cells or direct presentation of antigen leads to the activation of antigen-specific CD4+ and CD8+ T-cells. CD8+ T-cells exert direct cytotoxic effects on infected cells and produce interferon-γ (IFN-γ) and tumour necrosis factor (TNF), which activate macrophages. CD4+ T-helper (Th) cells polarise into different subsets. Th1 cells produce interleukin (IL)-2 for T-cell activation, and IFN-γ or TNF for macrophage activation. Th17 cells activate polymorphonuclear granulocytes (PNGs) via IL-17 production, while Th2 cells activate B-cells via IL-4. Additionally, some CD4+ T-cells express class I-restricted T-cell associated molecule (CRTAM) and have the potential to differentiate into various cytotoxic CD4+ T-cell subsets based on environmental cytokines. Solid granulomas form to contain M. tuberculosis, but if the bacterial load exceeds containment, the granuloma will undergo caseous necrosis and fail to contain the infection. PRR: pattern recognition receptors; MHC: major histocompatibility complex; CD: cluster of differentiation; CTL: cytotoxic T-lymphocyte; IEL: intraepithelial lymphocyte.
FIGURE 2.
Countries in the three high-burden country lists for tuberculosis (TB), TB/HIV and multidrug-resistant TB (MDR-TB) identified by the World Health Organization (WHO) in 2016–2020, and their areas of overlap. Countries with a smoking prevalence >20% of all adult men as of 2018 are highlighted in bold. Data taken from the WHO Global TB Report 2020 [1] and the Global Health Observatory (GHO) data repository [3]. ¶: GHO data not available.
Epidemiological links between CS and TB were first suspected in the early 1900s, but systematic investigation did not begin for several decades and was confounded by social factors such as socioeconomic status. In 1956, Lowe [4] reported a spike in TB incidence in men, but not women, over the age of 40 years in Birmingham, UK. He noted, “In industry men are often exposed to dust, fume, smoke and mist hazards in a bewildering variety… but one of the most profound differences between the pulmonary environment of males and females [is] the degree to which the lungs are exposed to tobacco smoke”. His investigation of 1200 TB patients and 979 age-matched general hospital patients found that non-smokers comprised 11.7% of the TB cohort compared to 21.0% of the control cohort, but did not include non-Europeans or adjust for any confounders [4]. In 1969, a study of 76 589 patients in Cheshire, UK, found that TB incidence was five times higher in current smokers compared to non-smokers, and twice as high in current smokers compared to ex-smokers, adjusted for age and sex [5].
In recent years, evidence for the links between CS and TB has continued to mount. A study of 78 000 deaths in India identified smoking as a cause of half the TB deaths reported in adult men, while a concurrent survey of 250 000 Indian men found that smokers were three times more likely to report a history of TB compared to non-smokers, adjusted for age, education level and tobacco chewing [6]. Another large-scale study of 42 655 elderly individuals in Hong Kong found pulmonary TB to be more common in smokers than non-smokers, with a dose-dependent relationship between smoking habits and disease development, adjusted for sex, age, alcohol use, marital status and education [7]. Since then, several meta-analyses and reviews of epidemiological studies have found that smoking is an independent risk factor for TB infection and disease, the development of severe TB, and mortality (table 1). In 2017, an estimated 17.6% of cases and 15.2% of deaths from TB in high-burden countries were directly attributable to smoking [17], while the WHO estimates that an estimated 0.9 million global TB cases were attributable to smoking as a major risk factor in 2018 [1].
TABLE 1.
Summary of systematic reviews on the effects of smoking on TB
| Systematic reviews on effects of smoking on TB | Confounders | Multivariate analysis |
|
Lin
et al., 2007 [8] Effect of smoking as a risk factor for TB infection, disease and mortality Confounders: TST cut-off for LTBI Alcohol consumption SES |
TST cut-off to diagnose LTBI | |
| Induration size of 5 mm | Pooled OR 2.08 (95% CI 1.53–2.83) |
|
| Induration size of 10 mm | Pooled OR 1.83 (95% CI 1.49–2.23) |
|
| Alcohol on risk of TB disease in current smokers | Summary estimate 1.62 (95% CI 1.15–2.29) |
|
| SES on risk of TB disease in current smokers | Summary estimate 1.95 (95% CI 1.45–2.61) |
|
| Alcohol on risk of TB disease in former smokers | Summary estimate 1.58 (95% CI 1.24–2.02) |
|
| SES on risk of TB disease in former smokers | Summary estimate 1.54 (95% CI 1.18–2.01) |
|
| Alcohol on risk of TB disease in ever-smokers | Summary estimate 2.00 (95% CI 1.55–2.57) |
|
| SES on risk of TB disease in ever-smokers | Summary estimate 3.28 (95% CI 2.25–4.76) |
|
| SES for risk of mortality from TB | Summary estimate 2.55 (95% CI 1.82–3.56) |
|
|
Slama
et al., 2007 [9] Effect of smoking as a risk factor for TB infection, disease and mortality Confounders: Quality of study as rated by three independent reviewers for 25 indicators of quality regarding study population, assessment of exposure to tobacco smoke, assessment of passive smoking, assessment of TB outcome, study design, analysis and data presentation |
Quality of study on risk of being infected with TB | |
| High-quality studies only | Pooled estimate 1.757 (95% CI 1.458–2.118) | |
| All studies | Pooled estimate 1.762 (95% CI 1.467–2.116) | |
| Quality of study on risk of TB disease | ||
| High-quality studies only | Pooled estimate 2.641 (95% CI 2.066–3.378) | |
| All studies | Pooled estimate 2.284 (95% CI 1.765–2.954) | |
| Quality of study on risk of TB mortality | ||
| High-quality studies only | Pooled estimate 1.347 (95% CI 1.107–1.638) | |
| All studies | Pooled estimate 2.236 (95% CI 1.340–3.732) | |
|
Wagnew
et al., 2018 [10] Effect of smoking on prevalence of TB Confounder: Diagnosis of diabetes |
Smoking on TB among diabetes patients | OR 7.6 (95% CI 1.46–39.53) |
|
Bates
et al., 2007 [11] Effect of smoking on TB infection, disease and mortality Confounders: None reported |
Effect of smoking on TB infection (no adjustment for confounders reported) |
Summary relative risk 1.73 (95% CI 1.46–2.04) |
| Effect of smoking on TB disease (no adjustment for confounders reported) |
Summary relative risk 2.29 (95% CI 1.93–2.71) | |
| Effect of smoking on TB mortality (no adjustment for confounders reported) |
Summary relative risk 1.60 (95% CI 1.31–1.95) | |
|
Burusie
et al., 2020 [12] Effect of smoking on TB treatment outcomes Confounders: Income category of the study country's economy (not individuals’ SES) |
Lower-middle income economy on effect of smoking on TB treatment outcomes | OR 1.74 (95% CI 1.31–2.30) |
| Upper-middle income economy on effect of smoking on TB treatment outcomes | OR 1.52 (95% CI 1.16–1.96) | |
| High-income economy on effect of smoking on TB treatment outcomes | OR 1.34 (95% CI 1.03–1.74) | |
|
Samuels
et al., 2018 [13] Effect of smoking on treatment outcomes in MDR-TB Confounders: None reported |
Effect of smoking on unsuccessful treatment outcomes for MDR-TB (no adjustment for confounders reported) | Pooled relative risk 0.94 (95% CI 0.75–1.19) |
|
Chaves Torres
et al., 2019 [14] Effect of smoking on TB treatment outcomes Confounders: None reported |
Effect of not smoking on favourable TB treatment outcomes (no adjustment for confounders reported) | OR 1.5 (95% CI 1.3–1.7) |
|
Patra
et al., 2015 [15] Effect of exposure to second-hand smoke on LTBI and active TB Confounders: Age Biomass fuel use SES Presence of TB patient in household |
Age on effect of exposure to second-hand smoke on LTBI | |
| Children | Summary relative risk 1.64 (95% CI 1.00–2.83) | |
| Adults | Summary relative risk 1.78 (95% CI 1.19–2.68) | |
| Age on effect of exposure to second-hand smoke on active TB disease | ||
| Children | Summary relative risk 3.41 (95% CI 1.81–6.45) | |
| Adults | Summary relative risk 1.32 (95% CI 1.04–1.68) | |
| Biomass fuel use on effect of exposure to second-hand smoke on LTBI | Summary relative risk 2.66 (95% CI 1.31–5.39) | |
| Biomass fuel use on effect of exposure to second-hand smoke on active TB disease | Summary relative risk 2.03 (95% CI 1.13–3.63) | |
| Presence of a TB patient in the household on effect of exposure to second-hand smoke on LTBI | Summary relative risk 2.03 (95% CI 1.25–3.28) | |
| Presence of a TB patient in the household on effect of exposure to second-hand smoke on active TB disease | Summary relative risk 1.87 (95% CI 1.30–2.69) | |
| Combined SES and age on effect of exposure to second-hand smoke on LTBI | Summary relative risk 1.11 (95% CI 0.54–2.31) | |
| Combined SES and age on effect of exposure to second-hand smoke on active TB disease | Summary relative risk 2.13 (95% CI 1.18–3.83) | |
|
Obore
et al., 2020 [16] Effect of smoking and exposure to second-hand smoke on contracting TB Confounders: None reported |
Effect of second-hand smoke exposure on risk of contracting TB (no adjustment for confounders reported) | Relative risk 2.15 (95% CI 1.419–3.242) |
| Effect of smoking on risk of contracting TB (no adjustment for confounders reported) | Relative risk 2.67 (95% CI 2.017–3.527) |
TB: tuberculosis; TST: tuberculin skin test; LTBI: latent tuberculosis infection; SES: socioeconomic status; OR: odds ratio; MDR: multidrug resistant.
Smoking is associated with a nine-fold greater risk of death associated with TB, which drops by as much as 65% upon smoking cessation [18]. The extent of an individual's smoking history also directly correlates with the risk of latent TB infection (LTBI) [19], while smoking cessation correlates with successful treatment of LTBI [20]. Smokers are at much greater risk of delayed diagnosis of TB [21], greater symptoms and bacterial load [22], unfavourable TB treatment outcomes [23], delayed recovery as measured via sputum conversion [24], more severe disease progression [25] and relapse after successful TB treatment [26]. An association between smoking and drug-resistant TB has recently been identified [27].
It is increasingly evident that there is a pressing need to evaluate interventions for TB in the context of smoking behaviour. The clinical impacts of CS exposure and TB are dependent on several complex factors, such as duration and severity of CS exposure, heterogeneity of TB disease, socioeconomic status and other demographic factors. Additionally, the timeframe required for the intersection of CS- and TB-damage can range from years to decades. These difficulties complicate attempts to elucidate the mechanisms by which CS exacerbates TB infection and disease. Furthermore, as a complex, multipartite mixture of >4500 components in both gaseous and particulate phases, CS acts variously as a damaging, pro-inflammatory stimulus and as an immunosuppressor. Many studies have examined the effects of individual components, such as nicotine, aryl hydrocarbon, acetylcholine and acrolein, but ultimately the biological impacts of CS exposure arise from prolonged exposure to all components combined. To best reflect clinically relevant interactions between CS and TB, this review gives primacy to investigations of the impact of whole CS on smokers, and CS or CS extract (CSE) on ex vivo human samples. Herein we summarise and evaluate current research to illuminate potential immune pathways underlying the interaction between CS and TB.
Smoking and innate immune responses to TB
Transmission and infection
TB is largely spread through the respiratory route via the inhalation of M. tuberculosis in droplets. The most common outcome is clearance of the mycobacteria, resulting in TB reactivity, which is asymptomatic and non-contagious. However, 5–10% of infected individuals progress to active disease. In active pulmonary TB, progressive aggregations of granulomas and tuberculous pneumonia form within the lungs. Over time, the granulomas caseate. While M. tuberculosis can infect most organs, pulmonary TB is the most significant form of the disease due to its high incidence, severity and infectious nature. Patients with active TB exhale droplets containing M. tuberculosis bacilli into the air that remain infectious for several hours, and only a few bacilli need to be inhaled for infection to develop. Larger TB droplets (>5 μm in diameter) land on the mucociliary escalator and are cleared from the airway without reaching the lungs. Smoking, however, results in slower mucociliary clearance, as well as ciliary dysfunction in the form of reduced cilia length, beat frequency and differentiation [28]. Subjects with primary ciliary dyskinesia experience recurrent respiratory infections, and sampling of their airways reveals an increased bacterial load of 104–109 CFU·mL−1 of species from >120 genera [29].
Smaller TB droplets (<5 μm) reach the alveoli, where M. tuberculosis infects airway alveolar macrophages (AMs) and airway epithelial cells (AECs). AMs have long undergone extensive investigation for their dual role in TB infection, being implicated in both the control and progression of TB disease, yet variable definitions of AMs have persisted in the literature over several decades, further complicating findings. For example, one notable study concluded that depletion of activated AMs in mice results in increased bacterial burden, but nonspecific AM depletion improves clinical outcomes [30], suggesting that AMs can only counteract TB infection under favourable conditions, and otherwise facilitate disease development. However, the method utilised depleted all activated macrophages, not simply AMs. A more recent study found that the selective depletion of AMs (Ly6G−MerTK+CD64+SiglecF+CD11c+) in mice results in a decrease in M. tuberculosis burden, while depletion of interstitial macrophages (Ly6G−MerTK+CD64+SiglecF−) results in an increase in bacterial burden [31].
M. tuberculosis is able to modulate macrophage polarisation and subvert their antimicrobial functions [32], which has recently been demonstrated in high resolution via single cell transcriptional analysis [33]. Thus, it has been shown that infected AMs (defined as CD11chighCD11blow cells from bronchoalveolar lavage (BAL) in [34] and adherence-purified cells from BAL in [35]) are able to localise to the lung interstitium and facilitate dissemination of M. tuberculosis to neutrophils, monocytes and dendritic cells (DCs) [34, 35]. Smoking greatly increases the number of AMs (defined via morphological examination) present in the lungs and airways [36], and this may exacerbate TB infection by providing additional niches for M. tuberculosis growth. Within this smoke-induced AM population, it is likely that the proportion of cells is skewed towards newly recruited macrophages compared to tissue-resident macrophages. This has been shown in a murine study of CS exposure, which revealed significant increases in monocyte-derived AMs (CD64/MertK+CD11c+CD11b+MHCII−) in both the BAL fluid (BALF) and lung tissue observable from 2 weeks of CS exposure and persisting to 24 weeks [37]. While tissue-resident macrophages may contain M. tuberculosis infection initially, mycobacterial phenolic glycolipids initiate monocyte chemoattractant protein-1 (MCP-1)-mediated recruitment of immature, permissive macrophages to the site of infection, which facilitate bacterial transmission and spread [35]. Increased MCP-1 levels are present in the sputum [38] and BALF from smokers [39], and, when comparing markers of macrophage maturity and differentiation in the BALF of smokers versus non-smokers, decreased CD31, CD91, CD44 and CD71 expression is evident on AMs (SSChighECAlow cells from BAL) [40]. Thus, it is likely that the population of activated and functional AMs in the lungs of smokers is diluted by the influx of permissive AMs that are amenable to M. tuberculosis infection. Interestingly, the mechanism by which smoking leads to such extensive and prolonged AM accumulation has been linked to inflammation [41], oxidative stress [42] and the action of CS-derived aldehydes and carbonyls on extracellular matrix in the lungs, leading to AM retention and entrapment [43]. It is evident that smoking results in wide-ranging changes to the lung microenvironment that impact on TB infection and disease progression.
Investigation of healthy young smokers via video bronchoscopy, mucosal biopsy and bronchial lavage has revealed significant increases in vascular hyperplasia, submucosal oedema, inflammatory cell infiltrates and goblet cell hyperplasia compared to non-smoking controls [44]. Within this inflamed environment, levels of ATP, caspase 1, interleukin (IL)-1 and IL-18 are elevated [45–47] and contribute to increased alveolar permeability. This increase in permeability associated with elevated levels of IL-1 results in the movement of infected AMs (SiglecF+CD11+) from the alveoli into the interstitial space [48]. Thus, smoking is likely to enrich intracellular niches for M. tuberculosis infection that facilitate pulmonary spread.
AM function
In addition to increasing AM retention and translocation to the interstitial space, smoking also impairs the function of AMs. It is important to note first, however, that the majority of studies using human BAL use adherence purification to identify AMs, and thus these populations can include recently migrated interstitial macrophages and adherent monocyte-derived macrophages (MDMs). An early investigation of human BAL cells revealed that AMs from smokers had impaired phagocytic ability, higher resting energy requirements and were larger, containing residual bodies with fibrous inclusions [49]. Intriguingly, smoke particles accumulate in the lysosomes of AMs, and lysosome-engorged AMs from the BALF of smokers exhibited impaired migration to M. tuberculosis in transwell assays in vitro [50]. This raises the possibility that despite increased AM numbers, the lungs of smokers may be more permissive to growth of M. tuberculosis. Later studies examined BAL-derived AMs from smokers, identified by light-scattering properties via flow cytometry, and revealed reduced expression of the adhesion molecules CD11a and CD54 and the proliferation/maturation marker CD71, and lower metabolic activity [51]. There was also reduced expression of CD31, CD91 and CD44 and reduced phagocytosis of apoptotic bronchial epithelial cells [40].
Two studies point towards impaired phagocytosis of M. tuberculosis as a result of smoking. One investigation demonstrated that circulating monocytes and macrophages from TB patients have reduced phagocytic ability, which is further reduced in TB patients who smoke [52]. A later study revealed reduced M. bovis bacille Calmette–Guérin (BCG) uptake in human MDMs from smoking TB patients compared to non-smoking TB patients [53]. Additionally, investigation of human MDMs and murine macrophages found that CSE and CS exposure impairs cytoskeletal changes of these cells and the actin rearrangement required for phagocytosis [54]. M. tuberculosis uptake by AMs is mediated by multiple receptors including surfactant protein (Sp)-A and Sp-D, Fc, complement and mannose receptors [55]. Studies in smokers with and without chronic obstructive pulmonary disease (COPD) have revealed reduced Sp-A and Sp-D in the BALF of both smoking groups [56, 57], but no significant impact of smoking on Fc receptors has been reported to date.
Because Sp-D-mediated M. tuberculosis uptake has been shown to enhance phago-lysosomal fusion and intracellular killing [58], this is one mechanism by which smoking could impair M. tuberculosis phagocytosis and control. Toll-like receptor-2 (TLR-2) is increased in the airways in experimental and human COPD [59] but is reduced in AMs isolated from smokers [60], supporting potentially reduced AM activation as a result of smoking. Of particular note are reported increases in complement receptor 3 (CR3) expression on the AMs of smokers [61, 62], because CR3-mediated phagocytosis of M. tuberculosis does not result in macrophage activation or phago-lysosomal fusion [63], thus facilitating increased intracellular M. tuberculosis survival. One recurring theme that emerges from examining the interaction of smoking and TB is the systematic impairment of early responses to infection, allowing M. tuberculosis to more easily establish an initial foothold.
Once macrophage receptors bind to their ligands on the surface of M. tuberculosis, invagination and budding of the cell membrane leads to uptake of the mycobacteria into the phagosome, where several M. tuberculosis surface lipids and secreted protein effectors prevent phago-lysosomal fusion [64]. This arrest allows M. tuberculosis to avoid hydrolytic degradation while retaining access to nutrients and endosomal transport systems to replicate and persist. Circumvention of phago-lysosomal fusion also delays normal processing and presentation of mycobacterial antigens to trigger adaptive immune responses [65]. There is little information on how smoking impacts the intracellular processing of M. tuberculosis; however, following phagocytosis of yeast, phago-lysosomal fusion in AMs (defined as adherence-purified cells from BAL) was halved in smoking versus non-smoking rats, although the mechanism involved was not elucidated [66].
Smoking further debilitates AM control of M. tuberculosis. A key study by O’Leary et al. [67] in 2014 demonstrated that AMs from smokers, isolated via adherence purification, were unable to inhibit the intracellular growth of the non-virulent H37Ra strain of M. tuberculosis in vitro. AMs from the BALF of smokers exhibited reduced expression and excretion of tumour necrosis factor (TNF), IL-1β, IL-6, RANTES and IL-8 following stimulation with TLR-2 and TLR-4 agonists, but not activation of TLR-3. Induction of IL-1R-associated kinase-1 (IRAK-1), p38 phosphorylation and NF-κB activation by TLR-2 and TLR-4 was also suppressed in smokers [68]. Therefore, both the expression of TLR-2 and TLR-4 and signalling through these receptors are downregulated by smoking. BALF from smokers contains higher levels of C-C motif chemokine ligand 22 (CCL22), IL-4, IL-13 and IL-10 compared to that of non-smokers, indicating an immuno-inhibitory cytokine profile that was recapitulated by mRNA analysis in a murine smoking model [69]. Furthermore, infected AMs from smokers produced significantly less interferon-γ (IFN-γ), TNF and IL-1β compared to those from non-smokers [67]. Similarly, a study of human AMs exposed to CSE in vitro found that the production of IFN-γ, TNF and IL-10 in response to BCG stimulation was impaired, as was the containment of BCG replication by AMs [70]. Both IFN-γ and TNF are essential for the control of M. tuberculosis infection, while IL-1β also contributes to the inhibition of growth [71]. IFN-γ induces autophagy, phagosomal maturation and the production of antimicrobial peptides against M. tuberculosis in human macrophages, and these activities are dependent on vitamin D [72]. Both TB [73] and smoking [74] are associated with vitamin D deficiency, which has far-reaching effects on adaptive immune responses. Interestingly, vitamin D deficiency is associated with the progression of LTBI to active disease [75]; however, vitamin D supplementation does not prevent the development of TB reactivity in children [76].
Smoking significantly influences macrophage phenotype switching and the ratio of M1 (CD68+) and M2 (CD163+Arg1+) macrophages in the lungs [69]. While M. tuberculosis infection alone predisposes macrophage polarisation to the M2 phenotype as infection progresses [77], a comparison of AMs from smoking and non-smoking TB patients revealed an even greater skewing towards M2 AMs (defined as cells from BAL) in smokers [69]. M1 macrophages activate inducible nitric oxide synthase (iNOS) that mediates bacterial killing, while M2 macrophages activate arginase pathways for cell proliferation, collagen biosynthesis and tissue repair/remodelling [78]. In TB, M1 macrophages promote bactericidal activity and granuloma formation and M2 macrophages suppress these effects [79]. In later stages of TB disease, M2 polarisation develops with higher levels of IL-4, IL-10, transforming growth factor-β and IL-13 that correlate with disease severity [80, 81]. Comparisons of patients with drug-susceptible TB and MDR-TB or extensively drug-resistant TB (XDR-TB) show that M2 polarisation is significantly higher in MDR-TB/XDR-TB patients [82]. It is likely that smoking may accelerate and intensify the switch from M1 to M2 phenotypes and worsen TB disease progression and outcomes.
Smoking also impairs autophagy, an intracellular process that recycles protein aggregates and turns over cytoplasmic organelles. A study of BAL AMs from smokers revealed extensive accumulation of autophagosomes that these cells were unable to traffic to the lysosome, leading to reduced intracellular protein clearance [83]. Autophagy is a significant defence mechanism for AM elimination of M. tuberculosis. Enhanced stimulation of autophagy in human MDMs can overcome the mycobacteria-induced blocking of phago-lysosomal fusion and reduce the intracellular survival of BCG [84]. Thus, smoking may further compromise AM defences against M. tuberculosis infection. Mitochondrial architecture and function are also disrupted as a result of this impaired recycling of cellular protein [83]. An examination of human AMs revealed that smoker AMs have reduced metabolic activity, glycolytic reserve and reserve respiratory capacity compared to non-smoker AMs. Glycolytic response is further reduced upon infection with M. tuberculosis [85]. This is significant because the transition from active to chronic TB in mice is accompanied by enhanced glucose uptake and glycolysis within granulomatous lesions [86]. Thus, reduced glycolytic reserve due to CS exposure may abrogate energy-intensive antimycobacterial responses to TB disease.
Exposure of macrophages to CS in vitro results in upregulation of glycolysis and increased production of reactive oxygen species (ROS) [87]. While M. tuberculosis is resistant to the oxidative bursts, these can cause damage to host lung tissue. AMs from smokers release more superoxide anion (O2−) than those from non-smokers, despite increased intracellular superoxide dismutase, and this increase in O2− is linked to AM-induced fibroblast lysis [88]. AMs from smokers also produce higher levels of ROS associated with reduced antioxidant activity, specifically Cu-superoxide dismutase, Zn-superoxide dismutase, glutathione S-transferase and glutathione peroxidase. This contributes to the pathogenesis of smoking-related respiratory tissue injury [89].
Oxidative stress not only induces lung tissue damage, but also impacts the function of AMs. Exposure of rats to CS resulted in increased levels of AM (defined as adherence-purified cells from BAL) cell death [90]. In the same study, further examination using mouse, rat and human BAL AMs treated with CSE in vitro revealed increased oxidative stress, mitochondrial dysfunction and cell death, but also showed that vitamin C and vitamin E treatment inhibited CSE-induced cell death [90]. These findings were recapitulated in a murine study that showed that antioxidant vitamin treatment reduced TNF and NFκB activation in AMs (defined morphologically from BAL cells) [91]. It is notable that antioxidant levels decrease in blood plasma during active TB disease and increase with treatment [92]. Furthermore, there is suggestive evidence that vitamin C supplementation (along with vitamin A and β-carotene) may reduce the risk of TB in current smokers [93]. Alveolar fluid from smokers is deficient in vitamin E compared to that of non-smokers [94], and vitamin E deficiency is associated with increased risk of TB disease among household contacts of TB patients [95]. These results, alongside the effects of vitamin D deficiency, highlight the intersection between smoking and malnutrition as risk factors for TB.
To summarise, AMs are positioned where the lung is exposed to M. tuberculosis and the toxic effects of CS. They are the first cells to harbour M. tuberculosis and represent an evolutionary battleground wherein host defence mechanisms are matched with pathogen evasion and subversion strategies. Smoking may tip the balance in favour of M. tuberculosis by influencing the accumulation and phenotype of AMs and impairing AM metabolism, maturation, activation, phagocytosis, cytokine production and bactericidal activities. Both the increase in AM number and their significant functional impairment primes for the proliferation and dissemination of M. tuberculosis.
Neutrophils
M. tuberculosis-infected AMs facilitate the spread of mycobacteria to neutrophils, monocytes and DCs within the alveoli and lung interstitial space. At this stage of infection, neutrophils directly inhibit or mediate clearance of M. tuberculosis in concert with AECs and AMs. Neutrophils are activated by M. tuberculosis and pro-inflammatory cytokines, triggering degranulation and release of ROS, proteases, antimicrobial peptides and DNA, and neutrophil extracellular traps (NETs). However, M. tuberculosis is able to evade or subvert many of these bactericidal activities. Following exposure to neutrophil ROS, M. tuberculosis induces neutrophil necrosis, triggering a permissive growth cascade. AMs and interstitial macrophages remove necrotic neutrophils via efferocytosis to protect surrounding tissue from bioactive neutrophil molecules, and the uptake of these infected, necrotic cells promotes intracellular M. tuberculosis growth [96]. Smoking downregulates AM efferocytosis of neutrophils via modification of extracellular matrix proteins with reactive carbonyls [97], and this promotes extracellular M. tuberculosis growth and spread, as well as tissue damage from spilled neutrophil cell contents.
Smoking is a major inducer of neutrophilic inflammation, and BAL and lung biopsies of smokers reveal significant increases in neutrophil influx [98]. One study using the human AEC line BEAS-2B and a murine model of CS exposure found that this influx of neutrophils was due to AEC necroptosis and damage-associated molecular pattern release, leading to pro-inflammatory CXCL8 and IL-6 production by neighbouring AECs [99]. Another showed that necroptosis was increased in human and experimental COPD, including in lung macrophages, in association with neutrophilic and granulocytic airway inflammation [100]. Genetic or pharmacological inhibition of necroptosis reduced airway inflammation, remodelling and emphysema. Smoke exposure also impairs neutrophil function and activity. In vitro exposure of human neutrophils to CSE compromised their bactericidal activity against Staphylococcus aureus, as well as increased degranulation and atypical cell death [101]. It also suppressed caspase-3 activity, leading to reduced neutrophil phagocytosis of heat-killed Escherichia coli. Further, in vitro exposure of murine neutrophils to total particulate matter from CS reduces neutrophil STAT1 activation, leading to decreased TNF, iNOS and bactericidal activity against E. coli [102].
These effects of smoking on neutrophils impact on their contribution to the control of TB infection. Neutrophils form NETs when incubated with M. tuberculosis in vitro, and both the sputum [103] and plasma [104] of active TB patients contain NETs. M. tuberculosis-induced NET formation is dependent on phagocytosis and ROS production, and leads to the activation of adjacent macrophages via Hsp72 [105]. Thus, NET formation is a component of neutrophil-mediated defence against TB infection. CS exposure impairs the translocation of the NADPH oxidase protein p67phox and ROS production in human neutrophils in vitro [106] and inhibits NET release, as well as reduces neutrophil speed, velocity and directionality [107]. Thus, it is likely that CS-induced NET dysfunction undermines neutrophil responses to M. tuberculosis. Moreover, during active TB, neutrophils are the most abundant and highly infected cell population within patient sputum and BAL [108], where they express high levels of programmed cell death 1 ligand 1 (PD-L1) [109] that interacts with programmed cell death 1 (PD-1) on lymphocytes to downregulate their function and cause programmed cell death. There has been one short report on the correlation between smoking and increased neutrophil surface PD-L1 density in HIV patients [110], which requires further examination, and may shed light on another means by which combined smoking and TB worsen patient outcomes.
Thus, CS exposure impairs both neutrophil antimycobacterial activities and the efferocytosis of necrotic M. tuberculosis-infected neutrophils, promoting extracellular M. tuberculosis growth and spread. However, few studies have demonstrated the direct impact of CS on neutrophils during TB in vivo, and the interaction between CS-exposed neutrophils and the adaptive immune system also requires more investigation.
Smoking and adaptive immune responses to TB
Dendritic cells
DCs are the intermediaries between the innate and adaptive immune systems, and are responsible for initiating T-cell-mediated responses to infection. DC quantity and quality in lymph nodes determine both the magnitude and type of T-cell response, or T-helper (Th) cell polarisation. In the context of TB, Th1 immunity is required for the control of M. tuberculosis infection, while Th2 immunity downregulates Th1 activities, such as phagocytosis, and favours asthma- and allergy-type inflammation. It is well established that smoking decreases DC numbers, impairs DC maturation and diminishes Ag-specific CD4+ T-cell proliferation in lung tissues [111, 112]. M. tuberculosis also manipulates the host DC response in detrimental ways. Activation of M. tuberculosis-specific T-cells is dependent on the migration of infected DCs from the lungs to the mediastinal lymph nodes, but this migration is delayed during TB infection [113]. This delay between infection and T-cell recruitment results in M. tuberculosis establishing a productive infection in the lung and disseminating to other tissues. Uptake of M. tuberculosis by DCs is mediated by DC-SIGN (CD209) and results in the downregulation of T-cell-activating IL-12 production in favour of immunosuppressive IL-10 production [114]. Nicotine also reduces IL-12 production in DCs [115]. The lack of IL-12 downregulates CCR7 expression in DCs and, thus, their migration to the lymph nodes [116]. Additionally, M. tuberculosis-infected DCs have reduced major histocompatibility complex class II (MHCII) antigen presentation [117] and inefficient CD4+ T-cell activation, so that only bystander DCs can effectively stimulate M. tuberculosis-specific T-cell responses [34].
This M. tuberculosis-mediated inhibition of DC function is exacerbated by smoking. CS-exposed mice challenged with M. tuberculosis infection had fewer IL-12-producing and TNF-producing DCs but more IL-10-producing DCs in their lungs compared to control mice. This was linked to reduced influx of IFN-γ-producing and TNF-producing CD4+ and CD8+ effector and memory T-cells. As a result, CS-exposed mice were more permissive to M. tuberculosis, with an increased bacterial load in their lungs and spleens compared to control mice [118]. Thus, M. tuberculosis replicates less effectively within DCs [119], but there is impaired DC initiation of T-cell-mediated immunity. Interestingly, smoking also shifts the ratio of CD4+ to CD8+ T-cell activation in mice, from 3:1 to 1.5:1 [120]. In vitro examination of murine bone marrow-derived DCs (BMDCs) demonstrated that CSE exposure induces CCL3 and C-X-C motif chemokine ligand 2 (CXCL2) production by DCs and significantly increases DC-induced proliferation of CD8+ T-cells [121]. The lower CD4+/CD8+ ratio correlates with a higher TB incidence rate in HIV-positive individuals [122], but the significance of this skewing in HIV-negative TB patients on the risk of TB is uncertain.
Once DCs migrate to the draining lymph nodes, Th cell polarisation is dictated by the cytokines and microenvironment present when naïve CD4+ T-cells are primed by DCs [123], and smoking also influences this process. In vitro exposure of human peripheral blood mononuclear cell (PBMC)-derived DCs to nicotine results in a decrease in Th1 differentiation and expansion [124], which is detrimental for host control of TB infection. Smoking also skews T-cell differentiation towards Th17 responses and IL-17A production [125]. This is intriguing because IL-17A is a potent neutrophil chemokine and is protective against M. tuberculosis infection [126] but IL-17A overproduction is also strongly associated with lung pathology and the development of COPD [127]. CS-exposed mice exhibit significant increases in Th17 cells, IL-17A production and IL-27 production in the lungs. Further, in vitro co-culture experiments revealed that BMDCs from CS-exposed mice enhanced the Th17 differentiation of CD4+ T-cells, and this was dependent on CD40 expression that was upregulated in BMDCs from CS-exposed mice [128]. Thus, long-term smoking and the resultant increase in IL-17A may result in damaging neutrophilia, which enhances lung pathology.
In summary, CS exposure decreases Th1 and increases Th2 cytokine secretion in DCs, limiting Th1 T-cell expansion and enhancing host susceptibility to M. tuberculosis. CS exposure of DCs also results in greater Th17 differentiation, which may underpin increased inflammation and lung damage. Direct evidence of the impact of CS on DCs in the context of TB is lacking, and additional investigations such as adoptive transfer studies can help to elucidate this.
T-cells
The critical role that T-cells play in the control of M. tuberculosis infection has been clearly demonstrated in non-human primate studies [129] and in HIV patients where the loss of CD4+ T-cells correlates with dramatically increased susceptibility to TB. Several different T-cell populations participate in the immune response to TB. CD4+ T-cells recognise MHCII-presented antigens and largely undergo Th1 polarisation, producing IFN-γ and TNF to activate other immune cells. Th1 CD4+ T-cells are essential for M1 macrophage activation, while Th17 CD4+ T-cells recruit neutrophils for early granuloma formation and M. tuberculosis control. CD8+ T-cells recognise MHCI-presented antigens, secreting perforins and granulysin to directly kill M. tuberculosis-infected cells.
CS exposure has several detrimental impacts on T-cell function and proliferation, and induces T-cell apoptosis [130]. Passive smoking correlates with increased prevalence of naïve T-cells in the blood of adolescents [131], and CD4+ T-cell numbers are lower in the blood and BALF of smokers than non-smokers [132]. Nicotine exposure alone in rats impairs antigen-mediated signalling in splenic T-cells, as evidenced by T-cell anergy, decreased T-cell receptor-induced Ca2+ elevation and decreased T-cell proliferation [133, 134]. These results have also been recapitulated in rats and mice exposed to CS [135]. In the case of CD4+ T-cells, smoking decreases IFN-γ, IL-2 and TNF production in the lungs [136, 137]. This effect is linked to the CS-induced downregulation of CD28 and upregulation of cytotoxic T-lymphocyte associated protein 4 (CTLA-4) expression in CD4+ T-cells in mice [138]. The negative effects of CS on T-cell function may affect the control of TB infection but, to date, few studies have examined this combination in vivo. One such study demonstrated that CS exposure followed by M. tuberculosis infection in mice decreased IFN-γ production by lung T-cells ex vivo, which was associated with an increased bacterial burden in the lungs [139].
This CS-induced dampening of Th1 responses to M. tuberculosis can be linked to IL-12, IL-4 and IL-13. In addition to supporting Th1 differentiation of CD4+ T-cells during DC stimulation, IL-12 is required for the maintenance of Th1 effector CD4+ T-cells during TB infection [140]. However, the BALF of current smokers contains significantly less IL-12 than that of controls [141]. A combined in vitro study of CSE-treated human monocyte-derived DCs and in vivo study of lung DCs from CS-exposed mice also observed impairment of DC IL-12 production [142]. This effect may also be systemic, because human PBMCs treated with CS-conditioned medium produce significantly less IL-12 upon stimulation than control cells [143]. Conversely, the Th2-associated cytokines IL-4 and IL-13 actively suppress Th1 functions, and these are upregulated in response to CS. Plasma IL-4 is elevated following exposure to passive smoking [144], and PBMCs from smokers produce more IL-4 upon stimulation, correlating with the number of cigarettes smoked per day [145]. A comparison of lung tissue sections found significantly increased IL-4 and IL-13 expression in the central airways of smokers compared to non-smokers, and this is further increased in smokers with chronic bronchitis [146]. Lung sections from CS-exposed rats revealed similar results, with increased IL-13 expression correlating with increased smooth airway actin and collagen content [147]. In M. tuberculosis infection, increased Th2 responses, such as IL-4 and IL-13, subvert bacterial containment and control, and result in increased lung damage [148]. One study found increased IL-4+CD4+ and reduced IFN-γ+CD4+ T-cells in the lungs of CS-exposed mice infected with BCG compared to control mice, and this was associated with increased bacterial burden in the lungs and spleens [149].
Smoking also enhances regulatory T-cell (Treg) activation. M. tuberculosis-specific FoxP3+ Tregs proliferate and accumulate early at sites of infection [150]. These cells are unable to restrict the growth of M. tuberculosis and in fact attenuate pulmonary immune responses to infection by suppressing T-cell proliferation and DC maturation. There are increased FoxP3+ Tregs in the large airways of smokers, which correlates with smoking pack-years, but reduced FoxP3+ Tregs in the smaller airways, which negatively correlates with airflow obstruction [151]. In addition, M. tuberculosis-infected AMs from both smokers and non-smokers induce increased FoxP3+ expression on naïve T-cells, but AMs are much more abundant in smokers and drive this regulatory phenotype in a dose-dependent manner [67]. FoxP3+ Tregs correlate with MDR-TB and increased IL-10 production, but decline during successful TB treatment [152]. Moreover, increased Tregs in guinea pigs leads to increased M. tuberculosis burden [153], and co-culture of Tregs with T-effector cells and M. tuberculosis-infected AMs significantly increases bacterial survival [154]. Therefore, the smoking-induced increase in Tregs could compromise host control of TB, although direct evidence is needed to support this.
CS-induced T-cell exhaustion may also influence the control of TB infection. There are significantly more circulating PD-1+ T-cells associated with T-cell exhaustion in smoking COPD patients than in healthy controls, and Tregs from these PBMCs express more CTLA-4 and suppress antigen-specific T-cell proliferation to a greater degree than those from healthy controls [155]. Expression of the immune exhaustion markers PD-1, T-cell immunoglobulin domain and mucin domain 3 (Tim3) and CTLA-4 is significantly higher in CD4+ and CD8+ T-cells of smoking compared to non-smoking HIV-infected subjects, and antigen-specific T-cell cytokine responses are significantly decreased in smoking HIV-infected subjects [156]. Nevertheless, PD-1+ T-cells are not exhausted or anergic in TB infection, and in fact proliferate extensively to maintain a productive, effector T-cell response to M. tuberculosis [157]. In fact, anti-PD-1-treated macaques succumb to TB quickly [158], and anti-PD-1 immunotherapy in humans can lead to reactivation and exacerbation of TB [159]. Therefore, the specific effects of CS-induced PD-1 upregulation on M. tuberculosis infection are yet to be defined.
With these multifaceted impacts on adaptive immunity during TB infection, the question of how smoking impacts vaccination against TB is a pressing one. Only one study has attempted to address this gap in knowledge. CS-exposed mice were administered a DNA vaccine expressing culture filtrate protein 10 (CFP10) and lysosomal integral membrane protein II, and this resulted in significantly fewer CFP10-responsive IFN-γ+ lung cells than in control mice [139], but the impact of smoking on vaccine-induced protection was not reported. There is an urgent need to update or augment the current BCG vaccine to induce longer-term protection against TB, which wanes during adolescence after infant immunisation. Considering the intersection of global TB burden and smoking incidence, it will be important to investigate the impacts of CS on vaccine-induced immunity against TB.
In summary, CS results in impairment of T-cell proliferation and signalling, and specific dampening of Th1 in favour of Th2 and Th17 responses, with implications for the effect of concurrent smoking on TB. Nevertheless, few studies have examined this interaction in vivo, and more studies are required to determine the connections between TB and CS-induced T-cell polarisation, exhaustion and regulation.
Future directions
Although TB models are well established and characterised, models of CS exposure in vivo are labour intensive and time-consuming, and lack standardisation regarding type of cigarette, dose of total suspended particles or total particulate matter, period of exposure and delivery system [160]. Perhaps as a result, in vivo studies directly examining combined CS exposure and TB are limited, with only three mouse studies published to date (table 2).
TABLE 2.
Comparison of combined CS exposure and M. tuberculosis infection experiments in vivo
| CS exposure | TB infection | Key findings | Reference |
| Female C57BL/6 Whole body exposure Teague Enterprises, Davis, CA, USA 1× 1R4F cigarette 2 h exposure twice per day 5 days per week for 6 weeks |
10–25 CFU Mtb Erdman via aerosol No CS exposure post-infection Harvest 28 days post-infection |
CS exposure results in: ↓ frequency of IFN-γ+ T-cells in lungs and spleens ↓ c-Jun, ATF2 and CREB expression in lungs ↑ Mtb burden in lungs ↓ Mtb antigen-specific T-cell responses post-vaccination | [139] |
| Female C57BL/6 mice Whole body exposure Teague Enterprises, Davis, CA, USA 170–180× 3R4F cigarettes 5 h exposure twice per day 5 days per week for 14 weeks |
10–25 CFU Mtb Erdman via aerosol No CS exposure post-infection Harvest 1, 7, 14 and 30 days post-infection |
CS exposure results in: ↓ number of lung macrophages and DCs producing TNF and IL-12 ↑ number of lung macrophages and DCs producing IL-10 ↓ number of CD4+ T-cells producing IFN-γ and TNF in lungs and spleens ↑ Mtb burden in lungs and spleen from day 14 post-infection ↑ Mtb lung lesion area in lungs | [118] |
| Female C57BL/6 mice Whole body exposure SIU-48, Promech Lab AB, Vintrie, Sweden 12× 2R4F cigarettes 50 min exposure twice per day 5 days per week for 6 weeks |
104 CFU Mtb H37Rv intranasally No CS exposure post-infection Harvest 28 and 42 days post-infection OR 0.5×106 CFU BCG intratracheally CS exposure post-infection Harvest 28 days post-infection |
CS exposure results in: ↓ number of CD4+IFN-γ+ T-cells in lungs ↑ frequency of CD4+IL-4+ T-cells in lungs ↓ RANTES concentration in BALF ↓ TNF, IL-12, IFN-γ and NO production by lung mononuclear cells ↑ Mtb burden in lungs and spleens ↓ Mtb granuloma size, number and cellular infiltration | [149] |
CS: cigarette smoke; TB: tuberculosis; CFU: colony-forming units; IFN: interferon; ATF2: activating transcription factor 2; CREB: cAMP response element-binding protein; Mtb: Mycobacterium tuberculosis; DC: dendritic cells; TNF: tumour necrosis factor; IL interleukin; CD: cluster of differentiation; BCG: bacille Calmette–Guérin; BALF: bronchoalveolar lavage fluid; NO: nitric oxide.
The major limitation of these studies is the lack of specialised smoking equipment housed within BSL3 facilities, and thus the inability to continue CS exposure post-TB infection. Additionally, no in vivo study has examined the impact of CS on TB treatment, the reactivation of LTBI or the protective efficacy of vaccination against TB. It is evident that further in vivo research is required, particularly multi-omics studies, to better and more holistically resolve the molecular mechanisms underpinning CS-induced susceptibility to TB disease, and the poorer outcomes experienced by smoking TB patients. Transcriptomic, proteomic and metabolomic analysis of CS-exposed, TB-infected animals will provide a much broader understanding of the widespread biological changes in response to both.
Beyond CS, many other sources of environmental damage, such as air pollutants and biomass smoke from burning fuel, have comparable impacts on TB [9]. The emergence of e-cigarettes and vaping is another facet of the interaction between smoking and TB [161] that requires further monitoring and investigation.
Concluding remarks
The significance of smoking cannot be overlooked when aiming to reduce the incidence and spread of TB. CS exposure is common in areas of high prevalence of TB. CS has complex and manifold impacts on every component of the pulmonary immune response to TB infection, but direct investigation of this interaction is severely limited regarding the adaptive immune response (figure 3). Although the reason that smoking so greatly exacerbates an individual's susceptibility to TB infection, disease, disease severity and treatment failure is outlined in broad strokes, further research is needed to elucidate the many gaps in knowledge regarding specific immunological mechanisms, which would lead to improved treatments.
FIGURE 3.
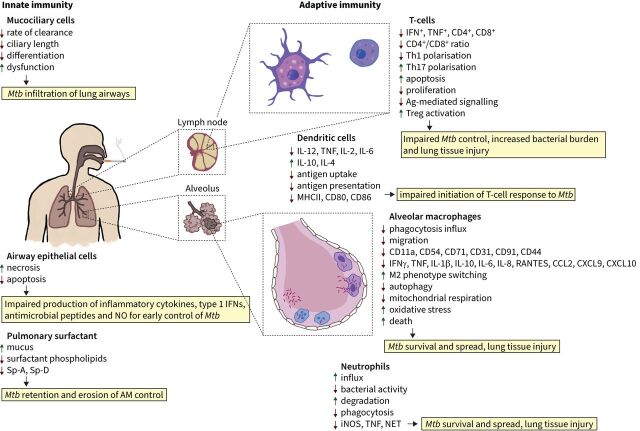
The effects of cigarette smoke (CS) exposure on innate and adaptive immunity that may influence the control of Mycobacterium tuberculosis (Mtb) infection. IFN: interferon; NO: nitric oxide; Sp: surfactant protein; AM: alveolar macrophage; TNF: tumour necrosis factor; CD: cluster of differentiation; Th: T-helper cell; Ag: antigen; Treg: regulatory T-cell; IL: interleukin; MHC: major histocompatibility complex; CCL: C-C motif chemokine ligand; CXCL: C-X-C motif chemokine ligand; iNOS: inducible nitric oxide synthase; NET: neutrophil extracellular traps.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of Interest: D.H. Quan reports grants from NHMRC Centre of Research Excellence in Tuberculosis Control (TB-CRE) Seed Funding “How does cigarette smoking impact the progression of TB disease and efficacy of TB vaccination?” (2019), and travel support from Centenary Institute Travel Award 2018, outside the submitted work.
Conflict of interest: A.J. Kwong has nothing to disclose.
Conflict of interest: P.M. Hansbro reports NHMRC APP1175134 Investigator Grant “Developing new preventions and treatments for chronic respiratory diseases” (2020–2024), for the present manuscript; and NHMRC APP1179092 Project grant “Development of a novel effective therapy for asthma and COPD” (2020–2022), NHMRC APP1156589 Project grant “Developing new treatments for COPD”, MRC-UK Project grant “New treatments for COPD” (2018–2021), NHMRC APP1137995 Project grant “Elucidating the role and potential for therapeutic targeting of TLR7 in emphysema and COPD” (2018–2022), NHMRC APP1138004 Project grant “Defining the roles and targeting interferon-epsilon as a new therapy for influenza in asthma and COPD” (2018–2022), outside the submitted work.
Conflict of interest: W.J. Britton reports grants from Australian National Health & Medical Research Council Centre of Research Excellence on TB Control 2018–2023 (APP1153493), for the present manuscript; grants from Australian Medical Research Future Fund (MRFF) MRF2007221 “A single dose, globally accessible vaccine to combat emerging SARS-CoV-2 variants” (2021–2023), MRFF RRDHI000011 “Integrating remote monitoring technology into digital healthcare” (2020–2021), MRFF APP1200755 “Pathway to the elimination of antibiotic-resistant and latent tuberculosis in the Pacific: the PEARL study” (2020–2024), Perpetual Trustees IPAP2019/1369 “Visualizing how the immune system controls infection and inflammation”, The Philip Bushell Foundation “New vaccines and biomarkers for the control of Tuberculosis” (2019–2023), Perpetual Trustees IPAP2019/1369 “Visualizing how the immune system controls infection and inflammation”, Australian Respiratory Council “Protecting the lungs against tuberculosis by pulmonary delivery of a novel TB vaccine” (2019); support for travel from BM Gates Foundation Collaboration for TB Vaccine Development Annual Scientific Symposium, Seattle, June 2019; patents filed for institution and investigators on Triccas JA, Pinto-Nadanachandran R, Britton WJ in June 2014 “Prevention and treatment of mycobacterium infection”, filed in EU (12859440.5), US (14/367854), AU (2012357637), China (CN 104271744A) and India (5865/DELNP/2014); and unpaid leadership role on Board of Trustees of The Leprosy Mission International (based in London), 2017 to present; outside the submitted work.
Support statement: We acknowledge support from the National Health and Medical Research Council Centre of Research Excellence in TB Control (APP1153493) to W.J. Britton and Investigator grant (APP1175134) to P.M. Hansbro. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.World Health Organization . Global Tuberculosis Report 2020. Geneva, World Health Organization, 2021. [Google Scholar]
- 2.Burki TK. The global cost of tuberculosis. Lancet Respir Med 2018; 6: 13. doi: 10.1016/S2213-2600(17)30468-X [DOI] [PubMed] [Google Scholar]
- 3.Global Health Observatory . Estimates of Current Tobacco Use, Tobacco Smoking and Cigarette Smoking (Age-standardized). Geneva, World Health Organization, 2018. [Google Scholar]
- 4.Lowe CR. An association between smoking and respiratory tuberculosis. Br Med J 1956; 2: 1081–1086. doi: 10.1136/bmj.2.5001.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rimington J. Chronic bronchitis, smoking and social class. A study among working people in the towns of Mid and East Cheshire. Br J Dis Chest 1969; 63: 193–205. doi: 10.1016/S0007-0971(69)80019-7 [DOI] [PubMed] [Google Scholar]
- 6.Gajalakshmi V, Peto R, Kanaka TS, et al. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35000 controls. Lancet 2003; 362: 507–515. doi: 10.1016/S0140-6736(03)14109-8 [DOI] [PubMed] [Google Scholar]
- 7.Leung CC, Li T, Lam TH, et al. Smoking and tuberculosis among the elderly in Hong Kong. Am J Respir Crit Care Med 2004; 170: 1027–1033. doi: 10.1164/rccm.200404-512OC [DOI] [PubMed] [Google Scholar]
- 8.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med 2007; 4: e20. doi: 10.1371/journal.pmed.0040020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slama K, Chiang CY, Enarson DA, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis 2007; 11: 1049–1061. [PubMed] [Google Scholar]
- 10.Wagnew F, Eshetie S, Alebel A, et al. Meta-analysis of the prevalence of tuberculosis in diabetic patients and its association with cigarette smoking in African and Asian countries. BMC Res Notes 2018; 11: 298. doi: 10.1186/s13104-018-3390-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bates MN, Khalakdina A, Pai M, et al. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med 2007; 167: 335–342. doi: 10.1001/archinte.167.4.335 [DOI] [PubMed] [Google Scholar]
- 12.Burusie A, Enquesilassie F, Addissie A, et al. Effect of smoking on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS One 2020; 15: e0239333. doi: 10.1371/journal.pone.0239333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuels JP, Sood A, Campbell JR, et al. Comorbidities and treatment outcomes in multidrug resistant tuberculosis: a systematic review and meta-analysis. Sci Rep 2018; 8: 4980. doi: 10.1038/s41598-018-23344-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaves Torres NM, Quijano Rodriguez JJ, Porras Andrade PS, et al. Factors predictive of the success of tuberculosis treatment: a systematic review with meta-analysis. PLoS One 2019; 14: e0226507. doi: 10.1371/journal.pone.0226507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patra J, Bhatia M, Suraweera W, et al. Exposure to second-hand smoke and the risk of tuberculosis in children and adults: a systematic review and meta-analysis of 18 observational studies. PLoS Med 2015; 12: e1001835. doi: 10.1371/journal.pmed.1001835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obore N, Kawuki J, Guan J, et al. Association between indoor air pollution, tobacco smoke and tuberculosis: an updated systematic review and meta-analysis. Public Health 2020; 187: 24–35. doi: 10.1016/j.puhe.2020.07.031 [DOI] [PubMed] [Google Scholar]
- 17.Amere GA, Nayak P, Salindri AD, et al. Contribution of smoking to tuberculosis incidence and mortality in high-tuberculosis-burden countries. Am J Epidemiol 2018; 187: 1846–1855. doi: 10.1093/aje/kwy081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen CP, Chan TC, Chan HT, et al. The reduction of tuberculosis risks by smoking cessation. BMC Infect Dis 2010; 10: 156. doi: 10.1186/1471-2334-10-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Xin H, Li X, et al. A dose-response relationship of smoking with tuberculosis infection: a cross-sectional study among 21008 rural residents in China. PLoS One 2017; 12: e0175183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eastment MC, McClintock AH, McKinney CM, et al. Factors that influence treatment completion for latent tuberculosis infection. J Am Board Fam Med 2017; 30: 520–527. doi: 10.3122/jabfm.2017.04.170070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altet N, Latorre I, Jimenez-Fuentes MA, et al. Assessment of the influence of direct tobacco smoke on infection and active TB management. PLoS One 2017; 12: e0182998. doi: 10.1371/journal.pone.0182998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adegbite BR, Edoa JR, Achimi Agbo P, et al. Epidemiological, mycobacteriological, and clinical characteristics of smoking pulmonary tuberculosis patients, in Lambarene, Gabon: a cross-sectional study. Am J Trop Med Hyg 2020; 103: 2501–2505. doi: 10.4269/ajtmh.20-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung CC, Yew WW, Chan CK, et al. Smoking adversely affects treatment response, outcome and relapse in tuberculosis. Eur Respir J 2015; 45: 738–745. doi: 10.1183/09031936.00114214 [DOI] [PubMed] [Google Scholar]
- 24.Visser ME, Stead MC, Walzl G, et al. Baseline predictors of sputum culture conversion in pulmonary tuberculosis: importance of cavities, smoking, time to detection and W-Beijing genotype. PLoS One 2012; 7: e29588. doi: 10.1371/journal.pone.0029588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altet-Gomez MN, Alcaide J, Godoy P, et al. Clinical and epidemiological aspects of smoking and tuberculosis: a study of 13,038 cases. Int J Tuberc Lung Dis 2005; 9: 430–436. [PubMed] [Google Scholar]
- 26.Yen YF, Yen MY, Lin YS, et al. Smoking increases risk of recurrence after successful anti-tuberculosis treatment: a population-based study. Int J Tuberc Lung Dis 2014; 18: 492–498. doi: 10.5588/ijtld.13.0694 [DOI] [PubMed] [Google Scholar]
- 27.Wang MG, Huang WW, Wang Y, et al. Association between tobacco smoking and drug-resistant tuberculosis. Infect Drug Resist 2018; 11: 873–887. doi: 10.2147/IDR.S164596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tilley AE, Walters MS, Shaykhiev R, et al. Cilia dysfunction in lung disease. Annu Rev Physiol 2015; 77: 379–406. doi: 10.1146/annurev-physiol-021014-071931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers GB, Carroll MP, Zain NM, et al. Complexity, temporal stability, and clinical correlates of airway bacterial community composition in primary ciliary dyskinesia. J Clin Microbiol 2013; 51: 4029–4035. doi: 10.1128/JCM.02164-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leemans JC, Thepen T, Weijer S, et al. Macrophages play a dual role during pulmonary tuberculosis in mice. J Infect Dis 2005; 191: 65–74. doi: 10.1086/426395 [DOI] [PubMed] [Google Scholar]
- 31.Huang L, Nazarova EV, Tan S, et al. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med 2018; 215: 1135–1152. doi: 10.1084/jem.20172020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan A, Singh VK, Hunter RL, et al. Macrophage heterogeneity and plasticity in tuberculosis. J Leukoc Biol 2019; 106: 275–282. doi: 10.1002/JLB.MR0318-095RR [DOI] [PubMed] [Google Scholar]
- 33.Pisu D, Huang L, Narang V, et al. Single cell analysis of M. tuberculosis phenotype and macrophage lineages in the infected lung. J Exp Med 2021; 218: e20210615. doi: 10.1084/jem.20210615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf AJ, Linas B, Trevejo-Nunez GJ, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol 2007; 179: 2509–2519. doi: 10.4049/jimmunol.179.4.2509 [DOI] [PubMed] [Google Scholar]
- 35.Cambier CJ, O'Leary SM, O'Sullivan MP, et al. Phenolic glycolipid facilitates mycobacterial escape from microbicidal tissue-resident macrophages. Immunity 2017; 47: 552–565. doi: 10.1016/j.immuni.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace WA, Gillooly M, Lamb D. Intra-alveolar macrophage numbers in current smokers and non-smokers: a morphometric study of tissue sections. Thorax 1992; 47: 437–440. doi: 10.1136/thx.47.6.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cass SP, Mekhael O, Thayaparan D, et al. Increased monocyte-derived CD11b(+) macrophage subpopulations following cigarette smoke exposure are associated with impaired bleomycin-induced tissue remodelling. Front Immunol 2021; 12: 740330. doi: 10.3389/fimmu.2021.740330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traves SL, Culpitt SV, Russell RE, et al. Increased levels of the chemokines GROα and MCP-1 in sputum samples from patients with COPD. Thorax 2002; 57: 590–595. doi: 10.1136/thorax.57.7.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capelli A, Di Stefano A, Gnemmi I, et al. Increased MCP-1 and MIP-1β in bronchoalveolar lavage fluid of chronic bronchitics. Eur Respir J 1999; 14: 160–165. doi: 10.1034/j.1399-3003.1999.14a27.x [DOI] [PubMed] [Google Scholar]
- 40.Hodge S, Hodge G, Ahern J, et al. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2007; 37: 748–755. doi: 10.1165/rcmb.2007-0025OC [DOI] [PubMed] [Google Scholar]
- 41.Beckett EL, Stevens RL, Jarnicki AG, et al. A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J Allergy Clin Immunol 2013; 131: 752–762. doi: 10.1016/j.jaci.2012.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dua K, Malyla V, Singhvi G, et al. Increasing complexity and interactions of oxidative stress in chronic respiratory diseases: an emerging need for novel drug delivery systems. Chem Biol Interact 2019; 299: 168–178. doi: 10.1016/j.cbi.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 43.Kirkham PA, Spooner G, Ffoulkes-Jones C, et al. Cigarette smoke triggers macrophage adhesion and activation: role of lipid peroxidation products and scavenger receptor. Free Radic Biol Med 2003; 35: 697–710. doi: 10.1016/S0891-5849(03)00390-3 [DOI] [PubMed] [Google Scholar]
- 44.Roth MD, Arora A, Barsky SH, et al. Airway inflammation in young marijuana and tobacco smokers. Am J Respir Crit Care Med 1998; 157: 928–937. doi: 10.1164/ajrccm.157.3.9701026 [DOI] [PubMed] [Google Scholar]
- 45.Eltom S, Stevenson CS, Rastrick J, et al. P2X7 receptor and caspase 1 activation are central to airway inflammation observed after exposure to tobacco smoke. PLoS One 2011; 6: e24097. doi: 10.1371/journal.pone.0024097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang MJ, Homer RJ, Gallo A, et al. IL-18 is induced and IL-18 receptor α plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol 2007; 178: 1948–1959. doi: 10.4049/jimmunol.178.3.1948 [DOI] [PubMed] [Google Scholar]
- 47.Cicko S, Lucattelli M, Muller T, et al. Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J Immunol 2010; 185: 688–697. doi: 10.4049/jimmunol.0904042 [DOI] [PubMed] [Google Scholar]
- 48.Cohen SB, Gern BH, Delahaye JL, et al. Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 2018; 24: 439–446. doi: 10.1016/j.chom.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris JO, Swenson EW, Johnson JE 3rd. Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest 1970; 49: 2086–2096. doi: 10.1172/JCI106426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berg RD, Levitte S, O'Sullivan MP, et al. Lysosomal disorders drive susceptibility to tuberculosis by compromising macrophage migration. Cell 2016; 165: 139–152. doi: 10.1016/j.cell.2016.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skold CM, Lundahl J, Hallden G, et al. Chronic smoke exposure alters the phenotype pattern and the metabolic response in human alveolar macrophages. Clin Exp Immunol 1996; 106: 108–113. doi: 10.1046/j.1365-2249.1996.d01-805.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aryanpur M, Mortaz E, Masjedi MR, et al. Reduced phagocytic capacity of blood monocyte/macrophages in tuberculosis patients is further reduced by smoking. Iran J Allergy Asthma Immunol 2016; 15: 174–182. [PubMed] [Google Scholar]
- 53.Yuan Y, Lin D, Feng L, et al. Upregulation of miR-196b-5p attenuates BCG uptake via targeting SOCS3 and activating STAT3 in macrophages from patients with long-term cigarette smoking-related active pulmonary tuberculosis. J Transl Med 2018; 16: 284. doi: 10.1186/s12967-018-1654-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minematsu N, Blumental-Perry A, Shapiro SD. Cigarette smoke inhibits engulfment of apoptotic cells by macrophages through inhibition of actin rearrangement. Am J Respir Cell Mol Biol 2011; 44: 474–482. doi: 10.1165/rcmb.2009-0463OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun 1998; 66: 1277–1281. doi: 10.1128/IAI.66.4.1277-1281.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.More JM, Voelker DR, Silveira LJ, et al. Smoking reduces surfactant protein D and phospholipids in patients with and without chronic obstructive pulmonary disease. BMC Pulm Med 2010; 10: 53. doi: 10.1186/1471-2466-10-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Honda Y, Takahashi H, Kuroki Y, et al. Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest 1996; 109: 1006–1009. doi: 10.1378/chest.109.4.1006 [DOI] [PubMed] [Google Scholar]
- 58.Ferguson JS, Voelker DR, McCormack FX, et al. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol 1999; 163: 312–321. [PubMed] [Google Scholar]
- 59.Haw TJ, Starkey MR, Pavlidis S, et al. Toll-like receptor 2 and 4 have opposing roles in the pathogenesis of cigarette smoke-induced chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2018; 314: L298–L317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Droemann D, Goldmann T, Tiedje T, et al. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res 2005; 6: 68. doi: 10.1186/1465-9921-6-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lofdahl JM, Wahlstrom J, Skold CM. Different inflammatory cell pattern and macrophage phenotype in chronic obstructive pulmonary disease patients, smokers and non-smokers. Clin Exp Immunol 2006; 145: 428–437. doi: 10.1111/j.1365-2249.2006.03154.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hodge S, Matthews G, Mukaro V, et al. Cigarette smoke-induced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. Am J Respir Cell Mol Biol 2011; 44: 673–681. doi: 10.1165/rcmb.2009-0459OC [DOI] [PubMed] [Google Scholar]
- 63.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 1998; 282: 1717–1721. doi: 10.1126/science.282.5394.1717 [DOI] [PubMed] [Google Scholar]
- 64.Simeone R, Sayes F, Lawaree E, et al. Breaching the phagosome, the case of the tuberculosis agent. Cell Microbiol 2021; 23: e13344. doi: 10.1111/cmi.13344 [DOI] [PubMed] [Google Scholar]
- 65.Johansen P, Fettelschoss A, Amstutz B, et al. Relief from Zmp1-mediated arrest of phagosome maturation is associated with facilitated presentation and enhanced immunogenicity of mycobacterial antigens. Clin Vaccine Immunol 2011; 18: 907–913. doi: 10.1128/CVI.00015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris JO, Gonzalez-Rothi RJ. Abnormal phagolysosome fusion in pulmonary alveolar macrophages of rats exposed chronically to cigarette smoke. Am Rev Respir Dis 1984; 130: 467–471. [DOI] [PubMed] [Google Scholar]
- 67.O'Leary SM, Coleman MM, Chew WM, et al. Cigarette smoking impairs human pulmonary immunity to Mycobacterium tuberculosis. Am J Respir Crit Care Med 2014; 190: 1430–1436. doi: 10.1164/rccm.201407-1385OC [DOI] [PubMed] [Google Scholar]
- 68.Chen H, Cowan MJ, Hasday JD, et al. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-κB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J Immunol 2007; 179: 6097–6106. doi: 10.4049/jimmunol.179.9.6097 [DOI] [PubMed] [Google Scholar]
- 69.Eapen MS, Hansbro PM, McAlinden K, et al. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci Rep 2017; 7: 13392. doi: 10.1038/s41598-017-13888-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Zyl-Smit RN, Binder A, Meldau R, et al. Cigarette smoke impairs cytokine responses and BCG containment in alveolar macrophages. Thorax 2014; 69: 363–370. doi: 10.1136/thoraxjnl-2013-204229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bourigault ML, Segueni N, Rose S, et al. Relative contribution of IL-1α, IL-1β and TNF to the host response to Mycobacterium tuberculosis and attenuated M. bovis BCG. Immun Inflamm Dis 2013; 1: 47–62. doi: 10.1002/iid3.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fabri M, Stenger S, Shin D-M, et al. Vitamin D is required for IFN-γ–mediated antimicrobial activity of human macrophages. Sci Transl Med 2011; 3: 104ra102. doi: 10.1126/scitranslmed.3003045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang SJ, Wang XH, Liu ZD, et al. Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des Devel Ther 2017; 11: 91–102. doi: 10.2147/DDDT.S79870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mousavi SE, Amini H, Heydarpour P, et al. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: evidence and potential mechanisms. Environ Int 2019; 122: 67–90. doi: 10.1016/j.envint.2018.11.052 [DOI] [PubMed] [Google Scholar]
- 75.Patterson B, Smith D, Telford A, et al. Vitamin D deficiency predicts latent TB reactivation independent of preventive therapy: a longitudinal study. Int J Tuberc Lung Dis 2020; 24: 916–921. doi: 10.5588/ijtld.19.0605 [DOI] [PubMed] [Google Scholar]
- 76.Ganmaa D, Uyanga B, Zhou X, et al. Vitamin D supplements for prevention of tuberculosis infection and disease. N Engl J Med 2020; 383: 359–368. doi: 10.1056/NEJMoa1915176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Refai A, Gritli S, Barbouche MR, et al. Mycobacterium tuberculosis virulent factor ESAT-6 drives macrophage differentiation toward the pro-inflammatory M1 phenotype and subsequently switches it to the anti-inflammatory M2 phenotype. Front Cell Infect Microbiol 2018; 8: 327. doi: 10.3389/fcimb.2018.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ley K. M1 means kill; M2 means heal. J Immunol 2017; 199: 2191–2193. doi: 10.4049/jimmunol.1701135 [DOI] [PubMed] [Google Scholar]
- 79.Huang Z, Luo Q, Guo Y, et al. Mycobacterium tuberculosis-induced polarization of human macrophage orchestrates the formation and development of tuberculous granulomas in vitro. PLoS One 2015; 10: e0129744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Almeida AS, Lago PM, Boechat N, et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol 2009; 183: 718–731. doi: 10.4049/jimmunol.0801212 [DOI] [PubMed] [Google Scholar]
- 81.Raju B, Hoshino Y, Belitskaya-Levy I, et al. Gene expression profiles of bronchoalveolar cells in pulmonary TB. Tuberculosis (Edinb) 2008; 88: 39–51. doi: 10.1016/j.tube.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho HJ, Lim YJ, Kim J, et al. Different macrophage polarization between drug-susceptible and multidrug-resistant pulmonary tuberculosis. BMC Infect Dis 2020; 20: 81. doi: 10.1186/s12879-020-4802-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Monick MM, Powers LS, Walters K, et al. Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol 2010; 185: 5425–5435. doi: 10.4049/jimmunol.1001603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gutierrez MG, Master SS, Singh SB, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004; 119: 753–766. doi: 10.1016/j.cell.2004.11.038 [DOI] [PubMed] [Google Scholar]
- 85.Gleeson LE, O'Leary SM, Ryan D, et al. Cigarette smoking impairs the bioenergetic immune response to Mycobacterium tuberculosis infection. Am J Respir Cell Mol Biol 2018; 59: 572–579. doi: 10.1165/rcmb.2018-0162OC [DOI] [PubMed] [Google Scholar]
- 86.Shi L, Salamon H, Eugenin EA, et al. Infection with Mycobacterium tuberculosis induces the Warburg effect in mouse lungs. Sci Rep 2015; 5: 18176. doi: 10.1038/srep18176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aridgides DS, Mellinger DL, Armstrong DA, et al. Functional and metabolic impairment in cigarette smoke-exposed macrophages is tied to oxidative stress. Sci Rep 2019; 9: 9624. doi: 10.1038/s41598-019-46045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoidal JR, Fox RB, LeMarbe PA, et al. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis 1981; 123: 85–89. [DOI] [PubMed] [Google Scholar]
- 89.Kondo T, Tagami S, Yoshioka A, et al. Current smoking of elderly men reduces antioxidants in alveolar macrophages. Am J Respir Crit Care Med 1994; 149: 178–182. doi: 10.1164/ajrccm.149.1.8111579 [DOI] [PubMed] [Google Scholar]
- 90.Aoshiba K, Tamaoki J, Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 2001; 281: L1392–L1401. doi: 10.1152/ajplung.2001.281.6.L1392 [DOI] [PubMed] [Google Scholar]
- 91.Silva Bezerra F, Valenca SS, Lanzetti M, et al. Alpha-tocopherol and ascorbic acid supplementation reduced acute lung inflammatory response by cigarette smoke in mouse. Nutrition 2006; 22: 1192–1201. doi: 10.1016/j.nut.2006.08.016 [DOI] [PubMed] [Google Scholar]
- 92.Wiid I, Seaman T, Hoal EG, et al. Total antioxidant levels are low during active TB and rise with anti-tuberculosis therapy. IUBMB Life 2004; 56: 101–106. doi: 10.1080/15216540410001671259 [DOI] [PubMed] [Google Scholar]
- 93.Soh AZ, Chee CBE, Wang YT, et al. Dietary intake of antioxidant vitamins and carotenoids and risk of developing active tuberculosis in a prospective population-based cohort study. Am J Epidemiol 2017; 186: 491–500. doi: 10.1093/aje/kwx132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pacht ER, Kaseki H, Mohammed JR, et al. Deficiency of vitamin E in the alveolar fluid of cigarette smokers. Influence on alveolar macrophage cytotoxicity. J Clin Invest 1986; 77: 789–796. doi: 10.1172/JCI112376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aibana O, Franke MF, Huang CC, et al. Vitamin E status is inversely associated with risk of incident tuberculosis disease among household contacts. J Nutr 2018; 148: 56–62. doi: 10.1093/jn/nxx006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dallenga T, Repnik U, Corleis B, et al. M. tuberculosis-induced necrosis of infected neutrophils promotes bacterial growth following phagocytosis by macrophages. Cell Host Microbe 2017; 22: 519–530.e3. doi: 10.1016/j.chom.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 97.Kirkham PA, Spooner G, Rahman I, et al. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem Biophys Res Commun 2004; 318: 32–37. doi: 10.1016/j.bbrc.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 98.Blidberg K, Palmberg L, Dahlen B, et al. Increased neutrophil migration in smokers with or without chronic obstructive pulmonary disease. Respirology 2012; 17: 854–860. doi: 10.1111/j.1440-1843.2012.02181.x [DOI] [PubMed] [Google Scholar]
- 99.Pouwels SD, Zijlstra GJ, van der Toorn M, et al. Cigarette smoke-induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol 2016; 310: L377–L386. doi: 10.1152/ajplung.00174.2015 [DOI] [PubMed] [Google Scholar]
- 100.Lu Z, Van Eeckhoutte HP, Liu G, et al. Necroptosis signaling promotes inflammation, airway remodeling, and emphysema in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2021; 204: 667–681. doi: 10.1164/rccm.202009-3442OC [DOI] [PubMed] [Google Scholar]
- 101.Guzik K, Skret J, Smagur J, et al. Cigarette smoke-exposed neutrophils die unconventionally but are rapidly phagocytosed by macrophages. Cell Death Dis 2011; 2: e131. doi: 10.1038/cddis.2011.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y, Geng S, Prasad GL, et al. Suppression of neutrophil antimicrobial functions by total particulate matter from cigarette smoke. Front Immunol 2018; 9: 2274. doi: 10.3389/fimmu.2018.02274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ong CW, Elkington PT, Brilha S, et al. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog 2015; 11: e1004917. doi: 10.1371/journal.ppat.1004917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van der Meer AJ, Zeerleder S, Blok DC, et al. Neutrophil extracellular traps in patients with pulmonary tuberculosis. Respir Res 2017; 18: 181. doi: 10.1186/s12931-017-0663-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Braian C, Hogea V, Stendahl O. Mycobacterium tuberculosis-induced neutrophil extracellular traps activate human macrophages. J Innate Immun 2013; 5: 591–602. doi: 10.1159/000348676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dunn JS, Freed BM, Gustafson DL, et al. Inhibition of human neutrophil reactive oxygen species production and p67phox translocation by cigarette smoke extract. Atherosclerosis 2005; 179: 261–267. doi: 10.1016/j.atherosclerosis.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 107.White PC, Hirschfeld J, Milward MR, et al. Cigarette smoke modifies neutrophil chemotaxis, neutrophil extracellular trap formation and inflammatory response-related gene expression. J Periodontal Res 2018; 53: 525–535. doi: 10.1111/jre.12542 [DOI] [PubMed] [Google Scholar]
- 108.Eum SY, Kong JH, Hong MS, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 2010; 137: 122–128. doi: 10.1378/chest.09-0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McNab FW, Berry MPR, Graham CM, et al. Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur J Immunol 2011; 41: 1941–1947. doi: 10.1002/eji.201141421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Psomas C, Tuaillon E, Marin G, et al. Increased neutrophil surface PD-L1 expression in tobacco smokers: consequences for anti-PD-1 treatment. J Acquir Immune Defic Syndr 2019; 80: e48–e49. doi: 10.1097/QAI.0000000000001897 [DOI] [PubMed] [Google Scholar]
- 111.Vassallo R, Tamada K, Lau JS, et al. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol 2005; 175: 2684–2691. doi: 10.4049/jimmunol.175.4.2684 [DOI] [PubMed] [Google Scholar]
- 112.Givi ME, Folkerts G, Wagenaar GT, et al. Cigarette smoke differentially modulates dendritic cell maturation and function in time. Respir Res 2015; 16: 131. doi: 10.1186/s12931-015-0291-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol 2011; 4: 288–293. doi: 10.1038/mi.2011.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Balboa L, Romero MM, Yokobori N, et al. Mycobacterium tuberculosis impairs dendritic cell response by altering CD1b, DC-SIGN and MR profile. Immunol Cell Biol 2010; 88: 716–726. doi: 10.1038/icb.2010.22 [DOI] [PubMed] [Google Scholar]
- 115.Yanagita M, Kobayashi R, Kojima Y, et al. Nicotine modulates the immunological function of dendritic cells through peroxisome proliferator-activated receptor-γ upregulation. Cell Immunol 2012; 274: 26–33. doi: 10.1016/j.cellimm.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 116.Khader SA, Partida-Sanchez S, Bell G, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med 2006; 203: 1805–1815. doi: 10.1084/jem.20052545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Noss EH, Harding CV, Boom WH. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell Immunol 2000; 201: 63–74. doi: 10.1006/cimm.2000.1633 [DOI] [PubMed] [Google Scholar]
- 118.Shang S, Ordway D, Henao-Tamayo M, et al. Cigarette smoke increases susceptibility to tuberculosis—evidence from in vivo and in vitro models. J Infect Dis 2011; 203: 1240–1248. doi: 10.1093/infdis/jir009 [DOI] [PubMed] [Google Scholar]
- 119.Tailleux L, Neyrolles O, Honore-Bouakline S, et al. Constrained intracellular survival of Mycobacterium tuberculosis in human dendritic cells. J Immunol 2003; 170: 1939–1948. doi: 10.4049/jimmunol.170.4.1939 [DOI] [PubMed] [Google Scholar]
- 120.Robbins CS, Dawe DE, Goncharova SI, et al. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol 2004; 30: 202–211. doi: 10.1165/rcmb.2003-0259OC [DOI] [PubMed] [Google Scholar]
- 121.Mortaz E, Kraneveld AD, Smit JJ, et al. Effect of cigarette smoke extract on dendritic cells and their impact on T-cell proliferation. PLoS One 2009; 4: e4946. doi: 10.1371/journal.pone.0004946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wolday D, Kebede Y, Legesse D, et al. Role of CD4/CD8 ratio on the incidence of tuberculosis in HIV-infected patients on antiretroviral therapy followed up for more than a decade. PLoS One 2020; 15: e0233049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaiko GE, Horvat JC, Beagley KW, et al. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology 2008; 123: 326–338. doi: 10.1111/j.1365-2567.2007.02719.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology 2003; 109: 365–373. doi: 10.1046/j.1365-2567.2003.01655.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shan M, Yuan X, Song LZ, et al. Cigarette smoke induction of osteopontin (SPP1) mediates TH17 inflammation in human and experimental emphysema. Sci Transl Med 2012; 4: 117ra9. doi: 10.1126/scitranslmed.3003041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Okamoto Yoshida Y, Umemura M, Yahagi A, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol 2010; 184: 4414–4422. doi: 10.4049/jimmunol.0903332 [DOI] [PubMed] [Google Scholar]
- 127.Yu Y, Zhao L, Xie Y, et al. Th1/Th17 cytokine profiles are associated with disease severity and exacerbation frequency in COPD patients. Int J Chron Obstruct Pulmon Dis 2020; 15: 1287–1299. doi: 10.2147/COPD.S252097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liang Y, Shen Y, Kuang L, et al. Cigarette smoke exposure promotes differentiation of CD4+ T-cells toward Th17 cells by CD40-CD40L costimulatory pathway in mice. Int J Chron Obstruct Pulmon Dis 2018; 13: 959–968. doi: 10.2147/COPD.S155754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin PL, Rutledge T, Green AM, et al. CD4 T-cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses 2012; 28: 1693–1702. doi: 10.1089/aid.2012.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hernandez CP, Morrow K, Velasco C, et al. Effects of cigarette smoke extract on primary activated T-cells. Cell Immunol 2013; 282: 38–43. doi: 10.1016/j.cellimm.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vardavas CI, Plada M, Tzatzarakis M, et al. Passive smoking alters circulating naive/memory lymphocyte T-cell subpopulations in children. Pediatr Allergy Immunol 2010; 21: 1171–1178. doi: 10.1111/j.1399-3038.2010.01039.x [DOI] [PubMed] [Google Scholar]
- 132.Forsslund H, Mikko M, Karimi R, et al. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest 2014; 145: 711–722. doi: 10.1378/chest.13-0873 [DOI] [PubMed] [Google Scholar]
- 133.Geng Y, Savage SM, Johnson LJ, et al. Effects of nicotine on the immune response. I. Chronic exposure to nicotine impairs antigen receptor-mediated signal transduction in lymphocytes. Toxicol Appl Pharmacol 1995; 135: 268–278. doi: 10.1006/taap.1995.1233 [DOI] [PubMed] [Google Scholar]
- 134.Geng Y, Savage SM, Razani-Boroujerdi S, et al. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T-cell anergy. J Immunol 1996; 156: 2384–2390. [PubMed] [Google Scholar]
- 135.Kalra R, Singh SP, Savage SM, et al. Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T-cells and depletes IP3-sensitive Ca2+ stores. J Pharmacol Exp Ther 2000; 293: 166–171. [PubMed] [Google Scholar]
- 136.Hagiwara E, Takahashi KI, Okubo T, et al. Cigarette smoking depletes cells spontaneously secreting Th1 cytokines in the human airway. Cytokine 2001; 14: 121–126. doi: 10.1006/cyto.2001.0860 [DOI] [PubMed] [Google Scholar]
- 137.Meuronen A, Majuri ML, Alenius H, et al. Decreased cytokine and chemokine mRNA expression in bronchoalveolar lavage in asymptomatic smoking subjects. Respiration 2008; 75: 450–458. doi: 10.1159/000114855 [DOI] [PubMed] [Google Scholar]
- 138.Zhang S, Petro TM. The effect of nicotine on murine CD4 T-cell responses. Int J Immunopharmacol 1996; 18: 467–478. doi: 10.1016/S0192-0561(96)00054-9 [DOI] [PubMed] [Google Scholar]
- 139.Feng Y, Kong Y, Barnes PF, et al. Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect Immun 2011; 79: 229–237. doi: 10.1128/IAI.00709-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Feng CG, Jankovic D, Kullberg M, et al. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol 2005; 174: 4185–4192. doi: 10.4049/jimmunol.174.7.4185 [DOI] [PubMed] [Google Scholar]
- 141.Burnham EL, Kovacs EJ, Davis CS. Pulmonary cytokine composition differs in the setting of alcohol use disorders and cigarette smoking. Am J Physiol Lung Cell Mol Physiol 2013; 304: L873–L882. doi: 10.1152/ajplung.00385.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kroening PR, Barnes TW, Pease L, et al. Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways. J Immunol 2008; 181: 1536–1547. doi: 10.4049/jimmunol.181.2.1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mian MF, Lauzon NM, Stampfli MR, et al. Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. J Leukoc Biol 2008; 83: 774–784. doi: 10.1189/jlb.0707481 [DOI] [PubMed] [Google Scholar]
- 144.Merghani TH, Saeed A, Alawad A. Changes in plasma IL4, TNFα and CRP in response to regular passive smoking at home among healthy school children in Khartoum, Sudan. Afr Health Sci 2012; 12: 41–47. [PMC free article] [PubMed] [Google Scholar]
- 145.Byron KA, Varigos GA, Wootton AM. IL-4 production is increased in cigarette smokers. Clin Exp Immunol 1994; 95: 333–336. doi: 10.1111/j.1365-2249.1994.tb06533.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miotto D, Ruggieri MP, Boschetto P, et al. Interleukin-13 and -4 expression in the central airways of smokers with chronic bronchitis. Eur Respir J 2003; 22: 602–608. doi: 10.1183/09031936.03.00046402 [DOI] [PubMed] [Google Scholar]
- 147.Cooper PR, Poll CT, Barnes PJ, et al. Involvement of IL-13 in tobacco smoke-induced changes in the structure and function of rat intrapulmonary airways. Am J Respir Cell Mol Biol 2010; 43: 220–226. doi: 10.1165/rcmb.2009-0117OC [DOI] [PubMed] [Google Scholar]
- 148.Heitmann L, Abad Dar M, Schreiber T, et al. The IL-13/IL-4Rα axis is involved in tuberculosis-associated pathology. J Pathol 2014; 234: 338–350. doi: 10.1002/path.4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shaler CR, Horvath CN, McCormick S, et al. Continuous and discontinuous cigarette smoke exposure differentially affects protective Th1 immunity against pulmonary tuberculosis. PLoS One 2013; 8: e59185. doi: 10.1371/journal.pone.0059185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guyot-Revol V, Innes JA, Hackforth S, et al. Regulatory T-cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med 2006; 173: 803–810. doi: 10.1164/rccm.200508-1294OC [DOI] [PubMed] [Google Scholar]
- 151.Isajevs S, Taivans I, Strazda G, et al. Decreased FOXP3 expression in small airways of smokers with COPD. Eur Respir J 2009; 33: 61–67. doi: 10.1183/09031936.00145307 [DOI] [PubMed] [Google Scholar]
- 152.Singh A, Dey AB, Mohan A, et al. Foxp3+ regulatory T-cells among tuberculosis patients: impact on prognosis and restoration of antigen specific IFN-γ producing T-cells. PLoS One 2012; 7: e44728. doi: 10.1371/journal.pone.0044728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shang S, Harton M, Tamayo MH, et al. Increased Foxp3 expression in guinea pigs infected with W-Beijing strains of M. tuberculosis. Tuberculosis (Edinb) 2011; 91: 378–385. doi: 10.1016/j.tube.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Semple PL, Binder AB, Davids M, et al. Regulatory T-cells attenuate mycobacterial stasis in alveolar and blood-derived macrophages from patients with tuberculosis. Am J Respir Crit Care Med 2013; 187: 1249–1258. doi: 10.1164/rccm.201210-1934OC [DOI] [PubMed] [Google Scholar]
- 155.Kalathil SG, Lugade AA, Pradhan V, et al. T-regulatory cells and programmed death 1+ T-cells contribute to effector T-cell dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 190: 40–50. doi: 10.1164/rccm.201312-2293OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Valiathan R, Miguez MJ, Patel B, et al. Tobacco smoking increases immune activation and impairs T-cell function in HIV infected patients on antiretrovirals: a cross-sectional pilot study. PLoS One 2014; 9: e97698. doi: 10.1371/journal.pone.0097698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reiley WW, Shafiani S, Wittmer ST, et al. Distinct functions of antigen-specific CD4 T-cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA 2010; 107: 19408–19413. doi: 10.1073/pnas.1006298107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kauffman KD, Sakai S, Lora NE, et al. PD-1 blockade exacerbates Mycobacterium tuberculosis infection in rhesus macaques. Sci Immunol 2021; 6: eabf3861. doi: 10.1126/sciimmunol.abf3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tezera LB, Bielecka MK, Ogongo P, et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-α. Elife 2020; 9: e52668. doi: 10.7554/eLife.52668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jones B, Donovan C, Liu G, et al. Animal models of COPD: what do they tell us? Respirology 2017; 22: 21–32. doi: 10.1111/resp.12908 [DOI] [PubMed] [Google Scholar]
- 161.Gomez AC, Rodriguez-Fernandez P, Villar-Hernandez R, et al. E-cigarettes: Effects in phagocytosis and cytokines response against Mycobacterium tuberculosis. PLoS One 2020; 15: e0228919. [DOI] [PMC free article] [PubMed] [Google Scholar]