Abstract
Multimorbidity is increasingly common and current healthcare strategies are not always aligned to treat this complex burden of disease. COPD, type-2 diabetes mellitus (T2D) and cardiovascular disease, especially atherosclerosis, occur more frequently together than expected, even when risk factors such as smoking, obesity, inactivity and poverty are considered. This supports the possibility of unifying mechanisms that contribute to the pathogenesis or progression of each condition.
Neutrophilic inflammation is causally associated with COPD, and increasingly recognised in the pathogenesis of atherosclerosis and T2D, potentially forming an aetiological link between conditions. This link might reflect an overspill of inflammation from one affected organ into the systemic circulation, exposing all organs to an increased milieu of proinflammatory cytokines. Additionally, increasing evidence supports the involvement of other processes in chronic disease pathogenesis, such as cellular senescence or changes in cellular phenotypes.
This review explores the current scientific evidence for inflammation, cellular ageing and cellular processes, such as reactive oxygen species production and phenotypic changes in the pathogenesis of COPD, T2D and atherosclerosis; highlighting common mechanisms shared across these diseases. We identify emerging therapeutic approaches that target these areas, but also where more work is still required to improve our understanding of the underlying cellular biology in a multimorbid disease setting.
Short abstract
Multimorbidity is increasingly common in our ageing population. Drawing together research across chronic inflammatory disease is vital to enable effective medical intervention and prevent research becoming siloed http://bit.ly/32l9hXs
Introduction
Multimorbidity is defined as the presence of two or more chronic conditions in the same person. It is increasingly common in our ageing population, affecting nearly 40% of European citizens [1] and is extremely costly in terms of healthcare resource use [2]. Some conditions co-occur more commonly than others and COPD is often present in multimorbid disease clusters with cardiovascular disease (CVD), especially atherosclerosis and type-2 diabetes mellitus (T2D) [3, 4], conditions associated with an immense global burden of ill health. These conditions share multiple risk factors, including age, smoking, obesity, physical inactivity and poverty [5, 6]. For example, smoking cigarettes is clearly associated with COPD pathogenesis, but also shares a robust association with diabetes and atherosclerosis after multivariable adjustment [7, 8]. However, smoking is neither necessary nor sufficient to cause any of these diseases and even after accounting for overlapping risk factors, the incidence of COPD, T2D and atherosclerosis presenting in the same person is still higher than expected [3, 4, 9].
There is great interest in understanding why multimorbidities cluster in individuals [10]. Network analyses have identified multiple shared molecular pathways and genetic associations between COPD, cardiovascular diseases and T2D [11, 12], perhaps alluding to common mechanisms. When present, these mechanisms might lower susceptibility to cause or increase the progression rate of components of the clustered diseases. This provides the opportunity for shared therapies that cut across traditional disease silos; a holistic approach to pathology permitting a holistic approach to patient care.
Multimorbidity is more common with increasing age and is associated with inflammation and cellular dysfunction. Targeting these processes (cellular ageing, inflammation and cellular dysfunction) may offer novel treatments across multimorbidities or allow us to utilise the drugs we already have for new indications (“repurposed” drugs). For example, statins, used to lower cholesterol as part of a CVD risk-reduction strategy are under investigation across a wide range of disease areas, including infections [13], COPD [14] and dementia [15]. However, research into shared mechanisms of multimorbidity are limited as most clinical observational studies and interventional trials focus on a single disease entity.
This review discusses known and emerging mechanisms of disease pathogenesis, which could be shared across multimorbidity, using COPD, atherosclerosis and T2D as exemplar diseases. While these are among the most common noncommunicable chronic diseases globally, they also present as risk factors for each other and there is an increased prevalence of COPD, atherosclerosis or T2D in patients with a diagnosis of any of these conditions [16–18]. In particular, this review will focus on the shared role of the neutrophil in these diseases. While neutrophils are not the only cells that play a role in COPD, atherosclerosis and T2D, they are the most abundant immune cells found in lung secretions of patients diagnosed with COPD and their products have been shown to cause all facets of lung disease in animal and cell models (from chronic bronchitis to emphysema); correlating with disease severity and decline [19, 20]. Recent advances in imaging technologies have also identified that neutrophils are present in the lipid core of human plaques and neutrophilia correlates with the number of rupture-prone lesions [21] and, although less studied, diabetic patients, rats and mice clearly demonstrate consistent defects of neutrophil chemotactic, phagocytic and microbicidal activities [22]. Together, this suggests neutrophils may provide a link between these three disease areas.
An overview of mechanisms for multimorbidity
There are multiple mechanisms that play a role in the development of COPD, atherosclerosis and T2D including: altered function of cells such as endothelial cell and neutrophils [23–25]; mitochondrial defects [26]; metabolic changes [27]; increased age [25, 28]; and increased inflammation [29, 30]. Despite overlapping mechanisms and risk factors, targeting a known manifestation of one disease does not always improve outcomes in another. For example, targeting glucose levels in patients with T2D [31] or improving airflow obstruction in COPD [32] does not necessarily improve cardiovascular mortality. There is an urgent need to further our understanding of the potential links between these disease areas to guide therapies for multimorbid patients. This review will focus on three main, yet overlapping, putative common mechanisms observed in COPD, atherosclerosis and T2D, while appreciating other mechanisms do exist as detailed above.
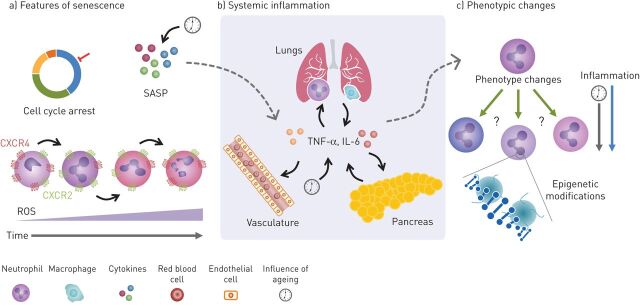
First, COPD, atherosclerosis and T2D are more common in an ageing host and “accelerated ageing” has been proposed as a pathogenic mechanism in these diseases, including ageing of individual cells resulting in a complex phenotype termed “senescence” (figure 1a) [33]. Cellular senescence was initially described as a process whereby cells entered permanent cell cycle arrest, allowing aged cells to be cleared. However, our understating of senescence has expanded, especially regarding nonreplicating cells, such as neutrophils (figure 1a) [34]. It is now recognised that senescent cells are diverse, and their function and fate may depend on the strength and duration of the stressor they are exposed to, including oxidative stress and DNA damage. These may lead to cell repair, immune clearance of the senescent cell or phenotypic changes in senescent cells that endure in tissues (as reviewed by van Deursen [35]).
FIGURE 1.
Potential common inflammatory mechanisms between COPD, type-2 diabetes mellitus (T2D) and cardiovascular disease (CVD). a) Senescence is described as changes that result in cell-cycle arrest and is associated with a senescence-associated secretory phenotype (SASP). The SASP leads to increases in inflammatory cytokines both locally and systemically (dotted arrow). This process in neutrophils, while slightly different to typical senescence, might be characterised by increases in chemokine receptor CXCR4 and decreases in CXCR2. This may change the way neutrophils behave and contribute to the damage seen in these diseases. These changes are also associated with increased reactive oxygen species (ROS) production, causing an oxidant imbalance and increasing damage. b) Inflammatory cytokines released by lung epithelial cells and immune cells can enter circulation from the lung in COPD. Likewise, these cytokines can also be released by immune cells in the pancreas in T2D and from endothelium and plaques in CVD. Increases of these cytokines also occur with age and can influence the cytokine milieu, leading to potential changes in neutrophil phenotype (dotted arrow). c) Changes in neutrophil subpopulations (or cell phenotype) caused by inflammation may also play a role in the crosstalk between COPD, CVD and T2D by contributing to tissue damage. There is also some evidence to suggest epigenetic changes may perpetuate the disease state causing long-term maladaptive changes to cellular phenotype and function, influenced by both inflammation and ageing. TNF: tumour necrosis factor; IL: interleukin.
Secondly, and linked to theme 1, atherosclerosis, COPD and T2D are associated with chronic systemic inflammation (figure 1b). Inflammation is both caused by and drives many chronic noncommunicable diseases, including COPD, atherosclerosis and T2D. Increased systemic inflammation has been reported in multimorbid patients when compared to those with a single disease or age-matched healthy volunteers [36, 37]. It is important to note that while similar patterns of inflammation, including neutrophilic inflammation, are observed in COPD, atherosclerosis and T2D [38–40], inflammation is a mechanism that plays a role in numerous chronic and acute diseases. Systemic crosstalk of inflammatory cytokines might occur through “overspill”, where inflammatory proteins produced in one organ can spread system-wide (figure 1b), impacting on the development or progression of other inflammatory diseases. The proximity of atherosclerotic plaques to the bloodstream provides obvious routes for systemic inflammation, but this mechanism has also been described in COPD and T2D [39, 41].
Thirdly, immune cell activation and dysfunction have been described in COPD, atherosclerosis and T2D, and linked with adverse disease outcomes (figure 1c). Clearly, neutrophils are not the only cell that show altered functionality in these three diseases, but the fact that neutrophil dysfunction occurs in COPD, atherosclerosis and T2D may provide novel links between these diseases [22, 42, 43]. This cellular dysfunction may represent a physiological response to a pathological event, but there is increasing evidence that chronic inflammation (as seen in COPD, atherosclerosis and T2D) can contribute to a shift in immune cell phenotype or subpopulation, changing the cell's usual response to an inflammatory insult, and increasing the potential for damage (figure 1c). This may or may not reflect cellular senescence, but epigenetic modification may be one potential mechanism for this long-term phenotypic shift [44], which could impact on multimorbidity.
These themes will be explored in more detail below. The overlapping nature of these proposed mechanisms have been are summarised in figure 1.
Theme 1: accelerated ageing in multimorbidity
Ageing-associated cellular senescence is also seen in the immune system, termed “immunosenescence”, with less effective pathogen containment and killing, enhanced inflammation and inefficient repair processes [25, 45].
The parallels between age and chronic disease are manifold, leading to the hypothesis that chronic conditions reflect a state of advanced or accelerated ageing. Both age and chronic disease are associated with primary hallmarks of ageing-related dysfunction: genetic instability, epigenetic alterations, loss of proteostasis and telomere shortening [28].
DNA instability has long been noted in COPD [46] and more recently described in T2D [47] and atherosclerosis [48]. Epigenetic modifications, including changes in histone deacetylase 2 activity, have been identified in all three conditions [49–51]. A loss of proteostasis and autophagy has been associated with emphysema in COPD [52] and also contribute to T2D [53] and atherosclerosis progression [54]. Studies have described a reduction in relative telomere length (RTL) in leukocytes in COPD, atherosclerosis and T2D, but this has not been universally replicated [55, 56]. A reduction in RTL was observed in peripheral lymphocytes in patients with COPD [57] and in lung epithelial cells exposed to cigarette smoke in vitro [58]. However, no significant difference in RTL was found in lung biopsies from patients with COPD compared to healthy controls [59], suggesting that these changes might be cell-type specific. Similarly, in T2D and atherosclerosis, RTL has been shown to be reduced in circulating leukocytes but with variable penetration across disease severity [60, 61].
These changes are thought to be due to the time-dependent accumulation of cellular damage; however, it remains unclear whether these changes occur before the onset of age-associated disease or as a result of disease, in all or only a subset of cells, and to what extent these changes are reversible.
Multimorbidity is associated with an increased burden of senescent cells, compared to health [62]. Senescent cells can be identified by senescence-associated β-galactosidase (SAβG) activity [63] (although not all senescent cells express SAβG [64]) or by the cocktail of inflammatory cytokines, growth hormones and proteinases they secrete, called the senescence-associated secretory phenotype (SASP) [65].
An increased presence of senescent cells, SAβG activity and the SASP have been described in atherosclerosis by both vascular smooth muscle cells and endothelial cells around atherosclerotic plaques in vitro and in vivo [66, 67]. In T2D, a high glucose level has been shown to drive premature senescence in vitro in endothelial cells, renal mesangial cells, adipose-derived stem cells and fibroblasts [68]. In COPD, a higher burden of senescent cells has been described in pulmonary vessels, especially those with vascular remodelling that has been associated with pulmonary hypertension, suggesting an interesting link between COPD and vascular disease [69]. A similar increase in senescence of the smooth muscle that surrounds pulmonary arteries in these patients was also observed, providing further links between vascular senescence, atherosclerosis and COPD [69]. Small sections of regulatory RNA, known as microRNAs (miRs) have been implicated in promoting cellular senescence and miR-34a has been associated with fibroblast senescence in COPD [70] atherosclerosis [71] and T2D [72], demonstrating a common link between diseases.
Circulating cells, such as neutrophils, may convey pathologies and enhance ageing processes between tissues, providing a link between multimorbid conditions. Moreover, there is evidence to support reverse transmigration of neutrophils from tissue into the blood, suggesting that any local senescent phenotype could be conferred systemically by neutrophils to other organs [73]. Neutrophil senescence in humans leads to distinct functional changes, including migration, phagocytosis and NETosis, which impair the host response to infection, while simultaneously increasing the potential for host damage through inflammation, reactive oxygen species (ROS) and proteases activity [74, 75]. Human neutrophil migratory accuracy reduces with age [25], a feature that is exaggerated in COPD [76] and potentially increases tissue damage through migration-associated release of proteases and ROS [25, 77]. Reduced neutrophil migratory accuracy and reduced bacterial killing has been identified in hyperglycaemia and replicated in vitro following neutrophil exposure to serum from diabetic patients [78]. In vitro, neutrophils have also been implicated in cell-to-cell crosstalk, with neutrophil microvesicles associated with inducing classical pathological changes in diabetic cardiovascular pathology [79] and COPD [80]. Furthermore, age-related changes seen in the muscle (e.g. sarcopenia) are also associated with an increase in neutrophil numbers: one study found a correlation between frailty and increased baseline neutrophil numbers [81] and a potential decline in neutrophil function [82], providing evidence for the role of neutrophil dysfunction to cause systemic ageing pathologies.
There is evidence that removal of senescent cells (senolysis) has a positive effect on age-related diseases [83] and age-related physical dysfunction in mice [84], including sarcopenia, cataracts and atherosclerosis via an inducible transgene that can deplete senescent cells [85, 86]. These transgenic approaches do not directly translate to a viable treatment in humans but targeting other features of senescence (such as SAβG activity) may be a therapeutic option. A first-in-human study has suggested that senolytics are safe and improved physical function in patients with idiopathic pulmonary fibrosis [87]. Preclinical studies in T2D identified that replicative-senescent β-cells actually contributed more to insulin production than nonsenescent cells [88], yet targeting senescent cells intermittently in mice still improved glucose tolerance and increased insulin sensitivity [89]. However, not all results are positive; in atherosclerosis, quercetin (a senolytic) was associated with nonsenescent cell death in primary human coronary artery endothelial cells [90], despite earlier studies suggesting potential benefits [91]. This suggests caution is required within this field and more trials are eagerly awaited.
Theme 2: systemic inflammation and overspill in multimorbidity
Driven by the primary hallmarks of ageing, there is low-grade inflammation in older individuals which is similar to the systemic inflammation seen in chronic disease, a phenomenon termed “inflammaging” [29]. In disease, this may be further exaggerated and although many inflammatory cytokines have been shown to be raised in COPD, atherosclerosis and T2D, the significant overlap present in inflammation means it is difficult to identify an initiating signal. Tumour necrosis factor (TNF)-α increases with increasing age [30] and has been repeatedly implicated in COPD, atherosclerosis and T2D (figure 2) and, therefore, will be used as example mediator of systemic inflammation; however, other cytokines, including interleukin (IL)-6 and IL-1β, have multiple systemic effects and are likely to be involved in multimorbidity as well (reviewed elsewhere [30]). The inflammasome (a multiprotein complex that regulates inflammatory cytokine production, including IL-1β) has also been described as being of importance in some models of COPD [92], atherosclerosis and T2D, and is again reviewed elsewhere [93].
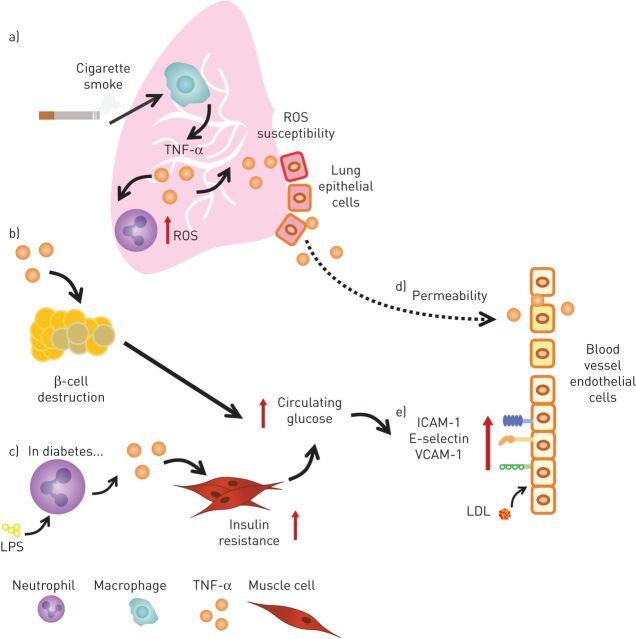
FIGURE 2.
A summary of the multiple influences of tumour necrosis factor (TNF)-α across COPD, type-2 diabetes mellitus (T2D) and atherosclerosis. TNF-α is produced by multiple cell types in various compartments of the body. a) Cigarette smoke increases TNF-α production by alveolar macrophages, leading to enhanced reactive oxygen species (ROS) production by neutrophils and increased ROS susceptibility of lung epithelial cells. b) From the perspective of T2D, circulating TNF-α can cause β-cell destruction that in turn leads to increases in blood glucose levels. c) Enhanced TNF-α production by neutrophils exposed to lipopolysaccharide (LPS) in patients with diabetes promotes insulin resistance, which also increases blood glucose levels. Both the mechanisms in b and c lead to glucose-driven adhesion molecule expression in the vasculature. d) TNF-α also increases vascular permeability and in COPD this can be from either TNF-α produced in circulation or diffusion of pulmonary TNF-α. e) In the vasculature, TNF-α increases adhesion molecules, such as intracellular adhesion molecule (ICAM)-1, E-selectin and vascular adhesion molecule (VCAM)-1, increasing the likelihood of immune cell infiltration. It also causes the promotion of low-density lipoprotein (LDL) uptake by endothelial cells.
Some studies have demonstrated an association with COPD severity and concentrations of systemic TNF-α, for example Singh et al. [38], although this has not been universally replicated [94], potentially indicating heterogeneity within patient populations. Macrophages produce TNF-α in response to cigarette smoke extract in vitro [95] and in vivo using smoke-exposed rats (figure 2a) [96]. Alveolar macrophages appear to require exposure to both a Toll-like receptor agonist and cigarette smoke extract in vitro to increase TNF-α production [97], a mechanism relevant in the lungs, where both smoke and bacteria are present [98]. While TNF-α is also present in smokers without COPD, multiple studies investigating inflammation in COPD and smoking do not account for the fact that only a proportion of smokers develop COPD. Despite this, TNF-α is associated with multiple facets of COPD in murine and cell models, including: mucus hypersecretion [99]; matrix remodelling [100]; repair and maintenance contributing to emphysema [100]; and corticosteroid insensitivity through the reduction of histone deacetylase-2 activity [101]. While these data suggest that TNF-α has a role in the pathogenesis of COPD, potentially exaggerated in patients with multiple lung infections, much of this evidence comes from animal models.
TNF-α concentrations are also elevated in the plasma of patients with an acute myocardial infarction [102] and in the adipose tissue and serum of obese patients with CVD [103]. TNF-α is implicated in insulin resistance and β-islet cell dysfunction in murine models, suggesting that IL-1β and TNF-α treatment result in a loss of the transcription factor FoxO1, which is required for maintaining β-cell identity (figure 2b) [104, 105]. TNF-α murine knockout models are more resistant to developing atherosclerosis [106], emphysema [107], endothelial dysfunction in diabetes [24] and late-stage diabetic retinopathy [108]. Neutrophils isolated from patients with T2D release larger amounts of proinflammatory cytokines, including TNF-α and IL-6 at baseline and in response to lipopolysaccharide (LPS) stimulation (figure 2c) [39]. It is possible that this response is similar to the enhanced TNF-α production in response to LPS seen in alveolar macrophages from patients with COPD [98], but further work is required to fully understand the impact of this neutrophil response to disease pathology. Furthermore, neutrophils isolated from patients with COPD released more proinflammatory cytokines in response to LPS in the presence of TNF-α [109], demonstrating a possible feedback loop between raised TNF-α production during infection and further proinflammatory signalling by neutrophils in COPD and T2D.
Collectively, these data suggest that TNF-α is elevated in COPD, atherosclerosis and T2D, and may contribute to individual diseases. One major caveat with many of the discussed studies is the lack of stratification of patients based on their comorbidities, and therefore it is likely that patients in these studies may already have a combination of COPD, atherosclerosis or T2D. Without this detailed information, it is impossible to fully understand the mechanisms underpinning pathology of a single disease, and to dissect the potential shared mechanisms in multimorbid patients. However, if inflammatory cytokines are to have a major role in multimorbidity, inflammation in one compartment needs to influence other compartments in the body. “Overspill” may be a means to achieve this, potentially unifying disease-related processes, and again although other cytokines are implicated in this, TNF-α is used as an example.
Evidence for overspill
Overspill is readily envisaged in atherosclerosis, where vascular plaques permit cytokines to enter the bloodstream causing systemic inflammation [36]. However, overspill is also highly likely during local tissue inflammation (e.g. in the lung) with increased vascular permeability permitting the spread of inflammation into the systemic circulation (figure 2d) [110, 111]. Increased circulating inflammatory cytokines also correlate with frailty and the development of sarcopenia in several epidemiological studies [82], providing a basis for chronically raised cytokine levels to influence multiple areas of the body.
In patients with COPD, the levels of IL-1β and TNF-α in the sputum of patients with COPD negatively correlated with body mass index, indicating a potential link between local disease in the lungs and systemic low-grade inflammation in the form of obesity [112]. However, in this study, sputum TNF-α concentrations did not correlate with plasma level [112], but this may reflect the short half-life of free TNF-α in biological fluids and the dilution of biological fluids, as seen in induced sputum and lavage samples [113], which are important considerations when interpreting systemic cytokine levels in disease. Despite the dilution of any cytokine overspill from the lungs into the blood, some studies suggest that systemic inflammation could drive the pathologies of COPD: a study of never-smoking patients with rheumatoid arthritis described some participants as having clear evidence of emphysema on computed tomography scans [114]. The exact mechanism of this observation has not been investigated but is likely to involve a combination of genetic and environmental factors alongside systemic inflammation. A study using a mouse model of COPD further supported the ability of lung inflammation to influence the vascular environment, as mice overexpressing IL-18, another inflammatory cytokine, developed impaired glucose tolerance over time [115].
A mechanism that could unify changes across diseases is the ability of TNF-α to increase the expression of capture receptors and adhesion molecules on the surface of blood vascular endothelial cells that are necessary to support leukocyte ingress into inflamed tissue [116]; a response that can be blocked using TNF-α inhibitors (figure 2e) [117]. Systemic changes in the expression of adhesion molecules by blood vascular endothelium also occur in patients with T2D, potentially preceding the onset of T2D [118] and modification of vascular adhesion molecule-1 has been linked to early atherosclerosis progression in cell lines from in vitro and in vivo studies [119, 120]. TNF-α also increased low-density lipoprotein uptake in vascular endothelium in vitro, a process known to promote atherosclerosis and further proinflammatory signalling (figure 2e) [40]. In patients with COPD, the influx of leukocytes into the lungs contributes to lung damage through protease and ROS, a process which TNF-α can enhance, further contributing to damage [121]. Furthermore, TNF-α increased the susceptibility of pulmonary vascular endothelium to ROS in vitro by reducing glutathione levels intracellularly, presenting a mechanism of enhanced damage of pulmonary endothelium in oxidative environments (figure 2a) [122].
Matrix remodelling promoted by TNF-α through matrix metalloproteinases (MMPs) may also provide a link between COPD, CVD and T2D, albeit through different mechanisms. Matrix remodelling through increased MMP-9 and MMP-2 activity have been linked to increased lung destruction and inflammation in COPD [100]. In CVD, MMPs have been shown to promote the occlusion of blood vessels during anthogenesis by enhancing blood clotting [123] and MMP-9 specifically has been linked with familial history of T2D and with increasing insulin resistance [124]. Matrix remodelling has also been associated with ageing in multiple studies, potentially due to age-related loss of functionality of cells responsible for maintaining the extracellular matrix [125].
These data present complex but unifying mechanisms of systemic inflammation in the pathogenesis of COPD, T2D and atherosclerosis. However, data from clinical studies of inflammatory processes have ambivalent results. Retrospective studies have identified a potential benefit for patients with T2D receiving anti-TNF-α therapy for rheumatoid arthritis [126], although no data currently exist to support the use of anti-TNF therapy for the sole purpose of treating T2D. Anti-TNF-α therapy (again used for rheumatoid arthritis) has also been reported to reduce hospitalisation due to COPD exacerbations [126], but a trial of specific anti-TNF-α therapy (infliximab) in unselected patients with COPD was not associated with improvements in health or lung function [127]. Patients on anti-TNF-α therapy for psoriasis were noted to have a reduced incidence of myocardial events in population studies [128] and patients with rheumatoid arthritis with similar treatment showed a reduction in aortic stiffness, linked to atherosclerosis progression [129]. Although etanercept, another TNF-α inhibitor, reduced systemic inflammation following an acute myocardial infarction, increased platelet activation was noted (a key feature of atherosclerosis) with no change in peripheral vasomotor or fibrinolytic function [130].
Trials of other interventions to reduce the activity of specific inflammatory cytokines have also been equivocal. Patients with a previous myocardial infarction treated with an anti-IL-1β medication, canakinumab, demonstrated a reduction in cardiovascular events over the study period, but with no reduction in all-cause mortality [131], and the same drug provided no clinical benefit in COPD [132]. Targeting the IL-1β receptor has also proved inefficacious in COPD, with the most common adverse event being worsening of COPD symptoms [133]. As with other re-purposed interventions, the timing, patient group, dose and duration of treatment may be fundamental to determining a response in multimorbidity and it might be that selected populations would benefit through offering a personalised medicine strategy, if they could be identified. Another important consideration is the bioavailability of TNF-α, or indeed other inflammatory cytokines, in the circulation. This could be influenced by free TNF-α (usually referred to as soluble TNF-α), membrane-bound or vesicular TNF-α, with each potentially influencing potency and the ability to be inhibited in the circulation [134].
Theme 3: altered cellular subpopulations and processes in multimorbidity – the lung as a central hub
Immune cell subtypes have been recognised for many years and have been implicated in chronic disease and multimorbidity. Initially, neutrophils were excluded from this concept, considered too short lived and too restricted in their responses to impact across disease clusters. However, emerging evidence has questioned this, and proposed a novel hypothesis to explain why neutrophils and damaged lungs may place the host at a particularly increased risk of other chronic inflammatory illnesses [73]. While other cell types are involved in the development of multimorbidity, the potential role of the neutrophil and the lungs will be explored here.
Neutrophils are known to exist in three states; quiescent, primed and activated, and in order to release proteases or build the reduced nicotinamide adenine dinucleotide phosphate oxidase (NOX) complex needed to generate ROS [135], neutrophils require significant stimulation to move from the quiescent to the activated state [136]. This system exists to prevent unwarranted host damage. Once cells become primed, activation occurs more readily and for several years it has been recognised that neutrophils can oscillate between these states, depending on the environment. Recent, elegant in vivo studies have shown that in health, human neutrophils do not coalesce in the lungs (as previously suggested) and instead pass through the pulmonary capillaries only marginally slower than red blood cells [137]. However, the transit time is much slower if neutrophils are primed, as has been shown in patients that have inflammatory lung diseases such as COPD [138] or even low-grade inflammation [139]. The reason for this slower transit time is thought to reflect that primed neutrophils adopt cytoskeletal changes which makes them stiffer and less deformable compared to quiescent cells, impacting on their ability to squeeze through the narrow capillaries. However, when neutrophils are artificially primed ex vivo and then reintroduced to the circulation, this retention signal in the lungs is steadily lost, suggesting these cells are either cleared from the body or are deprimed [137]. Forced mechanical deformation ex vivo of neutrophils has been shown to deprime neutrophils [140], and it has been suggested that the lungs may form an important site where neutrophils, primed through exposure to circulating inflammation, may return to the quiescent state because of mechanical modulation in the tight pulmonary capillaries. The narrow pulmonary vasculature might, therefore, have important functions for immunomodulation.
In chronic lung disease, the slower transit time persists, suggesting primed cells remain in the systemic circulation, perhaps caused by the pulmonary vasculature destruction and remodelling present in COPD (preventing depriming by mechanical manipulation of neutrophils) and the proinflammatory environment of the lungs [138]. These primed cells would be much more susceptible to respond to an inflammatory signal in another organ, perhaps linking lung disease with other chronic inflammatory pathologies. Furthermore, human neutrophils can reverse migrate in vitro through the endothelium and back into the circulation [73], after sampling inflamed tissues. These previously tissue-bound cells maintain a proinflammatory and apoptotic-resistant phenotype, which could provide a link between chronic inflammation and systemic damage in COPD, atherosclerosis and T2D as they circulate around the body [73]. This mechanism, however, would rely on COPD or associated lung damage being the initiator disease. While hypothetically low-grade inflammation may prime neutrophils, and subsequently add to the burden of disease and contribute to the pathogenesis of multimorbidity [139], this field is in its infancy and the evidence (although generally supportive) remains patchy.
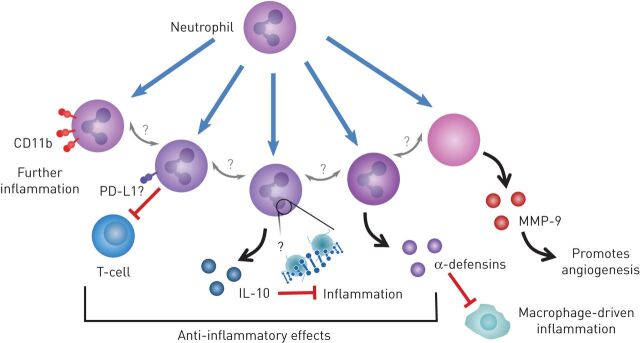
There is increasing evidence for neutrophil heterogeneity and plasticity in terms of cellular responses and surface expression of receptors, with both pro and anti-inflammatory subpopulations described (figure 3) [141, 142]. An anti-inflammatory and pro-resolving neutrophil phenotype have been described, where the secretion of α-defensins modifies the inflammatory response of macrophages [143] and neutrophils can also modify angiogenic processes with the secretion of MMP-9 [144], as well as their typical proinflammatory responses. It is unclear whether the presence of chronic inflammatory disease in an ageing host leads to a loss of neutrophil plasticity, a change in phenotype or merely a priming of cells, but there is clear evidence of increased neutrophil activity and dysfunction across COPD, atherosclerosis and T2D (as described earlier) and studies are describing differences in neutrophil populations between patients with COPD and controls [145] and in murine models of atherosclerosis [146], although this field is in its infancy.
FIGURE 3.
Neutrophil phenotypes can be altered, and these changes may impact the pathogenesis of other diseases. Several neutrophil phenotypes are described showing the plasticity of the neutrophil. Increasing surface expression of CD11b has been linked with further inflammation and therefore a proinflammatory phenotype. Several anti-inflammatory phenotypes have also been described: the suppression of T-cell function; the release of an anti-inflammatory cytokine interleukin (IL)-10; and the release of α-defensins shown to inhibit macrophage-driven inflammation by preventing mRNA translation. Neutrophils also release matrix metallopeptidase (MMP)-9, which contributes to matrix remodelling and promotes angiogenesis impacting on vascular remodelling in disease. This shows a broad array of inflammatory functions by the neutrophil. PD-L1: programmed-death ligand 1.
Key cellular processes may also link multimorbidities. ROS are known to cause damage in biological systems, including within settings of chronic inflammation [147], and are linked to ageing as described by the free radical theory of ageing [148]. Neutrophils have enhanced ROS responses in patients with COPD, especially with bacterially induced exacerbations [149, 150]. Raised markers of oxidative stress have been identified in the sputum and plasma of patients with COPD, related to increased ROS and also decreased antioxidant activity [151]. This increase in ROS production in the lungs presents a potential mechanism for local damage in patients with COPD. There is also evidence of systemic ROS activity in COPD, potentially predisposing to events seen in the pathogenesis of atherosclerosis and T2D [151, 152]. In atherosclerosis, upregulation of NOX2 expression has been described before atherosclerosis develops [153] and in diabetes, ROS production can contribute to β-cell destruction, as these cells are particularly sensitive to ROS-induced apoptosis [154]. NOX1 has also been shown to play a role in diabetes-associated complications from human in vitro and murine in vivo experiments: human aortic endothelial cells and cells in the renal cortex upregulate NOX1 levels in response to hyperglycaemic conditions [155] and NOX1 inhibition in vivo is associated with a reduction in atherosclerosis and reduced adhesion of inflammatory cells to the vascular wall [156]. Data sourced from the National Institutes of Health (ClinicalTrials.gov) show several trials of antioxidants in COPD, diabetes and atherosclerosis, suggesting there remains great interest in mitigating ROS-related damage in chronic disease.
Interwoven processes require an integrated approach
Current management strategies for COPD, T2D and atherosclerosis have improved outcomes for patients but have not halted disease processes. This might suggest we need a new therapeutic approach. The mechanistic themes outlined above (ageing and senescence, inflammation and cellular dysfunction) appear interwoven; each associated with each other. There are clear links with altered cellular functions, inflammation and senescence, with impaired immune cell surveillance associated with a higher burden of senescent cells [157], a greater burden of SASP and the associated systemic and tissue inflammation [36, 41, 112]. Once present, inflammation, including TNF-α, serves as an important priming agent for the oxidative burst [121]. ROS can damage cells and tissues, instigate further proinflammatory responses and exacerbate DNA damage. This proinflammatory milieu is associated with epigenetic modification and RTL shortening, a loss of proteostasis and a greater burden of senescent cells, in what is considered a continuing cycle of damage and disease progression. These processes are more apparent in our globally ageing population. However, more research is needed. Not all environmentally exposed individuals develop disease (for example, not all smokers develop COPD or atherosclerosis), Although there are clear clusters, not all chronic inflammatory conditions appear together, and age or environmental exposures alone do not predict those at most risk [10, 31]. It is unclear whether the biological hallmarks of ageing all present together in susceptible individuals, or whether one hallmark precedes the others. It is also unclear whether biological ageing is ubiquitous across tissues, or whether certain tissues, such as the lung with its high exposure to environmental toxins and bacteria, are more affected. The cellular dysfunction seen in COPD, atherosclerosis and T2D share common features, including altered neutrophil migratory dynamics and impaired phagocytic responses, but do not seem to only reflect a senescent cell. Perhaps it is the combination of chronic pulmonary and systemic inflammation, the presence of hyperglycaemia and the inability to deprime cells in damaged lungs in an ageing host that makes the combination of these conditions so dangerous for an individual.
A challenge for treating multimorbidity also lies in healthcare procedures, as the initial disease diagnosis usually dictates the treatment pathway. If these interwoven inflammatory mechanisms underly the disease process, identification of the predominant pathway, as opposed to the first diagnosed disease, may improve treatment outcomes. This is also met with a relatively poor understanding of early disease with increasing recognition that our diagnostic criteria may not identify early disease. For example, in COPD, approximately 70% of small airways are lost prior to the development of airflow obstruction; T2D is preceded with a period of “prediabetes” where blood sugars are raised, and atherosclerosis is usually only diagnosed when there is flow-limiting disease associated with symptoms. Identifying the predominant pathways in individual patients, while having the potential to provide therapeutic benefits, will require large, longitudinal and deeply phenotyped patient cohorts across disease and science silos.
Current studies of these mechanisms have often been conducted with one condition central to the study without considering the other diseases. Ongoing studies in each of the topics discussed in this article are summarised in table 1 and highlight the segregation of these studies, but also the lack of novel drugs and the current focus on diet and exercise intervention. To understand these processes in more depth will require exacting science in carefully characterised patients with a cross-disciplinary approach.
TABLE 1.
Key ongoing or recently completed interventional trials in COPD, type-2 diabetes mellitus (T2D) and cardiovascular disease (CVD) related to the mechanisms of ageing, inflammation and cellular phenotype
| Study name | Study identifier | Study overview | Disease area | Phase |
| Ageing | ||||
| HIV-related Accelerated Aging of the Airway Epithelium | NCT01974219 | Observational study investigating the role HIV may play in the accelerated ageing of the small airway endothelium and development of COPD | COPD | N/A |
| Pulmonary Rehabilitation Innovation and Microbiota in Exacerbations of COPD (PRIME) | NCT03701945 | An interventional study to investigate how the microbiota of the lungs contributes to pulmonary ageing and AECOPD, and the impact pulmonary rehabilitation has on AECOPD | COPD | N/A |
| In Utero Smoking and Premature Cellular Senescence | NCT01865435 | A trial to investigate telomere length as a surrogate for cellular senescence in peripheral lymphocytes obtained from the cord blood of newborns from mothers with absence of smoking or smoking >5 cigarettes per day | COPD | N/A |
| Nutritional and Functional Changes in Heart Failure and COPD | NCT01787682 | An interventional trial to investigate gut absorption in patients with CVD or COPD and if this can be improved with a high protein supplement to reduce muscle loss in these patients | CVD, COPD | N/A |
| Cardio-vascular Protective Effects of Wolfberry in Middle-aged and Older Adults | NCT03535844 | A single-blind 16-week study investigating the protective effects of Wolfberry in positively changing endothelial function and lipidomic profiles | CVD | N/A |
| Regulation of Endothelial Progenitor Cells by Short-Term Exercise (EPC-Ex) | NCT01169831 | An open interventional study investigating the effects of exercise on endothelial progenitor cell numbers in sedentary older adults and older endurance athletes | CVD | N/A |
| Prevention of Cardiovascular Stiffening With Aging and Hypertensive Heart Disease (LVH) | NCT03476785 | An open interventional trial to assess how exercise intervention prevents the age-related stiffening of the left ventricule and vasculature | CVD | N/A |
| n-3 PUFA for Vascular Cognitive Aging | NCT01953705 | A 3-year interventional study to investigate if omega 3 PUFA can support small blood vessels in the brain and promote brain health in adults >75 years of age with a high risk of cognitive decline | CVD | 2 |
| Impact of Ageing on Adipose, Muscle and Systemic Inflammation | NCT02777138 | Observational study of macrophage and T-cells in adipose tissue and inflammatory markers (mRNA and protein secretions) between a group of younger and older males with similar lifestyles | CVD, T2D | N/A |
| Cell Signaling and Resistance to Oxidative Stress: Effects of Aging and Exercise | NCT03419988 | An open interventional study investigating the effects of exercise on the expression of an antioxidant regulation protein nuclear erythroid-2-p45-related factor-2 (Nrf2) in peripheral blood mononuclear cells in healthy younger (18–28 years) and older (>60 years) adults | CVD, T2D | N/A |
| Resistance Exercise and Low-Intensity Physical Activity Breaks in Sedentary Time to Improve Muscle and Cardiometabolic Health (REALPA) | NCT03771417 | An interventional study to assess the impact of resistance exercise with low-intensity physical activity breaks on skeletal muscle and cardiometabolic health in adults aged 65–80 years | CVD, T2D | N/A |
| Dietary Reduction of AGEs to Prevent Cognitive Decline in Elderly Diabetics | NCT02739971 | An intervention pilot study to investigate if it is feasible to reduce dietary AGEs and therefore reduce cognitive decline | T2D | N/A |
| Inflammation | ||||
| Efficacy of Periodontal Treatment on Systemic Inflammation and for Prevention of Exacerbations in Patients With COPD (Expertention) | NCT03279718 | An interventional pilot study to investigate if periodontal treatment can reduce systemic inflammatory markers (including CRP and IL-1b and IL-6) | COPD | N/A |
| INvestigating COPD Outcomes, Genomics and Neutrophilic Inflammation With Tiotropium and Olodaterol (INCOGNITO) | NCT03152149 | An open-label interventional trial comparing treatment of patients with COPD with tiotropium and olodaterol reduces bacterial load and neutrophilic inflammation versus inhaled fluticasone furoate and vilanterol | COPD | 4 |
| Pivotal Study to Assess the Efficacy, Safety and Tolerability of Dupilumab in Patients With Moderate-to-severe COPD With Type 2 Inflammation (BOREAS) | NCT03930732 | An interventional study to assess the efficacy of dupilumab (an IL-4 receptor alpha antibody) in reducing the annual exacerbation rate in patients with moderate-to-severe COPD | COPD | 3 |
| Biological Effects of Quercetin in COPD | NCT03989271 | A study to determine if a dietary supplement, quercetin, can enhance anti-inflammatory effects in patients with COPD and reduce markers of oxidative stress and inflammation | COPD | 1/2 |
| Anti-ST2 (MSTT1041A) in COPD (COPD-ST2OP) | NCT03615040 | An interventional study to assess the use of an IL-33 inhibitor on the frequency of AECOPD events in patients with COPD over 48 weeks | COPD | 2 |
| A 12-week Study Treating Participants Who Have alpha1-antitrypsin-related COPD With Alvelestat (MPH966) or Placebo (ASTRAEUS) | NCT03636347 | An interventional study investigating the effects of a neutrophil elastase inhibitor in patients with α1-antitrypsin deficient Pizz or null phenotype with COPD. Outcomes include sputum and blood inflammatory biomarkers, including neutrophil elastase activity. | COPD | 2 |
| Effect of IL-1β Inhibition on Inflammation and Cardiovascular Risk | NCT02272946 | An interventional study to investigate the effects of IL-1β inhibitor canakinumab in reducing vascular inflammation in HIV-infected individuals | CVD | 2 |
| ASSessing the Effect of Anti-IL-6 Treatment in Myocardial Infarction: The ASSAIL-MI Trial (ASSAIL-MI) | NCT03004703 | An interventional study to assess the impact of a single administration of an anti-IL-6 antibody, tocilizumab, on myocardial damage following myocardial infarction | CVD | 2 |
| Effects of SGLT-2 Inhibition on Myocardial Fibrosis and Inflammation as Assessed by Cardiac MRI in Patients With DM2 | NCT03782259 | An interventional study to investigate how inhibition of a glucose transporter, SGLT-2, with dapagliflozin impacts cardiovascular health and inflammation in patients with T2D | CVD, T2D | 4 |
| Cellular processes | ||||
| Early iNO for Oxidative Stress, Vascular Tone and Inflammation in Babies With Hypoxic Respiratory Failure | NCT01891500 | An interventional study to investigate an already approved intervention, inhaled nitric oxide, in newborns with hypoxic respiratory failure to reduce biomarkers of oxidative injury | CVD | 4 |
| Impacts of Mitochondrial-targeted Antioxidant on Peripheral Artery Disease Patients | NCT03506633 | An interventional study to examine the impact of a mitochondrial antioxidant (MitoQ) on vascular endothelial function in patients with peripheral vascular disease | CVD | N/A |
| Effects of Saxagliptin on Adipose Tissue Inflammation in Humans | NCT02285985 | An interventional study to investigate the effects of saxagliptin on adipose tissue inflammation in obese participants | T2D | 4 |
AECOPD: acute exacerbations of COPD; PUFA: polyunsaturated fatty acid; AGEs: advanced glycation end-products; CRP: C-reactive protein; IL: interleukin; MRI: magnetic resonance imaging; DM2: type-2 diabetes; N/A: not available; iNO: inhaled nitric oxide.
It is also important to understand the relative contributions of each mechanism (ageing and senescence, inflammatory cytokines, altered cell processes) to each disease (COPD, CVD and T2D). The limited success of single cytokine therapies [129, 158–160], clearly demonstrates these diseases are complex and that each mechanism is just a piece of the puzzle. Identifying the relative contribution is challenging, as existing models usually allow either enough resolution to determine a single mechanistic effect, or identify broad changes without ascertaining the mechanism or the role of each disease in multimorbid patients. Addressing this challenge relies on a careful balance of in vivo models and in vitro human studies to stitch together the bigger picture, incorporating novel methods of network analysis and systems biology [161], and stratification of multimorbid patients in clinical studies, not just reporting them. This is further confounded by many studies not detailing the comorbidity of their patient populations; highlighted by one study that demonstrated the burden of comorbidity in COPD as of 8656 patients diagnosed with COPD, 1631 participants had an existing diagnosis of CVD or T2D and found associations with systemic inflammation and hospitalisation due to these comorbidities [162]. We might be missing important links in the mechanism of each disease because the multimorbid status of patients is not known or reported.
Conclusion
Finding treatments for multimorbidity is challenging but it is important to consider diseases holistically and not in isolation, as many pathological processes appear to be shared. There are multiple ways that COPD, atherosclerosis and T2D are linked, not just through shared risk factors but also through inflammation, ROS production, senescence and accelerated ageing and altered cellular functions and phenotypes. These processes are inter-related, each influencing the other and understanding the fine balance between positive cellular responses and damaging ones in health and across diseases may revolutionise therapy for chronic inflammatory diseases. Indeed, advancements in our understanding of chronic diseases are already suggesting new potential therapeutic strategies that bridge traditional disease silos; however, these are still in their infancy. The appreciation of neutrophil heterogeneity and the roles these cells play across chronic illness may also highlight potential treatment targets. Manipulation of T-cells and dendritic cells has yielded novel cancer therapies and the same concept might support new approaches for treating chronic inflammatory conditions [163, 164].
Most translational studies of COPD have focused on the lung disease and not fully characterised the burden of multimorbidity, but perhaps now is the time for a more thorough clinical assessment of the patients included in discovery science studies, detailing multimorbidity where it is present. It may be possible that systemic inflammation is simultaneously influencing the pathogenesis of COPD, atherosclerosis and T2D, yet limitations in diagnosis result in one being diagnosed first. Multimorbidity is also an important consideration for future clinical trials, as an intended target in one condition might improve an outcome for a related chronic disease, if we only had the foresight to look.
Footnotes
Conflict of interest: M.J. Hughes reports grants from Wellcome Trust, during the conduct of the study.
Conflict of interest: H.M. McGettrick has nothing to disclose.
Conflict of interest: E. Sapey reports grants from Wellcome Trust, British Lung Foundation, Medical Research Council, NIHR and Dunhill Trust, outside the submitted work.
Provenance: Submitted article, peer reviewed.
Support statement: This study was funded by the Wellcome Trust (203823/Z/16/Z). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Palladino R, Tayu Lee J, Ashworth M, et al. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing 2016; 45: 431–435. doi: 10.1093/ageing/afw044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37–43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 3.Lee CTC, Mao IC, Lin CH, et al. Chronic obstructive pulmonary disease: a risk factor for type 2 diabetes. A nationwide population-based study. Eur J Clin Invest 2013; 43: 1113–1119. [DOI] [PubMed] [Google Scholar]
- 4.Mullerova H, Agusti A, Erqou S, et al. Cardiovascular comorbidity in COPD: systematic literature review. Chest 2013; 144: 1163–1178. doi: 10.1378/chest.12-2847 [DOI] [PubMed] [Google Scholar]
- 5.Laniado-Laborín R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int J Environ Res Public Health 2009; 6: 209–224. doi: 10.3390/ijerph6010209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SC. Multiple risk factors for cardiovascular disease and diabetes mellitus. Am J Med 2007; 120: S3–11. doi: 10.1016/j.amjmed.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 7.Huang Z-S, Jeng J-S, Wang C-H, et al. Correlations between peripheral differential leukocyte counts and carotid atherosclerosis in non-smokers. Atherosclerosis 2001; 158: 431–436. doi: 10.1016/S0021-9150(01)00445-2 [DOI] [PubMed] [Google Scholar]
- 8.Foy CG, Bell RA, Farmer DF, et al. Smoking and incidence of diabetes among US adults: findings from the Insulin Resistance Atherosclerosis Study. Diabetes Care 2005; 28: 2501–2507. doi: 10.2337/diacare.28.10.2501 [DOI] [PubMed] [Google Scholar]
- 9.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, et al. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes 2014; 5: 444–470. doi: 10.4239/wjd.v5.i4.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ording AG, Sørensen HT. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol 2013; 5: 199–203. doi: 10.2147/CLEP.S45305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goh K-I, Cusick ME, Valle D, et al. The human disease network. Proc Natl Acad Sci USA 2007; 104: 8685–8690. doi: 10.1073/pnas.0701361104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosdidier S, Ferrer A, Faner R, et al. Network medicine analysis of COPD multimorbidities. Respir Res 2014; 15: 111. doi: 10.1186/s12931-014-0111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenwood H, Patel J, Mahida R, et al. Simvastatin to modify neutrophil function in older patients with septic pneumonia (SNOOPI): study protocol for a randomised placebo-controlled trial. Trials 2014; 15: 332. doi: 10.1186/1745-6215-15-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walton GM, Stockley JA, Griffiths D, et al. Repurposing treatments to enhance innate immunity. Can statins improve neutrophil functions and clinical outcomes in COPD? J Clin Med 2016; 5: E89. doi: 10.3390/jcm5100089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu C-S, Tseng P-T, Stubbs B, et al. Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta-analysis. Sci Rep 2018; 8: 5804. doi: 10.1038/s41598-018-24248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med 2013; 11: 117. doi: 10.1186/1741-7015-11-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuleta I, Farrag T, Busse L, et al. High prevalence of COPD in atherosclerosis patients. Int J Chron Obstruct Pulmon Dis 2017; 12: 3047–3053. doi: 10.2147/COPD.S141988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosny H, Abdel-Hafiz H, Moussa H, et al. Metabolic syndrome and systemic inflammation in patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc 2013; 62: 85–89. doi: 10.1016/j.ejcdt.2013.02.007 [DOI] [Google Scholar]
- 19.Liu J, Pang Z, Wang G, et al. Advanced role of neutrophils in common respiratory diseases. J Immunol Res 2017; 2017: 6710278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro SD, Goldstein NM, Houghton AM, et al. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol 2003; 163: 2329–2335. doi: 10.1016/S0002-9440(10)63589-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ionita MG, van den Borne P, Catanzariti LM, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol 2010; 30: 1842–1848. doi: 10.1161/ATVBAHA.110.209296 [DOI] [PubMed] [Google Scholar]
- 22.Fainsod-Levi T, Gershkovitz M, Völs S, et al. Hyperglycemia impairs neutrophil mobilization leading to enhanced metastatic seeding. Cell Rep 2017; 21: 2384–2392. doi: 10.1016/j.celrep.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 23.Meigs JB, Hu FB, Rifai N, et al. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004; 291: 1978–1986. doi: 10.1001/jama.291.16.1978 [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Lee S, Zhang H, et al. Interaction of IL-6 and TNF-α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS One 2017; 12: e0187189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sapey E, Greenwood H, Walton G, et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 2014; 123: 239–248. doi: 10.1182/blood-2013-08-519520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Integr Med (Encinitas) 2014; 13: 35–43. [PMC free article] [PubMed] [Google Scholar]
- 27.Naik D, Joshi A, Paul TV, et al. Chronic obstructive pulmonary disease and the metabolic syndrome: consequences of a dual threat. Indian J Endocrinol Metab 2014; 18: 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013; 153: 1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol Ser A Biol Sci Med Sci 2014; 69: S4–S9. doi: 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 30.Brüünsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am 2003; 23: 15–39. doi: 10.1016/S0889-8561(02)00056-5 [DOI] [PubMed] [Google Scholar]
- 31.Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015; 6: 1246–1258. doi: 10.4239/wjd.v6.i13.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brook RD, Anderson JA, Calverley PMA, et al. Cardiovascular outcomes with an inhaled beta2-agonist/corticosteroid in patients with COPD at high cardiovascular risk. Heart 2017; 103: 1536–1542. doi: 10.1136/heartjnl-2016-310897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front public Heal 2017; 5: 335. doi: 10.3389/fpubh.2017.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisel KC, Bautz F, Seitz G, et al. Modulation of CXC chemokine receptor expression and function in human neutrophils during aging in vitro suggests a role in their clearance from circulation. Mediators Inflamm 2009; 2009: 790174. doi: 10.1155/2009/790174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Deursen JM. The role of senescent cells in ageing. Nature 2014; 509: 439–446. doi: 10.1038/nature13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min X, Lu M, Tu S, et al. Serum cytokine profile in relation to the severity of coronary artery disease. Biomed Res Int 2017; 2017: 4013685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei J, Xiong X-F, Lin Y-H, et al. Association between serum interleukin-6 concentrations and chronic obstructive pulmonary disease: a systematic review and meta-analysis. PeerJ 2015; 3: e1199. doi: 10.7717/peerj.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh S, Verma SK, Kumar S, et al. Correlation of severity of chronic obstructive pulmonary disease with potential biomarkers. Immunol Lett 2018; 196: 1–10. doi: 10.1016/j.imlet.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 39.Hatanaka E, Monteagudo PT, Marrocos MSM, et al. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol 2006; 146: 443–447. doi: 10.1111/j.1365-2249.2006.03229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Yang X, Bian F, et al. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-κB and PPAR-γ. J Mol Cell Cardiol 2014; 72: 85–94. doi: 10.1016/j.yjmcc.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 41.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax 2010; 65: 930–936. doi: 10.1136/thx.2009.130260 [DOI] [PubMed] [Google Scholar]
- 42.Yoshikawa T, Dent G, Ward J, et al. Impaired neutrophil chemotaxis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175: 473–479. doi: 10.1164/rccm.200507-1152OC [DOI] [PubMed] [Google Scholar]
- 43.Sadhu C, Masinovsky B, Dick K, et al. Essential role of phosphoinositide 3-kinase in neutrophil directional movement. J Immunol 2003; 170: 2647–2654. doi: 10.4049/jimmunol.170.5.2647 [DOI] [PubMed] [Google Scholar]
- 44.Shanmugam MK, Sethi G. Role of epigenetics in inflammation-associated diseases. Subcell Biochem 2013; 61: 627–657. [DOI] [PubMed] [Google Scholar]
- 45.Ventura MT, Casciaro M, Gangemi S, et al. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy 2017; 15: 21. doi: 10.1186/s12948-017-0077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samara KD, Tzortzaki EG, Neofytou E, et al. Somatic DNA alterations in lung epithelial barrier cells in COPD patients. Pulm Pharmacol Ther 2010; 23: 208–214. doi: 10.1016/j.pupt.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 47.Palazzo RP, Bagatini PB, Schefer PB, et al. Genomic instability in patients with type 2 diabetes mellitus on hemodialysis. Rev Bras Hematol Hemoter 2011; 34: 31–35. doi: 10.5581/1516-8484.20120011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cervelli T, Borghini A, Galli A, et al. DNA damage and repair in atherosclerosis: current insights and future perspectives. Int J Mol Sci 2012; 13: 16929–16944. doi: 10.3390/ijms131216929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol 2009; 71: 451–464. doi: 10.1146/annurev.physiol.010908.163257 [DOI] [PubMed] [Google Scholar]
- 50.Kong X, Fang M, Li P, et al. HDAC2 deacetylates class II transactivator and suppresses its activity in macrophages and smooth muscle cells. J Mol Cell Cardiol 2009; 46: 292–299. doi: 10.1016/j.yjmcc.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 51.Noh H, Oh EY, Seo JY, et al. Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-β1-induced renal injury. Am J Physiol Physiol 2009; 297: F729–F739. doi: 10.1152/ajprenal.00086.2009 [DOI] [PubMed] [Google Scholar]
- 52.Bodas M, Patel N, Silverberg D, et al. Master autophagy regulator transcription factor EB regulates cigarette smoke-induced autophagy impairment and chronic obstructive pulmonary disease–emphysema pathogenesis. Antioxid Redox Signal 2017; 27: 150–167. doi: 10.1089/ars.2016.6842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukherjee A, Morales-Scheihing D, Butler PC, et al. Type 2 diabetes as a protein misfolding disease. Trends Mol Med 2015; 21: 439–449. doi: 10.1016/j.molmed.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hofmann C, Katus HA, Doroudgar S. Protein misfolding in cardiac disease. Circulation 2019; 139: 2085–2088. doi: 10.1161/circulationaha.118.037417 [DOI] [PubMed] [Google Scholar]
- 55.Sadr M, Noori Mugahi SMH, Hassanzadeh G, et al. Telomere shortening in blood leukocytes of patients with chronic obstructive pulmonary disease. Tanaffos 2015; 14: 10–16. [PMC free article] [PubMed] [Google Scholar]
- 56.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res 2012; 730: 68–74. doi: 10.1016/j.mrfmmm.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savale L, Chaouat A, Bastuji-Garin S, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 179: 566–571. doi: 10.1164/rccm.200809-1398OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walters MS, De BP, Salit J, et al. Smoking accelerates aging of the small airway epithelium. Respir Res 2014; 15: 94. doi: 10.1186/s12931-014-0094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Everaerts S, Lammertyn EJ, Martens DS, et al. The aging lung: tissue telomere shortening in health and disease. Respir Res 2018; 19: 95. doi: 10.1186/s12931-018-0794-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Y, Cui W, Zhang D, et al. The shortening of leukocyte telomere length relates to DNA hypermethylation of LINE-1 in type 2 diabetes mellitus. Oncotarget 2017; 8: 73964–73973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willeit P, Willeit J, Brandstätter A, et al. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol 2010; 30: 1649–1656. doi: 10.1161/atvbaha.110.205492 [DOI] [PubMed] [Google Scholar]
- 62.Childs BG, Durik M, Baker DJ, et al. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 2015; 21: 1424–1435. doi: 10.1038/nm.4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 1995; 92: 9363–9367. doi: 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee BY, Han JA, Im JS, et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006; 5: 187–195. doi: 10.1111/j.1474-9726.2006.00199.x [DOI] [PubMed] [Google Scholar]
- 65.Zhu Y, Armstrong JL, Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 2014; 17: 324–328. doi: 10.1097/mco.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 66.Vasile E, Tomita Y, Brown LF, et al. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J 2001; 15: 458–466. doi: 10.1096/fj.00-0051com [DOI] [PubMed] [Google Scholar]
- 67.Libby P, Ridker PM, Maseri A, et al. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–1143. doi: 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 68.Cramer C, Freisinger E, Jones RK, et al. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev 2010; 19: 1875–1884. doi: 10.1089/scd.2010.0009 [DOI] [PubMed] [Google Scholar]
- 69.Noureddine H, Gary-Bobo G, Alifano M, et al. Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ Res 2011; 109: 543–553. doi: 10.1161/circresaha.111.241299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wrench C, Baker J, Fenwick P, et al. MicroRNA-34a drives small airway fibroblast cellular senescence in COPD. Eur Respir J 2017; 50: Suppl. 61, OA289. doi: 10.1183/1393003.congress-2017.oa289 [DOI] [Google Scholar]
- 71.Han H, Qu G, Han C, et al. MiR-34a, miR-21 and miR-23a as potential biomarkers for coronary artery disease: a pilot microarray study and confirmation in a 32-patient cohort. Exp Mol Med 2015; 47: e138–e138. doi: 10.1038/emm.2014.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen Y, Xu H, Pan X, et al. miR-34a and miR-125b are upregulated in peripheral blood mononuclear cells from patients with type 2 diabetes mellitus. Exp Ther Med 2017; 14: 5589–5596. doi: 10.3892/etm.2017.5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buckley CD, Ross EA, McGettrick HM, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol 2006; 79: 303–311. doi: 10.1189/jlb.0905496 [DOI] [PubMed] [Google Scholar]
- 74.Kaplon J, Zheng L, Meissl K, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 2013; 498: 109–112. doi: 10.1038/nature12154 [DOI] [PubMed] [Google Scholar]
- 75.Drew W, Wilson D V, Sapey E. Inflammation and neutrophil immunosenescence in health and disease: targeted treatments to improve clinical outcomes in the elderly. Exp Gerontol 2018; 105: 70–77. doi: 10.1016/j.exger.2017.12.020 [DOI] [PubMed] [Google Scholar]
- 76.Sapey E, Stockley JA, Greenwood H, et al. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011; 183: 1176–1186. doi: 10.1164/rccm.201008-1285OC [DOI] [PubMed] [Google Scholar]
- 77.Naccache PH, Lefebvre JS. A straight neutrophil path to healthy aging? Blood 2014; 123: 154–156. doi: 10.1182/blood-2013-11-538256 [DOI] [PubMed] [Google Scholar]
- 78.Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci 2016; 351: 201–211. doi: 10.1016/j.amjms.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 79.Gomez I, Ward B, Souilhol C, et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat Commun 2020; 11: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garner J, Soni S, O'Dea K, et al. Late breaking abstract – Intra-alveolar neutrophil-derived microvesicles: a biomarker of COPD severity. Eur Respir J 2018; 52: Suppl, 62, OA4921. doi: 10.1183/13993003.congress-2018.OA4921 [DOI] [Google Scholar]
- 81.Baylis D, Bartlett DB, Syddall HE, et al. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Omaha) 2013; 35: 963–971. doi: 10.1007/s11357-012-9396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson D, Jackson T, Sapey E, et al. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev 2017; 36: 1–10. doi: 10.1016/j.arr.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 83.Kirkland JL, Tchkonia T, Zhu Y, et al. The clinical potential of senolytic drugs. J Am Geriatr Soc 2017; 65: 2297–2301. doi: 10.1111/jgs.14969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med 2018; 24: 1246–1256. doi: 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011; 479: 232–236. doi: 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Childs BG, Baker DJ, Wijshake T, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016; 354: 472–477. doi: 10.1126/science.aaf6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 2019; 40: 554–563. doi: 10.1016/j.ebiom.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Helman A, Avrahami D, Klochendler A, et al. Effects of ageing and senescence on pancreatic β-cell function. Diabetes Obes Metab 2016; 18: 58–62. doi: 10.1111/dom.12719 [DOI] [PubMed] [Google Scholar]
- 89.Palmer AK, Xu M, Zhu Y, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 2019; 18: e12950. doi: 10.1111/acel.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang H V, Tran DT, Rebuffatti MN, et al. Investigation of quercetin and hyperoside as senolytics in adult human endothelial cells. PLoS One 2018; 13: e0190374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roos CM, Zhang B, Palmer AK, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016; 15: 973–977. doi: 10.1111/acel.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang W, Ni H, Wang H, et al. NLRP3 inflammasome is essential for the development of chronic obstructive pulmonary disease. Int J Clin Exp Pathol 2015; 8: 13209–13216. [PMC free article] [PubMed] [Google Scholar]
- 93.Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res 2015; 8: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sapey E, Ahmad A, Bayley D, et al. Imbalances between interleukin-1 and tumor necrosis factor agonists and antagonists in stable COPD. J Clin Immunol 2009; 29: 508–516. doi: 10.1007/s10875-009-9286-8 [DOI] [PubMed] [Google Scholar]
- 95.Demirjian L, Abboud RT, Li H, et al. Acute effect of cigarette smoke on TNF-α release by macrophages mediated through the erk1/2 pathway. Biochim Biophys Acta - Mol Basis Dis 2006; 1762: 592–597. doi: 10.1016/j.bbadis.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 96.Wang W, Li X, Xu J. Exposure to cigarette smoke downregulates β2-adrenergic receptor expression and upregulates inflammation in alveolar macrophages. Inhal Toxicol 2015; 27: 488–494. doi: 10.3109/08958378.2015.1075628 [DOI] [PubMed] [Google Scholar]
- 97.Metcalfe HJ, Lea S, Hughes D, et al. Effects of cigarette smoke on toll-like receptor (TLR) activation of chronic obstructive pulmonary disease (COPD) macrophages. Clin Exp Immunol 2014; 176: 461–472. doi: 10.1111/cei.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dickson RP, Erb-Downward JR, Martinez FJ, et al. The microbiome and the respiratory tract. Annu Rev Physiol 2016; 78: 481–504. doi: 10.1146/annurev-physiol-021115-105238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lora JM, Zhang DM, Liao SM, et al. Tumor necrosis factor-alpha triggers mucus production in airway epithelium through an Iκβ kinase β-dependent mechanism. J Biol Chem 2005; 280: 36510–36517. doi: 10.1074/jbc.M507977200 [DOI] [PubMed] [Google Scholar]
- 100.Thomson EM, Williams A, Yauk CL, et al. Overexpression of tumor necrosis factor-α in the lungs alters immune response, matrix remodeling, and repair and maintenance pathways. Am J Pathol 2012; 180: 1413–1430. doi: 10.1016/j.ajpath.2011.12.020 [DOI] [PubMed] [Google Scholar]
- 101.Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol 2011; 163: 29–43. doi: 10.1111/j.1476-5381.2010.01199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fahim MR, Halim SM, Kamel I. Tumor necrosis factor alpha in patients with acute myocardial infarction. Egypt J Immunol 2004; 11: 31–37. [PubMed] [Google Scholar]
- 103.Bilgic Gazioglu S, Akan G, Atalar F, et al. PAI-1 and TNF-alpha profiles of adipose tissue in obese cardiovascular disease patients. Int J Clin Exp Pathol 2015; 8: 15919–15925. [PMC free article] [PubMed] [Google Scholar]
- 104.Nordmann TM, Dror E, Schulze F, et al. The role of inflammation in β-cell dedifferentiation. Sci Rep 2017; 7: 6285. doi: 10.1038/s41598-017-06731-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Talchai C, Xuan S, Lin H V, et al. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012; 150: 1223–1234. doi: 10.1016/j.cell.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohta H, Wada H, Niwa T, et al. Disruption of tumor necrosis factor-α gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005; 180: 11–17. doi: 10.1016/j.atherosclerosis.2004.11.016 [DOI] [PubMed] [Google Scholar]
- 107.Churg A, Wang RD, Tai H, et al. Tumor necrosis factor-α drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 2004; 170: 492–498. doi: 10.1164/rccm.200404-511OC [DOI] [PubMed] [Google Scholar]
- 108.Huang H, Gandhi JK, Zhong X, et al. TNFα is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investig Opthalmology Vis Sci 2011; 52: 1336. doi: 10.1167/iovs.10-5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blidberg K, Palmberg L, Dahlén B, et al. Chemokine release by neutrophils in chronic obstructive pulmonary disease. Innate Immun 2012; 18: 503–510. doi: 10.1177/1753425911423270 [DOI] [PubMed] [Google Scholar]
- 110.Mammoto A, Mammoto T, Kanapathipillai M, et al. Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics. Nat Commun 2013; 4: 1759. doi: 10.1038/ncomms2774 [DOI] [PubMed] [Google Scholar]
- 111.Ohmura T, Tian Y, Sarich N, et al. Regulation of lung endothelial permeability and inflammatory responses by prostaglandin A2: role of EP4 receptor. Mol Biol Cell 2017; 28: 1622–1635. doi: 10.1091/mbc.e16-09-0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sapey E, Bayley D, Ahmad A, et al. Inter-relationships between inflammatory markers in patients with stable COPD with bronchitis: intra-patient and inter-patient variability. Thorax 2008; 63: 493–499. doi: 10.1136/thx.2007.086751 [DOI] [PubMed] [Google Scholar]
- 113.Gane JM, Stockley RA, Sapey E. TNF-α autocrine feedback loops in human monocytes: the pro- and anti-inflammatory roles of the TNF-α receptors support the concept of selective TNFR1 blockade in vivo. J Immunol Res 2016; 2016: 1079851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jacob J, Song JW, Yoon H-Y, et al. Prevalence and effects of emphysema in never-smokers with rheumatoid arthritis interstitial lung disease. EBioMedicine 2018; 28: 303–310. doi: 10.1016/j.ebiom.2018.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takenaka S, Kawayama T, Imaoka H, et al. The progression of comorbidity in IL-18 transgenic chronic obstructive pulmonary disease mice model. Biochem Biophys Res Commun 2014; 445: 597–601. doi: 10.1016/j.bbrc.2014.02.052 [DOI] [PubMed] [Google Scholar]
- 116.Mackay F, Loetscher H, Stueber D, et al. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med 1993; 177: 1277–1286. doi: 10.1084/jem.177.5.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tak PP, Taylor PC, Breedveld FC, et al. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum 1996; 39: 1077–1081. doi: 10.1002/art.1780390702 [DOI] [PubMed] [Google Scholar]
- 118.Tabit CE, Chung WB, Hamburg NM, et al. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord 2010; 11: 61–74. doi: 10.1007/s11154-010-9134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ntaios G, Gatselis NK, Makaritsis K, et al. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis 2013; 227: 216–221. doi: 10.1016/j.atherosclerosis.2012.12.029 [DOI] [PubMed] [Google Scholar]
- 120.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 1999; 100: 2473–2476. doi: 10.1161/01.CIR.100.25.2473 [DOI] [PubMed] [Google Scholar]
- 121.Friedrichs B, Neumann U, Schüller J, et al. Cigarette-smoke-induced priming of neutrophils from smokers and non-smokers for increased oxidative burst response is mediated by TNF-α. Toxicol Vitr 2014; 28: 1249–1258. doi: 10.1016/j.tiv.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 122.Ishii Y, Partridge CA, Del Vecchio PJ, et al. Tumor necrosis factor-α-mediated decrease in glutathione increases the sensitivity of pulmonary vascular endothelial cells to H2O2. J Clin Invest 1992; 89: 794–802. doi: 10.1172/JCI115658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vacek TP, Rehman S, Neamtu D, et al. Matrix metalloproteinases in atherosclerosis: role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc Health Risk Manag 2015; 11: 173–183. doi: 10.2147/VHRM.S68415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nikołajuk A, Matulewicz N, Stefanowicz M, et al. Serum matrix metalloproteinase 9 and macrophage migration inhibitory factor (MIF) are increased in young healthy nonobese subjects with positive family history of type 2 diabetes. Int J Endocrinol 2018; 2018: 1–7. doi: 10.1155/2018/3470412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Freitas-Rodríguez S, Folgueras AR, López-Otín C. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res 2017; 1864: 2015–2025. doi: 10.1016/j.bbamcr.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 126.Suissa S, Ernst P, Hudson M. TNF-α antagonists and the prevention of hospitalisation for chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2008; 21: 234–238. doi: 10.1016/j.pupt.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 127.Rennard SI, Fogarty C, Kelsen S, et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175: 926–934. doi: 10.1164/rccm.200607-995OC [DOI] [PubMed] [Google Scholar]
- 128.Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol 2017; 76: 81–90. doi: 10.1016/j.jaad.2016.07.042 [DOI] [PubMed] [Google Scholar]
- 129.Angel K, Provan SA, Fagerhol MK, et al. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens 2012; 25: 644–650. doi: 10.1038/ajh.2012.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Padfield GJ, Din JN, Koushiappi E, et al. Cardiovascular effects of tumour necrosis factor α antagonism in patients with acute myocardial infarction: a first in human study. Heart 2013; 99: 1330–1335. doi: 10.1136/heartjnl-2013-303648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ridker PM, Everett BM, Thuren T, et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 132.Durham AL, Caramori G, Chung KF, et al. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl Res 2016; 167: 192–203. doi: 10.1016/j.trsl.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Calverley PMA, Sethi S, Dawson M, et al. A randomised, placebo-controlled trial of anti-interleukin-1 receptor 1 monoclonal antibody MEDI8968 in chronic obstructive pulmonary disease. Respir Res 2017; 18: 153. doi: 10.1186/s12931-017-0633-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fitzgerald W, Freeman ML, Lederman MM, et al. A system of cytokines encapsulated in extracellular vesicles. Sci Rep 2018; 8: 8973. doi: 10.1038/s41598-018-27190-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McGuinness AJA, Sapey E. Oxidative stress in COPD: sources, markers, and potential mechanisms. J Clin Med 2017; 6: E21. doi: 10.3390/jcm6020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.El-Benna J, Hurtado-Nedelec M, Marzaioli V, et al. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev 2016; 273: 180–193. doi: 10.1111/imr.12447 [DOI] [PubMed] [Google Scholar]
- 137.Summers C, Singh NR, White JF, et al. Pulmonary retention of primed neutrophils: a novel protective host response, which is impaired in the acute respiratory distress syndrome. Thorax 2014; 69: 623–629. doi: 10.1136/thoraxjnl-2013-204742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tregay N, Begg M, Cahn A, et al. Use of autologous 99m technetium-labelled neutrophils to quantify lung neutrophil clearance in COPD. Thorax 2019; 74: 659–666. doi: 10.1136/thoraxjnl-2018-212509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vogt KL, Summers C, Chilvers ER, et al. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur J Clin Invest 2018; 48: e12967. doi: 10.1111/eci.12967 [DOI] [PubMed] [Google Scholar]
- 140.Ekpenyong AE, Toepfner N, Fiddler C, et al. Mechanical deformation induces depolarization of neutrophils. Sci Adv 2017; 3: e1602536. doi: 10.1126/sciadv.1602536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol 2012; 2: 120134. doi: 10.1098/rsob.120134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Evrard M, Kwok IWH, Chong SZ, et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity 2018; 48: 364–379. doi: 10.1016/j.immuni.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 143.Brook M, Tomlinson GH, Miles K, et al. Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci USA 2016; 113: 4350–4355. doi: 10.1073/pnas.1601831113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kolaczkowska E, Kubes P. Angiogenic neutrophils: a novel subpopulation paradigm. Blood 2012; 120: 4455–4457. doi: 10.1182/blood-2012-09-457226 [DOI] [PubMed] [Google Scholar]
- 145.Loi ALT, Hoonhorst S, van Aalst C, et al. Proteomic profiling of peripheral blood neutrophils identifies two inflammatory phenotypes in stable COPD patients. Respir Res 2017; 18: 100. doi: 10.1186/s12931-017-0586-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Averill MM, Barnhart S, Becker L, et al. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation 2011; 123: 1216–1226. doi: 10.1161/CIRCULATIONAHA.110.985523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes Metab Res Rev 2015; 31: 113–126. doi: 10.1002/dmrr.2558 [DOI] [PubMed] [Google Scholar]
- 148.Harman D. Free radical theory of aging: an update – increasing the functional life span. Ann NY Acad Sci 2006; 1067: 10–21. doi: 10.1196/annals.1354.003 [DOI] [PubMed] [Google Scholar]
- 149.Vaitkus M, Lavinskiene S, Barkauskiene D, et al. Reactive oxygen species in peripheral blood and sputum neutrophils during bacterial and nonbacterial acute exacerbation of chronic obstructive pulmonary disease. Inflammation 2013; 36: 1485–1493. doi: 10.1007/s10753-013-9690-3 [DOI] [PubMed] [Google Scholar]
- 150.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest 2013; 144: 266–273. doi: 10.1378/chest.12-2664 [DOI] [PubMed] [Google Scholar]
- 151.Zeng M, Li Y, Jiang Y, et al. Local and systemic oxidative stress status in chronic obstructive pulmonary disease patients. Can Respir J 2013; 20: 35–41. doi: 10.1155/2013/985382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rahman I, Morrison D, Donaldson K, et al. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996; 154: 1055–1060. doi: 10.1164/ajrccm.154.4.8887607 [DOI] [PubMed] [Google Scholar]
- 153.Judkins CP, Diep H, Broughton BRS, et al. Direct evidence of a role for NOX2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE −/− mice. Am J Physiol Circ Physiol 2010; 298: H24–H32. doi: 10.1152/ajpheart.00799.2009 [DOI] [PubMed] [Google Scholar]
- 154.Lenzen S. Oxidative stress: the vulnerable β-cell. Biochem Soc Trans 2008; 36: 343–347. doi: 10.1042/BST0360343 [DOI] [PubMed] [Google Scholar]
- 155.Zhu K, Kakehi T, Matsumoto M, et al. NADPH oxidase NOX1 is involved in activation of protein kinase C and premature senescence in early stage diabetic kidney. Free Radic Biol Med 2015; 83: 21–30. doi: 10.1016/j.freeradbiomed.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 156.Gray SP, Di Marco E, Okabe J, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013; 127: 1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159 [DOI] [PubMed] [Google Scholar]
- 157.Ovadya Y, Landsberger T, Leins H, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 2018; 9: 5435. doi: 10.1038/s41467-018-07825-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Feldmann M, Maini RN. Anti-TNFα therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 2001; 19: 163–196. doi: 10.1146/annurev.immunol.19.1.163 [DOI] [PubMed] [Google Scholar]
- 159.Gupta-Ganguli M, Cox K, Means B, et al. Does therapy with anti-TNF-α improve glucose tolerance and control in patients with type 2 diabetes? Diabetes Care 2011; 34: e121. doi: 10.2337/dc10-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baldi S, Jose PE, Bruschi C, et al. The mediating role of cytokine IL-6 on the relationship of FEV1 upon 6-minute walk distance in chronic obstructive pulmonary disease. Int J COPD 2014; 9: 1091–1099. doi: 10.2147/COPD.S57845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Faner R, Cruz T, López-Giraldo A, et al. Network medicine, multimorbidity and the lung in the elderly. Eur Respir J 2014; 44: 775–788. doi: 10.1183/09031936.00078714 [DOI] [PubMed] [Google Scholar]
- 162.Thomsen M, Nordestgaard BG, Vestbo J, et al. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med 2013; 1: 543–550. doi: 10.1016/S2213-2600(13)70137-1 [DOI] [PubMed] [Google Scholar]
- 163.Labanieh L, Majzner RG, Mackall CL. Programming CAR-T-cells to kill cancer. Nat Biomed Eng 2018; 2: 377–391. doi: 10.1038/s41551-018-0235-9 [DOI] [PubMed] [Google Scholar]
- 164.Garg NK, Dwivedi P, Prabha P, et al. RNA pulsed dendritic cells: an approach for cancer immunotherapy. Vaccine 2013; 31: 1141–1156. doi: 10.1016/j.vaccine.2012.12.027 [DOI] [PubMed] [Google Scholar]