Abstract
MalF and MalG are the cytoplasmic membrane components of the binding protein-dependent ATP binding cassette maltose transporter in Escherichia coli. They are thought to form the transport channel and are thus of critical importance for the mechanism of transport. To study the contributions of individual transmembrane segments of MalF, we isolated 27 point mutations in membrane-spanning segments 3, 4, and 5. These data complement a previous study, which described the mutagenesis of membrane-spanning segments 6, 7, and 8. While most of the isolated mutations appear to cause assembly defects, L323Q in helix 5 could interfere more directly with substrate specificity. The phenotypes and locations of the mutations are consistent with a previously postulated structural model of MalF.
One of the most widely studied bacterial ATP binding cassette transporters is the binding protein-dependent system for maltose and maltodextrins, MalEFGK2 (for a review, see reference 11). It is composed of an extracytoplasmic substrate binding protein, MalE (MBP), two polytopic cytoplasmic membrane components, MalF and MalG, and the membrane-associated ATP binding subunits of MalK. MBP is responsible for the high affinity of the transport system, with a Kd of 1 μM. MBP consists of two nearly symmetrical lobes between which the binding site is formed. A heterodimer of the two integral membrane components MalF and MalG is thought to form the transport channel. MalF has eight transmembrane segments (TM) while MalG has six, and both proteins insert into the membrane with their termini in the cytoplasm. The penultimate cytoplasmic domain and the following TM carry the consensus sequence EAA-X(3)-G-X(9)-I-X-LP conserved in all integral membrane subunits of bacterial binding protein-dependent systems. This motif is one site of interaction with the ATPase subunit MalK (14, 20). MalK contains the classical Walker A and B consensus sequences found in ATP-hydrolyzing proteins and is present as a homodimer (15). The transport system is able to use maltodextrins up to maltoheptaose as substrates, but with decreasing affinities. Even longer dextrins are processed by a periplasmic amylase, the malS gene product (24, 25).
When the purified transport complex is reconstituted in liposomes, ATP-dependent active transport of maltose can be demonstrated, suggesting that the MalK dimer drives transport (6). These studies also indicated that the stoichiometry of the complex is MalFGK2. The findings that these three subunits copurify and that the purified maltose transporter is active in detergent solution (23) indicate a tight interaction of these proteins.
MBP-independent mutants that transport maltose in the absence of MalE were isolated for MalF and MalG (4). Surprisingly, wild-type MBP interferes with the activity of the MBP-independent MalFGK2 complex (28), and hydrolysis of ATP by the MalK subunit occurs constitutively, even in the absence of substrate (7). These and other data suggested that the MalFGK2 complex must be able to attain at least two conformations, only one of which is able to trigger ATP hydrolysis by the MalK subunit (1).
Topological models can be an informative basis for structure-function studies of polytopic membrane proteins such as MalF. Alkaline phosphatase (2, 10), β-galactosidase (13), and β-lactamase (22) reporter protein studies indicated the presence of eight TM. Reliable topological models allow dissection of membrane proteins into various subdomains. Seventeen segments were postulated for MalF, of which five are cytoplasmic, eight are transmembrane, and four are periplasmic segments. All of these segments, except the large second periplasmic loop, are small enough for site-specific mutagenesis using degenerate oligonucleotides. We have previously mutagenized TM 6, 7, and 8 of MalF and isolated up to 14 individual point mutations in each TM (8). The phenotypes of these and other mutations (4, 9) were used to model the MalFG transport channel (11). In order to produce a more complete set of point mutations in TM of MalF, we mutagenized TM 3, 4, and 5. These mutants allowed the identification of several new sites in MalF that are of structural and functional importance.
MATERIALS AND METHODS
Bacteria, plasmids and growth of cells.
Escherichia coli strains used were as follows. DHB4 recA::cat is araD139 Δ(ara-leu)7697 ΔlacX74 ΔphoA (PvuII) phoR ΔmalF3 galE galK thi rpsL recA::cat F′lacIq pro (2). RE10 is DHB4 malT(Con) (8). pDHB32 contains the E. coli malF gene and part of the malG gene expressed under tac promoter control (2).
Media were made according to the method of Miller (19). Unless otherwise indicated, liquid cultures were routinely grown at 37°C. Conditional phenotypes of isolated MalF mutants were checked at 28, 37, and 42°C.
Maltose transport assays.
Transport assays were done with overnight cultures grown in minimal medium A containing 0.2% glycerol as the carbon source and 0.2% maltose to induce the chromosomal mal genes. Prior to the assay, cultures were washed three times in minimal medium A–0.2% glycerol and were diluted to an optical density at 600 nm of approximately 0.1. The determination of the initial rate of [14C]maltose uptake was done as described by Brass et al. (3). Transport was assayed at a final concentration of 0.2 μM maltose.
Mutagenesis of TM 3, 4, and 5 of MalF and characterization of the individual point mutations.
Mutations in TM 3, 4, and 5 of MalF were isolated using degenerate oligonucleotides as described previously (8). Mutagenesis primers were synthesized containing 2% degenerate sequence, leading mainly to single base pair substitutions. To cover TM 3, 4, and 5 the following primers were used, respectively: 5′-TACCCGGAATGGCTGGAATGGGATTATTCGTCCTCTTCCCTCTGGTCTGCACCATCGCCATTGCCTTCACC-3′, 5′-GCACCAGAC ACGCCAGAACCATGCCGACCGCCACCGTTAAAAAGACAGTGAATC GCGAGAACACCACGGTCCAGACGAAAATGGCGAGGAACGG-3′, and 5′-CGTCCTGCTGATTCTGCCCTACGCGGTGCCATCGTTCATTTCAATCTTGATTTTCAAAGG-3′. After mutagenesis, transformants were plated on MacConkey maltose indicator plates, monitoring utilization of maltose as a carbon source. Proteolysis with proteinase K of MalF mutants in spheroplasts was done on ice in strain RE10 after growth at 37 or 42°C as described earlier (8).
RESULTS
Mutagenesis of TM 3, 4, and 5 of MalF.
The segments of the malF gene encoding the 3rd, 4th, and 5th TM were mutagenized using degenerate oligonucleotides (Fig. 1). Colonies exhibiting reduced maltose transport were identified as weakly positive or negative colonies on MacConkey maltose plates after growth at 37°C. These candidates were tested for expression of MalF protein by Western blotting. Six mutants produced either undetectable (A73G, F91Y, and F91C in TM 3) or reduced (I280F in TM 4 and S329L in TM 5) amounts of MalF protein or showed smaller molecular weight derivatives of MalF (P82S in strain RE10), which might be due to instability of these mutant proteins in vivo (data not shown). All others were made at wild-type levels. We isolated 10 point mutations each in TM 3 and 4 and 7 point mutations in TM 5 (Fig. 2 to 4).
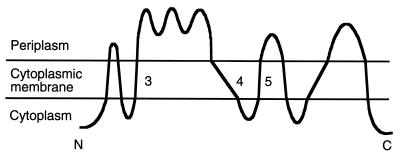
FIG. 1.
Topological model of MalF. TM 3, 4, and 5 are indicated.
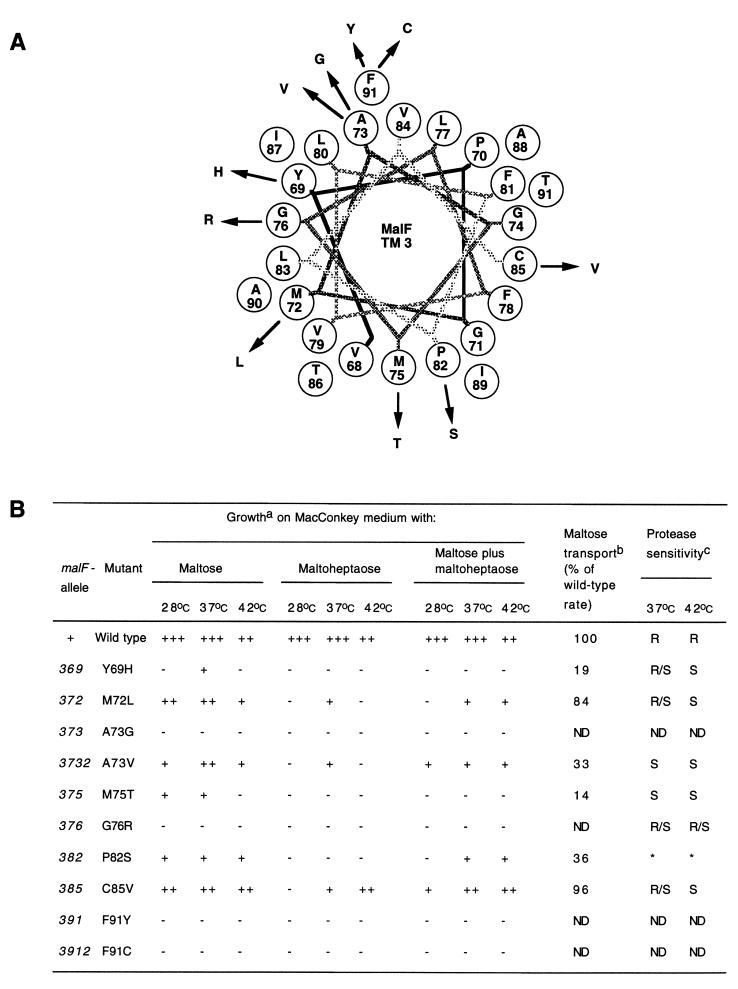
FIG. 2.
(A) Helical wheel projection of MalF TM 3. Point mutations in TM 3 of MalF are plotted as a canonical helix (3.6 residues per turn). TM 3 starts with V68 and ends with F91. (B) Phenotypes of mutations in TM 3. aGrowth of malF mutants on MacConkey agar plates containing 1% maltose or maltoheptaose or both at either 28, 37, or 42°C. +++, dark red colonies; ++, dark pink colonies; +, light pink colonies; −, white colonies. bThe initial rate of maltose transport after growth at 37°C was determined as described in Materials and Methods. The wild-type rate (100%) was 523 pmol/min/109 cells. ND, not done. cProteinase K assays were done as described previously (8). R, MalF resistant to protease treatment; S, cleavage by proteinase K; R/S, partial proteolysis. *The P82S mutant was expressed as a full-length protein in strain DHB4 but as a shorter fragment in RE10. ND, not done because these malF alleles were not expressed.
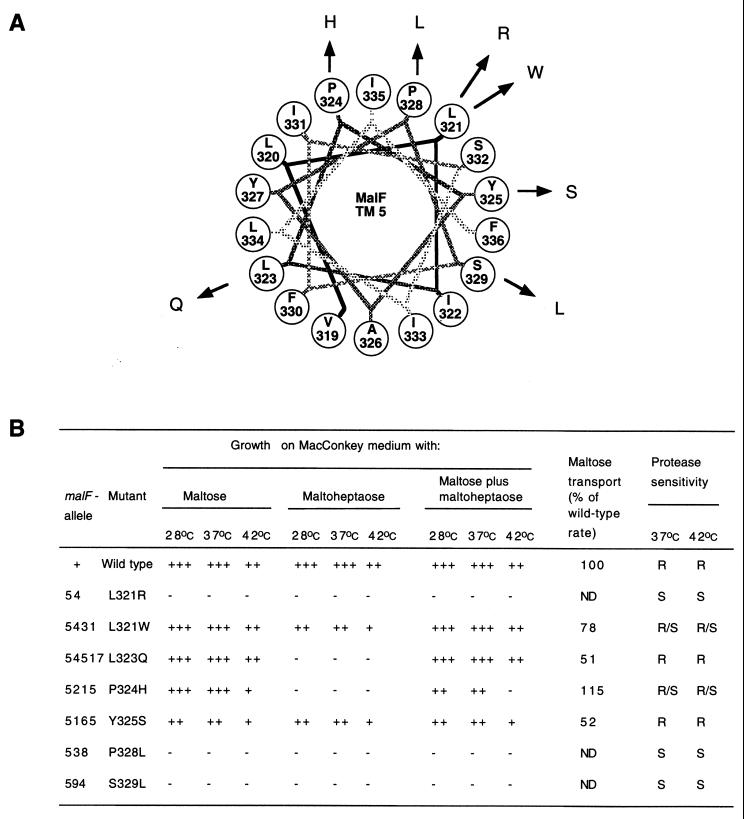
FIG. 4.
(A) Helical wheel projection of MalF TM 5. Point mutations in TM 5 of MalF are plotted as a canonical helix (3.6 residues per turn). TM 5 starts with V319 and ends with F336. (B) Phenotypes of mutations in TM 5. See the legend to Fig. 2B for further details.
Maltose transport activity of the isolated mutants.
Cells of mutants which did not exhibit a completely Mal-negative phenotype on indicator plates were subjected to transport assays to determine the severity of the transport defect (Fig. 2 to 4). Based on the initial rate of maltose transport, three classes of mutants could be distinguished. Five class I mutants were isolated, transporting maltose at ≥70% of the wild-type rates. This class contained two mutants in TM 3, M72L and C85V, one mutant in TM 4, F293I, and two mutants in TM 5, L321W and P324H. In the 11 mutants of class II, maltose transport was between 10 and 70% of the wild-type rates. These mutants were Y69H, A73V, M75T, and P82S in TM 3; F277I, F281S, T295M, C304L, and Q307R in TM 4; and L323Q and Y325S in TM 5. The 11 other mutants fell into class III, transporting maltose at levels which were below 10% of the wild-type.
Phenotypes of mutants on MacConkey maltose plates.
To further characterize the isolated mutants, we tested growth on MacConkey maltose plates at 28, 37, and 42°C (Fig. 2 to 4). Three types of phenotypes were detected. First, seven mutants showed growth comparable to the wild-type at all temperatures. These mutants were F277I, F293I, T295M, C304L, and Q307R in TM 4 and L321W and L323Q in TM 5. Second, 12 mutants exhibited reduced maltose utilization compared to the wild type at least at one of the temperatures tested. These mutants were Y69H, M72L, A73V, M75T, P82S, and C85V in TM 3; F281S, V282D, W283R, and G299C in TM 4; and P324H and Y325S in TM 5. The other eight mutants were Mal minus at all temperatures.
Mutations leading to reduced utilization of maltose as a carbon source could interfere either with transport function, protein structure, or both. We expected that conformational defects could lead to a conditional phenotype with respect to MalF function. Six mutations, M75T in TM 3, F281S, W283R, and G299C in TM 4, and P324H in TM 5 caused temperature sensitivity of MalF function. All mutants of this class were temperature sensitive for maltose utilization but did not use maltoheptaose at all.
As we had observed earlier, strain DHB4 recA carrying pDHB32 (wild-type malF) did not utilize maltose at 42°C as well as it did at 28 and 37°C (8), which was taken into account when temperature sensitivity was interpreted.
Phenotypes of mutants on MacConkey maltoheptaose plates.
Mutations altering substrate specificity of MalF were identified by comparing growth of the mutants on MacConkey maltose (Mal) and MacConkey maltoheptaose (Dex) plates (Fig. 2 to 4). There are four possible phenotypes, Mal+ Dex+, Mal+ Dex−, Mal− Dex+, and Mal− Dex−. Except for the Mal− Dex+ phenotype, we identified mutants in each of these groups. Mal− Dex+ mutants have so far only been isolated in TM 6 (8).
Eleven mutants were Mal+ Dex+; however, none of these grew as well as the wild type on both maltose and maltoheptaose plates. These mutants were M72L, A73V, and C85V in TM 3; F277I, V282D, F293I, T295M, C304L, and Q307R in TM 4; and L321W and Y325S in TM 5.
Eight mutants were Mal+ Dex−. These mutants were Y69H, M75T, and P82S in TM 3; F281S, W283R, and G299C in TM 4; and L323Q and P324H in TM 5. Partial Dex− phenotypes at some of the temperatures tested were detected for M72L, A73V, and C85V in TM 3.
The other eight mutants had a Mal− Dex− phenotype. Mutations exhibiting a Mal+ Dex− phenotype were further investigated for a dextrin dominant-negative phenotype by assaying growth of the mutants on MacConkey agar containing both maltose and maltoheptaose. Dextrin dominant-negative mutants grow on maltose media but not on media containing either maltoheptaose or maltoheptaose plus maltose. This phenotype may be explained by dextrins blocking the transport channel. This blockage could be due to structural alterations leading to a narrower or less flexible channel. Two mutants, M72L and M75T in TM 3, fell into this class.
Protease sensitivity of mutants in TM 3, 4, and 5 of MalF.
MalF is cleaved by various proteases such as trypsin, chymotrypsin, and proteinase K added to spheroplasts when either MalG or MalK is absent (8, 27). We used proteinase K assays in the presence of MalGK as an indication of conformational defects (Fig. 2 to 4) (8).
In contrast to previous observations for mutants in TM 6, 7, and 8, most mutants were sensitive to proteinase K. Except for two mutants in TM 5 (L323Q and Y325S), all mutants which expressed protein were at least partially protease sensitive. The term partial protease sensitivity was used when we detected proteolytic fragments together with full-length MalF. After growth at 42oC, all mutants which exhibited a temperature-sensitive phenotype for maltose transport were protease sensitive.
DISCUSSION
A similar study was done previously focusing on TM 6, 7, and 8 (8). It indicated that the C-terminal TM are significant for the structure and function of MalF. TM 6 and TM 7 were found to be important for substrate specificity and MalF assembly, respectively, while TM 8 is thought to contribute to the efficiency of transport. The contributions of MalF TM 3 and 4 for the function of the maltose transporter were largely unknown, while several mutations with an MBP-independent phenotype, one of which causes altered substrate specificity, have been isolated in TM 5 (4, 17). To learn more about the importance of these TM, we mutagenized the corresponding segments of the malF gene and characterized the relevant phenotypes.
All mutants in TM 3 were protease sensitive, and four mutants were not expressed as full-length proteins even at 37°C. In addition, mutations in TM 3 had more severe phenotypes than mutations in TM 4 or 5. We therefore speculate that TM 3 is important for MalF assembly. The nature of the assembly defects may be best explained by a negative effect of these mutations on other TM interacting with TM 3. The importance of TM 3 was unexpected since the membrane components of the histidine transporter have only five TM, which align with TM 4 through 8 of MalF. We had therefore hypothesized earlier that the N-terminal three TM of MalF would not be of central importance for maltose transport (11). It could be, however, that even if TM 3 is not directly involved in channel formation, it could provide conformational stability via its interactions with TM 2, 4, and 5 (Fig. 5).
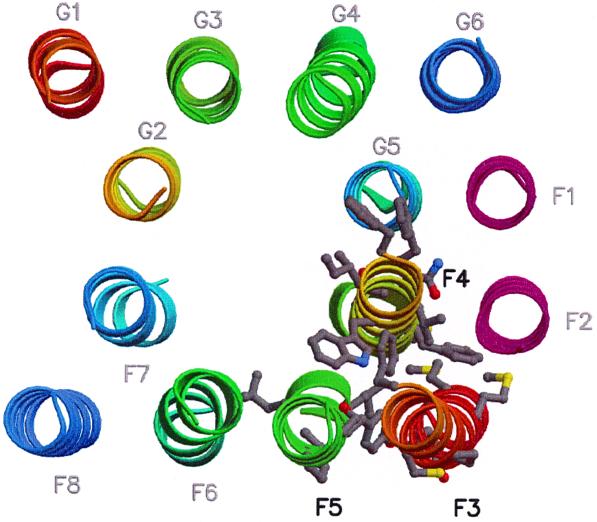
FIG. 5.
Location of mutations in the three-dimensional model of the MalFG core (11). Transmembrane helices (MalF, F1 to F8; MalG, G1 to G6) are shown as ribbons. Color coding is from red to blue along the sequence, except for F1 and F2 (in pink). Mutated residues on helices F3 to F5 are shown as grey ball-and-stick representations. Helices F3 and F4 were extended in the three-dimensional model to include all observed mutations. The figure was produced with Molscript (16) and Raster3D (18).
When the isolated mutations were included in helical wheel projections (Fig. 2 to 4), we found that most mutations in TM 3, 4, and 5 causing reduced maltose transport or interfering with proper assembly of MalF were localized mainly on predicted interfaces with other TM (Fig. 5). Mutations in TM 3 mainly affected the postulated interfaces with TM 2, 4, and 5 of MalF. A similar positioning of mutations was detected for TM 4. Our model predicts that most parts of TM 4 interact with other TM, except one helix face containing F277 and T295, which should be oriented towards the transport channel. Except for L323Q, which is discussed below, mutations in TM 5 were positioned in the interface with TM 3 and 4 of MalF. Therefore, the positions of the isolated mutations were largely in agreement with the postulated three-dimensional model.
The isolated mutations were also tested for functional defects by determining maltose uptake and the utilization of maltose and maltoheptaose as a carbon source. In most cases, mutations with a transport phenotype were proteinase K sensitive, which points to assembly defects. This finding is best explained by suggesting that these TM are of importance for assembly and that effects on, for example, substrate specificity, were probably indirect. Substrate specificity seems to be mainly affected by mutations in TM 6 of MalF, where we had previously isolated eight mutations tightly clustered on one face of the helix which all lead to changes in substrate specificity (8). An exception is L323Q in TM 5, which transported maltose at nearly wild-type levels but was fully impaired in dextrin uptake. Since this mutation had no proteinase K phenotype and L323 is predicted to be facing the transport channel, it could be that this residue contributes directly to substrate specificity of the maltose transporter. A further argument for an involvement of the corresponding helix face of TM 5 in substrate specificity is that an L334W mutation confers a change in substrate specificity from maltose to lactose (17). Like L323, L334 is also predicted to be exposed to the transport channel.
Our analysis of the point mutations in TM 3 through 8 indicates that many MalF mutations with strong phenotypes can be isolated, several of which are caused by conservative substitutions. This is in contrast to the results of extensive mutational studies done on LacY by Frillingos et al. which indicated an unexpected tolerance of LacY to changes in its primary sequence (12). This observation could be explained by the different genetic approaches used or by the complexity of the ATP binding cassette maltose transporter. The fact that MalF has to productively interact with MalG, MBP, and MalK could be responsible for the detected susceptibility to the introduced mutations.
Combined with the previous study there are now 68 individual point mutations in the TM of MalF available. These and other large collections of malFG mutants, isolated in the laboratories of Shuman (4), Traxler (21) and Dassa (5, 26), will prove useful in the future to address the question of mechanism by biochemical and biophysical means.
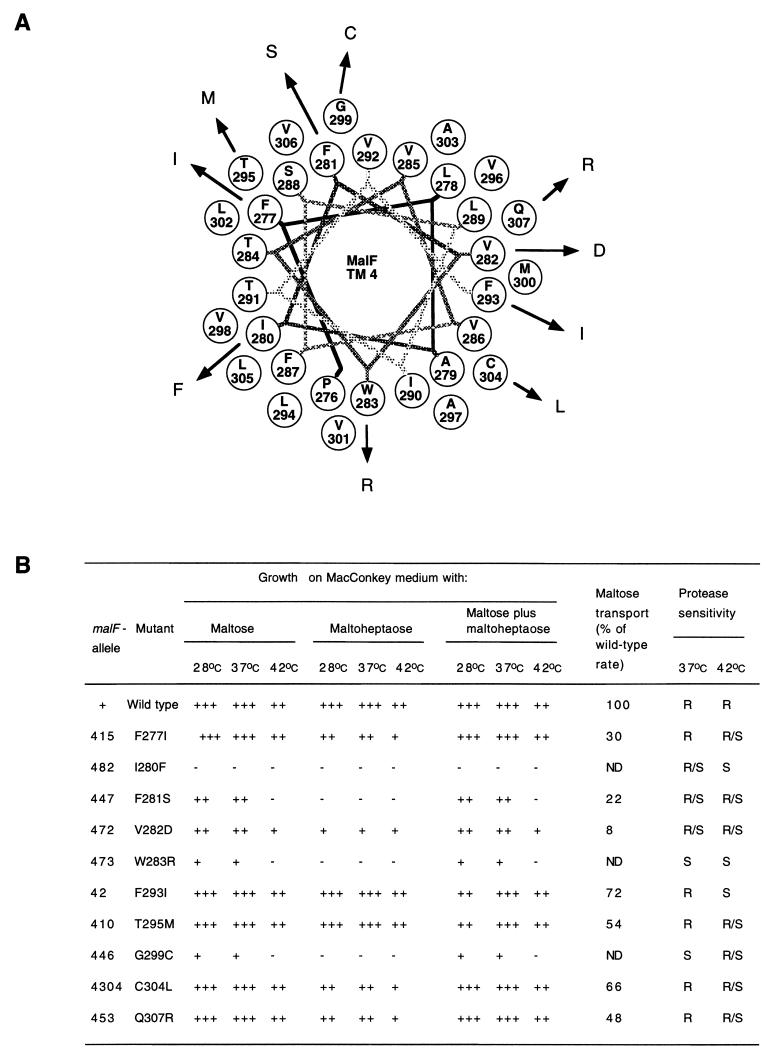
FIG. 3.
(A) Helical wheel projection of MalF TM 4. Point mutations in TM 4 of MalF are plotted as a canonical helix (3.6 residues per turn). TM 4 starts with P276 and ends with Q307. (B) Phenotypes of mutations in TM 4. See the legend to Fig. 2B for further details.
ACKNOWLEDGMENT
This work was supported by Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Boos W, Lucht J M. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, Curtiss R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1996. pp. 1175–1209. [Google Scholar]
- 2.Boyd D, Manoil C, Beckwith J. Determinants of membrane protein topology. Proc Natl Acad Sci USA. 1987;84:8525–8529. doi: 10.1073/pnas.84.23.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brass J, Ehmann U, Bukau B. Reconstitution of maltose transport in Escherichia coli: conditions affecting import of maltose-binding protein into the periplasm of calcium-treated cells. J Bacteriol. 1983;155:97–106. doi: 10.1128/jb.155.1.97-106.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covitz K-Y M, Panagiotidis C H, Hor L-I, Reyes M, Treptow N A, Shuman H A. Mutations that alter the transmembrane signalling pathway in an ATP binding cassette (ABC) transporter. EMBO J. 1994;13:1752–1759. doi: 10.1002/j.1460-2075.1994.tb06439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dassa E. Sequence-function relationships in MalG, an inner membrane protein from the maltose transport system in Escherichia coli. Mol Microbiol. 1993;7:39–47. doi: 10.1111/j.1365-2958.1993.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 6.Davidson A L, Nikaido H. Purification and characterization of the membrane-associated components of the maltose transport system from Escherichia coli. J Biol Chem. 1991;266:8946–8951. [PubMed] [Google Scholar]
- 7.Davidson A L, Shuman H A, Nikaido H. Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc Natl Acad Sci USA. 1992;89:2360–2364. doi: 10.1073/pnas.89.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrle R, Pick C, Ulrich R, Hofmann E, Ehrmann M. Characterization of transmembrane domains 6, 7, and 8 of MalF by mutational analysis. J Bacteriol. 1996;178:2255–2262. doi: 10.1128/jb.178.8.2255-2262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrmann M, Beckwith J. Proper insertion of a complex membrane protein in the absence of its amino-terminal export signal. J Biol Chem. 1991;266:16530–16533. [PubMed] [Google Scholar]
- 10.Ehrmann M, Boyd D, Beckwith J. Genetic analysis of membrane protein topology by a sandwich gene fusion approach. Proc Natl Acad Sci USA. 1990;87:7574–7578. doi: 10.1073/pnas.87.19.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrmann M, Ehrle R, Hofmann E, Boos W, Schlösser A. The ABC maltose transporter. Mol Microbiol. 1998;29:685–694. doi: 10.1046/j.1365-2958.1998.00915.x. [DOI] [PubMed] [Google Scholar]
- 12.Frillingos S, Sahin-Toth M, Wu J, Kaback H. Cys-scanning mutagenesis: a novel approach to structure function relationships in polytopic membrane proteins. FASEB J. 1998;12:1281–1299. doi: 10.1096/fasebj.12.13.1281. [DOI] [PubMed] [Google Scholar]
- 13.Froshauer S, Green G N, Boyd D, McGovern K, Beckwith J. Genetic analysis of the membrane insertion and topology of MalF, a cytoplasmic membrane protein of Escherichia coli. J Mol Biol. 1988;200:501–511. doi: 10.1016/0022-2836(88)90539-6. [DOI] [PubMed] [Google Scholar]
- 14.Hunke S, Mourez M, Jéhanno M, Dassa E, Schneider E. ATP modulates subunit-subunit interactions in an ATP-binding cassette transporter (MalFGK2) determined by site-directed chemical cross-linking. J Biol Chem. 2000;275:15526–15534. doi: 10.1074/jbc.275.20.15526. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy K, Traxler B. MalK forms a dimer independent of its assembly into the MalFGK2 ATP-binding cassette transporter of Escherichia coli. J Biol Chem. 1999;274:6259–6264. doi: 10.1074/jbc.274.10.6259. [DOI] [PubMed] [Google Scholar]
- 16.Kraulis P. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Cryst. 1991;24:946–950. [Google Scholar]
- 17.Merino G, Shuman H. Unliganded maltose-binding protein triggers lactose transport in an Escherichia coli mutant with an alteration in the maltose transport system. J Bacteriol. 1997;179:7687–7694. doi: 10.1128/jb.179.24.7687-7694.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merritt E, Bacon D. Raster3D: photorealistic molecular graphics. Methods Enzymol. 1997;277:505–524. doi: 10.1016/s0076-6879(97)77028-9. [DOI] [PubMed] [Google Scholar]
- 19.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 20.Mourez M, Hofnung M, Dassa E. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 1997;16:3066–3077. doi: 10.1093/emboj/16.11.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson B, Traxler B. Exploring the role of integral membrane proteins in ATP-binding cassette transporters: analysis of a collection of MalG insertion mutants. J Bacteriol. 1998;180:2507–2514. doi: 10.1128/jb.180.9.2507-2514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prinz W, Beckwith J. Gene fusion analysis of membrane protein topology: a direct comparison of alkaline phosphatase and β-lactamase fusions. J Bacteriol. 1994;176:6410–6413. doi: 10.1128/jb.176.20.6410-6413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reich-Slotky R, Panagiotidis C, Reyes M, Shuman H. The detergent-soluble maltose transporter is activated by maltose binding protein and verapamil. J Bacteriol. 2000;182:993–1000. doi: 10.1128/jb.182.4.993-1000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider E, Freundlieb S, Tapio S, Boos W. Molecular characterization of the MalT-dependent periplasmic alpha-amylase of Escherichia coli encoded by malS. J Biol Chem. 1992;267:5148–5154. [PubMed] [Google Scholar]
- 25.Spiess C, Happersberger H P, Glocker M O, Spiess E, Rippe K, Ehrmann M. Biochemical characterization and mass spectrometric disulfide bond mapping of periplasmic α-amylase MalS of Escherichia coli. J Biol Chem. 1997;272:22125–22133. doi: 10.1074/jbc.272.35.22125. [DOI] [PubMed] [Google Scholar]
- 26.Tapia M, Mourez M, Hofnung M, Dassa E. Structure-function study of MalF protein by random mutagenesis. J Bacteriol. 1999;181:2267–2272. doi: 10.1128/jb.181.7.2267-2272.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traxler B, Beckwith J. Assembly of a hetero-oligomeric membrane protein complex. Proc Natl Acad Sci USA. 1992;89:10852–10856. doi: 10.1073/pnas.89.22.10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treptow N A, Shuman H A. Allele-specific malE mutations that restore interactions between maltose-binding protein and the inner-membrane components of the maltose transport system. J Mol Biol. 1988;202:809–822. doi: 10.1016/0022-2836(88)90560-8. [DOI] [PubMed] [Google Scholar]