Abstract
Pulmonary arterial hypertension (PAH) is a dreaded complication of systemic sclerosis (SSc) that occurs in ∼10% of patients. Most individuals present with severe symptoms, significant functional impairment and severe haemodynamics at diagnosis, and survival after PAH diagnosis is poor. Therefore, early diagnosis through systematic screening of asymptomatic patients has the potential to identify PAH at an early stage. Current evidence suggests that early diagnosis and treatment of PAH in patients with SSc may lead to better clinical outcomes. Annual screening may include echocardiography, but this can miss some patients due to suboptimal visualisation or insufficient tricuspid regurgitation. Other options for screening include the DETECT algorithm or the use of a combination of pulmonary function testing (forced vital capacity/diffusing capacity of the lung for carbon monoxide ratio) and N-terminal-pro-brain natriuretic peptide levels. Symptomatic patients, those with an elevated tricuspid regurgitation velocity on echocardiogram with or without secondary echocardiographic features of PAH, and those who screen positive on the DETECT or other pulmonary function test algorithms should undergo right heart catheterisation. Exercise echocardiography or cardiopulmonary exercise testing, nailfold capillaroscopy and molecular biomarkers are promising but, as yet, unproven potential options. Future screening studies should employ systematic catheterisation to define the true predictive values for PAH.
Short abstract
Screening can detect PAH at an early stage of the disease, which permits earlier medical interventions and may improve outcomes in systemic sclerosis patients. bit.ly/2Q5akGu
Introduction
Pulmonary arterial hypertension (PAH) is a devastating condition that causes significant disability and often results in premature death. Pathologically, PAH is characterised by proliferative remodelling of the small pulmonary arteries, which increases resistance to blood flow through the pulmonary circulation [1]. Clinically, PAH is defined during right heart catheterisation (RHC) by an increase in the mean pulmonary arterial pressure (mPAP) >20 mmHg in the context of an elevated pulmonary vascular resistance (PVR) >3 Wood units and normal left heart pressures (pulmonary artery wedge and/or left ventricular end-diastolic pressure ≤15 mmHg) [2]. The haemodynamic definition of pulmonary hypertension (PH) used to be an elevation in mPAP ≥25 mmHg [3]; however, since the upper limit of normal for mPAP at rest is 20 mmHg, the definition of PH was recently changed [2, 4].
Most patients with PAH have advanced symptoms and severe haemodynamic derangement at the time of diagnosis [5–7]. Despite recent medical advances and effective therapies for PAH, annual mortality remains high at ∼10% in idiopathic PAH [8–11]. Prognosis is even worse in certain subgroups such as PAH associated with systemic sclerosis (SSc) [12–19]. Given such poor long-term outcomes, it is logical to aim to detect early disease manifestations before the onset of symptoms. There is a delay of 2–4 years between the onset of symptoms and diagnosis of PAH, underscoring the need to also consider PAH and establish the diagnosis expediently once those symptoms arise [5, 20, 21]. Unfortunately, the most recent studies from European PAH registries still observe that 72–85% of patients are in New York Heart Association (NYHA) functional class III or IV symptoms at diagnosis [12, 22, 23], which is unchanged from the National Institutes of Health registry cohort published over 30 years ago [21]. Furthermore, most patients still present with severe haemodynamics with right heart dysfunction or right heart failure at the time of PAH diagnosis. Therefore, earlier detection of PAH during a milder, asymptomatic period could allow early intervention and the opportunity to improve outcomes.
However, PAH is a rare disease with an estimated prevalence of only 15–50 per million inhabitants and an annual incidence of 2.4–7.6 per million [5, 24]. Therefore, systematic screening at the population level is not practical or feasible and could only be achieved in subpopulations of individuals at higher risk of developing PAH. There are several medical conditions associated with PAH [2]; however, patients with SSc are a group at higher risk of PAH in whom screening can be justified [3, 25–30]. The objectives of this review are to discuss the rationale, modalities, and future horizons for PAH screening in patients with SSc.
General considerations for PAH screening
By definition, screening is the systematic use of a test in individuals at risk, to detect disease prior to the onset of symptoms or overt manifestations [31, 32]. Implicitly, the act of screening requires that a test is available, and an intervention exists that can influence the outcome if instituted at an early pre-symptomatic stage. An ideal screening test has high sensitivity and specificity for the disease of interest, is reproducible, noninvasive, inexpensive and easily accessible. Additionally, screening for asymptomatic disease should be performed in settings where the results can be acted upon with further confirmative testing and with specific treatment or preventative interventions. The diagnosis of PAH requires RHC in an experienced centre, which is a relatively safe but invasive test [33].
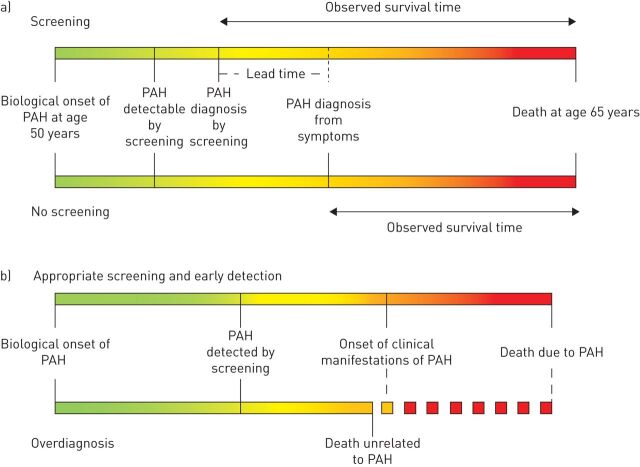
There are several important concepts to first consider in relation to screening for PAH in at-risk populations (figure 1). The first question is whether early detection and intervention actually improve outcomes as opposed to lead-time bias, wherein survival appears better only because a diagnosis is made earlier in the disease course with the patient being observed for a longer period of time, but actual life expectancy is unchanged. This is a possibility if PAH screening studies show improved survival with early detection and treatment, particularly since available PAH therapies have never been proven to prolong survival in patients with established SSc-PAH. A second consideration with screening is the potential for overdiagnosis [34]. Overdiagnosis could occur when PAH is detected at an early stage, but early detection does not affect the outcome if other factors, unrelated to PAH, result in death before clinical manifestations and PAH-related mortality would have otherwise occurred. This can be particularly relevant in elderly patients with SSc, those with severe unrelated comorbid medical conditions (i.e. cancer), or when there is other end-stage organ involvement due to SSc (i.e. severe fibrotic lung disease or severe gastrointestinal disease). Indeed, death is unrelated to PAH in a significant proportion of SSc-PAH patients [15]. In such situations, it is conceivable that the risks of testing or treatment could outweigh the benefit of early case identification in some circumstances. This is not to say that RHC is unnecessary in elderly patients with SSc or those with multiple comorbidities. RHC may still provide other useful information even when a diagnosis of PAH in not made, particularly when symptoms are present. For example, post-capillary PH due to left ventricular involvement and diastolic dysfunction may be discovered, or PH due to cardiac output (e.g. from anaemia) may be detected, which may cause symptoms and are managed differently than PAH. The third point to make with regard to PAH screening studies is to distinguish between a true screening population (those with no symptoms or manifestations of disease) as opposed to detecting PAH with mild symptoms and/or with early disease manifestations. As will be noted, many screening studies involved patients who had symptoms such as unexplained dyspnoea. The inclusion of symptomatic patients increases the pre-test probability and prevalence of disease in the population and potentially over-estimates the observed benefit–risk balance of a screening programme compared with one in a truly asymptomatic population. Thus, in “screening” studies where the majority of patients are symptomatic, the performance characteristics (i.e. positive and negative predictive values) of screening modalities should be interpreted with caution and should not be reported as, nor considered as, screening cohorts.
FIGURE 1.
Epidemiologic concepts for pulmonary arterial hypertension (PAH) screening programmes. a) Lead-time bias. b) Overdiagnosis.
Rationale for screening in SSc patients
The prevalence of PAH in SSc ranges between 7% to 19% so it is sufficiently common to justify systematic screening [35–39]. The prospective, multicentre ItinerAIR study in France reported a relatively low annual incidence of PAH at 0.61 per 100 patient-years (95% CI 0.26–1.20) [40], although more recent studies reported a higher annual incidence of ∼1.5% [37, 41, 42]. With longer disease duration, the cumulative incidence rises, with 18% of patients with diffuse cutaneous SSc and 24% of patients with limited cutaneous SSc developing PH over 15 years [42]. Early detection of PAH should be of paramount importance since it accounts for ∼30% of deaths in SSc patients [43]. As discussed previously, most individuals present with advanced symptoms and right heart dysfunction at PAH diagnosis, which predicts worse survival [12, 13, 16, 17, 44].
Even though PAH therapies are effective in the SSc-PAH population [45–47], many patients fail to improve in terms of symptoms, exercise capacity, haemodynamics or risk profiles [12, 23, 48] and overall long-term prognosis remains dismal. In contrast, with idiopathic PAH no studies have proven a mortality benefit of PAH therapy in SSc-PAH. A recent study from the French PH registry, which included 513 incident SSc-PAH patients, reported 1-, 3- and 5-year transplant-free survival rates of 87%, 55% and 35%, respectively [12]. This was similar to the REVEAL (Registry to Evaluate Early and Long-Term PAH Management) registry in the USA, where 3-year survival was 51.2% for newly diagnosed SSc-PAH and 61.4% in previously diagnosed patients [14]. In the COMPERA registry, even “low-risk” connective tissue disease-associated patients with PAH had only a 64% 3-year survival rate while the “high-risk” patients had a 34% 3-year survival [23].
More recently, the prospective PHAROS (Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma) study recently reported better survival rates at 1, 3 and 5 years of 95%, 75% and 63%, which may have been related to diagnosis at an earlier stage of the disease due to more widespread screening of patients with SSc [15]. Indeed, patients in the PHAROS study had less severe symptoms at diagnosis with 59% of patients in NYHA functional class I or II as opposed to only 31% in the REVEAL registry [14] and 27% in the recent French registry study [12]. This suggests that earlier diagnosis and treatment could translate to better long-term outcomes; however, better survival rates in PHAROS could also be potentially attributed to lead-time bias (figure 1). Nevertheless, the PHAROS results are consistent with a study by Humbert et al. [49] who compared survival between a cohort of 16 patients with SSc with newly diagnosed PAH enrolled in the French registry between 2002 and 2003, and a “detection” cohort of 16 patients with SSc who were screened with echocardiography and had subsequent RHC confirmation. In the early detection cohort, haemodynamics and symptoms were milder at the time of diagnosis (PVR 9.1±6.1 versus 16.2±5.4 Wood units). Long-term survival was also significantly better in the detection cohort with 64% of patients still alive at 8 years, compared with only 17% of SSc-PAH patients from the routine practice cohort. Although half of the detection cohort had NYHA III symptoms, indicating this was likely not a truly “asymptomatic” screening population, there is reasonable evidence that earlier detection of PAH might translate into better long-term outcome.
Screening modalities in SSc
Transthoracic echocardiography
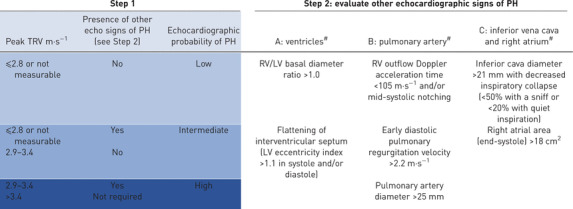
Transthoracic echocardiography (TTE) is a recommended option for annual screening for patients with SSc, meeting certain criteria in the 6th World Symposium on Pulmonary Hypertension and the 2015 European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines [3, 28], and supported by systematic reviews published in 2014 and 2018 [50, 51]. The tricuspid regurgitation velocity (TRV) and other indirect features suggestive of PH, such as right heart chamber enlargement, are used to assess the probability of PH (table 1). RHC is recommended when patients have an intermediate or high risk for PH based on TTE, defined as peak TRV >2.8 m·s−1 or TRV ≤2.8 m·s−1 (or not measurable) in combination with other variables suggestive of PH from at least two of the three different categories. Using slightly different TRV thresholds, the DETECT study found that TTE had a sensitivity of only 71% and specificity of 69% [39]. Composite screening algorithms (see below) increase the sensitivity and negative predictive value compared with TTE. There are other limitations of TTE, the most important being that TRV is unattainable due to an inadequate tricuspid regurgitation Doppler signal in up to 15% of individuals [50]. Other patient-related factors such as obesity, lung hyperinflation and chest wall deformity can also reduce quality of measurements and affect the diagnostic performance of TTE. Additionally, reliance on the TRV is hampered by its imprecision in estimating systolic pulmonary arterial pressure (sPAP), with over- or under-estimation in many cases [52–54].
TABLE 1.
Echocardiographic evaluation of probability of pulmonary hypertension (PH) according to the 2015 European Society of Cardiology/European Respiratory Society guidelines
|
TRV: tricuspid regurgitation velocity; RV: right ventricle; LV: left ventricle. #: echocardiographic signs from at least two different categories (A/B/C) from the list should be present to alter the level of echocardiographic probability of PH.
Two-dimensional speckle tracking on TTE allows measurement of right ventricle(RV) and right atrial strain. Strain can detect occult intrinsic right heart dysfunction or increased RV afterload when the TRV is not elevated and before cardiac chamber enlargement or other echocardiographic measures of RV function, such as tricuspid annular plane systolic excursion and RV fractional area change, become abnormal [55–57]. One study found that a peak longitudinal systolic strain threshold of −14.48% at the apical segment of the RV lateral wall had 100% specificity for PAH in patients with SSc [57]. Therefore, this could be a useful parameter in the future, particularly when TRV is not attainable. However, measurement of RV strain is also subject to the same limitations of two-dimensional image quality. Additionally, abnormal RV strain may reflect intrinsic myocardial involvement by fibrosis rather than elevated afterload in SSc [55]. Further prospective studies are needed to evaluate the performance, feasibility and validity of speckle-tracking TTE for detecting early PAH in the context of screening asymptomatic patients with SSc.
Pulmonary function tests
An isolated reduction in diffusing capacity of the lung for carbon monoxide (DLCO) with a relative preservation of the forced vital capacity (FVC) is associated with PAH in SSc, although a considerable proportion with an isolated low DLCO do not have PAH, will develop obstructive lung disease, interstitial lung disease or improve DLCO during follow-up [58]. The ratio of FVC % predicted to DLCO % predicted (FVC %/DLCO %) may help account for the fact that DLCO can be reduced in SSc because of pulmonary restriction due to interstitial lung disease. A declining DLCO during follow-up should strongly raise the suspicion of PAH, particularly in those with limited SSc and in those without interstitial lung disease [59]. A DLCO >60% predicted has good but imperfect ability to exclude PAH [60, 61], whereas DLCO values <50% predicted have higher specificity (90%) and positive predictive value (88%) [60]. Importantly, a normal DLCO does not entirely exclude PAH. DLCO values above the lower limit of normal had low sensitivity (72%) but high negative predictive value (97%) for PH in a multicentre study including 572 patients with SSc [61]. Studies have also examined the utility of partitioning the DLCO into the capillary and membrane conductance components with conflicting results as to whether this improves detection of PAH [61–63].
Exercise stress testing
Exercise stress echocardiography and cardiopulmonary exercise testing (CPET) are currently not recommended as PAH screening modalities [3]. Patients with SSc frequently exhibit abnormal haemodynamic responses to exercise even when resting haemodynamics are normal, which may reflect early pulmonary vascular disease or left heart disease [16, 64–66]. Detecting an abnormal exercise haemodynamic response with echocardiography requires an accurate estimate of sPAP from the TRV, as well as cardiac output, at each stage of exercise, since high pulmonary arterial pressure can be entirely due to flow and does not necessarily reflect pulmonary vascular disease [67, 68]. Codullo et al. [69] found that a change in sPAP on echocardiogram (ΔsPAP) and the ΔsPAP/Δcardiac index during exercise predicted future development of PH. Stress echocardiography may improve the sensitivity of resting echocardiography but has poor specificity for PAH [70]. A major limitation of exercise echocardiography is a lack of precision and measurement error for both sPAP and cardiac output, particularly at higher levels of exercise [71]. In one study, Kusunose et al. [72] used echo-derived estimates of mPAP immediately after a 6-min walk test and electric cardiometry to calculate cardiac output and found that ΔmPAP/Δcardiac output was predictive of future development of PAH in a cohort of patients with connective tissue disease (70% of whom had SSc). A more recent cross-sectional study performed CPET and RHC in 173 consecutive patients with SSc and found that a minute ventilation/carbon dioxide production nadir >45.5 was highly associated with PAH, whereas a peak oxygen uptake of >18.7 mL·kg−1·min−1 excluded PAH, which may help reduce unnecessary RHC [73]. However, prospective longitudinal studies are needed to clarify the role of CPET and stress echocardiography for PAH screening in the SSc population before it can be recommended.
Cardiac biomarkers
The myocardial natriuretic peptide N-terminal pro-brain natriuretic peptide (NT-proBNP) has been the most extensively evaluated biomarker in SSc and is the most widely available in clinical practice. NT-proBNP is inadequate as a lone screening tool due to low sensitivity (56–69%) and low negative predictive value, since it can be normal in patients with early disease who have not yet developed right heart strain [74, 75]. Furthermore, NT-proBNP is not specific for PAH or right ventricular dysfunction since it can also be elevated in patients with left heart dysfunction or renal insufficiency, both of which are common in SSc [76]. However, NT-proBNP may be a useful adjunct to other screening tools such as TTE and pulmonary function tests and is used in screening algorithms with levels between 204 and 395 ng·L−1 as thresholds for abnormal [39, 74, 75, 77–79]. In one cross-sectional study, the combination of a normal high-sensitivity troponin (<14 ng·L−1) and normal age- and sex-adjusted NT-proBNP had 92% negative predictive value for pre-capillary PH [77]. The combination of a DLCO <70% predicted and a NT-proBNP >97th percentile was associated with a 47-fold risk of developing PAH within the next 3 years [80].
Composite screening algorithms
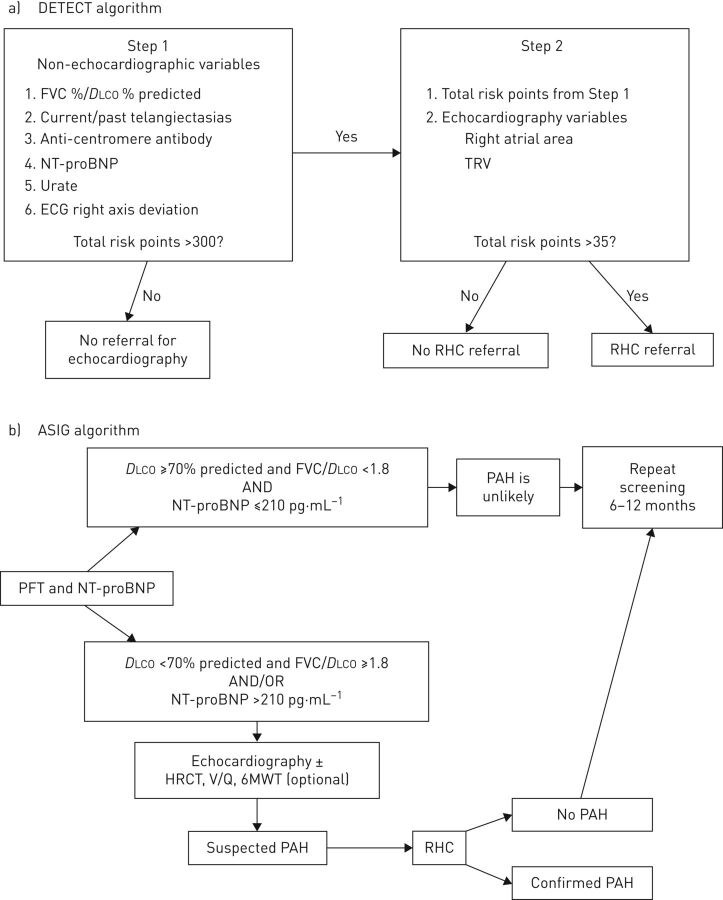
The DETECT study was a prospective, cross-sectional initiative across 18 countries in order to develop an evidence-based screening algorithm with the objective of minimising the number of missed PAH diagnoses [39]. Importantly, DETECT was limited to patients with a disease duration >3 years and a DLCO <60% predicted, which enriched the study population with patients at higher likelihood of having PAH. RHC and echocardiography were performed systematically in 466 patients in order to determine the true false-negative rate (missed PAH diagnosis) with noninvasive screening alone. The two-step DETECT algorithm is shown in figure 2. In the first step, six non-echocardiographic variables were considered, which intentionally aligned with variables that would be available in real-world practice for a non-PH specialist (i.e. rheumatologists). In step 2, the TRV and right atrial area were considered in addition to the risk points from step 1, to determine the need for RHC. In the DETECT population, the two-step algorithm had a lower false-negative rate of 4% (sensitivity 96%) compared with a 29% rate of missed diagnoses using echocardiography screening thresholds alone. Of the 87 PAH cases diagnosed in the DETECT study, most had mild or early disease as evidenced by 64% being in NYHA functional class I or II with an average mPAP of 32.5±8.3 mmHg and mean PVR of 4.6±2.8 Wood units. The DETECT algorithm has been externally validated [41, 81–83] and is a recommended option to screen patients with disease duration >3 years and a DLCO <60% predicted [3, 28]. The DETECT study PAH risk calculator is available online (www.detect-pah.com).
FIGURE 2.
Comparison of screening algorithms for pulmonary arterial hypertension (PAH) in systemic sclerosis. a) The DETECT algorithm and b) the Australian Scleroderma Interest Group (ASIG) algorithm. FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; NT-proBNP: N-terminal pro-brain natriuretic peptide; TRV: tricuspid regurgitation velocity; RHC: right heart catheterisation; PFT: pulmonary function test; HRCT: high-resolution computed tomography; V/Q: ventilation perfusion scan.
In an unselected cohort of 195 patients with SSc, including patients with disease duration <3 years and DLCO >60% predicted who would not have met inclusion for DETECT, Vandecasteele et al. [41] compared DETECT, the 2009 ESC/ERS echocardiography screening criteria [84], and the 2015 ESC/ERS echocardiographic criteria (table 1) [3]. All criteria detected the three patients who had PAH, but with a significantly higher rate of RHC referral using the DETECT algorithm (30% of all patients) compared with the 2009 (9%) and 2015 ESC/ERS guidelines (17%). They reported a considerably higher cost with the DETECT algorithm, which is expected given the application of multiple tests used in the algorithm to a lower risk population than DETECT was initially developed for. This study did confirm the utility of DETECT in lower risk, unselected populations and the use of TTE as a first-line screening tool for patients with SSc. Interestingly, 14 patients in this study who underwent RHC had a mPAP between 21 and 24 mmHg, a range previously referred to as being “borderline” elevated but which now falls within the definition of PH [2]. RHC was recommended by the DETECT algorithm in 13 (93%) of these 14 patients, by the 2015 ESC/ERS screening algorithm in 10 (71%) and the 2009 ESC/ERS guidelines only recommended RHC in four (29%) patients. Since patients with a resting mPAP between 21 and 24 mmHg have worse survival and a risk of progression to PAH [85–89], the DETECT algorithm warrants further evaluation for early detection in light of the new definition of PH as an mPAP >20 mmHg.
The Australian Scleroderma Interest Group (ASIG) performed a multicentre study at 13 sites in Australia with annual clinical assessments, TTE, pulmonary function tests and blood biomarkers [78, 79]. If the DLCO was ≥70% predicted, FVC %/DLCO % predicted was <1.8 and the NT-proBNP was ≤210 ng·L−1, patients were considered at a low likelihood of PAH and underwent repeat screening in the future. If DLCO was <70% predicted with FVC %/DLCO % ≥1.8 and/or NT-proBNP > 210 ng·L−1, patients underwent RHC and echocardiography, a high resolution computed tomography scan, ventilation/perfusion scanning and a 6-min walk test, as needed (figure 2). Unlike the DETECT study, systematic RHC was not performed in all patients in the ASIG study. Of the 49 patients who had an RHC, 17 (35%) had PAH. A total of 16 of these 17 patients screened positive on echocardiography with a sPAP >40 mmHg. One patient with PAH was missed by the algorithm; they had an unmeasurable TRV and had a DLCO <70% predicted as the only other positive screening test. In this study, the sensitivity, specificity, and positive and negative predictive value for PAH detection were 94.1%, 54.5%, 61.5% and 92.3%, respectively. In comparison, the sensitivity, specificity, PPV and NPV of the 2009 ESC/ERS echocardiography screening guidelines in this cohort were 94.1%, 31.8%, 51.6% and 87.5% [79].
There are some advantages of the ASIG algorithm, in that fewer variables are needed in the initial step and some inclusion criteria were less restrictive than DETECT (which only included patients with a disease duration >3 years and DLCO <60% predicted). However, the larger number of patients and countries involved in the DETECT study, as well as the systematic use of RHC, make it a more robustly validated tool for patients meeting those inclusion criteria. A study by Hao et al. [83] compared the DETECT, ASIG and 2009 ESC/ERS guideline criteria in a separate cohort of patients with SSc and found that DETECT and the ASIG algorithms performed similarly but ASIG reduced the referral rate for RHC without missing any cases of PAH. This direct comparison may nevertheless be biased against DETECT in favour of ASIG. The inclusion criteria for the Australian-based comparison cohort of Hao et al. [83] were an elevated TRV (estimated sPAP >40 mmHg) and DLCO <50% predicted with an FVC >85% predicted, which favourably improves the negative predictive value of the ASIG algorithm. The DETECT cohort was less restrictive, including all-comers with a disease duration >3 years, a DLCO <60% and FVC >40% for these parameters.
The DETECT and ASIG algorithms both incorporate TTE after the initial screening step, but the echocardiographic criteria are not essential in high-risk patients in order to proceed direct to RHC. For example, it is possible to accumulate enough risk points in step 1 of the DETECT algorithm, which carry over into step 2, such that the echocardiographic variables in step 2 do not change the decision to refer for RHC. Indeed, another study using high-risk patients from the PHAROS cohort and a French SSc cohort at Hôpital Cochin (Paris, France) found that TTE missed 7–13% of patients ultimately diagnosed with PAH, most of whom were detected using FVC %/DLCO % >1.6% [90].
The specific recommendations for screening in the 6th World Symposium of Pulmonary Hypertension are: “For patients with SSc and SSc spectrum with uncorrected DLCO <80% of predicted, annual screening should be considered. The appropriate screening tools include DETECT, the 2015 ESC/ERS recommendations for TTE or FVC/DLCO ratio >1.6 (assuming none-to-mild interstitial lung disease) and >2-fold upper limit of normal of NT-proBNP. If any of these screening tests are positive, these patients should be referred for RHC. For those with uncorrected DLCO ≥80% of predicted, screening may be considered with TTE” [28].
While guidelines recommend annual screening for PAH in certain patients with SSc, the efficiency and cost-effectiveness of an annual screening time-frame or of a serial screening approach is less clear. Morrisroe et al. [37] evaluated a large cohort of 1636 patients with SSc between 2007 and 2016 who were screened serially during follow-up with TTE and PFTs. Most patients (76%) were diagnosed with PAH at their first screening evaluation but others were detected as late as their ninth screening visit. Importantly, the advanced symptoms (64% in functional class III and 16% in functional class IV) of patients diagnosed at first screening indicates this was not a true screening population. Patients with PAH detected at their first screening had worse symptoms, higher PVR and had worse survival compared with patients detected in subsequent screening evaluations. This illustrates the difficult balance between diminishing yield and the higher cost of a serial screening programme versus the potential to detect earlier disease and improve outcomes [91]. Further studies on cost-effectiveness of serial screening are needed to help guide policies and recommendations for the optimal time interval and duration of PAH screening in asymptomatic patients with SSc.
Considerations for future screening studies
Future studies in this domain should be prospective, multicentre and multinational, and should aim to: 1) include unselected and truly asymptomatic or minimally symptomatic patients with SSc in order to understand test performance in a true screening population; 2) systematically perform the gold-standard test for PAH (i.e. RHC) in all patients so that disease prevalence and the true positive and negative predictive values can be definitively established and accurately reported; and 3) evaluate promising noninvasive test modalities with foreseeable advantages over the status quo.
Nailfold capillaroscopy is a method of assessing periungual microvascular involvement in SSc by allowing visualisation and measurement of capillary density and morphology. Abnormalities of nailfold capillaries correlate with the extent of pulmonary involvement and with haemodynamics [92]. Nailfold capillary density is lower in SSc-PAH patients compared with patients with SSc without PAH and capillary density correlates with symptoms and resting mPAP [93, 94]. Interestingly, capillary abnormalities are present in patients with idiopathic PAH compared with healthy controls but to a lesser extent than in SSc [95]. Voilliot et al. [96] found that nailfold capillaroscopy abnormalities were associated with a future risk of PH but they only used echocardiography-derived sPAP, not RHC confirmed cases. Sequential examinations can help predict progression in organ involvement, with a loss of nailfold capillaries during serial follow-up being associated with an 18-fold risk of developing new onset pre-capillary PH [97]. A well-designed prospective evaluation of nailfold capillaroscopy with systematic RHC would be interesting to evaluate this noninvasive technique for PAH screening.
Cardiac magnetic resonance imaging (cMRI) is a noninvasive imaging modality that is more sensitive than TTE for detecting cardiac abnormalities in patients with SSc [98]. However, there are currently no prospective studies evaluating cMRI as a screening tool for PAH in SSc. RV enlargement and fibrosis at the RV insertion point are commonly present in patients with SSc-PAH, yet most patients with SSc without PAH also have abnormalities on cMRI and, importantly, RV enlargement is not specific for PAH [98, 99]. In a highly selected cohort of patients with SSc referred for suspected PAH, ventricular mass index (calculated as right end-diastolic ventricular mass divided by left end-diastolic ventricular mass) strongly correlated with invasive haemodynamics and discriminated patients with PH from those without (AUC 0.92) [100]. Pulmonary blood volume can be quantified using cMRI and may be an early indicator of pulmonary vascular involvement [101]. It is not known whether changes in cMRI-derived pulmonary blood volume occur over time in patients with SSc or whether this predicts future development of PAH. Given the potentially higher sensitivity of cMRI over TTE, a future prospective screening study should compare serial cMRI to TTE and/or composite algorithms with systematic RHC performed in all patients. Certainly, the availability and cost-effectiveness of such a screening approach would also need to be considered.
There are several interesting and novel potential blood biomarkers for PAH in SSc that reflect endothelial dysfunction, inflammation, autoimmunity, epigenetic changes, extracellular matrix deposition and metabolite changes, which have recently been extensively reviewed by Odler et al. [102]. For example, one recent study found that levels of chemokine CCL21, which is involved in T-cell differentiation, were higher in patients with SSc-PAH from two independent cohorts versus controls, patients with SSc and interstitial lung disease, patients with SSc without PH and idiopathic PAH [103]. Furthermore, CCL21 levels were elevated years prior to the PAH diagnosis and were associated with survival after diagnosis. However, many biomarkers are limited to single studies or there are conflicting results for other potential blood biomarkers. Many are nonspecific for pulmonary vascular disease, and very few have been evaluated prospectively or validated as screening tools. This will be an area of intense research in coming years. The ongoing PVDomics study (ClinicalTrials.gov identifier: NCT02980887) may provide novel insights into biological markers of early pulmonary vascular disease in several populations, including patients with SSc.
Conclusions
PAH is a rare and life-threatening disease for which early detection with systematic screening could lead to earlier diagnosis and improved outcomes in at-risk populations. The recent 6th World Symposium on PH recommends the use of TTE, the DETECT algorithm or FVC %/DLCO % ratio with NT-proBNP to screen for PAH in patients with SSc spectrum disorders and a DLCO <80% predicted. For patients with a normal DLCO (>80% predicted), annual TTE can be considered. Additional studies are needed to determine the most cost-effective strategy and identify the optimal screening interval and duration. Identification of novel markers for early pulmonary vascular disease are future priorities for improving PAH screening and early detection in at-risk populations, including patients with SSc. Ideally, future screening studies should evaluate asymptomatic patients and ensure systematic catheterisation is performed to define the true prevalence of PAH and the true predictive values. When such a design is not feasible or practical in evaluating a new potential screening tool, it is important to explicitly note this as a limitation.
Footnotes
Publication of this peer-reviewed article was sponsored by Boehringer Ingelheim, Germany (principal sponsor European Respiratory Review issue 153).
Conflict of interest: J. Weatherald reports grants, personal fees and non-financial support from Actelion, personal fees and non-financial support from Bayer, personal fees from Novartis, and grants from the European Respiratory Society and Canadian Vascular Network, outside the submitted work.
Conflict of interest: D. Montani reports grants and personal fees from Actelion and Bayer, and personal fees from GSK, MSD and Pfizer, outside the submitted work.
Conflict of interest: M. Jevnikar has nothing to disclose.
Conflict of interest: X. Jais reports grants, personal fees and non-financial support from Actelion and Bayer, personal fees and non-financial support from MSD, and grants and non-financial support from GSK, outside the submitted work.
Conflict of interest: L. Savale reports grants, personal fees and non-financial support from Actelion, Bayer, MSD and GSK, outside the submitted work.
Conflict of interest: M. Humbert reports personal fees and non-financial support from Actelion, grants, personal fees and non-financial support from Bayer, grants and personal fees from GSK, and personal fees from Merck and United Therapeutics, outside the submitted work.
References
- 1.Humbert M, Guignabert C, Bonnet S, et al. . Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53: 1801887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. . Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Vachiery J-L, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs G, Berghold A, Scheidl S, et al. . Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34: 888–894. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Sitbon O, Chaouat A, et al. . Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 6.D'Alonzo GE, Barst RJ, Ayres SM, et al. . Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 7.Benza RL, Gomberg-Maitland M, Miller DP, et al. . The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 8.Benza RL, Miller DP, Barst RJ, et al. . An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Sitbon O, Chaouat A, et al. . Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 10.Humbert M, Sitbon O, Yaïci A, et al. . Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010; 36: 549–555. [DOI] [PubMed] [Google Scholar]
- 11.Benza RL, Miller DP, Gomberg-Maitland M, et al. . Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 12.Weatherald J, Boucly A, Launay D, et al. . Haemodynamics and serial risk assessment in systemic sclerosis associated pulmonary arterial hypertension. Eur Respir J 2018; 52: 1800678. [DOI] [PubMed] [Google Scholar]
- 13.Mukerjee D, St George D, Coleiro B, et al. . Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis 2003; 62: 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung L, Farber HW, Benza R, et al. . Unique predictors of mortality in patients with pulmonary arterial hypertension associated with systemic sclerosis in the REVEAL registry. Chest 2014; 146: 1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolstad KD, Li S, Steen V, et al. . Long-term outcomes in systemic sclerosis-associated pulmonary arterial hypertension from the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma registry (PHAROS). Chest 2018; 154: 862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condliffe R, Kiely DG, Peacock AJ, et al. . Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179: 151–157. [DOI] [PubMed] [Google Scholar]
- 17.Campo A, Mathai SC, Le Pavec J, et al. . Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 182: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubenfire M, Huffman MD, Krishnan S, et al. . Survival in systemic sclerosis with pulmonary arterial hypertension has not improved in the modern era. Chest 2013; 144: 1282–1290. [DOI] [PubMed] [Google Scholar]
- 19.Kawut SM, Taichman DB, Archer-Chicko CL, et al. . Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest 2003; 123: 344–350. [DOI] [PubMed] [Google Scholar]
- 20.Strange G, Gabbay E, Kermeen F, et al. . Time from symptoms to definitive diagnosis of idiopathic pulmonary arterial hypertension: the delay study. Pulm Circ 2013; 3: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich S, Dantzker DR, Ayres SM, et al. . Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987; 107: 216–223. [DOI] [PubMed] [Google Scholar]
- 22.Boucly A, Cottin V, Nunes H, et al. . Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J 2017; 50: 1700465. [DOI] [PubMed] [Google Scholar]
- 23.Hoeper MM, Kramer T, Pan Z, et al. . Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 24.Peacock AJ, Murphy NF, McMurray JJV, et al. . An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007; 30: 104–109. [DOI] [PubMed] [Google Scholar]
- 25.Lau EMT, Humbert M, Celermajer DS. Early detection of pulmonary arterial hypertension. Nat Rev Cardiol 2015; 12: 143–155. [DOI] [PubMed] [Google Scholar]
- 26.Diab N, Hassoun PM. Pulmonary arterial hypertension: screening challenges in systemic sclerosis and future directions. Eur Respir J 2017; 49: 1700522. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin VV, Archer SL, Badesch DB, et al. . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009; 119: 2250–2294. [DOI] [PubMed] [Google Scholar]
- 28.Frost A, Badesch D, Gibbs JSR, et al. . Diagnosis of pulmonary hypertension. Eur Respir J 2019; 53: 1801904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanna D, Gladue H, Channick R, et al. . Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65: 3194–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau EMT, Celermajer DS, Keogh A, et al. . Screening of pulmonary arterial hypertension. Semin Respir Crit Care Med 2017; 38: 596–605. [DOI] [PubMed] [Google Scholar]
- 31.Wald NJ. The definition of screening. J Med Screen 2001; 8: 1. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization . Cancer. Screening. www.who.int/cancer/prevention/diagnosis-screening/screening/en/ Date last updated: February 3, 2017. Date last accessed: February 5, 2019.
- 33.Hoeper MM, Lee SH, Voswinckel R, et al. . Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol 2006; 48: 2546–2552. [DOI] [PubMed] [Google Scholar]
- 34.Bulliard J-L, Chiolero A. Screening and overdiagnosis: public health implications. Public Health Rev 2015; 36: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hachulla E, Gressin V, Guillevin L, et al. . Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52: 3792–3800. [DOI] [PubMed] [Google Scholar]
- 36.Vonk MC, Broers B, Heijdra YF, et al. . Systemic sclerosis and its pulmonary complications in The Netherlands: an epidemiological study. Ann Rheum Dis 2009; 68: 961–965. [DOI] [PubMed] [Google Scholar]
- 37.Morrisroe K, Stevens W, Sahhar J, et al. . Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening programme. Arthritis Res Ther 2017; 19: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phung S, Strange G, Chung LP, et al. . Prevalence of pulmonary arterial hypertension in an Australian scleroderma population: screening allows for earlier diagnosis. Intern Med J 2009; 39: 682–691. [DOI] [PubMed] [Google Scholar]
- 39.Coghlan JG, Denton CP, Grünig E, et al. . Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73: 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hachulla E, de Groote P, Gressin V, et al. . The three-year incidence of pulmonary arterial hypertension associated with systemic sclerosis in a multicenter nationwide longitudinal study in France. Arthritis Rheum 2009; 60: 1831–1839. [DOI] [PubMed] [Google Scholar]
- 41.Vandecasteele E, Drieghe B, Melsens K, et al. . Screening for pulmonary arterial hypertension in an unselected prospective systemic sclerosis cohort. Eur Respir J 2017; 49: 1600227. [DOI] [PubMed] [Google Scholar]
- 42.Nihtyanova SI, Schreiber BE, Ong VH, et al. . Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014; 66: 1625–1635. [DOI] [PubMed] [Google Scholar]
- 43.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66: 940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Launay D, Sitbon O, Hachulla E, et al. . Survival in systemic sclerosis-associated pulmonary arterial hypertension in the modern management era. Ann Rheum Dis 2013; 72: 1940–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Badesch DB, Tapson VF, McGoon MD, et al. . Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med 2000; 132: 425–434. [DOI] [PubMed] [Google Scholar]
- 46.Coghlan JG, Galiè N, Barberà JA, et al. . Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaine S, Chin K, Coghlan G, et al. . Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J 2017; 50: 1602493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mercurio V, Diab N, Peloquin G, et al. . Risk assessment in scleroderma patients with newly diagnosed pulmonary arterial hypertension: application of the ESC/ERS risk prediction model. Eur Respir J 2018; 52: 1800497. [DOI] [PubMed] [Google Scholar]
- 49.Humbert M, Yaici A, de Groote P, et al. . Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum 2011; 63: 3522–3530. [DOI] [PubMed] [Google Scholar]
- 50.Gladue H, Altorok N, Townsend W, et al. . Screening and diagnostic modalities for connective tissue disease-associated pulmonary arterial hypertension: a systematic review. Semin Arthritis Rheum 2014; 43: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young A, Nagaraja V, Basilious M, et al. . Update of screening and diagnostic modalities for connective tissue disease-associated pulmonary arterial hypertension. Semin Arthritis Rheum 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher MR, Forfia PR, Chamera E, et al. . Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D'Alto M, Romeo E, Argiento P, et al. . Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int J Cardiol 2013; 168: 4058–4062. [DOI] [PubMed] [Google Scholar]
- 54.Greiner S, Jud A, Aurich M, et al. . Reliability of noninvasive assessment of systolic pulmonary artery pressure by Doppler echocardiography compared to right heart catheterization: analysis in a large patient population. J Am Heart Assoc 2014; 3: e001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukherjee M, Chung S-E, Ton VK, et al. . Unique abnormalities in right ventricular longitudinal strain in systemic sclerosis patients. Circ Cardiovasc Imaging 2016; 9: e003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durmus E, Sunbul M, Tigen K, et al. . Right ventricular and atrial functions in systemic sclerosis patients without pulmonary hypertension. Speckle-tracking echocardiographic study. Herz 2015; 40: 709–715. [DOI] [PubMed] [Google Scholar]
- 57.Hekimsoy V, Kaya EB, Akdogan A, et al. . Echocardiographic assessment of regional right ventricular systolic function using two-dimensional strain echocardiography and evaluation of the predictive ability of longitudinal 2D-strain imaging for pulmonary arterial hypertension in systemic sclerosis patients. Int J Cardiovasc Imaging 2018; 34: 883–892. [DOI] [PubMed] [Google Scholar]
- 58.Steen VD, Graham G, Conte C, et al. . Isolated diffusing capacity reduction in systemic sclerosis. Arthritis Rheum 1992; 35: 765–770. [DOI] [PubMed] [Google Scholar]
- 59.Steen V, Medsger TA. Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum 2003; 48: 516–522. [DOI] [PubMed] [Google Scholar]
- 60.Mukerjee D, St George D, Knight C, et al. . Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford) 2004; 43: 461–466. [DOI] [PubMed] [Google Scholar]
- 61.Degano B, Soumagne T, Delaye T, et al. . Combined measurement of carbon monoxide and nitric oxide lung transfer does not improve the identification of pulmonary hypertension in systemic sclerosis. Eur Respir J 2017; 50: 1701008. [DOI] [PubMed] [Google Scholar]
- 62.Sivova N, Launay D, Wémeau-Stervinou L, et al. . Relevance of partitioning DLCO to detect pulmonary hypertension in systemic sclerosis. PLoS One 2013; 8: e78001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Overbeek MJ, Groepenhoff H, Voskuyl AE, et al. . Membrane diffusion- and capillary blood volume measurements are not useful as screening tools for pulmonary arterial hypertension in systemic sclerosis: a case control study. Respir Res 2008; 9: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saggar R, Khanna D, Furst DE, et al. . Exercise-induced pulmonary hypertension associated with systemic sclerosis: four distinct entities. Arthritis Rheum 2010; 62: 3741–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steen V, Chou M, Shanmugam V, et al. . Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 2008; 134: 146–151. [DOI] [PubMed] [Google Scholar]
- 66.Kovacs G, Avian A, Wutte N, et al. . Changes in pulmonary exercise haemodynamics in scleroderma: a 4-year prospective study. Eur Respir J 2017; 50: 1601708. [DOI] [PubMed] [Google Scholar]
- 67.Naeije R, Vanderpool R, Dhakal BP, et al. . Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med 2013; 187: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herve P, Lau EM, Sitbon O, et al. . Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J 2015; 46: 728–737. [DOI] [PubMed] [Google Scholar]
- 69.Codullo V, Caporali R, Cuomo G, et al. . Stress Doppler echocardiography in systemic sclerosis: evidence for a role in the prediction of pulmonary hypertension. Arthritis Rheum 2013; 65: 2403–2411. [DOI] [PubMed] [Google Scholar]
- 70.Nagel C, Henn P, Ehlken N, et al. . Stress Doppler echocardiography for early detection of systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Res Ther 2015; 17: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kovacs G, Maier R, Aberer E, et al. . Assessment of pulmonary arterial pressure during exercise in collagen vascular disease: echocardiography vs right-sided heart catheterization. Chest 2010; 138: 270–278. [DOI] [PubMed] [Google Scholar]
- 72.Kusunose K, Yamada H, Hotchi J, et al. . Prediction of future overt pulmonary hypertension by 6-min walk stress echocardiography in patients with connective tissue disease. J Am Coll Cardiol 2015; 66: 376–384. [DOI] [PubMed] [Google Scholar]
- 73.Dumitrescu D, Nagel C, Kovacs G, et al. . Cardiopulmonary exercise testing for detecting pulmonary arterial hypertension in systemic sclerosis. Heart 2017; 103: 774–782. [DOI] [PubMed] [Google Scholar]
- 74.Mukerjee D, Yap LB, Holmes AM, et al. . Significance of plasma N-terminal pro-brain natriuretic peptide in patients with systemic sclerosis-related pulmonary arterial hypertension. Respir Med 2003; 97: 1230–1236. [DOI] [PubMed] [Google Scholar]
- 75.Williams MH, Handler CE, Akram R, et al. . Role of N-terminal brain natriuretic peptide (NT-proBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J 2006; 27: 1485–1494. [DOI] [PubMed] [Google Scholar]
- 76.Chighizola C, Meroni PL, Schreiber BE, et al. . Role of N-terminal pro-brain natriuretic peptide in detecting clinically significant cardiac involvement in systemic sclerosis patients. Clin Exp Rheumatol 2012; 30: Suppl. 71, S81–S85. [PubMed] [Google Scholar]
- 77.Avouac J, Meune C, Chenevier-Gobeaux C, et al. . Cardiac biomarkers in systemic sclerosis: contribution of high-sensitivity cardiac troponin in addition to N-terminal pro-brain natriuretic peptide. Arthritis Care Res (Hoboken) 2015; 67: 1022–1030. [DOI] [PubMed] [Google Scholar]
- 78.Thakkar V, Stevens WM, Prior D, et al. . N-terminal pro-brain natriuretic peptide in a novel screening algorithm for pulmonary arterial hypertension in systemic sclerosis: a case-control study. Arthritis Res Ther 2012; 14: R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thakkar V, Stevens W, Prior D, et al. . The inclusion of N-terminal pro-brain natriuretic peptide in a sensitive screening strategy for systemic sclerosis-related pulmonary arterial hypertension: a cohort study. Arthritis Res Ther 2013; 15: R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allanore Y, Borderie D, Avouac J, et al. . High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum 2008; 58: 284–291. [DOI] [PubMed] [Google Scholar]
- 81.Soukup T, Pudil R, Kubinova K, et al. . Application of the DETECT algorithm for detection of risk of pulmonary arterial hypertension in systemic sclerosis: data from a Czech tertiary centre. Rheumatology (Oxford) 2016; 55: 109–114. [DOI] [PubMed] [Google Scholar]
- 82.Castillo A G-D, Callejas-Moraga EL, García G, et al. . High sensitivity and negative predictive value of the DETECT algorithm for an early diagnosis of pulmonary arterial hypertension in systemic sclerosis: application in a single center. Arthritis Res Ther 2017; 19: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hao Y, Thakkar V, Stevens W, et al. . A comparison of the predictive accuracy of three screening models for pulmonary arterial hypertension in systemic sclerosis. Arthritis Res Ther 2015; 17: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galiè N, Hoeper MM, Humbert M, et al. . Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 85.Bae S, Saggar R, Bolster MB, et al. . Baseline characteristics and follow-up in patients with normal haemodynamics versus borderline mean pulmonary arterial pressure in systemic sclerosis: results from the PHAROS registry. Ann Rheum Dis 2012; 71: 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valerio CJ, Schreiber BE, Handler CE, et al. . Borderline mean pulmonary artery pressure in patients with systemic sclerosis: transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum 2013; 65: 1074–1084. [DOI] [PubMed] [Google Scholar]
- 87.Visovatti SH, Distler O, Coghlan JG, et al. . Borderline pulmonary arterial pressure in systemic sclerosis patients: a post-hoc analysis of the DETECT study. Arthritis Res Ther 2014; 16: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heresi GA, Minai OA, Tonelli AR, et al. . Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm Circ 2013; 3: 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kovacs G, Avian A, Tscherner M, et al. . Characterization of patients with borderline pulmonary arterial pressure. Chest 2014; 146: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 90.Gladue H, Steen V, Allanore Y, et al. . Combination of echocardiographic and pulmonary function test measures improves sensitivity for diagnosis of systemic sclerosis-associated pulmonary arterial hypertension: analysis of 2 cohorts. J Rheumatol 2013; 40: 1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quinlivan A, Thakkar V, Stevens W, et al. . Cost savings with a new screening algorithm for pulmonary arterial hypertension in systemic sclerosis. Intern Med J 2015; 45: 1134–1140. [DOI] [PubMed] [Google Scholar]
- 92.Ohtsuka T, Hasegawa A, Nakano A, et al. . Nailfold capillary abnormality and pulmonary hypertension in systemic sclerosis. Int J Dermatol 1997; 36: 116–122. [DOI] [PubMed] [Google Scholar]
- 93.Hofstee HMA, Vonk Noordegraaf A, Voskuyl AE, et al. . Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis 2009; 68: 191–195. [DOI] [PubMed] [Google Scholar]
- 94.Riccieri V, Vasile M, Iannace N, et al. . Systemic sclerosis patients with and without pulmonary arterial hypertension: a nailfold capillaroscopy study. Rheumatology (Oxford) 2013; 52: 1525–1528. [DOI] [PubMed] [Google Scholar]
- 95.Corrado A, Correale M, Mansueto N, et al. . Nailfold capillaroscopic changes in patients with idiopathic pulmonary arterial hypertension and systemic sclerosis-related pulmonary arterial hypertension. Microvasc Res 2017; 114: 46–51. [DOI] [PubMed] [Google Scholar]
- 96.Voilliot D, Magne J, Dulgheru R, et al. . Prediction of new onset of resting pulmonary arterial hypertension in systemic sclerosis. Arch Cardiovasc Dis 2016; 109: 268–277. [DOI] [PubMed] [Google Scholar]
- 97.Avouac J, Lepri G, Smith V, et al. . Sequential nailfold video capillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum 2017; 47: 86–94. [DOI] [PubMed] [Google Scholar]
- 98.Hachulla A-L, Launay D, Gaxotte V, et al. . Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68: 1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hesselstrand R, Scheja A, Wuttge DM, et al. . Enlarged right-sided dimensions and fibrosis of the right ventricular insertion point on cardiovascular magnetic resonance imaging is seen early in patients with pulmonary arterial hypertension associated with connective tissue disease. Scand J Rheumatol 2011; 40: 133–138. [DOI] [PubMed] [Google Scholar]
- 100.Hagger D, Condliffe R, Woodhouse N, et al. . Ventricular mass index correlates with pulmonary artery pressure and predicts survival in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology (Oxford) 2009; 48: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 101.Kanski M, Arheden H, Wuttge DM, et al. . Pulmonary blood volume indexed to lung volume is reduced in newly diagnosed systemic sclerosis compared to normal – a prospective clinical cardiovascular magnetic resonance study addressing pulmonary vascular changes. J Cardiovasc Magn Reson 2013; 15: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Odler B, Foris V, Gungl A, et al. . Biomarkers for pulmonary vascular remodeling in systemic sclerosis: a pathophysiological approach. Front Physiol 2018; 9: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoffmann-Vold A-M, Hesselstrand R, Fretheim H, et al. . CCL21 as a Potential serum biomarker for pulmonary arterial hypertension in systemic sclerosis. Arthritis Rheumatol 2018; 70: 1644–1653. [DOI] [PubMed] [Google Scholar]