Abstract
Chronic bronchitis is a chronic, progressive disease that is difficult to treat. Despite much effort, patients remain highly symptomatic. Currently, a number of innovative bronchoscopic treatments for this disease are under investigation. Liquid nitrogen metered cryospray, bronchial rheoplasty and balloon desobstruction all aim to destroy the hyperplastic goblet cells and excess submucous glands using different strategies. These therapies are in an early phase of clinical research and larger randomised controlled trials are needed to confirm the pilot data available and to evaluate the treatment durability. The fourth technique, targeted lung denervation (TLD), aims to decrease the release of acetylcholine, which regulates smooth muscle tone and mucus production by ablating the parasympathetic nerves running alongside the main bronchi. Evaluation of this treatment is at a more advanced stage and promising effects on exacerbation frequency have been shown. However, confirmation of the benefit in improvement in chronic bronchitis symptoms is still needed.
Short abstract
We provide an overview of four innovative bronchoscopic treatments for chronic bronchitis. These treatments show promising results, but future work should focus specifically on chronic bronchitis symptoms and the sustainability of the treatment effect. https://bit.ly/34DNBce
Introduction
In the past decade there has been a significant development in bronchoscopic treatments for emphysema. Multiple randomised controlled trials have been performed to evaluate bronchoscopic treatments such as coils, valves, vapour ablation, airway bypass stents and AeriSeal [1–14]. These efforts have led to the inclusion of both coils and endobronchial valves in the 2017 Global Initiative for Chronic Obstructive Lung Disease therapeutic guidelines and the vapour ablation therapy in the 2019 guidelines [15, 16]. Effectively, these treatments all aim to treat the emphysemateous phenotype of COPD, but their introduction has contributed to the knowledge necessary to develop innovative bronchoscopic approaches for the chronic bronchitis phenotype. In this article we review the current literature and discuss these very new bronchoscopic developments for the treatment of chronic bronchitis.
Chronic bronchitis
The classic definition of chronic bronchitis specifies a history of productive cough with sputum production for ≥3 months in two successive years [17]. Chronic bronchitis is characterised by airway inflammation, mucus hypersecretion and ineffective clearance due to ciliary dysfunction [18]. The main clinical features of chronic bronchitis, increased productive cough and dyspnoea, are associated with poor outcomes [19–23]. For example, COPD patients with chronic bronchitis symptoms were found to have worse airflow obstruction, greater burden of respiratory symptoms such as dyspnoea, cough, wheeze and nasal symptoms, more physical activity limitation and a worse quality of life [21–23]. Furthermore, it was shown that chronic cough and sputum production were associated with frequent COPD exacerbations including exacerbations requiring hospitalisations [19–22]. Chronic mucus hypersecretion was found to be associated with a faster decline in lung function [20]. According to a recently published review, the prevalence of chronic bronchitis in patients with COPD ranges from 12% to 35% [24]. The treatment of chronic bronchitis is aimed at reducing the overproduction of mucus, its tenacity, cough, restoring ciliary transport and attenuating inflammation. The current treatment options for chronic bronchitis are smoking cessation, physical measures (such as chest physical therapy, high-frequency chest wall oscillation or flutter valve) and drug therapy (expectorants and mucolytics, methylxanthines and short- and long-acting β-adrenergic receptor agonists, anticholinergics, glucocorticoids, phosphodiesterase (PDE)-4 inhibitors, antioxidants and macrolides) [18]. However, there are no currently approved interventions that effectively address the airway metaplasia and mucus hypersecretion of chronic bronchitis, and despite much effort patients remain highly symptomatic. There is a need for alternative therapeutic strategies. The mucus overproduction in chronic bronchitis is mostly generated by the goblet cells located in the first to fifth generations (up to the subsegmental bronchus) [25], an area which is easily accessible for bronchoscopic treatment.
Bronchoscopic treatments for chronic bronchitis
Search strategy and selection criteria
References for this review were identified through searches of PubMed, Scopus and Google Scholar for articles published until June 2020, by use of the terms “COPD”, “chronic bronchitis” and “bronchoscopy”. Articles, conference proceedings and relevant references cited in those articles were reviewed. All articles found were published in English. We also searched the clinical trial registration websites clinicaltrials.gov and the ISRCTN registry to identify ongoing or planned trials for the treatments we found in our literature search.
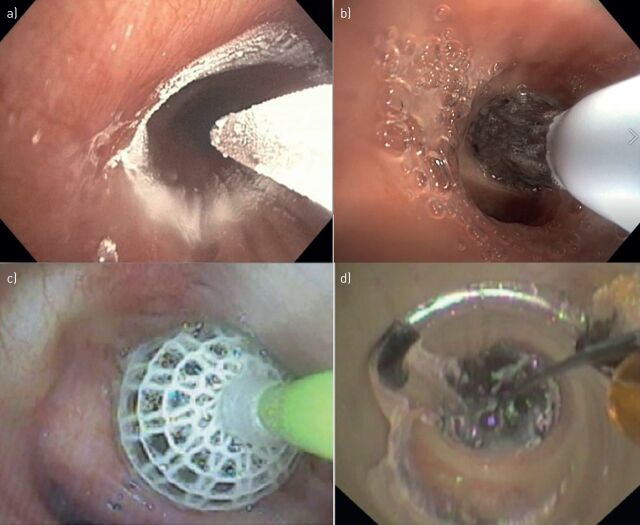
In our literature search, we identified three specific bronchoscopic treatments for chronic bronchitis: the RejuvenAir System delivering metered cryospray (CSA Medical, Lexington, MA, USA), RheOx bronchial rheoplasty (Gala Therapeutics, San Carlos, CA, USA) and the Karakoca balloon desobstruction treatment (Rezektor Balon, Istanbul, Turkey). In addition, we sourced the TLD treatment aimed at both COPD phenotypes (emphysema and chronic bronchitis), and included it in our review. Table 1 summarises the publications found. Figure 1 presents pictures of the different treatment catheters during treatment.
TABLE 1.
Number of publications per treatment identified by the literature search
| Subjects n | First author, year [reference] | |
| RejuvenAir System Metered Cryospray | ||
| Animal study (Yorkshire pigs) | 6 | Au, 2012 [26] |
| Patients scheduled for lobectomy (nonchronic bronchitis) | 21 | Krimsky, 2010 [27] |
| Patients scheduled for lobectomy/pneumonectomy (nonchronic bronchitis) | 16 | Slebos, 2017 [28] |
| Chronic bronchitis patients | 35 | Garner, 2020 [29] |
| RheOx bronchial rheoplasty | ||
| Chronic bronchitis patients | 30 | Valipour, 2020 [30] |
| Karakoca balloon desobstruction | ||
| COPD patients | 10 | Karacoka, 2015 [31] |
| COPD patients | 188 | Karacoka, 2018 [32] |
| Targeted lung denervation | ||
| Animal study (sheep and dogs) | 11/2 | Hummel, 2019 [33] |
| Animal study (sheep) | 19 | Mayse, 2020 [34] |
| COPD patients | 22 | Slebos, 2015 [35], Kistemaker, 2015 [36], Koegelenberg, 2016 [37] |
| COPD patients | 15 | Valipour, 2018 [38] |
| COPD patients | 46 | Valipour, 2019 [39] |
| COPD patients | 82 | Slebos, 2019 [40] |
FIGURE 1.
Bronchoscopic images of treatment catheters. a) RejuvenAir System delivering metered cryospray; b) RheOx bronchial rheoplasty, reproduced with permission from Gala Therapeutics (San Carlos, CA, USA); c) Karakoca balloon desobstruction, reproduced from [31] with permission; d) targeted lung denervation.
RejuvenAir System Metered Cryospray
Description of treatment
The aim of the RejuvenAir System Metered Cryospray is to destroy the hyperplasic goblet cells and excess of submucous glands with a liquid nitrogen spray, potentially inducing an airway tissue nonscarring healing response. The RejuvenAir System delivers a programmed metered cryospray of liquid nitrogen at −196°C. The dose is determined by real-time feedback from a thermocouple in the catheter. Each bronchial airway receives a standardised amount of nitrogen based on the airway size; the system delivers a 10-mm circular cryoablation zone with a depth ranging from 0.1 to 0.5 mm. In the first clinical trial, the cryospray procedure was performed in three separate treatments (right lower lobe, left lower lobe and both upper lobes and trachea separately) with ∼45 days in between. The treatment was performed under general anasethesia with an average procedure time of ∼30 min [29]. In future trials the treatment will be completed in two procedures.
Published results
Animal study
A published study of six Yorkshire pigs exposed to a range of doses of liquid nitrogen (varying duration and number of cycles) resulted in superficial necrosis extending from the mucosa to the cartilage, but not beyond [26].
Studies in nonchronic bronchitis patients
First, two safety and feasibility studies were performed in nonchronic bronchitis patients with a forced expiratory volume in 1 s (FEV1) ≥50% predicted. The first study by Krimsky et al. [27] performed cryotherapy with the CryoSpray Ablation System in 21 patients undergoing standard bronchoscopy in anticipation of a lobectomy for (non)small lung cancer, carcinoid tumour or recurrent mycobacterial infection. Bronchoscopic and histological examinations of the airways were performed between 1 and 106 days after treatment and showed cryonecrosis limited to the mucosal and submucosal layers and no damage to connective or cartilage tissue. For up to 106 days after treatment, complete re-epithelialisation of the airway mucosa and a thinned or absent smooth muscle layer were visible. Furthermore, no adverse events were observed [27]. The second safety study in nonchronic bronchitis patients used a next-generation system and was performed in 16 patients undergoing standard bronchoscopy in anticipation of a lobectomy or pneumonectomy for suspicious or cancerous peripheral lung lesions [28]. Two sprays were delivered into each of the lobar and first segmental bronchi. There were no intraoperative complications or technical difficulties nor device or treatment-related serious adverse events.
Study in chronic bronchitis patients
The RejuvenAir study (NCT02483637) was a prospective, single-arm, open-label trial investigating the safety and feasibility of the treatment for the first time in chronic bronchitis patients [29]. The treatment was completed in three separate procedures and the patients were fit for discharge the same day (except for two pre-planned overnight stays). In total, 35 patients were enrolled and 34 underwent all three procedures. In this study none of the serious adverse events were found to be related to the device or the procedure. Furthermore, the results showed a significant clinical improvement in quality of life and cough symptoms after 3 months. 12 months after the treatment, no clinically relevant improvements were found (table 2). However, in the first 11 patients, owing to safety precautions, there was a large time gap between the first and second treatments, which could have influenced the outcomes.
TABLE 2.
Results of the RejuvenAir System Metered Cryospray study [29]
| RCT | Subjects | Time point | Result | |
| Quality of life | ||||
| SGRQ total score change | No | 34 | 3 months | −6.4±14.4 |
| No | 31 | 12 months | −4.6±15.1 | |
| CAT total score change | No | 34 | 3 months | −3.8±7.1 |
| No | 31 | 12 months | −2.0±7.2 | |
| Chronic bronchitis symptoms | ||||
| LCQ total score change | No | 34 | 3 months | 21.6±32.2 |
| No | 31 | 12 months | 9.1±29.0 | |
| Lung function | ||||
| FEV1 mL change | No | 34 | 3 months | −33.2±167 |
| No | 31 | 12 months | −96.5±198 | |
| Exercise capacity | ||||
| 6MWD m change | No | 34 | 3 months | 1.1±55.4 |
| No | 31 | 12 months | 8.5±76.2 |
Data are presented as n or mean±sd. Statistically significant values compared to baseline (p<0.05) are presented in bold. RCT: randomised controlled trial; SGRQ: St George's Respiratory Questionnaire; CAT: COPD Assessment Test; LCQ: Leicester Cough Questionnaire; FEV1: forced expiratory volume in 1 s; 6MWD: 6-min walk distance.
Ongoing and planned trials
In 2020, two trials started: 1) a US Food and Drug Administration (FDA) sham-controlled randomised trial including 330 participants (NCT03893370) with a reduction in exacerbation rate as primary end-point and 2) a randomised controlled trial investigating in greater depth the mechanisms of action of the treatment in 32 patients (NCT03892694) with goblet cell density as the end-point.
RheOx bronchial rheoplasty
Description of treatment
Another novel treatment for chronic bronchitis is the RheOx bronchial rheoplasty. The RheOx catheter delivers short bursts of high-frequency electrical energy to the airway epithelium and submucosal tissue layers targeting abnormal goblet cells and glands and facilitating their replacement with healthier tissues. Bronchial rheoplasty is performed in two separate treatments (one lung per treatment) with 1 month in between, and treatment is delivered to the second- to seventh-generation airways. Treatment is performed under general anasethesia with an average procedure time of ∼30 min. Because the treatment uses a monopolar electrode, it is contraindicated in those individuals with implantable cardiac devices and the like and potentially limits the eligibility of this therapy in a population who have a high incidence of cardiovascular morbidity and mortality.
Published results
No publications were found with results of pre-clinical laboratory or animal studies.
Study in chronic bronchitis patients
Two combined multicentre single-arm clinical trials (Australian New Zealand Clinical Trials Registry ACTRN12617000330347; NCT03107494), investigated for the first time the treatment in 30 patients with chronic bronchitis [30]. The treatment was performed bilaterally in two separate procedures with on average 43±21 activations per lung and the median hospital stay was 1 day (range 0–4 days). Four procedure-related serious adverse events were reported up to 6 months (pneumonia n=1, mucosal scarring n=1 and COPD exacerbations n=2; all resolved without sequelae) and none between 6 and 12 months’ follow-up. 3, 6 and 12 months after treatment, significant improvements were found in quality of life measured by the COPD Assessment Test (CAT) and St George's Respiratory Questionnaire (SGRQ). Furthermore, the two CAT questions on phlegm and cough decreased (it was not stated whether this change was statistically significant). 3 months after treatment, a relative reduction of 39% in mean goblet cell hyperplasia score was found. Lung function parameters did not change (table 3).
TABLE 3.
Results of multicentre RheOx bronchial rheoplasty study [30]
| RCT | Subjects | Time point | Result | |
| Quality of life | ||||
| SGRQ total score change | No | 30 | 3 months | −16.9±20.0 |
| No | 29 | 12 months | −15.2±20.4 | |
| CAT total score change | No | 30 | 3 months | −8.8±7.6 |
| No | 29 | 12 months | −7.0±8.9 | |
| Chronic bronchitis symptoms# | ||||
| CAT change in question on phlegm | No | 30 | 3 months | −1.8±1.1 |
| No | 29 | 12 months | −1.7±1.5 | |
| CAT change in question on cough | No | 30 | 3 months | −1.4±1.3 |
| No | 29 | 12 months | −1.1±1.6 | |
| Lung function | No | 29/30 | 3 and 12 months | No sign changes |
| Exercise capacity | Not reported | |||
| Other | ||||
| Goblet cell hyperplasia scoring | No | 54 lungs | 90–120 days | Relative reduction of 39% |
Data are presented as n or mean±sd. Statistically significant values (p<0.05) are presented in bold. RCT: randomised controlled trial; SGRQ: St George's Respiratory Questionnaire; CAT: COPD Assessment Test. #: based on a single question; it was not clear whether these changes were statistically significant.
Ongoing and planned trials
According to clinicaltrials.gov, two early feasibility studies have started in the USA (NCT03631472; estimated n=30) and Canada (NCT03385616; estimated n=24).
Karakoca balloon desobstruction procedure
Description of treatment
The Karakoca resector balloon developed for the treatment of airway obstruction [41] has been found a place in the management of chronic bronchitis patients. The device consists of a latex balloon covered with a mesh structure of 0.2-mm polyurethane/lycra fibres, mounted onto the distal end of long single-lumen polyethylene tube. The maximum inflated diameter of the balloon is 10–24 mm and can reach 8–3-mm bronchi [31]. The balloon is inserted into the narrowed bronchial lumen and repeatedly inflated and deflated, resulting in a force applied to the bronchial mucosa of ∼2.0–2.5 bar [31], mechanically disrupting the hyperplasic goblet cells. The treatment is performed in a single session, and in total 100–300 segmental bronchi could be treated during a 60-min bronchoscopy [32].
Published results
One first pilot feasibility study was performed including 10 patients [31]. According to the publication, none of the patients developed complications between 1 and 3 months’ follow-up. Furthermore, patients improved in FEV1, Borg dyspnoea scale and peripheral oxygen saturation (SpO2) levels, although only individual data are shown in the manuscript and no statistical testing was performed. Biopsies after intervention showed decreases in the number of goblet cells and mucus-containing goblet cells (no actual numbers or statistical tests were reported). Later, the same authors published a larger single-centre case series investigating the technique including 188 COPD patients [32]. According to the authors, none of the 188 patients developed intraoperative, perioperative or post-operative complications. Clinical outcomes were only measured up to 1 month after treatment, but showed statistically significant improvements in FEV1, SpO2 and 6-min walk distance (table 4). Unfortunately, no prospective trial or randomised controlled trial with a longer follow-up than 1 month has been performed or published.
TABLE 4.
Results of the Karakoca balloon desobstruction procedure [32]
| RCT | Subjects | Time point | Result | |
| Quality of life | Not reported | |||
| Chronic bronchitis symptoms | Not reported | |||
| Lung function | ||||
| FEV1 L | No | 185 | 1 month | Baseline 0.77±0.26 Follow-up 1.28±0.47 |
| Exercise capacity | ||||
| 6MWD m | No | 185 | 1 month | Baseline 69±41 Follow-up 387±113 |
Data are presented as n or mean±sd. Statistically significant values (p<0.05) are presented in bold. RCT: randomised controlled trial; FEV1: forced expiratory volume in 1 s; 6MWD: 6-min walk distance.
Ongoing and planned trials
We could not find ongoing or planned trials on the trial registration websites.
Targeted lung denervation
Description of treatment
The TLD treatment (Nuvaira, Minneapolis, MN, USA) aims to treat both phenotypes of COPD, emphysema and chronic bronchitis. The aim is to disrupt parasympathetic nerve transmission to and from the lung. In COPD patients the basal parasympathetic tone mediated via the vagal nerve is elevated, increasing acetylcholine levels, airway smooth muscle contraction and mucus production [42]. The TLD uses a dual cooled catheter with radiofrequency energy to disrupt parasympathetic nerve transmission (both efferent and afferent) while minimising effects to the mucosal surface. The treatment is performed in one session under general anaesthesia with a median procedure time of 74 min [40].
Published results
Pre-clinical studies
Before patient testing, multiple bench and animal studies were performed, two using the same system and power used in the clinical trials [33, 34]. The first study was performed in 11 sheep and two dogs and showed that the treatment reduced pulmonary airway resistance, abolished the Hering–Breuer reflex and consistently resulted in bronchial wall fibrosis to a depth which engulfed nearly all pulmonary branches of the vagal nerve [33]. No adverse clinical events occurred during the 30-day follow-up in the animals. The second study was performed in 19 sheep with a follow-up to 640 days after TLD. In this study, efferent axon staining was decreased by >70% at 365 and 640 days. Furthermore, tissue structure 1 cm proximal and distal to the treated area remained normal and there were no serious effects in the pulmonary veins and arteries and the oesophagus [34].
Studies in COPD patients
The first in human study (NCT01483534; IPS-I), published in 2015, included in total 22 patients who were treated with two different doses, performed bilaterally in two separate procedures, and with a follow-up duration of 1 year [35]. This study showed that the treatment was technically feasible 93% of the time and that the treatment was safe and well tolerated at both doses used. During the study, one serious adverse event (gastroparesis) deemed to be related to the device/treatment occurred. The improvements in efficacy tended to be larger in the patients treated with the higher dose (20 W) (table 5). A small substudy of this cohort (n=7) showed that inflammatory markers measured in bronchial wash and brush specimens decreased 30 days after treatment, suggesting a mechanistic role of the treatment in modulating airways and possibly systemic inflammation [36]. Post hoc analyses of the same cohort showed that antimuscarinic medication provided a synergistic bronchodilatory effect to TLD alone, indicating the potential benefits of the combination of TLD treatment and inhaled long-acting muscarinic antagonists and highlighting the need for further research in this area [37]. The following clinical trial (NCT01716598; IPS-II) investigated the feasibility of performing the treatment in a single session and long-term safety [38]. In total, 15 patients underwent the TLD treatment (procedure time 89±16 min) and no procedural complications occurred. Up to 3 years after treatment, five respiratory serious adverse events were reported. None were deemed to be related to the treatment.
TABLE 5.
Results of targeted lung denervation
| RCT | Subjects | Dose | Time point | Result | First author, year [reference] | |
| Quality of life | ||||||
| SGRQ total score absolute change | No | 10 | 20 W | 1 year | −11.1±9.1 | Slebos, 2015 [35] |
| No | 10 | 15 W | 1 year | −0.9±8.6 | Slebos, 2015 [35] | |
| No | 15 | 15 W | 1 year | −1.85±20.8 | Valipour, 2018 [38] | |
| Yes | 15 | 32 W | 1 year | −7.5±10.3 | Valipour, 2019 [39] | |
| Yes | 15 | 29 W | 1 year | −1.9±12.5 | Valipour, 2019 [39] | |
| No | 16 | 32 W | 1 year | −6.1±21# | Valipour, 2019 [39] | |
| Yes | 82 | 26/32 W | 1 year | No sign. difference between treatment group and SoC group | Slebos, 2019 [40] | |
| Chronic bronchitis symptoms | Not reported | |||||
| Lung function | ||||||
| FEV1 % change | No | 10 | 20 W | 1 year | 11.6±32.3 | Slebos, 2015 [35] |
| No | 10 | 15 W | 1 year | 0.02±15.1 | Slebos, 2015 [35] | |
| No | 15 | 15 W | 1 year | 40.3±42.1 | Valipour, 2018 [38] | |
| FEV1 mL change | Yes | 15 | 32 W | 1 year | 94.2±228 | Valipour, 2019 [39] |
| Yes | 15 | 29 W | 1 year | 57±82 | Valipour, 2019 [39] | |
| No | 16 | 32 W | 1 year | 34±174# | Valipour, 2019 [39] | |
| Yes | 82 | 26/32 W | 1 year | No sign. difference between treatment group and SoC group | Slebos, 2019 [40] | |
| Exercise capacity | ||||||
| 6MWD m absolute change | No | 10 | 20 W | 1 year | 24.2±45.6 | Slebos, 2015 [35] |
| No | 10 | 15 W | 1 year | −9.3±70.6 | Slebos, 2015 [35] | |
| No | 15 | 15 W | 1 year | 53.7±74.4 | Valipour, 2018 [38] | |
| Cycle ergometry endurance time | Yes | 82 | 26/32 W | 1 year | No sign. difference between treatment group and SoC group | Slebos, 2019 [40] |
| Other | ||||||
| Inflammation markers (CCl4 protein, CXCL8 gene, TGF-β gene) | No | 7 | ? | 30 days | Significant decrease in all markers (p<0.05) | Kistemaker, 2015 [36] |
Data are presented as n or mean±sd. Statistically significant values (p<0.05) are presented in bold. RCT: randomised controlled trial; SGRQ: St George's Respiratory Questionnaire; FEV1: forced expiratory volume in 1 s; 6MWD: 6-min walk distance; TGF: transforming growth factor; SoC: standard of care. #: p-values were not reported.
A second-generation TLD device was developed and the next trial was a 1:1 randomised blinded trial to investigate safety, feasibility and dosing (29 versus 32 W) of the treatment in 30 patients (NCT02058459; AIRFLOW 1) [39]. In an early phase of the trial, five patients experienced serious adverse events, after which the trial was stopped. After adding an oesophageal balloon during treatment to facilitate accurate measurement of the oesophago-to-electrode distance to avoid inadvertent capture of the gastric parasympathetic plexi, the trial continued. Patients were not treated in locations close to the oesophagus. Due to the gastrointestinal adverse events that occurred during this trial, an additional 16 patients were treated. The conclusion of the trial was that the safety and feasibility of the treatment was acceptable. No significant differences were found in safety or efficacy between the two doses used, but there was a trend towards superior efficacy in the higher-energy dose group (32 W). Afterwards, the AIRFLOW 2 trial was executed, including 82 patients in a multicentre randomised sham-controlled double-blinded trial comparing the treatment with standard of medical care [40]. The results of the trial at 1 year showed that the TLD treatment group experienced a significant lower number of respiratory events and the risk of COPD exacerbation requiring hospitalisation was significantly lower compared to the sham group.
Ongoing and planned trials
A recently commenced FDA-sponsored trial will include up to 520 participants in Europe, Canada and the United States (NCT03639051; AIRFLOW-3 trial [43]).
Discussion
Currently, there are four novel bronchoscopic therapies undergoing research evaluation for patients with chronic bronchitis: the RejuvenAir System Metered Cryospray, the RheOx bronchial rheoplasty, the Karakoca balloon desobstruction procedure and the Nuvaira TLD. Figure 2 shows a schematic overview of the mechanisms of action of the treatment. Two distinct mechanisms of action can be distinguished. Metered cryospray, bronchial rheoplasty and Karakoca balloon desobstruction procedures are designed to ablate hyperplastic goblet cells and excess submucous glands, using three distinct strategies, and ultimately promoting healthy epithelial regeneration of the airway lining. TLD counteracts the elevated basal parasympathetic tone by interrupting the vagal nerve bilaterally, reducing mucus hypersecretion, smooth muscle contraction and airways inflammation.
FIGURE 2.
Schematic overview of mechanisms of action of bronchoscopic interventions for chronic bronchitis. a) Chronic bronchitis airway epithelium with an increased number of hyperplasic goblet cells causing increased mucus secretion. b) The Karakoca resector balloon consists of a latex balloon with a mesh structure of polyurethane/lycra fibres. Inside the airway the balloon is repeatedly inflated and deflated resulting in a force applied to the bronchial mucosa, which mechanically disrupts the hyperplasic goblet cells. c) The RheOx bronchial rheoplasty catheter delivers short bursts of high-frequency electrical energy to the airway epithelium and submucosal tissue layers targeting the goblet cells and glands, aiming to replace the destroyed cells with healthier tissue. d) The RejuvenAir System Metered Cryospray catheter delivers liquid nitrogen at −196°C to the airways, aiming to destroy the goblet cells and inducing a nonscarring healing response with healthier tissue regeneration as a result. e) The Nuvaira dual-cooled balloon catheter uses radiofrequency to disrupt the local vagal nerve branches and therefore the parasympathetic nerve transmission, aimed to decrease acetylcholine levels, resulting in a decreased airway smooth muscle contraction and mucus production.
The three treatments aimed at “rejuvenation” of the epithelial wall each use different techniques. The RejuvenAir system applies liquid nitrogen in a radial spray, bronchial rheoplasty delivers short-duration high-frequency energy using a basket electrode and the Karakoca balloon desobstruction applies mechanical pressure to the airway via an inflatable balloon. The published results of the RejuvenAir System Metered Cryospray treatment showed that the treatment is feasible and safe. This first study in COPD patients showed a promising improvement in cough symptoms measured using multidimensional questionnaires. The first results of the RheOx bronchial rheoplasty treatment showed that this treatment is technically feasible and has an acceptable safety profile. Furthermore, significant improvements in goblet cell hyperplasia and quality of life and were found. The Karakoca balloon desobstruction was published as single-centre; case-series are published with outcomes up to only 1 month after treatment. For all treatments, randomised controlled trials are needed to be able to draw conclusions regarding the safety, feasibility and efficacy of each treatment in the short and longer term. In the future, comparative trials will help to clarify which technique is the safest, most effective and results in a sustained regeneration of the epithelial wall that translates to clinically meaningful reduction in symptom burden. Additionally, if these treatments can indeed “heal” the injured epithelium, how durable are these benefits? Thus, potentially, repeated treatments may be needed.
The last treatment has a different mechanism of action, targeting the vagal nerve directly rather than the airway epithelial layer. Disruption of the vagal nerve may have effects on both efferent and afferent signalling downstream. Interruption of the efferent signal lowers the basal cholinergic tone, reducing smooth muscle contraction and airway resistance in addition to decreasing mucus production; effects that appear to be mediated by inflammatory modulation. Conversely, stimuli may induce local reflex-mediated contraction of airway smooth muscle [44] and disruption of this afferent signal may be blocked by the treatment as well, thus attenuating airway hyperresponsiveness. To date, studies of TLD have not focused on the chronic bronchitis phenotype, and the effects on symptoms, notably increased cough with mucus expectoration, are unknown and merit further enquiry. However, development of this innovative therapy is at a more advanced stage than other bronchoscopic treatments and the only one to have been evaluated in a double-blind sham-controlled randomised trial. This randomised controlled trial showed that patients who underwent TLD experienced a significantly lower number of respiratory events, and the risk of a COPD exacerbation requiring hospitalisation was significantly lower compared to the sham group. The planned FDA trial will be valuable in confirming these results in a larger cohort. Furthermore, the impact of nerve regeneration in re-establishing parasympathetic connections influencing the durability of treatment effect is unknown and will require longer-term surveillance.
A challenge in this field is the lack of a universally agreed definition of chronic bronchitis. As mentioned earlier, the prevalence of chronic bronchitis in patients with COPD is variably quoted as 12–35% [24], in part a consequence of no consensus and a poor disease entity definition. The classic Reid definition, published in 1965, states that chronic bronchitis is diagnosed when chronic cough and sputum persists for ≥3 months of the year for two consecutive years [24, 45]. Others include only the presence of bronchial hypersecretion or chronic cough or both [24]. Other options are the Reid index [46] or questionnaires, although to our knowledge no validated chronic bronchitis questionnaire is available. For example, defining chronic bronchitis using the SGRQ identified more subjects at risk of future exacerbations than the classic definition [47]. The definition used will have consequences for the study population selected for trials. Furthermore, the question arises which end-point(s) would be most suitable to measure treatment efficacy. Aside from confirming airflow limitation, there are no specific lung function tests for diagnosing chronic bronchitis, which remains largely a clinical diagnosis. In addition to exacerbation frequency, quantification of cough, sputum production, breathlessness and health-related quality of life using multidimensional validated disease-specific questionnaires represents the most valuable and accurate ways of characterising disease severity and treatment response. Furthermore, outcomes on a cellular level (for example bronchoscopic biopsies) could investigate the proof of principle of the proposed mechanism of action.
In conclusion, chronic bronchitis is a chronic, progressive and difficult-to-treat disease. Despite all efforts, patients remain highly symptomatic. The affected airways are centrally located and easily accessible to a bronchoscope. Currently, three bronchoscopic treatments directly targeting chronic bronchitis are in development, aiming to destroy hyperplastic goblet cells and excess submucous glands with three different techniques. These treatments are in the very early phase of research and improvements in quality of life have been reported, but little tangible effect on cough and sputum production. Another bronchoscopic treatment is TLD, which aims to decrease mucus production, reducing acetylcholine levels by ablating the vagal nerve. The development of this treatment is more advanced and promising effects are found on the frequency of COPD exacerbations. However, this treatment is not specifically aimed at chronic bronchitis, but at COPD in general, and the effects on chronic bronchitis symptoms are unknown. Future work is needed to include larger randomised controlled trials and should focus on the change in specific chronic bronchitis symptoms such as increased cough and sputum. Furthermore, investigating the sustainability of the effect and the potential of re-treatment needs to be included in these trials.
Acknowledgements
The authors would like to thank Judith Hartman for the help with producing figure 2.
Footnotes
Provenance: Submitted article, peer reviewed
Conflict of interest: J.E. Hartman has nothing to disclose.
Conflict of interest: J.L. Garner has nothing to disclose.
Conflict of interest: P.L. Shah reports personal fees from Boston Scientific, CSA Medical, Creo Medical, Nuvairia Olympus, Medtronic and PneumRX/BTG as consultant on scientific advisory boards, and sponsorship from Imperial College for a bronchoscopy course by from ERBE, Cook Medical, Medtronic, Boston Scientific, Broncus, Pulmonx, Olympus and PneumRX/BTG, outside the submitted work. He has been an investigator on clinical trials with bronchial thermoplasty, endobronchial valves, endobronchial coils, thermal ablation and the airway bypass procedure.
Conflict of interest: D-J. Slebos reports grants, non-financial support and other from Nuvaira Inc.; grants and non-financial support from CSA medical and PneumRx/BTG; and grants, personal fees and non-financial support from PulmonX Inc., outside the submitted work.
References
- 1.Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA 2016; 315: 175–184. doi: 10.1001/jama.2015.17821 [DOI] [PubMed] [Google Scholar]
- 2.Sciurba FC, Criner GJ, Strange C, et al. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trial. JAMA 2016; 315: 2178–2189. doi: 10.1001/jama.2016.6261 [DOI] [PubMed] [Google Scholar]
- 3.Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013; 1: 233–240. doi: 10.1016/S2213-2600(13)70047-X [DOI] [PubMed] [Google Scholar]
- 4.Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015; 373: 2325–2335. doi: 10.1056/NEJMoa1507807 [DOI] [PubMed] [Google Scholar]
- 5.Criner GJ, Sue R, Wright S, et al. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med 2018; 198: 1151–1164. doi: 10.1164/rccm.201803-0590OC [DOI] [PubMed] [Google Scholar]
- 6.Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015; 386: 1066–1073. doi: 10.1016/S0140-6736(15)60001-0 [DOI] [PubMed] [Google Scholar]
- 7.Kemp S V, Slebos DJ, Kirk A, et al. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med 2017; 196: 1535–1543. doi: 10.1164/rccm.201707-1327OC [DOI] [PubMed] [Google Scholar]
- 8.Valipour A, Slebos DJ, Herth F, et al. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT study. Am J Respir Crit Care Med 2016; 194: 1073–1082. doi: 10.1164/rccm.201607-1383OC [DOI] [PubMed] [Google Scholar]
- 9.van Agteren JE, Hnin K, Grosser D, et al. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; 2: CD012158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015; 46: 651–662. doi: 10.1183/09031936.00205614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah PL, Slebos DJ, Cardoso PF, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet 2011; 378: 997–1005. doi: 10.1016/S0140-6736(11)61050-7 [DOI] [PubMed] [Google Scholar]
- 12.Wood DE, Nader DA, Springmeyer SC, et al. The IBV Valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol 2014; 21: 288–297. doi: 10.1097/LBR.0000000000000110 [DOI] [PubMed] [Google Scholar]
- 13.Eberhardt R, Gompelmann D, Schuhmann M, et al. Complete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysema. Chest 2012; 142: 900–908. doi: 10.1378/chest.11-2886 [DOI] [PubMed] [Google Scholar]
- 14.Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 2016; 4: 185–193. doi: 10.1016/S2213-2600(16)00045-X [DOI] [PubMed] [Google Scholar]
- 15.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195: 557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 16.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019 Report. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf Date last accessed: August 19, 2020.
- 17.Ferris BG. Epidemiology standardization project (American Thoracic Society). Am Rev Respir Dis 1978; 118: 1–120. [PubMed] [Google Scholar]
- 18.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 228–237. doi: 10.1164/rccm.201210-1843CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgel P-R, Nesme-Meyer P, Chanez P, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 2009; 135: 975–982. doi: 10.1378/chest.08-2062 [DOI] [PubMed] [Google Scholar]
- 20.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996; 153: 1530–1535. doi: 10.1164/ajrccm.153.5.8630597 [DOI] [PubMed] [Google Scholar]
- 21.De Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J 2012; 40: 28–36. doi: 10.1183/09031936.00141611 [DOI] [PubMed] [Google Scholar]
- 22.Kim V, Han MLK, Vance GB, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest 2011; 140: 626–633. doi: 10.1378/chest.10-2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim V, Garfield JL, Grabianowski CL, et al. The effect of chronic sputum production on respiratory symptoms in severe COPD. COPD 2011; 8: 114–120. doi: 10.3109/15412555.2011.558546 [DOI] [PubMed] [Google Scholar]
- 24.Dotan Y, So JY, Kim V. Chronic bronchitis: where are we now? Chronic Obstr Pulm Dis 2019; 6: 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross MH, Pawlina W. Figure 19.22 Divisions of the bronchial tree and summary of its histological features. In: Histology: a Text and Atlas: With Correlated Cell and Molecular Biology. 7th Edn. Baltimore, Wolters Kluwer, 2015. [Google Scholar]
- 26.Au JT, Carson J, Monette S, et al. Spray cryotherapy is effective for bronchoscopic, endoscopic and open ablation of thoracic tissues. Interact Cardiovasc Thorac Surg 2012; 15: 580–584. doi: 10.1093/icvts/ivs192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krimsky WS, Broussard JN, Sarkar SA, et al. Bronchoscopic spray cryotherapy: assessment of safety and depth of airway injury. J Thorac Cardiovasc Surg 2010; 139: 781–782. doi: 10.1016/j.jtcvs.2009.03.051 [DOI] [PubMed] [Google Scholar]
- 28.Slebos DJ, Breen D, Coad J, et al. Safety and histological effect of liquid nitrogen metered spray cryotherapy in the lung. Am J Respir Crit Care Med 2017; 196: 1351–1352. doi: 10.1164/rccm.201611-2220LE [DOI] [PubMed] [Google Scholar]
- 29.Garner JL, Shaipanich T, Hartman JE, et al. A prospective safety and feasibility study of metered cryospray (MCS) for patients with chronic bronchitis in COPD. Eur Respir J 2020; 56: 00556-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valipour A, Fernandez-Bussy S, Ing AJ, et al. Bronchial rheoplasty for treatment of chronic bronchitis: twelve-month results from a multicenter clinical trial. Am J Respir Crit Care Med 2020; 202: 681–689. doi: 10.1164/rccm.201908-1546OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karakoca Y, Karaagac Gogus G, Yapicier O. Use of resector balloon desobstruction in patients with severe chronic obstructive pulmonary disease: a pilot feasibility study on a novel desobstruction technique. J Bronchology Interv Pulmonol 2015; 22: 209–214. doi: 10.1097/LBR.0000000000000178 [DOI] [PubMed] [Google Scholar]
- 32.Karakoca Y, Gogus G, Akduman S, et al. Follow-up outcomes of chronic obstructive pulmonary disease patients who underwent dilatation and curettage with the Karakoca resector balloon: a 188-case series over 5 years. Medicine 2018; 97: e13400. doi: 10.1097/MD.0000000000013400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hummel JP, Mayse ML, Dimmer S, et al. Physiologic and histopathologic effects of targeted lung denervation in an animal model. J Appl Physiol 2019; 126: 67–76. doi: 10.1152/japplphysiol.00565.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayse ML, Norman HS, Peterson AD, et al. Targeted lung denervation in sheep: durability of denervation and long-term histologic effects on bronchial wall and peribronchial structures. Respir Res 2020; 21: 117. doi: 10.1186/s12931-020-01383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slebos DJ, Klooster K, Koegelenberg CF, et al. Targeted lung denervation for moderate to severe COPD: a pilot study. Thorax 2015; 70: 411–419. doi: 10.1136/thoraxjnl-2014-206146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kistemaker LE, Slebos DJ, Meurs H, et al. Anti-inflammatory effects of targeted lung denervation in patients with COPD. Eur Respir J 2015; 46: 1489–1492. doi: 10.1183/13993003.00413-2015 [DOI] [PubMed] [Google Scholar]
- 37.Koegelenberg CF, Theron J, Slebos DJ, et al. Antimuscarinic bronchodilator response retained after bronchoscopic vagal denervation in chronic obstructive pulmonary disease patients. Respiration 2016; 92: 58–60. doi: 10.1159/000447641 [DOI] [PubMed] [Google Scholar]
- 38.Valipour A, Asadi S, Pison C, et al. Long-term safety of bilateral targeted lung denervation in patients with COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 2163–2172. doi: 10.2147/COPD.S158748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valipour A, Shah PL, Pison C, et al. Safety and dose study of targeted lung denervation in moderate/severe COPD patients. Respiration 2019; 98: 329–339. doi: 10.1159/000500463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slebos DJ, Shah PL, Herth FJF, et al. Safety and adverse events after targeted lung denervation for symptomatic moderate to severe chronic obstructive pulmonary disease (AIRFLOW). A multicenter randomized controlled clinical trial. Am J Respir Crit Care Med 2019; 200: 1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karakoca Y, Karaagac G, Aydemir C. Therapeutic bronchoscopic intervention with resector balloon. J Bronchology Interv Pulmonol 2009; 16: 78–80. [DOI] [PubMed] [Google Scholar]
- 42.Gosens R, Zaagsma J, Meurs H, et al. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 2006; 7: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slebos DJ, Degano B, Valipour A, et al. Design for a multicenter, randomized, sham-controlled study to evaluate safety and efficacy after treatment with the Nuvaira® lung denervation system in subjects with chronic obstructive pulmonary disease (AIRFLOW-3). BMC Pulm Med 2020; 20: 41. doi: 10.1186/s12890-020-1058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol 2006; 101: 971–985. doi: 10.1152/japplphysiol.00313.2006 [DOI] [PubMed] [Google Scholar]
- 45.Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A Report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet 1965; 1: 775–779. [PubMed] [Google Scholar]
- 46.Reid L. Measurement of the bronchial mucous gland layer: a diagnostic yardstick in chronic bronchitis. Thorax 1960; 15: 132–141. doi: 10.1136/thx.15.2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim V, Zhao H, Regan E, et al. The St. George's Respiratory Questionnaire definition of chronic bronchitis may be a better predictor of COPD exacerbations compared with the classic definition. Chest 2019; 156: 685–695. doi: 10.1016/j.chest.2019.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]