Abstract
Acute exacerbation of interstitial lung disease (ILD) is associated with a poor prognosis and high mortality. Numerous studies have documented acute exacerbation in idiopathic pulmonary fibrosis (IPF), but less is known about these events in other ILDs that may present a progressive-fibrosing phenotype. We propose defining acute exacerbation as an acute, clinically significant respiratory deterioration, typically less than 1 month in duration, together with computerised tomography imaging showing new bilateral glass opacity and/or consolidation superimposed on a background pattern consistent with fibrosing ILDs. Drawing on observations in IPF, it is suspected that epithelial injury or proliferation and autoimmunity are risk factors for acute exacerbation in ILDs that may present a progressive-fibrosing phenotype, but further studies are required. Current acute exacerbation management strategies are based on recommendations in IPF, but no randomised controlled trials of acute exacerbation management have been performed. Although there are no formal strategies to prevent the development of acute exacerbation, possible approaches include antifibrotic drugs (such as nintedanib and pirfenidone), and minimising exposure to infection, airborne irritants and pollutants. This review discusses the current knowledge of acute exacerbation of ILDs that may present a progressive-fibrosing phenotype and acknowledges limitations of the data available.
Short abstract
Acute exacerbation can occur in ILDs associated with a progressive-fibrosing phenotype, other than IPF; and are associated with significant morbidity. There are pressing needs to identify patients at risk of AE and for therapies that reduce this risk. http://ow.ly/tEA330mNE0r
Introduction
Fibrosing interstitial lung diseases (ILDs) remain a significant therapeutic and diagnostic challenge. Some patients with fibrosing ILDs develop a progressive phenotype [1]. Progressive-fibrosing ILD (PF-ILD) is a terminology recently used to describe these patients [1]. Idiopathic pulmonary fibrosis (IPF) may be regarded as the prototype PF-ILD; all patients with this condition develop a progressive-fibrosing phenotype. Progressive fibrosis occurs in other ILDs; however, unlike in IPF, it only affects a proportion of patients [1, 2]. The clinical course and diagnosis of ILDs with a progressive-fibrosing phenotype are discussed by Cottin et al. [3] in this issue of European Respiratory Review. Acute exacerbation (AE) of ILDs (AE-ILD) can occur at any point during the course of disease and is characterised by rapid respiratory deterioration (marked increase in dyspnoea and hypoxaemia) associated with new widespread alveolar abnormality [4–7]. The features of AE-ILD are not uniformly defined throughout the literature; however, in most cases, AE-ILD manifests radiographically as new diffuse, bilateral, ground-glass opacification (with or without consolidation) superimposed on a background of chronic fibrotic changes consistent with fibrosing ILDs on high-resolution computed tomography scans [6, 7]. Histopathologically, AE-ILD most commonly manifests as diffuse alveolar damage superimposed on a background of fibrosing ILD (e.g. usual interstitial pneumonia), but other patterns have been described (e.g. organising pneumonia) [4, 8–13].
It is well documented that AE of IPF can be both unpredictable and often fatal, but less is known about these events in patients with other ILDs that may present a progressive-fibrosing phenotype. This review describes the current body of knowledge, drawing from experience in IPF where appropriate.
Impact of AEs in ILDs that may present a progressive-fibrosing phenotype
In most ILDs that may present a progressive-fibrosing phenotype, it is difficult to assess the impact of AE as there are limited epidemiologic data on their incidence and prevalence [14]. Furthermore, the overall incidence of AE varies widely between studies, depending on the method of analysis and disease definitions used. The annual incidence of AE in IPF is reported to be 5–19% and is less common in milder forms of IPF [8, 15–25]. In other ILDs where a percentage of patients develop a progressive-fibrosing phenotype, the highest AE rates are reported in patients with a histological or radiological pattern of usual interstitial pneumonia (UIP). This can be observed in patients with chronic hypersensitivity pneumonitis, asbestosis, fibrosing nonspecific interstitial pneumonia (NSIP), and connective tissue disease (CTD)-ILDs (e.g. rheumatoid arthritis-associated ILD (RA-ILD) or systemic sclerosis-associated ILD (SSc-ILD)) [4, 6, 10, 11, 13, 26–34].
AE in IPF and other fibrosing ILDs that may present a progressive phenotype is associated with a poor prognosis and a high rate of mortality; which in turn contributes to a high economic burden, primarily from a high rate of hospitalisation [7, 25, 35, 36]. The post-exacerbation mortality in ILDs is reported to range from 33–83% [6, 28]; with hospital mortality rates of 50–100% in CTD-ILDs and 75–100% in patients with hypersensitivity pneumonitis [6, 10, 11, 13]. It is proposed that lower baseline lung function parameters, impaired oxygenation and a higher fibrosis score/more extensive radiological disease may increase the risk of mortality in IPF patients with an AE [23, 36–39]. In addition, the marked acceleration in the progression of fibrosis and lung function decline, in combination with a significant worsening in respiratory signs and symptoms (including dyspnoea and hypoxaemia), can substantially impact quality of life and the ability of patients to carry out their activities of daily living [40].
Proposed definition of AE in ILDs that may present a progressive-fibrosing phenotype
At present, there is not a widely accepted single definition of AE for all ILDs that may present a progressive-fibrosing phenotype. The International Working Group defined AE in IPF as an acute clinically significant respiratory deterioration, typically less than 1 month in duration, and can be categorised as extraparenchymal (e.g. pulmonary embolism, pneumothorax, pleural effusion) or parenchymal [7, 41]. Although initially defined to be caused by unknown and unidentifiable aetiologies, AE of IPF has now been recognised to include both idiopathic and triggered events (e.g. infection, drug toxicity, aspiration). In line with this definition, an AE in ILDs that may present a progressive-fibrosing phenotype is proposed to be an acute, clinically significant, respiratory deterioration characterised radiologically by new widespread alveolar abnormality typically less than 1 month in duration.
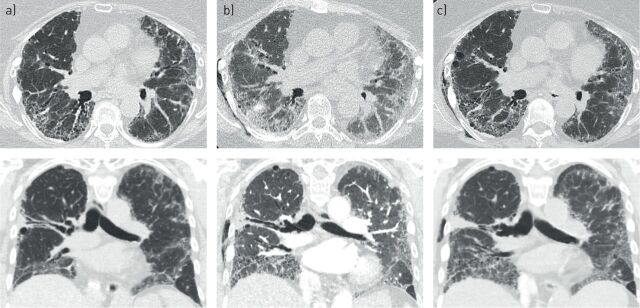
In terms of diagnostics, patients present with acute worsening or development of dyspnoea, typically less than 1 month in duration. Thromboembolic disease should be excluded using computed tomography (CT) imaging with a pulmonary embolism protocol. The CT image of patients with AE should show new bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with fibrosing ILD (including, but not limited to the presence of a UIP pattern, honeycombing) (figure 1); and deterioration not fully explained by cardiac failure (or fluid overload). This definition is being used in an ongoing, randomised, double-blind, placebo-controlled, phase III trial of nintedanib in PF-ILD (excluding IPF) (ClinicalTrials.gov identifier: NCT02999178; INBUILD), where time to first AE or death over 52 weeks is a pre-specified key secondary end-point [1].
FIGURE 1.
A 63-year-old woman with rheumatoid arthritis-associated interstitial lung disease who presented to clinic with 2 weeks of increasing shortness of breath. Axial and coronal images show a) baseline computed tomography 1 year before presentation showing reticulation and honeycombing in a definite usual interstitial pneumonia pattern; b) presentation of acute exacerbation of interstitial lung disease with new onset bilateral ground-glass opacities on background of usual interstitial pneumonia and c) follow-up computed tomography 1 week after treatment with high-dose steroids showing some improvement in ground-glass opacification.
The diagnosis of AE-ILD relies solely on clinical and radiological findings. In patients with IPF, bronchoscopy with bronchoalveolar lavage (BAL) had been considered necessary to exclude an infectious aetiology, but there are findings to suggest that the outcome is similar in exacerbations with an identifiable trigger or in idiopathic cases. There remains an argument for selective use of bronchoscopy with BAL in certain patients, particularly those who are receiving immunosuppression at the time of presentation that increases the risk of atypical and opportunistic infections [42, 43]. Lung biopsies have limited value, as it is difficult to determine if pathological changes are due to acute lung injury or caused by the underlying disease; in addition, nonelective lung biopsy in patients with ILDs is associated with a high inpatient mortality and rarely influences treatment decisions [44–46]. While pulmonary function tests show a progressive decline in forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO) in ILDs that may present a progressive-fibrosing phenotype, AEs are thought to be more likely to show abrupt worsening in both these metrics [16, 22, 26, 47].
Risk factors associated with AE
Contrary to AE-IPF, it is uncertain whether AE in ILDs that may present a progressive-fibrosing phenotype can be the first presentation of the disease. Based on observations in IPF, it is proposed that epithelial injury or proliferation, coagulation abnormalities, and autoimmunity are contributing factors [7]. As described above, AE of other ILDs that may present a progressive-fibrosing phenotype is not caused by thromboembolic disease, cardiac failure, fluid overload or an alternative noninfectious pulmonary condition (such as pulmonary embolism, pneumothorax, or pleural effusion) [7, 17].
Data from retrospective studies in IPF suggest that AE is more commonly observed in nonsmoking, older patients with more advanced disease (e.g. those with a low and/or recent decline in FVC, low DLCO or those requiring supplemental oxygen) [6, 7, 23, 48–51]. However, it is important to remain vigilant, as AE-ILD can occur in patients with well-maintained physiological and clinical status across all ages. In many cases of AE in IPF, no external triggers are identified [7]; however, there is limited evidence to suggest that it may be precipitated by infection, microaspiration, surgical lung biopsy, surgical resection, bronchoscopy (BAL, cryobiopsy), air pollution, prior exacerbation events and some medications (e.g. methotrexate or tocilizumab for RA-ILD or α-interferon) [5, 6, 10, 28, 38, 41, 52–57]. AE is also more frequently observed during the winter season, reflecting an increased rate of viral infections [41].
Blood-based biomarkers can play an important role in predicting the clinical course and outcomes of ILDs [58]. However, while some blood-based biomarkers of AE-ILD have been suggested (e.g. lactate dehydrogenase, C-reactive protein, Krebs von den Lungen-6, pro-calcitonin, circulating fibrocytes, elevated interleukin-17 and anti-heat shock protein 70 autoantibodies, syndecan-4), only a limited number have been validated in prospective studies [51, 59, 60].
Management of AE
There are no randomised studies on which to base optimal management of AE in ILDs. In IPF, international management guidelines recommend the use of supportive care with symptom palliation and long-term oxygen therapy (for patients with resting hypoxaemia) [17]. Beyond supportive care in IPF, corticosteroids are prescribed, based on anecdotal evidence of benefit (particularly when organising pneumonia is present) and the high mortality rate [4, 7, 17, 61]. In other ILDs that may present a progressive-fibrosing phenotype, limited retrospective studies suggest that corticosteroids may confer a benefit in AE of idiopathic interstitial pneumonia (IIP), CTD-ILDs and in selected cases of sarcoidosis and hypersensitivity pneumonitis. However, the reported post-exacerbation mortality rates remain high and the optimal regimen has yet to be defined [7, 11, 13, 31, 62, 63]. It is particularly important to note that high-dose steroids should be used with caution in patients with SSc-ILD due to the associated risk of SSc renal crisis [64, 65]. In cases of suspected AE, it is recommended to identify and eliminate potential exposure to causative toxic agents on a case-by-case basis, particularly in cases of hypersensitivity pneumonitis. Empiric broad-spectrum antibiotics are often administered to patients with AEs to rule out difficult-to-identify infectious agents and antiviral therapy can be used during periods of heightened risk (e.g. oseltamivir during the influenza season) [7]. Immunosuppressive agents (such as cyclosporine A, cyclophosphamide, tacrolimus or azathioprine) can also be used in combination with corticosteroids, but, while some efficacy signals have been observed in small uncontrolled studies in IPF, there is no conclusive evidence to support their use [6, 31, 66–68].
Patients with AE of IPF may develop hypoxic respiratory failure requiring intervention with mechanical ventilation; however, this is associated with a high mortality rate and has to be assessed on a case-by-case basis as the risk may outweigh the benefit. For example, mechanical ventilation can be used as a bridge to lung transplant in eligible patients [17, 69]. An alternative approach is to use nasal cannula oxygen, either conventional or high-flow, which is suggested by a small number of studies to potentially support breathing and avoid the need for intubation/mechanical ventilation [70–72]. Use of noninvasive ventilation may also be considered [73, 74]. The last resort for some patients following an acute worsening of IPF is lung transplantation, but given the nature of the disease, a minority of patients are likely to be considered eligible [17].
The use of extracorporeal membrane oxygenation (ECMO) is emerging to become an effective management method. ECMO has the possibility of minimising the risk of “triggering” underlying chronic processes that can lead to fatal deterioration of the lungs by providing extracorporeal lung support, which can allow one to reduce the invasiveness of ventilation. ECMO may also bridge the period necessary for registering selected patients for a lung transplant [26, 75, 76].
Although the discussion here has focused on AE of IPF, the management approaches are likely to be applicable to patients with other ILDs that may present a progressive-fibrosing phenotype. It is important to note, however, that further controlled studies are required, as the available data are extremely limited and although IPF is considered a prototype of other PF-ILDs, there are some differences between these ILDs. For example, those with other PF-ILDs may be more likely to have elements of an inflammatory process during an AE (i.e. organising pneumonia admixed with dense fibrosis) and therefore may be more likely to benefit from acute immunomodulatory therapy than in AE-IPF.
Preventive strategies
There are no formal recommendations for the treatment of ILDs that may present a progressive-fibrosing phenotype or the prevention of AEs. Data from studies in patients with IPF suggest that antifibrotic drugs (such as nintedanib and pirfenidone) could have a role in preventing AE, but it is not clear whether these agents should be withheld or continued during an exacerbation event [77].
Two phase II trials have reported contrasting results for pirfenidone as a preventive treatment for AE of IPF, with one study showing a benefit and the other failing to demonstrate an effect [21, 78]. In a meta-analysis of five randomised trials, pirfenidone was associated with decreases in all-cause mortality and IPF-related mortality, but there was no significant decrease in the incidence of AEs [79]. There is some evidence that perioperative use of pirfenidone in IPF patients may prevent post-operative exacerbations [80, 81]. While exacerbations were not included as an end-point in phase III trials of pirfenidone, a pooled analysis of data from the three pivotal studies showed that patients treated with pirfenidone had a lower risk of respiratory-related hospitalisations than those in the placebo arms of these trials [82–84].
In a phase II study, nintedanib appeared to delay the time to first AE in patients with IPF (according to investigator assessment), but only one of the two pivotal INPULSIS phase III trials showed a statistically significant effect of nintedanib to reduce AE of IPF versus placebo (p=0.02) [20, 85]. In both phase III trials, nintedanib reduced the decline in FVC, which is consistent with slowing the progression of IPF [20]. Pooled analyses of phase II and phase III data have since suggested a prolongation of the time to first AE of IPF with nintedanib [86–88]. Nintedanib may prolong survival after an AE, but the small number of events included in the analysis does not allow definitive conclusions [7]. Also, the delay in time to first AE may have resulted from a delay in loss of lung function, which potentially would make these patients statistically less likely to suffer with an AE.
Considering other ILDs, a small retrospective study has suggested that pirfenidone may reduce inflammation in patients with an AE of interstitial pneumonia who are undergoing corticosteroid treatment [89]. However, the drug did not improve the survival rate at 30 and 90 days compared with untreated patients. As stated previously, the phase III INBUILD trial (ClinicalTrials.gov identifier: NCT02999178 ) is currently investigating the efficacy of nintedanib versus placebo on outcomes including the incidence of AEs in patients with PF-ILD [1].
Other potential preventive measures in AE of IPF and other ILDs that may present a progressive-fibrosing phenotype may include influenza and pneumococcal vaccination, hand washing and avoidance of sick contacts, avoidance of airborne irritants and pollutants, and strategies to minimise mechanical ventilator-induced lung injury [59]. Despite the observation that gastro-oesophageal reflux disease may be a potential risk factor for the progression of IPF, it is not clear whether anti-acid treatment has a preventive effect on the development of AE of IPF and other IPF-related outcomes due to conflicting data [87, 90]. A recent study suggests that anti-acid treatment may reduce IPF-related mortality but notes that randomised trials are required [91]. Furthermore, anti-acid treatment may increase the risk of infection in patients with more advanced disease [87].
Conclusions
AE of ILDs that may present a progressive-fibrosing phenotype is an unpredictable serious life-threatening event that can occur at any time during the disease course. Data on the incidence of AE in ILDs that may present a progressive-fibrosing phenotype is limited, but for most of these diseases the rate is thought to be lower than that for IPF despite similarities in the clinical presentation and course. The diagnosis of AE in ILDs that may present a progressive-fibrosing phenotype is reliant on clinical and radiological findings, but the choice of method should be considered on a case-by-case basis. The risk factors associated with the development of AE are poorly defined; therefore, there is a need for biomarkers to identify at-risk patients. Treatment of AE is based on scant evidence in IPF and further studies are required to determine the optimal approach. Although further data are required, nintedanib and pirfenidone may prove to have a role in the prevention of AE of ILDs that may present a progressive-fibrosing phenotype.
Acknowledgements
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. Writing assistance was provided by Bethany Degg of GeoMed, an Ashfield company, part of UDG Healthcare plc, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Footnotes
Provenance: Publication of this peer-reviewed article was sponsored by Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, CT, USA (principal sponsor, European Respiratory Review issue 150).
Conflict of interest: M. Kolb reports grants and personal fees from Roche, Boehringer Ingelheim and Prometic, personal fees from Gilead and Genoa, and grants from Actelion, Respivert, Alkermes and Pharmaxis, outside the submitted work.
Conflict of interest: B. Bondue reports grants and personal fees from Boehringer Ingelheim and Le Roche-Hoffmann-La Roche, outside the submitted work.
Conflict of interest: A. Pesci has nothing to disclose.
Conflict of interest: Y. Miyazaki reports grants and personal fees from Nippon Boehringer Ingelheim and grants from Shionogi, outside the submitted work.
Conflict of interest: J.W. Song has nothing to disclose.
Conflict of interest: N.Y. Bhatt has nothing to disclose.
Conflict of interest: J.T. Huggins reports grants and other fees from Roche/Genentech and Boehringer Ingelheim, during the conduct of the study.
Conflict of interest: J.M. Oldham reports other support from Boehringer Ingelheim for writing fees, during the conduct of the study; and personal fees (for speakers fees and advisory board fees) from Boehringer Ingelheim and Genentech, outside the submitted work.
Conflict of interest: M.L. Padilla reports personal fees from Boehringer Ingelheim and Genentech, outside the submitted work.
Conflict of interest: J. Roman reports grants and personal fees from Boehringer Ingelheim and is a member of the Medical Advisory Board of Pulmonary Fibrosis Foundation.
Conflict of interest: S. Shapera reports personal fees from AstraZeneca, grants, personal fees (participation in clinical trials) and other from Boehringer Ingelheim, Canada and Hoffman La-Roche, Canada, and other (participation in clinical trials) from Medimmune, ProMetic Canada and Sanofi-Aventis, outside the submitted work.
Support statement: The authors received no direct compensation related to the development of the manuscript. Writing assistance was contracted and funded by Boehringer Ingelheim Pharmaceuticals Inc. (BIPI).
References
- 1.Flaherty KR, Brown KK, Wells AU, et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir Res 2017; 4: e000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells AU, Brown KK, Flaherty KR, et al. What's in a name? That which we call IPF, by any other name would act the same. Eur Respir J 2018; 51: 1800692. [DOI] [PubMed] [Google Scholar]
- 3.Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive fibrosing interstitial lung diseases. Eur Respir Rev 2017; 26: 180076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churg A, Wright JL, Tazelaar HD. Acute exacerbations of fibrotic interstitial lung disease. Histopathology 2011; 58: 525–530. [DOI] [PubMed] [Google Scholar]
- 5.Leuschner G, Behr J. Acute exacerbation in interstitial lung disease. Front Med (Lausanne) 2017; 4: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med 2009; 103: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med 2016; 194: 265–275. [DOI] [PubMed] [Google Scholar]
- 8.Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006; 27: 143–150. [DOI] [PubMed] [Google Scholar]
- 9.Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest 2005; 128: 3310–3315. [DOI] [PubMed] [Google Scholar]
- 10.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 2007; 132: 214–220. [DOI] [PubMed] [Google Scholar]
- 11.Olson AL, Huie TJ, Groshong SD, et al. Acute exacerbations of fibrotic hypersensitivity pneumonitis: a case series. Chest 2008; 134: 844–850. [DOI] [PubMed] [Google Scholar]
- 12.Oda K, Ishimoto H, Yamada S, et al. Autopsy analyses in acute exacerbation of idiopathic pulmonary fibrosis. Respir Res 2014; 15: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki Y, Tateishi T, Akashi T, et al. Clinical predictors and histologic appearance of acute exacerbations in chronic hypersensitivity pneumonitis. Chest 2008; 134: 1265–1270. [DOI] [PubMed] [Google Scholar]
- 14.Olson AL, Gifford AH, Inase N, et al. Epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur Respir Rev 2018; 27: 180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryerson CJ, Cottin V, Brown KK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: shifting the paradigm. Eur Respir J 2015; 46: 512–520. [DOI] [PubMed] [Google Scholar]
- 16.Hyzy R, Huang S, Myers J, et al. Acute exacerbation of idiopathic pulmonary fibrosis. Chest 2007; 132: 1652–1658. [DOI] [PubMed] [Google Scholar]
- 17.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FJ, de Andrade JA, Anstrom KJ, et al. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zisman DA, Schwarz M, Anstrom KJ, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med 2010; 363: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone Clinical Study Group in J. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35: 821–829. [DOI] [PubMed] [Google Scholar]
- 22.Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2010; 27: 103–110. [PubMed] [Google Scholar]
- 23.Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011; 37: 356–363. [DOI] [PubMed] [Google Scholar]
- 24.Kolb M, Richeldi L, Behr J, et al. Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume. Thorax 2017; 72: 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu YF, Wu N, Chuang CC, et al. Patterns and economic burden of hospitalizations and exacerbations among patients diagnosed with idiopathic pulmonary fibrosis. J Manag Care Spec Pharm 2016; 22: 414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Disayabutr S, Calfee CS, Collard HR, et al. Interstitial lung diseases in the hospitalized patient. BMC Med 2015; 13: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto S. Histopathological features of pulmonary asbestosis with particular emphasis on the comparison with those of usual interstitial pneumonia. Osaka City Med J 1997; 43: 225–242. [PubMed] [Google Scholar]
- 28.Hozumi H, Nakamura Y, Johkoh T, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open 2013; 3: e003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration 2012; 83: 20–27. [DOI] [PubMed] [Google Scholar]
- 30.Usui Y, Kaga A, Sakai F, et al. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open 2013; 3: e002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toyoda Y, Hanibuchi M, Kishi J, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia associated with connective tissue disease. J Med Invest 2016; 63: 294–299. [DOI] [PubMed] [Google Scholar]
- 32.Song JW, Lee HK, Lee CK, et al. Clinical course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis 2013; 30: 103–112. [PubMed] [Google Scholar]
- 33.Tomiyama F, Watanabe R, Ishii T, et al. High prevalence of acute exacerbation of interstitial lung disease in Japanese patients with systemic sclerosis. Tohoku J Exp Med 2016; 239: 297–305. [DOI] [PubMed] [Google Scholar]
- 34.Kradin RL, Digumarthy SR, Baggish AL, et al. Case records of the Massachusetts General Hospital. Case 12–2010. An 89-year-old man with progressive dyspnea. N Engl J Med 2010; 362: 1522–1531. [DOI] [PubMed] [Google Scholar]
- 35.Cottin V, Schmidt A, Catella L, et al. Burden of idiopathic pulmonary fibrosis progression: a 5-year longitudinal follow-up study. PLoS One 2017; 12: e0166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moua T, Westerly BD, Dulohery MM, et al. Patients with fibrotic interstitial lung disease hospitalized for acute respiratory worsening: a large cohort analysis. Chest 2016; 149: 1205–1214. [DOI] [PubMed] [Google Scholar]
- 37.Johannson K, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis: a proposal. Curr Respir Care Rep 2013; 2: 10.1007/s13665-013-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Judge EP, Fabre A, Adamali HI, et al. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J 2012; 40: 93–100. [DOI] [PubMed] [Google Scholar]
- 39.Simon-Blancal V, Freynet O, Nunes H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration 2012; 83: 28–35. [DOI] [PubMed] [Google Scholar]
- 40.Koyama K, Sakamoto S, Isshiki T, et al. The activities of daily living after an acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2017; 56: 2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007; 176: 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arcadu A, Moua T. Bronchoscopy assessment of acute respiratory failure in interstitial lung disease. Respirology 2017; 22: 352–359. [DOI] [PubMed] [Google Scholar]
- 43.Zink A, Manger B, Kaufmann J, et al. Evaluation of the RABBIT Risk Score for serious infections. Ann Rheum Dis 2014; 73: 1673–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qureshi RA, Ahmed TA, Grayson AD, et al. Does lung biopsy help patients with interstitial lung disease? Eur J Cardiothorac Surg 2002; 21: 621–626. [DOI] [PubMed] [Google Scholar]
- 45.Han Q, Luo Q, Xie JX, et al. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2015; 149: 1394–401, e1. [DOI] [PubMed] [Google Scholar]
- 46.Hutchinson JP, Fogarty AW, McKeever TM, et al. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016; 193: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 47.Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med 2006; 174: 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collard HR, Richeldi L, Kim DS, et al. Acute exacerbations in the INPULSIS trials of nintedanib in idiopathic pulmonary fibrosis. Eur Respir J 2017; 49: 1601339. [DOI] [PubMed] [Google Scholar]
- 49.Kakugawa T, Sakamoto N, Sato S, et al. Risk factors for an acute exacerbation of idiopathic pulmonary fibrosis. Respir Res 2016; 17: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mura M, Porretta MA, Bargagli E, et al. Predicting survival in newly diagnosed idiopathic pulmonary fibrosis: a 3-year prospective study. Eur Respir J 2012; 40: 101–109. [DOI] [PubMed] [Google Scholar]
- 51.Ohshimo S, Ishikawa N, Horimasu Y, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med 2014; 108: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 52.Bando M, Ohno S, Hosono T, et al. Risk of acute exacerbation after video-assisted thoracoscopic lung biopsy for interstitial lung disease. J Bronchology Interv Pulmonol 2009; 16: 229–235. [DOI] [PubMed] [Google Scholar]
- 53.Chida M, Kobayashi S, Karube Y, et al. Incidence of acute exacerbation of interstitial pneumonia in operated lung cancer: institutional report and review. Ann Thorac Cardiovasc Surg 2012; 18: 314–317. [DOI] [PubMed] [Google Scholar]
- 54.Johannson KA, Vittinghoff E, Lee K, et al. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J 2014; 43: 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molyneaux PL, Cox MJ, Wells AU, et al. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res 2017; 18: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakamoto S, Homma S, Mun M, et al. Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: a retrospective study. Intern Med 2011; 50: 77–85. [DOI] [PubMed] [Google Scholar]
- 57.Wootton SC, Kim DS, Kondoh Y, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 183: 1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guiot J, Moermans C, Henket M, et al. Blood biomarkers in idiopathic pulmonary fibrosis. Lung 2017; 195: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kondoh Y, Cottin V, Brown KK. Recent lessons learned in the management of acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir Rev 2017; 26: 170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato Y, Tanino Y, Wang X, et al. Baseline serum syndecan-4 predicts prognosis after the onset of acute exacerbation of idiopathic interstitial pneumonia. PLoS One 2017; 12: e0176789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazor R, Vandevenne A, Pelletier A, et al. Cryptogenic organizing pneumonia. Characteristics of relapses in a series of 48 patients. The Groupe d'Etudes et de Recherche sur les Maladles “Orphelines” Pulmonaires (GERM“O”P). Am J Respir Crit Care Med 2000; 162: 571–577. [DOI] [PubMed] [Google Scholar]
- 62.Arai T, Tachibana K, Sugimoto C, et al. High-dose prednisolone after intravenous methylprednisolone improves prognosis of acute exacerbation in idiopathic interstitial pneumonias. Respirology 2017; 22: 1363–1370. [DOI] [PubMed] [Google Scholar]
- 63.McKinzie BP, Bullington WM, Mazur JE, et al. Efficacy of short-course, low-dose corticosteroid therapy for acute pulmonary sarcoidosis exacerbations. Am J Med Sci 2010; 339: 1–4. [DOI] [PubMed] [Google Scholar]
- 64.Steen VD, Medsger TA Jr. Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum 1998; 41: 1613–1619. [DOI] [PubMed] [Google Scholar]
- 65.Denton CP, Lapadula G, Mouthon L, et al. Renal complications and scleroderma renal crisis. Rheumatology (Oxford) 2009; 48: Suppl. 3, iii32–35. [DOI] [PubMed] [Google Scholar]
- 66.Ota M, Iwasaki Y, Harada H, et al. Efficacy of intensive immunosuppression in exacerbated rheumatoid arthritis-associated interstitial lung disease. Mod Rheumatol 2017; 27: 22–28. [DOI] [PubMed] [Google Scholar]
- 67.Sakamoto S, Homma S, Miyamoto A, et al. Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2010; 49: 109–115. [DOI] [PubMed] [Google Scholar]
- 68.Homma S, Sakamoto S, Kawabata M, et al. Cyclosporin treatment in steroid-resistant and acutely exacerbated interstitial pneumonia. Intern Med 2005; 44: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 69.Gaudry S, Vincent F, Rabbat A, et al. Invasive mechanical ventilation in patients with fibrosing interstitial pneumonia. J Thorac Cardiovasc Surg 2014; 147: 47–53. [DOI] [PubMed] [Google Scholar]
- 70.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372: 2185–2196. [DOI] [PubMed] [Google Scholar]
- 71.Horio Y, Takihara T, Niimi K, et al. High-flow nasal cannula oxygen therapy for acute exacerbation of interstitial pneumonia: a case series. Respir Investig 2016; 54: 125–129. [DOI] [PubMed] [Google Scholar]
- 72.Koyauchi T, Hasegawa H, Kanata K, et al. Efficacy and tolerability of high-flow nasal cannula oxygen therapy for hypoxemic respiratory failure in patients with interstitial lung disease with do-not-intubate orders: a retrospective single-center study. Respiration 2018: 1–7. [DOI] [PubMed] [Google Scholar]
- 73.Vianello A, Arcaro G, Battistella L, et al. Noninvasive ventilation in the event of acute respiratory failure in patients with idiopathic pulmonary fibrosis. J Crit Care 2014; 29: 562–567. [DOI] [PubMed] [Google Scholar]
- 74.Yokoyama T, Kondoh Y, Taniguchi H, et al. Noninvasive ventilation in acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2010; 49: 1509–1514. [DOI] [PubMed] [Google Scholar]
- 75.Moerer O, Quintel M. Bridge or abyss: extracorporeal membrane oxygenation for acute respiratory failure in interstitial lung disease. Am J Respir Crit Care Med 2016; 193: 474–476. [DOI] [PubMed] [Google Scholar]
- 76.Todd EM, Biswas Roy S, Hashimi AS, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: a single-center experience in the present era. J Thorac Cardiovasc Surg 2017; 154: 1798–1809. [DOI] [PubMed] [Google Scholar]
- 77.Richeldi L, Varone F, Bergna M. Pharmacological management of progressive fibrosing interstitial lung disease: a review of the current evidence. Eur Respir Rev 2017; 26: 180074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005; 171: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 79.Aravena C, Labarca G, Venegas C, et al. Pirfenidone for idiopathic pulmonary fibrosis: a systematic review and meta-analysis. PLoS One 2015; 10: e0136160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iwata T, Yoshida S, Nagato K, et al. Experience with perioperative pirfenidone for lung cancer surgery in patients with idiopathic pulmonary fibrosis. Surg Today 2015; 45: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 81.Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711L (PEOPLE Study). Respir Res 2016; 17: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 83.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 84.Ley B, Swigris J, Day BM, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2017; 196: 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011; 365: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 86.Costabel U, Inoue Y, Richeldi L, et al. Efficacy of nintedanib in idiopathic pulmonary fibrosis across prespecified subgroups in INPULSIS. Am J Respir Crit Care Med 2016; 193: 178–185. [DOI] [PubMed] [Google Scholar]
- 87.Kreuter M, Wuyts W, Renzoni E, et al. Antacid therapy and disease outcomes in idiopathic pulmonary fibrosis: a pooled analysis. Lancet Respir Med 2016; 4: 381–389. [DOI] [PubMed] [Google Scholar]
- 88.Richeldi L, Cottin V, du Bois RM, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS((R)) trials. Respir Med 2016; 113: 74–79. [DOI] [PubMed] [Google Scholar]
- 89.Matsumura T, Tsushima K, Abe M, et al. The effects of pirfenidone in patients with an acute exacerbation of interstitial pneumonia. Clin Respir J 2018; 12: 1550–1558. [DOI] [PubMed] [Google Scholar]
- 90.Lee JS, Collard HR, Anstrom KJ, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med 2013; 1: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fidler L, Sitzer N, Shapera S, et al. Treatment of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest 2018; 153: 1405–1415. [DOI] [PubMed] [Google Scholar]