Abstract
LuxR is the transcriptional activator for quorum-sensing control of luminescence in Vibrio fischeri. A series of alanine-scanning mutants spanning a predicted helix-turn-helix region in the DNA binding domain of LuxR was constructed, and the activity of each of the LuxR mutant proteins in recombinant Escherichia coli was investigated. The region covered by the mutagenesis spanned residues 190 to 224. About half of the alanine-scanning mutants showed activities similar to that of the wild-type LuxR: at least two were positive-control mutants, four appeared to be defective in DNA binding, and several others were characterized as DNA binding affinity mutants. This analysis, taken together with information about other bacterial transcription factors, provides insights into amino acid residues in LuxR that are involved in DNA binding and transcriptional activation.
Many bacteria regulate the expression of specific genes in a cell density-dependent fashion. This phenomenon has been given the term quorum sensing (12). Quorum sensing involves the production of intercellular signals. A number of gram-negative bacteria use acyl-homoserine lactones (acyl-HSLs) as quorum-sensing signals. Acyl-HSL signaling was first recognized in the marine bacterium Vibrio fischeri, where it controls expression of luminescence. Quorum sensing requires two V. fischeri genes: luxI, which encodes an acyl-HSL synthase, and luxR, which encodes an acyl-HSL-dependent transcriptional activator. For quorum-sensing control of V. fischeri luminescence, the specific signal is N-(3-oxohexanoyl)-homoserine lactone (3-oxo-C6-HSL) (for reviews, see references 11, 12, and 23). The luxR gene is adjacent to but transcribed divergently from the lux operon. The first gene in the lux operon is luxI, and the next five genes code for polypeptides involved in the light-emitting reaction (7, 8). The luxI promoter, a ς70 RNA polymerase (RNAP)-dependent promoter (28), contains a lux box (3, 29), a 20-bp inverted repeat centered −42.5 bp from the luxI transcriptional start site (6). Other gram-negative genera possess LuxR and LuxI homologs. Different genes are controlled by acyl-HSL homologs in different bacteria (10, 11, 21, 23).
The functional regions of LuxR have been defined primarily on the basis of molecular genetic studies (for a recent review, see reference 29). LuxR is a modular protein composed of 250 amino acid residues. The N-terminal two-thirds of the protein constitutes a 3-oxo-C6-HSL binding domain. The C-terminal one-third of the protein contains a helix-turn-helix (HTH) motif and is responsible for lux gene activation. In the absence of 3-oxo-C6-HSL, the N-terminal domain blocks the function of the C-terminal domain. When 3-oxo-C6-HSL is bound to the N-terminal domain, LuxR binds to the lux box and activates transcription of the lux operon (5). The available evidence is consistent with the hypothesis that LuxR is an ambidextrous activator that makes contact with the C-terminal domain of the RNAP α subunit and with another region of RNAP (6).
Although it has proven difficult to purify active, full-length LuxR (27, 29), the LuxR homologs TraR from Agrobacterium tumefaciens and ExpR from Erwinia chrysanthemi have been purified recently (22, 32). These proteins bind to DNA containing lux-box-like elements. Binding of full-length LuxR has not been studied in vitro, but recently we described an in vivo DNA binding assay (5). This assay involves the measurement of β-galactosidase activity in E. coli containing p35LB10, a plasmid with an artificial lacZ promoter containing a lux box positioned between the −35 and −10 hexamers. In this system, lacZ expression is repressed by LuxR in a 3-oxo-C6-HSL-dependent fashion.
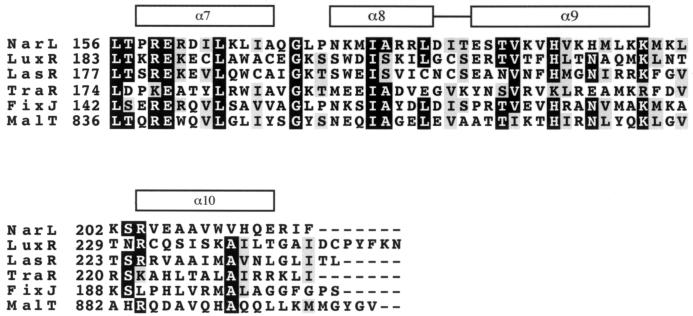
About 2 dozen LuxR homologs have now been identified (29). These polypeptides show sequence identity in the N-terminal acyl-HSL binding domain and in the C-terminal DNA binding domain. In addition the C-terminal DNA binding domain shows significant identity to an HTH-containing domain of a larger group of transcription factors, the LuxR-FixJ superfamily (12). This family includes the Escherichia coli response regulator NarL, for which a crystal structure has been described (1). The C-terminal DNA binding domain of NarL is composed of four α-helices. The central helices, α8 and α9, form the HTH motif, which is supported by a hydrophobic core composed of the flanking helices α7 and α10. A sequence alignment with LuxR, NarL, and other members of the superfamily suggested to us that the HTH motif of LuxR is between residues 200 and 224 (Fig. 1). To begin to identify LuxR residues important in DNA binding and in activity of DNA-bound LuxR, we have performed an alanine-scanning mutagenesis over the HTH region and determined the function of each mutant LuxR with respect to DNA binding by means of the repressor assay described above. We have also analyzed the lux operon activator function of each mutant LuxR. We have identified mutant LuxR polypeptides that appear to bind DNA but do not activate transcription (positive-control mutants). We have identified mutants that do not bind to the lux box. We have also identified mutants that bind poorly but that under appropriate conditions retain an ability to activate lux operon transcription.
FIG. 1.
Sequence alignment of the NarL HTH region with other members of the LuxR-FixJ superfamily of transcription factors. The black boxes indicate identity in a minimum of 4 of the 6 sequences, and the gray boxes indicate similarity in 4 of the 6 sequences. The boxes over the alignment delineate α-helices 7 to 10 of NarL. The HTH of NarL consists of the eighth and ninth α-helices. The numbers to the left of each sequence indicate the residue number of the adjacent amino acid.
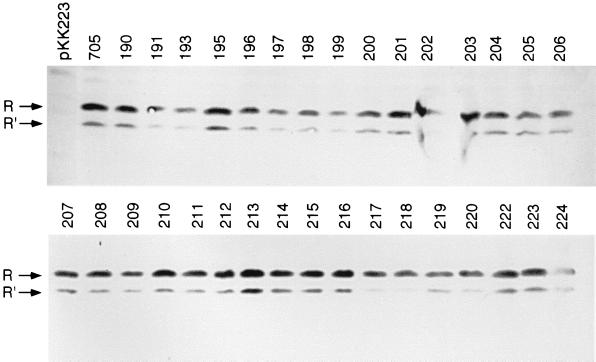
We constructed genes coding for 32 alanine-substitution mutants that spanned amino acid residues 190 to 224 (3 positions have an alanine in this region of the wild-type LuxR). The wild-type luxR was in pKE705 (Table 1). This LuxR expression vector was constructed by cloning a PCR-amplified luxR with its native Shine-Dalgarno sequence from pHK705 with the primers 5′-CAGGAAACAGCTATGACC-3′ and 5′-CTCGAGTTAATTTTTAAAGTATGGGCAATC-3′ (which introduces an XhoI site at the 3′ end of luxR) and cloning the PCR product into EcoRI- and SmaI-digested pKK223-3. The luxR mutations were generated by replacing a 580-bp XbaI-XhoI restriction fragment of pKE705 containing the 3′ 576 bp of luxR with overlap extension PCR products (15) that encode the appropriate alanine substitution. The PCR fragments were ligated with XbaI- and XhoI-digested pKE705 to generate the LuxR alanine-scanning mutant plasmid series described in Table 1. As a confirmation of our constructs, the sequence of each luxR gene was determined at the University of Iowa DNA Core Facility. We also performed a Western immunoblot analysis with antiserum raised against LuxR as described elsewhere (26). This analysis showed that all of the alanine-scanning mutant plasmids directed E. coli to synthesize LuxR polypeptides of the correct molecular mass (Fig. 2).
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| pKK223-3 | Contains ptac, Apr | 2 |
| pHK705 | pUC18 with 1-kb luxR fragment, Apr | 17 |
| pKE705 | ptac-luxR Apr | This study |
| pKE190 to pKE224 | ptac-luxR series with alanine substitutions at the amino acid position indicated by the plasmid number, Apr | This study |
| pKE555 | luxICDABE, Cmr | 6 |
| p35LB10 | 35LB10-controlled lacZ, Smr/Spr Gmr | 5 |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Gmr, gentamicin resistance; Smr/Spr, streptomycin/spectinomycin resistance
FIG. 2.
Western immunoblot analysis of E. coli containing p35LB10 and a luxR plasmid. The first lane on top is the vector pKK223. The next lane is pKE705, which contains a wild-type luxR. The alanine-scanning mutant pKE plasmids are shown in the remaining lanes, as indicated by the plasmid number. R, 28-kDa luxR product; R′, a 25-kDa antigen described previously (26).
We used E. coli JM109 (30), which has the genotype lacIq recA1 supE44. Cultures were grown at 30°C in Luria-Bertani broth containing the appropriate antibiotics for plasmid maintenance, 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for induction of ptac-luxR genes, and 3-oxo-C6-HSL (100 nM for experiments with pKE555 and 200 nM for experiments with p35LB10) unless otherwise indicated. Luciferase and β-galactosidase were measured in cells harvested at an optical density of 1.0 at 600 nm by techniques described elsewhere (4, 19).
The LuxR alanine-substitution mutants were assessed with respect to their ability to activate transcription of the lux genes. This was done by measuring luciferase activity in E. coli containing a luxR plasmid and the lux operon plasmid pKE555 (Tables 1 and 2). The mutants were also assessed with respect to their ability to bind the lux box. This was done by measuring β-galactosidase activity in E. coli containing a luxR plasmid and p35LB10, which has a LuxR-repressible lacZ gene (Tables 1 and 2), as described previously (5). One-half of the alanine-substitution mutant proteins showed considerable binding activity (5- to 13-fold repression compared to a 10-fold repression by the wild-type luxR) and an ability to activate the luxI promoter (81 to 182% of the wild-type luxR). We presume that the residues at the positions defined by these mutants do not participate in DNA binding or in an interaction with RNAP, directly. Four of the substitution mutants, L191A, W193A, R212A, and H217A, showed essentially no ability to activate transcription of the lux operon or to repress lacZ. These probably represent mutant proteins that cannot interact with the lux regulatory DNA. A third group including W201A, I206A, and, perhaps, K198A retained some repressor function (>2-fold) but had very little lux activator function (<1% of wild-type activity). These can be considered positive-control mutants—mutants that can bind lux regulatory DNA but nevertheless fail to activate transcription of the lux operon. Many of the mutant LuxR proteins showed little or no repressor activity, but they maintained an ability to function as an activator (for example E196A and K224A; Table 2). These mutant proteins presumably have a decreased affinity for the lux regulatory DNA, and they require synergistic binding with RNAP. A map of the different mutations is shown in Fig. 3.
TABLE 2.
Repression and activation by LuxR alanine substitution mutants
| LuxR | Activity (relative units)a
|
|
|---|---|---|
| β-Galactosidase | Luciferase | |
| No LuxR plasmid | 100 | 0.1 |
| Wild type | 10 | 100 |
| C190A | 24 | 148 |
| L191A | 107 | 0.1 |
| A192 | 10 | 100 |
| W193A | 103 | 0.1 |
| A194 | 10 | 100 |
| C195A | 9 | 81 |
| E196A | 116 | 57 |
| G197A | 100 | 1.4 |
| K198A | 48 | 0.5 |
| S199A | 41 | 160 |
| S200A | 15 | 162 |
| W201A | 8 | 8 |
| D202A | 10 | 105 |
| I203A | 100 | 5 |
| S204A | 10 | 106 |
| K205A | 21 | 133 |
| I206A | 30 | 0.7 |
| L207A | 74 | 73 |
| G208A | 22 | 182 |
| C209A | 91 | 59 |
| S210A | 12 | 120 |
| E211A | 10 | 102 |
| R212A | 104 | 0.2 |
| T213A | 67 | 50 |
| V214A | 68 | 143 |
| T215A | 13 | 145 |
| F216A | 105 | 4 |
| H217A | 107 | 0.04 |
| L218A | 105 | 12 |
| T219A | 8 | 122 |
| N220A | 94 | 67 |
| A221 | 10 | 100 |
| Q222A | 15 | 120 |
| M223A | 9 | 92 |
| K224A | 95 | 66 |
Relative units of β-galactosidase represent the percentage of activity in E. coli (p35LB10) without a luxR plasmid. Relative units of luciferase are the percentage of activity in extracts from E. coli (pHK555 and pKE705) grown in the presence of 3-oxo-C6-HSL. Values are means of two independent experiments, each done in triplicate. In all cases, the standard deviations were <15%.
FIG. 3.
A diagram of the region of LuxR probed by alanine-scanning mutagenesis. Large red amino acid residues indicate those required for DNA binding. Changes of small red residues to alanines appeared to reduce, but not eliminate, binding to the target DNA. Changes of the large blue residues to alanines resulted in a strong reduction in the positive control, but not in DNA binding, and the small blue residue appears to have a weaker influence on the positive control. Changes of the black residues to alanines did not affect the activity of LuxR appreciably.
Those proteins that showed no appreciable activity as a repressor or as an activator were presumptive DNA-binding mutants. Two of the four presumptive DNA-binding mutants, L191A and W193A, had alanine substitutions in a region adjacent to the HTH predicted by our alignment with NarL (Fig. 1 and 3). We suggest that the wild-type hydrophobic residues L191 and W193 may stabilize the structure of the HTH. In support of this hypothesis, the two corresponding residues in NarL, L164 and L166, stabilize the HTH. L164 interacts with helix 9, and L166 interacts with helix 8 (1). L191 and W193 are highly conserved in the LuxR family (29). The other two substitutions, R212A and H217A, may define residues involved in DNA recognition and binding. The alanine substitutions in these mutant proteins are for basic residues in what we predict represents the recognition helix (based on the alignment with NarL; Fig. 1). For other transcriptional regulators, basic residues in the recognition helix make direct contact with the regulatory DNA. For example, arginine residues in the recognition helix regions of catabolite activator protein (CAP) and the bacteriophage 434 Cro protein make direct contact with DNA (20, 24). A histidine in the TyrR recognition helix and an arginine on a loop adjacent to the E. coli PurR recognition helix make contact with DNA (16, 25). Thus, we suggest that R212 and H217 in LuxR make contact with the DNA.
The positive-control mutants W201A and I206A (and perhaps the weaker positive-control mutant K198A) may represent an activating patch in the vicinity of the first helix of the HTH. This suggestion finds support from studies of other transcription factors. For example, positive-control mutations in CAP, λcI, and FIS also map to this area, and there is evidence that the residues defined by these mutations interact with RNAP directly (9, 13, 14, 31). The LuxR residues W201 and I206 align with surface-exposed residues of NarL (1) (Fig. 1). A surface-exposed location of these residues is consistent with the hypothesis that they make direct contact with RNAP. Again by analogy to NarL, G208 would represent a surface-exposed residue on the same face of the helix as W201 and I206. Of interest, the G208A mutant showed almost twice the wild-type activation of lux transcription. Perhaps G208A is better able to interact with RNAP than the wild type.
We previously proposed that LuxR makes contact with two different regions of RNAP (6). Presumably the promoter-distal subunit of a LuxR dimer interacts with the α-CTD, and the promoter-proximal subunit interacts with another region of RNAP. Our best positive-control mutant, W201A is on the first helix of the HTH motif (Fig. 1 and 3). Because W201 corresponds to a critical residue in λcI that interacts with the RNAP ς subunit (14), we suggest that W201 might interact with the ς subunit.
Positive-control mutants have been reported in one other LuxR homolog, A. tumefaciens TraR. The TraR positive-control mutants were obtained by random mutagenesis. Changes in the N-terminal TraR residues D10 and G123 to N and R or Q, respectively, have been shown to eliminate positive control (18). Because the position of these residues is in the signal-binding domain of TraR and because the amino acid substitutions are not conservative, it is difficult to speculate as to what their role in gene activation may be.
A number of the LuxR alanine-scanning mutants (for example, E196A) showed little or no ability to repress p35LB10 lacZ, but they retained function as an activator of the pKE555 luminescence operon (Table 2 and Fig. 3). This phenotype is similar to that described previously for a truncation mutant of LuxR that consisted of the N-terminal methionine followed by residues 157 to 250 (5). Because in vitro DNA binding studies indicate that the truncated LuxR does not interact with the lux promoter in the absence of RNAP (27), it was hypothesized that this mutant LuxR has a low binding affinity for the target DNA. Thus, it must be recruited to the promoter by RNAP (5). We believe that this is also the case for the alanine-scanning mutants with little or no repressor activity but with appreciable activator function.
In conclusion, we have analyzed a series of alanine-scanning mutant LuxR proteins. This analysis has led to predictions about specific amino acid residues involved in DNA binding and other residues that interact with RNAP to allow transcriptional activation. Other types of investigations—for example, suppressor analyses or studies of LuxR structure—will provide tests of our hypotheses. The studies described here do, however, provide a conceptual framework to guide future studies.
Acknowledgments
This work was supported by a grant from the National Science Foundation (MCB 9808308). K.A.E. was supported by Public Health Service training grant T32-AI07343.
REFERENCES
- 1.Baikalov I, Schroder I, Kaczor-Grzeskowiak M, Grzeskowiak K, Gunsalus R P, Dickerson R E. Structure of the Escherichia coli response regulator. NarL Biochemistry. 1996;35:11053–11061. doi: 10.1021/bi960919o. [DOI] [PubMed] [Google Scholar]
- 2.de Boer H A, Comstock L J, Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci USA. 1983;80:21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devine J H, Shadel G S, Baldwin T O. Identification of the operator of the lux regulon from Vibrio fischeri ATCC7744. Proc Natl Acad Sci USA. 1989;86:5688–5692. doi: 10.1073/pnas.86.15.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlap P V, Greenberg E P. Control of Vibrio fischeri luminescence gene expression in Escherichia coli by cyclic AMP and cyclic AMP receptor protein. J Bacteriol. 1985;164:45–50. doi: 10.1128/jb.164.1.45-50.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egland K A, Greenberg E P. Conversion of the Vibrio fischeri transcriptional activator, LuxR, to a repressor. J Bacteriol. 2000;182:805–811. doi: 10.1128/jb.182.3.805-811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egland K A, Greenberg E P. Quorum sensing in Vibrio fischeri: elements of the luxI promoter. Mol Microbiol. 1999;31:1197–1204. doi: 10.1046/j.1365-2958.1999.01261.x. [DOI] [PubMed] [Google Scholar]
- 7.Engebrecht J, Nealson K H, Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 8.Engebrecht J, Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci USA. 1984;81:4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eschenlauer A C, Reznikoff W S. Escherichia coli catabolite gene activator protein mutants defective in positive control of lac operon transcription. J Bacteriol. 1991;173:5024–5029. doi: 10.1128/jb.173.16.5024-5029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuqua C, Greenberg E P. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr Opin Microbiol. 1998;1:183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 11.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 12.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosink K K, Gaal T, Bokal IV A J, Gourse R L. A positive control mutant of the transcription activator protein FIS. J Bacteriol. 1996;178:5182–5187. doi: 10.1128/jb.178.17.5182-5187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochschild A, Irwin N, Ptashne M. Repressor structure and the mechanism of positive control. Cell. 1983;32:319–325. doi: 10.1016/0092-8674(83)90451-8. [DOI] [PubMed] [Google Scholar]
- 15.Horton R M, Pease R L. Recombination and mutagenesis of DNA sequences using PCR. In: McPherson M J, editor. Directed mutagenesis: a practical approach. Oxford, United Kingdom: Oxford University Press; 1991. pp. 217–247. [Google Scholar]
- 16.Hwang J S, Yang J, Pittard A J. Critical base pairs and amino acid residues for protein-DNA interaction between the TyrR protein and tyrP operator of Escherichia coli. J Bacteriol. 1997;179:1051–1058. doi: 10.1128/jb.179.4.1051-1058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan H B, Greenberg E P. Overproduction and purification of the luxR gene product: transcriptional activator of the Vibrio fischeri luminescence system. Proc Natl Acad Sci USA. 1987;84:6639–6643. doi: 10.1073/pnas.84.19.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Z, Farrand S K. Signal-dependent DNA binding and functional domains of the quorum-sensing activator TraR as identified by repressor activity. Proc Natl Acad Sci USA. 1999;96:9009–9014. doi: 10.1073/pnas.96.16.9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 20.Mondragon A, Harrison S C. The phage 434 Cro/OR1 complex at 2.5 Å resolution. J Mol Biol. 1991;219:321–334. doi: 10.1016/0022-2836(91)90568-q. [DOI] [PubMed] [Google Scholar]
- 21.Pesci E C, Pearson J P, Seed P C, Iglewski B H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reverchon S, Bouillant M L, Salmond G, Nasser W. Integration of the quorum-sensing system in the regulatory networks controlling virulence factor synthesis in Erwinia chrysanthemi. Mol Microbiol. 1998;29:1407–1418. doi: 10.1046/j.1365-2958.1998.01023.x. [DOI] [PubMed] [Google Scholar]
- 23.Salmond G P C, Bycroft B W, Stewart G S A B, Williams P. The bacterial ‘enigma’: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 24.Schultz S C, Shields G C, Steitz T A. Crystal structure of CAP-DNA complex: the DNA is bent by 90°. Science. 1991;253:1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- 25.Schumacher M A, Choi K Y, Zalkin H, Brennan R G. Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by α helices. Science. 1994;266:763–770. doi: 10.1126/science.7973627. [DOI] [PubMed] [Google Scholar]
- 26.Slock J, VanRiet D, Kolibachuk D, Greenberg E P. Critical regions of the Vibrio fischeri LuxR protein defined by mutational analysis. J Bacteriol. 1990;172:3974–3979. doi: 10.1128/jb.172.7.3974-3979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens A M, Dolan K M, Greenberg E P. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc Natl Acad Sci USA. 1994;91:12619–12623. doi: 10.1073/pnas.91.26.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens A M, Greenberg E P. Quorum sensing in Vibrio fischeri: essential elements for activation of the luminescence genes. J Bacteriol. 1997;179:557–562. doi: 10.1128/jb.179.2.557-562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens A M, Greenberg E P. Transcriptional activation by LuxR. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 231–242. [Google Scholar]
- 30.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Busby S, Ebright R H. Identification of the functional subunit of a dimeric transcription activator protein by use of oriented heterodimers. Cell. 1993;73:375–379. doi: 10.1016/0092-8674(93)90236-j. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Winans S C. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc Natl Acad Sci USA. 1999;96:4832–4837. doi: 10.1073/pnas.96.9.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]