Abstract
Matrikines are bioactive fragments of the extracellular matrix (ECM) that are fundamental in regulating a diverse array of physiological processes. The tripeptide Proline-Glycine-Proline (PGP) is a collagen-derived matrikine that has classically been described as a neutrophil chemoattractant. In this article, we describe our current understanding of the pathways that generate, degrade and modify PGP to dictate its bioavailability and stability. Additionally, we discuss our expanding appreciation of the capacity of PGP to regulate diverse cell types and biological processes, independent of its activity on neutrophils, including a putative role in wound repair. We argue that PGP functions as a primitive and conserved damage-associated molecular pattern, which is generated during infection or injury and subsequently acts to shape ensuing inflammatory and repair processes. As a fragment of the ECM that accumulates at the epicentre of the action, PGP is perfectly positioned to focus neutrophils to the exact site required and direct a localised repair response. However, it is essential that PGP is efficiently degraded, as if this matrikine is allowed to persist then pathology can ensue. Accordingly, we discuss how this pathway is subverted in chronic lung diseases giving rise to persistent inflammation and pathological tissue remodelling.
Short abstract
The matrikine Pro-Gly-Pro regulates pulmonary inflammation and repair, but its persistence can drive pathology http://ow.ly/vUbo30kswVO
Introduction
The extracellular matrix (ECM) is a non-cellular component of all tissues and organs, which serves as a scaffold for constituent cells. The individual components and three-dimensional structure of the ECM imparts signals to resident cells to shape fundamental processes such tissue development, maintenance of homeostasis, inflammation and wound repair. The ECM is a dynamic entity that undergoes continuous degradation and resynthesis. This can result in the proteolytic liberation of bioactive fragments termed “matrikines” that function to further modulate cell behaviour. Classically, we consider the complex interplay of mediators such as cytokines, chemokines, growth factors and eicosanoids in regulating fundamental physiological processes but overlook the significance of matrikines. However, matrikines, derived from ECM macromolecules such as collagen, elastin, laminin and hyaluronan, have been implicated in the regulation of diverse biological processes [1]. Localised perturbations within a tissue will inevitably result in alterations to the ECM and frequently the release of matrikines. Consequently, matrikines are perfectly positioned to sense microenvironmental fluctuations and dictate an appropriate response to the exact location and at the precise time when required to meet the immediate needs of the tissue. However, it is mandatory that ECM turnover be tightly regulated as an aberrant ECM and dysregulated matrikine production are implicated in the pathology of many chronic diseases. This article will focus on our current understanding of the collagen-derived matrikine Proline-Glycine-Proline (PGP). We detail pathways that define its bioavailability, its central role in regulating basic biological processes, and how dysregulation of the pathway is implicated in disease states.
PGP: a multifaceted matrikine
Collagen represents in excess of 90% of the total protein mass of the ECM in mammals, and proteolytic processing of native collagen can yield the matrikine PGP. The prevalence of the PGP sequence within collagen molecules (28 PGP sequences per type I collagen fibril, 43 per type III collagen, 25 per type IV collagen and 44 per type V collagen) results in a potentially abundant bioactive signalling moiety that remains cryptic within the triple helical structure of collagen until liberated by proteolytic processing. Once released, the small and hydrophilic nature of PGP means that it can readily diffuse through the dense ECM. Furthermore, its unusual structure owing to the cycling back of the proline side chains onto the backbone amino group results in a matrikine that is resistant to generic protease degradation.
Pfister et al. [2] first identified N-terminal acetylated PGP (AcPGP) and N-terminal methylated PGP in an alkali eye injury model in rabbits, demonstrating their capacity to drive neutrophil recruitment and ensuing corneal ulceration [3–5]. Whilst conventional glutamic acid-leucine-arginine+ (ELR+) CXC neutrophil chemokines are functional at nanomolar levels, AcPGP was subsequently demonstrated to operate at a micromolar level [6] The unacetylated peptide (PGP) also evokes neutrophil chemotaxis but is 4–7-fold less potent [7]. Weathington et al. [6] convincingly demonstrated that PGP functioned as a neutrophil chemoattractant by mimicking key sequences found in classical neutrophil chemokines and signalling through CXCR1/2. Accordingly, intratracheal instillation of AcPGP dose dependently elicited neutrophilic inflammation in the airways of mice that was abolished in cxcr2-/- animals [6, 8]. Subsequent studies have highlighted downstream signalling events following CXCR1/2 engagement by AcPGP [9, 10], and demonstrated the capacity of AcPGP to drive neutrophil superoxide production [6] and matrix metalloproteinase (MMP)-9 [9] and CXCL8 release [10]. More recently, Afonso et al. [11] demonstrated that collagen engagement of discodin domain receptor 2 on the surface of neutrophils promoted the release of PGP-generating enzymes at the leading edge of the neutrophil and the generation of a localised PGP gradient. This acted as a compass to focus neutrophil migration through a three-dimensional matrix [11], rationalising how this pathway may operate physiologically.
Increasingly, it is apparent that PGP can have profound effects on other cells independent of its classical activity as a neutrophil chemoattractant. Hahn et al. [12] demonstrated that PGP and AcPGP could function at nanomolar concentrations to promote paracellular permeability in endothelial cells both in vitro and in vivo, in a process that was CXCR2 dependent. Subsequently, the same group demonstrated that AcPGP could also drive the release of pro-inflammatory mediator endothelin-1 from aortic endothelial cells both via ligation of CXCR2. Additionally, recent studies have suggested a role for PGP in facilitating wound repair. AcPGP was demonstrated to drive CXCR2-dependent migration, proliferation and tube-forming activity in endothelial progenitor cells at nanomolar concentrations. Subsequently, topical application of AcPGP promoted cutaneous neovascularisation and wound healing [13]. Furthermore, our group have recently demonstrated a profound capacity of PGP/AcPGP to promote proliferation, radial spreading and prominent lamellipod formation in human lung bronchial epithelial cells [14], cardinal features of epithelial wound repair responses.
Pathways defining PGP bioavailability
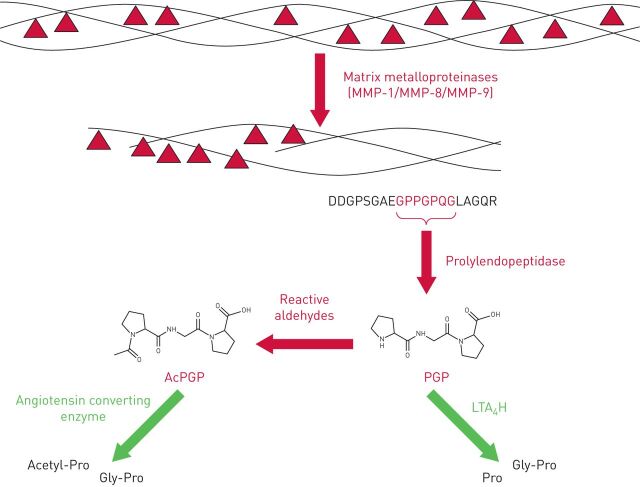
Generation of PGP from collagen is a multistep enzymatic process that requires the concerted action of specific MMPs and prolylendopeptidase (figure 1). The initial cleavage of native collagen by MMP-1, MMP-8 or MMP-9 yields fragments that are a suitable substrate size for prolylendopeptidase, which subsequently acts to liberate PGP [7, 15]. MMPs can be derived from a variety of cellular sources [16], whilst prolylendopeptidase has been reported to be expressed by neutrophils [15], airway macrophages [17] and epithelial cells [17, 18]. Since neutrophils are an abundant source of the proteases that generate PGP it is anticipated that this pathway can drive a self-sustaining cycle of neutrophilic inflammation [9].
FIGURE 1.
Schematic of the proteolytic cascade that defines the availability of collagen-derived matrikines Pro-Gly-Pro (PGP) and acetyl-Pro-Gly-Pro (AcPGP). PGP generation from collagen is mediated via a sequential enzymatic cascade: initial cleavage of native collagen is by specific matrix metalloproteinases (MMP-1, MMP-8 or MMP-9), which yield fragments of collagen that are a suitable substrate size for a second enzyme, prolylendopeptidase, that subsequently cuts out the PGP sequence. Liberated PGP can subsequently be degraded by the action of extracellular leukotriene A4 hydrolase (LTA4H) to resolve inflammation. Conversely, the N-terminal proline of PGP can be chemically acetylated through the action of reactive aldehydes to yield a species (AcPGP) that is resistant to LTA4H-mediated degradation. The AcPGP can, however, be degraded by the action of angiotensin converting enzyme.
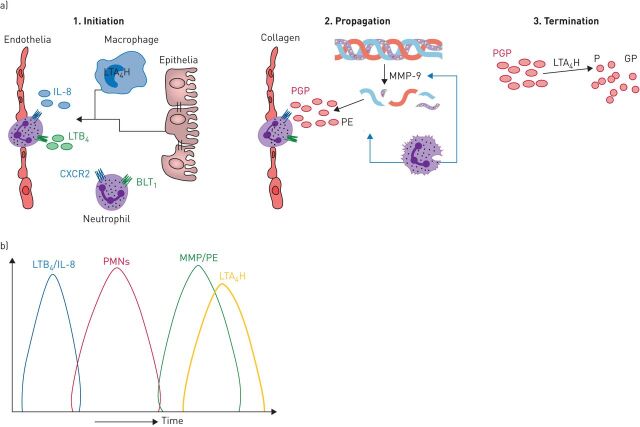
We rationalised that pathways must be in place to ensure that PGP-mediated inflammation is resolved. Accordingly, we identified an anti-inflammatory pathway whereby PGP is degraded during episodes of acute neutrophilic inflammation by a novel extracellular aminopeptidase activity of the enzyme leukotriene A4 hydrolase (LTA4H) [19]. LTA4H classically functions intracellularly within myeloid cells as an epoxide hydrolase to convert leukotriene A4 to pro-inflammatory lipid mediator LTB4 [20, 21]. LTB4 drives the recruitment and activation of an array of cells including neutrophils and is implicated in protection against invading microorganisms but also in the pathology of a multitude of chronic diseases [22]. Thus LTA4H represents a highly unusual enzyme with directly opposing pro- and anti-inflammatory activities that dictate the amplitude and persistence of neutrophilic inflammation (figure 2) [23]. Whilst the epoxide hydrolase activity of LTA4H operates intracellularly primarily within myeloid cells, the cellular source of the extracellular enzyme is less clear.
FIGURE 2.
a) The opposing roles of leukotriene A4 hydrolase (LTA4H) operate to promote and then resolve neutrophilic inflammation. 1. Initiation: in response to microbial infection, lung resident cells can generate leukotriene B4 (LTB4) via the classical intracellular activity of LTA4H. LTB4 can subsequently function to promote neutrophil recruitment into the tissue. IL: interleukin; BLT1: Leukotriene B4 receptor 1. 2. Propagation: neutrophils are a rich source of the enzymes that generate Pro-Gly-Pro (PGP) from collagen (matrix metalloproteinases (MMPs) and prolylendopeptidase (PE)), and thus can function to accentuate their own recruitment. 3. Termination: to counteract a potential PGP-mediated vicious circle of neutrophilic inflammation, LTA4H operates in an extracellular environment to degrade PGP. b) In acute self-resolving neutrophilic inflammation, the release of PGP-generating enzymes (MMPs/PE) is coordinated with the release of the PGP-degrading enzyme (LTA4H) to ensure that PGP is unable to persist. PMN: polymorphonuclear cell.
Whilst PGP is readily broken down by LTA4H, AcPGP is completely protected from degradation [19] and thus the acetylation process is seemingly a key event. Acetylation of peptides is classically the function of N-acetyl transferases. However, the small size and N-terminal proline of PGP seemingly preclude it from being a favourable substrate for such enzymes [24, 25]. Subsequently, we have demonstrated that PGP can be chemically acetylated through the action of reactive aldehydes, such as acrolein and acetaldehyde [26], which can be generated physiologically during inflammation [27, 28]. Whilst AcPGP is resistant to degradation via LTA4H, we have recently demonstrated that it can be degraded through a previously unidentified action of angiotensin converting enzyme (ACE) [29]. ACE classically functions to convert angiotensin I into the vasoactive peptide hormone angiotensin II [30, 31], although it has also been demonstrated to be capable of degrading a variety of other peptides [32]. Thus in a scenario remarkably analogous to the PGP–LTA4H axis, AcPGP can be degraded by another multipurpose enzyme.
PGP in health
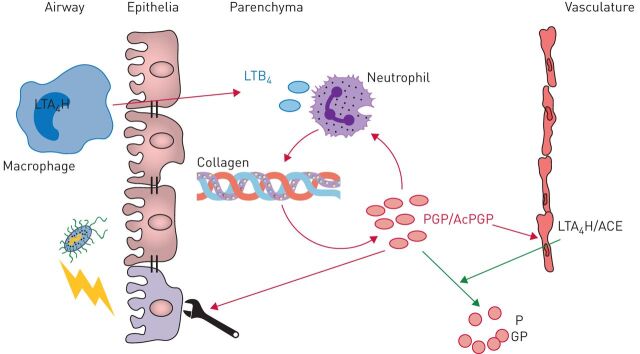
In response to infection/injury, we believe that PGP essentially functions as a damage-associated molecular pattern, operating to fine-tune neutrophil recruitment to the specific site required (figure 3). Subsequently, as neutrophils navigate through the ECM they generate “breadcrumbs” of PGP to guide other proximal neutrophils. Indeed, it is feasible that whilst neutrophil-derived proteases function to breach the collagen basement membrane during extravasation that they are concomitantly generating PGP to attract neutrophils to a focal point of exit from the vasculature. More recent studies pertaining to endothelial progenitor cells [13] and bronchial epithelial cells [14], suggests that PGP also possesses a previously unheralded function in wound healing and may repair demarcated airway epithelium and promote neo-angiogenesis. Thus, in essence, a fragment of the ECM liberated during inflammation and injury could remarkably be functioning to direct a localised repair response at the specific site of the inflammatory reaction (figure 3).
FIGURE 3.
Pro-Gly-Pro (PGP) is a damage-associated molecular pattern that senses and directs localised inflammation and repair processes. Respiratory infections may result in damage to the airway epithelium and activation of resident cells leading to the generation of leukotriene B4 (LTB4) through the classical intracellular activity of leukotriene A4 hydrolase (LTA4H). LTB4, in combination with other neutrophil chemoattractants, can drive neutrophil recruitment out of the vasculature and into the tissue. As neutrophils navigate through the dense extracellular matrix they generate PGP/acetyl-Pro-Gly-Pro (AcPGP) at their leading edge to stabilise neutrophil directionality and persistence. Additionally, this PGP/AcPGP can act as “breadcrumbs” to guide other proximal neutrophils to the specific site where they are required. Concomitantly, localised PGP/AcPGP can act to repair proximal airway epithelium to seal the breach to the external environment and promote neo-angiogenesis to facilitate wound healing. PGP/AcPGP can ultimately contribute indirectly to their own degradation by promoting vascular permeability and enabling an influx of extracellular plasma LTA4H and angiotensin converting enzyme (ACE); thus resolving inflammation, terminating remodelling and restoring homeostasis.
A constant dynamic exists between generation and degradation of PGP/AcPGP. In acute self-resolving settings it is absolutely the norm for PGP/AcPGP to be efficiently degraded and not persist [19, 23, 33]. High levels of ACE are present in the plasma [34], and we have demonstrated that airway ACE levels are elevated during episodes of inflammation as consequence of increased vascular permeability and an influx of plasma enzyme [29]. We have also observed very high levels of extracellular LTA4H circulating in plasma, and thus airway levels may again be dictated, in part, by vascular permeability. It is noteworthy, therefore, that neutrophils and PGP itself impart changes in endothelial cells to promote vascular permeability, which would in turn enable an influx of extracellular LTA4H and ACE to degrade PGP/AcPGP. Thus PGP/AcPGP can potentiate their own degradation to facilitate the efficient resolution of inflammation and restoration of homeostasis.
PGP in chronic lung disease
A protease–anti-protease imbalance is a hallmark of many chronic lung diseases, resulting in disruption of tissue architecture and excessive matrikine production that can perpetuate inflammation and remodelling. There is accumulating evidence that PGP persistence can drive pathologies observed in chronic lung diseases (figure 4). We have demonstrated that a failure to degrade PGP in a murine model of acute self-resolving airway inflammation resulted in substantially augmented neutrophils and macrophages, a general protease imbalance and ECM degradation [33]; hallmark features of many chronic lung diseases. Furthermore, whilst PGP may function to promote bronchial epithelial repair, we have seen that a failure to degrade PGP in a mouse model of allergic airways disease results in pathological epithelial remodelling, mucus hypersecretion and exacerbated airway hyperresponsiveness [14]. Given the capacity of PGP to promote endothelial permeability [12] and neovascularisation [13], we believe that persistence of this matrikine could also contribute to oedema and pathological angiogenesis observed in some chronic lung diseases.
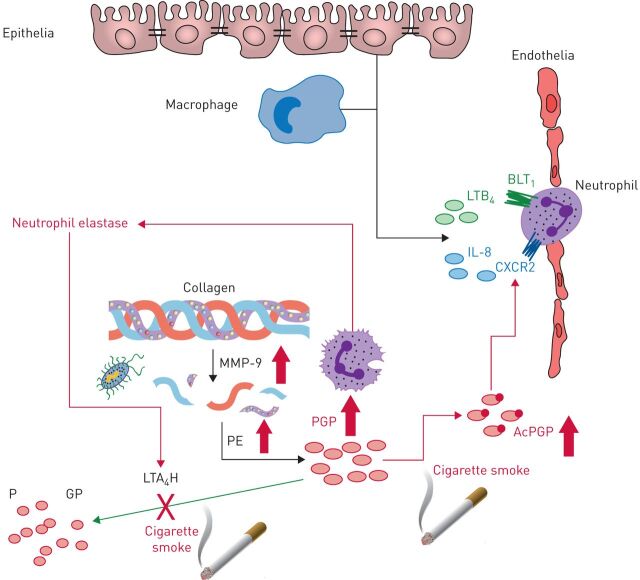
FIGURE 4.
Leukotriene A4 hydrolase (LTA4H)-mediated degradation of Pro-Gly-Pro (PGP) is disturbed in chronic lung diseases. Neutrophils recruited to the lung release enzymes (matrix metalloproteinases (MMPs) and prolylendopeptidase (PE)) that proteolytically target collagen to generate PGP. To counteract this, LTA4H is released to degrade PGP and resolve neutrophilic inflammation. If this balance between PGP-generating and PGP-degrading enzymes is disturbed then PGP can persist and drive pathology. Cigarette smoke, a major risk factor for the development of chronic obstructive pulmonary disease, can promote the expression and/or release of MMPs and PE and ensuing PGP generation. Furthermore, cigarette smoke disturbs the LTA4H-mediated anti-inflammatory pathway in two ways: 1) chemically acetylating PGP (AcPGP) and protecting it from degradation by LTA4H; and 2) selective abrogation of LTA4H PGP-degrading activity. A protease imbalance is a hallmark of many chronic lung diseases, with neutrophil elastase prominently implicated in disease pathologies. Neutrophil elastase can promote MMP activation to facilitate PGP generation, whilst degrading extracellular LTA4H. Respiratory infections act to further spike PGP generation in a setting where degradation is impaired. As a result, AcPGP/PGP can drive neutrophilic inflammation and pathology. LTB4: leukotriene B4; IL: interleukin; BLT1: Leukotriene B4 receptor 1.
Chronic obstructive pulmonary disease (COPD) describes a progressive, irreversible and predominantly smoking-induced small airway and/or emphysematous disease associated with airflow limitation. Chronic exposure of mice to cigarette smoke causes PGP/AcPGP accumulation, neutrophilic inflammation and emphysema. Reducing/neutralising PGP/AcPGP in this cigarette smoke model ameliorates inflammation and pathology [17, 19, 35, 36], whilst promoting PGP degradation reduces inflammation and emphysema [37]. Furthermore, it is noteworthy that chronic administration of AcPGP alone into the airways of mice is sufficient to elicit an emphysematous phenotype [6, 8]. Elevated PGP/AcPGP have been reported in sputum, bronchoalveolar lavage fluid and serum of COPD patients, often correlating with neutrophil surrogate marker myeloperoxidase (MPO) [6, 15, 35, 38, 39]. This is true for all Global Initiative for Chronic Obstructive Pulmonary Disease stages of disease and in patients that have stopped smoking [35]. Preliminary studies suggest that sputum PGP levels are highest around the time of COPD exacerbation and decline with successful treatment [38], potentially supportive of PGP/AcPGP being utilised as a biomarker for disease severity. Intriguingly, a recent study has also highlighted that AcPGP accumulation can drive tumour cell dissemination to lung parenchyma [40], potentially rationalising the association between inflammatory processes in smokers and an increased risk of lung cancer.
Rationalising the accumulation of PGP/AcPGP in COPD patients, we have demonstrated that LTA4H-mediated PGP degradation is perturbed by cigarette smoke. Cigarette smoke contains large amounts of reactive aldehydes that chemically acetylate PGP, enhancing its chemotactic activity and protecting it from degradation by LTA4H [19, 23, 26]. Additionally, cigarette smoke selectively abrogates the peptidase activity of LTA4H [19, 35, 41], again seemingly driven by intrinsic reactive aldehydes. Reactive aldehyde scavengers such as carbocisteine are able to prevent cigarette smoke-induced PGP acetylation and LTA4H inhibition. Thus, it is pertinent that these compounds have demonstrated clinical benefit in COPD patients and reduced inflammation in animal models of emphysema [42–44]. ACE levels and activity in COPD patients remain largely unexplored though there are suggestions that it too may be reduced [29].
Cystic fibrosis (CF) is a lethal genetic disorder caused by defective function of the CF transmembrane conductance regulator, which predisposes patients to infection, chronic airway inflammation (with a prominent neutrophilia) and airway remodelling. PGP/AcPGP is elevated in CF patients, correlating with PGP-generating enzymes and neutrophil surrogate MPO [7]. Importantly, PGP levels were shown to spike with disease exacerbation, decline with inpatient therapy and inversely correlate with forced expiratory volume in 1 s. Whilst lung transplantation is the therapeutic modality frequently used for end-stage lung disease, recipients often have a poor prognosis due to the development of bronchiolitis obliterans syndrome (BOS), a condition characterised by a neutrophil influx and ECM remodelling. BOS patients have elevated levels of PGP in their bronchoalveolar lavage fluid, again showing a striking correlation with levels of MMP-9, prolylendopeptidase and MPO [45]. Importantly, Hardison et al. [45] demonstrated that the capacity of BOS bronchoalveolar lavage fluid to elicit neutrophil chemotaxis ex vivo was predominantly attributable to the intrinsic PGP component, which was relatively more important as a driver of the neutrophil chemotaxis than the CXCL8 present. Asthma is a heterogeneous disease with variable inflammatory, remodelling and clinical phenotypes. Severe asthma phenotypes have been described that exhibit pronounced neutrophilic inflammation [46], and pathological epithelial remodelling that contributes to progressive decline in lung function [47]; hallmark features of PGP persistence. Intriguingly, we have recently demonstrated that AcPGP accumulates specifically in the sputum of severe asthmatics [14]. The rationale for why PGP/AcPGP is able to accumulate in CF, BOS and severe asthma patients is the subject of ongoing investigation.
An over-exuberant or persistent PGP response is clearly implicated in the pathologies of certain chronic lung diseases, but a recent study paradoxically suggests that too little PGP can yield a distinct pathology. Idiopathic pulmonary fibrosis (IPF) and COPD are anticipated to share similar aetiologies, with cigarette smoke the major risk factor for the development of both. However, IPF displays quite distinct clinical and pathological features manifesting as an interstitial fibrosis and honeycombing [48]. Whilst PGP/AcPGP is prevalent in COPD patients, it is undetectable in patients with IPF [29]. Furthermore, therapeutic administration of AcPGP/PGP in a murine belomycin model of fibrosis completely abrogated pulmonary fibrosis [29]. Accordingly, we demonstrated the pathogenic function attributed to ACE in fibrosis to be a consequence of overzealous AcPGP degradation [29]. Thus PGP/AcPGP can seemingly have very divergent roles: pathogenic in their capacity to drive neutrophilic inflammation and ECM degradation in the context of COPD, but protective in their capacity to limit fibrosis in IPF. Accordingly, we believe that disparate perturbations of the PGP–LTA4H/AcPGP–ACE axis contribute to these distinct pathologies.
Conclusions
We anticipate that PGP essentially functions as a primitive and conserved damage-associated molecular pattern, which is generated during infection or injury and acts to shape ensuing inflammatory and repair processes. As a fragment of the ECM that accumulates at the epicentre of the action, PGP is perfectly positioned to focus neutrophils to exactly where they are required, as well as initiate proximal repair responses. However, it is critical the PGP is efficiently degraded as its persistence can drive pathological features of chronic lung diseases. We have previously discussed strategies that could be utilised to target this matrikine therapeutically to potentially ameliorate chronic lung disease pathologies; be it neutralisation of the peptide itself, inhibition of PGP-generating enzymes or receptor blockade [49]. However, our increasing realisation of the potential role of PGP in wound repair processes and the surprising recent developments that it is protective against pulmonary fibrosis, highlights the necessity to maintain homeostatic levels and questions the feasibility of targeting this matrikine. Furthermore, we should now consider implications and opportunities for LTA4H and ACE inhibitors, which have been developed to reduce LTB4 and angiotensin II, respectively, if they inadvertently cause PGP and AcPGP accumulation [31, 50].
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: None declared.
Support statement: Funding was received from the Wellcome trust (209458/Z/17/Z). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Gaggar A, Weathington N. Bioactive extracellular matrix fragments in lung health and disease. J Clin Invest 2016; 126: 3176–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister RR, Haddox JL, Sommers CI, et al. . Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-proline-glycine-proline and N-methyl-proline-glycine-proline. Invest Ophthalmol Vis Sci 1995; 36: 1306–1316. [PubMed] [Google Scholar]
- 3.Haddox JL, Pfister RR, Muccio DD, et al. . Bioactivity of peptide analogs of the neutrophil chemoattractant, N-acetyl-proline-glycine-proline. Invest Ophthalmol Vis Sci 1999; 40: 2427–2429. [PubMed] [Google Scholar]
- 4.Haddox JL, Pfister RR, Sommers CI, et al. . Inhibitory effect of a complementary peptide on ulceration in the alkali-injured rabbit cornea. Invest Ophthalmol Vis Sci 2001; 42: 2769–2775. [PubMed] [Google Scholar]
- 5.Pfister RR, Haddox JL, Sommers CI. Injection of chemoattractants into normal cornea: a model of inflammation after alkali injury. Invest Ophthalmol Vis Sci 1998; 39: 1744–1750. [PubMed] [Google Scholar]
- 6.Weathington NM, van Houwelingen AH, Noerager BD, et al. . A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 2006; 12: 317–323. [DOI] [PubMed] [Google Scholar]
- 7.Gaggar A, Jackson PL, Noerager BD, et al. . A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol 2008; 180: 5662–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Houwelingen AH, Weathington NM, Verweij V, et al. . Induction of lung emphysema is prevented by L-arginine-threonine-arginine. FASEB J 2008; 22: 3403–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Jackson PL, Tanner S, et al. . A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PloS One 2011; 6: e15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overbeek SA, Henricks PA, Srienc AI, et al. . N-acetylated proline-glycine-proline induced G-protein dependent chemotaxis of neutrophils is independent of CXCL8 release. Eur J Pharmacol 2011; 668: 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afonso PV, McCann CP, Kapnick SM, et al. . Discoidin domain receptor 2 regulates neutrophil chemotaxis in 3D collagen matrices. Blood 2013; 121: 1644–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn CS, Scott DW, Xu X, et al. . The matrikine N-alpha-PGP couples extracellular matrix fragmentation to endothelial permeability. Sci Adv 2015; 1: e1500175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon YW, Heo SC, Lee TW, et al. . N-acetylated proline-glycine-proline accelerates cutaneous wound healing and neovascularization by human endothelial progenitor cells. Sci Rep 2017; 7: 43057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel DF, Peiró T, Shoemark A, et al. . An extracellular matrix fragment drives epithelial remodeling and airway hyper-responsiveness. Sci Transl Med 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Reilly P, Jackson PL, Noerager B, et al. . N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res 2009; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 2006; 61: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braber S, Koelink PJ, Henricks PA, et al. . Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am J Physiol Lung Cell Mol Physiol 2011; 300: L255–L265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szul T, Bratcher PE, Fraser KB, et al. . Toll-like receptor 4 engagement mediates prolyl endopeptidase release from airway epithelia via exosomes. Am J Respir Cell Mol Biol 2016; 54: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snelgrove RJ, Jackson PL, Hardison MT, et al. . A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science 2010; 330: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001; 294: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 21.Haeggstrom JZ. Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. J Biol Chem 2004; 279: 50639–50642. [DOI] [PubMed] [Google Scholar]
- 22.Di Gennaro A, Haeggstrom JZ. The leukotrienes: immune-modulating lipid mediators of disease. Adv Immunol 2012; 116: 51–92. [DOI] [PubMed] [Google Scholar]
- 23.Snelgrove RJ. Leukotriene A4 hydrolase: an anti-inflammatory role for a proinflammatory enzyme. Thorax 2011; 66: 550–551. [DOI] [PubMed] [Google Scholar]
- 24.Varland S, Osberg C, Arnesen T. N-terminal modifications of cellular proteins: the enzymes involved, their substrate specificities and biological effects. Proteomics 2015; 15: 2385–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnesen T. Towards a functional understanding of protein N-terminal acetylation. PLoS Biol 2011; 9: e1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardison MT, Brown MD, Snelgrove RJ, et al. . Cigarette smoke enhances chemotaxis via acetylation of proline-glycine-proline. Front Biosci 2012; 4: 2402–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchida K, Kanematsu M, Morimitsu Y, et al. . Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem 1998; 273: 16058–16066. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MM, Hazen SL, Hsu FF, et al. . Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest 1997; 99: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Reilly PJ, Ding Q, Akthar S, et al. . Angiotensin-converting enzyme defines matrikine-regulated inflammation and fibrosis. JCI Insight 2017; 2: 91923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skeggs LT Jr, Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J Exp Med 1956; 103: 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaman MA, Oparil S, Calhoun DA. Drugs targeting the renin-angiotensin-aldosterone system. Nat Rev Drug Discov 2002; 1: 621–636. [DOI] [PubMed] [Google Scholar]
- 32.Rieger KJ, Saez-Servent N, Papet MP, et al. . Involvement of human plasma angiotensin I-converting enzyme in the degradation of the haemoregulatory peptide N-acetyl-seryl-aspartyl-lysyl-proline. Biochem J 1993; 296: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akthar S, Patel DF, Beale RC, et al. . Matrikines are key regulators in modulating the amplitude of lung inflammation in acute pulmonary infection. Nat Commun 2015; 6: 8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei L, Alhenc-Gelas F, Soubrier F, et al. . Expression and characterization of recombinant human angiotensin I-converting enzyme. Evidence for a C-terminal transmembrane anchor and for a proteolytic processing of the secreted recombinant and plasma enzymes. J Biol Chem 1991; 266: 5540–5546. [PubMed] [Google Scholar]
- 35.Wells JM, O'Reilly PJ, Szul T, et al. . An aberrant leukotriene A4 hydrolase-proline-glycine-proline pathway in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 190: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdul Roda M, Sadik M, Gaggar A, et al. . Targeting prolyl endopeptidase with valproic acid as a potential modulator of neutrophilic inflammation. PloS One 2014; 9: e97594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paige M, Wang K, Burdick M, et al. . Role of leukotriene A4 hydrolase aminopeptidase in the pathogenesis of emphysema. J Immunol 2014; 192: 5059–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Reilly PJ, Jackson PL, Wells JM, et al. . Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations. BMJ Open 2013; 3: e004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells JM, Jackson PL, Viera L, et al. . A randomized, placebo-controlled trial of roflumilast. Effect on proline-glycine-proline and neutrophilic inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bekaert S, Fillet M, Detry B, et al. . Inflammation-generated extracellular matrix fragments drive lung metastasis. Cancer Growth Metastasis 2017; in press [ 10.1177/1179064417745539]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snelgrove R, Kheradmand F. Leukotriene A4 hydrolase: the Janus enzyme shows its ugly side in smokers. Am J Respir Crit Care Med 2014; 190: 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanaoka M, Droma Y, Chen Y, et al. . Carbocisteine protects against emphysema induced by cigarette smoke extract in rats. Chest 2011; 139: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 43.Zheng JP, Kang J, Huang SG, et al. . Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet 2008; 371: 2013–2018. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda H, Yamaya M, Sasaki T, et al. . Carbocisteine reduces frequency of common colds and exacerbations in patients with chronic obstructive pulmonary disease. J Am Geriatr Soc 2006; 54: 378–380. [DOI] [PubMed] [Google Scholar]
- 45.Hardison MT, Galin FS, Calderon CE, et al. . The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J Immunol 2009; 182: 4423–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore WC, Hastie AT, Li X, et al. . Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 2014; 133: 1557–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen L, Xueping E, Tarsi J, et al. . Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med 2007; 176: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000; 161: 646–664. [DOI] [PubMed] [Google Scholar]
- 49.Snelgrove RJ. Targeting of a common receptor shared by CXCL8 and N-Ac-PGP as a therapeutic strategy to alleviate chronic neutrophilic lung diseases. Eur J Pharmacol 2011; 667: 1–5. [DOI] [PubMed] [Google Scholar]
- 50.Low CM, Akthar S, Patel DF, et al. . The development of novel LTA4H modulators to selectively target LTB4 generation. Sci Rep 2017; 7: 44449. [DOI] [PMC free article] [PubMed] [Google Scholar]