Abstract
Pulmonary hypertension related to chronic lung disease, mainly represented by COPD and idiopathic pulmonary fibrosis, is associated with a worse outcome when compared with patients only affected by parenchymal lung disease. At present, no therapies are available to reverse or slow down the pathological process of this condition and most of the clinical trials conducted to date have had no clinically significant impact. Nevertheless, the importance of chronic lung diseases is always more widely recognised and, along with its increasing incidence, associated pulmonary hypertension is also expected to be growing in frequency and as a health burden worldwide. Therefore, it is desirable to develop useful and reliable tools to obtain an early diagnosis and to monitor and follow-up this condition, while new insights in the therapeutic approach are explored.
Short abstract
New prospective in the management of pulmonary hypertension related to chronic lung diseases http://bit.ly/2lW4CfE
Introduction
Pulmonary hypertension (PH) in chronic lung disease (CLD), mainly represented by COPD and idiopathic pulmonary fibrosis (IPF), is associated with a reduced functional status and worse outcomes [1–3].
To date, PH is defined by the presence of a mean pulmonary artery pressure (mPAP) ≥25 mmHg [4]. During the 6th World Symposium on Pulmonary Hypertension, held in Nice in 2018, a new mPAP cut-off value was suggested, as an mPAP of 20 mmHg is widely above the upper limit of normal value [5]. Furthermore, an increased risk of disease progression has been found in patients affected by pulmonary vascular diseases with an mPAP between 21 and 24 mmHg [5–7]. To define precapillary PH, as in the case of PH-CLD, the presence of an mPAP value >20 mmHg has to be associated to a pulmonary artery wedge pressure ≤15 mmHg and a pulmonary vascular resistance ≥3 WU. However, more studies are needed in order to improve the management of this group of patients [8].
According to the updated recent clinical classification of PH (table 1), PH related to CLD and to systemic disease with lung involvement belongs to groups 3 and 5, respectively. PH associated with lymphangioleiomyomatosis (LAM) has been moved from group 5 and now is classified with other PH related to parenchymal lung diseases [8]. The prevalence of PH in both COPD and IPF is generally linked to the severity of the parenchymal involvement and to the hypoxaemia caused by the underlying pulmonary disease [9]. However, the real prevalence is difficult to define because the definition given in different studies and the tools used for its assessment are very heterogeneous [10–14]. Although the correlation between PH and CLD has been well established, it is still unclear whether PH is an independent disease or rather a consequence of the severity of the CLD. A peculiar genetic pattern seems to be related to the severity of PH [15, 16] and Hoffmann et al. [17] found differences in the gene expression in PH-IPF and COPD-PH patients suggesting the presence of different molecular pathways in the development of PH in the two diseases and the need for specific treatments.
TABLE 1.
Updated clinical classification of pulmonary hypertension (PH)
| 1 PAH |
|
|
|
|
|
|
|
|
|
|
|
|
| 2 PH due to left heart disease |
|
|
|
|
| 3 PH due to lung diseases and/or hypoxia |
|
|
|
|
|
| 4 PH due to pulmonary artery obstructions |
|
|
| 5 PH with unclear and/or multifactorial mechanisms |
|
|
|
|
PAH: pulmonary arterial hypertension; PVOD: pulmonary veno-occlusive disease; PCH: pulmonary capillary haemangiomatosis; LVEF: left ventricular ejection fraction. Reproduced from [8] with permission from the publisher.
Due to the high prevalence of CLD and the increase in mortality and morbidity associated with these diseases, the active search for possible early diagnostic tools along with improvements in the management and research field of PH related to CLD are required. The search for biomarkers and echocardiographic measurements for a noninvasive and early diagnosis, the improvement in functional tests useful for management purposes and new clinical trials, and the improvement in specific imaging tools might be promising starting points and will be highlighted in this article.
Biomarkers research for early diagnosis and prognosis
Although right heart catheterisation (RHC) is the gold standard for the diagnosis of PH [4], and echocardiography is a useful tool for screening and monitoring patients at risk, the research of a biomarker that can be detected by single blood sample collection or in exhaled breath would be a useful noninvasive tool in patients affected by CLD.
The ideal biomarker should show good sensitivity, specificity and reproducibility. Moreover, it should be safe, cheap and easy to be obtained and should also have value oscillations wide enough to be easily measured. Furthermore, it should reflect the clinical course of the disease and the response to treatment [18].
Biomarkers involved in PH progression have been divided into different groups: markers of vascular dysfunction, inflammation, myocardial stress, low cardiac output and/or tissue hypoxia [18].
Among endothelial cell markers, asymmetric dimetilarginine (ADMA) is a natural amino acid and endogenous inhibitor of nitric oxide (NO) that is generated from the methylation of arginine residues by the enzyme arginine methyltransferases with subsequent proteolysis. It has been shown that the endothelial injury can cause an increase in plasmatic ADMA concentration and was proven to be associated with a lower NO concentration and the consequent increase in the vascular tone [19]. ADMA seems to induce pulmonary dysfunction changing the expression and the activity of connexion 43, a transmembrane protein involved in the gap junction on the cell membrane, allowing the transfer of signalling molecules between cytoplasm and extracellular space [20]. Furthermore, a higher concentration of this amino acid was found in paediatric patients affected by congenital heart diseases and PH when compared to those without PH [21]. Even though this pathological mechanism is more specific for pulmonary artery hypertension (PAH), recently a higher concentration of ADMA has been found in COPD-PH patients when compared to healthy controls and stable COPD patients without PH. Interestingly, ADMA was negatively related to oxygen saturation [22]. However, these data and the fact that ADMA could play a role in the pathogenesis of PH in these groups of patients should be confirmed in a larger population.
Gene expression studies, such as microarrays and RNA sequencing, provide accessible and fast screening technologies to detect single genes, groups of co-regulated genes or pathways involved in remodelling processes. In addition to the identification of coding RNA, the expression of non-coding RNA, such as microRNAs (miRNAs), can also be analysed. miRNAs are not translated into proteins, but their role is important in the regulation of mRNA at the transcriptional and post-transcriptional level [23]. A number of miRNAs [24–28] have been found in PAH but their role in PH group 3 pathogenesis should be further investigated. A group of miRNAs, called hypoxamirs, shows dynamic alterations after exposure to hypoxic condition [29]. By means of the regulation of the target gene expression, they seem to play a role in the development of hypoxia-induced PH [30, 31]. Moreover, miR-190a, a hypoxamir, is also involved in the upregulation of Ca2+ influx and plays an important role in the pulmonary vascular vasoconstriction related to hypoxaemia [32]. Recently, a correlation between the severity of COPD-PH and circulating levels of miR-190a were found, suggesting a potential role of these molecules in the early diagnosis and prognosis in patients affected by PH related to CLD [33].
So far, the majority of studies assessing biomarkers by mean of microarray assay related to CLD-PH have been conducted on explanted lungs or biopsy samples.
However, the easiest but less refined approach in the analysis of gene expression in PH is the analysis of samples obtained from lung homogenate [34]. Unfortunately, as intrapulmonary arteries represent only a minor portion of the lung tissue, their expression of genes may be masked. Furthermore, the severe parenchymal remodelling in CLD may disguise relevant findings. Using a gene wide microarray analysis, Rajkumar et al. [35] identified RNA expression profiles from lung tissue homogenates in patients affected by PAH, PH-IPF and controls. A different gene expression signature was found in the PAH group compared to PH-IPF, suggesting the absence of a specific gene profile for PH. In another study, genes involved in inflammation and activation of innate immunity from lung samples of patients affected by PH associated with systemic sclerosis, IPF and idiopathic PAH (iPAH) did not have any relationship with the presence of PH. In this study, a specific gene signature was found in the iPAH group that included genes involved in antigen presentation and chemokine activity [16]. Mura et al. [15] analysed the gene profile from lung samples of IPF, PH-IPF and PAH patients. All subjects were divided into three groups: severe PH, intermediate PH and no PH. In the PH groups a peculiar gene signature was found and it was linked to extracellular matrix remodelling and fibroblast proliferation/migration. All these data from studies of lung homogenate samples in PAH and IPF-PH suggest a common PH gene expression panel of genes involved in cell proliferation, inflammatory mechanisms and extracellular matrix remodelling. The presence of inflammatory genes is not peculiar for PH as it has also been found in subjects with no PH affected by IPF.
The analysis of circulating cells can provide a useful tool to detect novel biomarkers of disease progression. Due to their clinical accessibility, circulating blood cells represent the most convenient cell source. Although gene expression varies in different cell type compartments, many of the regulated pathways are common, suggesting that similar signalling patterns are involved in PH pathogenesis. However, it is still unclear if these are adaptive or pathological responses and the mechanisms and kinetics of their regulation has yet not been elucidated [36].
Plasma receptor for advanced glycation end products (RAGE) are immunoglobulins involved in immune and inflammatory responses in several pathophysiological conditions. Due to their presence in the alveolar space and in the blood during lung injury, they have been suggested as prognostic biomarkers in several lung diseases [37]. Alveolar cells produce different surfactant-derived proteins (SPs) in order to maintain the surfactant function and structure [38]. Specific SPs have been used as lung injury markers, predictors of alveolar damage or a cardiovascular prognostic marker of mortality and morbidity [39–43]. Both RAGE and SPs are associated with alteration of alveolar membranes in various cardiorespiratory diseases and for this reason they might play a role as prognostic markers in this group of PH.
Volatile organic compounds (VOCs) are produced during normal physiological activity and are detected in low concentrations in the exhaled breath. Pathophysiological events affecting the lung can increase or modify the presence of VOCs in the exhaled breath changing its profile, called exhaled volatome. As they are not detected in the peripheral blood system, they are not metabolised or stored in the fat compartment [44, 45]. Given these considerations, VOCs have been suggested as noninvasive biomarkers in numerous diseases, included PH.
Significant changes in exhaled breath have been reported in many respiratory diseases, such as lung cancer [44], COPD [46], asthma [47] and cystic fibrosis [48].
Several studies [49–51] have explored volatomic compound changes in PAH patients with advanced disease, but at this stage many anatomical and physiological changes occur in the lung, heart, immune/inflammatory system and circulation, affecting the exhaled volatolome [52]. Therefore, it is still debated if the reported change in volatomic compounds could be helpful in the early diagnosis of iPAH.
In cases of PAH associated with connective tissue diseases or HIV infection, or PH related to CLD, the research approach should be different because of the complexity of the pathophysiological process. It is still not clear how the pattern of volatolome could be present in two different diseases (i.e. COPD and PH), as a portion of these compounds could be affected by molecular pathways involved in the pathological processes of each disease. Volatolomic alteration in “pure” COPD patients has been demonstrated, so an evaluation in the subgroup with COPD and PH is required in order to show a specific pattern associated with the combination of the two diseases. Studies of volatolome in pure interstitial lung disease (ILD) and PH-ILD are required [52].
The 6-min walk test and cardiopulmonary exercise test
The 6-min walk test (6MWT) is a useful tool for the measurement of exercise capacity and it is largely used for the evaluation of cardiac and pulmonary diseases. In COPD and IPF patients some values recorded during this test are related to a bad prognosis as summarised in tables 2 and 3. As is well known, it consists of the longest walk possible over a 6 min recorded on a hard, flat surface. In order to standardise the protocol, a 30 m aisle was suggested in the 2002 American Thoracic Society guidelines, together with the measurement of heart rate and of the perception of dyspnoea (Borg scale) at the beginning and end of the test. Oxygen saturation measurement was not mandatory and the measurement of arterial oxygen saturation at the beginning and end of the test was optional [53]. However, the 2014 European Respiratory Society/American Thoracic Society technical standard guidelines recommended the continuous measurement of oxygen saturation measured by pulse oximetry (SpO2) during the test. The standardisation of the procedure is still unclear and new guidelines should be drafted [54].
TABLE 2.
Principle variables in 6-min walk related to a poor prognosis in patients affected by idiopathic pulmonary fibrosis
| Value predictors of a poor prognosis | |
| Distance m | <250 |
| SpO2 nadir % | <88 |
| HRR bpm | <13 |
| DSP %·m | <200 |
SpO2: arterial oxygen saturation measured by pulse oximetry; HRR: heart rate recovery after 1 min following the test; DSP: distance saturation product, the product of the 6-min walk distance and the lowest registered SpO2.
TABLE 3.
Principle variables in 6-min walk test related to a poor prognosis in patients affected by COPD
| Value predictors of a poor prognosis | |
| Distance m | ≤334 |
| 6MWspeed m·s−1 | ≤0.8 |
| 6MWW m·kg−1 | ≤20 000 |
| DSP %·m | ≤290 |
6MWspeed: calculated by dividing the 6-min walk distance by the total walking time; 6MWW: 6-min walking work, the product of the 6-min walking distance and the patient weight; DSP: distance saturation product, the product of the 6-min walk distance and the lowest registered arterial oxygen saturation measured by pulse oximetry.
Although the test was originally designed for the study of COPD patients, it has been used for clinical and research purposes in different diseases, including PAH and IPF. In particular, for PAH, the successful use of the changes in the 6-min walking distance (6MWD) as a primary end-point in the first PAH trial allowed the use of this parameter as a primary end-point for other PAH trials.
The measurement of 6MWD allows the indirect quantification of shortness of breath and fatigue, the most common symptoms reported by PAH, COPD and IPF patients. In each disease the positive change in 6MWD from baseline after an intervention, such as new treatments or rehabilitation training, is associated with improvements in symptoms and the patient's ability to perform daily activities [55].
In patients affected by PH-IPF, a significative decrease in the 6MWD was observed. In particular, a distance <250 m covered and a 24-week decline in 6MWD >50 m were associated with an increased risk of death [56]. From the analysis of data drawn from a clinical trial, a significant decrease in 6MWD and oxygen saturation at rest and a higher desaturation during the 6MWT were found in PH-IPF patients when compared to patients without PH [57]. However, a retrospective report in a small groups showed different results [58].
Taken together, all these data support the idea that even though the 6MWT is a useful, cheap and simple tool for the study and clinical assessment of CLD-PH, standardised guidelines for its use in this condition are currently lacking.
In addition to 6MWD, which may be affected by age, sex and comorbidities, other parameters may be derived from the 6MWT in order to improve prognostic value in CLD-PH patients.
In a 3-year prospective study, 2010 COPD patients were evaluated with the 6MWT. In addition to 6MWD, other derived parameters were considered such as mean walking-speed, 6-min walking work (the product of 6MWD in meters and body weight in kilograms), distance saturation product (the product of the 6MWD in meters and the lowest registered SpO2 %), exercise-induced oxygen desaturation (defined as the nadir SpO2 in the 6MWT) [59]. All these derived variables were confirmed to have an additional predicting value on mortality in this group of patients.
Also for IPF patients, 6MWD has been considered an important measure for the evaluation of the assessment of the disease. However, other derived parameters should be considered. According to the last updated European Respiratory Society/American Thoracic Society guidelines [55], continuous oxygen saturation monitoring is recommended. It allows not only the nadir SpO2 to be recorded but also continuous heart rate monitoring. Another important point in this group of patients is the measurement of heart rate recovery, defined as the difference between the heart rate at the end of the 6MWT and at the first minute of the recovery period, with a lower value associated with worse outcomes in patients with IPF [56, 58]. Furthermore, a heart rate recovery ≤13 at the first minute of recovery seems to be an indicator of IPF associated PH [60, 61].
In a group of 81 IPF patients, the distance saturation product predicted the 12-month mortality earlier that either 6MWD and SpO2 nadir alone [62]. Other variables may be considered, for example, the change in SpO2 from baseline to the nadir value, the time to recovery of SpO2 after a walk and the distance desaturation product. The speed recorded during the entire walk or in the first half versus the second half of the walk may also be a reliable marker of disease progression [63].
All these considerations emphasise the importance of further investigations in PH related to the CLD group. It should be important to investigate how 6MWT variables may be related to functional decline and to the associated mortality risk. A composite score which takes into consideration all those different measures could be a useful option.
Although the 6MWT is a practical and simple test which provides a global measurement of functional capacity, it does not distinguish among all the elements involved in exercise limitation, which may include pulmonary parenchymal and vessel, cardiovascular and neuromuscular factors.
The cardiopulmonary exercise test (CPET) is a noninvasive technique of proven help in the assessment of exercise limitation, providing information about all the elements involved in the physical effort.
CPET is performed using different protocols according to the goal of the exercise evaluation. The exercise may be performed on a treadmill or a cycle ergometer, preferred in patients affected by respiratory diseases. Using the incremental testing protocol, subjects are asked to exercise until exhaustion while the workload is increased (usually every 1–2 min). The ramp protocol exercise is useful for the diagnosis and risk stratification in chronic respiratory disease. In the constant work rate exercise test, based on an initial incremental exercise test evaluation, patients are asked to exercise to the same workload. The use of work rate exercise test, pre- and post-interventions, is increasing as it allows the response to exercise training or rehabilitation to be measured, which is an indication for long-term oxygen therapy and lung volume reduction [64–66].
Its use is recommended in all patients affected by PH, who are clinically stable and able to perform exercise testing. It is usually performed without the assessment of arterial blood gases even though a proper differential diagnosis between group 1 (PAH) and group 3 (PH secondary to lung disease) might require an evaluation of changes in arterial carbon dioxide tension during exercise and of cardiac output/oxygen uptake (V′O2) ratio [2]. In PAH and chronic thromboembolic PH patients, a high ventilation (V′E)/carbon dioxide production ratio, due to excessive hyperventilation, seems to be caused by an increased death space, V′E/perfusion mismatch and chemoreceptor mismatch [67].
The use of CPET in the study and evaluation of CLD-PH raises some issues. Although the 6MWT is considered a submaximal exercise test when compared to the CPET, where patients achieve maximal exercise capacity, peak V′O2 in patients with IPF undertaking a 6MWT and CPET was found to be similar and 6MWD was strongly correlated with peak V′O2 during CPET, suggesting that 6MWT can be considered a maximal exercise test in this group of patients [68].
As PH in CLD is generally associated with an advanced stage of the diseases, patients in this group usually need oxygen supplementation at rest or during intense physical exertion, therefore the exercise tests may require the use of a more sophisticated technology, a properly equipped laboratory and trained staff. Few data are available on the use of CPET in the assessment of PH related to CLD as its use is limited to few referral centres with sufficiently extensive experience in this field. However, its use should be encouraged since it can be a reliable noninvasive test for the early detection of pulmonary vascular impairment, and therefore it can help in defining a more accurate prognostic assessment in CLD patients.
Echocardiography
Although RHC remains the gold standard for the assessment of CLD-PH, several studies have confirmed the agreement between the findings of invasive studies and echocardiographic measurements. However, in this group of patients, the correlation between the echocardiographic estimated systolic pulmonary artery pressure (sPAP) and the RHC measured sPAP is lower (range 0.65–0.73) [69–72] than the same values obtained in a population of patients affected by cardiac diseases (range 0.93–0.97) [73–75], suggesting that a greater variability between estimated and measured pressure in patients affected by lung disease may be due to factors limiting the accurate visualisation and measurement of the tricuspid regurgitant jet. In the case of obstructive lung disease, it might be related to the increase of intrathoracic gas, expansion of the thoracic ribcage and modifications in the position of the heart. It is not clear whether changes in chest wall and/or in the position of the heart might be responsible for difficult sPAP measurement in ILD patients [72].
Among patients affected by IPF, Van der Veerdonk et al. [76] found that the most important determinant of long-term survival was the adaptation of the right ventricle (RV) to the increase of the pulmonary vascular resistances. Due to the complexity of RV geometry related to the predominant distribution of its muscular fibres on the longitudinal plane, the transverse/radial diameters functional measurements may not directly reflect the most important components of RV systolic action and the conventional measures of RV, for example tricuspidal annular displacement may be subject to errors that don't reflect the RV systolic dysfunction.
Over the past few years, two-dimensional speckle-tracking strain echocardiography has enabled an angle-independent technique to be obtained to quantify myocardial deformation, including the assessment of RV function in pulmonary hypertension. The RV global longitudinal strain has been already demonstrated to be an important determinant of outcome in advanced PAH patients [77]. Although transthoracic echocardiography is the standard practice for the evaluation of RV dysfunction at rest, stress echocardiography should be considered as a diagnostic tool to assess PH during exercise, as well as RV contractile reserve. Recently, D'Andrea et al. [78] investigated the RV contractile reserve at rest and during exercise in IPF patients at early stages of IPF. They found that this dysfunction is already present at rest in this group of patients and that it worsens during the exercise. Further investigations are required to evaluate the role of these measurements in predicting mortality among the CLD-PH patients, most of all in patients affected by combined pulmonary fibrosis and emphysema, where PH is very common and is consistently related to a worse prognosis.
The diagnosis of PH in COPD patients is challenging. In fact, although echocardiography is an available and low-cost tool for noninvasive screening of PH, the current standard echocardiographic measurements have not proven to be reliable in this group of patients. Secondary to the typical lung hyperinflation in COPD, it is more difficult to obtain an accurate measure of the tricuspidal regurgitation velocity (TRV) since its correct measurement is highly linked to the angle of acquisition of the image. So, a poor correlation has been found between TRV and invasive haemodynamic measurements in this group of patients. Compared to RHC measurements, the RV strain is not related to mPAP but it correlates with pulmonary vascular pressure, suggesting how a noninvasive tool that is able to assess increased pulmonary vascular resistances independently from mPAP could provide a promising and more sensitive measure useful for the early detection of pulmonary vascular diseases [79]. Further investigations would be necessary to understand if RV strain is representative of the functional status and if it can be used as a clinical tool for the diagnosis and prognostic assessment of patients affected by COPD-PH.
In LAM patients, echocardiographic measurements have been collected during exercise, showing that exercise-induced PH in LAM seems to be related not only to the hypoxic pulmonary vascular constriction, but also to a diastolic dysfunction as demonstrated by an increase in estimated pulmonary capillary wedge pressure [80].
Imaging
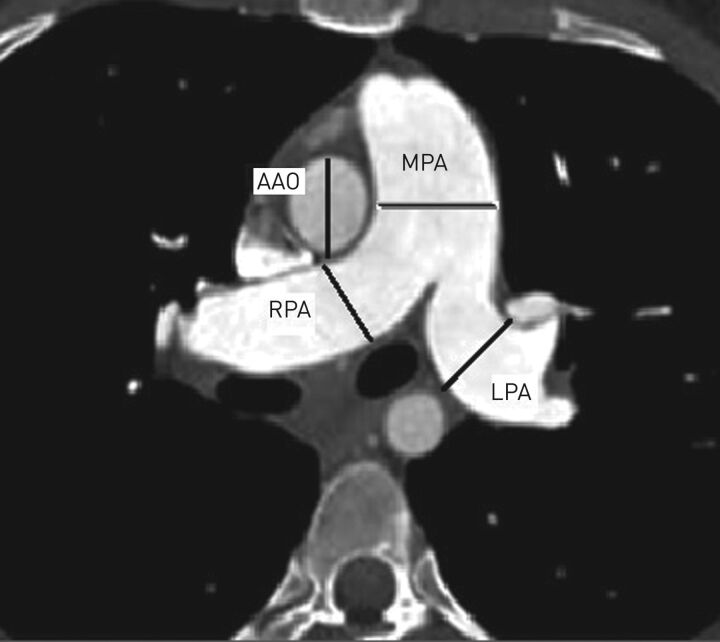
The traditional tool for radiological diagnosis in CLD-PH is the computed tomography (CT) scan. This examination is essential in order to exclude other causes of PH, such as chronic thromboembolic disease, left heart acquired or congenital heart disease. The CT evaluation can be divided into two levels: the measurement of pulmonary central arteries, particularly the maximum diameter of the main pulmonary artery (cut off value: 29 mm for males, 27 mm for females) and the ratio between this value and the diameter of the ascending aorta, pathological if >1 (figure 1). On CT angiography, only the vascular lumen is measured, while on non-contrast exams the vessel wall is included [81]. For younger patients, the pulmonary artery/ascending aorta ratio correlates more strongly with PA pressure than with the diameter of the main pulmonary artery, while the reverse is true in patients aged >50 years [82, 83]. Other indexes to consider are the ratio between segmental pulmonary arteries diameter and the related bronchi in different lobes (cut off value: 1.25) and the ratio between the main pulmonary artery and the height of the dorsal vertebra measured at the same level (pathological if >1.5). These simple measurements are based on the evidence that PH is associated with pulmonary artery branch enlargement, which is more evident in some diseases (such as Eisenmenger syndrome or post-embolic PH or in some types of iPAH). The correlation between pulmonary artery diameter and haemodynamics may vary in different types of PH but remains moderate to strong [81, 84–86]. According to several studies, the CT measurements are reliable, with 96% sensitivity and >95% positive predictive value [86–88]. On the other hand, normal pulmonary artery dimensions cannot exclude mild PH. In patients with advanced fibrosing lung disease, a threshold of 32 mm is considered more specific [89, 90]. The combination of the CT measurements with the echocardiographic values in a composite score could increase the accuracy when compared to the single measurements. The RV overload is associated with these measurements, as it has already been demonstrated with echocardiography (thickening of the RV free wall, RV dilatation, bowing of the interventricular septum, increased septal angle and pericardial effusion) [91].
FIGURE 1.
Measures obtained from the computed tomography scan that are useful for the radiological suspicion of pulmonary hypertension (PH). Main pulmonary artery (MPA) dilatation values >29 mm in men and >27 mm in women are strongly related to PH (sensitivity 87%, specificity 89%), when its value is >35 mm the specificity reaches 100%. Right and left pulmonary artery (RPA and LPA, respectively) diameter>18 mm is abnormal. MPA dilatation and an ascending aorta (AAO) diameter ratio >1, in individuals <50 years old and with diffuse pulmonary fibrosis, is highly specific for hypertension (specificity >90%, sensitivity 70%). In patients with severe COPD this ratio is more accurate than echocardiography.
Advanced CT studies requiring the i.v. injection of a contrast agent and cardiac gating, such as the evaluation of pulmonary artery distensibility and increased transition time, are also available. The dual energy CT scan allows not only the evaluation of the pulmonary artery, but also the presence of alterations in perfusion, information that can be particularly useful in chronic thromboembolic pulmonary hypertension. Unfortunately, these techniques are not often used, since generally the pulmonary artery studies are performed without the use of the cardiac gating. Finally, in patients with dilated pulmonary arteries on CT scans, the evaluation of left atrial area and RV dimensions is always useful to distinguish underlying left cardiac disease from other causes of PH [92].
High-resolution non-contrast CT can identify parenchymal lung disease and discriminate between PH lung disease and idiopathic pulmonary artery (PAH) group 3 versus group 1 [9, 93]. The indirect evaluation of PH through the determination of the underlying diffuse parenchymal disease and its severity (spread), is traditionally performed by means of a visual score. In cardiology, a visual score of emphysema or diffuse parenchymal disease >40% is generally considered sufficient to justify the presence of PH, although there are not enough studies in order to confirm this hypothesis. Concerning the diffuse parenchymal disease diagnosis, the opinion of the radiologist or, in specific cases, a multidisciplinary discussion is usually reliable, but it suffers from a certain level of interobserver variability. Moreover, a precise correlation between disease extent and haemodynamic severity has not, to date, been found. In some studies, functional and radiological scores in COPD and ILD obtained through a visual score have been suggested. Basically, according to some previous studies [94, 95], the visual scores are related to functional impairment and prognosis [96]. However, although these visual evaluations are still useful, further improvements have been made, especially in the quantification of COPD (emphysema, air trapping, bronchial remodelling) with automatic scores which are available for all the modern CT. Recently, a new subgroup in COPD phenotypes, called pulmonary vascular phenotype has been described [3]. In order to identify this subgroup, software packages able to quantify the vascular pruning and pulmonary blood volume by CT without contrast are under investigation. Pulmonary blood volume seems to be a strong prognostic index related to the enlargement of the right ventricle and it can represent a possible direct expression of the residual smoking injury.
Concerning fibrosing ILDs [97], only CT automatic quantification of lung disease extent by means of a density mask is considered insufficient. To date, different methods for recognising a parenchymal pattern and its quantification have been suggested. Some methods already use artificial intelligence and deep learning [98]. Among these methods the most important are the measurements based on the density histogram, skewness and kurtosis called AMFM (Adaptive Multiple Feature Method), QLF (Quantitative Lung Fibrosis), DTA (Data Driven textural Analysis) and FRI (Functional Respiratory Imaging). The strongest and more reproducible one is CALIPER (Computer Aided Lung Informatics for Pathology Evaluation and Rating), which is not yet available on the market. CALIPER analyses the parenchymal texture, suggesting a diagnosis and quantifying the different components with an accuracy which is slightly higher when compared to the majority of human expert observers. CALIPER allows a measurement which is impossible for the human observer: the lung vascular-related structures and the evaluation of perivascular fibrosis, which seems to have a prognostic value in IPF, cardiovascular disease in ILD, hypersensitivity pneumonitis and unclassifiable ILDs [99]. Peculiar problems are related to diseases that directly involve pulmonary vessels and increase their resistance, even in the presence of a minimum parenchymal involvement, such as in Langerhans cells histiocytosis, sarcoidosis and systemic sclerosis.
Analysing the measurement of central pulmonary arteries with CT scans in ILDs, the role of the enlargement of central pulmonary arteries is less specific when compared with those found in PH-COPD, due to the effect of the fibrotic traction on compliant structures that may cause the detection of false positives.
In summary, the most relevant novelties in imaging of CLD-PH are the development of artificial intelligence or machine learning, which represents a promising tool to integrate new advanced imaging techniques, and magnetic resonance imaging (4-dimensional) evolution [100] with the introduction of dynamic sequences that are able to quantify the blood flow and its velocity with good accuracy through the use of new visual tools (streamlines and speed vectors). The whirling blood flow is a reliable sign of PH. 4-Dimensional magnetic resonance imaging is able to quantify the wall shear stress in pulmonary arteries and further advances in this technique could, in the future, avoid the performance of RHC. Furthermore, magnetic resonance imaging has proven to be able to evaluate and quantify local morphological and structural right ventricle alterations more precisely than echocardiography. However, this tool is still not of widespread use because of its complexity and limited time availability in non-specialised radiological units. Moreover, further clinical trials are still necessary in order to confirm these initial results.
Treatment
To date, specific treatment for CLD-PH is not available. The optimal therapy of the underlying lung disease is recommended according to the more recent guidelines. Hypoxaemic patients should be treated with oxygen supplementation, although its benefit has only been properly and prospectively evaluated in PH-COPD. In this group of patients, oxygen supplementation prevented the progressive increase of mPAP when used for at least 15 h per day and a slight decrease of this value was observed if oxygen was supplemented for at least 18 h per day [101, 102]. Long-term oxygen supplementation is not effective in the normalisation of PAP or in pulmonary vascular remodelling reversal. Drugs approved for group 1 PH (PAH) have been tested in patients with PH due to parenchymal lung diseases. However, randomised clinical trials have only been conducted in COPD, ILD and sarcoidosis. The use of PAH drugs in PH-CLD is still being debated [103]. Several studies both in COPD and IPF patients reported controversial or negative results [104, 105]. In PH-COPD, the use of drugs used in PAH, in particular sildenafil and bosentan, has shown a limited positive effect on haemodynamic measurements not associated with an improvement in symptoms and quality of life [106, 107]. A clinical trial on ambrisentan in patients affected by PH-IPF has been interrupted because of the increase in hospitalisation and mortality in the treatment arm [108]. The same results were found in a trial on the use of riociguat in patients affected by PH-ILD [109]. Recently, the add-on therapy with sildenafil in severe IPF patients treated with nintedanib has not shown any significant improvement in symptoms [110]. Post hoc patients showing echocardiographic signs of right heart dysfunction were compared to the others. No significant differences in symptoms and forced vital capacity were found; however, the stabilisations of levels of brain natriuretic peptide was more prominent in patients with right heart disfunction compared to the others [111]. A phase IIb randomised controlled trial is ongoing and aims to evaluate the efficacy, safety and tolerability of pirfenidone in combination with sildenafil in patients with advanced IPF and intermediate or high probability of group 3 PH (https://clinicaltrials.gov/ct2/show/NCT02951429).
Therefore, the use of PAH drugs in PH-COPD and PH-ILD patients is now not recommended and sometimes could have detrimental effects, it could only be considered in expert centres on a case by case evaluation. Data concerning the use of PAH drugs in PH associated with sarcoidosis are also controversial. In a retrospective analysis of 22 patients affected by sarcoidosis associated with PH, detected at RHC, the use of bosentan and/or sildenafil significantly increased the 6MWD [112]. However, no significant change of the 6MWD was observed in an open label prospective study assessing the efficacy of inhaled iloprost [113]. However, only one randomised clinical trial was performed in this group of patient showing a significant improvement in pulmonary haemodynamics, although no significant change in 6MWD was observed [114].
Pulmonary rehabilitation
As is well known, many CLDs are characterised by the presence of dyspnoea, fatigue, reduced exercise tolerance and peripheral muscle dysfunction. Pulmonary rehabilitation in COPD patients has been demonstrated to be a successful tool in the improvement of exercise capacity, quality of life and symptoms, at the same time reducing hospitalisation rates [115–118]. Patients affected by other CLDs show the same symptoms and peripheral muscle dysfunction, suggesting that pulmonary rehabilitation may represent an additional treatment in these group of patients as well. However, a specific rehabilitation protocol may be necessary for every disease in consideration of the differences in underlying respiratory pathophysiology, symptom kinetics and disease courses.
Pulmonary rehabilitation in ILD has been investigated in two randomised trials in a group with mixed ILD and one with IPF showing only a short-term benefit in terms of exercise tolerance, dyspnoea and quality of life [119–121].
In the past, patients affected by PAH were advised to avoid exercise. The derived increase in pulmonary pressures could have, in fact, exacerbated right heart failure. Nowadays, recent studies have demonstrated important benefits of exercise training in this group of patients. In a large study on 183 PH patients (group 1: n=103, group 2: n=8, group 3: n=11, and group 4: n=31), an improvement in 6MWD and maximum V′O2 at peak of CPET was demonstrated. However, 13% of the patients experienced adverse events, although not severe, mostly syncope and presyncopal episodes [122]. This highlights the importance of an appropriate symptom monitoring protocol during exercise training.
All the studies suggest that pulmonary rehabilitation may have clinically significant benefits in patients with PH, including PAH and CLD-PH. Since CLD includes different diseases with peculiar characteristics, more specific studies should be performed to design specific rehabilitation programmes for each group of patients. Furthermore, it should be important to investigate the correct timing to initiate pulmonary rehabilitation. In iPAH, recent data suggest that early referral to pulmonary rehabilitation is necessary to obtain the maximum benefits, while in patients with other ILDs the benefits seem to not be related to disease severity [11]. Currently no data are available for patients affected by CLD-PH.
Conclusion
It has been well established that the presence of PH in CLD is associated with a worse functional status and a bad prognosis. As PH-CLD is increasing in morbidity and mortality, further investigations are required in order to improve early diagnosis and provide better clinical management. The discovery of different biomarkers, possibly easy to collect from blood samples or exhaled breath, may lead to a significant improvement in the management of this disease.
Exercise capacity assessment is important for prognostic evaluation in this group of patients. Although 6MWT is a useful and easy tool, further studies should be run in order to draw a composite index that might take into consideration several values obtained by this test and not only the distance covered. CPET should be encouraged, especially at an early stage of the disease, when patients are not affected by severe respiratory failure.
Further studies should be carried out in order to improve the use of radiographic and echocardiographic tests for the noninvasive diagnosis of PH-CLD and to support the possibility of screening programmes in this group of patients.
In addition to the implementation of trials aimed at evaluating the use of specific drugs in PH-CLD, specific rehabilitation programmes should be investigated in order to improve the quality of life and exercise performance in this group of patient.
Footnotes
Provenance: Publication of this peer-reviewed article was sponsored by Boehringer Ingelheim, Germany (principal sponsor European Respiratory Review issue 153).
Conflict of interest: D. Elia has nothing to disclose.
Conflict of interest: A. Caminati reports personal fees from Roche and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: M. Zompatori has nothing to disclose.
Conflict of interest: R Cassandro has nothing to disclose.
Conflict of interest: C. Lonati has nothing to disclose.
Conflict of interest: F. Luisi has nothing to disclose.
Conflict of interest: G. Pelosi has nothing to disclose.
Conflict of interest: S. Provencher reports grants and personal fees from Actelion Pharmaceuticals, and grants from Boehringer Ingelheim and Resverlogix Corp, outside the submitted work.
Conflict of interest: S. Harari reports grants and personal fees from Roche, Actelion and Boehringer Ingelheim, outside the submitted work.
References
- 1.Adir Y, Harari S. Pulmonary hypertension associated with chronic obstructive lung disease and idiopathic pulmonary fibrosis. Curr Opin Pulm Med 2014; 20: 414–420. doi:10.1097/MCP.0000000000000084 [DOI] [PubMed] [Google Scholar]
- 2.Seeger W, Adir Y, Barberà JA, et al. . Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: 25 Suppl., D109–D116. doi:10.1016/j.jacc.2013.10.036 [DOI] [PubMed] [Google Scholar]
- 3.Nathan SD, Barbera JA, Gaine SP, et al. . Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914. doi:10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. doi:10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 5.Douschan P, Kovacs G, Avian A, et al. . Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med 2018; 197: 509–516. doi:10.1164/rccm.201706-1215OC [DOI] [PubMed] [Google Scholar]
- 6.Valerio CJ, Schreiber BE, Handler CE, et al. . Borderline mean pulmonary artery pressure in patients with systemic sclerosis: transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum 2013; 65: 1074–1084. doi:10.1002/art.37838 [DOI] [PubMed] [Google Scholar]
- 7.Coghlan JG, Wolf M, Distler O, et al. . Incidence of pulmonary hypertension and determining factors in patients with systemic sclerosis. Eur Respir J 2018; 51: 1701197. doi:10.1183/13993003.01197-2017 [DOI] [PubMed] [Google Scholar]
- 8.Simonneau G, Montani D, Clermajer DS, et al. . Haemodynamic definitions and update clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi:10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caminati A, Cassandro R, Harari S. Pulmonary hypertension in chronic lung interstitial disease. Eur Respir Rev 2013; 22: 292–301. doi:10.1183/09059180.00002713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thabut G, Dauriat G, Stern JB, et al. . Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005; 127: 1531–1536. doi:10.1378/chest.127.5.1531 [DOI] [PubMed] [Google Scholar]
- 11.Shorr AF, Wainright JL, Cors CS, et al. . Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J 2007; 30: 715–721. doi:10.1183/09031936.00107206 [DOI] [PubMed] [Google Scholar]
- 12.Chaouat A, Bugnet AS, Kadaoui N, et al. . Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 189–194. doi:10.1164/rccm.200401-006OC [DOI] [PubMed] [Google Scholar]
- 13.Lettieri CJ, Nathan SD, Barnett SD, et al. . Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006; 129: 746–752. doi:10.1378/chest.129.3.746 [DOI] [PubMed] [Google Scholar]
- 14.Raghu G, Nathan SD, Behr J, et al. . Pulmonary hypertension in idiopathic pulmonary fibrosis with mild to moderate restriction. Eur Respir J 2015; 46: 1370–1377. doi:10.1183/13993003.01537-2014 [DOI] [PubMed] [Google Scholar]
- 15.Mura M, Anraku M, Yun Z, et al. . Gene expression profiling in the lungs of patients with pulmonary hypertension associated with pulmonary fibrosis. Chest 2012; 141: 661–673. doi:10.1378/chest.11-0449 [DOI] [PubMed] [Google Scholar]
- 16.Hsu E, Shi H, Jordan RM, et al. . Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum 2011; 63: 783–794. doi:10.1002/art.30159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann J, Wilhelm J, Marsh LM, et al. . Distinct differences in gene expression patterns in pulmonary arteries of patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis with pulmonary hypertension. Am J Respir Crit Care Med 2014; 190: 98–111. doi:10.1164/rccm.201401-0037OC [DOI] [PubMed] [Google Scholar]
- 18.Maniscalco M, Paris D, Carone M, et al. . Is there a role for biomarkers in pulmonary rehabilitation? Biomark Med 2018; 12: 1069–1072. doi:10.2217/bmm-2018-0219 [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Yang T, Xu X, et al. . Oxidative stress and nitric oxide signaling related biomarkers in patients with pulmonary hypertension: a case control study. BMC Pulm Med 2015; 15: 50. doi:10.1186/s12890-015-0045-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannakoulas G, Mouratoglou S-A, Gatzoulis MA, et al. . Blood biomarkers and their potential role in pulmonary arterial hypertension associated with congenital heart disease: a systematic review. Int J Cardiol 2014; 174: 618–623. doi:10.1016/j.ijcard.2014.04.156 [DOI] [PubMed] [Google Scholar]
- 21.Sanli C, Oguz D, Olgunturk R, et al. . Elevated homocysteine and asymmetric dimethyl arginine levels in pulmonary hypertension associated with congenital heart disease. Pediatr Cardiol 2012; 33: 1323–1331. doi:10.1007/s00246-012-0321-9 [DOI] [PubMed] [Google Scholar]
- 22.Telo S, Kırkıl G, Kuluöztürk M. Can ADMA play a role in determining pulmonary hypertension related to chronic obstructive pulmonary disease? Clin Respir J 2018; 12: 1433–1438. doi:10.1111/crj.12675 [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Ba Y, Ma L, et al. . Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008; 18: 997–1006. doi:10.1038/cr.2008.282 [DOI] [PubMed] [Google Scholar]
- 24.Baptista R, Marques C, Catarino S, et al. . MicroRNA-424(322) as a new marker of disease progression in pulmonary arterial hypertension and its role in right ventricular hypertrophy by targeting SMURF1. Cardiovasc Res 2018; 114: 53–64. doi:10.1093/cvr/cvx187 [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Li S. Circulating microRNA as a novel biomarker for pulmonary arterial hypertension due to congenital heart disease. Pediatr Cardiol 2017; 38: 86–94. doi:10.1007/s00246-016-1487-3 [DOI] [PubMed] [Google Scholar]
- 26.Jin P, Gu W, Lai Y, et al. . The circulating microRNA-206 level predicts the severity of pulmonary hypertension in patients with left heart diseases. Cell Physiol Biochem 2017; 41: 2150–2160. doi:10.1159/000475569 [DOI] [PubMed] [Google Scholar]
- 27.Sarrion I, Milian L, Juan G, et al. . Role of circulating miRNAs as biomarkers in idiopathic pulmonary arterial hypertension: possible relevance ofmiR-23a. Oxid Med Cell Longev 2015; 2015: 792846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulin R, Sutendra G, Gurtu V, et al. . A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res 2015; 116: 56–69. doi:10.1161/CIRCRESAHA.115.303910 [DOI] [PubMed] [Google Scholar]
- 29.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic Biol Med 2013; 64: 20–30. doi:10.1016/j.freeradbiomed.2013.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greco S, Martelli F. MicroRNAs in hypoxia response. Antioxid Redox Signal 2014; 21: 1164–1166. doi:10.1089/ars.2014.6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Yin T, Yan W, et al. . Dysregulation of microRNA-214 and PTEN contributes to the pathogenesis of hypoxic pulmonary hypertension. Int J Chron Obstruct Pulmon Dis 2017; 12: 1781–1791. doi:10.2147/COPD.S104627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SS, Ran YJ, Zhang DD, et al. . MicroRNA-190 regulates hypoxic pulmonary vasoconstriction by targeting a voltage-gated K+ channel in arterial smooth muscle cells. J Cell Biochem 2014; 115: 1196–1205. doi:10.1002/jcb.24771 [DOI] [PubMed] [Google Scholar]
- 33.Jiang J X, Liang Y, Yang M, et al. . miR-190a-5p participates in the regulation of hypoxia-induced pulmonary hypertension by targeting KLF15 and can serve as a biomarker of diagnosis and prognosis in chronic obstructive pulmonary disease complicated with pulmonary hypertension. Int J Chron Obstruct Pulmon Dis 2018; 13: 3777–3790. doi:10.2147/COPD.S182504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwapiszewska G, Wilhelm J, Wolff S, et al. . Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension. Respir Res 2005; 6: 109. doi:10.1186/1465-9921-6-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajkumar R, Konishi K, Richards TJ, et al. . Genome wide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol 2010; 298: H1235–H1248. doi:10.1152/ajpheart.00254.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann J, Wilhelm J, Olschewski A. Microarray analysis in pulmonary hypertension. Eur Respir J 2016; 48: 229–241. doi:10.1183/13993003.02030-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calfee CS, Ware LB, Eisner MD, et al. . Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008; 63: 1083–1089. doi:10.1136/thx.2008.095588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serrano AG, Perez-Gil J. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem Phys Lipids 2006; 141: 105–118. doi:10.1016/j.chemphyslip.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 39.Swenson ER, Maggiorini M, Mongovin S, et al. . Pathogenesis of high altitude pulmonary edema: inflammation is not an etiologic factor. JAMA 2002; 287: 2228–2235. doi:10.1001/jama.287.17.2228 [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi H, Kanoh S, Motoyoshi K. Serum surfactant protein-A, but not surfactant protein-D or KL-6, can predict preclinical lung damage induced by smoking. Biomarkers 2008; 13: 385–392. doi:10.1080/13547500801903651 [DOI] [PubMed] [Google Scholar]
- 41.Hill J, Heslop C, Man SF, et al. . Circulating surfactant protein-D and the risk of cardiovascular morbidity and mortality. Eur Heart J 2011; 32: 1918–1925. doi:10.1093/eurheartj/ehr124 [DOI] [PubMed] [Google Scholar]
- 42.Winkler C, Atochina-Vasserman EN, Holz O, et al. . Comprehensive characterisation of pulmonary and serum surfactant protein D in COPD. Respir Res 2011; 12: 29. doi:10.1186/1465-9921-12-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gargiulo P, Banfi C, Ghilardi S, et al. . Surfactant-derived proteins as markers of alveolar membrane damage in heart failure. PLoS One 2014; 9: e115030. doi:10.1371/journal.pone.0115030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haick H, Broza YY, Mochalski P, et al. . Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev 2014; 43: 1423–1449. doi:10.1039/C3CS60329F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haick H, Cohen-Kaminsky S. Detecting lung infections in breath prints: empty promise or next generation diagnosis of infections. Eur Respir J 2015; 45: 21–24. doi:10.1183/09031936.00183714 [DOI] [PubMed] [Google Scholar]
- 46.Allers M, Langejuergen J, Gaida A, et al. . Measurement of exhaled volatile organic compounds from patients with chronic obstructive pulmonary disease (COPD) using closed gas loop GC-IMS and GC-APCI-MS. J Breath Res 2016; 10: 026004. doi:10.1088/1752-7155/10/2/026004 [DOI] [PubMed] [Google Scholar]
- 47.Grob NM, Laskowski D, Dweik RA. A technical report on exhaled nitric oxide measurement: asthma monitoring in ahletes. J Breath Res 2008; 2: 37027. doi:10.1088/1752-7155/2/3/037027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith D, Sovová K, Dryahina K, et al. . Breath concentration of acetic acid vapour is elevated in patients with cystic fibrosis. J Breath Res 2016; 10: 021002. doi:10.1088/1752-7155/10/2/021002 [DOI] [PubMed] [Google Scholar]
- 49.Mansoor JK, Schelegle ES, Davis CE, et al. . Analysis of volatile compounds in exhaled breath condensate in patients with severe pulmonary arterial hypertension. PLoS One 2014; 9: e95331. doi:10.1371/journal.pone.0095331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cikach FS Jr, Tonelli AR, Barnes J, et al. . Breath analysis in pulmonary arterial hypertension. Chest 2014; 145: 551–558. doi:10.1378/chest.13-1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen-Kaminsky S, Nakhleh M, Perros F, et al. . A proof of concept for the detection and classification of pulmonary arterial hypertension through breath analysis with a sensor array. Am J Respir Crit Care Med 2013; 188: 756–759. doi:10.1164/rccm.201303-0467LE [DOI] [PubMed] [Google Scholar]
- 52.Nakhleh MK, Haick H, Humbert M. Volatolomics of breath as an emerging frontier in pulmonary arterial hypertension. Eur Respir J 2017; 49: 1601897. doi:10.1183/13993003.01897-2016 [DOI] [PubMed] [Google Scholar]
- 53.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. doi:10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 54.Gaine S, Simonneau G. The need to move from 6-minute walk distance to outcome trials in pulmonary arterial hypertension. Eur Respir Rev 2013; 22: 487–494. doi:10.1183/09059180.00006213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holland AE, Spruit MA, Troosters T, et al. . An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–1446. doi:10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 56.du Bois RM, Weycker D, Albera C, et al. . Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med 2011; 183: 1231–1237. doi:10.1164/rccm.201007-1179OC [DOI] [PubMed] [Google Scholar]
- 57.Lama VN, Flaherty KR, Toews GB, et al. . Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003; 168: 1084–1090. doi:10.1164/rccm.200302-219OC [DOI] [PubMed] [Google Scholar]
- 58.Harari S, Caminati A, Cassandro R, et al. . Pulmonary hypertension in idiopathic pulmonary fibrosis does not influence six-minute walk distance: results from a retrospective study. Sarcoidosis Vasc Diffuse Lung Dis 2015; 31: 297–305. [PubMed] [Google Scholar]
- 59.Andrianopulos V, Wouters EF, Pinto-Plata VM. Prognostic value of variables derived from the six-minute walking test in patients with COPD: results from the ECLIPSE study. Respir Med 2015; 109: 1138–1146. doi:10.1016/j.rmed.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 60.Swigris JJ, Swick J, Wamboldt FS, et al. . Heart rate recovery after 6-min walk test predicts survival in patients with idiopathic pulmonary fibrosis. Chest 2009; 136: 841–848. doi:10.1378/chest.09-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swigris JJ, Olson AL, Shlobin OA, et al. . Heart rate recovery after six-minute walk test predicts pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respirology 2011; 16: 439–445. doi:10.1111/j.1440-1843.2010.01877.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lettieri CJ, Nathan SD, Browning RF, et al. . The distance-saturation product predicts mortality in idiopathicpulmonary fibrosis. Respir Med 2006; 100: 1734–1741. doi:10.1016/j.rmed.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 63.Lancaster L. Utility of the six-minute walk test in patients with idiopathic pulmonary fibrosis. Multidisciplinary Resp Med 2018; 13: 45. doi:10.1186/s40248-018-0158-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wasserman K, Hansen JE, Sietsema K, et al.. Exercise testing and interpretation. 5th Edn. Philadelphia, Lippincott Williams and Wilkins, 2011; pp. 141–146. [Google Scholar]
- 65.Porszasz J, Stringer W, Casaburi R. Equipment, measurements and quality control. In: Palange P, Laveneziana P, Neder JA, et al.. Clinical Exercise Testing (ERS Monograph). Sheffield, European Respiratory Society; 2018, pp. 58–81. [Google Scholar]
- 66.Weisman IM, Zeballos RJ. Clinical exercising testing progress. Clin Chest Med 2001; 4: 679–701. [DOI] [PubMed] [Google Scholar]
- 67.Farina S, Bruno N, Agalbato C, et al. . Physiological insights of exercise hyperventilation in arteriale and thromboembolic pulmonary hypertension. Int J Cardiol 2018; 258: 178–182. doi:10.1016/j.ijcard.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 68.Holland AE, Dowman L, Fiore J Jr, et al. . Cardiorespiratory responses to 6-minute walk test in interstitial lung disease: not always a submaximal test. BMC Pulm Med 2014; 14: 136. doi:10.1186/1471-2466-14-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laaban J-P, Diebold B, Zelinski R, et al. . Noninvasive estimation of systolic pulmonary artery pressure using Doppler echocardiography in patients with chronic obstructive pulmonary disease. Chest 1989; 96: 1258–1262. doi:10.1378/chest.96.6.1258 [DOI] [PubMed] [Google Scholar]
- 70.Bach DS, Curtis JL, Christensen PJ, et al. . Preoperative echocardiographic evaluation of patients referred for lung volume reduction surgery. Chest 1998; 114: 972–980. doi:10.1378/chest.114.4.972 [DOI] [PubMed] [Google Scholar]
- 71.Tramarin R, Torbicki A, Marchandise B, et al. . Doppler, echocardiographic evaluation of pulmonary artery pressure in obstructive pulmonary disease: a European multicentre study. Eur Heart J 1991; 12: 103–111. doi:10.1093/oxfordjournals.eurheartj.a059855 [DOI] [PubMed] [Google Scholar]
- 72.Arcasoy SM, Christie JD, Ferrari VA, et al. . Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167: 735–740. doi:10.1164/rccm.200210-1130OC [DOI] [PubMed] [Google Scholar]
- 73.Berger M, Haimowitz A, Van Tosh A, et al. . Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol 1985; 6: 359–365. doi:10.1016/S0735-1097(85)80172-8 [DOI] [PubMed] [Google Scholar]
- 74.Currie PJ, Seward JB, Chan K-L, et al. . Continuous wave Doppler estimation right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol 1985; 6: 750–756. doi:10.1016/S0735-1097(85)80477-0 [DOI] [PubMed] [Google Scholar]
- 75.Yorck PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 1984; 70: 657–662. doi:10.1161/01.CIR.70.4.657 [DOI] [PubMed] [Google Scholar]
- 76.Van de Veerdonk MC, Kind T, Marcus JT, et al. . Progressive right ventriculare dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. doi:10.1016/j.jacc.2011.06.068 [DOI] [PubMed] [Google Scholar]
- 77.Sachdev A, Villarraga HR, Frantz RP, et al. . Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 2011; 139: 1299–1309. doi:10.1378/chest.10-2015 [DOI] [PubMed] [Google Scholar]
- 78.D'Andrea A, Stanziola AA, Saggar R. Right ventricular functional reserve in early-stage idiopathic pulmonary fibrosis an exercise two-dimensional speckle tracking Doppler echocardiography study. Chest 2019; 155: 297–306. doi:10.1016/j.chest.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 79.Rice JL, Stream AR, Fox DL, et al. . Speckle tracking echocardiography to evaluate for pulmonary hypertension in chronic obstructive pulmonary disease. COPD 2016; 13: 595–600. doi:10.3109/15412555.2015.1134468 [DOI] [PubMed] [Google Scholar]
- 80.Sonaglioni A, Baravelli M, Cassandro R, et al. . Hemodynamic mechanisms of exercise-induced pulmonary hypertension in patients with lymphangioleiomyomatosis: the role of exercise stress echocardiography. J Am Soc Echocardiogr 2018; 31: 888–901. doi:10.1016/j.echo.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 81.Altschul E, Remy-Jardin M, Machnicki S, et al. . Imaging of pulmonary hypertension: pictorial essay. Chest 2019; 156: 211–227. doi:10.1016/j.chest.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 82.Barbosa EJ Jr, Gupta NK, Torigian DA, et al. . Current role of imaging in the diagnosis and management of pulmonary hypertension. AJR Am J Roentgenol 2012; 198: 1320–1331. doi:10.2214/AJR.11.7366 [DOI] [PubMed] [Google Scholar]
- 83.Truong U, Fonseca B, Dunning J, et al. . Wall shear stress measured by phase contrast cardiovascular magnetic resonance in children and adolescents with pulmonary arterial hypertension. J Cardiovasc Magn Reson 2013; 15: 81. doi:10.1186/1532-429X-15-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis G, Hoey ET, Reynolds JH, et al. . Multi-detector CT assessment in pulmonary hypertension: techniques, systematic approach to interpretation and key findings. Quant Imaging Med Surg 2015; 5: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan RT, Kuzo R, Goodman LR, et al. . Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Chest 1998; 113: 1250–1256. doi:10.1378/chest.113.5.1250 [DOI] [PubMed] [Google Scholar]
- 86.Grosse A, Grosse C, Lang I. Evaluation of the CT imaging findings in patients newly diagnosed with chronic thromboembolic pulmonary hypertension. PLoS One 2018; 13: e0201468. doi:10.1371/journal.pone.0201468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ascha M, Renapurkar RD, Tonelli AR. A review of imaging modalities in pulmonary hypertension. Ann Thorac Med 2017; 12: 61–73. doi:10.4103/1817-1737.203742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raymond TE, Khabbaza JE, Yadav R, et al. . Significance of main pulmonary artery dilation on imaging studies. Ann Am Thorac Soc 2014; 11: 1623–1632. doi:10.1513/AnnalsATS.201406-253PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alhamad EH, Al-Boukai AA, Al-Kassimi FA, et al. . Prediction of pulmonary hypertension in patients with or without interstitial lung disease: reliability of CT findings. Radiology 2011; 260: 875–883. doi:10.1148/radiol.11103532 [DOI] [PubMed] [Google Scholar]
- 90.Auti OB, Kansal K, Ashok KG, et al. . MDCT assessment of pulmonary arterial hypertension. Curr Radiol Rep 2017; 5: 18. doi:10.1007/s40134-017-0212-1 [Google Scholar]
- 91.Iyer AS, Wells JM, Vishin S, et al. . CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest 2014; 145: 824–832. doi:10.1378/chest.13-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katikireddy CK, Singh M, Muhyieddeen K, et al. . Left atrial area and right ventricle dimensiond in non gated axial chest CT can differentiate pulmonary hypertension due to left heart disease from other causes. J Cardiovasc Comput Tomogr 2016; 10: 246–250. doi:10.1016/j.jcct.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 93.Frost A, Badesch D, Gibbs SR, et al. . Diagnosis of pulmonary hypertension. Eur Respir J 2019; 53: 1801904. doi:10.1183/13993003.01904-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lynch DA, Godwin JD, Safrin S, et al. . High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnostic and prognosis. Am J Resp Crit Care Med 2005; 172: 488–493. doi:10.1164/rccm.200412-1756OC [DOI] [PubMed] [Google Scholar]
- 95.Flaherty KR, Mumford JA, Murray S, et al. . Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Resp Crit CareMed 2003; 168: 543–548. doi:10.1164/rccm.200209-1112OC [DOI] [PubMed] [Google Scholar]
- 96.King CS, Nathan SD. Pulmonary hypertension due to interstitial lung disease. Curr Opin Pulm Med 2019; 25: 459–467. doi:10.1097/MCP.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 97.Yagi M, Taniguchi H, Kondoh Y. CT determined pulmonary artery to aorta ratio as a predictor of elevated pulmonary artery pressure and survival in idiopathic pulmonary fibrosis. Respirology 2017; 22: 1393–1399. doi:10.1111/resp.13066 [DOI] [PubMed] [Google Scholar]
- 98.Wu J, Qian T. A survey of pulmonary nodule detection, segmentation and classification in computed tomography with deep learning techniques. A survey of pulmonary nodule detection, segmentation and classification in computed tomography with deep learning techniques. J Med Artif Intell 2019; 2: 1–12. [Google Scholar]
- 99.Jacob J, Bartholmai BJ, Rajagopalan S, et al. . Predicting outcomes in idiopathic pulmonary fibrosis using automated computed tomographic analysis. Am J Respir Crit Care Med 2018; 198: 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cann C M, Gopalan D, Sheares K, et al. . Imaging in pulmonary hypertension, part 1. Clinical perspectives, classification, imaging techniques and imaging algorithm. Postgrad Med J 2012; 88: 271–279. doi:10.1136/postgradmedj-2011-130292 [DOI] [PubMed] [Google Scholar]
- 101.Timms RM, Khaja FU, Williams GW. Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med 1985; 102: 29–36. doi:10.7326/0003-4819-102-1-29 [DOI] [PubMed] [Google Scholar]
- 102.Weitzenblum E, Sautegeau A, Ehrhart M, et al. . Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985; 131: 493–498. [DOI] [PubMed] [Google Scholar]
- 103.Harari S, Elia D, Humbert M. Pulmonary hypertension in parenchymal lung diseases: any future for new therapies? Chest 2018; 153: 217–223. doi:10.1016/j.chest.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 104.Chen X, Tang S, Liu K, et al. . Therapy in stable chronic obstructive pulmonary disease patients with pulmonary hypertension: a systematic review and meta-analysis. J Thorac Dis 2015; 7: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prins KW, Duval S, Markowitz J, et al. . Chronic use of PAH-specific therapy in World Health Organization Group III pulmonary hypertension: a systematic review and meta-analysis. Pulm Circ 2017; 7: 145–155. doi:10.1086/690017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vitulo P, Stanziola A, Confalonieri M, et al. . Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant 2017; 36: 166–174. doi:10.1016/j.healun.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 107.Valerio G, Bracciale P, Grazia DA. Effect of bosentan upon pulmonary hypertension in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2009; 3: 15–21. doi:10.1177/1753465808103499 [DOI] [PubMed] [Google Scholar]
- 108.Raghu G, Behr J, Brown KK, et al. . Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med 2013; 158: 641–649. doi:10.7326/0003-4819-158-9-201305070-00003 [DOI] [PubMed] [Google Scholar]
- 109.Nathan SD, Behr J, Collard HR, et al. . Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomised, placebo-controlled phase 2b study. Lancet Respir Med 2019; 7: 780–790. doi: 10.1016/S2213-2600(19)30250-4 [DOI] [PubMed] [Google Scholar]
- 110.Kolb M, Raghu G, Wells AU, et al. . Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med 2018; 379: 1722–1731. doi:10.1056/NEJMoa1811737 [DOI] [PubMed] [Google Scholar]
- 111.Behr J, Kolb M, Song JW, et al. . Nintedanib and Sildenafil in Patients with Idiopathic Pulmonary Fibrosis and Right Heart Dysfunction (INSTAGE): a pre-specified sub-group analysis of a double-blind, randomized clinical trial. Am J Respir Crit Care Med 2019. doi: 10.1164/rccm.201903-0488OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barnett CF, Bonura EJ, Nathan SD, et al. . Treatment of sarcoidosis-associated pulmonary hypertension. A two-center experience. Chest 2009; 135: 1455–1461. doi:10.1378/chest.08-1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baughman RP, Judson MA, Lower EE, et al. . Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2009; 26: 110–120. [PubMed] [Google Scholar]
- 114.Baughman RP, Culver DA, Cordova FC, et al. . Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest 2014; 145: 810–817. doi:10.1378/chest.13-1766 [DOI] [PubMed] [Google Scholar]
- 115.McNamara RJ, McKeough ZJ, McKenzie DK, et al. . Water-based exercise in COPD with physical comorbidities: a randomised controlled trial. Eur Respir J 2013; 41: 1284–1291. doi:10.1183/09031936.00034312 [DOI] [PubMed] [Google Scholar]
- 116.Gouzi F, Prefaut C, Abdellaoui A, et al. . Blunted muscle angiogenic training-response in COPD patients versus sedentary controls. Eur Respir J 2013; 41: 806–814. doi:10.1183/09031936.00053512 [DOI] [PubMed] [Google Scholar]
- 117.Bronstad E, Rognmo O, Tjonna AE, et al. . High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur Respir J 2012; 40: 1130–1136. doi:10.1183/09031936.00193411 [DOI] [PubMed] [Google Scholar]
- 118.Burtin C, Saey D, Saglam M, et al. . Effectiveness of exercise training in patients with COPD: the role of muscle fatigue. Eur Respir J 2012; 40: 338–344. doi:10.1183/09031936.00111811 [DOI] [PubMed] [Google Scholar]
- 119.Holland AE, Hill CJ, Conron M, et al. . Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax 2008; 63: 549–554. doi:10.1136/thx.2007.088070 [DOI] [PubMed] [Google Scholar]
- 120.Nishiyama O, Kondoh Y, Kimura T, et al. . Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 2008; 13: 394–399. doi:10.1111/j.1440-1843.2007.01205.x [DOI] [PubMed] [Google Scholar]
- 121.Grüning E, Lichtblau M, Ehlken N. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 2012; 40: 84–92. doi:10.1183/09031936.00123711 [DOI] [PubMed] [Google Scholar]
- 122.Holland AE, Hill CJ, Glaspole I, et al. . Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med 2012; 106: 429–435. doi:10.1016/j.rmed.2011.11.014 [DOI] [PubMed] [Google Scholar]