Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) results from incomplete resolution of acute pulmonary emboli, organised into fibrotic material that obstructs large pulmonary arteries, and distal small-vessel arteriopathy. Pulmonary endarterectomy (PEA) is the treatment of choice for eligible patients with CTEPH; in expert centres, PEA has low in-hospital mortality rates and excellent long-term survival. Supportive medical therapy consists of lifelong anticoagulation plus diuretics and oxygen, as needed.
An important recent advance in medical therapy for CTEPH is the arrival of medical therapies for patients with inoperable disease or persistent/recurrent pulmonary hypertension after PEA. The soluble guanylate cyclase stimulator riociguat is licensed for the treatment of CTEPH in patients with inoperable disease or with recurrent/persistent pulmonary hypertension after PEA. Clinical trials of this agent have shown improvements in patients' haemodynamics and exercise capacity. Phosphodiesterase-5 inhibitors, endothelin receptor antagonists and prostanoids have been used in the treatment of CTEPH, but evidence of benefit is limited. Challenges in the future development of medical therapy for CTEPH include better understanding of the underlying pathology, end-points to monitor the condition's progress, and the optimisation of pulmonary arterial hypertension therapies in relation to diverse patient characteristics and emerging options such as balloon pulmonary angioplasty.
Short abstract
Targeted medical therapy with riociguat is beneficial for inoperable CTEPH or persistent/recurrent CTEPH after PEA http://ow.ly/pdRg3091AiF
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a subclass of pulmonary hypertension. To distinguish CTEPH from subacute pulmonary embolism, diagnosis is made after ≥3 months of therapeutic anticoagulation [1]. Diagnosis includes a mean pulmonary arterial pressure (mPAP) ≥25 mmHg with pulmonary capillary wedge pressure (PCWP) ≤15 mmHg, mismatched perfusion defects on lung ventilation/perfusion scan and/or specific diagnostic signs of chronic thromboembolism on angiography [1, 2].
CTEPH is considered a two-compartment disease: there is initial occlusion of the proximal major vessels by fibrotic material as a consequence of nonresolution of a single or recurrent pulmonary embolism, accompanied by a distal pulmonary arteriopathy and microvascular disease in the nonobstructed areas [3–6]. The proximal obstructions are suitable for pulmonary endarterectomy (PEA), which is the treatment of choice for eligible patients [7, 8]. Perioperative mortality is now <5% in highly experienced surgical centres and most patients experience substantial functional improvement and near normalisation of haemodynamic parameters [6, 9–13]. Therefore, all patients should be assessed for PEA eligibility in an expert centre [6].
Not all patients with CTEPH are eligible for surgery. Patients with distal lesions are unlikely to be candidates for PEA [1, 14]. Based on international registry data, 36% of patients are considered ineligible for surgery [15]. Furthermore, ∼50% of patients will have persistent or recurrent pulmonary hypertension after surgery [9, 16] and may require further treatment. In patients with CTEPH ineligible for PEA, balloon pulmonary angioplasty (BPA) is a promising new experimental approach, but it is not yet clear which patients are best suited for this procedure; it should only be performed in experienced high-volume CTEPH centres [1].
Recently, options for the medical management of CTEPH have been widened by the application of drugs initially developed for the treatment of pulmonary arterial hypertension (PAH) and the advent of riociguat, the only targeted medical therapy currently licensed for the treatment of CTEPH.
This review discusses current medical therapies for CTEPH and emerging treatment options.
Supportive medical therapy
Optimal medical treatment for CTEPH consists of anticoagulants, plus diuretics and oxygen in cases of heart failure or hypoxaemia [1]. The aim of anticoagulation in CTEPH is to prevent in situ pulmonary artery thrombosis and recurrent venous thromboembolism [14]. Treatment should be continued throughout the patient's life, even after PEA [1]. Although no randomised controlled trials of anticoagulants have been conducted in patients with CTEPH, experience with vitamin K antagonists indicates that risk of recurrent pulmonary embolism or venous thromboembolism is low. Major limitations of vitamin K antagonists include their narrow therapeutic window, their interactions with food and other drugs and the need for repeated blood testing [17]. Today, many patients are receiving new oral anticoagulants, but to date there have been no clinical trials of these agents in patients with CTEPH.
PAH therapies
Rationale for use of PAH therapies in CTEPH
Drugs that target key pathways involved in the pathology of PAH are now established in the treatment of this condition [18, 19]. The similarities in pathological features between the two conditions provide a rationale for evaluating PAH therapies in patients with CTEPH [14].
Endothelin-1 is a powerful vasoconstrictor and smooth muscle mitogen synthesised and secreted by vascular endothelial cells. Levels of endothelin-1 are elevated in PAH and CTEPH [20–24], and recent evidence suggests a potential role for endothelin-1 in smooth muscle cell proliferation within the chronic clot in CTEPH, as well as in the distal arteriopathy [25]. Endothelin receptor antagonists (ERAs) act by blocking the type A endothelin receptor selectively, or both the type A and type B endothelin receptors nonselectively, preventing the vasoconstrictive and proliferative actions of endothelin-1 [18, 19, 26].
Prostacyclin, a highly potent vasodilator that can inhibit platelet aggregation and smooth muscle cell proliferation, is produced by endothelial cells [27]. Levels of prostacyclin are reduced in PAH, and thus prostanoid medications have been used [18, 19, 27].
Nitric oxide (NO), an endogenous vasodilator produced by the endothelium, inhibits platelet aggregation and growth of smooth muscle cells [28, 29]. NO activates soluble guanylate cyclase (sGC) to synthesise cyclic guanosine monophosphate (cGMP), a secondary signalling molecule that ultimately leads to decreased intracellular calcium and smooth muscle relaxation. Levels of endogenous NO synthase and NO metabolites are diminished in patients with PAH [28, 29], and thus the pathway is a key target for treatment. Inhibitors of phosphodiesterase (PDE)-5 prevent degradation of cGMP, while stimulators of sGC, such as riociguat, augment cGMP synthesis [18, 19]. There are no studies of prostacyclin or NO pathways specifically in CTEPH and the absence of an animal model greatly hampers research into the physiology of this condition. The rationale for the use of prostacyclin analogues, PDE-5 inhibitors and riociguat in CTEPH depends on its pathophysiological resemblance to PAH.
Open-label studies
Small, open-label trials of PAH therapies, including bosentan [30–32], treprostinil [33], epoprostenol [34–36] and sildenafil [37] have been conducted in patients with CTEPH. These trials are limited by their small patient cohorts, lack of randomisation and blinding, and, in many studies, by the absence of a control arm.
Randomised controlled trials
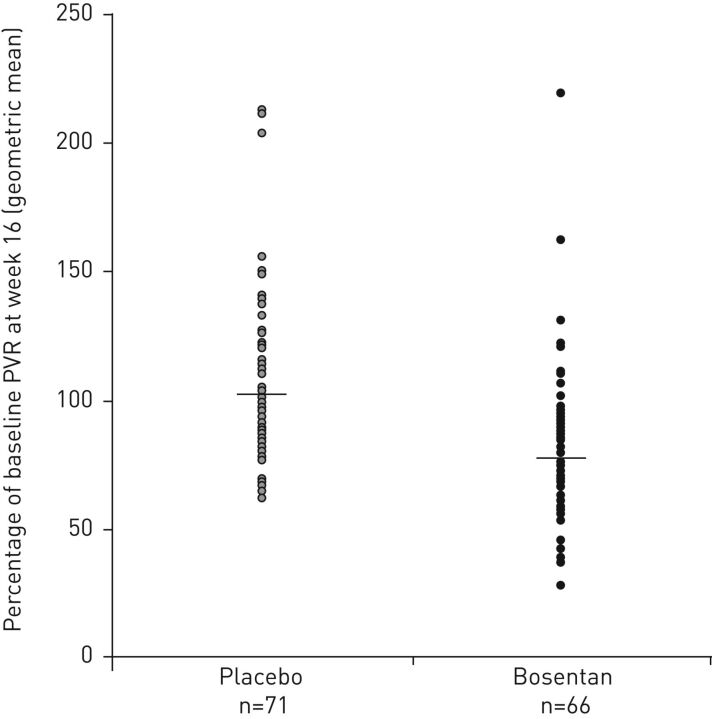
Efficacy data for randomised controlled trials of targeted therapy in patients with CTEPH [38–41] are summarised in table 1; safety results are in table 2. The efficacy and safety of the dual ERA bosentan were evaluated in the randomised, double-blind, placebo-controlled BENEFIT study [39]. Eligible patients (n=157) had symptomatic CTEPH (World Health Organization functional class (WHO FC) II–IV), 6-min walking distance (6MWD) <450 m, mPAP ≥25 mmHg, PCWP <15 mmHg and pulmonary vascular resistance (PVR) at rest ≥300 dyn·s·cm−5. Patients were randomised to placebo or bosentan at an initial dose of 62.5 mg twice daily for 4 weeks, subsequently increased to the target dose of 125 mg twice daily (patients weighing <40 kg were maintained at 62.5 mg twice daily). After 16 weeks of treatment, mean PVR (co-primary end-point) decreased from baseline in bosentan-treated patients and increased in the placebo arm (figure 1) [39]. There was no statistically significant effect of bosentan on 6MWD, the other co-primary end-point (bosentan +2.9 m, placebo +0.8 m; mean treatment effect +2.2 m, 95% CI −22.5–26.8 m). Statistically significant improvements for bosentan over placebo on haemodynamic parameters are presented in table 1. There was no statistically significant decrease in time to clinical worsening with bosentan versus placebo (hazard ratio 0.63, 95% CI 0.15–2.64), with few clinical worsening events in either treatment group (bosentan n=5 (6%), placebo n=3 (4%)). The most frequent adverse events in the bosentan arm were peripheral oedema, abnormal liver function test, headache, right ventricular failure, nasopharyngitis, vertigo and palpitations (table 2). Two patients (3%) in the bosentan group and four (5%) in the placebo group withdrew as a result of adverse events, and one death occurred in each treatment arm (both judged to be unrelated to study treatment).
TABLE 1.
Efficacy data for randomised controlled trials of targeted therapy in patients with chronic thromboembolic pulmonary hypertension
| BENEFIT [39] | Sildenafil study [41] | CHEST-1 [38] | CHEST-2 [40] | |||||
| Bosentan | Placebo | Sildenafil | Placebo | Riociguat | Placebo | Prior riociguat | Prior placebo | |
| Patients n | 77 | 80 | 9 | 10 | 173 | 88 | 155 | 82 |
| Study design | Multicentre, randomised, double-blind, placebo-controlled | Randomised, double-blind, placebo-controlled | Multicentre, randomised, double-blind, placebo-controlled | Long-term, open-label extension | ||||
| Primary end-point | Change in PVR and 6MWD after 16 weeks | Change in 6MWD after 12 weeks | Change in 6MWD after 16 weeks | None | ||||
| 6MWD m | +3 (−13–19) | +1 (−18–20) | +18±34# | +0±49# | +39±79*** | −6±84 | +591±589 | +37±69 |
| PVR dyn·s·cm−5 | −146+ (−207–−85) | +30 (−25–85) | −179 (245)* | +18 (76) | −226±248*** | +23±274 | ||
| mPAP mmHg | −6±7# | +0±6# | −4±7*** | +1±7 | ||||
| TPR dyn·s·cm−5 | + | |||||||
| PAOP mmHg | +0±3# | −0±3# | ||||||
| mRAP mmHg | −0±5# | −1±6# | −1±5 | −1±5 | ||||
| PCWP mmHg | +1±4 | +0±4 | ||||||
| mPa mmHg | −9±12*** | −0±12 | ||||||
| Cardiac output L·min−1 | +1±1*** | −0±1 | ||||||
| Cardiac index L·min−1·m−2 | *** | −0±1# | −0±0# | |||||
| SvO2 % | ||||||||
| SaO2 % | −2±4 | −3±8 | ||||||
| PaO2 mmHg | −3±15 | −5±12 | ||||||
| Heart rate beats·min−1 | +2±12 | +1±12 | ||||||
| NT-proBNP pg·mL−1 | −200**,¶ | +400¶ | −355±648# | −77±130# | −291±1717*** | +76±1447 | −375±1182 | −505±1591 |
| WHO FC % | ||||||||
| Improved | 14 | 11 | 44 | 0 | 33 | 15 | 50 | 39 |
| No change | 83 | 80 | 56 | 80 | 62 | 78 | 45 | 59 |
| Worsened | 3 | 9 | 0 | 20 | 5 | 7 | 4 | 2 |
| Clinical worsening events % | 4 | 6 | 2 | 6 | 16 | |||
| Borg dyspnoea score | −0 (−1–0)* | +0 (−0–1) | −1±1# | +0±2# | −1±2** | +0±2 | −1±2 | −1±2 |
| EQ-5D score | +0.06±0.28 | −0.08±0.34 | +0.12±0.29 | +0.01±0.30 | ||||
| LPH score | −7±19 | −2±19 | ||||||
| CAMPHOR score | ||||||||
| Symptoms | −2±5# | −0±3# | ||||||
| Activity | −3±2# | −1±3# | ||||||
| Quality of life | −2±7# | −0±4# | ||||||
Data are presented as mean±sd or mean (95% CI), unless otherwise stated. Data represent change from baseline at 16 weeks (CHEST-1 and BENEFIT), 12 weeks (sildenafil study) or 1 year (CHEST-2). 6MWD: 6-min walking distance; PVR: pulmonary vascular resistance; mPAP: mean pulmonary arterial pressure; TPR: total pulmonary resistance; PAOP: pulmonary artery occlusion pressure; mRAP: mean right atrial pressure; PCWP: pulmonary capillary wedge pressure; mPa: mean arterial pressure; SvO2: mixed venous oxygen saturation; SaO2: arterial oxygen saturation; PaO2: arterial oxygen tension; NT-proBNP: N-terminal of the pro-brain natriuretic peptide; WHO FC: World Health Organization functional class; EQ-5D: EuroQoL 5-dimensions quality-of-life questionnaire; LPH: Living with Pulmonary Hypertension questionnaire; CAMPHOR: Cambridge Pulmonary Hypertension Outcome Review. #: mean±se; ¶: estimated from graph. *: p<0.05; **: p<0.01; ***: p<0.001; +: p<0.0001 versus placebo.
TABLE 2.
Adverse-event data for randomised controlled trials of targeted therapy in patients with chronic thromboembolic pulmonary hypertension
| BENEFIT [39] | Sildenafil study [41] | CHEST-1 [38] | CHEST-2 [40] | |||||
| Bosentan | Placebo | Sildenafil | Placebo | Riociguat | Placebo | Prior riociguat | Prior placebo | |
| Patients n | 77 | 80 | 9 | 10 | 173 | 88 | 155 | 82 |
| All adverse events # | ||||||||
| Headache | 7 | 1 | 22 | 10 | 25 | 14 | ||
| Dizziness/vertigo | 5.2 | 1.3 | 23 | 12 | 19 | 20 | ||
| Dyspepsia | <5 | <5 | 33 | 10 | 18 | 8 | ||
| Peripheral oedema | 13 | 8 | 16 | 20 | 15 | 23 | ||
| Nasopharyngitis | 5 | 3 | 15 | 9 | 24 | 22 | ||
| Alanine aminotransferase increase¶ | 14 | 4 | ||||||
| Diarrhoea | 10 | 5 | 14 | 15 | ||||
| Cough | 5 | 18 | 13 | 15 | ||||
| Dyspnoea | 5 | 14 | 12 | 11 | ||||
| Upper respiratory tract infection | 6 | 5 | 12 | 10 | ||||
| Serious adverse events+ | ||||||||
| Right ventricular failure | 3 | 4 | 0 | 0 | 3 | 3 | ||
| Syncope | 0 | 0 | 2 | 3 | ||||
| Haemoptysis | 0 | 0 | 2 | 0 | 4 (2) | |||
| Worsening pulmonary hypertension | 3 | 1 | 0 | 0 | ||||
| Urticarial rash | 11 | 0 | ||||||
Data are presented as % of patients (rounded to nearest whole number unless otherwise stated) or n (%). #: adverse events occurring in ≥10% of patients in the active treatment arm in any study; ¶: three times the upper limit of normal; +: “most frequent serious adverse events”, as presented in the respective publications.
FIGURE 1.
Percentage of baseline pulmonary vascular resistance (PVR) at week 16 in patients receiving bosentan or placebo in the BENEFIT study [39]. Reproduced and modified from [39] with permission.
The ERA macitentan (10 mg·day−1) is currently under investigation in the randomised, double-blind, placebo-controlled MERIT-1 study, which plans to enrol 78 patients with surgically inoperable CTEPH and is expected to report in the near future (ClinicalTrials.gov NCT02021292). The primary outcome is PVR at week 16. The results from this trial are expected soon. The study will be followed by an open-label extension, MERIT-2, which concentrates on safety and is expected to complete in 2018 (ClinicalTrials.gov NCT02060721). The ERA ambrisentan (5 mg·day−1) was to have been investigated in patients with inoperable CTEPH in the AMBER-I study (ClinicalTrials.gov NCT01884675) and its open-label follow-up, AMBER-II (ClinicalTrials.gov NCT01894022), but only 33 out of 160 planned patients were enrolled (ambrisentan n=17, placebo n=16). The available data from AMBER-I have been published on the ClinicalTrials.gov website, showing a median change in 6MWD at 16 weeks (primary end-point) of +25 m in the ambrisentan arm versus −10 m in the placebo arm (outcome 1). Median change from baseline in PVR was −130 dyn·s·cm−5 with ambrisentan and −103 dyn·s·cm−5 with placebo (outcome 2).
Sildenafil, a PDE-5 inhibitor, was evaluated in a randomised, placebo-controlled pilot study in 19 patients with inoperable or persistent CTEPH (WHO FC II/III) and a 6MWD of 100–450 m [41]. Patients were randomised to sildenafil 40 mg three times daily or placebo for 12 weeks, at which time all placebo patients were switched to sildenafil, with a repeat assessment after 1 year. At 12 weeks the change from baseline in 6MWD (primary end-point) did not differ significantly between the sildenafil and placebo arms, but an improvement in PVR was seen (table 1). At 1 year (n=17), there were significant improvements from baseline in the sildenafil arm for 6MWD (+36 m, 95% CI 8–64 m; p=0.014) and PVR (−149 dyn·s·cm−5, 95% CI −228–−71 dyn·s·cm−5; p<0.001). It should be noted that the overall severity of CTEPH at baseline was lower in the placebo arm than in the sildenafil arm.
The Aerosolized Iloprost Randomized Study included 203 patients with severe pulmonary hypertension, including 57 with CTEPH, randomised to receive inhaled iloprost or placebo [42]. The study reported an overall positive treatment effect, but the subgroup analyses described results for primary pulmonary hypertension and a “nonprimary” population that included CTEPH, collagen vascular disease and use of appetite suppressants. The implications of this trial for CTEPH are unclear, because the results for CTEPH patients alone were not reported.
Real-world data
Single centres and registries have described the management of CTEPH: examples are summarised in table 3 [10, 43–48]. The patients reported may be postoperative, technically inoperable, those who refuse surgery and those with operable disease but who are not suitable for PEA because of comorbid conditions. Drug use, effectiveness and outcomes can be expected to vary between these groups. The national centre for PEA surgery in the UK [44], an international registry [10, 11] and several national pulmonary hypertension registries [43, 45–48] indicate that targeted medical therapy is used in both nonoperated and operated patients, but more frequently in the former. ERAs (particularly bosentan) are the most frequently used targeted therapy, the exception being in Latvia, where 90% of patients receive sildenafil [48]. Most patients receiving targeted therapy are treated with a single agent, particularly when treatment is initiated, although some receive combination therapy during the course of the disease [10, 43, 45–47]. The coadministration of riociguat with PDE-5 inhibitors is contraindicated, because excessive reductions of systemic blood pressure can occur with this combination [49]. Published outcomes data from registries are limited, but the most recent results show that 1-year survival with medical treatment for nonoperated patients with CTEPH is ≥88% (table 3). Recent data from the international CTEPH registry show that nonoperated patients who received targeted therapy had more severe disease at baseline, in terms of a shorter time from symptoms to diagnosis, a higher frequency of WHO FC III/IV, shorter 6MWD and a more severe haemodynamic profile (higher mPAP and PVR; lower cardiac output and PCWP) [10]. Survival is consistently worse among nonoperated than operated patients. Interpretation of this finding should consider differences in baseline characteristics and the diverse reasons for a patient being considered inoperable (e.g. comorbidity or distal disease).
TABLE 3.
Published registry data for pulmonary arterial hypertension therapies in patients with chronic thromboembolic pulmonary hypertension (CTEPH)
| Country (year) [ref.] | Functional class | Therapies | Main outcomes | |
| UK (2008)# [44] | WHO II–IV |
Surgical n=321¶ Targeted therapy 65 |
Nonsurgical n=148¶ Targeted therapy 90 ERA 56 PDE-5 inhibitor 33 Prostanoid 11 |
Survival at 1 and 3 years+: 88% and 76%, respectively, for surgical patients; 82% and 70%, respectively for nonsurgical patients (p=0.023) |
| International (2016) [10] | NYHA I–IV |
Operated n=404 Overall 29 ERA 13 PDE-5 inhibitor 15 Prostanoid 1 |
Nonoperated n=275 Overall 61 ERA§ 24 PDE-5 inhibitor 17 ERA + PDE-5 inhibitor 18 Prostanoid 2 |
Estimated survival at 1, 2 and 3 years
|
| Latvia (2016) [48] | NYHA II–IV |
Overall n=31 Sildenafil 90 Ambrisentan 7 Bosentan 1 |
Cross-sectional analysis only | |
| Portugal (2013) [43] | WHO II–IV |
Overall n=33 Any 67 Monotherapy 36 Two drugs 15 Three drugs 7 Clinical trial 3 ERA 52 Sildenafil 39 Prostanoid 6 |
|
|
| Spain (2016) [45] | WHO I–IV |
PEA n=122 Any 43 |
Non-PEA n=269 Any 82 ERA 38 PDE-5 inhibitor 31 ERA/PDE-5 inhibitor 8 Prostanoid (oral) 3 Prostanoid (i.v.) 1 Prostanoid (inhaled) <1 |
|
| Switzerland (2008) [46] | NYHA II–IV |
Baseline n=70 Any 31 Iloprost (inhaled) 22 Bosentan 5 Bosentan/sildenafil 1 Iloprost (i.v.) 1 Last visit n=46 Any 81 Iloprost (inhaled) 24 Bosentan 21 Sildenafil 7 Bosentan/iloprost 6 Sildenafil/iloprost 11 Bosentan/sildenafil 6 ≥3 drugs 2 |
|
|
| Switzerland (2015) [47] | NYHA 3.0±0.7 |
Overall (n=100)ƒ Started within 3 months Any 74 Monotherapy 64 Two drugs 9 Three drugs 0 ERA 63 PDE-5 inhibitor 20 Prostanoid 0 Maximal therapy Any 82 Monotherapy 49 Two drugs 30 Three drugs 2 |
|
|
Data are presented as % or mean±sd, unless otherwise stated. WHO: World Health Organization; ERA: endothelin receptor antagonist; PDE: phosphodiesterase; New York Heart Association; PEA: pulmonary endarterectomy; 6MWD: 6-min walking distance; mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance. #: study from UK national PEA surgery centre; ¶: data from 2003 onward; +: data from 2001 onward; §: mostly bosentan, with some sitaxsentan; ƒ: data for 2009–2012.
CTEPH-targeted medical therapy
The CHEST-1 study
The efficacy and safety of the dual-action sGC stimulator riociguat were assessed in CHEST-1, a phase III, multicentre, randomised, double-blind, placebo-controlled study [38]. Patients (n=261) had inoperable CTEPH, as assessed prospectively by an adjudication committee of experienced PEA surgeons, or persistent/recurrent pulmonary hypertension after PEA, with a 6MWD of 150–450 m, PVR >300 dyn·s·cm−5 and mPAP ≥25 mmHg. Eligible patients were randomised to receive placebo or riociguat at an initial dose of 1 mg three times daily. In the first 8 weeks of the study, the dose was adjusted according to systolic systemic arterial pressure and signs/symptoms of hypotension to a final individualised dose of up to 2.5 mg three times daily. Patients then received their individualised dose for a further 8 weeks. At week 16, 77% of patients still participating in the study were taking the maximal riociguat dose of 2.5 mg three times daily. During the study, the dose of the study drug was decreased in 18 patients (10%) in the riociguat group compared with three (3%) in the placebo group. At week 16, 6MWD (the primary end-point) had increased from baseline by a mean of 39 m in riociguat-treated patients compared with a 6 m decrease in the placebo arm (least-squares mean difference 46 m, 95% CI 25–67 m; p<0.001). In addition, significantly more patients experienced improvement/stabilisation of their WHO FC in the riociguat arm (33%/62%, respectively) compared with the placebo arm (15%/78%, respectively; p=0.003), although there was no statistically significant difference in the incidence of clinical worsening events between the two arms (riociguat 2%, placebo 6%). Significant benefits for riociguat versus placebo on key efficacy parameters are shown in table 1. The most frequently occurring adverse events in the riociguat arm were headache, dizziness, dyspepsia, peripheral oedema and nasopharyngitis (table 2). The most frequently occurring serious adverse events were right ventricular failure, syncope and haemoptysis (table 2). Deaths related to adverse events occurred in two patients (1%) in the riociguat group (heart failure n=1 and acute renal failure n=1) and in three patients (3%) in the placebo group (respiratory insufficiency n=1, circulatory arrest n=1 and cardiac arrest n=1). With 89% of patients receiving the higher doses of 2.5 and 2.0 mg three times daily at week 16, dose-dependent efficacy and safety have not been analysed.
Based on the results of the CHEST-1 trial, riociguat was licensed in Europe for the treatment of adult patients with WHO FC II/III and inoperable CTEPH or persistent/recurrent pulmonary hypertension after surgical treatment [49]. The adjudication committee excluded 164 (37%) out of the 446 patients screened for CHEST-1 because they were considered “operable” in terms of disease distribution. This observation illustrates the importance of thorough evaluation of CTEPH patients and the use of pharmacological therapy only in patients not eligible for PEA.
Riociguat responder analysis
For patients with PAH, several parameters that indicate response to treatment have been identified, and threshold values for these parameters which correlate with survival have been defined. These “responder thresholds” form part of treatment goals for patients with PAH [1, 50, 51]. These values include 6MWD ≥380 m, WHO FC I/II, cardiac index ≥2.5 L·min−1·m−2, mixed venous oxygen saturation (SvO2) ≥65% and N-terminal pro-brain natriuretic peptide (NT-proBNP) <1800 pg·mL−1.
An exploratory analysis from CHEST-1 examined the proportion of patients who achieved the responder thresholds described earlier at baseline and at the end of the study [52]. In addition, the analysis examined the proportions of patients achieving PVR <500 dyn·s·cm−5 because values above this level are strongly correlated with increased mortality in patients with CTEPH [53]. Riociguat increased the proportion of patients with 6MWD ≥380 m, WHO FC I/II and PVR <500 dyn·s·cm−5 from 37%, 34% and 25%, respectively, at baseline to 58%, 57% and 50%, respectively, at week 16, whereas there was little change in placebo-treated patients. Similar changes were observed for thresholds for cardiac index, SvO2, NT-proBNP level and right atrial pressure. Additionally, riociguat was associated with an increase in the proportion of patients meeting the combination of response criteria for 6MWD, WHO FC, cardiac index, SvO2 and NT-proBNP level. These results suggest that riociguat increased the proportion of patients achieving criteria defining a positive response to therapy.
Riociguat long-term data
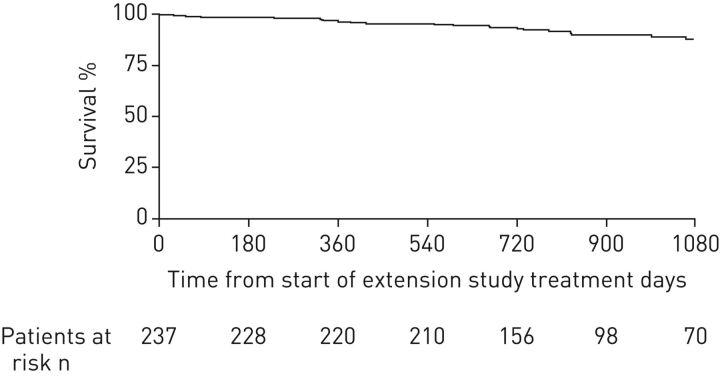
Patients who completed CHEST-1 were eligible to enter a long-term open-label extension study (CHEST-2) [40, 54]. Overall, 237 patients (98%) entered CHEST-2 and received riociguat (patients who received placebo in CHEST-1 were initiated at a dose of 1 mg three times daily and adjusted to an individual dose as in the CHEST-1 study). The primary objective of CHEST-2 was to evaluate the long-term safety of riociguat (mean treatment duration was 83 weeks, median 75 weeks). Of 157 patients treated for 1 year in CHEST-2, 12 (8%) were receiving additional PAH therapies (ERAs n=8, prostanoids n=4). The most common adverse events in the extension study were nasopharyngitis (23%), dizziness (19%), peripheral oedema (18%) and diarrhoea (14%). For adverse events of special interest, hypotension was reported in 6% of patients and syncope in 7%. Overall, exposure-adjusted rates of adverse events were lower in CHEST-2 than in CHEST-1. The most common drug-related serious adverse events were syncope (2%) and hypotension (1%), which resolved in all cases. Exploratory efficacy analyses showed that the increase in 6MWD seen in patients originally randomised to riociguat was maintained, and 6MWD also increased in patients originally randomised to placebo, to an extent comparable to the former group. The estimated rate of clinical worsening-free survival at 1 year was 88% (95% CI 83–92%), with an estimated overall survival rate at 1 year of 97% (95% CI 93–98%). 2-year data from CHEST-2 have recently been published. The mean±sd change from baseline in 6MWD was +52±66 m at 1 year (n=209) and +50±68 m at 2 years (n=162) [54]. The estimated survival rate was 93% (95% CI 89–96%) at 2 years (figure 2) [54], with no new safety signals detected with the additional duration of treatment.
FIGURE 2.
Kaplan–Meier analysis of survival over 2 years of treatment with riociguat in the CHEST-2 study [54]. Overall survival was 97% (95% CI 93–98%) at 1 year and 93% (95% CI 89–96%) at 2 years. If the worst-case scenario was assumed, in which patients who dropped out were assumed to have died, survival was 92% (95% CI 88–95%) at 1 year and 87% (95% CI 82–91%) at 2 years. Reproduced and modified from [54] with permission.
Other potential uses of medical therapy in CTEPH
Bridging therapy
One potential use of medical therapy in CTEPH is as a bridge to PEA [14, 55]. Registry data show that a substantial proportion of operable patients receive such therapy (table 3), but no randomised trials have been performed in this patient population. The first report of bridging therapy was from 12 patients with severe CTEPH (PVR >1200 dyn·s·cm−5) who received continuous i.v. prostacyclin before undergoing PEA [35]. Treatment was associated with a 28% decrease in mean PVR (from 1510 to 1088 dyn·s·cm−5; p<0.001) and a marked decrease in plasma BNP level (from 547 to 188 pg·mL−1; p<0.01). Operative mortality was 8%. In a retrospective analysis of nine patients who received continuous i.v. epoprostenol before PEA, six patients experienced either clinical stability or improvement, with a mean reduction in PVR of 28%, and three experienced clinical deterioration [56]. In contrast, in a study of inhaled iloprost before PEA, there were no significant changes in mPAP, cardiac index or PVR; the authors concluded that bridging therapy may have detrimental effects on systemic haemodynamics [57].
In a single-blind randomised study, 25 patients with CTEPH were randomised to standard of care with or without bosentan for 16 weeks before surgery [58]. After 16 weeks, there were statistically significant benefits in favour of bosentan in total pulmonary resistance (–299 dyn·s·cm−5; p=0.004), 6MWD (+33 m; p=0.014) and mPAP (−11 mmHg; p=0.005). Postoperative mortality was four (31%) out of 13 patients who received bosentan versus three (25%) out of 12 patients who did not.
A retrospective analysis compared 111 patients with CTEPH who received targeted therapy, including bosentan, sildenafil, epoprostenol and combination therapy, before undergoing PEA with 244 patients who did not receive targeted therapy [59]. Patients receiving medical therapy had little or no improvement in haemodynamic or post surgical outcomes, and referral of operable patients for PEA was delayed compared with patients who did not receive medical therapy. In the international CTEPH registry there were no differences in PEA complications between operated patients who received bridging therapy compared with those who did not, but multivariable analysis indicated that bridging therapy was associated with increased mortality (hazard ratio 2.62, 95% CI 1.30–5.28; p=0.0072) [10].
At present there is no evidence to recommend bridging therapy before PEA, and all eligible patients should proceed to surgery without delay.
Operable patients not undergoing surgery
Medical treatment may be appropriate for patients who are technically operable in terms of disease distribution, but for whom surgery is refused or is considered to be too high risk because of comorbid conditions. Their risk–benefit ratio may be unacceptable because of an excessive surgical risk or because the anticipated benefit is small, as in patients with mild symptoms and good functional capacity: the effects of medical therapy may differ greatly between these populations. There are no robust data in this population, and treatment is based on anecdotal evidence and consensus. The 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines state that “Medical treatment of CTEPH with targeted therapy may be justified […] in the presence of an unacceptable surgical risk–benefit ratio” [1]. Other authors affirm that all operable patients should proceed to PEA without delay [55].
Patients receiving BPA
Studies, mainly undertaken in Japan, have described use of targeted medical therapy for “bridging” prior to BPA or in patients whose haemodynamic parameters did not normalise after BPA [60–68]. The implications for the use of targeted PAH therapies are difficult to assess in patients undergoing a series of BPA procedures. These studies were uncontrolled, and in European practice, patients in the Japanese cohorts would have been referred for PEA assessment first rather than medical therapy or BPA.
Summary and future directions
The development of further medical therapies for CTEPH depends on improving our understanding of the pathological mechanisms of this disease, including the pathways involved in progression of an acute thrombus into an obstructive fibrous mass, and how the additional distal arterial vasculopathy develops. The lack of an animal model for CTEPH is a barrier to research in this area.
The 2015 ESC/ERS guidelines for the treatment of pulmonary hypertension state that targeted medical therapy may be justified in technically nonoperable patients or those with persistent or recurrent pulmonary hypertension after PEA [1], but the optimal use and benefits of most therapies are unclear, and randomised controlled trials and/or consensus are needed. Riociguat is currently the only targeted medical therapy licensed for the treatment of adults with inoperable or persistent/recurrent CTEPH and recommended by ESC/ERS guidelines [1, 49].
There has been no “head to head” clinical trial to compare efficacy of the PAH therapies either in PAH or CTEPH. With this limitation, the short-term haemodynamic effects of riociguat, bosentan and sildenafil in CTEPH are similar (table 1), but their effects on 6MWD are different [38, 39, 41]. In contrast, in PAH the three classes have similar effects on haemodynamic parameters and exercise capacity [69–71]. The poor correlation between haemodynamic and functional outcomes highlights the need to improve study design and end-points in CTEPH. A recent study reported that patients with inoperable CTEPH (which might be considered “more PAH-like”) experienced significant improvements in peak oxygen uptake and gas exchange after treatment with PAH drugs, but there were no such effects in patients with operable CTEPH (which might be considered “less PAH-like”) [72]. The authors concluded that drug effects on exercise function in inoperable CTEPH cannot be translated to all forms of the condition. In addition, further studies are required to confirm the role of targeted medical therapies before and after BPA and to characterise the patient populations suitable for the two options. The RACE study (ClinicalTrials.gov NCT02634203), in which patients with CTEPH who are not eligible for PEA will be randomised to riociguat or BPA, may throw light on this question.
With the excellent results now achieved in expert centres, PEA remains the treatment of choice for CTEPH, and all patients should be managed at an expert centre in which the assessment of operability should be made. Riociguat, PAH-targeted therapy or BPA should only be offered to patients ineligible for PEA or with persistent/recurrent CTEPH after surgery [73, 74]. Future developments in targeted medical therapy may demand more refined definitions of CTEPH (e.g. distribution of disease, operability or eligibility for medical therapy) so that treatments can be individualised to provide the most appropriate treatment and the best possible outcome.
Disclosures
H-A. Ghofrani ERR-0107-2016_Ghofrani (1.2MB, pdf)
M.M. Hoeper ERR-0107-2016_Hoeper (1.2MB, pdf)
J. Pepke-Zaba ERR-0107-2016_Pepke-Zaba (1.2MB, pdf)
Acknowledgements
Editorial assistance was provided by Adelphi Communications Ltd (Bollington, UK), supported by Bayer AG (Berlin, Germany).
Footnotes
Conflict of interest: Disclosures can be found alongside this article at err.ersjournals.com
Provenance: Publication of this peer-reviewed article was sponsored by Bayer AG, Berlin, Germany (principal sponsor, European Respiratory Review issue 143).
References
- 1.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 2.Gopalan D, Delcroix M, Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok FA, Mos IC, van Kralingen KW, et al. Chronic pulmonary embolism and pulmonary hypertension. Semin Respir Crit Care Med 2012; 33: 199–204. [DOI] [PubMed] [Google Scholar]
- 4.Lang I. Chronic thromboembolic pulmonary hypertension: a distinct disease entity. Eur Respir Rev 2015; 24: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014; 130: 508–518. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins D, Madani M, Fadel E, et al. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madani MM, Jamieson SW. Technical advances of pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Semin Thorac Cardiovasc Surg 2006; 18: 243–249. [DOI] [PubMed] [Google Scholar]
- 8.Mayer E. Surgical and post-operative treatment of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2010; 19: 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom National Cohort. Circulation 2016; 133: 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation 2016; 133: 859–871. [DOI] [PubMed] [Google Scholar]
- 11.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 12.Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012; 94: 97–103. [DOI] [PubMed] [Google Scholar]
- 13.Taboada D, Pepke-Zaba J, Jenkins DP, et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J 2014; 44: 1635–1645. [DOI] [PubMed] [Google Scholar]
- 14.Pepke-Zaba J, Jansa P, Kim NH, et al. Chronic thromboembolic pulmonary hypertension: role of medical therapy. Eur Respir J 2013; 41: 985–990. [DOI] [PubMed] [Google Scholar]
- 15.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. [DOI] [PubMed] [Google Scholar]
- 16.Hoeper MM. Pharmacological therapy for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2015; 24: 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roca B, Roca M. The new oral anticoagulants: reasonable alternatives to warfarin. Cleve Clin J Med 2015; 82: 847–854. [DOI] [PubMed] [Google Scholar]
- 18.O'Callaghan DS, Savale L, Montani D, et al. Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol 2011; 8: 526–538. [DOI] [PubMed] [Google Scholar]
- 19.Rosenblum WD. Pulmonary arterial hypertension: pathobiology, diagnosis, treatment, and emerging therapies. Cardiol Rev 2010; 18: 58–63. [DOI] [PubMed] [Google Scholar]
- 20.Bauer M, Wilkens H, Langer F, et al. Selective upregulation of endothelin B receptor gene expression in severe pulmonary hypertension. Circulation 2002; 105: 1034–1036. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Yung GL, Marsh JJ, et al. Endothelin mediates pulmonary vascular remodelling in a canine model of chronic embolic pulmonary hypertension. Eur Respir J 2000; 15: 640–648. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Yung GL, Marsh JJ, et al. Pulmonary vascular remodeling distal to pulmonary artery ligation is accompanied by upregulation of endothelin receptors and nitric oxide synthase. Exp Lung Res 2000; 26: 287–301. [DOI] [PubMed] [Google Scholar]
- 23.Langer F, Bauer M, Tscholl D, et al. Circulating big endothelin-1: an active role in pulmonary thromboendarterectomy? J Thorac Cardiovasc Surg 2005; 130: 1342–1347. [DOI] [PubMed] [Google Scholar]
- 24.Reesink HJ, Meijer RC, Lutter R, et al. Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ J 2006; 70: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 25.Southwood M, MacKenzie Ross RV, Kuc RE, et al. Endothelin ETa receptors predominate in chronic thromboembolic pulmonary hypertension. Life Sci 2016; 159: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madonna R, Cocco N, De Caterina R. Pathways and drugs in pulmonary arterial hypertension – focus on the role of endothelin receptor antagonists. Cardiovasc Drugs Ther 2015; 29: 469–479. [DOI] [PubMed] [Google Scholar]
- 27.Lang IM, Gaine SP. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respir Rev 2015; 24: 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klinger JR, Abman SH, Gladwin MT. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188: 639–646. [DOI] [PubMed] [Google Scholar]
- 29.Tonelli AR, Haserodt S, Aytekin M, et al. Nitric oxide deficiency in pulmonary hypertension: pathobiology and implications for therapy. Pulm Circ 2013; 3: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoeper MM, Kramm T, Wilkens H, et al. Bosentan therapy for inoperable chronic thromboembolic pulmonary hypertension. Chest 2005; 128: 2363–2367. [DOI] [PubMed] [Google Scholar]
- 31.Seyfarth HJ, Hammerschmidt S, Pankau H, et al. Long-term bosentan in chronic thromboembolic pulmonary hypertension. Respiration 2007; 74: 287–292. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich S, Speich R, Domenighetti G, et al. Bosentan therapy for chronic thromboembolic pulmonary hypertension. A national open label study assessing the effect of bosentan on haemodynamics, exercise capacity, quality of life, safety and tolerability in patients with chronic thromboembolic pulmonary hypertension (BOCTEPH-Study). Swiss Med Wkly 2007; 137: 573–580. [DOI] [PubMed] [Google Scholar]
- 33.Lang I, Gomez-Sanchez M, Kneussl M, et al. Efficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertension. Chest 2006; 129: 1636–1643. [DOI] [PubMed] [Google Scholar]
- 34.Cabrol S, Souza R, Jais X, et al. Intravenous epoprostenol in inoperable chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2007; 26: 357–362. [DOI] [PubMed] [Google Scholar]
- 35.Nagaya N, Sasaki N, Ando M, et al. Prostacyclin therapy before pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Chest 2003; 123: 338–343. [DOI] [PubMed] [Google Scholar]
- 36.Scelsi L, Ghio S, Campana C, et al. Epoprostenol in chronic thromboembolic pulmonary hypertension with distal lesions. Ital Heart J 2004; 5: 618–623. [PubMed] [Google Scholar]
- 37.Reichenberger F, Voswinckel R, Enke B, et al. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur Respir J 2007; 30: 922–927. [DOI] [PubMed] [Google Scholar]
- 38.Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 39.Jaïs X, D'Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol 2008; 52: 2127–2134. [DOI] [PubMed] [Google Scholar]
- 40.Simonneau G, D'Armini AM, Ghofrani HA, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J 2015; 45: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 41.Suntharalingam J, Treacy CM, Doughty NJ, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 2008; 134: 229–236. [DOI] [PubMed] [Google Scholar]
- 42.Olschewski H, Simonneau G, Galiè N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322–329. [DOI] [PubMed] [Google Scholar]
- 43.Baptista R, Meireles J, Agapito A, et al. Pulmonary hypertension in Portugal: first data from a nationwide registry. Biomed Res Int 2013; 2013: 489574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Condliffe R, Kiely DG, Gibbs JS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2008; 177: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 45.Escribano-Subías P, Del Pozo R, Román-Broto A, et al. Management and outcomes in chronic thromboembolic pulmonary hypertension: from expert centers to a nationwide perspective. Int J Cardiol 2016; 203: 938–944. [DOI] [PubMed] [Google Scholar]
- 46.Fischler M, Speich R, Dorschner L, et al. Pulmonary hypertension in Switzerland: treatment and clinical course. Swiss Med Wkly 2008; 138: 371–378. [DOI] [PubMed] [Google Scholar]
- 47.Mueller-Mottet S, Stricker H, Domenighetti G, et al. Long-term data from the Swiss pulmonary hypertension registry. Respiration 2015; 89: 127–140. [DOI] [PubMed] [Google Scholar]
- 48.Skride A, Sablinskis K, Rudzitis A, et al. First data from Latvian chronic thromboembolic pulmonary hypertension registry. Eur J Intern Med 2016; 32: e23–e24. [DOI] [PubMed] [Google Scholar]
- 49.Bayer AG. Adempas©: EU Summary of Product Characteristics. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034.pdf Date last accessed: February 28 2017. Date last updated: February 24, 2017.
- 50.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013; 62: Suppl. 25, D73–D81. [DOI] [PubMed] [Google Scholar]
- 51.Nickel N, Golpon H, Greer M, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2012; 39: 589–596. [DOI] [PubMed] [Google Scholar]
- 52.D'Armini AM, Ghofrani HA, Kim NH, et al. Use of responder threshold criteria to evaluate the response to treatment in the phase III CHEST-1 study. J Heart Lung Transplant 2015; 34: 348–355. [DOI] [PubMed] [Google Scholar]
- 53.Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg 2003; 76: 1457–1462. [DOI] [PubMed] [Google Scholar]
- 54.Simonneau G, D'Armini AM, Ghofrani HA, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 372–380. [DOI] [PubMed] [Google Scholar]
- 55.Kim NH, Mayer E. Chronic thromboembolic pulmonary hypertension: the evolving treatment landscape. Eur Respir Rev 2015; 24: 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bresser P, Fedullo PF, Auger WR, et al. Continuous intravenous epoprostenol for chronic thromboembolic pulmonary hypertension. Eur Respir J 2004; 23: 595–600. [DOI] [PubMed] [Google Scholar]
- 57.Kramm T, Eberle B, Krummenauer F, et al. Inhaled iloprost in patients with chronic thromboembolic pulmonary hypertension: effects before and after pulmonary thromboendarterectomy. Ann Thorac Surg 2003; 76: 711–718. [DOI] [PubMed] [Google Scholar]
- 58.Reesink HJ, Surie S, Kloek JJ, et al. Bosentan as a bridge to pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2010; 139: 85–91. [DOI] [PubMed] [Google Scholar]
- 59.Jensen KW, Kerr KM, Fedullo PF, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation 2009; 120: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 60.Fukui S, Ogo T, Goto Y, et al. Exercise intolerance and ventilatory inefficiency improve early after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol 2015; 180: 66–68. [DOI] [PubMed] [Google Scholar]
- 61.Inami T, Kataoka M, Shimura N, et al. Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: a breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv 2014; 7: 1297–1306. [DOI] [PubMed] [Google Scholar]
- 62.Inami T, Kataoka M, Ando M, et al. A new era of therapeutic strategies for chronic thromboembolic pulmonary hypertension by two different interventional therapies; pulmonary endarterectomy and percutaneous transluminal pulmonary angioplasty. PLoS One 2014; 9: e94587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–762. [DOI] [PubMed] [Google Scholar]
- 64.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. [DOI] [PubMed] [Google Scholar]
- 65.Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012; 76: 485–488. [DOI] [PubMed] [Google Scholar]
- 66.Tatebe S, Sugimura K, Aoki T, et al. Multiple beneficial effects of balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ J 2016; 80: 980–988. [DOI] [PubMed] [Google Scholar]
- 67.Velázquez Martín M, Albarrán González-Trevilla A, Alonso Charterina S, et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Preliminary experience in Spain in a series of 7 patients. Rev Esp Cardiol 2015; 68: 535–537. [DOI] [PubMed] [Google Scholar]
- 68.Yanagisawa R, Kataoka M, Inami T, et al. Safety and efficacy of percutaneous transluminal pulmonary angioplasty in elderly patients. Int J Cardiol 2014; 175: 285–289. [DOI] [PubMed] [Google Scholar]
- 69.Galiè N, Ghofrani AH. New horizons in pulmonary arterial hypertension therapies. Eur Respir Rev 2013; 22: 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 71.Rubin LJ, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension: a long-term extension study (PATENT-2). Eur Respir J 2015; 45: 1303–1313. [DOI] [PubMed] [Google Scholar]
- 72.Charalampopoulos A, Gibbs JS, Davies RJ, et al. Exercise physiological responses to drug treatments in chronic thromboembolic pulmonary hypertension. J Appl Physiol 2016; 121: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoeper MM. Chronic thromboembolic pulmonary hypertension at the crossroad. Eur Respir J 2014; 43: 1230–1232. [DOI] [PubMed] [Google Scholar]
- 74.Hoeper MM. Residual pulmonary hypertension after pulmonary endarterectomy: the fog is clearing. Circulation 2016; 133: 1731–1733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H-A. Ghofrani ERR-0107-2016_Ghofrani (1.2MB, pdf)
M.M. Hoeper ERR-0107-2016_Hoeper (1.2MB, pdf)
J. Pepke-Zaba ERR-0107-2016_Pepke-Zaba (1.2MB, pdf)